Abstract
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) of cervids now detected in 19 states of the United States, three Canadian provinces, and South Korea. Whether noncervid species can be infected by CWD and thereby serve as reservoirs for the infection is not known. To investigate this issue, we previously used serial protein misfolding cyclic amplification (sPMCA) to demonstrate that CWD prions can amplify in brain homogenates from several species sympatric with cervids, including prairie voles (Microtus ochrogaster) and field mice (Peromyscus spp.). Here, we show that prairie voles are susceptible to mule deer CWD prions in vivo and that sPMCA amplification of CWD prions in vole brain enhances the infectivity of CWD for this species. Prairie voles inoculated with sPMCA products developed clinical signs of TSE disease approximately 300 days prior to, and more consistently than, those inoculated with CWD prions from deer brain. Moreover, the deposition patterns and biochemical properties of protease-resistant form of PrP (PrPRES) in the brains of affected voles differed from those in cervidized transgenic (CerPrP) mice infected with CWD. In addition, voles inoculated orally with sPMCA products developed clinical signs of TSE and were positive for PrPRES deposition, whereas those inoculated orally with deer-origin CWD prions did not. These results demonstrate that transspecies sPMCA of CWD prions can enhance the infectivity and adapt the host range of CWD prions and thereby may be useful to assess determinants of prion species barriers.
INTRODUCTION
Chronic wasting disease (CWD) is a fatal transmissible spongiform encephalopathy (TSE) of cervids, including deer, elk, and moose. CWD is the only TSE found in free-ranging wildlife; its spread in nature may reflect the dissemination of prions from saliva and excreta of infected cervids (10, 20, 22, 28, 36) and/or the persistence of infectious prions in the environment (15, 23, 30). While the known natural host range for CWD is limited to cervids, recent studies have expanded the number of noncervid species (ferrets, mink, hamsters, cattle, sheep, Peromyscus spp. mice, and voles) that can be infected experimentally (3, 11–14, 26, 32). It is not known whether noncervid species may contribute to the spread of CWD as reservoirs or vectors; however, interspecies transmission of prion diseases (e.g., from deer to noncervids) is less efficient than intraspecies transmission, a phenomenon commonly referred to as a species barrier. Species barriers may be mediated by differences in cellular PrP (PrPC) sequence (5, 13, 16), inoculum titer, route of inoculation, and other still unknown factors (4, 13, 16, 24, 25).
We recently demonstrated (18) that PrPC from several noncervid species, including prairie voles (Microtus ochrogaster) and prairie deer mice (Peromyscus maniculatus bairdii), can be converted to the protease-resistant form of PrP (PrPRES) in vitro by serial protein misfolding cyclic amplification (sPMCA). Heisey et al. (14) have since shown that other vole and Peromyscus species are indeed susceptible to intracerebral (i.c.) inoculation with CWD. To determine whether sPMCA may enhance interspecies TSE transmission, we inoculated prairie voles (M. ochrogaster) with sPMCA products consisting of deer PrPRES amplified in normal vole brain versus brain homogenate from a CWD-infected deer not subjected to sPMCA. Our studies suggest that sPMCA can facilitate the transmission of CWD to noncervid species and thereby may be useful in assessing species barriers to CWD infection.
MATERIALS AND METHODS
Sources and preparation of brain homogenates for sPMCA.
Donor animals were housed and euthanized according to IACUC-approved protocols. Transgenic mice encoding cervid PrP [Tg(CerPrP)1536+/−] (5) were housed at Colorado State University (CSU). Prairie voles (M. ochrogaster) were obtained from Thomas Curtis (University of Oklahoma) and were housed at CSU. Normal brain homogenates (NBHs) were prepared as previously described (17). CWD-positive brain homogenate D10 was prepared from a CWD-infected mule deer (generously provided by Michael Miller, Colorado Division of Wildlife) and has been used previously in several CWD bioassay and sPMCA studies (5, 17, 20, 21).
sPMCA procedure.
To prevent possible contamination, NBH was thawed on ice and loaded into 96-well plates (TempPlate III; USA Scientific) or 0.2-ml PCR tubes (Nunc/ThermoFisher Scientific) in a laboratory that was never used for prion research. The samples were then transported to the prion research laboratory, where CWD-positive brain homogenate was diluted into the NBH to a total volume of 50 μl (unseeded, NBH-only controls also comprised 50 μl). Equivalent dilutions not subjected to sPMCA were frozen at −70°C for the duration of the experiment and used for quantification of amplified samples. The samples were placed in a Misonix 4000 sonicator containing 200 ml of distilled water and subjected to 40-s bursts at power level 7 followed by 30-min incubations at 37°C for 48 h (these steps comprise one round of PMCA), and the samples were diluted 1:2 into fresh NBH for each new round. These settings yielded the most efficient amplification of PrPRES in our experiments.
Inocula.
All experimental procedures involving animals proceeded according to Colorado State University IACUC-approved protocols. For intracerebral inoculations, 10% (wt/vol) brain homogenate samples containing 1% Triton X-100 were diluted 1:10 in sterile saline containing penicillin-streptomycin (100 U/ml) to reduce detergent concentration to 0.1%. Thus, animals were inoculated intracerebrally with 25 μl of 1% (wt/vol) brain homogenate using a Kendall 3/10-ml insulin syringe inserted into the left parietal lobe. Animals were sedated using ketamine-xylazine anesthesia according to approved IACUC protocols. Upon euthanasia, the contralateral brain hemisphere was removed and fixed for histology, and the inoculated (left) hemisphere was frozen for Western blot analysis. For oral inoculation, unanesthetized animals were hand fed brain homogenate from the tip of a micropipette. Male and female animals in all studies were between 1 and 5 months old at inoculation. Animals were monitored weekly for the appearance of neurological dysfunction (ataxia, hind-limb paresis, inactivity, or hyperactivity) and more frequently upon detection of any of these symptoms.
Transgenic mice encoding cervid PrP.
Transgenic mice expressing cervid PrP [Tg(CerPrP)1536, or Tg1536 mice] (5) have been used previously in sPMCA and CWD studies (9, 17, 18, 21). Protein sequences throughout the manuscript are numbered according to the mouse sequence.
Histology and immunohistochemistry.
Brains were fixed in 10% formalin for at least 48 h. Each was then cut into six coronal sections of equal widths, treated with 88% formic acid for 1 h, rinsed continuously with water for at least 2 h, and embedded in paraffin. Paraffin-embedded sections (6 μm) were mounted onto positively charged glass slides and then heated at 65°C for 45 min, deparaffinized, and rehydrated through graded ethanol. Antigen retrieval was performed using a Retriever system and Dako Target Retrieval Solution (Dako, Hamburg, Germany). Tissues were blocked in 3% hydrogen peroxide in methanol for 1 h followed by TNB buffer (0.1 M Tris-HCl, 0.15 M NaCl, 0.5% blocking reagent [Perkin-Elmer]) for 1 h. Tg1536 mouse tissues were stained for PrP using horseradish peroxidase (HRP)-conjugated monoclonal antibody (MAb) Bar224 at a 1:1,000 dilution and tyramide signal amplification (Perkin-Elmer). Vole tissues were stained using unconjugated monoclonal antibody Bar224 at a 1:1,000 dilution followed by HRP-conjugated EnVision plus anti-mouse secondary antibody. PrPRES was visualized by incubation in aminoethylcarbazole (AEC+; Dako) for 30 min with hematoxylin counterstain and bluing reagent (0.1% sodium bicarbonate). Images were then captured using an Olympus Vanox-S microscope (Olympus) with DP70 digital camera.
Electrophoresis and Western blotting.
For in vivo studies, brains were removed, and the left hemispheres were homogenized (10%, wt/vol) in phosphate-buffered saline (PBS) containing 1% Triton X-100 before centrifugation at 2,000 × g for 1 min and removal of the supernatant for analysis by Western blotting. For sPMCA studies, samples containing Tg(CerPrP)1536+/− mouse, cat, or coyote brain homogenate were brought to a final SDS concentration of 0.25% prior to digestion with 100 μg/ml proteinase K (PK) for 30 min at 37°C followed by 10 min at 45°C. Vole samples were digested with 100 μg/ml PK for 30 min at 37°C unless otherwise noted. The different PK digestion protocols were developed to address the innate differences in PK sensitivity of PrPC between different species (18). Samples had a final volume of 10 μl after addition of PK and were boiled in 0.33% NLS (N-lauroyl sarcosine) buffer (Invitrogen) for 3 min after PK digestion.
Electrophoresis and Western blotting of sPMCA samples were performed as previously described (17, 18). After transfer, membranes were incubated in blocking solution (0.5% nonfat dry milk in PBS with 0.05% Tween 20) and antibodies using the Millipore SNAP-i.d. system. Membranes for evaluation of Tg1536 mouse, vole, mink, cat, and coyote samples were incubated in Bar224 MAb (Cayman Chemical) conjugated directly to horseradish peroxidase, except where noted. All membranes were washed using distilled H2O (dH2O) containing 0.2% Tween 20 before application of ECL-Plus chemiluminescent reagents (Amersham). Data were generated using a digital Fuji-Doc gel documentation system (Fuji) with automated detection of saturation limits, and densitometric analyses were performed using ImageGauge (Fuji) and Photoshop (Adobe) programs.
Proteinase K degradation and glycoform analyses.
For proteinase K degradation assays, 8-μl samples of 10% brain homogenate were digested with 500, 1,000, 2,000, and 4,000 μg/ml PK at 37°C for 30 min. The sizes and densities of the protease-resistant Western blot bands that resulted were measured using the Photoshop (Adobe) histogram function, and these values were plotted using Excel (Microsoft). A linear trend line was fit to the data and used to calculate the concentration of PK that would result in 50% of the signal intensity (PK50) of the most intense sample (i.e., the one digested with 500 μg/ml PK). Four experiments were used to find the average PK50 for each prion isolate. Outliers were defined as any PK50 value that differed from the others for that prion isolate by greater than 1,000 μg/ml. The results were then compared using two-tailed t tests with an α value of 0.05.
For glycoform analysis the Western blot bands corresponding to di-, mono-, and unglycosylated PrPRES were encircled with equivalently sized boxes, and signal was quantified by densitometry as described above. The fraction of total signal density corresponding to each glycoform was recorded for each sample. Sixteen separate samples from five different voles were used to evaluate vole PrPRES, and 12 separate samples were used to evaluate CWD-positive (CWD+) deer PrPRES.
Transspecies sPMCA amplification assays.
For transspecies sPMCA, 10% brain homogenates from infected voles were combined to make a pooled 10% brain homogenate. This homogenate was diluted 1:10 into 10% NBH from several species, including BALB/c mice, mink, prairie dogs, transgenic mice expressing human PrPC [Tg(HuPrP) mice], domestic cats, and coyote and subjected to sPMCA and Western blotting as described previously (18). Samples were subjected to three rounds of sPMCA with 1:2 dilutions into fresh NBH at each subsequent round. Unseeded, NBH-only negative-control samples were processed in tandem to confirm that spontaneous PrPRES formation did not occur and that protease digestion was complete.
RESULTS
Prairie voles are susceptible to CWD.
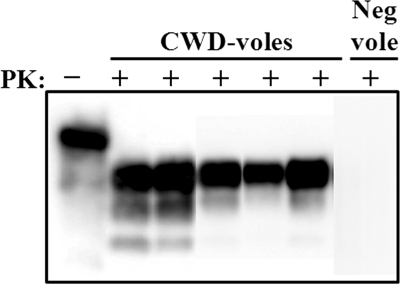
To investigate whether prairie voles (Microtus ochrogaster) might be susceptible to mule deer-origin CWD, we inoculated animals (in cohorts of 9) with 25 μl of 1% (wt/vol) CWD+ mule deer (D10) brain homogenate by the intracerebral (i.c.) route. The D10 brain homogenate has been used in several previous bioassay and sPMCA studies (5, 17, 18, 20, 21). One of the 9 voles inoculated with D10 deer brain homogenate developed distinct clinical symptoms of neurological disease, including hyperactivity, ataxia, and hind limb paresis, at 463 days postinoculation (dpi) and was euthanized. The other animals displayed less-specific signs of illness, such as lethargy and ataxia, between 221 and 734 dpi and were euthanized in accordance with CSU IACUC guidelines. Subsequent Western blot analysis demonstrated PrPRES in 5 of 6 animals (83.33%) that survived more than 221 dpi (Fig. 1). For simplicity, these animals are referred to as CWD-voles in later analyses. Two additional animals that were killed by their cage mates at 168 and 173 dpi were negative for PrPRES by Western blotting. One animal that died 6 days after inoculation was not analyzed. Negative-control animals were inoculated i.c. with brain homogenate from a CWD-negative deer and survived more than 700 dpi. All negative-control voles were negative for PrPRES by Western blotting.
Fig. 1.
Prairie voles are susceptible to CWD. Ten percent brain homogenates from voles (CWD-voles) inoculated with CWD-positive mule deer brain D10 after digestion with 100 μg/ml PK. Neg vole, brain homogenate from a vole inoculated with CWD-negative deer brain; +, PK-digested samples; −, samples not digested with PK. This image is a composite of three Western blots.
Immunohistochemistry (IHC) revealed PrPRES deposition throughout the brain of three voles (Fig. 2A to H). PrPRES staining was heavy in the thalamus (Fig. 2E), and scant to minimal PrPRES was seen in the hippocampus (Fig. 2C and D). In the cerebellum, cell-associated and clumped PrPRES was identified within the granular layer (Fig. 2F). Large (“florid”) PrPRES plaques were not observed. Spongiform degeneration, with vacuoles ∼5 to 20 μm in diameter (Fig. 3), appeared to generally colocalize with PrPRES deposition. These IHC data contrasted with those from Tg(CerPrP)1536 mice (Fig. 2S to ZZ), in which PrPRES was found primarily as plaques in the hippocampus. These results are consistent with the findings of Heisey et al. (14), who recently demonstrated that other North American vole species (i.e., Microtus pennsylvanicus and Myodes gapperi) are susceptible to CWD infection.
Fig. 2.
Prairie voles accumulate PrPRES in diverse brain regions. (A to H) Prairie voles inoculated with CWD-infected deer brain D10. In the forebrain, PrPRES (red staining) was heaviest in the ventral and medial gray matter of the cerebral cortex and was multifocal to regionally extensive (A). Clumped aggregates of PrPRES were seen in the tunica adventitia of many, mostly meningeal, vascular channels in the forebrain of 2/3 IHC-positive (IHC+) animals (B). Scant to minimal PrPRES was seen in the hippocampi of 3/3 IHC+ animals, with fine to coarsely granular aggregates in the CA1-CA3 layers (C) and in the stratum granulosum (D). Coarsely granular to clumped, consistently cell-associated aggregates of PrPRES are shown in the thalamus and hypothalamus (E). In the cerebellum, PrPRES was identified in 3/3 IHC+ animals and was consistently cell associated and clumped within the granular layer (F). In the brain stem, multifocal to regionally extensive accumulations of PrPRES were identified in 3/3 IHC+ animals, which was both cell and neuropil associated (G). In 1/3 IHC+ animals, heavy amounts of granular to clumped PrPRES was identified in the ependymal epithelial cells (H). (I to R) Prairie voles inoculated with vPMCA-PrPRES. In the forebrain, PrPRES was identified in 8/8 IHC+ animals and was heaviest in the gray matter of the cerebral cortex including the ventral and medial gray matter (I). In 4/8 IHC+ animals, scattered mild to moderate, fine to coarsely granular PrPRES was identified within the putamen and caudate nucleus (J). In the hippocampus, scant to minimal PrPRES was seen in 5/8 IHC+ animals with fine to coarsely granular aggregates seen in the CA1-CA3 layers (K) and in the stratum granulosum (L). In the thalamus and hypothalamus, coarsely granular to clumped, consistently cell-associated aggregates of PrPRES were identified in 8/8 IHC+ animals (M). In 4/8 IHC+ animals, coarsely granular to clumped aggregates of PrPRES were identified in the medial habenular nucleus (N). In 8/8 IHC+ animals, cell-associated and clumped PrPRES was consistently identified in the granular layer of the cerebellum (O). In 2/8 animals, sparse depositions of PrPRES were seen in the molecular and Purkinje layers (P). In 3/8 IHC+ animals, heavy amounts of granular to clumped PrPRES were identified in the ependymal epithelial cells (Q). In the brain stem, multifocal to regionally extensive accumulations of granular to clumped PrPRES were identified in 8/8 IHC+ animals, which was both cell associated and regionally disseminated throughout the neuropil (R). (S to ZZ) Tg(CerPrP)1536 mice inoculated with CWD-infected deer brain D10. Similar patterns of PrPRES immunoreactivity were observed in 7/7 evaluated animals. In the forebrain, PrPRES was extensively distributed throughout the gray matter of the cerebral cortex (S and T). Heavy amounts of clumped PrPRES were seen in the caudoputamen, with the densest aggregates within the periventricular gray matter and ependymal epithelial cells (U). Heavy, clumped, and largely extracellular PrPRES aggregates were observed throughout the entirety of the hippocampal formation, including the pyramidal layer (V), the stratum radiatum of field CA1 (W), and field CA2 (X). Moderate to marked, finely granular to clumped PrPRES deposits were seen in the periaqueductal gray matter (Y). Cell and non-cell-associated PrPRES was observed in the granular (Z) and molecular (ZZ) layers of the cerebellum. Scale bars, 25 μm (A, C, D, I, J, L, and R), 40 μm (F, G, K, M, O, P, Q, Z, and ZZ), 60 μm (E, N, S, T, U, V, W, X, and Y), and 75 μm (B and H).
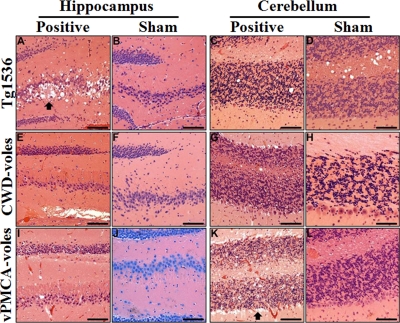
Fig. 3.
Neuropathology in infected prairie voles and Tg1536 mice. Hematoxylin- and eosin-stained hippocampal and cerebellar sections from terminal CWD (Positive) and sham-inoculated (Sham) Tg(CerPrP)1536 mice (A to D) and voles (E to L). (A to D) Neuropil spongiosis and plaque formation (arrow) were observed only in the hippocampi of CWD-inoculated Tg(CerPrP)1536 mice (A) but not sham-inoculated animals (B). No degeneration was seen in the cerebellum of CWD-infected (C) or sham-inoculated (D) Tg(CerPrP)1536 mice. (E to H) Prairie voles (CWD-voles) inoculated with CWD-infected deer brain D10 (Positive) or with negative deer brain (Sham). Significant neurodegeneration was not identified in hippocampal or cerebellar sections. (I to L) Prairie voles (vPMCA-voles) inoculated with vPMCA product generated by PMCA (Positive) or with NBH from PMCA (Sham). No degeneration was seen in the hippocampus of infected (I) or sham-inoculated (J) voles. Marked loss of granular cell neurons and Purkinje cells (arrow) was noted in the cerebellar cortex of infected vPMCA-voles (K) but not sham-inoculated voles (L). Scale bars, 500 μm (A to G, I, K, and L), 150 μm (H), and 300 μm (J).
sPMCA enhances CWD infection in prairie voles.
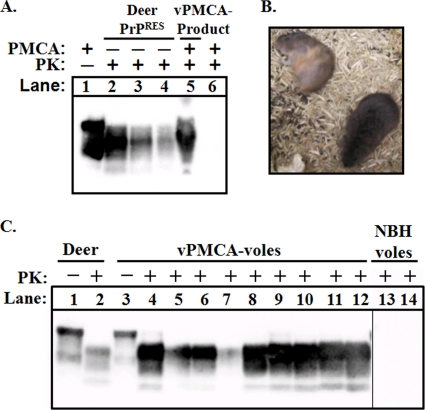
We previously demonstrated that normal brain homogenates (NBH) from prairie voles support amplification of mule deer CWD prions by sPMCA (18). To investigate whether sPMCA can be used to generate infectious prions in vitro, we inoculated prairie voles with the products from PMCA amplification of D10 in prairie vole NBH. This procedure resulted in PrPRES material we refer to as vPMCA-product. Briefly, to generate vPMCA-product, D10 was diluted 1:200 into vole NBH and amplified for nine rounds of sPMCA with 1:2 dilutions into fresh NBH at each round. The final concentration of D10 in vPMCA-product was 1:51,200 after nine rounds of sPMCA and 1:512,000 after the material was diluted 1:10 for i.c. inoculation. We used densitometric analysis to estimate the total PrPRES concentration of vPMCA-product relative to D10, and found it to be equivalent to an ∼1:600 dilution of D10 brain (Fig. 4A). Thus, the vPMCA-product would have been equivalent to an ∼1:6,000 dilution of D10 after 1:10 dilution for i.c. inoculation of voles. No PrPRES was detected in unseeded vole NBH during nine rounds of sPMCA.
Fig. 4.
Voles are susceptible to PrPRES generated by transspecies PMCA. (A) CWD-positive deer brain D10 was diluted 1:200 into prairie vole NBH and subjected to nine rounds of PMCA. The final dilution of D10 in the amplified sample (lane 5) was 1:51,200. Lanes 2 to 4 show 1:400, 1:800, and 1:1600 dilutions of D10 not subjected to PMCA (−), respectively. Dilutions of D10 greater than 1:10,000 are not detectable by Western blotting. Lane 1 is 1/10 the volume of D10 brain not subjected to PMCA or PK digestion. Lane 6 is unseeded vole NBH after PMCA and PK digestion. This panel is representative of three independent experiments. (B) Infected voles displayed signs of neurological disease including frequently rolling into dorsal recumbency (animal in upper left). (C) All nine voles (vPMCA-voles) inoculated with PrPRES generated by transspecies PMCA (vPMCA-product) (see legend of Fig. 2A) were positive for PrPRES by Western blotting (lanes 4 to 12). Lanes 1 and 2, samples from CWD-infected deer D10 shown for comparison; lanes 13 and 14, brain homogenate from voles inoculated with unseeded, NBH-only samples from the PMCA experiment shown in Fig. 2A. These samples were subjected to the same PK conditions and were present on the same blot (but separated by additional lanes).
All nine voles i.c. inoculated with 25 μl of vPMCA-product (vPMCA-voles) developed symptoms of neurological disease between 230 and 282 dpi, including rapid movement and ataxia, frequently rolling into dorsal recumbency with difficulty in regaining normal, sternal posture (Fig. 4B). The presence of PrPRES in all vPMCA-inoculated voles was confirmed by Western blotting (Fig. 4C) and IHC (Fig. 2I to R). In vPMCA-voles, PrPRES was evident throughout the brain, with notable accumulation in the thalamus (Fig. 2 M). PrPRES deposits were primarily diffuse and granular and associated with vacuoles ∼5 to 20 μm in diameter. Compared with Tg(CerPrP)1536 mice infected with CWD, brains of vPMCA-voles had less overall PrPRES staining in the hippocampus and no large plaque-like deposits.
Another histological feature in vPMCA-voles was the presence of cell-associated and clumped PrPRES consistently identified in the granular layer of the cerebellum (Fig. 2O), together with prominent Purkinje cell loss in the cerebellar cortex (Fig. 3K). These IHC findings were also present in CWD-voles, but PrPRES deposition was greater and more consistent. Two negative-control groups comprised voles that were inoculated with unseeded prairie vole NBH that had been subjected to nine rounds of sPMCA or D10 diluted 1:51,200 (the equivalent of the final D10 concentration of vPMCA-product). No animals in these groups demonstrated clinical signs of prion disease or PrPRES in the brain after 700+ dpi (Fig. 4C shows representative examples). The quantity of PrPRES in the vPMCA-product inoculum the vPMCA-voles received was determined by densitometry to correspond to an ∼1:6,000 dilution of D10 with respect to the PrPRES in D10. These vPMCA-voles were euthanized by 239 ± 17 dpi due to signs of neurologic disease (Table 1). This survival period compares with that produced by i.c. inoculation of Tg(CerPrP)1536 mice with a 1:500 dilution of D10 (greater than 1,000-fold the D10 concentration and greater than 10-fold the PrPRES present in the inoculum received by the vPMCA-voles), in which all animals became symptomatic and were euthanized by 259 ± 20 dpi (Table 1).
Table 1.
Attack rates and incubation periods
| Species (group) | Inoculum | Inoculation route | Western blotting result (no. of positive animals/no. tested) | Survival time (dpi)d |
|---|---|---|---|---|
| Prairie vole (CWD-voles) | CWD-positive deer brain | Intracerebral | 5/8c | 540 ± 204 |
| Prairie vole (vPMCA-voles) | vPMCA-product | Intracerebral | 9/9 | 239 ± 17e |
| Tg(CerPrP)1536 mouse | CWD-positive deer brain | Intracerebral | 9/9 | 259 ± 20 |
| Prairie vole | vPMCA-producta | Oral | 5/9 | 462+f |
| Prairie vole | CWD-positive deer brain D10b | Oral | 0/9 | 500–833 |
| Prairie vole and Tg(CerPrP)1536 | Unseeded NBH from PMCA or CWD-negative deer brain | Intracerebral or oral | 0/9 | >600 |
Final dilution of D10 equivalent to 1.5 × 10−6.
Final dilution equivalent to 1:10.
The attack rate is 5/6 if two voles that died before 200 dpi are excluded from the analysis.
Standard deviations are indicated for some values.
This incubation period was significantly different from that of CWD-voles (P < 0.05) and Tg(CerPrP)1536 mice (P < 0.05).
One animal in this group showed neurologic signs at 462 dpi, and another showed signs at 646 dpi. The other animals in this group did not show distinct clinical signs, but 5/9 animals in total were positive by Western blotting.
Glycoform ratios and migration patterns.
No differences in glycoform ratios between the vPMCA-voles and CWD-voles were noted. PrPRES from vPMCA-voles displayed a significantly higher proportion of the diglycosylated (P = 0.0002, two-tailed t test) and less of the monoglycosylated (P = 0.004, two-tailed t test) and unglycosylated (P = 0.0002, two-tailed t test) PrPRES forms than CWD-infected deer brain (data not shown).
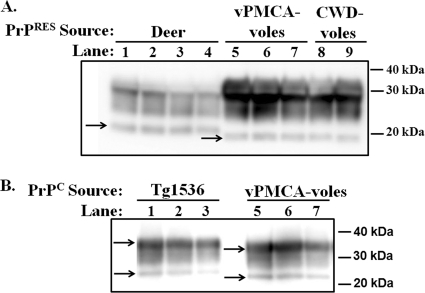
The Western blot migration patterns of PrPRES from CWD-voles and vPMCA-voles were indistinguishable; however, PrPRES from both groups migrated faster than mule deer PrPRES (Fig. 5A). This difference appeared to be due to innate differences in the electrophoretic mobility of vole versus cervid [i.e., Tg(CerPrP)1536 mouse] PrPC (Fig. 5B). This result was confirmed using multiple antibodies, including Bar224, SAF32 (shown in Fig. 5B), SAF84, and 8G8. Semiquantitative estimation of PrPC concentration using multiple antibodies revealed that Tg(CerPrP)1536 NBH contained at least two to five times more PrPC than vole NBH (not shown).
Fig. 5.
Electrophoretic mobility of vole versus deer PrP. (A) Lanes 1 to 4, samples from CWD-positive deer D10; lanes 5 to 7, samples from voles inoculated with PrPRES generated by amplification of D10 in vole NBH; lanes 8 and 9, samples from voles inoculated with CWD-positive deer brain D10 not subjected to PMCA. All samples were subjected to PK digestion prior to Western blotting. Arrows indicate the unglycosylated band of PrPRES. (B) Samples from Tg(CerPrP)1536 mice and vPMCA-voles, as indicated, not digested with PK. Arrows indicate diglycosylated (top band) and unglycosylated (bottom band) PrPC from each isolate.
Proteinase K degradation assays.
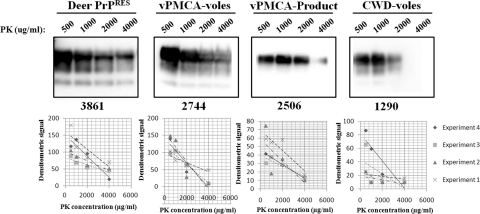
For proteinase K (PK) degradation assays, 8-μl samples of 10% brain homogenate were digested with 500, 1,000, 2,000 and 4,000 μg/ml PK (Fig. 6, upper panels) and the resulting protease-resistant Western blot bands were measured by densitometry. A linear trend line was fit to the data (Fig. 6, lower panels) and used to calculate the concentration of PK that would result in Western blot bands containing 50% the signal intensity (PK50) of the most intense sample (i.e., the sample digested with 500 μg/ml PK). The average PK50 was 3,860 μg/ml (standard deviation [SD], 703) for deer PrPRES, 2,744 μg/ml (SD, 1,012) for vPMCA-vole PrPRES, 2,505 μg/ml (SD, 964) for vPMCA-product, and 1,289 μg/ml (SD, 340) for CWD-vole PrPRES (Fig. 6). Interestingly, the PK degradation profiles for vPMCA-product, vPMCA-voles, and CWD-voles were significantly different from those for deer PrPRES (P = 0.035, 0.029, 0.00007, respectively, two-tailed t test using average PK50 from four experiments for each isolate). While the degradation profile of vPMCA-product was not significantly different from either the PrPRES of CWD-voles (P = 0.10, two-tailed t test) or vPMCA-voles (P = 0.733, two-tailed t test), the PrPRES from CWD-voles was significantly different from that of vPMCA-voles (P = 0.04, two-tailed t test). These results indicate that in vitro passage of deer PrPRES in vole brain homogenate resulted in a product with a PK degradation profile that was intermediate to PrPRES from voles inoculated with deer CWD and voles inoculated with vPMCA-product, suggesting that sPMCA may assist adaptation of certain prion strains to a new host species.
Fig. 6.
Proteinase K degradation assay. Brain homogenates (10%, wt/vol) from CWD-positive deer D10 (Deer PrPRES), D10 amplified in vole NBH by PMCA (vPMCA-product), voles inoculated with vPMCA-Product (vPMCA-voles), and voles inoculated with D10 (CWD-voles) were subjected to digestion with 500 to 4,000 μg/ml PK. The PK degradation assay was repeated at least four times for each isolate. The results were plotted on a graph (below each blot), and average PK concentration required to decrease the Western blot signal by half, relative to the 500 μg/ml lane, was found using the linear regression lines (given as a whole numeral under each representative Western blot).
Transspecies sPMCA assays.
We previously demonstrated (18) that mule deer prions (D10 isolate) do not amplify efficiently in normal brain homogenate from several species, including BALB/c mice, mink, transgenic mice expressing human PrPC [Tg(HuPrP) mice], cats, coyotes, and prairie dogs. In further attempts to differentiate deer CWD PrPRES from PrPRES generated by transspecies sPMCA followed by in vivo passage in prairie voles, we tested whether NBH from any of the noncervid species above would support amplification of PrPRES from vPMCA-voles. Three differences were detected. In contrast to deer-origin D10, PrPRES from vPMCA-voles consistently amplified in cat, coyote, and mink NBH (Fig. 7). Neither the vPMCA-vole PrPRES nor D10 prions amplified in NBH from any of the other species mentioned above (data not shown). As previously noted (18), when amplification did not occur, the Western blot signal of input D10 PrPRES decreased with successive rounds of sPMCA (Fig. 7).
Fig. 7.
PrPRES from infected voles amplifies in cat, coyote, and mink NBH by transspecies PMCA. CWD-positive deer brain D10 (PrPRES source deer) and pooled brain homogenate from vPMCA-voles were diluted 1:100 into cat (A), coyote (B), and mink (C) NBH. Two to three replicate samples were subjected to PK digestion (PK+) after three rounds of PMCA (PMCA+). The final dilution of each sample was 1:400, represented by a nonamplified (PMCA−) 1:400 dilution for reference (lanes 2 and 7 in panels A and B; lanes 1 and 5 for panel C). Unseeded NBH subjected to three rounds of PMCA followed by PK digestion (NBH) was used in lanes 5 and 6 and 10 and 11 in panels A and B and lanes 9 to 11 in panel C. Samples not digested with PK (PK−) demonstrate PrPC present in the NBH. Results are representative of three experiments.
We previously showed that position 170 of the substrate PrPC plays an important role in determining the ability to support amplification of PrPRES by sPMCA (18). To investigate whether this position affects amplification of PrPRES from vPMCA-voles, we diluted brain homogenate from D10 or from vPMCA-voles in NBH from red-backed voles (Myodes gapperi) for sPMCA because red-backed voles are polymorphic at position 170 of PrP and may express asparagine or serine (or both, if heterozygous) at this position. As pictured, D10 amplified most efficiently in NBH from animals homozygous for asparagine at PrP position 170 (170 N/N animals) (Fig. 8A, top panel), and amplified very poorly in NBH from animals that were homozygous for serine at this position (170 S/S animals). In contrast, PrPRES from vPMCA-voles (prairie voles are homozygous for asparagine at position 170) amplified equally well in NBH from animals of any of the three genotypes (Fig. 8A, bottom panel). For D10, average densitometric signal was approximately five times greater when amplified in 170 N/N voles than in 170 S/S voles (Fig. 8B), whereas for vPMCA-voles, average densitometric signal was less than 1.2 times greater in 170 N/N than in 170 S/S voles (Fig. 8B).
Fig. 8.
The presence of serine at PrP position 170 is associated with poor amplification of deer, but not vole, PrPRES. (A) CWD-positive deer brain D10 (Deer brain homog.) and pooled brain homogenate from vPMCA-voles were diluted 1:100 into red-backed vole (Myodes gapperi) NBH. Two replicate samples were subjected to three rounds of PMCA (PMCA+), followed by PK digestion (PK+). The final dilution of each sample was 1:400, represented by a nonamplified (PMCA−) 1:400 dilution for reference (lane 2). Unseeded NBH subjected to three rounds of PMCA followed by PK digestion (NBH) was used in lanes 11 and 12. Samples not digested with PK (PK −, lane 1) demonstrate PrPC present in the NBH. Results are representative of three experiments. (B) Average densitometric scores (arbitrary units) from panel A were plotted on a graph. Results from experiments using vPMCA-vole brain as seed are represented by triangles and a solid linear regression line, and experiments using deer brain as seed are represented by circles and a dashed linear regression line.
PMCA enables oral transmission of CWD prions to prairie voles.
To investigate whether prairie voles might be orally susceptible to CWD, we inoculated animals (in cohorts of 9) with either (i) 50 μl of 1% (wt/vol) CWD+ mule deer D10 brain homogenate or (ii) 35 μl of PrPRES generated by amplification of D10 in prairie vole NBH (vPMCA-product). To generate this vPMCA-product, D10 was diluted 1:18,750 into vole NBH and amplified for three rounds of sPMCA with 1:2 dilutions into fresh NBH at each round. The final concentration of D10 in the vPMCA-product was 1.5 × 10−6 after three rounds of sPMCA. Control animals were orally inoculated with unseeded NBH from the same PMCA experiment and with deer brain from a CWD-negative deer. No control animals developed clinical signs of neurologic disease, nor were any of these animals positive for PrPRES by Western blotting after 600 to 700+ dpi. Furthermore, none of the animals inoculated orally with 1:100 D10 CWD+ deer brain was positive after 500 or more dpi (Table 1). Three animals in this group were observed for 833 dpi and did not show clinical signs of neurologic disease or PrPRES by Western blotting.
In contrast, the brains of 5 of 9 (Table 1) animals orally inoculated with PrPRES generated by amplification of D10 in prairie vole NBH, a final D10 dilution of 1.5 × 10−6, were positive for PrPRES by Western blotting (Fig. 9). One of these animals showed clinical signs of neurologic disease including lethargy and hindlimb paresis and was euthanized at 462 dpi (animal 2266). A second animal showed clinical signs and was euthanized at 646 dpi (Fig. 9, animal 2269). The last animal to show clinical signs was euthanized at 735 dpi, at which point any remaining animals were also euthanized and analyzed by Western blotting (Fig. 9).
Fig. 9.
Prairie voles inoculated orally with brain homogenate from PMCA accumulate PrPRES. Nine prairie voles (animal numbers 2266 to 2275) were inoculated orally (PO) with 35 μl of brain homogenate produced by cyclic amplification of D10 CWD+ deer brain in vole NBH (vPMCA-product). The final dilution of D10 in the inoculum was 1.5 × 10−6. Animal 2266 showed clinical signs and was euthanized at 462 dpi; animal 2269 showed clinical signs and was euthanized at 646 dpi. The others were euthanized by 735 dpi. Animals 2267 and 2270 showed evidence of PrPRES on immunoblots that were markedly overexposed (data not shown). Brain samples were subjected to 25 μg/ml PK digestion, except for the sample in lane 10, which shows vole PrPC. All samples contained a total volume of 20 μl of 10% brain homogenate, except lanes 1 and 2 and lane 10, which contained 1/10 that volume.
DISCUSSION
In the present study we demonstrate that PrPRES generated in vitro by transspecies sPMCA is infectious. Prairie voles are an outbred species with the probability of exposure to CWD in nature. All nine voles inoculated with sPMCA-generated prions, nominally equivalent to a 1:512,000 dilution of the initial mule deer brain D10 seed, developed clinical signs of TSE and were positive for PrPRES by Western blotting by 239 ± 17 dpi. This contrasted with voles inoculated with a 1:512,000 dilution of D10 not subjected to sPMCA, which developed neither clinical signs of CWD nor detectable levels of PrPRES in the brain. We also demonstrate that in vitro amplification of deer CWD PrPRES in prairie vole brain homogenates enhances transmission of disease to that species. Prairie voles inoculated with vPMCA-product developed neurologic disease more rapidly than Tg(CerPrP)1536 mice (239 ± 17 dpi versus 259 ± 20 dpi, respectively, P < 0.05), despite the lower PrPRES titer of the vPMCA-product inoculum. Densitometric analysis revealed that the vPMCA-PrPRES inoculum was equivalent to an ∼1:6,000 dilution of D10 with respect to PrPRES, whereas Tg(CerPrP)1536 mice received a 1:500 dilution of D10. Thus, voles received ∼10-fold less PrPRES than the Tg(CerPrP)1536 mice yet still succumbed to disease more rapidly than the Tg(CerPrP)1536 mice. The amount of seed D10 in the vPMCA-product is about 1/1,000 (500/512,000) of the D10 in the inoculum the Tg(CerPrP)1536 received. Furthermore, PrPC levels in the central nervous system (CNS) of Tg(CerPrP)1536 mice are four to five times higher than in deer (17) or wild-type FVB mice (5) and at least two to five times higher than prairie voles (not shown). Castilla et al. (6) recently demonstrated that mouse prions could be amplified in normal brain homogenate substrate from hamsters, and vice versa, and that this was followed by enhanced transmission to the species used for substrate. Similarly, Green et al. (9) demonstrated that amplification of mouse scrapie in NBH from Tg(CerPrP)1536 mice generated prions that were transmissible to Tg(CerPrP)1536 mice. Our studies reinforce these findings using CWD prions and prairie voles, an outbred species whose geographic range overlaps that of CWD-infected cervids and that thereby may be exposed to CWD prions in nature (2, 38). Importantly, our results suggest that if prairie voles or other rodent species become naturally infected with CWD, the resultant oral infectivity may be enhanced for that species.
The sPMCA adaptation of CWD for the prairie vole M. ochrogaster presented in this report is qualitatively consistent with in vivo adaptation results we are observing in the meadow vole (M. pennsylvanicus), a closely related species. Heisey et al. (14) previously reported a median survival time of 270 dpi for M. pennsylvanicus i.c. challenged with CWD-positive Wisconsin white-tailed deer brain homogenate. With a single additional i.c. passage, the median survival dropped to 190 dpi (95% confidence interval [CI], 111 to 228), and similar decreases continue to be observed on successive serial passages out to the fifth passage. Of 8 meadow voles orally challenged with the same CWD isolate used by Heisey et al. (14), no animals ever displayed clinical signs, and all were negative by Western blotting up to 484 dpi. In contrast, oral challenge of 14 meadow voles with a PrPRES-positive brain homogenate from meadow voles clinically affected with CWD yielded two clinical and immunoblot-positive animals at 344 and 507 dpi. In addition to meadow voles, preliminary results from the three other rodent species described by Heisey et al. (14) indicate similar patterns of adaptation upon serial passage; for example, the initial median survival time of 595 dpi for P. maniculatus dropped to 305 dpi (95% CI, 193 to 376) with a single additional passage.
The inability of deer-origin CWD isolate D10 to efficiently amplify in NBH from 170 S/S Myodes animals in the sPMCA reaction is intriguing. It might seem reasonable to argue that this arises because the genotype of the D10 PrP is 170 N/N (true for cervids in general), and thus the inefficiency reflects a simple mismatch. However, this fails to explain why PrPRES from prairie voles, which also have the 170 N/N genotype, efficiently amplifies in brain homogenate from 170 S/S Myodes animals by sPMCA. This sPMCA result is, however, consistent with the findings of Heisey et al., who observed that the susceptibility of Myodes 170 S/S animals to intracerebral CWD challenge, as measured by survival time, was intermediate between that of M. pennsylvanicus and P. maniculatus, both of which possess the 170 N/N genotype (14). These results suggest that the effect of a PrP 170 S/N mismatch on CWD amplification is not absolute and that adaption can eventually lead to efficient conversion even in the presence of a 170 S/N mismatch.
The pattern of PrPRES deposition in the CNS of infected prairie voles was distinct from that in deer and Tg(CerPrP)1536 mice (5, 8, 31, 34, 37). PrPRES accumulation was principally in the thalamus, hippocampus, cerebral cortex, and granular layer of the cerebellar cortex. Deposits were generally granular and diffuse, and no large plaques were observed. Purkinje cell loss was detected in the cerebellar cortex, with only sparse PrPRES deposition in the Purkinje layer. In contrast, CWD-infected Tg(CerPrP)1536 mice displayed high levels of PrPRES in the hippocampus and cerebral cortex, with less accumulation in the thalamus and cerebellum. PrPRES deposits in the Tg(CerPrP)1536 mice typically occurred as large, patchy plaques with no loss of Purkinje cells. However, the latter difference may be due to the lack of PrPC expression by Purkinje cells in this transgenic mouse strain (7, 27). The differences in neuropathological features between CWD-infected voles and Tg(CerPrP)1536 mice are consistent with the alterations in strain properties sometimes observed upon transspecies passage of CWD and other TSEs (26, 35, 39).
The unique pathological features of infected voles may also provide some insights into prion disease mechanisms. Diseased voles displayed significant PrPRES accumulation in the granule cell layer but not in the Purkinje or molecular layers of the cerebellum, suggesting that Purkinje cell loss in these animals may be associated with an indirect mechanism of neurodegeneration or non-PrPRES conformers of the prion protein. Interestingly, certain strains of PrP-deficient mice (Ngsk, Zürich II, and Rcm0) exhibit marked Purkinje cell death although this phenotype appears to be due to overexpression of the PrP-like Doppel protein versus the absence of PrPC expression in these cells (1, 27, 29, 33). Further studies may elucidate the mechanisms underlying this aspect of neurodegeneration in voles.
We used PK degradation assays to further compare vole versus cervid PrPRES and found that the PK degradation profiles for all of the vole-associated PrPRES isolates were significantly different from our deer PrPRES isolate (P < 0.05, two-tailed t test). Although we used one mule deer CWD isolate (D10), this CWD isolate is the source of all of our in vitro and in vivo vole experiments, and we performed the assays several times to increase the accuracy of our results. Interestingly, the degradation profile of vPMCA-product appeared to be intermediate to PrPRES from voles inoculated with deer CWD versus voles inoculated with vPMCA-product, a finding that corroborates the results of Meyerett et al. (21) comparing the stability of D10 after in vivo versus in vitro passage (i.e., sPMCA) in Tg(CerPrP)1536 mouse brain. These results suggest that both in vivo and in vitro passage affect the stability of PrPRES.
We also used transspecies sPMCA to distinguish vole and deer PrPRES in vitro, a novel application of this technology similar to the cell panel assay (19). We show that in vivo vole-passaged PrPRES amplifies in normal brain homogenates from cats, coyotes, and mink, whereas mule deer CWD isolate D10 does not. To our knowledge, this is the first demonstration of prion amplification in canid brain homogenate. We attribute this finding to changes in CWD prion strain properties subsequent to adaption to a new host species (prairie voles). Furthermore, vole-passaged prions amplified efficiently in NBH from red-backed voles that express asparagine or serine at PrP position 170, whereas mule deer CWD isolate D10 amplified efficiently only in Pr 170 N/N substrate. These results indicate that, in contrast to deer-origin CWD prions, vole-passaged CWD prions have altered physical properties that, in turn, alter the species barriers to transmission. Thus, sPMCA may be useful in typing and characterizing prion strains. The conversion of cat and coyote PrPC to PrPRES is particularly interesting in that it suggests that these carnivore species may be somewhat susceptible to prions from other CWD-infected noncervid species because amplification in these species gives rise to prions with broadened host range and transmissibility.
Finally, we demonstrate for the first time that sPMCA enhances transspecies transmission of prions by the oral route—a less-well-characterized but perhaps more important criterion for species adaptation. In the present study we inoculated voles orally with prions generated by three rounds of sPMCA in vole NBH (resulting in a 1.5 × 10−6 final dilution of deer-origin D10). A second cohort of voles was inoculated orally with prions from CWD deer brain D10 not subjected to sPMCA. Whereas 5 of 9 voles receiving CWD prions amplified in vole brain homogenate were positive for intracerebral accumulation of PrPRES by 462 to 735 dpi, none of the voles that were inoculated with a 10% homogenate of CWD+ deer brain developed signs of disease or were any positive for PrPRES during a comparable or longer time period. The differences in clinical signs in orally versus intracerebrally inoculated voles may have been due to route of infection and brain regions affected, a question we plan to address in subsequent studies comparing incubation periods, attack rates, and neuropathology in the new vole host versus the well-characterized cervidized mouse.
In conclusion, we show that transspecies sPMCA amplification of CWD prions in vole brain substrate enhances their infectivity for voles in vivo—an adaptation phenomenon historically requiring serial subpassages in animals. Concurrently, transspecies sPMCA also has the capacity to alter the CWD prion species barrier. Thus, sPMCA offers insight into prion strain properties, transmission barriers, and species adaptation.
ACKNOWLEDGMENTS
This work was supported by contract NO1-AI-25491 from NIH/NIAID, grant D07ZO-072 from the Morris Animal Foundation, and grant NCRR-T32-RR07072.
We thank Candace Mathiason, Mark Zabel, and Nicholas Haley for help and advice and Jeanette Hayes-Klug and Sheila Hays for excellent animal care.
Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Anderson L., Rossi D., Linehan J., Brandner S., Weissmann C. 2004. Transgene-driven expression of the Doppel protein in Purkinje cells causes Purkinje cell degeneration and motor impairment. Proc. Natl. Acad. Sci. U. S. A. 101:3644–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker R. 1983. Michigan mammals. Wayne State University, Detroit, MI [Google Scholar]
- 3. Bartz J. C., Marsh R. F., McKenzie D. I., Aiken J. M. 1998. The host range of chronic wasting disease is altered on passage in ferrets. Virology 251:297–301 [DOI] [PubMed] [Google Scholar]
- 4. Bartz J. C., McKenzie D. I., Bessen R. A., Marsh R. F., Aiken J. M. 1994. Transmissible mink encephalopathy species barrier effect between ferret and mink: PrP gene and protein analysis. J. Gen. Virol. 75:2947–2953 [DOI] [PubMed] [Google Scholar]
- 5. Browning S. R., et al. 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J. Virol. 78:13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castilla J., et al. 2008. Crossing the species barrier by PrP(Sc) replication in vitro generates unique infectious prions. Cell 134:757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischer M., et al. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255–1264 [PMC free article] [PubMed] [Google Scholar]
- 8. Fox K. A., Jewell J. E., Williams E. S., Miller M. W. 2006. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J. Gen. Virol. 87:3451–3461 [DOI] [PubMed] [Google Scholar]
- 9. Green K. M., et al. 2008. Accelerated high fidelity prion amplification within and across prion species barriers. PLoS Pathog. 4:e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haley N. J., Seelig D. M., Zabel M. D., Telling G. C., Hoover E. A. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamir A. N., et al. 2006. Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. J. Vet. Diagn. Invest. 18:558–565 [DOI] [PubMed] [Google Scholar]
- 12. Hamir A. N., Miller J. M., Kunkle R. A., Hall S. M., Richt J. A. 2007. Susceptibility of cattle to first-passage intracerebral inoculation with chronic wasting disease agent from white-tailed deer. Vet. Pathol. 44:487–493 [DOI] [PubMed] [Google Scholar]
- 13. Harrington R. D., et al. 2008. A species barrier limits transmission of chronic wasting disease to mink (Mustela vison). J. Gen. Virol. 89:1086–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heisey D. M., et al. 2010. Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J. Virol. 84:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson C. J., Pedersen J. A., Chappell R. J., McKenzie D., Aiken J. M. 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog. 3:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong Q., et al. 2005. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J. Neurosci. 25:7944–7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurt T. D., et al. 2007. Efficient in vitro amplification of chronic wasting disease PrPRES. J. Virol. 81:9605–9608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurt T. D., Telling G. C., Zabel M. D., Hoover E. A. 2009. Trans-species amplification of PrPCWD and correlation with rigid loop 170N. Virology 387:235–243 [DOI] [PubMed] [Google Scholar]
- 19. Mahal S. P., et al. 2007. Prion strain discrimination in cell culture: the cell panel assay. Proc. Natl. Acad. Sci. U. S. A. 104:20908–20913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mathiason C. K., et al. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136 [DOI] [PubMed] [Google Scholar]
- 21. Meyerett C., et al. 2008. In vitro strain adaptation of CWD prions by serial protein misfolding cyclic amplification. Virology 382:267–276 [DOI] [PubMed] [Google Scholar]
- 22. Miller M. W., Williams E. S. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 425:35–36 [DOI] [PubMed] [Google Scholar]
- 23. Miller M. W., Williams E. S., Hobbs N. T., Wolfe L. L. 2004. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 10:1003–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piening N., et al. 2006. Conversion efficiency of bank vole prion protein in vitro is determined by residues 155 and 170, but does not correlate with the high susceptibility of bank voles to sheep scrapie in vivo. J. Biol. Chem. 281:9373–9384 [DOI] [PubMed] [Google Scholar]
- 25. Raymond G. J., et al. 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 19:4425–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raymond G. J., et al. 2007. Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. J. Virol. 81:4305–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossi D., et al. 2001. Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. EMBO J. 20:694–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Safar J. G., et al. 2008. Transmission and detection of prions in feces. J. Infect. Dis. 198:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakaguchi S., et al. 1996. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature 380:528–531 [DOI] [PubMed] [Google Scholar]
- 30. Seidel B., et al. 2007. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One 2:e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sigurdson C. J. 2008. A prion disease of cervids: chronic wasting disease. Vet. Res. 39:41. [DOI] [PubMed] [Google Scholar]
- 32. Sigurdson C. J., et al. 2008. Experimental chronic wasting disease (CWD) in the ferret. J. Comp. Pathol. 138:189–196 [DOI] [PubMed] [Google Scholar]
- 33. Silverman G. L., et al. 2000. Doppel is an N-glycosylated, glycosylphosphatidylinositol-anchored protein. Expression in testis and ectopic production in the brains of Prnp0/0 mice predisposed to Purkinje cell loss. J. Biol. Chem. 275:26834–26841 [DOI] [PubMed] [Google Scholar]
- 34. Spraker T. R., et al. 2002. Distribution of protease-resistant prion protein and spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. Vet. Pathol. 39:546–556 [DOI] [PubMed] [Google Scholar]
- 35. Tamguney G., et al. 2009. Transmission of scrapie and sheep-passaged bovine spongiform encephalopathy prions to transgenic mice expressing elk prion protein. J. Gen. Virol. 90:1035–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamguney G., et al. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams E. S., Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J. Wildl. Dis. 16:89–98 [DOI] [PubMed] [Google Scholar]
- 38. Wilson D. E., Reeder D. M. 2005. Mammal species of the world. A taxonomic and geographic reference, 3rd ed The Johns Hopkins University Press, Baltimore, MD [Google Scholar]
- 39. Yokoyama T., Masujin K., Iwamaru Y., Imamura M., Mohri S. 2009. Alteration of the biological and biochemical characteristics of bovine spongiform encephalopathy prions during interspecies transmission in transgenic mice models. J. Gen. Virol. 90:261–268 [DOI] [PubMed] [Google Scholar]