Abstract
One goal of vaccination is to promote development of mucosal effector cells that can immediately respond to peripheral infection. This is especially important for protection against viruses that enter the host through the respiratory tract. We show that targeting the OX40 costimulatory receptor (CD134) strongly promotes mucosal memory in the CD8 T cell compartment. Systemic injection of an agonist antibody to OX40 strongly enhanced development of polyfunctional effector CD8 T cells that were induced after intraperitoneal infection with a highly virulent strain of vaccinia virus. These cells were located in lymphoid organs and also the lung, and importantly, long-term memory CD8 T cells were maintained in the lung over 1 year. Anti-OX40 also boosted memory development when mice were vaccinated subcutaneously with viral peptide. These CD8 T cells were sufficient to provide protection from lethal respiratory infection with live vaccinia virus independent of CD4 T cells and antibody. Again, the CD8 T cell populations that were induced after secondary infection displayed polyfunctionality and were maintained in the lung for over a year. These data suggest that agonists to the OX40 costimulatory receptor represent potential candidates for incorporation into vaccines for respiratory viruses.
INTRODUCTION
Antibodies can provide protection against viruses and other infectious agents, but it is recognized that cellular memory provided by T cells is important for limiting infection. Many pathogens enter the body via mucosal surfaces. Current ideas support the notion that more centralized memory T cells that circulate throughout secondary lymphoid organs will not respond, expand in number, or relocate sufficiently quickly to provide immediate protection against disease caused by reinfection. In contrast, memory T cells that populate peripheral organs, such as the lung and gut, sometimes referred to as effector memory cells, have been suggested to be the cells that can provide this first line of defense (17, 19). Being able to elicit long-lived memory CD8 T cell populations that are not only cytolytic but polyfunctional in making high levels of gamma interferon (IFN-γ) and tumor necrosis factor (TNF) may also be essential for protection (7, 14, 45, 54). Therefore, molecules that induce high-frequency persisting polyfunctional CD8 T cell populations that localize in mucosal tissues are likely a key factor in generating effective cellular immunity and might offer considerable advantages in terms of protection if incorporated into a vaccine (1, 45).
The initial activation and priming of naïve CD8 T cells is likely one stage at which this type of protective memory population is generated. From studies of CD4 helper activity, it is becoming increasingly apparent that signals provided to antigen-presenting cells, or directly to CD8 T cells, can induce a program that dictates the quality of memory and the ability to respond to recall antigen. Most interestingly, a number of costimulatory interactions in the TNF/TNF receptor (TNFR) superfamily, such as those between CD40-CD40L, CD27-CD70, and TRAILR-TRAIL, have been implicated in this process (41). This suggests that targeting these or similar types of molecules might hold promise for generating the desirable mucosa-associated memory T cells.
Vaccinia virus (VACV) is a good model pathogen for investigating factors that control mucosal immunity and for studying the development of protective vaccines. Variants of VACV are being used as vaccine vehicles for infectious diseases, such as HIV and herpes simplex virus (HSV) infection, severe acute respiratory syndrome (SARS), influenza, tuberculosis (TB), and malaria (2, 12, 13, 35). Furthermore, VACV infection itself via the lung can target multiple cell types, including dendritic cells, and result in disseminated disease. The level of protection needed to combat mucosal VACV infection might be greater than with a virus such as influenza virus that is much more restricted in the cells in the lung that it can enter and replicate in and in how it then spreads. Recently, we demonstrated that the endogenous interaction of OX40 with OX40L, two additional members of the TNF/TNFR superfamily, was required for generating CD8 T cell memory for the Western Reserve strain of vaccinia virus (VACV-WR) and, most interestingly, that OX40-deficient mice could not generate protective CD8 T cells that were located in the lung and controlled an intranasal infection (43). More recently, we assessed the requirement for OX40 after infection with the attenuated poxvirus vaccine strains VACV-Lister and -NYCBOH. In striking contrast to VACV-WR, little difference in priming of virus-specific CD8 T cells was observed in OX40−/− mice (42). Therefore, a large cytokine-competent VACV-reactive memory CD8 T cell pool forms only when OX40 becomes active in response to persisting virus and as a consequence of virulence mechanisms of vaccinia. Importantly, in our published studies, we showed that the frequency of memory CD8 T cells elicited in the lung, and the use of endogenous OX40-OX40L interactions, determined the ability of the host to protect against subsequent infection (42, 43). This suggested that OX40 signals may induce the periphery-localizing memory T cells that are desirable for immunity against mucosal reinfection and that targeting OX40 in a vaccine strategy might effectively boost mucosal CD8 T cell memory. Here, we show this is the case, with an agonist antibody to OX40 strongly promoting mucosa-located protective memory CD8 T cell populations after virus infection or in a vaccination protocol with viral peptide. Moreover, these memory CD8 T cells were long-lived and were maintained in mucosal tissues, providing a means to immediately respond to secondary infection.
MATERIALS AND METHODS
Mice.
The studies reported here conform to the Animal Welfare Act and the NIH guidelines for the care and use of animals in biomedical research. All experiments were done in compliance with the regulations of the La Jolla Institute Animal Care Committee in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. Eight- to 12-week-old female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). OX40−/− mice on the BL/6 background were bred in house.
Peptides and tetramers.
The peptides used were B8R (20 to 27; TSYKFESV), A3L (270 to 227; KSYNYMLL), A8R (189 to 196; ITYRFYLI), B2R (54 to 62; YSQVNKRYI), and A23R (297 to 305; IGMFNLTFI) (32). Major histocompatibility complex (MHC)/peptide tetramers for the epitope B8R (20 to 27; TSYKFESV)/H-2Kb, conjugated to allophycocyanin (APC), were obtained from the National Institutes of Health Tetramer Core facility (Emory University, Atlanta, GA).
Viruses.
The VACV Western Reserve strain was purchased from the ATCC (Manassas, VA) and grown in HeLa cells, and the titer was determined on VeroE6 cells (43).
Immunization protocols.
Mice were infected intraperitoneally (i.p.) with 2 ×105 PFU of VACV as described previously (39, 43). In other experiments, mice were immunized subcutaneously (s.c.) at the base of the tail with either 10 or 2 μg/mouse of B8R peptide in incomplete Freund adjuvant (IFA), together with a hepatitis B virus (HBV) core 128 to 140 (TPPAYRPPNAPIL) epitope. Effector responses were analyzed between days 4 and 15 postinfection, while memory responses were analyzed 21 or more days after infection, after restimulation in vitro with VACV peptides. Anti-OX40 (150 μg/mouse; OX86, generated in-house) was injected i.p. on day 1 postinfection or postimmunization.
Vaccinia virus intranasal challenge.
Mice were anesthetized by inhalation of isoflurane and inoculated by the intranasal (i.n.) route with 3.5 ×106 PFU of VACV-WR. The mice were weighed daily for 2 weeks following challenge and euthanized when they lost 25% of their initial body weight. Tissues were collected in 10% neutral-buffered Formalin for hematoxylin and eosin (H&E) staining as described previously (44). Body weight was calculated as a percentage of the mean weight for each group on the day of challenge.
CD4 and CD8 depletion.
Mice were depleted of CD4+ or CD8+ T cells with anti-CD4 (clone GK1.5; 200 μg/mouse) or anti-CD8 (clone 2.43; 200 μg/mouse) given in one intravenous (i.v.) injection 3 days prior to and one i.p. injection every 3 days after intranasal challenge with VACV-WR. CD4 and CD8 depletion was confirmed by flow cytometry of spleens and lungs of treated mice.
Flow cytometry.
Cells (1 × 106 to 2 × 106 in 200 μl) were incubated with medium or VACV peptides at 1 μg/ml for 1 h at 37°C. GolgiPlug (BD Biosciences) was added, and the incubation continued for 7 h. The cells were stained with anti-CD8 (peridinin chlorophyll protein [PerCP]; 53-6.7), followed by fixation with cytofix-cytosperm (BD Biosciences) for 20 min at 4°C. The fixed cells were subjected to intracellular cytokine staining in BD Perm/Wash buffer for 30 min at 4°C. Anti-TNF (fluorescein isothiocyanate [FITC]; MP6-XT22) and anti-IFN-γ (APC; XMG1.2) were from e-Biosience and were used at a 1:100 dilution. Samples were analyzed with a FACSCalibur flow cytometer using CellQuest (BD Biosciences) and FlowJo (Tree Star, San Carlos, CA) software.
VACV titer assay.
Tissues from individual mice were homogenized and sonicated for 0.5 min with a pause every 10 s using an ultrasonic cleaner (1210; Branson, Danbury, CT). Serial dilutions were made, and the virus titers were then determined by plaque assay on confluent VeroE6 cells.
Statistics.
Statistical significance was analyzed by Student's t test. Unless otherwise indicated, data represent the mean ± standard error of the mean (SEM), with a P value of <0.05 considered statistically significant.
RESULTS
Anti-OX40 treatment enhances generation of virus-specific CD8 T cells in response to virulent VACV-WR.
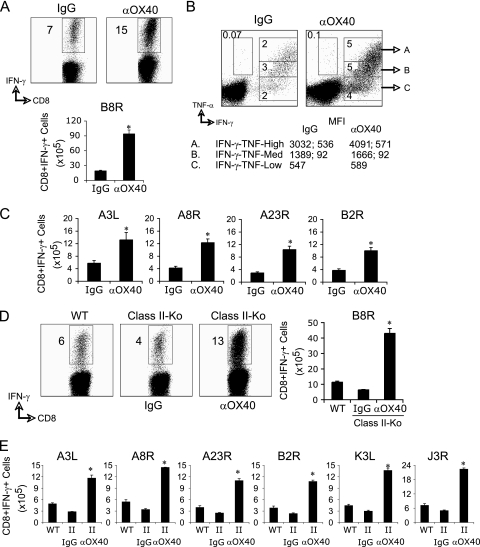
To assess the potential of targeting OX40 to promote protective tissue-resident virus-specific memory CD8 T cell populations, we focused on systemic priming so it would not provide a bias to generating mucosa-associated T cells. Additionally, many vectors that are being used as vaccine vehicles are being tested systemically because of possible safety concerns regarding mucosal immunization, as well as simple logistical issues associated with the efficiency of mucosal as opposed to systemic vaccination. We previously showed that an agonist antibody to OX40 enhanced the generation of effector CD8 T cells when given following infection with an attenuated vaccinia virus strain (VACV-Lister) that normally elicits a weak CD8 T cell response that does not involve endogenous OX40/OX40L interactions (42). We then focused on a nonattenuated strain, VACV-Western Reserve, that elicits much stronger CD8 T cell priming when dominant and subdominant epitope-specific populations are taken into account. Although endogenous OX40 interactions are required for generating high frequencies of effector CD8 T cells in response to VACV-WR (42, 43), the agonist antibody strongly promoted many more functional effector cells making IFN-γ in the spleen after 1 week. This applied to both the immunodominant population reactive with the epitope B8R and less dominant populations, so that each was boosted in number by a minimum of 3-fold (Fig. 1A to C). Thus, even with a strongly replicating virus that normally results in engagement of OX40/OX40L to drive CD8 T cell priming, supplementing endogenous OX40 signals can still further enhance the generation of effector T cells.
Fig. 1.
Enhanced CD8 T cell responses to VACV following anti-OX40 treatment. Wild-type C57BL/6 (A to C) or class-II deficient (Class-II-Ko) (D and E) mice were infected i.p. with 2 × 105 PFU/mouse of VACV-WR. One day later, the mice were treated with 150 μg of control rat IgG or anti-OX40 (αOX40). Eight days after infection, IFN-γ- and TNF-secreting CD8 cells were assessed in the spleen by intracellular cytokine staining after ex vivo stimulation with VACV peptides as indicated. The data are either representative plots of IFN-γ and TNF staining in gated CD8 T cells, with the percent positive indicated, or total numbers plus SEM of CD8+ IFN-γ+ T cells per spleen from four individual mice. (B) Representative plots for cytokine staining, gating on CD8+ CD62L-low cells. The percentages that stained positive for IFN-γ (gate C) or TNF (gate B) and IFN-γ/TNF (gate A) are indicated. (B) The MFI for IFN-γ (left) and TNF (right) staining in the gated populations are shown. *, P < 0.05 (IgG versus αOX40 treated) as determined by Student's t test. Similar results were obtained in three separate experiments.
Next, costaining for intracellular IFN-γ and TNF was performed on B8R-specific effector CD8 T cells to determine if there were entirely different populations of virus-specific T cells or if there were subsets within the IFN-γ-producing population. In general, the majority of B8R-specific CD8 T cells produced IFN-γ, with a minor population that produced TNF only (Fig. 1B). The B8R-specific CD8 T cells that produced IFN-γ could be divided into three subsets based on the TNF cytokine pattern: IFN-γ+ only (single producers; gate C), IFN-γ+ TNF-medium (double producers; gate B), and IFN-γ+ TNF+-high (double producers; gate A). Anti-OX40 treatment increased the frequency of all three populations (Fig. 1B; top). Using mean fluorescence intensity (MFI) analysis, Precopio et al. (36) found an association between the MFI of IFN-γ and the degree of polyfunctionality in VACV-specific CD8 T cells. Specifically, cells with increasing function (ability to produce TNF, interleukin 2 [IL-2], and MIP1-α) had higher MFIs of IFN-γ staining and thus produced more cytokine per cell (36). Analysis of the MFI staining within each gated population revealed that anti-OX40 treatment increased the MFI of IFN-γ, but not TNF, in the population that produced the highest levels of both IFN-γ and TNF (gated population A).
Lastly, the effect of targeting OX40 on eliciting primary VACV-specific CD8 T cell populations was not dependent on CD4+ T cells, as it occurred equivalently in MHC class II knockout animals (Fig. 1D and E). Signaling through OX40, therefore, represents a strong exogenous stimulus for promoting systemic primary effector CD8 T cells regardless of whether infection is with attenuated or nonattenuated vaccinia virus.
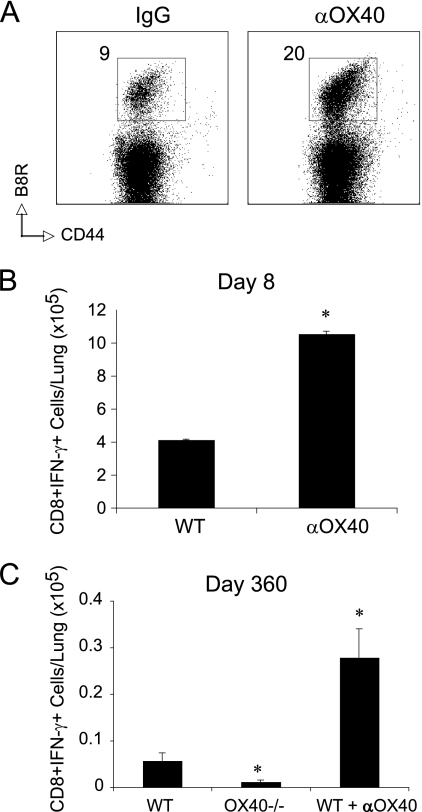
Most significantly, we observed a strong accumulation of VACV-reactive effector CD8 T cells in the lungs after 8 days, even though the virus infection and anti-OX40 injection were in the peritoneal cavity (Fig. 2A and B). This substantiated the possibility that OX40 signals might promote mucosal memory, and in line with this, we observed markedly enhanced numbers of virus-specific CD8 T cells 360 days after infection and anti-OX40 treatment (Fig. 2C). This correlated with the fact that very few memory CD8 T cells were present in the lungs of VACV-infected OX40-deficient mice at this time (Fig. 2C). Thus, OX40 signaling is a dominant driving force in generating long-lived CD8 T cell memory that is located in the lung.
Fig. 2.
Anti-OX40 treatment enhances the number of effector and memory CD8 T cells in the lung. Wild-type (A to C) or OX40−/− (C) mice were infected i.p. with 2 × 105 PFU/mouse of VACV-WR. One day later, mice were treated with 150 μg of control rat IgG or anti-OX40 (αOX40). Eight (A and B) or 360 (C) days after infection, virus-specific CD8 T cells were assessed in the lungs by tetramer staining (A) or intracellular cytokine staining (B and C) after ex vivo stimulation with VACV B8R peptide. The data are either representative plots of B8R tetramer staining in gated CD8 T cells, with the percent positive indicated, or total numbers plus SEM of CD8+ IFN-γ+ T cells per lung from four individual mice. *, P < 0.05 (IgG versus αOX40 treated) as determined by Student's t test. Similar results were obtained in three separate experiments.
Anti-OX40 treatment enhances the frequency and polyfunctionality of VACV-specific effector CD8 T cells in the lungs and spleen after peptide vaccination.
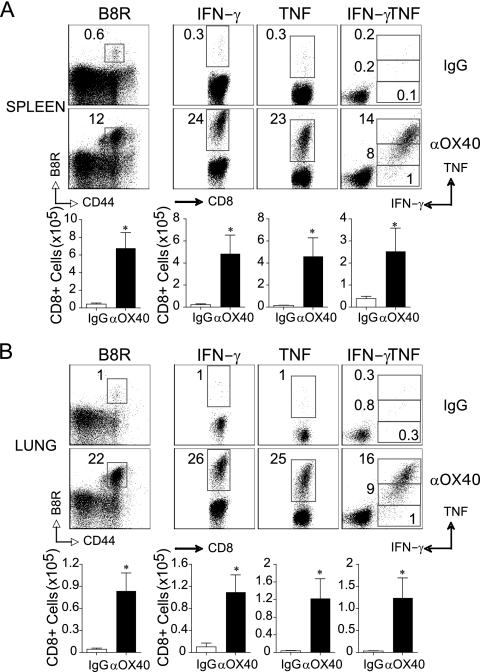
To pursue this activity of OX40, we focused on priming CD8 T cells by immunization with a peptide of VACV, as this does not result in antibody responses and allows evaluation of the protective capacity of the elicited T cell populations. MHC class I-restricted VACV peptide can induce CD8 T cells that prevent mortality after intranasal infection with live virus, but the level of protection is variable and dependent on the dose used for immunization (33, 43, 47). Mice were injected s.c. with the immunodominant peptide B8R20-27, given in IFA, and anti-OX40 was given 1 day later. Memory is the most important feature of any vaccination protocol, but before focusing on this aspect of the response, we determined if targeting OX40 with peptide immunization replicated the systemic and peripheral effector responses observed with whole-virus immunization (Fig. 1 and 2). Correlating with the prior data, anti-OX40 strongly boosted the number of VACV-specific primary effector CD8 T cells and, in particular, the polyfunctional subset that made high levels of TNF and IFN-γ, and this was observed not only in the spleen (Fig. 3A), but also markedly in the lungs (Fig. 3B).
Fig. 3.
Anti-OX40 treatment enhances the frequency and polyfunctionality of VACV-specific effector CD8 T cells in the lungs and spleen after systemic vaccination. (A) Wild-type mice were immunized s.c. at the base of the tail with 2 μg of B8R peptide in IFA. One day later, the mice were treated with 150 μg of control rat IgG or anti-OX40 (αOX40). Eight days postvaccination, spleen (A) and lung (B) cells were stained with B8R tetramer (left). CD8 T cell polyfunctionality was assessed by intracellular IFN-γ and TNF staining after stimulation with B8R peptide (right). (Top) Representative plots of tetramer or cytokine staining, gating on CD8+ T cells. The percentages that stained positive for B8R tetramer, IFN-γ alone, or TNF and IFN-γ/TNF are indicated. (Bottom) Total numbers of B8R tetramer-positive, CD8+ IFN-γ+, CD8+ TNF+, and CD8+ IFN-γ+ TNF+ cells per tissue. The results are means and SEM (n = 4 mice/group) from one experiment. *, P < 0.05 (IgG versus αOX40 treated) as determined by Student's t test. Similar results were obtained in two separate experiments.
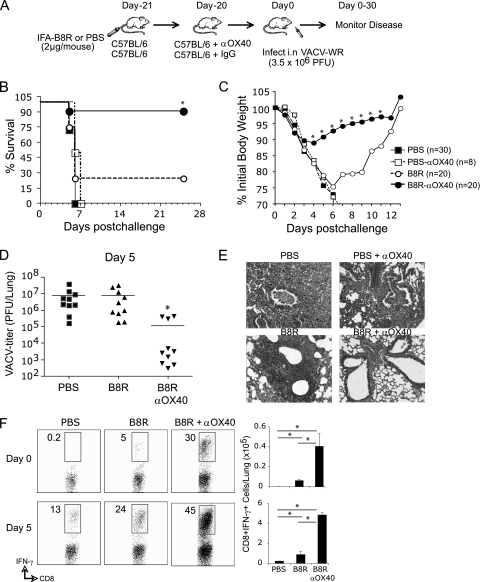
Anti-OX40 treatment combined with a single CD8 T cell peptide epitope of VACV protects mice against lethal respiratory challenge.
To determine if targeting OX40 resulted in protective mucosal memory, immunized and anti-OX40-treated mice were infected intranasally with VACV-WR 3 weeks later (Fig. 4A). Upon infection with a normally lethal dose of virus, 90% of the anti-OX40-treated mice survived with only moderate loss of body weight, whereas only 20% survived when OX40 was not targeted, and loss of body weight was far more severe (Fig. 4B and C). Correspondingly, at the peak of the T cell response at day 5, viral titers were reduced only in mice that had previously received anti-OX40 (Fig. 4D), and the inflammatory response in the lung that is a consequence of virus persistence was dramatically reduced (Fig. 4E). Significantly, 5- to 8-fold more functional (IFN-γ-secreting) memory CD8 T cells were present in the lungs 3 weeks after immunization in mice that received anti-OX40, representing approximately 30% of the CD8 T cells being reactive with the priming VACV peptide before virus infection (Fig. 4F, day 0). This directly correlated with the total number of VACV peptide-reactive secondary effector CD8 T cells generated in the lungs, which were at a frequency 10-fold higher than before reinfection. Moreover, the difference in frequency between the groups that did or did not receive anti-OX40 was maintained (Fig. 4F, day 5). Thus, exogenous OX40 signals given at the time of activation of naïve VACV-specific CD8 T cells promote a large population of CD8 T cells that have the ability to localize at mucosal surfaces and fully protect against subsequent virus infection via the lungs.
Fig. 4.
Anti-OX40 treatment combined with a single CD8 T cell peptide epitope of VACV completely protects mice against lethal mucosal VACV infection. (A) Wild-type mice were immunized s.c. at the base of the tail with 2 μg of B8R peptide in IFA. One day later, the mice were treated with 150 μg of control rat IgG or anti-OX40 (αOX40). Some controls received adjuvant but no peptide (PBS) or adjuvant plus anti-OX40 (PBS + αOX40). Three weeks postvaccination, the mice were infected i.n. with a lethal dose of VACV-WR (3.5 × 106 PFU/mouse [300 times the 50% lethal dose {LD50}]). The animals were weighed daily and euthanized if weight loss was greater than 25% of body weight. (B and C) Mean percent survival (B) and percentage of initial body weight (C) from the indicated numbers of mice. Mean weight data in some cases were not plotted beyond the point at which the mice died, and beyond day 7, the data reflect only mice that survived infection. (D) On day 5 post-VACV-WR challenge, lungs were removed from representative mice in panels C and D, and VACV titers were determined as described in Materials and Methods. (E) Lung sections stained with hematoxylin and eosin from representative mice in panel C. Magnification, ×100. (F) On the day of (top, day 0) or 5 days after (bottom) intranasal challenge, the numbers of IFN-γ-secreting CD8 cells were assessed in lungs from representative mice in panels B and C by intracellular cytokine staining after stimulation with B8R peptide. The results are means and SEM for each group (n = 10 mice/group) from two experiments. *, P < 0.05 (B8R versus B8R plus αOX40 treated).
Anti-OX40 treatment elicits long-lived polyfunctional VACV-specific CD8 memory T cells in the lung.
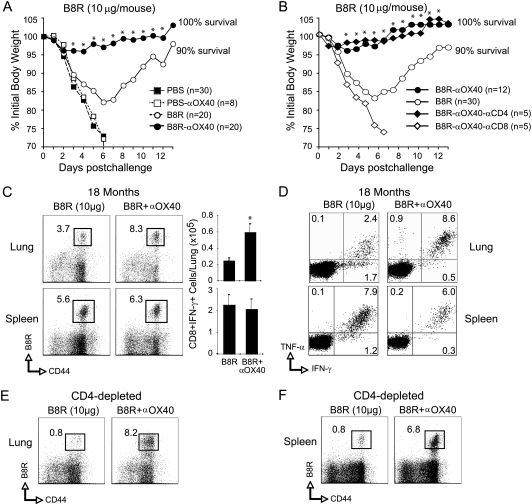
Lastly, we addressed whether this population of memory CD8 T cells was long-lived, even after a secondary infection, and if anti-OX40 would be active even if the initial immunization were optimized. Mice were primed with a 5-fold-higher dose of peptide in adjuvant with or without anti-OX40; 3 weeks later, a normally lethal intranasal infection was given, and again, targeting OX40 resulted in essentially full protection with little evidence of weight loss (Fig. 5A) and no death. Protection was confirmed to be mediated by CD8 T cells, as depletion of these cells, but not CD4 T cells, resulted in lethal weight loss (Fig. 5B). These animals were left for 18 months, and the frequency of VACV peptide-reactive CD8 T cells was again determined in the lungs and spleen. Quite strikingly, the number of CD8 T cells remained elevated in the anti-OX40-treated mice, and specifically in the lung, as analyses of the spleen revealed no difference in the frequency of VACV-specific CD8 T cells surviving (Fig. 5C). Our previous studies (42, 43), which included direct adoptive transfer of memory CD8 T cells into the lungs, together with those shown in Fig. 4, demonstrate that the numbers of CD8 T cells in the lung prior to intranasal challenge directly correlate with the degree of protection. These data suggested that minimally 104 CD8 T cells should be present in the lungs in order to allow the survival of the majority of animals infected with a normally lethal dose of VACV-WR. This threshold was clearly achieved in the lung over an 18-month period following anti-OX40 treatment (Fig. 5C). Furthermore, these cells in the lung retained their polyfunctionality, being capable of making high levels of IFN-γ and TNF, whereas the long-lived population present in the lung that resulted from vaccination in the absence of anti-OX40 contained a much lower frequency capable of exhibiting this phenotype (Fig. 5D). CD4 T cells have variably been shown to be important for maintenance of effective CD8 T cell memory, but they were not required for the persistence of the cells found in virus-challenged mice previously immunized with anti-OX40 (compare Fig. 5C to E and F). In contrast, the long-lived CD8 memory populations in the control mice that were not immunized with anti-OX40 did exhibit some CD4 T cell dependence (compare Fig. 5C to E and F). Therefore, targeting OX40 promotes long-lived CD4-independent mucosal CD8 T cell memory for vaccinia virus epitopes.
Fig. 5.
Anti-OX40 treatment enhances polyfunctionality and persistence of VACV-specific memory CD8 T cells in the lung independently of CD4 T cells. Wild-type mice were immunized s.c. at the base of the tail with 10 μg of B8R peptide in IFA. One day later, the mice were treated with 150 μg of control rat IgG or anti-OX40 (αOX40). Some controls received adjuvant but no peptide (PBS) or adjuvant plus anti-OX40 (PBS + αOX40). Three weeks postvaccination, the mice were infected i.n. with a lethal dose of VACV-WR (3.5 × 106 PFU/mouse [300 × the LD50]). The animals were weighed daily and euthanized if weight loss was greater than 25% of body weight. (A and B) Mean percentages of initial body weight from the indicated numbers of mice. The mean weight data in some cases were not plotted beyond the point at which the mice died, and data beyond day 7 reflect only mice that survived infection. (B) As indicated, groups of mice were depleted of CD4 (αCD4) or CD8 (αCD8) T cells prior to intranasal challenge with VACV. (C to F) eighteen months after intranasal challenge, the numbers of B8R tetramer-positive (C, E, and F) and B8R-specific IFN-γ- and TNF-secreting (D) CD8 T cells were assessed in lungs and spleens from representative mice in panels A and B. The results are means and SEM for each group (the numbers of mice per group are shown in panels A and B) from two experiments. *, P < 0.05 (B8R versus B8R plus αOX40 treated).
DISCUSSION
Here, we show that stimulating the OX40 receptor during the initiation of CD8 T cell responses not only can boost the frequency of memory T cells that are formed, but importantly, it efficiently boosts T cell memory that is located at mucosal surfaces, specifically the lung. We demonstrated this when priming of CD8 T cells was induced by live virus, as well as when priming was induced with viral peptide. These data suggest that incorporating agonist reagents to OX40 in a vaccine vehicle may represent an effective way to immunize against viruses that primarily infect the respiratory tract, where a lack of pathogenicity or mortality relies on a preexisting population of effector memory CD8 T cells that can immediately respond and reduce virus replication.
OX40 was reported to be a costimulatory receptor for CD4 T cells many years ago and was found to provide signals to amplify the proliferation, as well as survival, of this subset (6, 40). This correlated with reports showing that blocking endogenous OX40/OX40L interactions could suppress the generation of CD4 T cell memory and that targeting OX40 exogenously with agonist reagents could promote memory generation in the CD4 compartment. OX40 may promote these activities by several means, including direct signals via NF-κB and phosphatidylinositol 3-kinase (PI3K)/Akt that target cell cycle-related proteins, such as survivin and cyclin A, and that target antiapoptotic molecules, such as Bcl-xL and Bcl-2 (37, 48–51). Further indirect activities upregulating cytokine receptors, such as IL-2R and IL-12R, may amplify the effects of ligating OX40 (21, 38, 53). The activity of OX40 on CD8 T cells was initially questioned (8, 20). However, a number of studies have now shown that OX40 signaling can positively impact the generation of CD8 T cell responses in several situations (3, 10, 22, 23), most notably in tumor settings (reviewed in reference 6). Moreover, several recent publications have demonstrated a role for OX40 in promoting CD8 T cell populations after virus infection (15, 16, 41, 43). In some cases, the activity of OX40 has been to augment CD4 help for CD8 priming, depending on the model system analyzed, whereas in other cases a direct activity of OX40 on CD8 T cells has been evident (41).
Collectively, these studies have suggested that OX40 is a good target for enhancing the generation of T cell memory and that agonist reagents to OX40, or the gene encoding OX40L, expressed in a vector, might be useful in vaccination regimens (41). Although the tumor literature is quite extensive in this regard (6), there have been few studies related to vaccination against viral disease. Furthermore, whether OX40 signaling simply promotes central memory that resides primarily in the lymphoid organs or whether it can equally or preferentially promote peripheral tissue memory (so-called effector memory) has not been clear. Studies in vitro showed that OX40L in an adenoviral vector transfected into monocytes could promote expansion of human flu-specific memory CD8 T cells (46) and also that an agonist OX40L.Fc construct could augment expansion of memory CD8 T cells specific for HIV and EBV (56), but arguably, these studies had very little relevance to vaccination per se. However, an interesting study in vivo did show that OX40L in a plasmid vector also encoding hepatitis B virus surface antigen could boost CD4 and CD8 T cell responses to this antigen in the spleen, when used in a vaccination protocol injecting the plasmid intramuscularly 3 times over 4 weeks (9). Furthermore, intramuscular vaccination with a plasmid encoding foot and mouth disease virus (FMDV) VP1 peptide plus a plasmid encoding OX40L had a moderate effect on promoting short-term CD4 and CD8 T cell priming in spleens of mice and a partial effect in reducing replication of FMDV in guinea pigs when challenged subcutaneously with the virus (55). Most relevant to our study are experiments with an HIV-1 canarypox vaccine vector coadministered with OX40L-expressing canarypox, also given intramuscularly. This resulted in enhanced expansion of HIV-specific CD8 T cells detected after 6 weeks in the spleen, although again no analysis of memory in other sites was performed (24).
Our experiments strongly extend these reports by first showing that targeting OX40 can promote enhanced CD8 T cell memory populations that are maintained for very long periods (up to 18 months) and that this can be seen following infection with live virulent virus, as well as with viral peptide vaccination. Arguably most important, we found that systemic vaccination and anti-OX40 treatment resulted in memory residing in mucosal tissues. One goal of vaccine development that can be applied to a variety of pathogens is the induction of mucosal immunity that can effectively block extensive pathogen replication and that, critically, is long-lived. Many investigators have suggested that this can be achieved only by mucosal vaccination because of trafficking programs of lymphocytes, possible imprinting dependent on the site of priming, and potential compartmentalization of immune responses in distinct niches (4, 5, 11, 29, 30, 34). However, there are studies of CD8 T cell vaccination that have argued against this (27, 28), for example, showing intramuscular or subcutaneous immunization with virus or vaccine vectors can result in strong responses at mucosal effector sites (18, 25, 26). Our data effectively support the latter and show that even though CD8 T cell priming with virus or peptide was in the peritoneal cavity or subcutaneous and the antibody to OX40 was also injected in the peritoneal cavity, memory CD8 T cell populations were induced that were maintained in the lung over many months. Most notably, these were sufficient to provide strong protection against intranasal reinfection with high doses of live virus.
It is not clear that promoting mucosal memory is specific to OX40 stimulation, nor is it indicative of OX40 promoting a different migratory pattern in memory cells, and this needs to be examined in the future. However, interestingly, in our previous studies of OX40-deficient mice (42, 43) and those here (Fig. 2), the lack of endogenous OX40 signals severely restricted the VACV-specific memory CD8 T cell population that was found in the lungs. This also correlated with a study of CD4 T cells in which OX40-deficient mice displayed a profound defect in the number of polyclonal memory cells found in peripheral tissues, including the lung and lamina propria. Furthermore, in several immunization protocols, a severe defect was observed in generating CD4 T cell populations displaying an effector memory phenotype that is most commonly associated with localization to peripheral tissues (52). Similarly, it was found during CD8 T cell priming to Listeria monocytogenes that so-called memory precursor effector cell development was strongly impaired in OX40-deficient mice, resulting in very few memory T cells being generated (31). Therefore, regardless of whether OX40 signaling directly or specifically promotes T cell memory that can locate in peripheral tissues, these results, together with our data here on vaccinia virus-specific memory CD8 T cell populations, suggest that targeting OX40 in vaccines may be highly desirable to promote protective memory for poxviruses and likely other viruses that may enter the body through mucosal tissues.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI77079 and AI087734 to S.S.-A. and CA91837 and AI67341 to M.C. and by a fellowship from the Center for Infectious Disease at the La Jolla Institute for Allergy and Immunology to S.S.-A.
This is publication 1380 from the La Jolla Institute for Allergy and Immunology.
We declare that no conflict of interest exists.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Ahlers J. D., Belyakov I. M. 2010. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood 115:1678–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Artenstein A. W. 2008. New generation smallpox vaccines: a review of preclinical and clinical data. Rev. Med. Virol. 18:217–231 [DOI] [PubMed] [Google Scholar]
- 3. Bansal-Pakala P., Halteman B. S., Cheng M. H., Croft M. 2004. Costimulation of CD8 T cell responses by OX40. J. Immunol. 172:4821–4825 [DOI] [PubMed] [Google Scholar]
- 4. Belyakov I. M., Ahlers J. D. 2009. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J. Immunol. 183:6883–6892 [DOI] [PubMed] [Google Scholar]
- 5. Calzascia T., et al. 2005. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 22:175–184 [DOI] [PubMed] [Google Scholar]
- 6. Croft M. 2010. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28:57–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davenport M. P., Belz G. T., Ribeiro R. M. 2009. The race between infection and immunity: how do pathogens set the pace? Trends Immunol. 30:61–66 [DOI] [PubMed] [Google Scholar]
- 8. Dawicki W., Bertram E. M., Sharpe A. H., Watts T. H. 2004. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J. Immunol. 173:5944–5951 [DOI] [PubMed] [Google Scholar]
- 9. Du X., et al. 2007. The adjuvant effects of co-stimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J. Gene Med. 9:136–146 [DOI] [PubMed] [Google Scholar]
- 10. Fujita T., Ukyo N., Hori T., Uchiyama T. 2006. Functional characterization of OX40 expressed on human CD8+ T cells. Immunol. Lett. 106:27–33 [DOI] [PubMed] [Google Scholar]
- 11. Gallichan W. S., Rosenthal K. L. 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 184:1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gherardi M. M., Esteban M. 2005. Recombinant poxviruses as mucosal vaccine vectors. J. Gen. Virol. 86:2925–2936 [DOI] [PubMed] [Google Scholar]
- 13. Gómez C. E., Najera J. L., Krupa M., Esteban M. 2008. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr. Gene Ther. 8:97–120 [DOI] [PubMed] [Google Scholar]
- 14. Harty J. T., Badovinac V. P. 2008. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8:107–119 [DOI] [PubMed] [Google Scholar]
- 15. Humphreys I. R., et al. 2007. Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J. Exp. Med. 204:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Humphreys I. R., et al. 2007. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T Cells: A CD4-dependent mechanism. J. Immunol. 179:2195–2202 [DOI] [PubMed] [Google Scholar]
- 17. Jameson S. C., Masopust D. 2009. Diversity in T cell memory: an embarrassment of riches. Immunity 31:859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaufman D. R., et al. 2008. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol. 181:4188–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohlmeier J. E., Woodland D. L. 2009. Immunity to respiratory viruses. Annu. Rev. Immunol. 27:61–82 [DOI] [PubMed] [Google Scholar]
- 20. Kopf M., et al. 1999. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity 11:699–708 [DOI] [PubMed] [Google Scholar]
- 21. Lathrop S. K., et al. 2004. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J. Immunol. 172:6735–6743 [DOI] [PubMed] [Google Scholar]
- 22. Lee S. J., et al. 2004. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J. Immunol. 173:3002–3012 [DOI] [PubMed] [Google Scholar]
- 23. Lee S. J., et al. 2007. CD134 costimulation couples the CD137 pathway to induce production of supereffector CD8 T cells that become IL-7 dependent. J. Immunol. 179:2203–2214 [DOI] [PubMed] [Google Scholar]
- 24. Liu J., Ngai N., Stone G. W., Yue F. Y., Ostrowski M. A. 2009. The adjuvancy of OX40 ligand (CD252) on an HIV-1 canarypox vaccine. Vaccine 27:5077–5084 [DOI] [PubMed] [Google Scholar]
- 25. Liu L., Fuhlbrigge R. C., Karibian K., Tian T., Kupper T. S. 2006. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity 25:511–520 [DOI] [PubMed] [Google Scholar]
- 26. Masopust D., et al. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207:553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masopust D., Vezys V., Marzo A. L., Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413–2417 [DOI] [PubMed] [Google Scholar]
- 28. Masopust D., et al. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 172:4875–4882 [DOI] [PubMed] [Google Scholar]
- 29. Mora J. R., et al. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424:88–93 [DOI] [PubMed] [Google Scholar]
- 30. Mora J. R., et al. 2005. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201:303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mousavi S. F., et al. 2008. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J. Immunol. 181:5990–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moutaftsi M., et al. 2006. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 24:817–819 [DOI] [PubMed] [Google Scholar]
- 33. Moutaftsi M., et al. 2009. Correlates of protection efficacy induced by vaccinia virus-specific CD8+ T-cell epitopes in the murine intranasal challenge model. Eur. J. Immunol. 39:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neutra M. R., Kozlowski P. A. 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6:148–158 [DOI] [PubMed] [Google Scholar]
- 35. Perkus M. E., Tartaglia J., Paoletti E. 1995. Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious diseases. J. Leukoc. Biol. 58:1–13 [DOI] [PubMed] [Google Scholar]
- 36. Precopio M. L., et al. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 204:1405–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rogers P. R., Song J., Gramaglia I., Killeen N., Croft M. 2001. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 15:445–455 [DOI] [PubMed] [Google Scholar]
- 38. Ruby C. E., Montler R., Zheng R., Shu S., Weinberg A. D. 2008. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J. Immunol. 180:2140–2148 [DOI] [PubMed] [Google Scholar]
- 39. Salek-Ardakani S., et al. 2009. Preferential use of B7.2 and not B7.1 in priming of vaccinia virus-specific CD8 T cells. J. Immunol. 182:2909–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salek-Ardakani S., Croft M. 2006. Regulation of CD4 T cell memory by OX40 (CD134). Vaccine 24:872–883 [DOI] [PubMed] [Google Scholar]
- 41. Salek-Ardakani S., Croft M. 2010. Tumor necrosis factor receptor/tumor necrosis factor family members in antiviral CD8 T-cell immunity. J. Interferon Cytokine Res. 30:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salek-Ardakani S., et al. 2011. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J. Clin. Invest. 121:296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salek-Ardakani S., Moutaftsi M., Crotty S., Sette A., Croft M. 2008. OX40 drives protective vaccinia virus-specific CD8 T cells. J. Immunol. 181:7969–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salek-Ardakani S., et al. 2003. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 198:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seder R. A., Darrah P. A., Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258 [DOI] [PubMed] [Google Scholar]
- 46. Serghides L., et al. 2005. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J. Immunol. 175:6368–6377 [DOI] [PubMed] [Google Scholar]
- 47. Snyder J. T., Belyakov I. M., Dzutsev A., Lemonnier F., Berzofsky J. A. 2004. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J. Virol. 78:7052–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song A., Tang X., Harms K. M., Croft M. 2005. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J. Immunol. 175:3534–3541 [DOI] [PubMed] [Google Scholar]
- 49. Song J., et al. 2004. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat. Immunol. 5:150–158 [DOI] [PubMed] [Google Scholar]
- 50. Song J., Salek-Ardakani S., So T., Croft M. 2007. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat. Immunol. 8:64–73 [DOI] [PubMed] [Google Scholar]
- 51. Song J., So T., Croft M. 2008. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J. Immunol. 180:7240–7248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soroosh P., Ine S., Sugamura K., Ishii N. 2007. Differential requirements for OX40 signals on generation of effector and central memory CD4+ T cells. J. Immunol. 179:5014–5023 [DOI] [PubMed] [Google Scholar]
- 53. Williams C. A., Murray S. E., Weinberg A. D., Parker D. C. 2007. OX40-mediated differentiation to effector function requires IL-2 receptor signaling but not CD28, CD40, IL-12Rbeta2, or T-bet. J. Immunol. 178:7694–7702 [DOI] [PubMed] [Google Scholar]
- 54. Wong P., Pamer E. G. 2003. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 21:29–70 [DOI] [PubMed] [Google Scholar]
- 55. Xiao C., Rajput Z. I., Hu S. 2007. Improvement of a commercial foot-and-mouth disease vaccine by supplement of Quil A. Vaccine 25:4795–4800 [DOI] [PubMed] [Google Scholar]
- 56. Yu Q., et al. 2006. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J. Immunol. 176:2486–2495 [DOI] [PubMed] [Google Scholar]