Abstract
Although bats are important reservoirs of diverse viruses that can cause human epidemics, little is known about the presence of picornaviruses in these flying mammals. Among 1,108 bats of 18 species studied, three novel picornaviruses (groups 1, 2, and 3) were identified from alimentary specimens of 12 bats from five species and four genera. Two complete genomes, each from the three picornaviruses, were sequenced. Phylogenetic analysis showed that they fell into three distinct clusters in the Picornaviridae family, with low homologies to known picornaviruses, especially in leader and 2A proteins. Moreover, group 1 and 2 viruses are more closely related to each other than to group 3 viruses, which exhibit genome features distinct from those of the former two virus groups. In particular, the group 3 virus genome contains the shortest leader protein within Picornaviridae, a putative type I internal ribosome entry site (IRES) in the 5′-untranslated region instead of the type IV IRES found in group 1 and 2 viruses, one instead of two GXCG motifs in 2A, an L→V substitution in the DDLXQ motif in 2C helicase, and a conserved GXH motif in 3C protease. Group 1 and 2 viruses are unique among picornaviruses in having AMH instead of the GXH motif in 3Cpro. These findings suggest that the three picornaviruses belong to two novel genera in the Picornaviridae family. This report describes the discovery and complete genome analysis of three picornaviruses in bats, and their presence in diverse bat genera/species suggests the ability to cross the species barrier.
INTRODUCTION
Picornaviruses are positive-sense single-stranded RNA viruses found in humans and a wide variety of animals, in which they can cause respiratory, cardiac, hepatic, neurological, mucocutaneous, and systemic diseases of various levels of severity (28, 46). Based on genotypic and serological characterization, picornaviruses currently are divided into 12 genera, namely, Enterovirus, Cardiovirus, Aphthovirus, Hepatovirus, Parechovirus, Erbovirus, Kobuvirus, Teschovirus, Sapelovirus, Senecavirus, Tremovirus, and Avihepatovirus. In the last few years, there has been a surge in the number of novel picornaviruses discovered and genomes sequenced (5, 20, 23, 24, 29, 34). In 2007, we performed complete genome analysis on a novel human rhinovirus species, human rhinovirus C, which belongs to the genus Enterovirus (28, 29). Recently, we also described the discovery and comparative genome analysis of three novel picornaviruses, proposed species “turdivirus 1, 2, and 3,” in wild dead birds in the Hong Kong Special Administrative Region (HKSAR) (52).
Bats possess unique characteristics that render this group of mammals both major natural reservoirs and dissemination vehicles for viruses (2). Before the severe acute respiratory virus (SARS) epidemic, bats were not known to be hosts of coronaviruses (CoV). In 2005, we and others reported the discovery of SARS-CoV-like viruses in horseshoe bats in HKSAR and other provinces of China (32, 36). In the subsequent few years, we have further described a huge diversity of previously undescribed bat coronaviruses, including two novel subgroups in Betacoronavirus (49, 51). Recently, we also have described the identification of novel paramyxoviruses in bats from China, supporting the presence of many previously unknown viruses in these flying mammals (31). Since only short sequences of picornaviruses have been reported recently in bats (35), we hypothesized that previously unrecognized picornaviruses are present in bats. To test this hypothesis, we carried out a territory-wide molecular epidemiology study of bats for picornaviruses. In this article, we report the discovery of three novel picornaviruses (groups 1, 2, and 3) in bats of diverse genera and species. Genome sequencing and comparative analysis showed that the three viruses fell into three distinct clusters in the Picornaviridae family. Based on their phylogenetic analysis and distinct genome features, the three viruses may belong to two novel genera in the Picornaviridae family.
MATERIALS AND METHODS
Bat surveillance and sample collection.
A total of 1,108 bats, of 18 species, were captured from 20 different locations in rural areas of HKSAR, including water tunnels, abandoned mines, sea caves, and forested areas, during two 1-year periods (January to December 2005 and January to December 2008) (Table 1; also see supplemental Table 1 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html). Their respiratory and alimentary specimens were collected using procedures described previously (32, 56).
Table 1.
Bat species captured and novel picornaviruses discovered in the present study
| Scientific name | Common name | No. of bats tested | No. (%) of bats positive for picornavirus(es) | Group (no.) of picornavirus(es) (n) detecteda | Sampling locations for the novel picornavirus(es) (n)a |
|---|---|---|---|---|---|
| Cynopterus sphinx | Greater short-nosed fruit bat | 19 | 0 (0) | ||
| Hipposideros armiger | Great round-leaf bat | 68 | 1 (1.5) | 3 | C |
| Hipposideros pomona | Pomona round-leaf bat | 86 | 0 (0) | ||
| Miniopterus pusillus | Lesser bent-winged bat | 78 | 2 (2.6) | 1 (1), 2 (1) | D |
| Miniopterus schreibersii | Greater bent-winged bat | 222 | 7 (3.2) | 1 (1), 2 (6) | Group 1, B; group 2, A (4) and B (2) |
| Myotis chinensis | Large mouse-eared bat | 46 | 0 (0) | ||
| Myotis horsfieldii | Horsfield's bat | 4 | 0 (0) | ||
| Myotis muricola | Nepalese whiskered myotis | 2 | 0 (0) | ||
| Myotis ricketti | Rickett's big-footed bat | 23 | 0 (0) | ||
| Nyctalus noctula | Common noctule | 27 | 0 (0) | ||
| Pipistrellus abramus | Japanese pipistrelle | 61 | 1 (1.6) | 2 | A |
| Pipistrellus tenuis | Least pipistrelle | 2 | 0 (0) | ||
| Rhinolophus affinis | Intermediate horseshoe bat | 80 | 0 (0) | ||
| Rousettus leschenaulti | Leschenault's rousette | 28 | 0 (0) | ||
| Rhinolophus sinicus | Chinese horseshoe bat | 309 | 1 (0.3) | 3 | C |
| Scotophilus kuhlii | Lesser asiatic yellow bat | 5 | 0 (0) | ||
| Tylonycteris pachypus | Lesser bamboo bat | 47 | 0 (0) | ||
| Tylonycteris robustula | Greater bamboo bat | 1 | 0 (0) |
n, number of bats positive for the indicated virus.
RNA extraction.
Viral RNA was extracted from the respiratory and alimentary specimens using a viral RNA minikit (QIAgen, Hilden, Germany). The RNA was eluted in 50 μl of RNase-free water and was used as the template for reverse transcription-PCR (RT-PCR).
RT-PCR of 3Dpol gene of picornaviruses using conserved primers and DNA sequencing.
Initial picornavirus screening was performed by amplifying a 571-bp fragment of the 3Dpol gene of picornaviruses using conserved primers (5′-CYTATHTRAARGATGAGCTKAGA-3′ and 5′-GCAATNACRTCATCKCCRTA-3′), which were designed by multiple alignment of the nucleotide sequences of the 3Dpol genes of various picornavirus species using previously described protocols (52, 55). Reverse transcription was performed using the SuperScript III kit (Invitrogen, San Diego, CA), and the reaction mixture (10 μl) contained RNA, first-strand buffer (50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2), 5 mM dithiothreitol (DTT), 50 ng random hexamers, 500 μM each deoxynucleoside triphosphate (dNTP), and 100 U SuperScript III reverse transcriptase. The mixtures were incubated at 25°C for 5 min, followed by 50°C for 60 min and 70°C for 15 min. The PCR mixture (25 μl) contained cDNA, PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, 2 mM MgCl2, and 0.01% gelatin), 200 μM each dNTP, and 1.0 U Taq polymerase (Applied Biosystems, Foster City, CA). The mixtures were amplified with 40 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min, with a final extension at 72°C for 10 min in an automated thermal cycler (Applied Biosystems, Foster City, CA). Standard precautions were taken to avoid PCR contamination, and no false-positive result was observed in negative controls.
All PCR products were gel purified using the QIAquick gel extraction kit (QIAgen, Hilden, Germany). Both strands of the PCR products were sequenced twice with an ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA) using the two PCR primers. The sequences of the PCR products were compared with known sequences of the 3Dpol genes of picornaviruses in the GenBank database.
RT-PCR of 2C genes of novel bat picornaviruses using conserved primers and DNA sequencing.
As the initial RT-PCR of the 3Dpol gene revealed potential novel picornaviruses in four alimentary tract specimens, all 1,108 alimentary tract specimens were rescreened using two sets of primers (designed by the multiple alignment of the nucleotide sequences obtained from the four strains during genome sequencing) by amplifying a 144-bp (5′-AACCCTCAAARGATTAYCTYAAA-3′ and 5′-CCAGTTCTAAGATCTTTCAAA-3′) and a 182-bp (5′-CATGAGGTKSAKAMVGAYTAT-3′ and 5′-TTGACACCAYYTCATCDAGTG-3′) fragment of the 2C gene of the novel picornaviruses. The components of the PCR mixtures and the cycling conditions were the same as those described above. The purification of the PCR products and DNA sequencing were performed as described above using the corresponding PCR primers. The sequences of the PCR products were compared to known sequences of the 2C genes of picornaviruses in the GenBank database.
Viral culture.
Two samples from each group of the three novel bat picornaviruses were cultured in LLC-Mk2 (rhesus monkey kidney; ATCC CCL-7), MRC-5 (human lung fibroblast; a gift from Wilina Lim, ViroMed Laboratories, Centre for Health Protection, Hong Kong), FRhK-4 (rhesus monkey kidney; ATCC CRL-1688), RD (human rhabdomyosarcoma; ATCC CCL-136), and Vero E6 (African green monkey kidney; ATCC CRL-1586) cells.
Genome sequencing.
Two genomes from each group of the three novel bat picornaviruses were amplified and sequenced using strategies we previously used for the complete genome sequencing of other picornaviruses and coronaviruses, with the RNA extracted from the alimentary specimens as templates (29, 32, 50, 52). The RNA was converted to cDNA by a combined random priming and oligo(dT) priming strategy. As initial results showed that the three novel bat picornaviruses are more closely related to porcine sapelovirus, simian sapelovirus, and avian sapelovirus, the cDNA was amplified by degenerate primers designed by the multiple alignment of the genomes of these three viruses (GenBank accession no. NC_003987, NC_004451, and NC_006553) and additional primers designed from the results of the first and subsequent rounds of sequencing. These primer sequences are shown in supplemental Table 1 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html. The 5′ ends of the viral genomes were confirmed by the rapid amplification of cDNA ends (RACE) using the 5′/3′ RACE kit (ClonTech). Sequences were assembled and manually edited to produce final sequences of the viral genomes.
Genome analysis.
The nucleotide sequences of the genomes and the deduced amino acid sequences of the open reading frame were compared to those of other picornaviruses. The unrooted phylogenetic trees of 2C and 3Dpol fragments were constructed using the neighbor-joining method for aligned nucleotide sequences in ClustalX1.83. The maximum-likelihood phylogenetic trees of P1, P2 (excluding 2A), and P3 (excluding 3A) were constructed using the TREEFINDER program (19), with bootstrap values calculated from 1,000 trees. Secondary-structure prediction in the 5′-untranslated region (UTR) was performed using Mfold (57).
Nucleotide sequence accession numbers.
The nucleotide sequences of the genomes of novel bat picornavirus groups 1, 2, and 3 have been deposited in the GenBank sequence database under accession numbers HQ595340 to HQ595345.
RESULTS
Bat surveillance and identification of three novel picornaviruses.
A total of 2,205 respiratory and alimentary specimens from 1,108 bats of 18 different species were obtained from various locations in HKSAR (Table 1 and supplemental Table 1 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html). The initial screening on 1,097 respiratory and 1,108 alimentary specimens by RT-PCR for a 571-bp fragment in the 3Dpol genes of picornaviruses was positive in four alimentary specimens from four bats of three different species. The sequences from these four positive samples had <70% nucleotide identities to the corresponding parts of the 3Dpol genes in all other known picornaviruses (Fig. 1A).
Fig. 1.
(A) Phylogenetic analysis of nucleotide sequences of 3Dpol of picornaviruses identified from bats in the present study. The four strains identified in the present study are shaded gray. (B) Phylogenetic analysis of nucleotide sequences of the 113-bp fragment of the 2C gene of picornaviruses identified from bats in the present study. The 12 novel picornavirus strains identified in the present study are shown in boldface. The six strains with genomes completely sequenced are shaded gray. The trees were constructed by the neighbor-joining method, and bootstrap values were calculated from 1,000 trees. The scale bar indicates the estimated number of substitutions per 20 nucleotides. AEV, avian encephalomyelitis virus (NC_003990); AiV, Aichi virus (NC_001918); BkV, bovine kobuvirus (NC_004421); DHV1, duck hepatitis virus 1 (NC_008250); DHVAP, duck hepatitis virus AP (NC_009750); DPV, avian sapelovirus (NC_006553); EMCV, encephalomyocarditis virus (NC_001479); ERAV, equine rhinitis A virus (NC_003982); ERBV, equine rhinitis B virus 1 (NC_003983); ERV3, equine rhinovirus 3 (NC_003077); FMDV-C, foot-and-mouth disease virus type C (NC_002554); HAV, hepatitis A virus (NC_001489); HEVC, human enterovirus C (NC_002058); HRV, human rhinovirus (FJ445142); HPeV, human parechovirus (NC_001897); LV, Ljungan virus (NC_003976); PEVA, porcine sapelovirus (NC_003987); PTV, porcine teschovirus (NC_003985); SePV1, seal picornavirus type 1 (NC_009891); SVV, Seneca Valley virus (NC_011349); SpV1, simian sapelovirus (NC_004451); ThV, theilovirus (NC_001366); TV1, turdivirus 1 (NC_014411); TV2, turdivirus 2 (NC_014412); TV3, turdivirus 3 (NC_014413).
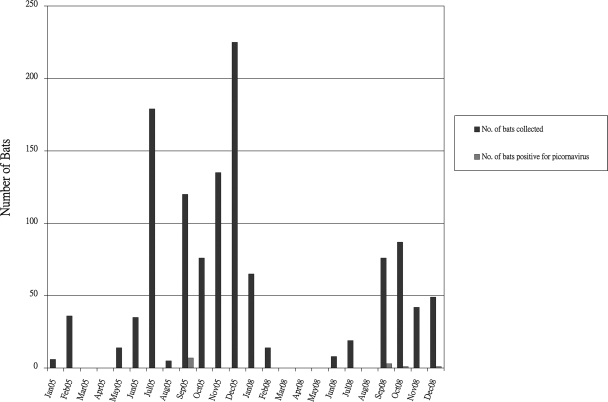
The rescreening of the 1,108 alimentary specimens by specific RT-PCR for the 2C gene of the novel bat picornaviruses was positive in a total of 12 alimentary specimens (including the previous four positive specimens) from 12 bats of five species, including Miniopterus pusillus (two bats), Miniopterus schreibersii (seven bats), Hipposideros armiger (one bat), Pipistrellus abramus (one bat), and Rhinolophus sinicus (one bat) (Table 1). The sequences from the 12 samples had <60% nucleotide identities to the corresponding parts of the 2C genes in all other known picornaviruses (Fig. 1B). Moreover, these 12 sequences fell into three distinct clusters, suggesting the presence of three novel picornaviruses (groups 1, 2, and 3) (Table 1 and Fig. 1B). All three novel bat picornaviruses were detected during autumn and early winter (September to December) in both years (Fig. 2). However, as very few specimens were collected in spring to protect the bats for reproduction after hibernation, the prevalence during spring cannot be ascertained.
Fig. 2.
Prevalence of novel bat picornaviruses (blue bars) among all bats (orange bars) collected in different seasons.
Viral culture.
No cytopathic effect was observed in any of the cell lines inoculated with the samples that were positive for novel bat picornavirus group 1, 2, or 3 by RT-PCR. Quantitative RT-PCR using the culture supernatants and cell lysates for monitoring the presence of viral replication also showed negative results.
Genome organization and coding potential of the novel bat picornaviruses.
The sizes of the genomes of bat picornavirus groups 1, 2, and 3 are 7,625 to 7,737 bases (strains LMH22A and NC16A), 7,609 to 7,677 bases (strains SK17F and MH9F), and 7,659 to 7,731 bases (strains TLC21F and TLC5F), respectively, after excluding the polyadenylated tract, and their G+C contents are 45, 43, and 50%, respectively (Table 2). The genome sizes of some strains may be larger, as the further sequencing of the ends may have been hampered by secondary structures. Their genome organizations are similar to those of other picornaviruses, with the characteristic gene order 5′-L, VP4, VP2, VP3, VP1, 2A, 2B, 2C, 3A, 3B, 3Cpro, 3Dpol-3′ (Fig. 3). Both 5′ (305 to 410, 331 to 419, and 424 to 496 bases) and 3′ (171 to 175, 139 to 174, and 218 bases) ends of the genomes contain untranslated regions (UTR). Downstream of the leader protein (L), each genome contains a large open reading frame of 7,149 to 7,152, 7,104 to 7,119, and 7,017 bases, respectively, which encodes potential polyprotein precursors of 2,382 to 2,383, 2,367 to 2,372, and 2,338 amino acids, respectively. The hypothetical protease cleavage sites of the polyproteins, as determined by multiple alignments with other picornaviruses with complete genomes available, are shown in supplemental Table 2 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html.
Table 2.
Genomic features of group 1, 2, and 3 bat picornaviruses and representatives of other generaa
| Picornavirus |
Genome features |
Pairwise amino acid identity (%) for virus group: |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | Name | Accession no. | Size (bp) | G+C content | 1 (strain NC16A) |
2 (strain MH9F) |
3 (strain TLC5F) |
||||||||||||
| P1 | P2a | P3b | 3Cpro | 3Dpol | P1 | P2b | P3c | 3Cpro | 3Dpol | P1 | P2b | P3c | 3Cpro | 3Dpol | |||||
| Aphthovirus | Foot-and-mouth disease virus C | NC_002554 | 8115 | 0.54 | 24.3 | 28.7 | 27.9 | 20.4 | 33.1 | 23.2 | 29.9 | 27.6 | 21.6 | 32.9 | 24.8 | 27.7 | 29.1 | 24.2 | 32.7 |
| Avihepatovirus | Duck hepatitis virus 1 | NC_008250 | 7687 | 0.43 | 18.1 | 24.7 | 26.3 | 23.4 | 27.9 | 19.3 | 22.4 | 26.7 | 22.4 | 28.5 | 18.9 | 22.9 | 26.7 | 21.5 | 27.3 |
| Cardiovirus | Encephalomyocarditis virus | NC_001479 | 7835 | 0.49 | 28.6 | 26.9 | 27.3 | 19.2 | 30.7 | 28.3 | 27.1 | 27.1 | 21.1 | 29.2 | 27.5 | 24.8 | 27.5 | 18.5 | 30.8 |
| Enterovirus | Human enterovirus C | NC_002058 | 7440 | 0.46 | 34.5 | 36.1 | 49.5 | 38.0 | 54.6 | 35.8 | 37.2 | 50.1 | 38.6 | 54.6 | 34.2 | 36.1 | 52.4 | 39.1 | 58.3 |
| Erbovirus | Equine rhinovirus 3 | NC_003077 | 8821 | 0.50 | 24.6 | 24.7 | 28.8 | 20.4 | 33.1 | 24.9 | 22.1 | 29.2 | 19.9 | 34.0 | 26.7 | 22.7 | 29.0 | 22.8 | 32.2 |
| Hepatovirus | Hepatitis A virus | NC_001489 | 7478 | 0.38 | 19.3 | 24.4 | 25.8 | 20.7 | 26.8 | 20.1 | 22.5 | 26.0 | 23.1 | 26.9 | 19.2 | 24.7 | 26.9 | 21.6 | 28.5 |
| Kobuvirus | Aichi virus | NC_001918 | 8251 | 0.59 | 20.5 | 26.1 | 30.8 | 22.0 | 33.3 | 22.2 | 26.7 | 30.6 | 21.6 | 33.3 | 20.2 | 25.6 | 32.3 | 27.3 | 34.6 |
| Orthoturdivirus (proposed) | Turdivirus 1 (00356) (proposed) | GU182406 | 8019 | 0.58 | 22.9 | 25.6 | 30.3 | 18.8 | 33.7 | 21.9 | 26.2 | 30.3 | 20.1 | 34.7 | 21.8 | 24.9 | 31.7 | 25.2 | 36.7 |
| Paraturdivirus (proposed) | Turdivirus 2 (10717) (proposed) | GU182408 | 7625 | 0.47 | 22.9 | 24.7 | 31.2 | 20.5 | 35.1 | 21.4 | 24.3 | 30.3 | 21.7 | 33.6 | 22.7 | 25.1 | 30.1 | 21.4 | 33.5 |
| Parechovirus | Human parechovirus | NC_001897 | 7348 | 0.39 | 18.6 | 23.7 | 26.3 | 16.9 | 26.2 | 20.7 | 23.1 | 27.2 | 19.6 | 29.3 | 15.5 | 25.7 | 25.9 | 17.5 | 26.3 |
| Senecavirus | Seneca valley virus | NC_011349 | 7310 | 0.51 | 24.4 | 27.4 | 27.5 | 20.1 | 29.9 | 24.7 | 28.6 | 27.1 | 19.8 | 29.9 | 26.6 | 28.2 | 27.5 | 22.3 | 29.8 |
| Teschovirus | Porcine teschovirus 1 | NC_003985 | 7117 | 0.45 | 25.2 | 26.3 | 31.3 | 22.8 | 34.9 | 23.2 | 28.0 | 30.3 | 21.2 | 34.1 | 23.5 | 27.1 | 31.7 | 22.1 | 36.5 |
| Tremovirus | Avian encephalomyelitis virus | NC_003990 | 7055 | 0.45 | 19.5 | 26.7 | 27.6 | 19.3 | 29.6 | 19.5 | 27.2 | 28.2 | 20.2 | 31.3 | 20.4 | 26.4 | 28.6 | 23.0 | 31.8 |
| Sapelovirus | Avian sapelovirus (duck picornavirus) | NC_006553 | 8289 | 0.43 | 40.1 | 41.9 | 53.9 | 41.8 | 58.5 | 38.7 | 42.5 | 54.7 | 41.7 | 59.4 | 40.1 | 39.1 | 55.1 | 44.4 | 58.8 |
| Sapelovirus | Porcine sapelovirus (porcine enterovirus A) | NC_003987 | 7491 | 0.41 | 42.8 | 38.8 | 54.7 | 43.2 | 58.4 | 39.3 | 39.4 | 54.7 | 42.6 | 59.3 | 44.3 | 41.5 | 58.0 | 48.1 | 62.0 |
| Sapelovirus | Simian sapelovirus (simian picornavirus 1) | NC_004451 | 8126 | 0.40 | 41.5 | 46.1 | 60.8 | 51.6 | 64.6 | 39.0 | 45.2 | 59.1 | 51.6 | 62.3 | 43.7 | 49.2 | 62.2 | 51.6 | 66.5 |
| Unclassified | Unclassified (group 1 virus strain NC16A) | HQ595340 | 7737 | 0.45 | 62.7 | 72.2 | 86.2 | 92.3 | 84.2 | 53.8 | 57.4 | 65.7 | 62.3 | 68.1 | |||||
| Unclassified | Unclassified (group 2 virus strain MH9F) | HQ595342 | 7677 | 0.43 | 62.7 | 72.2 | 86.2 | 92.3 | 84.2 | 51.9 | 54.5 | 66.3 | 62.3 | 68.3 | |||||
| Unclassified | Unclassified (group 3 virus strain TLC5F) | HQ595344 | 7731 | 0.50 | 53.8 | 57.4 | 65.7 | 62.3 | 68.1 | 51.9 | 54.5 | 66.3 | 62.3 | 68.3 | |||||
Shown is a comparison of genomic features of group 1, 2, and 3 bat picornaviruses, representative species of other genera, and amino acid identities between the predicted P1, P2 (excluding 2A), P3 (excluding 3A), 3Cpro, and 3Dpol proteins of bat picornavirus groups 1, 2, and 3 and the corresponding proteins of representative species of other picornavirus genera.
P2 region excluding 2A.
P3 region excluding 3A.
Fig. 3.
Genome organizations of group 1, 2, and 3 bat picornaviruses.
Phylogenetic analyses.
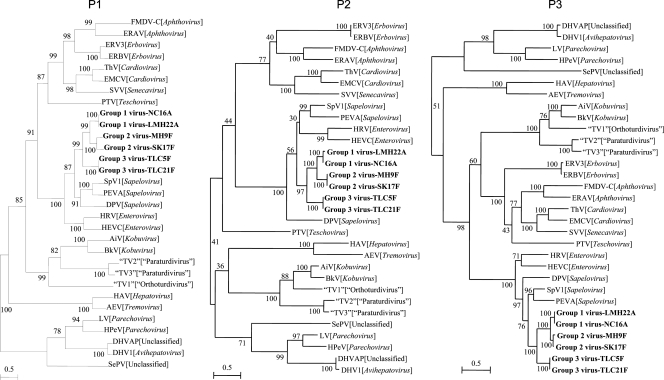
The phylogenetic trees constructed using the amino acid sequences of P1, P2 (excluding 2A), and P3 (excluding 3A) of bat picornavirus groups 1, 2, and 3 and other picornaviruses are shown in Fig. 4, and the corresponding pairwise amino acid identities are shown in Table 2. For all three regions, the three novel bat picornaviruses possess higher amino acid identities to the homologous genes in sapeloviruses than to those of other picornaviruses (Table 2). While in the P1 and P3 (excluding 3A) trees all strains of the three novel bat picornaviruses also were most closely related to the sapeloviruses, this phenomenon was not as evident in the P2 (excluding 2A) tree (Fig. 4). Group 1 and 2 viruses clustered together with high bootstrap support of ≥99 in all three trees, and they clustered with group 3 viruses with high bootstrap support of ≥97 in all three trees (Fig. 4).
Fig. 4.
Phylogenetic analyses of the P1, P2 (excluding 2A), and P3 (excluding 3A) regions of bat picornavirus groups 1, 2, and 3. Bootstrap values expressed as percentages are shown at the nodes, and the scale reflects the estimated number of substitutions per two amino acids along the branches. The novel bat picornaviruses are in boldface.
Genome analyses.
The 5′UTR of bat picornavirus group 1 (strain NC16A) and group 2 (strain MH9F) are 410 and 419 nucleotides (nt) in length, respectively, and share more than 80% nucleotide identity. They possess an IRES structure similar to that of type IV (hepatitis C virus-like) IRES with stem-loop domains II and III (Fig. 5). The putative translation initiation site of group 1 virus is contained in an optimal Kozak context (RNNAUGG), but that of group 2 virus has one nucleotide substitution, from G to U after AUG (AAGAUGU). Upstream of the putative translation initiation site of group 2 virus, another in-frame AUG presents at position 321, which is surrounded by an optimal Kozak context. Based on the sequence alignment between the 5′UTR of group 1 and 2 viruses and type IV IRES sequences, we believe that AUG at position 420 is the true initiation site (Fig. 5). The 5′UTR of group 1 and 2 viruses also contains a stretch of 17 nucleotides (UACUGCCUGAUAGGGUC) identical to that of porcine sapelovirus (formerly porcine enterovirus A [PEVA]). Within this stretch of 17 nucleotides are the most conserved domain, IIIe, and the four upstream nucleotides of the type IV IRES (16). No obvious pyrimidine tract can be found near the translation initiation site.
Fig. 5.
(A and B) Predicted type IV IRES structures of bat picornavirus groups 1 (A) and 2 (B). Domain numbers and pseudoknot stem elements were labeled according to Hellen et al. (16). The AUG start codon is in boldface. (C) Predicted type I IRES structure of bat picornavirus group 3. The pyrimidine tract and AUG in motif Y8-X19-AUG are in boldface and are underlined. The AUG start codon is in boldface.
The 5′UTR of group 3 virus (strain TLC5F) is 496 nt in length. The putative translation initiation site of this virus also has one nucleotide substitution, from G to C after AUG (AAGAUGC), which is different from the optimal Kozak context (RNNAUGG) (25). The 5′UTR of group 3 virus has a fragment (from position 251 to 403) with high nucleotide identity (79%) to the corresponding region of poliovirus (PV), implicating that this virus has an IRES structure to similar that of PV (Fig. 5), which has a type I IRES with six stem-loops (38). In PV, the Yn-Xm-AUG motif is separated by several nucleotides from the authentic initiation codon AUG, which is characteristic of type I IRES in picornaviruses. The predicted IRES structure of group 3 virus has a structure similar to that of PV with stem-loop domains III to VI. However, the cloverleaf structure of enterovirus type I IRES was not found, which may be due to incomplete 5′UTR sequences. The pyrimidine tract (UUUCUUUU) of the Y8-X19-AUG motif is located between stem-loop domains V and VI from position 396 to 403 (Fig. 5). The distance between the Y8-X19-AUG motif and the putative translation initiation site is 71 nucleotides.
Similarly to the aphthoviruses, cardioviruses, erboviruses, kobuviruses, sapeloviruses, senecavirus, and teschoviruses, the L protein is present in each of the polyproteins of the three bat picornaviruses (see supplemental Table 3 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html). However, their L proteins exhibit very low (≤21%) amino acid identities to those of other known picornaviruses. While the L proteins of group 1 and 2 viruses share 49.3 to 58.1% amino acid identities with each other, they showed only 12.3 to 16.4% amino acid identities to that of group 3 virus. The lengths of L proteins of different picornaviruses were highly variable, ranging from 55 to 451 amino acids. The L proteins of group 1, 2, and 3 viruses were 73 to 74, 73, and 55 amino acids in length, respectively, with that of group 3 virus being the shortest within Picornaviridae. Unlike those of aphthoviruses, erboviruses, and avian sapelovirus, the L proteins of bat picornavirus groups 1, 2, and 3 do not possess the characteristic amino acid residues important for proteolytic activity. The catalytic dyad Cys and His, conserved in papain-like thiol protease, has been found in the L proteins of aphthoviruses and erboviruses, which are able to self-cleave C terminally (17). The L proteins of aphthoviruses also can induce the cleavage of eukaryotic initiation factors 4GI and 4GII (15, 17). Although the L proteins of bat picornavirus groups 1, 2, and 3 possess cysteine and histidine residues and that of group 3 virus possesses histidine but not cysteine residues, we cannot determine their proteolytic ability because of poor sequence alignment and lack of experimental support. The L proteins of bat picornavirus groups 1, 2, and 3 also do not possess the GXCG motif, which is located in the active site of chymotrypsin-like proteases, suggesting that they do not have chymotrypsin-like protease activity (47). Moreover, they do not contain the putative zinc finger motif [C-X-H-X-(5)-C-X-(2)-C], which is found in Theiler's murine encephalomyelitis virus (TMEV), Saffold virus, and human TMEV-like cardiovirus, or [C-X-H-X-(6)-C-X-(2)-C], which is found in encephalomyocarditis virus (EMCV) (4). The L protein of cardiovirus, which binds zinc and is phosphorylated during infection, plays a role in the regulation of viral genome translation (10). As for kobuvirus, teschovirus, and senecavirus, the functions of the L proteins of the three novel bat picornaviruses remain to be determined.
The P1 (capsid-coding) regions in the genomes of the three bat picornaviruses encode the capsid genes VP4, VP2, VP3, and VP1. Similarly to aphthoviruses, cardioviruses, enteroviruses, erboviruses, hepatoviruses, sapeloviruses, senecavirus, teschoviruses, and tremovirus, the VP0 of the three novel bat picornaviruses probably are cleaved into VP4 and VP2 based on sequence alignment (see supplemental Table 3 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html). Similarly to the enteroviruses, sapeloviruses, and senecavirus, the VP1 of the three novel bat picornaviruses possess the [PS]ALXAXETG motif (see supplemental Table 3).
The P2 regions in the genomes of the three bat picornaviruses encode nonstructural proteins 2A, 2B, and 2C. Interestingly, there are two potential cleavage sites at the junction of 2A and 2B in group 1 and 2 viruses (strain LMH22A, EEQ|GPAEEQ|G [group 1]; strain NC16A, FEQ|GPDAEEQ|G [group 1] and EEQ|GPDADEQ|G [group 2]) but only one at the junction of 2A and 2B in group 3 virus (PLAEEQ|G). The 2A protein of picornaviruses, similarly to the L protein, also has a highly variable region, with lengths ranging from 9 to 305 amino acids. The 2A proteins of the three novel bat picornaviruses (sizes ranging from 208 to 228 amino acids) exhibit very low (≤22%) amino acid identities to those of other picornaviruses. While the 2A proteins of group 1 and 2 viruses shared 62.4 to 65.9% amino acid identities, they only showed 27.3 to 29.5% amino acid identities to group 3 virus. Similarly to enteroviruses and sapeloviruses, 2A of the three novel bat picornaviruses possesses the characteristic chymotrypsin-like structures with cysteine-reactive catalytic sites (see supplemental Table 1 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html). A putative catalytic triad of His, Asp, and Cys identified in 2A proteinases of enteroviruses and rhinoviruses also is found in the 2A proteins of the three bat picornaviruses, except that His is replaced by Leu (18, 43). The GXCG motif conserved in chymotrypsin-like protease has been found in enterovirus and rhinovirus 2A proteins which can autocleave P1 polyprotein at its N terminus and mediate the cleavage of the p220 component of the cap-binding complex eIF-4γ, leading to the shutoff of host cellular protein synthesis (18, 27). The GXCG motif also has been identified in the 2A proteins of sapeloviruses, including simian and porcine sapeloviruses but not avian sapelovirus (47). The 2A proteins of bat picornavirus groups 1 and 2 contain two potential motifs, GHCG and GICG, while that of group 3 virus contains one motif, GLCG. In aphthovirus and cardiovirus, the conserved amino acids Asn-Pro-Gly (NPG) at the C terminus of 2A, together with a proline (P) at the N terminus of 2B, are required for cotranslational cleavage (43). This NPGP motif also is identified in the 2A proteins of erbovirus, teschovirus, avihepatovirus, senecavirus, and seal picornavirus (8, 23, 48, 53) but is absent from those of the three novel bat picornaviruses. The 2A proteins of aviheptovirus, kobuvirus, parechovirus, tremovirus, and the proposed genus “Orthoturdivirus” also contain the conserved H-box/NC motifs characteristic of a family of cellular proteins involved in cell proliferation control (18, 48, 52). In contrast, the 2A proteins of the three novel bat picornaviruses do not possess the H-box/NC motifs.
Similarly to all other picornaviruses, 2C of the three bat picornaviruses possesses the GXXGXGKS motif for NTP binding (13). Similarly to most picornaviruses, 2C of group 1 and 2 viruses possesses the DDLXQ motif for putative helicase activity (14). As for group 3 virus, the L in the DDLXQ motif of its 2C is replaced by valine (see supplemental Table 3 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html). This is similar to the case of a minority of picornaviruses, in which the L can be replaced by another nonpolar amino acid in some if not all strains, such as alanine in Ljungan virus (a parechovirus) (37), phenylalanine in duck hepatitis virus 1 (an avihepatovirus) (48), isoleucine in hepatitis A virus (a hepatovirus) (7), human enterovirus C (an enterovirus) (42), and Aichi virus (a kobuvirus) (54).
The P3 regions in the genomes of the three bat picornaviruses encode 3A, 3B (VPg, small genome-linked protein), 3Cpro (protease), and 3Dpol (RNA-dependent RNA polymerase). Similarly to enteroviruses, kobuviruses, and sapelovirus, 3Cpro of the three bat picornaviruses contain the catalytic triad of H-E-C, whereas other picornaviruses contain the catalytic triad of H-D-C (see supplemental Table 3 at http://147.8.74.24/supplementary_files/Bat_picornavirus_JV/index.html) (1). Similarly to other picornaviruses, they also contain the conserved GXCG motif (GQCG in all three bat picornaviruses), which is considered to form part of the active site of the protease (see supplemental Table 3) (12). Similarly to enterovirus, hepatovirus, sapelovirus, senecavirus, and tremovirus, they contain a motif (NFRDI for group 1 and 2 viruses and KYRDI for group 3 virus) which is similar to that of a conserved RNA-binding motif (KFRDI). As in other picornaviruses, 3Cpro of group 3 virus contains the conserved GXH motif, which forms part of the substrate binding pocket of the protease (see supplemental Table 3). On the other hand, the glycine in the GXH motif of 3Cpro in group 1 and 2 viruses is uniquely replaced by alanine. Similarly to other picornaviruses, 3Dpol of the three novel bat picornaviruses contains the conserved KDE[LI]R, GG[LMN]PSG, YGDD, and FLKR motifs (see supplemental Table 1) (22).
DISCUSSION
We report the discovery and describe the first complete genomes of three distinct novel picornaviruses from diverse bat species. Despite the increasing recognition of bats as natural reservoirs of many important zoonotic viruses, such as SARS-CoV, Nipah virus, Hendra virus, and Ebola virus, the role of bats in the evolution and biology of picornaviruses remains unknown. A recent study on bat guano virome from the United States revealed the presence of viral sequences belonging to diverse families, including Picornaviridae (35). However, the largest contig of 1,998 bp covered only about 70% of the P1 region, which displayed 46% amino acid similarity to the corresponding region of human Aichi virus within the genus Kobuvirus (35). Therefore, the sequence likely represents a virus different from the present three picornaviruses, although further molecular characterization is required to ascertain the taxonomy of the kobuvirus-related virus. Among the six bat picornavirus strains with complete genomes sequenced in this study, three distinct groups were observed in all three phylogenetic trees constructed using the P1, P2 (excluding 2A), and P3 (excluding 3A) regions (Fig. 4). In addition to their phylogenetic clustering, the three groups also displayed other distinct genome features. Within each group, the two strains were always clustered with a very high bootstrap support of 100. In addition, the two strains in each group possessed high amino acid identities to each other and identical genomic characteristics. Based on these analyses, we used the designations of group 1, 2, and 3 viruses to describe these three novel picornaviruses. Unlike bat coronaviruses, these bat picornaviruses do not exhibit much bat genus specificity. For bat coronaviruses, a particular coronavirus species in general infects bats of only one genus (51). In our recent study on the discovery of three novel bat paramyxoviruses, Tuhokovirus 1, 2, and 3, only the bat species Rousettus leschenaulti was found to harbor these viruses among the 11 studied species (31). On the other hand, from the results of the present study, it seems that a particular picornavirus species was able to infect bats of different genera. For example, group 2 viruses were found in lesser bent-winged bat (genus Miniopterus), common bent-winged bat (genus Miniopterus), and Japanese pipistrelle (genus Pipistrellus), and group 3 viruses were found in great round-leaf bat (genus Hipposideros) and Chinese horseshoe bat (genus Rhinolophus) (Table 1). In contrast, highly similar strains of the present bat picornaviruses were found in different bat genera/species (Fig. 4). This suggests that these bat picornaviruses can easily cross the species or even genus barrier among the bats, a characteristic that may allow the emergence of epidemics in new hosts. It would be interesting to know whether these bat picornaviruses use the same or different receptors for cell entry in bats of different genera and species.
Bat group 3 picornavirus may belong to a novel genus in the family Picornaviridae. In the present study, the L proteins of bat picornavirus groups 1, 2, and 3 possessed very low homology (5.1 to 20% amino acid identities) to those of sapeloviruses, with those of group 3 viruses being the shortest L protein among those of all picornaviruses. Moreover, unlike that of avian sapelovirus, their L proteins do not contain the GXCG motif important for chymotrypsin-like protease activity. Similarly, the 2A protein of bat picornavirus groups 1, 2, and 3 also had very low homology (2.6 to 22% amino acid identities) to 2A protein of sapeloviruses. Furthermore, the P1, P2 (excluding 2A), and P3 (excluding 3A) regions of bat picornavirus groups 1, 2, and 3 possessed only 38.7 to 40.1%, 39.1 to 42.5%, and 53.9 to 55.1% amino acid identities to the corresponding regions in the avian sapelovirus, respectively (Table 2). Therefore, the three novel bat picornaviruses may belong to a new genus separate from Sapelovirus (26, 39, 47). Group 3 viruses also possessed other genomic characteristics distinct from those of the sapeloviruses. For example, it had a type I IRES but the sapeloviruses had a type IV IRES, and it had a DDVGQ motif but the sapeloviruses had a DDLGQ motif in the helicase.
It is arguable that bat picornavirus groups 1 and 2 belong to a separate genus from group 3 viruses. The P1, P2 (excluding 2A), and P3 (excluding 3A) of group 1 and 2 viruses possessed 51.9 to 53.8%, 54.5 to 57.4%, and 65.1 to 66.7% amino acid identities to the P1, P2 (excluding 2A), and P3 (excluding 3A) of group 3 viruses, respectively. Therefore, from the criterion of amino acid identity, group 1 and 2 viruses may belong to the same genus as group 3 viruses. However, the former two virus groups possessed genomic features very different from those of the latter. For example, they had a type IV IRES but the latter had a type I IRES. Their L and 2A proteins also possessed very low homology (12.3 to 16.4% amino acid identities in L and 27.3 to 29.5% amino acid identities in 2A) to those of the latter. While only one GXCG motif is present in the 2A proteins of bat picornaviruses of group 3 and simian and porcine sapeloviruses and none in avian sapelovirus, the 2A proteins of bat picornavirus groups 1 and 2 contain two potential GXCG motifs. They, but not group 3 viruses and the sapeloviruses, had two potential cleavage sites at the junction of 2A and 2B. They had a DDLGQ motif, but group 3 viruses had a DDVGQ motif in the helicase. Most uniquely, they possessed AMH in the GXH motif of 3Cpro, a characteristic not observed in other picornaviruses. Owing to these genomic features, bat picornavirus groups 1 and 2 should be classified in a genus separate from that of group 3 viruses.
The pathogenicity of the present novel picornaviruses to diverse species remains to be determined. Members of the closely related genus Sapelovirus are not very pathogenic to their hosts. Duck sapelovirus, first isolated in Taiwan from the intestines of ducks with signs of hepatitis in 1990, does not cause mortality in day-old ducklings but may have inhibited their growth (47). Porcine sapelovirus, first assigned as group II porcine enterovirus and later as enterovirus species PEVA, generally causes asymptomatic enteric infections of swine (26). Simian sapelovirus was isolated in the 1950s and 1960s from primate cell cultures or tissues or from clinical specimens derived from captive or wild-caught primates (21). Little is known regarding its pathogenesis. In the present study, the bats positive for bat picornavirus groups 1, 2, and 3 apparently were healthy. In our recent study on the ecoepidemiology of severe acute respiratory syndrome-related Rhinolophus bat coronavirus (SARSr-Rh-BatCoV), Chinese horseshoe bats harboring this virus also appeared asymptomatic during sampling and tagging (30). However, their body weights were found to be significantly lower than those for bats negative for SARSr-Rh-BatCoV irrespective of their carrier state for another coronavirus, Rhinolophus bat coronavirus HKU2, suggesting that SARSr-Rh-BatCoV causes acute, self-limiting infection in Chinese horseshoe bats (30). Therefore, further studies are required to determine if the present three novel bat picornaviruses cause any diseases in the different bat species.
Viruses of at least 13 families have been found in bats. Before the end of the SARS epidemic, viruses of nine families, including Arenaviridae, Bunyaviridae, Flaviviridae, Orthomyxoviridae, Paramyxoviridae, Reoviridae, Retroviridae, Rhabdoviridae, and Togaviridae, were known to be present in bats (2). Shortly after the end of the SARS epidemic, bats were discovered to be the reservoir of SARS-related coronavirus (32, 36). In the past 5 years, more than 10 viruses of the family Coronaviridae have been discovered in various species of bats (3, 9, 11, 40, 41, 45, 49, 51). In addition to coronaviruses, viruses of the families Filoviridae, Adenoviridae, and Astroviridae also have been discovered first in bats in the past 5 years (6, 33, 44). The high diversity of viruses in bats may be a result of their species diversity, ability to fly, environmental pressures, and habits of roosting (2). As bats are the major reservoirs of many lethal viruses capable of transmission to humans, such as coronaviruses (SARS-related coronavirus), paramyxoviruses (Hendra virus and Nipah virus), rhabdoviruses (rabies virus), and filoviruses (Marburg virus and Ebola virus), continuous surveillance of viruses in bats is important in preparation for emerging viral infections.
ACKNOWLEDGMENTS
We thank Alan Chi-Kong Wong, Siu-Fai Leung, Chik-Chuen Lay, Ping-Man So, and K. F. Chan (HKSAR Department of Agriculture, Fisheries, and Conservation [AFCD]) and the Hong Kong Police Force for facilitation and support; Cynthia S. M. Chan and Joseph W. K. So from AFCD; and King-Shun Lo (Laboratory Animal Unit) and Cassius Chan for their excellent technical assistance and collection of animal specimens. We are grateful for the generous support of Carol Yu, Richard Yu, Hui Hoy, and Hui Ming in the genomic sequencing platform.
This work was partly supported by the Research Grant Council Grant, University Grant Council, Strategic Research Theme Fund, Committee for Research and Conference Grant, and University Development Fund, The University of Hong Kong; The Tung Wah Group of Hospitals Fund for Research in Infectious Diseases; the HKSAR Research Fund for the Control of Infectious Diseases of the Health, Welfare and Food Bureau; the Providence Foundation Limited in memory of the late Lui Hac Minh; and the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the HKSAR Department of Health.
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Bazan J. F., Fletterick R. J. 1988. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proc. Natl. Acad. Sci. U. S. A. 85:7872–7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calisher C. H., Childs J. E., Field H. E., Holmes K. V., Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19:531–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrington C. V., et al. 2008. Detection and phylogenetic analysis of group 1 coronaviruses in South American bats. Emerg. Infect. Dis. 14:1890–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen H. H., Kong W. P., Roos R. P. 1995. The leader peptide of Theiler's murine encephalomyelitis virus is a zinc-binding protein. J. Virol. 69:8076–8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu C. Y., et al. 2008. Identification of cardioviruses related to Theiler's murine encephalomyelitis virus in human infections. Proc. Natl. Acad. Sci. U. S. A. 105:14124–14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu D. K., Poon L. L., Guan Y., Peiris J. S. 2008. Novel astroviruses in insectivorous bats. J. Virol. 82:9107–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. 1987. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J. Virol. 61:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doherty M., Todd D., McFerran N., Hoey E. M. 1999. Sequence analysis of a porcine enterovirus serotype 1 isolate: relationships with other picornaviruses. J. Gen. Virol. 80:1929–1941 [DOI] [PubMed] [Google Scholar]
- 9. Dominguez S. R., O'Shea T. J., Oko L. M., Holmes K. V. 2007. Detection of group 1 coronaviruses in bats in North America. Emerg. Infect. Dis. 13:1295–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dvorak C. M., et al. 2001. Leader protein of encephalomyocarditis virus binds zinc, is phosphorylated during viral infection, and affects the efficiency of genome translation. Virology 290:261–271 [DOI] [PubMed] [Google Scholar]
- 11. Gloza-Rausch F., et al. 2008. Detection and prevalence patterns of group I coronaviruses in bats, northern Germany. Emerg. Infect. Dis. 14:626–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gorbalenya A. E., Donchenko A. P., Blinov V. M., Koonin E. V. 1989. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 243:103–114 [DOI] [PubMed] [Google Scholar]
- 13. Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. 1989. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 17:4713–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorbalenya A. E., Koonin E. V., Wolf Y. I. 1990. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 262:145–148 [DOI] [PubMed] [Google Scholar]
- 15. Gradi A., et al. 2004. Cleavage of eukaryotic translation initiation factor 4GII within foot-and-mouth disease virus-infected cells: identification of the L-protease cleavage site in vitro. J. Virol. 78:3271–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hellen C. U., de Breyne S. 2007. A distinct group of hepacivirus/pestivirus-like internal ribosomal entry sites in members of diverse picornavirus genera: evidence for modular exchange of functional noncoding RNA elements by recombination. J. Virol. 81:5850–5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hinton T. M., Ross-Smith N., Warner S., Belsham G. J., Crabb B. S. 2002. Conservation of L and 3C proteinase activities across distantly related aphthoviruses. J. Gen. Virol. 83:3111–3121 [DOI] [PubMed] [Google Scholar]
- 18. Hughes P. J., Stanway G. 2000. The 2A proteins of three diverse picornaviruses are related to each other and to the H-rev107 family of proteins involved in the control of cell proliferation. J. Gen. Virol. 81:201–207 [DOI] [PubMed] [Google Scholar]
- 19. Jobb G., von Haeseler A., Strimmer K. 2004. TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evol. Biol. 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Jones M. S., Lukashov V. V., Ganac R. D., Schnurr D. P. 2007. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J. Clin. Microbiol. 45:2144–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalter S. S. 1982. Enteric viruses of nonhuman primates. Vet. Pathol. 19:33–43 [PubMed] [Google Scholar]
- 22. Kamer G., Argos P. 1984. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 12:7269–7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kapoor A., et al. 2008. A highly divergent picornavirus in a marine mammal. J. Virol. 82:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapoor A., et al. 2008. A highly prevalent and genetically diversified Picornaviridae genus in South Asian children. Proc. Natl. Acad. Sci. U. S. A. 105:20482–20487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kozak M. 1986. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44:283–292 [DOI] [PubMed] [Google Scholar]
- 26. Krumbholz A., et al. 2002. Sequencing of porcine enterovirus groups II and III reveals unique features of both virus groups. J. Virol. 76:5813–5821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamphear B. J., et al. 1993. Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J. Biol. Chem. 268:19200–19203 [PubMed] [Google Scholar]
- 28. Lau S. K., et al. 2009. Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J. Infect. Dis. 200:1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lau S. K., et al. 2007. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J. Clin. Microbiol. 45:3655–3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau S. K., et al. 2010. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as reservoir for acute, self-limiting infection that allows recombination events. J. Virol. 84:2808–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lau S. K., et al. 2010. Identification and complete genome analysis of three novel paramyxoviruses, Tuhoko virus 1, 2 and 3, in fruit bats from China. Virology 404:106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lau S. K., et al. 2005. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U. S. A. 102:14040–14045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leroy E. M., et al. 2005. Fruit bats as reservoirs of Ebola virus. Nature 438:575–576 [DOI] [PubMed] [Google Scholar]
- 34. Li L., et al. 2009. A novel picornavirus associated with gastroenteritis. J. Virol. 83:12002–12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li L., et al. 2010. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 84:6955–6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li W., et al. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676–679 [DOI] [PubMed] [Google Scholar]
- 37. Lindberg A. M., Johansson S. 2002. Phylogenetic analysis of Ljungan virus and A-2 plaque virus, new members of the Picornaviridae. Virus Res. 85:61–70 [DOI] [PubMed] [Google Scholar]
- 38. Malnou C. E., Poyry T. A., Jackson R. J., Kean K. M. 2002. Poliovirus internal ribosome entry segment structure alterations that specifically affect function in neuronal cells: molecular genetic analysis. J. Virol. 76:10617–10626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oberste M. S., Maher K., Pallansch M. A. 2003. Genomic evidence that simian virus 2 and six other simian picornaviruses represent a new genus in Picornaviridae. Virology 314:283–293 [DOI] [PubMed] [Google Scholar]
- 40. Pfefferle S., et al. 2009. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerg. Infect. Dis. 15:1377–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poon L. L., et al. 2005. Identification of a novel coronavirus in bats. J. Virol. 79:2001–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Racaniello V. R., Baltimore D. 1981. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc. Natl. Acad. Sci. U. S. A. 78:4887–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ryan M. D., Flint M. 1997. Virus-encoded proteinases of the picornavirus super-group. J. Gen. Virol. 78:699–723 [DOI] [PubMed] [Google Scholar]
- 44. Sonntag M., Muhldorfer K., Speck S., Wibbelt G., Kurth A. 2009. New adenovirus in bats, Germany. Emerg. Infect. Dis. 15:2052–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang X. C., et al. 2006. Prevalence and genetic diversity of coronaviruses in bats from China. J. Virol. 80:7481–7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tracy S., Chapman N. M., Drescher K. M., Kono K., Tapprich W. 2006. Evolution of virulence in picornaviruses. Curr. Top. Microbiol. Immunol. 299:193–209 [DOI] [PubMed] [Google Scholar]
- 47. Tseng C. H., Tsai H. J. 2007. Sequence analysis of a duck picornavirus isolate indicates that it together with porcine enterovirus type 8 and simian picornavirus type 2 should be assigned to a new picornavirus genus. Virus Res. 129:104–114 [DOI] [PubMed] [Google Scholar]
- 48. Tseng C. H., Knowles N. J., Tsai H. J. 2007. Molecular analysis of duck hepatitis virus type 1 indicates that it should be assigned to a new genus. Virus Res. 123:190–203 [DOI] [PubMed] [Google Scholar]
- 49. Woo P. C., et al. 2007. Comparative analysis of 12 genomes of three novel group 2c and group 2d coronaviruses reveals unique group and subgroup features. J. Virol. 81:1574–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Woo P. C., et al. 2009. Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. J. Virol. 83:908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Woo P. C., et al. 2006. Molecular diversity of coronaviruses in bats. Virology 351:180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Woo P. C., et al. 2010. Comparative analysis of six genome sequences of three novel picornaviruses, turdiviruses 1, 2 and 3, in dead wild birds, and proposal of two novel genera, Orthoturdivirus and Paraturdivirus, in the family Picornaviridae. J. Gen. Virol. 91:2433–2448 [DOI] [PubMed] [Google Scholar]
- 53. Wutz G., et al. 1996. Equine rhinovirus serotypes 1 and 2: relationship to each other and to aphthoviruses and cardioviruses. J. Gen. Virol. 77:1719–1730 [DOI] [PubMed] [Google Scholar]
- 54. Yamashita T., et al. 1998. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J. Virol. 72:8408–8412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yip C. C., et al. 2010. Emergence of enterovirus 71 “double-recombinant” strains belonging to a novel genotype D originating from southern China: first evidence for combination of intratypic and intertypic recombination events in EV71. Arch. Virol. 155:1413–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yob J. M., et al. 2001. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg. Infect. Dis. 7:439–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]