Abstract
Whole-body bioimaging was employed to study the effects of passive immunotherapies on lethality and viral dissemination in BALB/c mice challenged with recombinant vaccinia viruses expressing luciferase. WRvFire and IHD-J-Luc vaccinia viruses induced lethality with similar times to death following intranasal infection, but WRvFire replicated at higher levels than IHD-J-Luc in the upper and lower respiratory tracts. Three types of therapies were tested: licensed human anti-vaccinia virus immunoglobulin intravenous (VIGIV); recombinant anti-vaccinia virus immunoglobulin (rVIG; Symphogen, Denmark), an investigational product containing a mixture of 26 human monoclonal antibodies (HuMAbs) against mature virion (MV) and enveloped virion (EV); and HuMAb compositions targeting subsets of MV or EV proteins. Bioluminescence recorded daily showed that pretreatment with VIGIV (30 mg) or with rVIG (100 μg) on day −2 protected mice from death but did not prevent viral replication at the site of inoculation and dissemination to internal organs. Compositions containing HuMAbs against MV or EV proteins were protective in both infection models at 100 μg per animal, but at 30 μg, only anti-EV antibodies conferred protection. Importantly, the t statistic of the mean total fluxes revealed that viral loads in surviving mice were significantly reduced in at least 3 sites for 3 consecutive days (days 3 to 5) postchallenge, while significant reduction for 1 or 2 days in any individual site did not confer protection. Our data suggest that reduction of viral replication at multiple sites, including respiratory tract, spleen, and liver, as monitored by whole-body bioluminescence can be used to predict the effectiveness of passive immunotherapies in mouse models.
INTRODUCTION
Following a massive vaccination campaign led by the World Health Organization, smallpox was declared eradicated in 1980. Vaccination of the general public in the United States was discontinued in 1972. However, in light of concerns that variola virus, the causative agent of smallpox, can be used as a bioterrorist weapon, strategies have been initiated worldwide, including in the United States, to develop medical countermeasures. The Preparedness and Response Program at the U.S. Centers for Disease Control and Prevention (CDC) recommended building stockpiles of smallpox vaccines for vaccination against smallpox, and anti-vaccinia virus immunoglobulin (VIG) for the management of serious adverse reactions to vaccination and postexposure treatment (5, 33). Currently, inoculation of military personnel with smallpox vaccine is a policy of the U.S. Department of Defense (http://www.smallpox.army.mil/resource/policies.asp).
One of the vaccines used in the global smallpox eradication program was Dryvax, a live vaccinia virus vaccine manufactured by Wyeth Pharmaceuticals, Inc. Dryvax was a derivative of the New York City Board of Heath strain of vaccinia virus, was prepared in calf lymph, and was the only licensed smallpox vaccine in the United States for many years following the discontinuation of routine smallpox vaccination. Dryvax was replaced with ACAM2000 (Acambis, Inc.), a plaque-purified clonal isolate of Dryvax that is manufactured in Vero cells using serum- and protein-free cell culture medium under controlled good manufacturing practices (25, 26). ACAM2000 was licensed as a smallpox vaccine by the United States in 2007. It is indicated for vaccination against smallpox in designated individuals, and it was shown to cause a reactogenicity similar to that of Dryvax (10, 12).
The management of some of the adverse events associated with live smallpox vaccines includes the use of VIG therapy (37). VIG may also be needed as prophylaxis in patients for whom the live attenuated smallpox vaccine is contraindicated, such as those with eczema or pregnant women. The intramuscular (i.m.) form of VIG was first obtained from plasma of hyperimmunized U.S. Army recruits in the 1950s and was used to treat complications of smallpox vaccinations. Later on, the intravenous (i.v.) formulations were found to have higher tolerability and better pharmacokinetic profiles (38). Licensed human anti-vaccinia virus immunoglobulin intravenous (VIGIV) manufactured by Cangene Corporation (U.S. Civilian Stockpile) is an IgG fraction of plasma taken from healthy donors that exhibited high titers of anti-vaccinia virus antibodies following vaccination with Dryvax (38).
Several animal models of orthopoxvirus infections have been developed and are currently used for evaluation of the efficacy and safety of vaccinia virus immunoglobulins and vaccine candidates. Most frequently, infections of mice with the mouse-adapted vaccinia virus Western Reserve strain (WR) and in some cases, infection of mice with ectromelia virus, the causative agent of mousepox, are used as surrogate models for the evaluation of vaccines and therapeutics against smallpox. Data derived from these animal models showed that human VIGIV protected mice from lethality when used before challenge and in some instances up to 4 days postchallenge (19, 21). However, due to the specific methods employed to evaluate the efficacy of VIGIV in these and other studies (lethality and weight loss), the mechanism of protection conferred by VIGIV has not been elucidated.
Mature virion (MV) and enveloped virion (EV) are two major, structurally distinct forms of poxviruses (vaccinia virus, variola virus, and monkeypox virus), each with its own distinct biology (34). The nucleoprotein core in MV and EV is surrounded with either one (MV) or two (EV) lipoprotein bilayers. The MV is the most abundant and stable particle in the environment, it remains intracellular until cell lysis, and it was suggested to play a primary role in person-to-person transmission (34). The EV forms only a small fraction of total infectious particles, yet EV has higher specific infectivity than MV (i.e., a lower particle/PFU ratio) (28). EVs are released rapidly and mediate efficient cell-to-cell spread in part due to a specific mechanism involving the repulsion of infectious virions by infected cells toward uninfected cells (7). Attempts to develop an effective subunit vaccine against smallpox showed that good protection from infection in animal models requires antibodies to both MV and EV (9, 14, 17). VIGIV was shown to contain anti-MV activity that was much higher than the anti-EV activity (19). However, the contribution of VIGIV to the protection of animals infected with vaccinia virus isolates that generate different amounts of EV during infection has not been investigated.
Previously, our laboratory successfully employed whole-body bioimaging to follow dissemination of recombinant WR vaccinia virus expressing luciferase (WRvFire). We showed that replication of WRvFire measured by detecting light emitted by the luciferase in the presence of d-luciferin substrate administered to infected animals correlated with viral loads measured ex vivo using a plaque assay and accurately predicted lethality (39). Bioimaging allowed us to demonstrate that Dryvax vaccination 2 to 3 weeks before challenge significantly reduced WRvFire replication at the site of inoculation (nasal cavity) and lungs and blocked virus dissemination to internal organs. These findings correlated with protection from lethality and minimal weight loss (39). This level of protection reflects the fact that active vaccination can induce strong cellular immunity that, in addition to neutralizing antibodies, contributes to the rapid clearance of infected cells.
Many studies have shown that treatments based on antibody products such as VIGIV and polyclonal sera from vaccinated animals confer protection from lethality. However, whether such protection correlates with reduction in the initial viral replication and prevents viral dissemination to internal organs of individual animals has not been shown yet.
In the current study, we used two strains of recombinant vaccinia virus expressing luciferase: WRvFire and International Health Department-J-Luc (IHD-J-Luc), which were generated from WR and IHD-J strains, previously shown to produce similar amounts of MV but to differ in the quantity of the released EV particles (4). Bioluminescence was recorded daily in a large cohort of live mice (>80 mice per strain) to compare the kinetics of viral dissemination. Using both strains, we tested the protective activity of VIGIV and a new product, a recombinant VIG (rVIG) containing 26 human monoclonal antibodies (HuMAbs) targeting proteins in MV and EV (18). The data showed that in both challenge models, the protection of mice from infection-induced lethality was associated with significant reduction in viral loads in the upper and lower respiratory tracts and in spleen and liver for 3 consecutive days.
MATERIALS AND METHODS
Viruses.
The thymidine kinase-positive (TK+) recombinant Western Reserve (WR) vaccinia virus expressing the luciferase reporter gene under the control of a synthetic E/L promoter (WRvFire) has been described previously (35) and was kindly provided by Bernard Moss (NIAID, NIH). WRvFire was propagated in HeLa cells and tittered in BSC-1 cells. A recombinant vaccinia virus expressing luciferase (IHD-J-Luc) was constructed based on the International Health Department J (IHD-J) strain of vaccinia virus. The luciferase gene (under the control of the same synthetic E/L promoter as in WRvFire) was inserted at a truncated-host-range gene locus equivalent to the cowpox gene, CP77. Details of the construction and characterization of sucrose gradient purified IHD-J-Luc will be described elsewhere (C. A. Meseda, M. Merchlinsky, and J. P. Weir, unpublished data). IHD-J-Luc remains pathogenic in the intranasal challenge model in BALB/c mice and has a 50% lethal dose (LD50) value similar to the wild-type IHD-J. Single stocks of WRvFire and of IHD-J-Luc vaccinia viruses containing 1.0 × 108 and 3.9 × 109 PFU/ml, respectively, were used throughout the study. The numbers of particles in viral stocks were determined by transmission electron microscopy (TEM) and were 2.4 × 109 and 3.3 × 1010 particles/ml for WRvFire and IHD-J-Luc, respectively; thus, both stocks contained similar ratios of noninfectious to infectious viral particles (20:1 and 10:1, respectively).
Infection of mouse 3T3 cells with WRvFire and IHD-J-Luc vaccinia viruses in vitro.
A confluent monolayer of BALB/3T3 (clone A31) mouse fibroblast cells (ATCC CCL-163) was infected with 70 PFU of WRvFire or IHD-J-Luc vaccinia viruses. After 2 h of incubation of cells with the viruses, infected cells were overlaid with Dulbecco's modified essential medium (DMEM) containing 0.5% methylcellulose (M-cellulose) or liquid DMEM. Cells were fixed and stained with crystal violet solution after 48 h of incubation.
Mice and protocols for in vivo treatments.
Five-week-old female BALB/c mice (National Cancer Institute, Frederick, MD) were used in all experiments. Immediately prior to challenge, mice were anesthetized using 2,2,2-tribromoethanol dissolved in tertiary amyl alcohol and diluted in sterile phosphate-buffered saline (PBS) according to the manufacturer's instructions. The anesthesia was administered at 20 μl/g of body weight by intraperitoneal (i.p.) injection. Mice were challenged via the intranasal (i.n.) route with 105 PFU (2 LD50) of WRvFire or of IHD-J-Luc in a 10-μl volume delivered in one nostril.
For prophylactic passive immunizations, animals were given VIGIV (lot 173601, 56 mg/ml; 6,675 U/ml [Cangene Corporation, Winnipeg, MB, Canada]) at 3 mg/animal (357 units) or 30 mg/animal (3,576 units) in 600 μl of PBS i.p. 2 days prior to challenge (39). In some experiments, mice received administration of rVIG (Symphogen A/S Lyngby, Denmark), a new investigational product containing a mixture of 26 HuMAbs (18) at 3, 30, or 100 μg/animal in 200 μl of PBS i.p. 2 days prior to challenge. In some studies, HuMAb subset compositions were administered at 30 μg/200 μl/animal. Control mice received the same volumes of PBS. The handling of mice and experimental procedures were approved by the CBER animal study review committee.
In vivo measurements of luciferase activity.
Mice were injected i.p. with 150 μg/g of body weight of d-luciferin potassium salt (Caliper Life Sciences, Hopkinton, MA) 10 to 15 min prior to imaging. Mice were anesthetized in an oxygen-rich induction chamber with 2% isoflurane and were imaged in dorsal and ventral positions by using an IVIS 50 cooled charge-coupled-device camera system (Caliper) as previously described (39). Images were collected daily on days 1 to 10 postchallenge at optimized exposure times to avoid saturation of the camera and were analyzed with the LivingImage 3.02 software (Caliper). To quantify the amount of light emission in specific organs, a single region of interest (ROI) was established for each organ and used throughout analysis as recommended by the manufacturer. The background bioluminescence was determined using images of d-luciferin-injected animals 1 day prior to infections. In all calculations, the background bioluminescence was subtracted from experimental values.
Statistical analysis.
Kaplan-Meier survival curves of times to death following infection were generated using standard GraphPad Prism V5 software. We used the t statistic to compare mean total fluxes between infected control mice (PBS treated) and infected and treated mice (VIGIV, rVIG, or HuMAb compositions) with Microsoft Excel.
RESULTS
Kinetics of viral dissemination in mice infected with WRvFire or IHD-J-Luc vaccinia viruses.
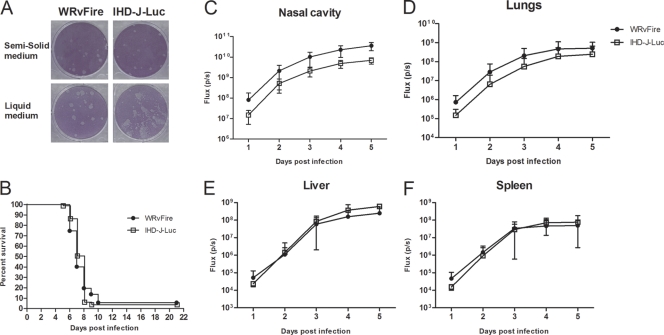
Two infectious vaccinia virus isoforms, EV and MV, have been shown to play predominant roles in in-host and interhost viral spread, respectively. The ratio of MV to EV particles produced during viral replication depends on the virus strain and the cell line used to propagate virus (27). While WR and IHD-J viruses produce similar amounts of MV viral particles, they differ in the quantity of released EV. In WR, the EV particles remain in the form of cell-associated enveloped (CEV) vaccinia virus, while in the case of IHD-J, the EV is efficiently released (28). To confirm that the viral stocks used in our study have the expected characteristics, the mouse BALB/c 3T3 fibroblasts were infected with WRvFire or IHD-J-Luc virus stocks. To distinguish between the appearances of MV and EV plaques, infected cells were overlaid with DMEM with or without 0.5% methylcellulose. The plaques formed by WRvFire appeared round and did not differ in the presence of methylcellulose medium (semisolid) or liquid medium. In contrast, comet-like plaques were noted in IHD-J-Luc-infected cells in the presence of liquid but not semisolid (M-cellulose) medium (Fig. 1A). These data showed that both WRvFire and IHD-J-Luc produce MV viral particles, but EV particles are effectively released only from IHD-J-Luc-infected cells.
Fig. 1.
In vitro infection of mouse cells and infection of mice in vivo with WRvFire and IHD-J-Luc vaccinia viruses. (A) Visualization of plaques formed by WRvFire and IHD-J-Luc viruses. Monolayers of 3T3 mouse cells were infected with WrvFire (left panels) or with IHD-J-Luc (right panels), overlaid with solid (top panels) or liquid (bottom panels) medium, and stained with crystal violet. (B to F) Lethality outcome and bioluminescence measurements of recombinant WRvFire and IHD-J-Luc vaccinia viruses in the internal organs of BALB/c mice. Eighty-seven and 81 BALB/c mice were infected i.n. with WRvFire (circles) and with IHD-J-Luc (squares) vaccinia viruses, respectively. Mice were observed for survival (B) and were subjected to whole-body bioimaging (C to F). Total fluxes in the nasal cavity (C), lungs (D), liver (E), and spleen (F) were determined as described in materials and methods and used to calculate mean total flux ± SD using t test.
To determine whether the kinetics of lethality and viral dissemination is affected by the levels of EV particles released during infection, BALB/c mice were infected with 105 PFU of WRvFire or IHD-J-Luc viruses (87 and 81 animals, respectively) and were imaged daily for 5 days using the IVIS 50 instrument. There was no significant difference between the survival curves (P = 0.91); in both infections, mice succumbed to death within 10 days postchallenge with ≤5 mice surviving in both groups (Fig. 1B).
Images of infected mice were processed using Living Image software to quantify total photon fluxes emitted by infected organs in individual mice, and mean total fluxes were calculated as previously described (39). Mean total fluxes detected in the nasal cavity and lungs of WRvFire-infected mice were significantly higher than in IHD-J-Luc-infected animals starting from day 1 and remained higher for 5 days (P < 0.0001) (Fig. 1C and D). In the liver and spleen, the mean total fluxes between the two strains were not visually different on any given day (Fig. 1E and F).
Prophylactic treatments with VIGIV reduced virus replication and dissemination and protected animals from lethality following intranasal challenge.
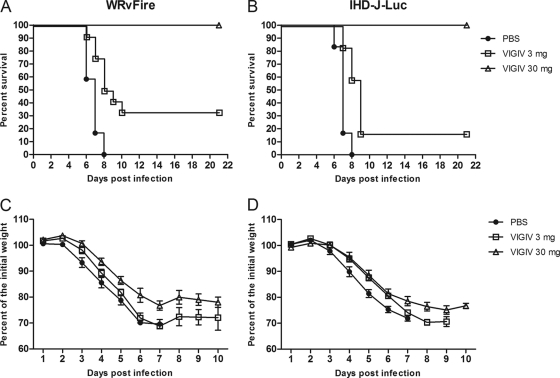
To investigate the effects of VIGIV on the dissemination of vaccinia virus to internal organs, BALB/c mice were inoculated with VIGIV at low and high doses of 3 and 30 mg/animal, respectively, and 2 days later were challenged i.n. with 105 PFU (2 LD50) of WRvFire or IHD-J-Luc (Fig. 2). Following infection, mice were observed for lethality for 21 days. All mice in the challenge-only groups succumbed to death between days 6 and 8 postchallenge. All infected mice that received 30 mg of VIGIV survived, while the 3-mg VIGIV dose protected 33% and 17% of mice from WRvFire- and IHD-J-Luc-induced lethality, respectively (Fig. 2A and B). The higher dose of VIGIV significantly reduced weight loss in WRvFire-infected mice on days 2 to 5 and both low and high doses of VIGIV significantly reduced weight loss in IHD-J-Luc-infected mice on days 4 to 6 (Fig. 2C and D). Interestingly, even animals that were fully protected (i.e., in the 30-mg VIGIV dose) did lose weight during the 10 days after infection.
Fig. 2.
VIGIV protected mice from lethality following WRvFire or IHD-J-Luc infections in a dose-dependent manner. Mice were inoculated i.p. with VIGIV at 3 mg/animal (squares), 30 mg/animal (triangles), or PBS (closed circles) 2 days before i.n. challenge with WRvFire (A and C) and with IHD-J-Luc (B and D) vaccinia viruses. Mice were observed for survival (A and B) for 21 days postinfection; weight measurements were performed daily for 10 days and used to calculate mean percentages of initial weight ± SD (C and D). For the VIGIV (30 mg)-treated and IHDJ-Luc-infected groups, n = 11; for all other groups, n = 12. Results are representative of three experiments.
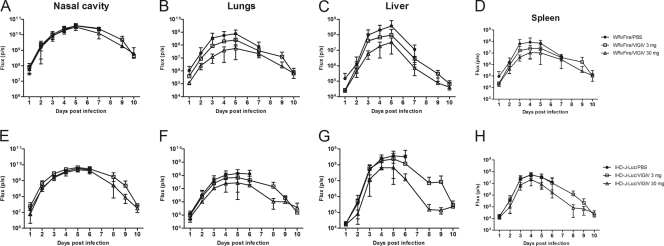
To assess the effects of VIGIV on viral dissemination by bioimaging, we first established mean total fluxes emitted by internal organs of uninfected animals by collecting images of 14 BALB/c mice following d-luciferin injections (data not shown). The results showed that on average, the mean background total fluxes ± standard deviation (SD) detected in the nasal cavity, lungs, liver, and spleen were 4.9 × 104 ± 2.2 × 104, 1.7 × 105 ± 1.4 × 105, 3.7 × 104 ± 1.4 × 104, and 4.3 × 104 ± 3.6 × 104 photons/second, respectively. After background fluxes were established, we recorded bioluminescence daily in individual animals infected with WRvFire or with IHD-J-Luc following treatments with VIGIV and calculated total fluxes in the nasal cavity, lungs, liver, and spleen. Bioluminescence in control mice (treated with PBS) that were infected with either WRvFire or with IHD-J-Luc peaked between days 3 and 5 in all organs (Fig. 3, closed circles in each panel). At 24 h postchallenge, bioluminescence in the nasal cavity was 2 logs over the background levels recorded in mice prior to challenge (Fig. 3A and E). VIGIV treatments did not reduce the total fluxes recorded in the nasal cavity of either WRvFire- or IHD-J-Luc-challenged mice on days 2 to 6 postinfection. By day 10 postinfection, bioluminescence in the nasal cavity of surviving animals was 2 to 2.5 logs reduced from the peak levels on day 5 but was still higher than background (Fig. 3A and E). These data suggested that during the first 24 h, WRvFire and IHD-J-Luc viruses continued to replicate rapidly at the site of infection and were unaffected by VIGIV prophylaxis.
Fig. 3.
Bioluminescence in organs of mice pretreated with VIGIV prior to infections with WRvFire or IHD-J-Luc. Mice were inoculated i.p. with VIGIV (day −2) at 3 mg/animal (squares), 30 mg/animal (triangles), or with PBS (closed circles), and were infected i.n. (day 0) with WRvFire (A-D) or IHD-J-Luc vaccinia viruses (E-H). Animals were subjected to whole-body imaging for 10 days. Bioluminescence in the nasal cavity (A, E), lungs (B, F), liver (C, G), and spleen (D, H) was recorded and used to calculate mean total flux ± SD. For the numbers of animals per groups see legend to Fig. 2.
In the lungs, liver, and spleen of mice infected with WRvFire, a slight increase over the background bioluminescence was noted on day 1 postinfection, but the differences were not statistically significant (Fig. 3B to D). No increases above background levels were detected in the same organs of mice infected with IHD-J-Luc (Fig. 3F to H). During days 2 to 5 postinfection, bioluminescence increased in the lungs, liver, and spleen in all infected animals, including mice that received high and low doses of VIGIV on day −2, suggesting that VIGIV did not completely prevent the dissemination of vaccinia virus to internal organs (Fig. 3). However, total fluxes were reduced in VIGIV-treated mice infected with either virus compared with control animals, and the reduction in bioluminescence was more pronounced in animals that received 30 mg compared with 3 mg of VIGIV. Total fluxes recorded in the lungs, liver, and spleen returned to background levels by day 10 in surviving mice (Fig. 3B to D and F to H). The dramatic reduction in bioluminescence in the organs of VIGIV-protected mice correlated with 1- to 3-log reduced titers (in relative light units per gram of tissue) (39) of WRvFire in the lungs, liver, and spleen as determined by subjecting the extracted organs to an in vitro luciferase reporter assay (data not shown). In addition, measurements of viral loads by a standard plaque reduction assay did not reveal viral replication in the organs of VIGIV (30 mg)-treated mice collected at day 10 postinfection (data not shown). These data confirmed that reduction of total fluxes observed in mice protected by a high dose of VIGIV correlated with the results of ex vivo measurements of viral loads.
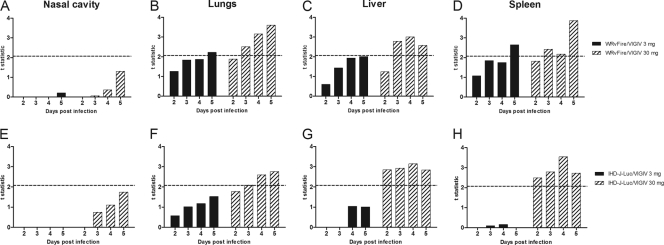
To determine whether the observed reductions in total fluxes in the organs of the VIGIV-treated mice were significant at any time point postinfection, we calculated the t statistic (Fig. 4). Mean total fluxes recorded from organs of 12 mice in each VIGIV treatment group were compared with mean total fluxes in 12 control animals. A t value of ≥2.07, corresponding to a two-sided P value of <0.05, is indicative of significant differences in means between two groups of 12 mice (Fig. 4A to D, dashed horizontal lines). Similarly, t values of ≥2.08 (two-sided P < 0.05) is indicative of significant differences between two groups of 11 or 12 mice (Fig. 4E to H, dashed lines). For the nasal cavity, t values were below the significance threshold at all time points in both infection models (Fig. 4A and E). At the same time, t values above the threshold were calculated for mean total fluxes in the lungs, liver, and spleen on days 3 to 5 postinfection in all mice that received 30 mg of VIGIV, indicating a significant difference in mean total fluxes from control mice (Fig. 4B to D and F to H, hatched bars). In mice treated with 3 mg of VIGIV (Fig. 4, closed bars), t values above the threshold were calculated for mean total fluxes in the lungs and spleen of mice infected with WRvFire only on day 5 (Fig. 4B and D) and not in any of the IHD-J-Luc-infected animals (Fig. 4F to H, closed bars). These data suggested that significant reductions in viral loads in 3 internal organs (lungs, liver, and spleen) conferred by VIGIV treatment correlated with protection from lethality in WRvFire- and in IHD-J-Luc-infected mice in a dose-dependent manner.
Fig. 4.
Statistical analysis of total fluxes recorded in organs of WRvFire- or IHD-J-Luc-infected control mice and mice treated with VIGIV. Total fluxes were recorded in the nasal cavity (A and E), lungs (B and F), liver (C and G), and spleen (D and H) of mice infected with WRvFire (A to D) or with IHD-J-Luc (E to H). The y axis shows t values that depict differences between the means of the total fluxes in control mice (PBS treated) and the means of the total fluxes in animals that received 3 mg/animal (closed bars) or 30 mg/animal (hatched bars) of VIGIV. Dashed horizontal lines depict the critical t values of 2.07 (12 mice per group) (A to D) and 2.08 (11 or 12 mice per group) (E to H). All values on or above the dashed lines are significant at α = 0.05 (two tailed).
Protection from lethality and reduction in viral dissemination by fully human recombinant VIG.
rVIG is a new investigational product containing a mixture of 26 human monoclonal antibodies (HuMAbs). The antibodies in this product were isolated from vaccinated human volunteers by the Symplex technology (24) as described by Lantto et al. (18). The antibodies were produced in Chinese hamster ovary (CHO) cells as full-length IgG1, either as individual HuMAbs or as a polyclonal composition. The HuMAbs in the rVIG recognize a broad range of MV and EV antigens, including A10, A14, A27, A33, A56, B5, D8, H3, H5, I1, WR148 (ATI protein), and the secreted vaccinia virus complement control protein (VCP), as described in detail elsewhere (18). As the rVIG was manufactured using a polyclonal cell line (18), it does not contain exactly equal amounts of each antibody. Relative quantitation by mass spectrometry (29) has indicated that the antibodies targeting neutralizing antigens on MV (A14, A27, D8, and H3) and EV (A33 and B5) constitute approximately equal portions, 40% each, of the total rVIG. The exact epitopes recognized by each antibody have not been determined.
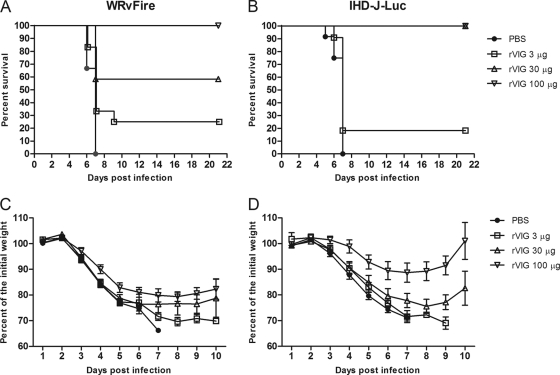
We first sought to determine the dose at which the rVIG protected mice from vaccinia virus-induced lethality. BALB/c mice were inoculated with rVIG at 3, 30, and 100 μg/animal i.p. and 2 days later were challenged i.n. with WRvFire or IHD-J-Luc viruses. Only the highest dose of rVIG (100 μg) completely protected animals from WRvFire challenge (Fig. 5A). In the case of IHD-J-Luc virus, mice were fully protected by pretreatment with rVIG at either a 100-μg or a 30-μg dose (Fig. 5B). The 100-μg dose significantly reduced weight loss in mice infected with WRvFire and IHD-J-Luc on days 4 to 6 and 3 to 6, respectively, whereas the 30-μg dose significantly reduced weight loss in IHD-J-Luc-infected mice only on days 5 and 6 (Fig. 5C and D); untreated animals all succumbed to infection by day 6. The lowest dose of rVIG (3 μg/animal) had no significant effect on weight loss.
Fig. 5.
rVIG protects mice from lethal challenge with WRvFire and IHD-J-Luc in a dose-dependent manner. BALB/c mice were inoculated with rVIG at 3 μg/animal (squares), 30 μg/animal (triangles), or 100 μg/animal (inverted triangles) or with PBS (closed circles) and 2 days later challenged with WRvFire (A) or IHD-J-Luc (B). Mice were observed for lethality for 21 days; weight measurements were performed daily for 10 days and used to calculate mean percentages of initial weight ± SD (C and D). For the PBS-treated and WRvFire-challenged groups, n = 15. For all rVIG-treated groups infected with WRvFire, n = 12. For the PBS-treated and for rVIG (30 μg)-treated and IHDJ-Luc-challenged groups, n = 12. For the 3-μg and 100-μg rVIG-treated IHDJ-Luc-infected groups, n = 11. Results are representative of three experiments.
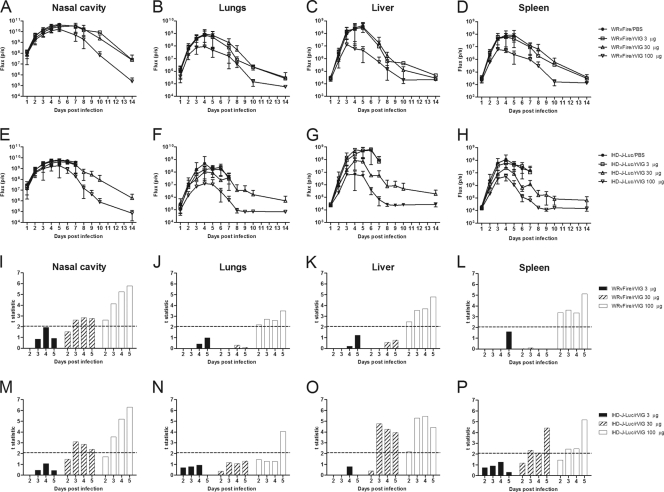
All infected mice were subjected to whole-body bioimaging, and the observed kinetics of total fluxes in control animals was similar to the total fluxes recorded in control groups in the experiments with VIGIV treatments (compare Fig. 6A to H and Fig. 3A to H). Reduction in bioluminescence throughout the course of infection was clearly noticeable in the lungs, liver, and spleen of mice that received 100 μg of rVIG (Fig. 6B to D and F to H). To establish whether rVIG significantly reduced viral loads in internal organs, mean total fluxes recorded in control and treatment groups were compared using the t statistic (Fig. 6I to P). At the fully protective dose of 100 μg, mean total fluxes were significantly different between rVIG-treated and control animals in the nasal cavity, lungs, liver, and spleen on days 2 to 5 after WRvFire challenge (Fig. 6I to L) and in the nasal cavity, liver, and spleen on days 3 to 5 and in the lungs on day 5 after IHD-J-Luc challenge (Fig. 6M to P). The intermediate dose of 30 μg significantly reduced mean total fluxes in the nasal cavity, liver, and spleen on days 3 to 5 in IHD-J-Luc in agreement with the survival outcome (Fig. 5B). In the case of WRvFire, the 30-μg dose of rVIG was not protective (Fig. 5A), and the bioluminescence data showed statistically significant reduction in fluxes only in the nasal cavity but not in the lungs and other internal organs (Fig. 6I to L, hatched bars). The 3-μg dose of rVIG did not significantly reduce mean viral loads at any time point in either infection model (Fig. 6I to P, closed bars). These data demonstrated that complete protection from lethality conferred by rVIG correlated with significant reduction in viral loads in at least three organs (nasal cavity, liver, and spleen) and for at least 3 consecutive days.
Fig. 6.
Bioluminescence in mice pretreated with rVIG prior to infections with WRvFire or IHD-J-Luc and statistical analysis of total fluxes in organs. (A to H) Bioluminescence of infected mice. Mice were inoculated i.p. with rVIG at 3 μg/animal (squares), 30 μg/animal (triangles), or 100 μg/animal (inverted triangles) or with PBS (closed circles) 2 days before i.n. challenge with WRvFire (A to D) or IHDJ-Luc (E to H). Animals were subjected to whole-body imaging for 10 days to record bioluminescence and calculate mean total flux ± SD in the nasal cavity (A and E), lungs (B and F), liver (C and G), and spleen (D and H). (I to P) Statistical analysis. Mean total fluxes were used to calculate the t statistic between groups of mice treated with 3 μg/animal (solid bars), 30 μg/animal (hatched bars), or 100 μg/animal (open bars) of rVIG and PBS. The dashed horizontal line depicts the critical t values of 2.06 in the WRvFire challenge (12 to 15 mice per group) (I to L) and 2.08 in the IHD-J-Luc challenge (11 or 12 mice per group) (M to P).
Antibodies against vaccinia virus EV proteins play a major role in the protective activity of rVIG.
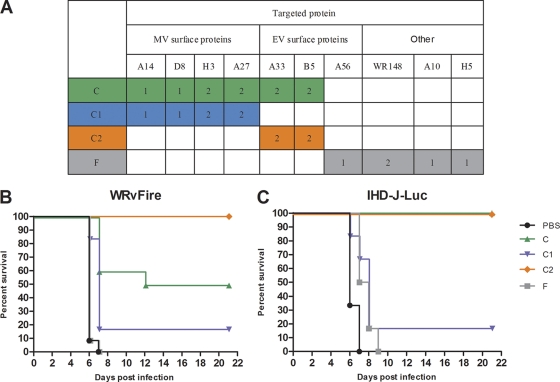
To further elucidate the role of antibodies against individual proteins in the protection from viral dissemination and lethality conferred by the rVIG, we next tested subsets of HuMAbs from the rVIG product that were prepared by Symphogen for these experiments (Fig. 7A). These mixtures were composed of HuMAbs against either MV or EV surface proteins (C1 and C2, respectively), or against both (C); composition F contained HuMAbs targeting nonenvelope MV proteins in addition to the EV A56 protein (Fig. 7A). The HuMAb subset compositions were composed of equal amounts of each antibody, i.e., composition C contains 1/10 of each HuMAb, composition C1 one-sixth of each antibody, etc. (Fig. 7A). In preliminary experiments, compositions C, C1, and C2, but not F, protected animals from lethality in both WRvFire and IHD-J-Luc challenge models when used at 100 μg/animal (data not shown). To allow for better differentiation, only 30 μg of the HuMAb compositions were used for pretreatment of mice prior to challenge with WRvFire or with IHD-J-Luc in subsequent experiments. As expected, mice treated with composition F succumbed to infection at the same rates as did untreated animals (Fig. 7B and C). Composition C, containing 10 HuMAbs targeting EV proteins A33 and B5 (2 MAbs each), MV proteins H3, A27 (2 MAbs each), and A14 and D8 proteins (1 MAb each), provided 50% and 100% protection from lethality in WRvFire and in IHD-J-Luc infections, respectively (Fig. 7B and C). The C1 composition containing 6 MAbs targeting only MV proteins protected less than 20% of mice in both infections. In contrast, composition C2, which contained 4 MAbs targeting EV surface proteins A33 and B5, completely protected animals from lethal challenge with both WRvFire and IHD-J-Luc viruses (Fig. 7B and C).
Fig. 7.
Effects of individual HuMAb compositions on lethality in WRvFire challenge model. (A) Specificities of HuMAbs targeting MV, EV surface vaccinia virus proteins and nonsurface (other) proteins within individual compositions are shown in the table. Numbers of MAbs against a specific target are indicated in colored cells. (B and C) BALB/c mice were inoculated i.p. with HuMAb compositions C (green triangles), C1 (blue inverted triangles), C2 (orange diamonds), and F (gray squares) at 30 μg/animal or with PBS (black circles) and were challenged with WRvFire (B) or with IHD-J-Luc (C) viruses 2 days later. Six to 12 mice per group were used in the experiment. Mice were observed for lethality and survival for 21 days. Results are representative of three experiments.
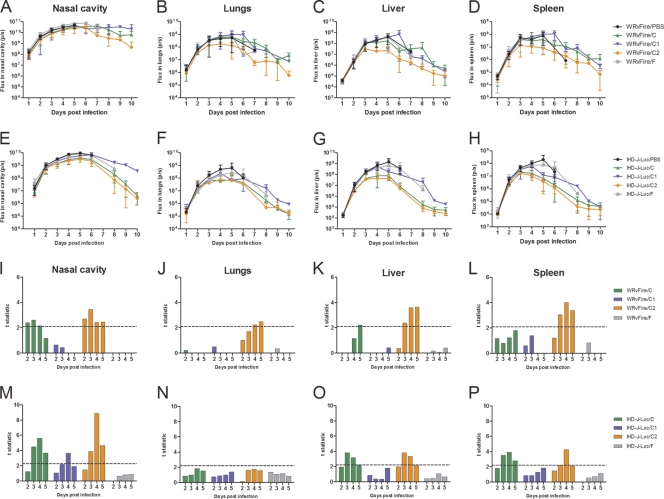
In the same study, mice were subjected to bioimaging and total fluxes were used to determine the effects of the HuMAb compositions on viral dissemination to internal organs (Fig. 8A to H). Statistical analysis of mean total fluxes recorded in organs revealed that composition F (primarily targeting nonenvelope proteins) did not significantly reduce bioluminescence in internal organs at any time point (Fig. 8I to P, gray bars). Composition C1 (MV-specific HuMAbs) reduced total fluxes in the nasal cavity on days 3 and 4 but not in other organs in IHD-J-Luc-infected mice and had no effect on total fluxes in WRvFire-infected mice (Fig. 8I to P, blue bars). In contrast, in mice treated with C2 (EV-specific HuMAbs) significant reductions in bioluminescence (compared with control animals) were observed in the nasal cavity, liver, and spleen on days 3 to 5 in both WRvFire- and IHD-J-Luc-infected mice, as well as in the lungs of WRvFire-challenged mice on days 4 and 5 (Fig. 8I to M, O, and P, orange bars). Composition C, which contained a combination of the HuMAbs in C1 and C2, significantly reduced bioluminescence in the nasal cavity (days 2 to 4), and liver (day 5) of WRvFire-infected animals, and in the nasal cavity, liver, and spleen (days 3 to 5) of IHD-J-Luc-infected mice (Fig. 8I, K, M, O, and P, green bars), similar to the effect of rVIG in IHD-J-Luc infections (Fig. 6M to P).
Fig. 8.
Bioluminescence and statistical analysis of total fluxes in mice that received prophylactic treatment with HuMAb compositions and were challenged with WRvFire or with IHD-J-Luc vaccinia virus. (A to H) Bioluminescence of infected mice. BALB/c mice were inoculated i.p. with HuMAb compositions C (green triangles), C1 (blue inverted triangles), C2 (orange diamonds), and F (gray squares) at 30 μg/animal or with PBS (black circles) and were challenged with WRvFire (A-D) or with IHD-J-Luc (E-H) viruses 2 days later. Animals were subjected to whole-body imaging for 10 days to record bioluminescence and calculate mean total flux ± SD in the nasal cavity (A and E), lungs (B and F), liver (C and G), and spleen (D and H). (I to P) Statistical analysis. Mean total fluxes were used to calculate the t statistic between groups of mice treated with compositions C (green bars), C1 (blue bars), C2 (orange bars), and F (gray bars) and PBS-treated mice. The dashed horizontal lines depict critical t values of 2.09 in the WRvFire challenge (10 to 12 mice per group) (I to L) and 2.28 in the IHD-J-Luc challenge (6 mice per group) (M to P).
The comparative analysis of the outcomes of the infection versus mean total fluxes further confirmed that full protection from lethality achieved with the dose of 30 μg of composition C2 in both challenge models, and by composition C in the IHD-J-Luc challenge model, correlated with a significant reduction in viral loads in at least three organs (nasal cavity, liver, and spleen) on days 3 to 5. In contrast, significant reduction of viral loads in only one organ, or reduction of viral loads in two organs but for less than 3 days in each (i.e., composition C1 in IHD-J-Luc and WRvFire models and composition C in the WRvFire challenge model), was not sufficient to prevent mortality.
These experiments demonstrated a predominant contribution of anti-EV HuMAbs in the rVIG product to protection from WRvFire and IHD-J-Luc lethal challenge. Treatment with 30 μg of composition C1 did not reduce viral dissemination and replication in the lungs and internal organs, while anti-EV antibodies in C2 significantly reduced replication of WRvFire and IHD-J-Luc virus in the nasal cavity, liver, and spleen and facilitated viral clearance by day 10 (Fig. 8). The lack of effectiveness by the HuMAbs in composition C1 may reflect the absence of antibodies targeting key protective epitopes in other MV proteins (such as L1). However, at the higher dose of 100 μg, C1 protected animals from lethality (data not shown), indicating that it is also dose dependent.
DISCUSSION
Using whole-body bioimaging, we followed the dissemination of recombinant vaccinia viruses in live mice and analyzed the mechanism of protection conferred by prophylaxis with anti-vaccinia virus IgG. Three types of human immunoglobulin therapies were tested: VIGIV, an anti-vaccinia virus IgG licensed for human use (Cangene, Canada); a new investigational fully human rVIG (Symphogen, Denmark); and subset antibody compositions of this product targeting different combinations of MV and EV proteins. The main findings were as follows: (i) BALB/c mice challenged with vaccinia virus strains that differ in the quantity of released EV particles exhibited similar lethality curves; (ii) prophylactic treatments with either VIGIV (30 mg per animal) or rVIG (100 μg per animal) protected animals from lethality following challenge with either WRvFire or IHD-J-Luc viruses; (iii) using daily bioimaging of individual mice, we demonstrated that neither prophylactic treatment prevented initial virus replication at the site of inoculation (nasal cavity) or dissemination to the lower respiratory tract (lungs) and internal organs (spleen and liver), even in surviving animals; (iv) protection from lethality correlated with significant reductions in viral loads (as measured by total fluxes) in at least three organs on days 3 to 5 postchallenge, while transient reduction for 1 or 2 days did not correlate with survival; and (v) at a 30-μg dose, a subset of HuMAbs targeting only key proteins of EV (A33 and B5) protected animals from lethal challenge with both viruses, whereas at a 100-μg dose, subsets of both anti-EV and anti-MV HuMAbs were protective (data not shown).
VIGIV and the rVIG target proteins in both MV and EV isoforms of vaccinia virus. Therefore, WRvFire and IHD-J-Luc were both used in the current study, since they both produce similar levels of MV but differ in the amounts of released EV. The more stable MV was suggested to play a key role in the virus spread between animals, whereas EV is important for dissemination within the infected host (34). In our experiments, in the absence of antibody treatment, total fluxes in WRvFire-infected animals were 0.5 to 1.0 log higher in the nasal cavity and lungs than in IHD-J-Luc-infected mice (Fig. 1C and D), thus confirming that WRvFire replicated at higher levels than IHD-J-Luc in the upper and lower respiratory tracts but not in spleen and liver. Since the times to death were identical after WRvFire and IHD-J-Luc challenge, the data suggested that the levels of viral replication in internal organs might play a primary role in determining the kinetics of lethality. For both viruses, bioluminescence measurements showed that the level of viral replication in the nasal cavity 24 h postinfection was 2 to 3 log higher than in the lungs, liver, and spleen. Thus, whole-body bioimaging revealed that the nasal cavity is a primary site of vaccinia virus replication following intranasal challenge and not only the lungs as previously reported (19).
We used two different sources of anti-vaccinia virus IgG; a licensed VIGIV from Cangene (human IgG purified from plasma of subjects vaccinated with Dryvax vaccine), and fully human recombinant VIG generated by single-cell PCR using B cells taken from human volunteers that had been vaccinated with the Lister vaccine (18). In preliminary studies, the neutralizing activities of both immunoglobulin preparations were evaluated using the β-galactosidase (β-Gal) reporter gene assay for MV neutralization (WR strain) (23) and the comet assay for EV neutralization (IHD-J strain) (A. Garcia, personal communication). In the MV neutralization assay, the 50% infectious dose (ID50) for rVIG was 0.2 μg/ml compared with 18 μg/ml for VIGIV (90-fold difference). In the comet assay, the lowest inhibitory doses were 0.23 μg/ml and 62 μg/ml, for rVIG and VIGIV, respectively (270-fold difference). These in vitro results were predictive of the higher in vivo activity of the rVIG reported in the current study. The two products were evaluated as prophylactic treatments for protection from lethality, weight loss, reduction in viral replication at the site of inoculation, and dissemination of virus to the lungs, spleen, and liver. Our results showed that both VIGIV and rVIG protected mice from lethality following WRvFire and IHD-J-Luc challenge. In the case of VIGIV, a complete protection from lethality in both viral infections was achieved at 30 mg/animal dose (or 178,800 U/kg) and was in the same range as the dose used for treatment of progressive vaccinia virus in a military smallpox vaccinee who received a cumulative dose of 186,000 U/kg given by multiple injections over a period of time (20). At the same time, 100 μg/animal of rVIG exerted full protection irrespective of the challenge virus. The difference between these two immunoglobulin preparations primarily reflects the fact that VIGIV is a preparation of total IgG, with only a fraction of vaccinia virus-specific antibodies. The 26 HuMAbs in the rVIG are vaccinia virus specific, targeting vaccinia virus MV and EV epitopes (Fig. 7) (18). Interestingly, at the fully protective dose, the rVIG and VIGIV had opposite effects on viral replication in the respiratory tract: the rVIG significantly reduced viral loads in the nasal cavity (but not in the lungs), while VIGIV was effective in reducing viral loads in the lungs but not in the nasal cavity (Fig. 4 and 6). The reason for the difference in the activities of these products in the upper and lower respiratory tracts is not clear at this point.
Several vaccinia virus proteins have been mapped as targets of neutralizing antibodies. The EV B5 and A33 proteins were identified as the main targets for protective antibodies in animal models (1, 9, 11, 15), and only B5 was confirmed as a target for the EV-neutralizing activity in sera derived from humans vaccinated with the Lister strain of vaccinia virus (31). In MV, five membrane proteins were identified as targets for neutralizing antibodies: A27, L1, H3, D8, and A17. Immunizations with A27 (13, 16, 30), L1 (9, 15), H3 (30), and D8 (30) conferred protection in mice and/or macaques. A subunit vaccine targeting the L1 (MV) and B5 (EV) protected mice against lethal intranasal challenge, confirming the benefit of combined anti-MV- and anti-EV-specific responses (17, 21). In agreement with studies in animal models, Dryvax vaccination has been shown to induce neutralizing Abs against membrane glycoproteins of both MV (L1, A27, and H3) and EV (B5 and A33) (6, 11, 21).
The rVIG contains a mixture of 26 HuMAbs targeting proteins in both MV and EV, and we showed that the 100-μg dose fully protected mice from WRvFire- and IHD-J-Luc-induced lethality. The 30-μg dose of the rVIG completely protected animals from lethality following IHD-J-Luc challenge, but only partially protected animals from WRvFire challenge (Fig. 5). These differences in the effects of rVIG could not be attributed to the difference in the composition of the WRvFire and IHD-J-Luc viral stocks, as both of them contained similar 1:10 ratios of infections to noninfectious particles (see Materials and Methods). Therefore, it was possible that in our experiments rVIG more efficiently controlled IHD-J-Luc than WRvFire dissemination. To further address this possibility, antibody compositions that contained subsets of HuMAbs in the rVIG were evaluated. The selected HuMAbs were targeting major neutralizing epitopes in MV (composition C1), EV (composition C2), or both (composition C) (Fig. 7A). At the 30-μg dose, composition C2 containing four HuMAbs targeting A33 and B5 proteins in EV protected mice from lethality following infection with both WRvFire and IHD-J-Luc. Therefore, in the case of WRvFire infection, C2 was fully protective at a dose 3-fold lower than that for the original rVIG product containing a mixture of MV and EV MAbs. These findings are in agreement with a previous report showing that protection against lethal challenge was improved when EV-specific antibodies were present along with anti-MV antibodies (19). It was somewhat unexpected that the anti-MV cocktail (composition C1) did not protect from challenge with WRvFire at the 30-μg dose. It should be noted that 30 μg is a very low dose (∼1.5 mg/kg of body weight), and as the C1 composition protected at the higher dose of 100 μg (not shown), the lack of protection at this low dose likely reflects the dose dependency of the anti-MV-mediated protection. Another contributing factor might be the lack of anti-L1 antibodies in the C1 mixture. However, other investigators have shown that there is a significant degree of redundancy in the neutralizing antibody response toward MV: following depletion of one or more neutralizing specificities, such as anti-L1, other specificities have been found to compensate for the loss (3, 13, 31). Since C1 was protective at a higher (100 μg/animal) but not at a lower (30 μg/animal) dose, future studies using fractionated composition C1 will help identify protective MAbs that might be diluted in the composition C1 by other MAbs that are not effective.
Antibodies play a crucial role in preventing secondary infection and even primary infection after active vaccination. Several mechanisms have been identified through which antibodies curtail viral replication, including blocking of the viral attachment to the cell membrane (anti-MV), inhibition of the release of EV from infected cells (8, 36), complement-dependent lysis of the EV membrane with a subsequent exposure of MV to neutralizing antibody (22), complement-dependent neutralization of the virus, and/or complement-directed lysis of infected cells (anti-EV) (2). In preclinical animal models, the protective efficacy of antibodies is traditionally evaluated by indirect assays, such as protection from weight loss and/or from lethality and by measuring of viral loads in infected organs using sensitive cell lines. Here we took advantage of bioimaging of live animals that allowed us to follow dissemination of the virus in individual animals throughout the observation period. This approach eliminates the animal-to-animal variability related to the collection of organs from different animals at multiple time points. Using bioluminescence recorded from internal organs of individual mice, we compared the mean total fluxes between IgG-treated and control mice by using the t statistic. It was found that in all cases when mice were protected from lethality, mean viral loads (fluxes) were significantly reduced in three organs simultaneously: in the respiratory tract (nasal cavity or lungs) and in the spleen and liver on days 3 to 5 postinfection. Therefore, our data indirectly confirmed that the administration of VIGIV or rVIG 2 days before virus challenge resulted in the distribution of the antibodies to all key sites of viral replication. On the other hand, our data also suggested that there is no single organ where the infection can be completely blocked using passively transferred antibodies, and initial virus dissemination could not be prevented. This finding is different from active vaccination, where dissemination to the internal organs was completely blocked, although the dose used for challenge was the same as that in the current study (39). In addition, at the fully protective doses, all three types of passive immunoglobulin treatments significantly reduced viral loads in spleen and liver, in agreement with our earlier study showing that the viral load at these sites provided the most accurate prediction of lethality versus survival outcome (39).
Eradication of smallpox was a major public health achievement that led to cessation of the immunization of general public against vaccinia virus. Nevertheless, the need for protection against smallpox has not been eliminated. An efficient and safe vaccine against smallpox requires the continuation of immunization of designated personnel in case of a bioterror attack, and as recently shown, could be useful in protection against monkeypox in populations in central Africa, who are at risk of infections due to frequent contact with animals (32).
Together, these findings provide valuable information on the impact of anti-vaccinia virus immunoglobulins on virus replication in internal organs. The same tools will be applied for the evaluation of new antiviral products (alone and in combinations) for pre- and postexposure treatments.
ACKNOWLEDGMENTS
We thank Carol Wise and Surender Khurana for critical reading of the manuscript and we thank Yamei Gao for excellent technical assistance.
This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under IAA 224-06-1322.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Bell E., et al. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425–431 [DOI] [PubMed] [Google Scholar]
- 2. Benhnia M. R., et al. 2009. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 83:1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benhnia M. R., et al. 2008. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J. Virol. 82:3751–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blasco R., Moss B. 1992. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66:4170–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cono J., Casey C. G., Bell D. M. 2003. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm. Rep. 52:1–28 [PubMed] [Google Scholar]
- 6. Davies D. H., et al. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79:11724–11733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doceul V., Hollinshead M., van der Linden L., Smith G. L. 2010. Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science 327:873–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engelstad M., Howard S. T., Smith G. L. 1992. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 188:801–810 [DOI] [PubMed] [Google Scholar]
- 9. Fogg C., et al. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230–10237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frey S. E., et al. 2009. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax in healthy vaccinia-naive adults. Vaccine 27:1637–1644 [DOI] [PubMed] [Google Scholar]
- 11. Galmiche M. C., Goenaga J., Wittek R., Rindisbacher L. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71–80 [DOI] [PubMed] [Google Scholar]
- 12. Greenberg R. N., Kennedy J. S. 2008. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin. Invest. Drugs 17:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He Y., et al. 2007. Antibodies to the A27 protein of vaccinia virus neutralize and protect against infection but represent a minor component of Dryvax vaccine–induced immunity. J. Infect. Dis. 196:1026–1032 [DOI] [PubMed] [Google Scholar]
- 14. Heraud J. M., et al. 2006. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 177:2552–2564 [DOI] [PubMed] [Google Scholar]
- 15. Hooper J. W., Custer D. M., Schmaljohn C. S., Schmaljohn A. L. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329–339 [DOI] [PubMed] [Google Scholar]
- 16. Hooper J. W., et al. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaufman D. R., et al. 2008. Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice. J. Virol. 82:6829–6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lantto J., et al. 2011. Capturing the natural diversity of the human antibody response against vaccinia virus. J. Virol. 85:1820–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Law M., Putz M. M., Smith G. L. 2005. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J. Gen. Virol. 86:991–1000 [DOI] [PubMed] [Google Scholar]
- 20. Lederman E., Groff H., Warkentien T., Reese A., Hruby D., Bolken T., Grosenbach D., Yan S., Painter W., Trost L., Lampert B., Cohen J., Engler R., Davidson W., Smith S., Wilkins K., Braden Z., Li Y., Damon I. 2009. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:532–536 [PubMed] [Google Scholar]
- 21. Lustig S., et al. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 79:13454–13462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lustig S., Fogg C., Whitbeck J. C., Moss B. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30–35 [DOI] [PubMed] [Google Scholar]
- 23. Manischewitz J., et al. 2003. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J. Infect. Dis. 188:440–448 [DOI] [PubMed] [Google Scholar]
- 24. Meijer P. J., et al. 2006. Isolation of human antibody repertoires with preservation of the natural heavy and light chain pairing. J. Mol. Biol. 358:764–772 [DOI] [PubMed] [Google Scholar]
- 25. Meseda C. A., Weir J. P. 2010. Third-generation smallpox vaccines: challenges in the absence of clinical smallpox. Future Microbiol. 5:1367–1382 [DOI] [PubMed] [Google Scholar]
- 26. Monath T. P., et al. 2004. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)–a second-generation smallpox vaccine for biological defense. Int. J. Infect. Dis. 8(Suppl. 2):S31–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Payne L. G. 1979. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J. Virol. 31:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Payne L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 50:89–100 [DOI] [PubMed] [Google Scholar]
- 29. Persson P., Engstrom A., Rasmussen L. K., Holmberg E., Frandsen T. P. 2010. Development of mass spectrometry based techniques for the identification and determination of compositional variability in recombinant polyclonal antibody products. Anal. Chem. 82:7274–7282 [DOI] [PubMed] [Google Scholar]
- 30. Pulford D. J., Gates A., Bridge S. H., Robinson J. H., Ulaeto D. 2004. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine 22:3358–3366 [DOI] [PubMed] [Google Scholar]
- 31. Putz M. M., Midgley C. M., Law M., Smith G. L. 2006. Quantification of antibody responses against multiple antigens of the two infectious forms of vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 12:1310–1315 [DOI] [PubMed] [Google Scholar]
- 32. Rimoin A. W., et al. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. U. S. A. 107:16262–16267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rotz L. D., Dotson D. A., Damon I. K., Becher J. A. 2001. Vaccinia (smallpox) vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2001. MMWR Recomm. Rep. 50:1–25; quiz CE1–CE7 [PubMed] [Google Scholar]
- 34. Smith G. L., Vanderplasschen A., Law M. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915–2931 [DOI] [PubMed] [Google Scholar]
- 35. Townsley A. C., Weisberg A. S., Wagenaar T. R., Moss B. 2006. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J. Virol. 80:8899–8908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vanderplasschen A., Hollinshead M., Smith G. L. 1997. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J. Gen. Virol. 78:2041–2048 [DOI] [PubMed] [Google Scholar]
- 37. Wharton M., Strikas R. A., Harpaz R., Rotz L. D., Schwartz B., Casey C. G., Pearson M. L., Anderson L. J. 2003. CDC. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Morb. Mortal. Wkly. Rep. 52:1–16 [PubMed] [Google Scholar]
- 38. Wittek R. 2006. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int. J. Infect. Dis. 10:193–201 [DOI] [PubMed] [Google Scholar]
- 39. Zaitseva M., et al. 2009. Application of bioluminescence imaging to the prediction of lethality in vaccinia virus-infected mice. J. Virol. 83:10437–10447 [DOI] [PMC free article] [PubMed] [Google Scholar]