Abstract
Eph family receptor tyrosine kinases (including EphA3, EphB4) direct pathfinding of neurons within migratory fields of cells expressing gradients of their membrane-bound ligands. Others (EphB1 and EphA2) direct vascular network assembly, affecting endothelial migration, capillary morphogenesis, and angiogenesis. To explore how ephrins could provide positional labels for cell targeting, we tested whether endogenous endothelial and P19 cell EphB1 (ELK) and EphB2 (Nuk) receptors discriminate between different oligomeric forms of an ephrin-B1/Fc fusion ligand. Receptor tyrosine phosphorylation was stimulated by both dimeric and clustered multimeric ephrin-B1, yet only ephrin-B1 multimers (tetramers) promoted endothelial capillary-like assembly, cell attachment, and the recruitment of low-molecular-weight phosphotyrosine phosphatase (LMW–PTP) to receptor complexes. Cell–cell contact among cells expressing both EphB1 and ephrin-B1 was required for EphB1 activation and recruitment of LMW–PTP to EphB1 complexes. The EphB1-binding site for LMW–PTP was mapped and shown to be required for tetrameric ephrin-B1 to recruit LMW–PTP and to promote attachment. Thus, distinct EphB1-signaling complexes are assembled and different cellular attachment responses are determined by a receptor switch mechanism responsive to distinct ephrin-B1 oligomers.
Keywords: Eph receptors, LMW–PTP, tyrosine kinases, signal transduction, ephrin oligomers
Eph family receptor tyrosine kinases have drawn increasing attention as signaling molecules that direct the targeting behavior of migratory neurons during development, vascular cell assembly, and angiogenesis (Cheng et al. 1995; Drescher et al. 1995; Pandey et al. 1995; Winslow et al. 1995; Tessier-Lavigne and Goodman 1996; Wang and Anderson 1997). In each of these functions Eph receptors direct cell–cell attachment and aggregation events through their engagement of membrane-bound ligands, ephrins. The Eph family is comprised of at least 14 different structurally related receptors expressed in tissue-restricted distributions consistent with their participation in developmental pattern formation (Gale et al. 1996). Eight different ephrins have been identified to date. They are membrane proteins of either glycerophosphatidylinositol (GPI)-linked (ephrin A) or transmembrane (ephrin B) types (Eph Nomenclature Committee 1997). On the basis of their overlapping affinity for ligands within each of these subgroups, Eph receptors have been catalogued into either EphA or EphB subclasses among orthologs expressed in mammals, avian, and amphibian species (Eph Nomenclature Committee 1997).
Juxtacrine interactions between EphB (receptors) and ephrin-B (ligands) on apposing cell membranes appear to direct specialized cell–cell recognition and targeting through signaling processes that involve ligand oligomerization. Neural crest cell migration and motor axon outgrowth avoid caudal somite halves in which ephrins-B1 and B2 are expressed, and cultured neurons show similar avoidance of surfaces coated with clustered, but not unclustered, forms of these ligands (Wang and Anderson 1997). Studies in transfected reporter cell systems show that ligand oligomerization is an important determinant of Eph receptor activation. Tyrosine phosphorylation of EphB1 requires presentation of ephrin-B1 in either clustered or membrane-attached forms (Davis et al. 1994). The carboxy-terminal 33 amino acids of three different ephrin-B proteins, ephrin-B1, ephrin-B2, and ephrin-B3, show remarkable sequence identity, suggesting a critical role for this domain in interactions with cytoplasmic compartment proteins (Cerretti et al. 1996; Tang et al. 1997). The ephrin-B1 cytoplasmic domain is subject to regulated tyrosine phosphorylation in cells stimulated with platelet-derived growth factor (PDGF) in cells overexpressing c-src and in response to forced aggregation by clustered EphB2 ectodomains (Holland et al. 1996; Bruckner et al. 1997). Tyrosine-phosphorylated ephrin-B1 has been recovered from developing embryos (Holland et al. 1996). Thus, regulated phosphorylation of conserved ephrin-B cytoplasmic tyrosine residues is anticipated to generate sites for ephrin-B interaction with adapter proteins containing SH2 or phosphotyrosine-binding PTB domains (Koch et al. 1991; van der Geer and Pawson 1995; Yajnik et al. 1996). Moreover, the conserved carboxy-terminal sequence, YYKV, suggests possible interactions with PDZ domain proteins that function as adapters regulating oligomerization of membrane proteins (Harrison 1996; Songyang et al. 1997).
Motivated by these findings, and the unexplained targeting responses of Eph receptor expressing cells within migratory gradient fields of membrane-bound ligands, we asked whether EphB1-mediated cell attachment and cell–cell assembly responses could be regulated by ephrin-B oligomerization. Here, we used two different cultured cell systems that express endogenous EphB1 receptors to evaluate whether different oligomerized forms of ephrin-B1 may direct alternative responses. Striking differences were observed in attachment and capillary-like assembly of primary microvascular endothelial cells and attachment of teratocarcinoma-derived P19 cells, despite the capacity of both dimeric and multimeric ephrin-B1 to promote tyrosine phosphorylation of EphB1 and EphB2. These differences in cellular response led us to explore mechanisms by which EphB subclass receptors discriminate between different ephrin-B1 oligomers. Increased attachment or endothelial cell–cell assembly required activation of an EphB receptor switch mechanism that (1) discriminates tetrameric from dimeric EphB1, and (2) regulates recruitment of low-molecular-weight protein tyrosine phosphatase (LMW–PTP) (Zhang et al. 1995) to receptor complexes. Site-directed mutation of a carboxy-terminal domain tyrosine residue required for this interaction eliminated LMW–PTP recruitment and downstream attachment responses to multimeric ligand. Finally, cell–cell contact with ephrin-B1 expressing cells promoted recruitment of LMW–PTP to EphB1 complexes during capillary-like endothelial assembly, in a process sensitive to an EphB1 competitor.
Results
Multimeric ephrin-B1/Fc promotes cellular assembly and attachment
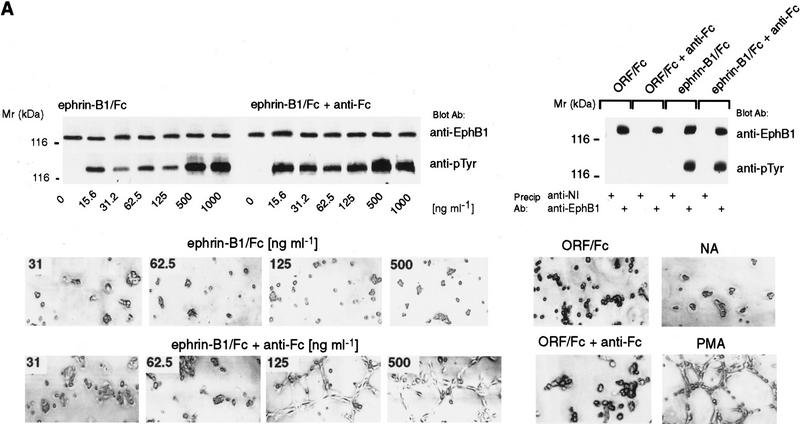
To test if variably oligomerized forms of ephrin-B1 evoke alternative signals through Eph receptors, we used a disulfide-linked immunoglobulin Fc fusion form of ephrin-B1 (ephrin-B1/Fc) (Beckmann et al. 1994). Ephrin-B1/Fc dimers, or anti-Fc preclustered multimers, were presented to each of two cell types that express endogenous EphB1 receptors, human renal microvascular endothelial cells (HRMEC) (Martin et al. 1997), and teratocarcinoma-derived, pluripotent murine P19 cells (Bain et al. 1994). As shown in Figure 1A, concentrations from 15–1000 ng/ml of either dimeric (ephrin-B1/Fc) or multimeric (ephrin-B1/Fc+anti-Fc) promoted tyrosine phosphorylation of endothelial EphB1, whereas an irrelevant open reading frame Fc fusion control (ORF/Fc) did not.
Figure 1.
Ephrin-B1/Fc dimers and multimers elicit different responses. (A) In vitro angiogenesis assay (Martin et al. 1997) HRMEC were plated on Matrigel-coated dishes in defined medium in the absence (NA), or presence of PMA (20 ng/ml), ephrin-B1/Fc (at the indicated concentrations, ng/ml) or a control Fc fusion protein, ORF/Fc (Beckmann et al. 1994) (500 ng/ml). Fc fusion proteins were presented as either dimers, or as preclustered multimers (+ anti-Fc). Cells were photographed 8 hr after plating. (Top) Complexes recovered with EphB1 (anti-EphB1) or nonimmune (anti-NI) antibodies from HRMEC stimulated for 15 min with indicated concentrations of ephrin-B1/Fc dimers (ephrin-B1/Fc), preclustered ephrin-B1/Fc multimers (ephrin-B1/Fc plus anti-Fc) or control fusion dimers (ORF/Fc, 500 ng/ml) or multimers (ORF/Fc plus anti-Fc ng/ml), were evaluated by immunoblot for receptor recovery (anti-EphB1) and activation (anti-pTyr). (B) P19 cells were plated on fibronectin coated dishes in the presence of the indicated concentrations of dimeric or multimeric forms of ephrin-B1/Fc or ORF/Fc, as described in Materials and Methods. The percentage of total cells attached after 90 min is displayed. (Mean ±s.e.m. of three independent determinations). In the insert, EphB1 receptors were immunoprecipitated from P19 cells plated on fibronectin-coated dishes and stimulated with 500 ng/ml of the indicated agents for 20 min. EphB1 recovery (anti-EphB1) and activation (anti-pTyr) were evaluated by immunoblot.
In sharp contrast to this similarity in EphB1 tyrosine phosphorylation, marked differences in cellular behavior were observed (Fig. 1A). Preclustered, multimeric ephrin-B1/Fc promoted endothelial capillary-like assembly in a two-dimensional in vitro assay (Martin et al. 1997), whereas dimeric ephrin-B1/Fc did not. These differences were observed at concentrations of each ligand form that stimulated qualitatively similar receptor tyrosine phosphorylation. Dimeric and preclustered multimeric forms of an irrelevant ORF/Fc were inactive at these concentrations (Fig. 1A). Independent experiments showed that ephrin-B1/Fc multimers promoted HRMEC attachment to Matrigel- and fibronectin-coated surfaces, whereas dimers did not (Fig. 4C, below; data not shown).
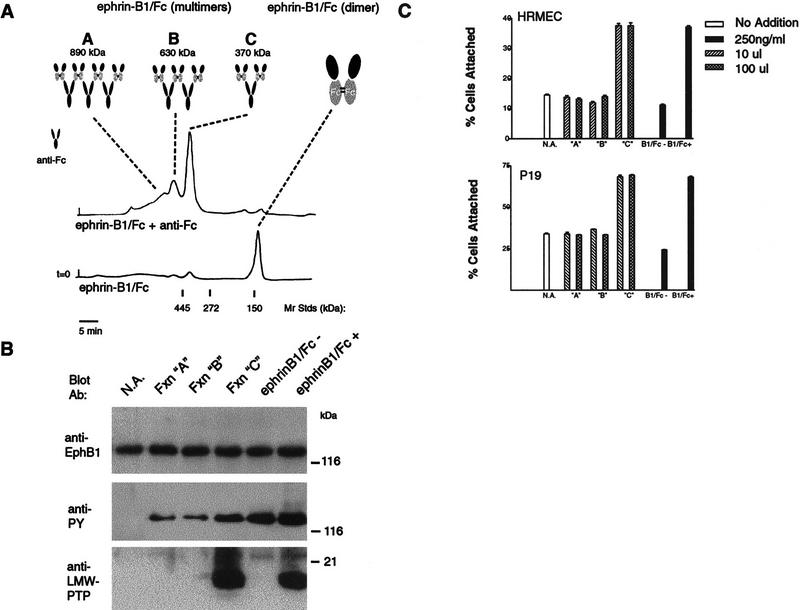
Figure 4.
Ephrin-B1/Fc tetramers recruit LMW–PTP to EphB1 and promote attachment. (A) Ephrin-B1/Fc dimers (ephrin-B1/Fc, 50 μg) or preclustered multimers (ephrin-B1/Fc plus 50 μg of anti-Fc 5 μg) were separated by exclusion chromatogrpahy in PBS on a Superose 6 column (Pharmacia) precalibrated with standards of 445 kD (ferritin), 272 kD (urease), and 150 kD (IgG) as indicated. Fractions containing 500 ng/ml of protein from the indicated A278 peaks (A,B,C) were analyzed for activity to promote tyrosine phosphorylation of EphB1 and recruitment of LMW–PTP to EphB1 complexes (B) and to promote attachment of HRMEC and P19 cells (C). Fractions containing complexes of size consistent with tetramers (one anti-Fc molecule complexing two ephrin-B1/Fc dimers) promoted LMW–PTP recruitment and attachment.
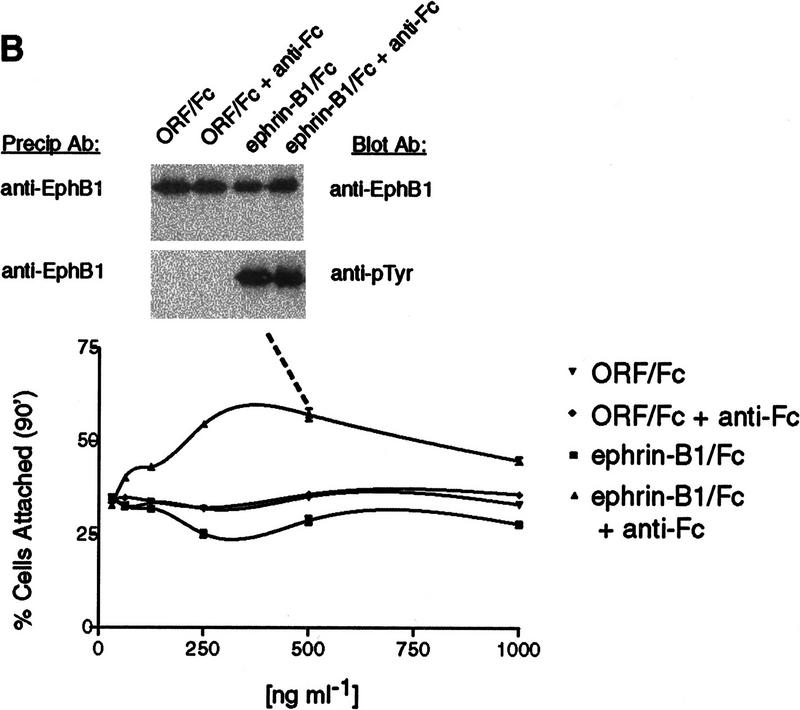
Next, we evaluated effects of ephrin-B1 multimerization on endogenous EphB1 receptors and responses in a second cell type, P19 cells (Fig. 1B). Preclustered ephrin-B1/Fc multimers promoted P19 cell attachment to fibronectin, whereas ephrin-B1/Fc dimers had a modest effect to decrease fibronectin attachment of a small subpopulation of cells, notably at ephrin-B1 concentrations at which multimers increased attachment. These different cell attachment responses were accompanied by similar EphB1 activation and tyrosine phosphorylation (Fig. 1B, inset). Thus, differential cell attachment or cell–cell assembly responses were observed in two different cell types, depending on the oligomeric form of ephrin-B1/Fc presented.
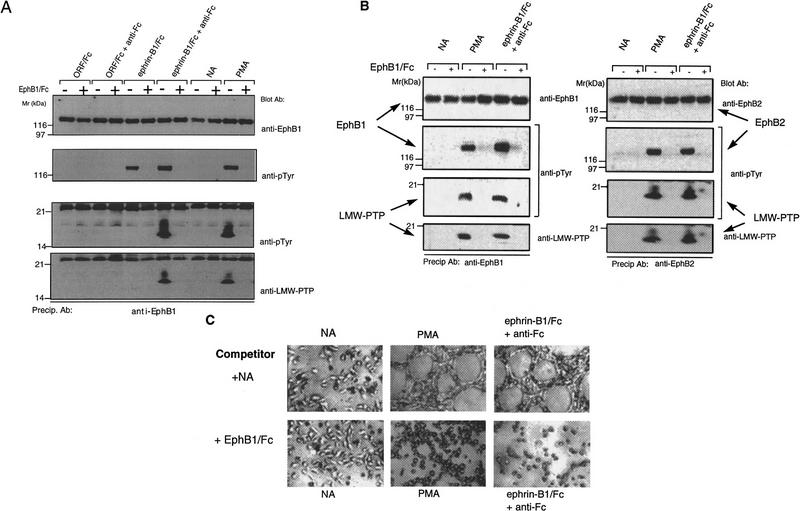
Given that other EphB subclass receptors share a similar affinity for ephrin-B1 (Brambilla et al. 1996), we evaluated whether ephrin-B1 dimers and multimers could variably activate or recruit other EphB subclass receptors (Brambilla et al. 1996). We screened HRMEC for expression of EphB2, EphB3, and EphB4 mRNA, using sequence-specific primers and RT–PCR (not shown). Among these receptors, only EphB2 was expressed at detectable levels. As predicted by its overlapping affinity for ephrin-B1 (Cerretti et al. 1996), EphB2 tyrosine phosphorylation was activated in response to either ephrin-B1/Fc dimers (−anti-Fc) or preclustered multimers (+anti-Fc), similar to the EphB1 responses (Fig. 2, right panel). This activation suggested the possibility that different oligomeric forms of ephrin-B1 could variably recruit heteromeric complexes that include both EphB1 and EphB2, as has been described for EGF receptor family members HER2/HER3 (Wallasch et al. 1995). Several lines of evidence, however, argue against this conclusion.
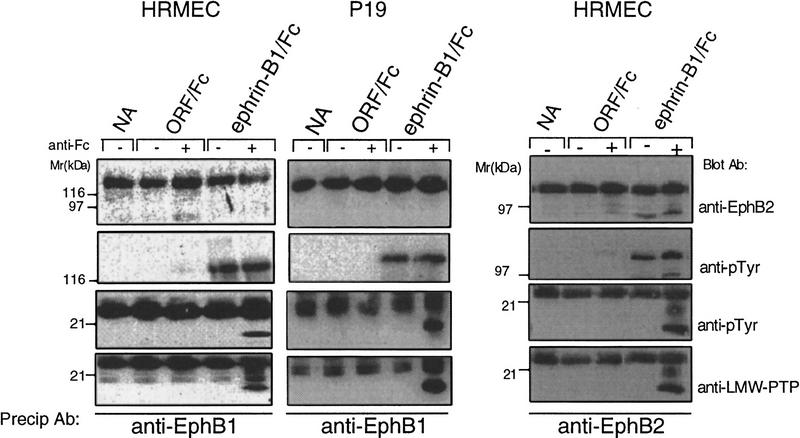
Figure 2.
Ephrin-B1/Fc multimers recruit LMW–PTP to EphB1 and EphB2 complexes. EphB1 immunoprecipitates from HRMEC (left) or P19 cells (center) or EphB2 immunoprecipitates (from HRMEC, right) were recovered from cells exposed to no addition (NA), ORF/Fc or ephrin-B1/Fc (500 ng/ml) as either dimers (1−anti-Fc) or as preclustered multimers (+anti-Fc). Receptor complex immunoblots were analyzed to show receptor recovery (anti-EphB1), left and center, or anti-EphB2, right, receptor activation (130 kD, anti-pTyr), an 18-kD tyrosine phosphoprotein (anti-pTyr) that is recognized by antibodies to LMW–PTP (anti-LMW–PTP). The 25-kD band in all samples is immunoglobulin light chain from the immunoprecipitation. LMW–PTP is recruited to both EphB1 and EphB2 receptor complexes only by ephrin-B1/Fc multimers (+anti-Fc).
Immunoprecipitation of either EphB1 or EphB2 failed to recover the other receptor, or the activating ephrin-B1/Fc, in a stable complex (not shown). Furthermore, different attachment responses to dimeric and multimeric ephrin-B1 were observed in transfected P19 cells that express EphB1 at 20- to 40-fold endogenous levels (Fig. 3C; and unpubl.). Under these conditions, the level of EphB1 overexpression is sufficiently high to obviate substantial formation of heteromeric receptor complexes (EphB1–EphB2) through which alternative signals could be generated, as has been described in the EGF-R/HER2-4 system (Wallasch et al. 1995; Tzahar et al. 1996). Together, these findings support the capacity of homo-oligomers of EphB1 to discriminate between oligomeric forms of ephrin-B1.
Figure 3.
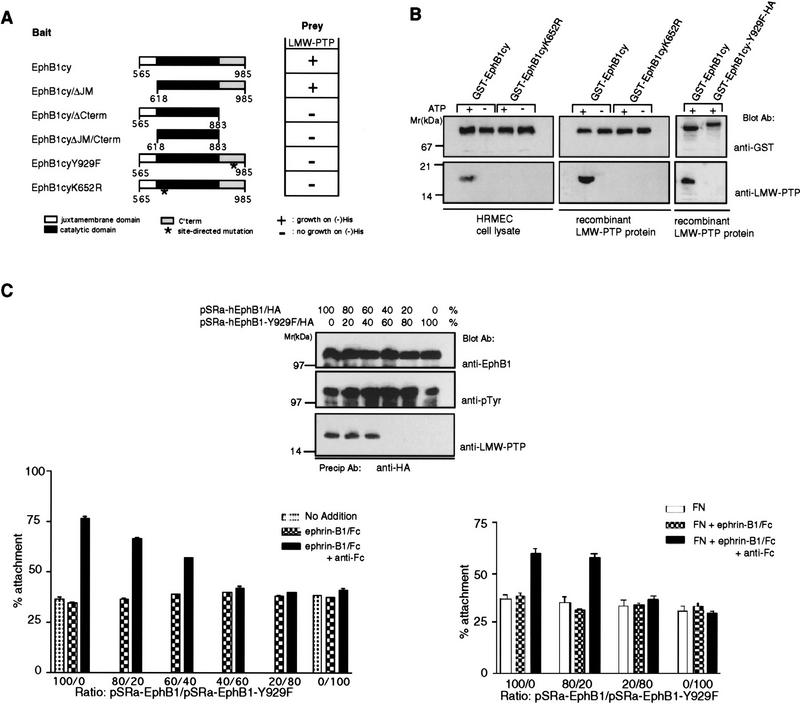
Reconstitution of EphB1 binding of LMW–PTP requires Y929 and an active EphB1 tyrosine kinase. (A) A yeast two-hybrid system (Stein et al. 1996) was used to analyze interactions between LMW–PTP and the wild-type EphB1 cytoplasmic domain (EphB1cy), truncations of indicated subdomains (EphB1cy/ΔJM, EphB1cy/ΔCterm) or point mutants (EphB1cyY929F, or EphB1cyK652R). Two-hybrid dependent growth (+) on histidine deficient selection media was eliminated (−) by deletion of the carboxy-terminal domain, inactivation of tyrosine-kinase function (K652R), or point mutation of Y929 (Y929F). (B) Recombinant GST fusions comprising the wild-type EphB1 cysoplasmic domain (GST–EphB1cy), a kinase-inactive mutant (GST–EphB1cyK652R) or GST–EphB1cy–Y929F/HA, were expressed in Sf9 cells, immobilized on glutathione-sepharose, incubated in kinase buffer in the presence (+) or absence (−) of ATP (Stein et al. 1996) as described in Materials and Methods. Glutathione–Sepharose-immobilized, phoshphorylated (+ATP), or unphosphorylated (−ATP) GST fusion proteins were incubated with HRMEC extracts or recombinant LMW–PTP protein, washed extensively, then analyzed by immunoblot, by use of the indicated antibodies. (C) P19 cells were transfected with the indicated ratios of pSRα expression constructs encoding wild-type EphB1 (pSRα-hEphB1/HA) or mutant (pSRα-hEphB1–Y929F/HA). Cells were stimulated with 500 ng/ml of ephrin-B1/Fc (dimers) or precomplexed ephrin-B1/Fc (multimers, +anti-Fc), or no addition, as indicated. (Top) Cells were stimulated with ephrin-B1/Fc multimers (+anti-Fc) and EphB1 immunoprecipitates (anti-HA) were analyzed for EphB1 activation (anti-pTyr) and recruitment of LMW–PTP (anti-LMW–PTP) by immunoblot. (Left) Transfected cells were exposed to indicated agonists in solution and attachment to fibronectin coated dishes was assayed (Materials and Methods). (Right) Alternatively, nitrocellulose-coated dishes were precoated with either fibronectin alone (FN), or in combination with ephrin-B1/Fc dimers or multimers (+anti-Fc), and cell attachment was assayed (Materials and Methods).
Ephrin-B1 multimers recruit LMW–PTP to EphB1 signaling complexes
On the basis of these findings, we reasoned that this alternative signaling may reflect differential recruitment of signaling complex components to receptors activated by ephrin-B1 dimers or multimers. To explore this issue, we assayed EphB1 complexes recovered after stimulation with dimeric or multimeric ephrin-B1/Fc. SH2-containing adapter proteins, Grb2, Grb10 (Stein et al. 1996), and Nck (Stein et al. 1998) were recruited to EphB1 complexes with indistinguishable efficiency by ephrin-B1 dimers and multimers (not shown). Phosphotyrosine immunoblots, however, identified a low-molecular-weight phosphoprotein (18 kD) that was selectively recruited by both EphB1 and EphB2 receptors following activation by multimeric ephrin-B1 (Fig. 2). A yeast two-hybrid screen of an E9.5–E10.5 murine library for EphB1-interative proteins (Stein et al. 1996) did not provide 18-kD candidates. A recent report, however, showed that the human LMW–PTP is itself a substrate for v-Src tyrosine phosphorylation (Rigacci et al. 1996). Accordingly, we analyzed EphB1 immunoprecipitates for LMW–PTP immunoreactivity (Wo et al. 1992; Zhang et al. 1994). We found that affinity-purified LMW–PTP antibodies recognized the 18-kD tyrosine phosphoprotein in both EphB1 and EphB2 signaling complexes following activation by multimeric, but not dimeric, ephrin-B1 (Fig. 2).
LMW–PTP is structurally distinct from the more widely studied tyrosine phosphatases (Zhang et al. 1995), is expressed in a wide range of cell types, and structural homologs are expressed in yeast (Ostantin et al. 1995). A catalytically inactive LMW–PTP functions as a dominant-negative protein that binds and precipitates tyrosine-phosphorylated PDGF receptors and functions to promote cell proliferation (Chiarugi et al. 1995). At least two alternatively spliced LMW–PTP isoforms exist (Bryson et al. 1995). The A isoform is the dominant species expressed in HRMEC and P19 and is the predominant form precipitating with EphB1 in response to multimeric, but not dimeric, ephrin-B1 (not shown). EphB1 receptor tyrosine kinase phosphorylates LMW–PTP in vitro, and EphB1 immunoprecipitation time courses have shown that immunoreactive LMW–PTP is tyrosine phosphorylated as soon as it is detected in EphB1 complexes (within 10 min); it remains detectable in EphB1 coprecipitable fractions for 90 min (E. Stein and T.O. Daniel, unpubl.)
LMW–PTP/EphB1 interaction requires Y929 and an active receptor tyrosine kinase
We used a yeast two-hybrid system (Stein et al. 1996) to define the site of interaction between LMW–PTP and EphB1cy. As shown in Figure 3A, deletion of the carboxy-terminal domain (EphB1 residues 883–984) eliminated the interaction. More specifically, inactivation of the EphB1 tyrosine kinase (K652R) and site-directed mutation of the only tyrosine residues within that carboxy-terminal domain, Y929, each eliminated the interaction. These findings are consistent with an important role for Y929 phosphorylation in creating the LMW–PTP-binding site. Residue Y929 is also required to create the binding site through which the SH2 domain of Grb10 binds EphB1 (Stein et al. 1996), suggesting that phosphorylation of this residue may provide a critical determinant of structure within this EphB1 subdomain. It is noteworthy that EphB receptors share remarkable sequence identity within the carboxy-terminal subdomain, including that tyrosine residue, in what has been recognized recently as a sterile alpha motif (Schultz et al. 1997).
We further characterized determinants of the EphB1/LMW–PTP interaction using in vitro coprecipitation assays of recombinant GST fusion proteins. A recombinant GST–EphB1 cytoplasmic domain fusion protein (GST–EphB1cy) was expressed in Sf9 cells, bound to glutathione–agarose, washed exhaustively, and then incubated in kinase buffer conditions that promote self-phosphorylation of kinase-active EphB1cy. In vitro self-phosphorylation of glutathione-bound GST–EphB1cy generates a binding site to which purified recombinant LMW–PTP (A isoform) and endogenous LMW–PTP from crude endothelial cell lysates bind (Fig. 3B). Kinase inactive (GST–EphB1cyK652R) and unphosphorylated EphB1 cytoplasic domains [GST–EphB1cy, (-ATP)] did not bind LMW–PTP (Fig. 3B). Finally, in vitro self-phosphorylation of the recombinant mutant protein, GST–EphB1cyY929F, failed to create the LMW–PTP-binding site. This result provided the opportunity for us to use mutant EphB1 receptors (Y929F) to explore the function of this subdomain in communicating the attachment response to multimeric ephrinB1.
A dominant-negative EphB1 mutant fails to recruit LMW–PTP and promote attachment
To evaluate whether LMW–PTP recruitment is required for EphB1 to mediate the increases in P19 adhesion, we transfected P19 cells with different ratios of expression plasmids to force high-level expression of epitope-tagged (hemagglutinin) versions of either wild-type or mutant (Y929F) EphB, or combinations of both proteins (Fig. 3C). The high transfection efficiency (>70%) of 19 cells permitted us to detect functional responses in pooled populations of transfected cells. Several observations are noteworthy. High-level expression of epitope-tagged wild-type receptor promotes the atachment response to multimeric ephrin-B1, consistent with the capacity of a single EphB receptor, EphB1, to mediate this response. Moreover, exogenously expressed EphB1/HA receptors recruit LMW–PTP when expressed in stoichiometric excess of the mutant (Y929F) EphB1/HA. Expression of increasing ratios of mutant (Y929F) to wild-type EphB1 blocked both LMW–PTP recruitment to immunoprecipitated EphB1 complexes and the attachment response to multimeric ephrin-B1/Fc (Fig. 3C, left panel). This dominant-negative effect assigns a critical function to phosphorylation of this tyrosine residue and implicates LMW–PTP in coupling of EphB1 receptor activation with increased attachment.
Surface-displayed ephrin-B1/Fc multimers promote attachment to fibronectin
To extend these findings, we asked whether ephrin-B1 dimers or multimers affixed to a substratum could evoke different P19 cell attachment responses, as they may on juxtacrine presentation to EphB receptor-bearing cells. Ligands presented in this format have been shown to affect axonal migration (Wang and Anderson 1997). Transfected cells were plated on nitrocellulose-coated plastic dishes to which a mixture of fibronectin and either ephrin-B1 dimers or multimers had been preadsorbed. As in the experiments in which ephrin-B1 was provided in solution, cells transfected with the wild-type EphB1 receptor showed increased attachment to surfaces coated with multimeric, but not dimeric, ephrin-B1. Cotransfection with high ratios (80:20 or 100:0) of the mutant EphB1-Y929F abrogated this discriminatory response, similar to results obtained in experiments in which ephrin-B1 was presented in solution. These data confirm that the P19 system is responsive to ephrin-B1 affixed to surfaces, that EphB1 receptors remain capable of discriminating dimeric from multimeric ephrin-B1 on a solid surface, and that residue Y929 plays a critical role in the attachment response.
Ephrin-B1 tetramers promote LMW–PTP recruitment and cell attachment
On the basis of additional observations showing that the ratio of preclustering monoclonal anti-Fc antibody to ephrin-B1/Fc was a critical determinant of cellular responses (data not shown), we examined which ephrin-B1/Fc multimeric species determines recruitment of LMW–PTP to EphB1 complexes to promote cellular adhesion. Gel filtration chromatography separated two distinct peaks of size and composition consistent with ephrin-B1 tetramers and hexamers (peaks B and C), as well as a broad peak containing higher order multimers (Fig. 4A; peak A). Exposure of HRMEC and P19 cells to 10-fold dilutions of each fraction (bracketing concentration differences among the peaks) showed that each was active to promote EphB1 receptor tyrosine phosphorylation. Ephrin-B1/Fc multimers from fractions of peak C (complexes of size consistent with two ephrin-B1/Fc dimers and one anti-Fc monoclonal antibody), however, uniquely promoted LMW–PTP recruitment (Fig. 4B) and increased adhesion in both HRMEC and P19 cells (Fig. 4C). These findings lead us to conclude that tetrameric complexes comprised of two ephrin-B1 dimers are the dominant active species capable of stimulating LMW–PTP recruitment and P19 attachment.
Endogenous ephrin-B ligands promote EphB1 activation and LMW–PTP recruitment
We conducted a final series of experiments to determine whether activation of endogenous EphB1 by juxtacrine contact with endogenous ephrin-B1 invokes assembly of signaling complexes that include LMW–PTP. Earlier studies of transfected EphB3 showed juxtacrine activation on contact with ephrin-B1-expressing cells (Bohme et al. 1996). Previously, we had shown that HRMEC express native forms of two ligands for EphB1, ephrin-B1 and ephrin-B2, at high constitutive levels (Daniel et al. 1996). Because these primary cultured endothelial cells (HRMEC) express both ligand and receptor, we were able to evaluate (1) whether endogenous EphB1 is activated (tyrosine phosphorylated) by engaging endogenous ephrin-B’s in a cell–cell contact dependent manner, (2) whether the EphB1 cmplexes that form during juxtacrine activation include LMW–PTP, and (3) whether competitive disruption of juxtacrine EphB1–ephrinB1 engagement affects cell–cell capillary-like assembly.
Recent reports show that PDGF stimulates tyrosine phosphorylation of transfected ephrin-B1 (Bruckner et al. 1997). This led us to confirm that phorbol myristate acetate (PMA), a general activator of migration, proliferation, and capillary-like assembly in endothelial cells (Fig. 1), also promotes tyrosine phosphorylation of endogenous ephrin-B1 (E. Stein, unpubl.). Anticipating that ephrin-B1 tyrosine phosphorylation promotes its interaction with adapter and signaling proteins capable of oligomerizing it, we reasoned that juxtacrine engagement with EphB1 receptors should recruit LMW–PTP in a cell–cell contact dependent manner. In Figure 5, identical numbers of endothelial cells were plated on culture dishes of different surface areas (p35, p60, p100, and p150) to alter cell–cell contact. EphB1 was unactivated in the absence of agonists, yet became tyrosine phosphorylated on exposure to exogenous (added) ephrin-B1/Fc, in both dimeric and multimeric forms, independent of the density at which cells were plated. EphB1 activation was seen in cells stimulated with PMA, yet this activation was dependent on cell–cell contact (in p35 and p60 dishes), consistent with a requirement for juxtacrine engagement. As shown before, LMW–PTP was recruited to EphB1 complexes activated by exogenous multimeric ephrin-B1/Fc. Notably, EphB1 from PMA stimulated cells plated in p35 and p60 dishes also recruited LMW–PTP.
Figure 5.
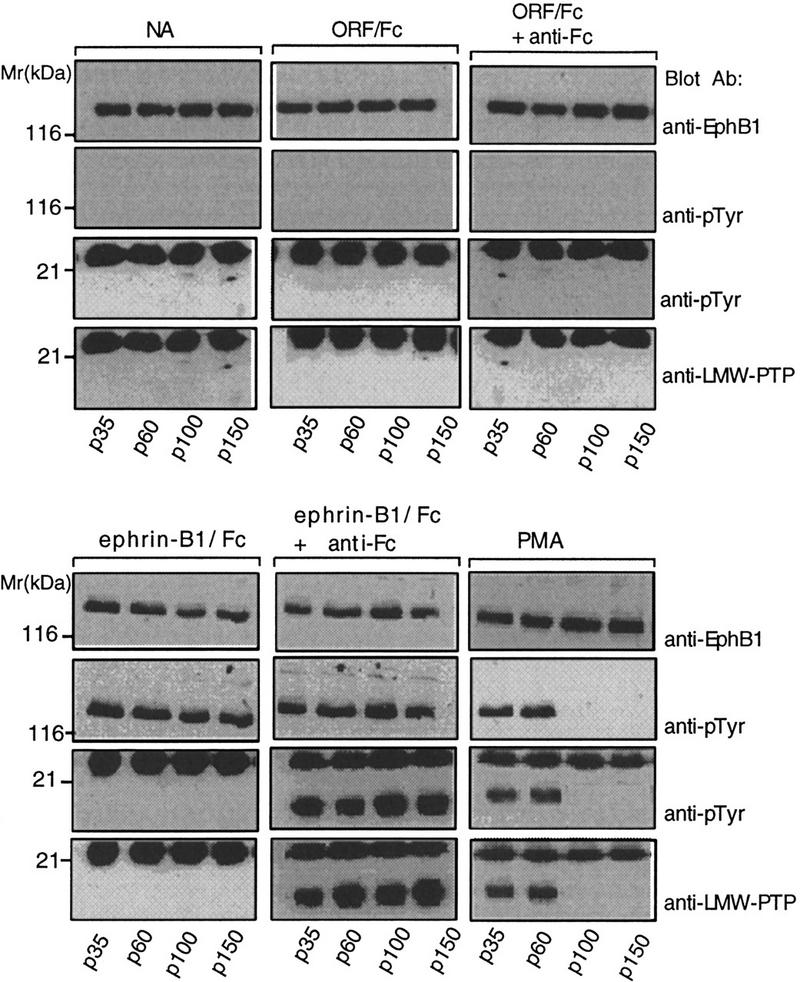
Endogenous ligand activation of EphB1 requires cell–cell contact and promotes LMW–PTP recruitment. Equal numbers of HRMEC (5 × 105 cells) were plated on Matrigel-coated 35 (p35)-, 60-, (p60)-,100-, (p100)-, or 150 (p150)-mm-diam. dishes, representing 1×, 3.1×, 8.6×, or 19.5× unit surface areas, in medium supplemented with 1% bovine albumin. Cells were incubated for 2 hr at 37°C, then stimulated for 15 min with either no addition (NA), control Fc fusion (ORF/Fc, 250 ng/ml), preclustered ORF/Fc (250 ng/ml) plus anti-Fc (25 ng/ml), ephrin-B1/Fc (250 ng/ml), preclustered ephrin-B1/Fc (250 ng/ml) plus anti-Fc (25 ng/ml), or phorbol myristate acetate (PMA, 2 ng/ml). Immunoprecipitated EphB1 receptor complexes were analyzed for receptor activation (anti-pTyr, 130-kD band) and recruitment of the 18-kD LMW–PTP (anti-pTyr and anti-LMW–PTP). The 25-kD band is immunoglobulin light chain from the immunoprecipitation. Notably, PMA-stimulated cell density-dependent activation of EphB1 and recruitment of LMW–PTP to receptor complexes.
An EphB1 ectodomain competitor (EphB1/Fc) blocks LMW–PTP recruitment and capillary-like assembly
To ascertain whether the cell density-dependent EphB1 activation was the consequence of EphB1 engagement by endogenous ephrin-Bs, and not the effect of PMA to provoke EphB1 tyrosine phosphorylation through some other means, we added EphB1/Fc (1 nm) as a soluble inhibitor of juxtacrine interaction. As shown in Figure 6A, EphB1/Fc blocked juxtacrine activation of EphB1 by endogenous ephrin-Bs, coincident with its interruption of LMW–PTP recruitment. Finally, we asked whether endothelial capillary-like assembly may be significantly affected by the soluble EphB1/Fc ectodomain competitor. In Figure 6, B and C, inclusion of EphB1/Fc (1.0 μg/ml) blocked the cell density-dependent EPhB1 tyrosine phosphorylation and LMW–PTP recruitment of cells plated on Matrigel, coincident with its effect of interrupting formation of cellular tubes and cords characteristic of in vitro angiogenesis. Together, our findings suggest a model in which: (1) PMA promotes tyrosine phosphorylation and oligomerization of endogenous ephrin-B1, (2) these oligomers stimulate EphB1 to assemble signaling complexes similar to those assembled in response to exogenously added tetrameric ephrin-B1, and (3) cell–cell contact is required for this juxtacrine signaling. It also appears that EphB1 activation is an important component of cell–cell recognition required for capillary-like endothelial assembly.
Figure 6.
EphB1/Fc blocks juxtacrine activation of EphB1 and EphB2 by PMA and blocks capillary-like endothelial assembly. (A) HRMEC were plated on Matrigel-coated 35-mm dishes and stimulated as above with agonists added in the absence (−) or presence (+) or a competitive EphB1 ectodomain antagonist, EphB1/Fc (500 ng/ml). As before, EphB1 immunoprecipitates were analyzed for receptor recovery (anti-EphB1), activation (anti-pTyr, 130kD), and recruitment of LMW–PTP (anti-pTyr and anti-LMW–PTP, 18 kD). EphB1/Fc blocked the PMA-stimulated EphB1 activation and LMW–PTP recruitment. (B) EphB1 (left) or EphB2 (right) immunoprecipitates were recovered from HRMEC plated on Matrigel at high density and stimulated for 15 min with no addition (NA), PMA (20 ng/ml), or preclustered ephrin-B1/Fc (250 ng/ml) plus anti-Fc (25 ng/ml), as above, in the absence (−) or presence (+) or EphB1/Fc (500 ng/ml). (C) Cells were plated as in Fig. 1 in medium containing no addition (no addition), PMA (20 ng/ml) or preclustered ephrin-B1/Fc (250 ng/ml) plus anti-Fc (25 ng/ml), in the absence (NA) or presence (+EphB1/Fc, 500 ng/ml) of receptor ectodomain competitor. EphB1/Fc blocked capillary-like endothelial assembly responses to PMA and clustered ephrin-B1/Fc.
Discussion
These data show the surprising capacity of endogenous EphB1 receptors to discriminate multimeric from dimeric ephrin-B1. Evidence for this discrimination is both biochemical and functional. Traditional models of receptor tyrosine kinase function have implicated ligand-induced dimerization of receptor monomers as a critical determinant of activation, permitting receptors to phosphorylate their dimeric partners in trans to initiate formation of signaling complexes (Lemmon and Schlessinger 1994). Our findings show that higher order ephrin-B1 oligomers, specifically tetramers (Fig. 4), determine the formation of alternative EphB1 and EphB2-signaling complexes that are marked by recruitment of LMW–PTP (Fig. 2). These complexes signal different cellular responses and raise questions about the molecular basis for differential recruitment of LMW–PTP.
Earlier work has highlighted a requirement for ephrin-B1 oligomerization to activate exogenously expressed EphB receptors (Davis et al. 1994). EphB1 tyrosine phosphorylation was stimulated by ephrin-B1 (ELK-L) in membrane attached or antibody-clustered, epitope-tagged forms, but not by soluble, unclustered ephrin-B1. We speculate that antibody clustering of the myc-tagged ephrin-B1 (Davis et al. 1994) formed a ligand dimer that is functionally comparable with the ephrin-B1/Fc dimers formed through interchain disulfide linkage of the Fc domain fusions in our studies. Both of these ephrin-B1 forms activate EphB1 tyrosine phosphorylation. The monovalent myc epitope likely limited the form of the antibody-clustered ephrin-B1 oligomers to dimers in the earlier study (Davis et al. 1994), whereas the Fc portion of our ephrin-B1/Fc dimers provided a multivalent epitope for multimer assembly by clustering antibodies (Fig. 4A).
One of the GPI-linked ephrin-A subclass ligands, ephrin-A1 (B61), plays an important role in TNFα-induced angiogenesis (Pandy et al. 1995). The oligomeric state of the active species, however, has not been clearly defined. Recombinant ephrin-A1/Fc-stimulated Eph-A2 (Eck) tyrosine phosphorylation, endothelial chemotaxis, and corneal angiogenesis (Pandey et al. 1995). On the basis of evidence that monomeric ephrin-A1 is inactive on EphA5 (Davis et al. 1994), and on the basis of the anticipated dimerization of Fc fusion proteins, the endothelial and angiogenic responses were most likely evoked by ephrin-A1 dimers. It is not yet clear whether ephrin-A1 is released by phospholipase C from its GPI membrane linkage (Davis et al. 1994) during angiogenesis, and if so, whether its oligomeric state would promote endothelial responses through a paracrine mechanism. The effect of neutralizing antibodies to interrupt TNF-induced corneal angiogenesis does suggest that active ephrin-A1 oligomers participate (Pandey et al. 1995).
Recently, we reported that dimeric and clustered multimeric ephrin-A1/Fc (LERK-1/Fc) are comparably active in promoting capillary-like assembly of umbilical vein endothelial cells, yet both forms are inactive on renal microvascular endothelial cells in this assay (Daniel et al. 1996). Moreover, multimeric ephrin-B1/Fc did not promote capillary-like assembly of umbilical vein endothelial cells. Together, these findings show vascular-bed-of-origin specificity in the endothelial responses to ephrins of A and B subclasses and point out that the ephrin-B1 dimer/tetramer recognition switch we describe here is not a functionally significant property of primary endothelial cells recovered from all vascular sites. In our view, this is consistent with the well-established tissue-restricted expression of specific Ephs and ephrins and their functions to direct cell targeting in tissue-restricted contexts.
Several features of the test systems we used to evaluate the interaction between LMW–PTP and EphB1 were important in reconstituting the interaction and may shed additional light on its structural determinants. We showed that wild-type GST–EphB1 fusion proteins, attached to glutathione beads, generate LMW–PTPbinding sites after self-phosphorylation (Fig. 3). Recent definition of a GST structure shows that a single functional binding site for glutathione is formed by GST dimers (McTigue et al. 1995). We anticipate that these GST–EphB1 dimers are closely packed when bound on the surface of glutathione–agarose and that their tyrosine kinase domains phosphorylate dimer partners in trans under in vitro kinase conditions. Only if packing of binding-competent receptor domains is sufficiently close on bead surfaces would one anticipate that a binding site requiring higher order oligomers could be generated. We have tested this by mixing stoichiometric amounts of binding-incompetent, mutant GST–EPhB1cy(Y929F) with wild-type GST-EphB1cy prior to loading glutathione–agarose. Although tyrosine self-phosphorylation of both forms is seen under kinase conditions, LMW–PTP-binding sites are not formed (E. Stein and T.O. Daniel, unpubl.). We view this as consistent with a requirement for two EphB1cy dimer pairs to be in close proximity, packed on the glutathione matrix, to form a LMW–PTP-binding site.
Features of the yeast two-hybrid interaction system also force higher order oligomerization of the LexA–EphB1cy bait. LexA fusion proteins oligomerize on binding to tandem recognition sites in the reporter gene promoters (Schnarr et al. 1991). Our findings suggest that the LexA–EphB1 fusion protein oligomerizes to permit tyrosine self-phosphorylation in trans with formation of the higher order oligomers required for LMW–PTP binding. These reconstitution system results establish that heteromeric EphB receptor complexes are not required for the recruitment of LMW–PTP, and they suggest that different EphB1 oligomers, formed in response to variably oligomerized ephrin-B1, are competent to signal alternative responses.
Recently, we reported that the SH2 domain of Grb10 binds to activated EphB1 through an interaction dependent on tyrosine phosphorylation and residue Y929 (Stein et al. 1996). Careful evaluation has shown that this interaction is typical of SH2 binding to phosphotyrosine-containing peptide motifs and shows no dependency on oligomerization of receptor domain (E. Stein and T.O. Daniel, unpubl.). Moreover, recruitment of Grb10 to EphB1 receptor complexes in intact cells is not affected by ephrin-B1 multimerization. At present, we interpret these differences to indicate that Grb10 binds directly to an SH2 recognition motif including phosphorylated residue Y929. In contrast, it appears that LMW–PTP binds as a consequence of a conformational switch that requires both phosphorylation of this residue and higher order receptor oligomerization. At present, the phosphorylation status of Y929 in cellular EphB1 receptors activated by tetrameric and dimeric ephrin-B1 remains untested. Yet, the reconstitution systems described here provide means for further defining the molecular determinants of LMW–PTP recruitment and downstream responses.
Available data do not yet paint a clear picture of the targets of LMW–PTP that may be important in downstream attachment and assembly responses. Nevertheless, definition of its site of recruitment permitted us to determine whether recruitment was functionally significant in mediating cell attachment responses. Not only did mutation of Y929 abrogate LMW–PTP recruitment to EphB1 complexes, but high-level expression of mutant receptors also served as dominant-negative proteins that disrupted P19 attachment responses to multimeric ephrin-B1. We conclude that additional, yet unidentified, EphB1 signaling complex components may play important roles in the oligomerization-dependent effects we report here, but phosphorylation of Y929 appears critical in both the oligomerization-dependent recruitment of LMW–PTP and increases in attachment to fibronectin.
The implications of these findings for cell–cell recognition, cell targeting, and assembly of specialized multicellular structures may be considerable. Gradients of membrane-bound ephrins (A and B classes) are expressed in developmental patterns that suggest they direct migration and targeting behavior (Wang and Anderson 1997; Nakamoto et al. 1996; Cheng et al. 1995; Brennan et al. 1997). In vitro evidence confirms capability for explanted cells to respond variably to differentially oligomerized ephrin-B2 (Wang and Anderson 1997). At least for the B class ephrins, cytoplasmic structural determinants are highly conserved and are subject to regulated covalent modification (tyrosine phosphorylation) known to recruit adapter molecules. We suggest that consensual cell–cell recognition is likely an important threshold condition that must be met before migrating cells cease migration and assemble specialized structures with targeted partners, whether in the context of developmental neural targeting, angiogenesis, or in repair processes. EphB-bearing cells may discriminate among potential target partners on the basis of their capacity to display appropriately oligomerized ligands such as ephrin-B1. The signals that govern ligand oligomerization and the adapter proteins that accomplish this process may provide a highly discriminatory code capable of determinating cell–cell interactions. Their definition promises to amplify on this intriguing process.
In closing, it should be noted that findings from recent studies suggest that ectodomain-driven ephrin-B1 oligomerization may evoke outside-in signaling to direct cell targeting (Henkemeyer et al. 1996; Holland et al. 1996; Bruckner et al. 1997). Although our findings focus on alternative receptor responses to different oligomeric ephrin-B1 forms, they do not address the possibility that receptor clustering may evoke outside-in signaling through ephrin-B1. At present, it is clear that oligomerization of receptors and ligands in this system are critical determinants of targeting response.
Materials and methods
Reagents
Anti-EphB1 (Stein et al. 1996) and anti-EphB2 (Henkemeyer et al. 1994) sera were described previously. Monoclonal phosphotyrosine antibody (4G10) was from Upstate Biotechnology Inc. (Lake Placid, NY). Monoclonal anti-human IgG1 (anti-Fc) antibody was from The Binding Site (Birmingham, UK). Polyclonal rabbit antiserum to LMW–PTP was generated against the entire coding region (Wo et al. 1992) and affinity-purified antibodies were recovered by adsorption to recombinant LMW–PTP protein immobilized on Immobilin P membranes, followed by elution with 2 m glycine at pH 2.5, as described (Koenig et al. 1993). These affinity-purified antibodies recognize LMW–PTP in crude lysates obtained from P19 cells stimulated with either dimeric or multimer ephrin-B1/Fc.
Ligand stimulation and immunoprecipitation of endogenous EphB receptors
P19 cells were passaged as recommended (ATCC, Rockville, MD) and incubated for 5 hr prior to experiments in 0.5 mm sodium suramin to eliminate any prior engagement of EphB receptors by endogenous ligands. Cells were then washed three times in serum-free medium and incubated for 1 hr in OPTI-MEM (Hyclone), prior to stimulation. Primary HRMEC were used at passages 3–6, as described (Martin et al. 1997). Ninety minutes prior to ligand stimulation, HRMEC (2 × 105 cells/60-mm dish) were plated on Matrigel-coated (Collaborative Biomedical Products, Becton-Dickinson, Bedford, MA) or fibronectin-coated dishes (Ingber 1990).
Ligand (ephrin-B1/Fc) or control (ORF/Fc) (Cerretti et al. 1996) multimers were generated by preincubation with anti-Fc at a fixed ratio of 0.1× the indicated concentrations of Fc fusion proteins (50 ng/ml of anti-Fc for 500 ng/ml of Fc fusion protein). If not otherwise indicated, Fc fusion proteins (ephrin-B1/Fc or ORF/Fc) were used at 500 ng/ml in the absence or presence of preclustering anti-Fc at 50 ng/ml. Cells were incubated with agonists at 37°C for 10 min, or as described in the figure legends to Figures 1, 5, and 6. Receptors were immunoprecipitated from either cleared cell lysates (P19) or Tricum vulgaris lectin fractions of HRMEC, as described (Stein et al. 1996). Precipitated proteins were analyzed by immunoblot (Stein et al. 1996).
Endothelial assembly into capillary-like structures
Twelve-well plates (Falcon) were coated with thin layers of Matrigel. HRMEC were plated at a density of 4 × 104 cells/well in DME containing 1% fetal bovine serum. Agonists or control peptides, alone, or in combination with an EphB1 ectodomain competitor, EphB1/Fc (1000 ng/ml) were added at the time of plating. Cells were incubated at 37°C for 8 hr and photographed by phase microscopy (magnification, 100×) (Diaphot-TMD, Nikon).
Cell-attachment assays
Six-well plates (Falcon) were coated with thin layers of Matrigel, or with 0.5 μg/cm2 fibronectin (Ingber 1990). Growth medium was replaced 48 hr before harvest with binding medium, DMEM (HRMEC) or α-MEM (P19), containing 1% bovine albumin. Cells were recovered by trypsinization (HRMEC) or vigorous trituration (P19), washed three times with binding medium, then plated at 1 × 105 cells/well. Ligands were added coincident with plating. After 90 min, unattached cells were dislodged by applying four brisk slaps of the plate on a horizontal surface. The attached cell layer was carefully washed once with PBS containing calcium and magnesium to collect the remaining unattached cells. Adherent cells were collected by incubation in Dispase, as described (Collaborative Biomedical Products), recovered by centrifugation, washed and viable cells counted. The ratio of attached to total number of cells recovered were calculated for each of three wells. Data are expressed as the mean ±SEM and are representative of three independent experiments.
Transfections
P19 cells were transfected with a total of 10 μg of plasmid DNA per P100 plate by the Lipofectamine method (GIBCO/BRL). As indicated, pSRα–EphB1/HA or pSRα–EphB1–Y929F/HA were mixed prior to transfection at the ratio indicated in Figure 4. Forty hours after transfection, cells from the same transfections were used in attachment assays and immunoprecipitation experiments. The data displayed are representative of three independent experiments.
GST–EphB1 affinity binding assays
Recombinant GST–EphB1cy and GST–EphB1–Y929F/HA proteins were generated as described and expressed in Sf9 cells. Purified proteins were premixed in defined ratios where indicated, bound to glutathione–Sepharose, and self-phosphorylated by incubation in kinase buffer, as described previously (Stein et al. 1996). Proteins bound to glutathione–agarose were then used as an affinity matrix to evaluate LMW–PTP binding, as described in the legend to Figure 3.
Solid phase attachment assay
Twelve-well plates (Falcon) were coated with a thick layer of nitrocellulose solution (Schleicher & Schuell) and allowed to air dry, as described (Wang and Anderson 1997). Coated wells were incubated overnight at 4°C with 0.8 ml of PBS containing fibronectin (1.9 μg) alone (no addition, NA), or in combination with dimeric or antibody-clustered multimeric ephrin-B1/Fc (0.4 μg). Two hours prior to assay, wells were washed twice and then incubated at 37°C with PBS containing 1% bovine albumin. P19 cells were recovered by vigorous trituration, washed once with OPTI-MEM, and plated at a density of 3–5 × 105 cells/well in binding medium. Ninety minutes after plating, cells were harvested and counted as described above.
Acknowledgments
We thank T. Pawson and S. Holland (Toronto, CA) for the kind gift of the EphB2 antibodies. This work was supported by PHS awards DK38517 and DK47078 (T.O.D.), GM27003 (R.L.V.), the Vanderbilt Cancer Center/Ingram Fund, a National Cancer Institute award (CA 68485) and the Deutsche Forschungsgemeinschaft (H.O.S.). We also thank Brigid Hogan, Stanley Cohen, Lee Limbird, Roger Chalkley, Steve Hanks, and Tony Weil for comments and critical input.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL tom.daniel@mcmail.vanderbilt.edu; FAX (615) 343-7156.
References
- Bain G, Ray WJ, Yao M, Gottlieb DI. From embryonal carcinoma cells to neurons: The P19 pathway. BioEssays. 1994;15:343–348. doi: 10.1002/bies.950160509. [DOI] [PubMed] [Google Scholar]
- Beckmann MP, Cerretti DP, Baum P, Vanden Bos T, James L, Farrah T, Kozlosky C, Hollingsworth T, Shilling H, Marashovsky E, et al. Molecular characterization of a family of ligands for eph-related tyrosine kinase receptors. EMBO J. 1994;13:3757–3762. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohme B, VandenBos T, Cerretti DP, Park LS, Hotrich U, Ruebsamen-Waigmann H, Strebhardt K. Cell-cell adhesion mediated by binding of membrane-anchored ligand LERK-2 to the EPH-related receptor human embryonal kinase 2 promotes tyrosine kinase activity. J Biol Chem. 1996;271:24747–24752. doi: 10.1074/jbc.271.40.24747. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Brueckner K, Orioli D, Bergemann AD, Flanagan JG, Klein R. Similarities and differences in the way transmembrane-type ligands interact with the ELK subclass of Eph receptors. Mol Cell Neurosci. 1996;8:199–209. doi: 10.1006/mcne.1996.0057. [DOI] [PubMed] [Google Scholar]
- Brennan C, Monschau B, Lindberg R, Guthrie B, Drescher U, Bonhoeffer F, Holder N. Two Eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development. 1997;124:655–664. doi: 10.1242/dev.124.3.655. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for EPH receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Bryson GLM, Massa H, Trask BJ, Van Etten RL. Gene structure, sequence, and chromosomal localization of the human red cell-type L-M-W acid phoshotyrosyl phosphatase gene, ACP1. Genomics. 1995;30:133–140. doi: 10.1006/geno.1995.9893. [DOI] [PubMed] [Google Scholar]
- Cerretti DP, VandenBos T, Nelson N, Kozlosky CJ, Reddy P, Maraskovsky E, Park LS, Lyman SD, Copeland NG, Gilbert DJ, et al. Isolation of LERK-5: A ligand of the eph-related receptor tyrosine kinases. Mol Immun. 1996;32:1197–1205. doi: 10.1016/0161-5890(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann A, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek 4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–378. doi: 10.1016/0092-8674(95)90426-3. , [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Cirri P, Raugei G, Camici G, Dolfi F, Berti A, Ramponi G. PDGF receptor as a specific in vivo target for low M(r) protein tyrosine phosphatase. FEBS Lett. 1995;372:49–53. doi: 10.1016/0014-5793(95)00947-8. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Stein E, Cerretti DP, St. John PL, Robert BL, Abrahamson DR. ELK and LERK-2 in developing kidney and microvascular endothelial assembly. Kidney Int. 1996;50:S-73–S-81. [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for Eph-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;226:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- Eph Nomenclature Committee. Unified nomenclature for Eph receptors and their ligands, the ephrins. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuele DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Harrison SC. Peptide-surface association: The case of PDZ and PTB domains. Cell. 1996;86:341–343. doi: 10.1016/s0092-8674(00)80105-1. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Marengene LEM, McGlade J, Olivier JP, Conlon RA, Holmyard DP, Letwin K, Pawson T. Immunolocalization for the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene. 1994;9:1001–1014. [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder S, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signaling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CA, Anderson D, Moran MF, Ellis C, Pawson T. SH2 and SH3 domains: Elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Koenig DW, Barley-Maloney L, Daniel TO. A Western blot assay detects autoantibodies to cryptic endothelial antigens in thrombotic microangiopathies. J Clin Immuno. 1993;13:204–211. doi: 10.1007/BF00919973. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem Sci. 1994;19:459–463. doi: 10.1016/0968-0004(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Martin MM, Schoecklmann HO, Foster G, Barley-Maloney L, McKanna J, Daniel TO. Identification of a subpopulation of human renal microvascular endothelial cells with capacity to form capillary-like cord & tube structures. In Vitro Cell Dev Biol. 1997;33:261–269. doi: 10.1007/s11626-997-0045-y. [DOI] [PubMed] [Google Scholar]
- McTigue MA, Williams DR, Tainer JA. Crystal structures of a schistosomal drug and vaccine target: Glutathione S-transferase from Schistosoma japonicia and its complex with the leading antischistosomal drug praziquantel. J Mol Biol. 1995;246:21–27. doi: 10.1006/jmbi.1994.0061. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Cheng H-J, Friedman GC, McLaughlin T, Hansen MJ, Yoon CH, O’leary DM, Flanagan JG. Topographically specific effects of ELK-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell. 1996;86:755–766. doi: 10.1016/s0092-8674(00)80150-6. [DOI] [PubMed] [Google Scholar]
- Ostantin K, Pokalsky C, Wang S, VanEtten RL. Cloning and characterization of a Saccharomyces cerevisiae gene encoding the low molecular weight protein-tyrosine phosphatase. J Biol Chem. 1995;270:18491–18499. doi: 10.1074/jbc.270.31.18491. [DOI] [PubMed] [Google Scholar]
- Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-α-induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- Rigacci S, Degl’Innocenti D, Bucciantini M, Cirri P, Berti A, Ramponi G. pp60v-src phosphorylates and activates low molecular weight phosphotyrosine-protein phosphatase. J Biol Chem. 1996;271:1278–1281. doi: 10.1074/jbc.271.3.1278. [DOI] [PubMed] [Google Scholar]
- Schnarr M, Oertel-Buchheit P, Kazmaier M, Granger-Schnarr M. DNA binding properties of the LexA repressor. Biochimie. 1991;73:423–431. doi: 10.1016/0300-9084(91)90109-e. [DOI] [PubMed] [Google Scholar]
- Schultz J, Ponting CP, Hofman K, Bork P. SAM as a protein interaction domain involved in developmental regulation. Protein Sci. 1997;6:249–253. doi: 10.1002/pro.5560060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Stein E, Cerretti DP, Daniel TO. Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem. 1996;271:23588–23593. doi: 10.1074/jbc.271.38.23588. [DOI] [PubMed] [Google Scholar]
- Stein E, Huynh-Do, Lane AA, Cerretti DP, Daniel TO. Nck recruitment to Eph receptor, EphB1/ELK, couples ligand activation to c-Jun kinase. J Biol Chem. 1998;273:1303–1308. doi: 10.1074/jbc.273.3.1303. [DOI] [PubMed] [Google Scholar]
- Tang XX, Pleasure DE, Ikegaki N. cDNA cloning, chromosomal localization, expression pattern of EPLG8, a new member of the EPLG gene family encoding ligands of EPH related protein-tyrosine kinase receptors. Genomics. 1997;41:17–24. doi: 10.1006/geno.1997.4615. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P, Pawson T. The PTB domain: A new protein module implicated in signal transduction. Trends Biochem Sci. 1995;20:277–280. doi: 10.1016/s0968-0004(00)89043-x. [DOI] [PubMed] [Google Scholar]
- Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Anderson DJ. EPH family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1996;18:383–396. doi: 10.1016/s0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- Winslow JW, Moran P, Valverde J, Shih A, Yuan JQ, Wong SC, Tsai SP, Goddard A, Henzel WJ, Hefti F, et al. Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron. 1995;14:973–981. doi: 10.1016/0896-6273(95)90335-6. [DOI] [PubMed] [Google Scholar]
- Wo Y-Y, McCormack AL, Shabanowitz J, Hunt DF, Davis JP, Mitchell GL, Van Etten RL. Sequencing, cloning and expression of human red cell-type acid phosphatase, a cytoplasmic phosphotyrosyl protein phosphatase. J Biol Chem. 1992;267:10856–10865. [PubMed] [Google Scholar]
- Yajnik V, Blaikie P, Bork P, Margolis B. Identification of residues within the SHC phosphotyrosine binding/phosphotyrosine interaction domain crucial for phosphopeptide interaction. J Biol Chem. 1996;271:1813–1816. doi: 10.1074/jbc.271.4.1813. , [DOI] [PubMed] [Google Scholar]
- Zhang M, Stauffacher CV, VanEtten RL. The three dimensional structure, chemical mechanism and function of the low molecular weight protein tyrosine phosphatases. Adv Prot Phosphatases. 1995;9:1–23. [Google Scholar]
- Zhang M, VanEtten RL, Stauffacher CV. Crystal structure of bovine heart phosphotyrosyl phosphatase at 2.2-A resolution. Biochemistry. 1994;33:11097–11105. doi: 10.1021/bi00203a006. [DOI] [PubMed] [Google Scholar]