Abstract
Following ocular herpes simplex virus 1 (HSV-1) infection of C57BL/6 mice, HSV-specific (HSV-gB498–505 tetramer+) CD8+ T cells are induced, selectively retained in latently infected trigeminal ganglia (TG), and appear to decrease HSV-1 reactivation. The HSV-1 latency-associated transcript (LAT) gene, the only viral gene that is abundantly transcribed during latency, increases reactivation. Previously we found that during latency with HSV-1 strain McKrae-derived viruses, more of the total TG resident CD8 T cells expressed markers of exhaustion with LAT+ virus compared to LAT− virus. Here we extend these findings to HSV-1 strain 17syn+-derived LAT+ and LAT− viruses and to a virus expressing just the first 20% of LAT. Thus, the previous findings were not an artifact of HSV-1 strain McKrae, and the LAT function involved mapped to the first 1.5 kb of LAT. Importantly, to our knowledge, we show here for the first time that during LAT+ virus latency, most of the HSV-1-specific TG resident CD8 T cells were functionally exhausted, as judged by low cytotoxic function and decreased gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) production. This resulted in LAT− TG having more functional HSV-gB498–505 tetramer+ CD8+ T cells compared to LAT+ TG. In addition, LAT expression, in the absence of other HSV-1 gene products, appeared to be able to directly or indirectly upregulate both PD-L1 and major histocompatibility complex class I (MHC-I) on mouse neuroblastoma cells (Neuro2A). These findings may constitute a novel immune evasion mechanism whereby the HSV-1 LAT directly or indirectly promotes functional exhaustion (i.e., dysfunction) of HSV-specific CD8+ T cells in latently infected TG, resulting in increased virus reactivation.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) establishes lifelong latency in its host. After a primary acute infection of the eye, HSV-1 travels up sensory neurons to the trigeminal ganglia (TG), where it maintains latent infections (2, 16, 18, 42, 48). Recurrent corneal disease results from the reactivation of latent virus from neurons of the TG, anterograde transportation to nerve termini, and reinfection of the cornea (2, 42). In addition to latency-associated transcript (LAT) and other viral factors, recent reports suggest that CD8+ T cells in the TG may contribute to the establishment, maintenance, and/or reactivation of latent HSV-1 in TG (2, 42). However, the mechanisms remain to be fully elucidated.
During HSV-1 latency, the HSV-1 latency-associated transcript (LAT) gene is the only viral gene that is consistently detected to be abundantly transcribed. LAT+ viruses (i.e., wild-type [wt] HSV-1) have a significantly higher reactivation phenotype (explant TG induced reactivation in mice and spontaneous reactivation in rabbits) than their LAT− mutant counterparts (32, 43, 54, 55, 67), and this appears to be due in large part to LAT's antiapoptosis activity (1, 8, 22, 55, 57, 58, 60, 72).
HSV-specific CD8+ T cells are selectively activated and retained in latently infected TG (2, 16, 42). They can significantly reduce explant TG-induced reactivation in mice (42, 48), apparently by interfering with virus replication and spread following the initial molecular events of reactivation. Thus, CD8+ T cells may help to decrease or delay HSV-1 reactivations (2, 42, 48) but do not eliminate the latent virus. This CD8+ T-cell activity is suggested by mouse, rabbit, and human studies (37, 42, 70, 75).
How does HSV-1 manage to reactivate in the face of an often sizable pool of virus-specific CD8+ T cells in the TG (16, 33, 42)? One possibility is CD8+ T-cell exhaustion. CD8+ T-cell exhaustion is usually the result of the prolonged high-level presence of antigen (Ag), as occurs during productive chronic infections (49). Chronic viral infections, such as with lymphocytic choriomeningitis virus (LCMV), HIV-1, hepatitis B virus (HBV), hepatitis C virus (HCV), and human T-lymphotropic virus (HTLV), appear to sustain their productivity by inducing functional impairment (exhaustion) of virus-specific CD8+ T cells (4, 21, 49). Functional exhaustion is characterized by progressive loss of production of cytokines, such as interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ), and other functions, such as cytotoxic activity (2, 38, 41, 49, 66, 74). Phenotypic exhaustion refers to the presence of markers of exhaustion on CD8 T cells, most commonly PD-1 (4, 25, 38, 62, 65, 66, 78). We recently showed that markers of exhaustion are present on CD8 T cells during HSV-1 latent infection of mouse trigeminal ganglia (TG) (2) and that more CD8 T cells have these markers in TG of mice latently infected with wild-type (wt) LAT+ virus compared to mutant LAT− virus.
In this article, we present the following findings. (i) During latency, LAT+ viruses caused significantly more functional exhaustion of total and HSV-1-specific TG resident CD8+ T cells than LAT− viruses. (ii) This was not due to an artifact of the HSV-1 strain used, since similar results were obtained with LAT+ viruses from two different HSV-1 strains, McKrae and 17syn+. (iii) LAT3.3A, a mutant that expresses just the first 1.5 kb of the primary 8.3-kb LAT gene, gave the same result as wt McKrae and wt 17syn+, mapping the LAT “exhaustion” function to the same region of LAT that is responsible for LAT's antiapoptosis activity and ability to support the wt reactivation phenotype (59). (iv) The number of functional (not exhausted) CD8+ T cells was significantly higher during latency with LAT− compared to LAT+ virus. (v) In mouse neuroblastoma cells stably expressing LAT, in the absence of other viral genes (as occurs during latency), both PD-L1 and major histocompatibility complex class I (MHC-I) were significantly upregulated. Our findings suggest that LAT may be involved in an immune evasion mechanism that reduces the number of functional HSV-specific CD8+ T cells within latently infected TG, thus allowing for increased viral reactivation of LAT+ virus compared to LAT− mutants.
MATERIALS AND METHODS
Mice.
Female C57BL/6 (B6) mice, 5 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal studies conformed to the Guide for the Care and Use of Laboratory Animals (50a).
Virus.
Throughout the study, wild-type HSV-1 strains and LAT-rescued mutants expressing LAT (e.g., dLAT2903R) are designated LAT+. LAT-null virus mutants are designated LAT−. Plaque-purified HSV-1 strains McKrae (wild type) and its derived mutants, LAT− dLAT2903, LAT+ dLAT2903R, and LAT+ LAT3.3A, were grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum (FCS), as described previously (39, 55, 58). The wild-type HSV-1 strain 17syn+ and its LAT− mutant and LAT+ rescuants were generously provided by Nigel Fraser (10).
Ocular infection.
Mice were infected ocularly with 2 × 105 PFU of either LAT+ or LAT− virus. All McKrae-derived viruses were suspended in 5 μl of tissue culture medium and administered as an eye drop without prior corneal scarification. For 17syn+ strain-derived viruses, corneal scarification was performed just prior to infection (10). Mock-infected mice received sterile tissue culture media and were used as negative controls in all experiments.
Preparation of single-cell suspensions from mouse TG.
Mice were euthanized and TG were harvested, pooled, and digested in DMEM–5% fetal bovine serum (FBS) containing collagenase I (Life Technologies, Carlsbad, CA), as we previously described (5, 16, 17, 37). The digested tissue suspension was passed through a 70-μm nylon cell strainer followed by a 40-μm nylon cell strainer. Cell suspensions were spun down at 1,400 rpm for 5 min at 4°C and then washed and suspended in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS]–0.01% NaN3–0.1% bovine serum albumin [BSA], 2 mM EDTA) for FACS acquisition and analysis. Ten TG were pooled and digested with collagenase type I. To obtain the number of cells per TG, the total number of cells obtained from a 10-TG cell suspension was divided by 10. Cells were stained with monoclonal antibodies (MAbs) and tetramers, as described below. The lymphocyte populations in the TG suspension were gated from other nonlymphocyte cells (i.e., glial cells, Schwann cells, neurons, fibroblasts, etc.) based on their specific size and granularity. The total number of CD8+ T cells was calculated based on the number of gated lymphocytes and used for normalization. The percentage of CD8+ T cells that were tetramer+ or PD-1+ was calculated based on the following formula: [(no. of events in CD3+ CD8+ tetramer+/PD-1+ cells) × (no. of events in gated lymphocytes)/(no. of total events acquired)] × 100.
Tetramer assay.
TG cell suspensions were analyzed for the frequency of CD8+ T cells specific to the HSV-gB498–505 epitope using the HSV-gB498–505 tetramer, which is H-2Kb restricted, as we previously described (82). The cells were first incubated with 2 μl of phycoerythrin (PE)-labeled HSV-gB498-505/H-2Kb tetramer at 4°C for 30 to 45 min. The cells were washed twice and stained with 1 μl fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8 antibody (clone 53-6.7; eBioscience). After two additional washings, cells were fixed with 1% formaldehyde in PBS. A total of 400,000 events were acquired by FACScan (Becton Dickinson) followed by analysis using Cell Quest software (BD Biosciences, San Jose, CA). The absolute numbers of HSV-gB498-505-specific CD8+ T cells were calculated using the following formula: no. of CD8+ tetramer+ cells in the test − no. of CD8+ tetramer+ cells in the negative control. The anti-mouse CD8 MAb was pretested prior to use to confirm that it did not interfere with altered tetramer binding.
Intracellular cytokine assay.
Intracellular cytokines produced by HSV-gB498–505 CD8+ T cells were measured using an intracellular cytokine assay kit (BD-Biosciences). Freshly isolated TG-derived CD8+ T cells were either unstimulated or were stimulated in triplicate with HSV-gB498–505 peptide or phorbol myristate acetate plus ionomycin (PMA + Iono) (positive control) at 37°C for 6 h, as recommended by the manufacturer. The cells were surface stained with peridinin chlorophyll protein (PerCP)-conjugated anti-mouse CD8 for 30 min at 4°C. For subsequent intracellular staining, cells were permeabilized and stained with PE-conjugated anti-mouse IFN-γ and TNF-α MAbs, as recommended by the manufacturer. The cells were washed twice and analyzed using a FACScan (BD Biosciences).
CD107 cytotoxicity assay.
The CD107 assay was performed as recently described (15–18, 37) with a few modifications. Freshly isolated TG-derived cells were left unstimulated or were stimulated with gB498–505 or phytohemagglutinin (PHA) (positive control) at 37°C for 5 to 6 h in the presence of BD GolgiStop (BD Biosciences) and 10 μl of FITC-conjugated CD107 antibody. Cells were washed and stained with 2 μl of PE-labeled HSV-gB498–505/H-2Kb tetramer and 1 μl of PerCP-conjugated anti-mouse CD8 for 30 min at 4°C. The cells were then washed again and analyzed using a FACScan (BD Biosciences).
Neuronal cell lines.
The mouse neuroblastoma cell lines C1300 and Neuro2A have been widely used as neuronal tissue culture models to study various aspects of herpes simplex virus. The LAT+ DC-LAT6 and LAT− DC-ΔLAT311 C1300-derived cell lines and the LAT+ JWLAT and LAT− JW-ΔLAT Neuro2A-derived cell lines have been described previously (12, 13, 37). JWLAT expresses a shorter region of LAT, LAT nucleotides (nt) 1 to 1499, compared to LAT nt 1 to 3225 for DC-LAT6. C1300-derived cell lines were grown in MEM containing 10% (vol/vol) heat-inactivated fetal calf serum, penicillin (10 U/ml), streptomycin (100 mg/ml), 1 mM sodium pyruvate, 1× MEM nonessential amino acids, and 1 mg/ml G-418. The Neuro2A-derived cell lines were grown in the same medium, but with 1 μg/ml puromycin in place of the G-418.
RNA expression levels.
For real-time PCR, the RNeasy minikit from Qiagen was used to isolate RNA from Neuro2A cells according to the manufacturer's instructions. The quality (optical density at 260/280 nm [OD260/280]) and quantity (concentration) of RNA were analyzed by Nanodrop spectrophotometer (ND-1000). To prepare cDNA, the RNA was reverse transcribed by reverse transcriptase following the instructions provided in the Qiagen Sensiscript synthesis kit. The cDNA was stored at −20°C until use. cDNA from each sample (equivalent to 50 to 100 ng input RNA) was diluted with RNase-free water and subjected to PCR using specific primers. The primer pair PD-L1 (catalog no. MP201906) and PD-L2 (catalog no. MP211705) were purchased from OriGene Technologies, Inc., and were used as recommended by the manufacturer. SYBR green master mix (Applied Biosystems) was used in a final volume of 25 μl. The cycling parameters were 95°C for 10 min, followed by 40 cycles of 95°C for 30s and 60°C for 60s. Samples were normalized to 16S RNA and were amplified in triplicate using an MJ Research Opticon thermal cycler. PCR controls without reverse transcriptase (water control) or with normal human genomic DNA as a template were routinely negative. The levels of cytokine expression were determined using a threshold cycle (2−[supb]ΔΔCT) method as described previously (51). The parental Neuro2A cell line was used as the reference for the expression of PD-L1 and PD-L2 in the LAT+ and LAT− cell lines.
Western blotting.
Total proteins from whole-cell lysates of LAT+ and LAT− Neuro2A cell lines were separated on NuPAGE 4 to 12% Bis-Tris gels. After Western transfer, the membrane was probed with 1 μg/ml anti-PD-L1 (catalog no. AP30655PU-N; Acris Antibodies). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Invitrogen) at a 1:2,000 dilution was used as the secondary antibody. Super Signal West Femto maximum sensitivity substrate (Thermo Scientific) was used to detect PD-L1.
Statistical analysis.
Student's t test and analysis of variance (ANOVA) were performed using the computer program Instat (GraphPad, San Diego). Results were considered statistically significant when the P value was <0.05.
RESULTS
Increased number of total TG resident CD8 T cells on days 11 and 35 postocular infection of mice with LAT+ compared to LAT− virus.
In a pilot experiment, we compared the percentages and numbers of total T cells in TG of mice latently infected with wild-type HSV-1 (strain McKrae) that had been perfused or not perfused prior to euthanasia. The numbers and percentages of HSV-1-specific CD4+ and CD8+ T cells were similar in perfused and nonperfused TG (not shown). This was expected, since during HSV-1 latency there are few if any HSV-1-specific T cells in the circulating blood. Subsequent experiments were carried out using nonperfused mice.
Groups of five B6 mice were infected ocularly with 2 × 105 PFU/eye of the LAT-null virus mutant dLAT2903 (LAT−) or its marker-rescued virus, dLAT2903R (LAT+) (55). Both viruses are derived from HSV-1 strain McKrae. TG were harvested 11 days postinfection (p.i.) (just after acute infection) or 35 days p.i. (i.e., after latency was well established). The percentage and number of CD3+ CD8+ T cells in TG were determined on both day 11 (Fig. 1A) and day 35 (Fig. 1B) postinfection. Representative experiments, each consisting of cells pooled from 10 TG, show the percentage of total TG resident CD8+ T cells (i.e., CD3+ CD8+) (left panels). The average ± standard deviation (SD)/TG of seven independent experiments is shown in the right panels. Both the percentage and average number of CD8+ T cells were higher in LAT+ TG compared to LAT− TG. As expected, more TG resident CD8+ T cells were present on day 11 than on day 35. On both day 11 and day 35, there were no differences in the percentages or numbers of total CD3+ CD8+ T cells in spleens from LAT+- compared to LAT−-infected mice (data not shown), indicating that the effect of LAT on CD8+ T cells was TG specific.
Fig. 1.
Higher percentages and numbers of total CD8+ T cells detected in LAT+ TG compared to LAT− TG during latent herpesvirus infection. Three groups of B6 mice (n = 10) were either left uninfected (Mock) or were ocularly infected with 2 × 105 PFU/eye of (i) LAT-null virus mutant dLAT2903 (LAT−) or with (ii) its marker-rescued virus, dLAT2903R (LAT+) (53). Both TG were harvested from each mouse either 11 days postinfection (5 mice) or 35 days postinfection (5 mice). The 10 TG were pooled and treated with collagenase I, and the numbers (nbr) and percentages of CD3+ T cells and CD3+ CD8+ T cells were determined in TG cell suspensions by FACS. (A). Dot plot showing percentages of CD3+ CD8+ T cells (left panel) and the average number ± SD/TG (right panel) of CD3+ CD8+ T cells per TG on day 11 postinfection. (B) Dot plot of the percentages of CD3+ CD8+ T cells (left panel) and the average number ± SD/TG (right panel) of CD3+ CD8+ T cells per TG on day 35 postinfection. Each histogram and dot plot represents seven consecutive independent experiments. The bar graphs show the means and SD of the seven independent experiments at each time point. *, P < 0.05 for cell numbers in LAT+ TG versus LAT− TG.
High numbers of CD8+ T cells were detected in LAT+ TG regardless of the mutant or strain of the virus.
To verify that the higher number of CD8+ T cells in LAT+ TG seen in Fig. 1 was not due to an artifact linked to the mutant virus used, we infected additional groups of 5 B6 mice with a different LAT+ mutant that has the first 1.5 kb of LAT expressed from an ectopic location in an otherwise LAT-null mutant (LAT3.3A) (58). LAT3.3A is a McKrae strain mutant and has a wild-type latency and reactivation phenotype similar to those of both LAT+ dLAT2903R and wild-type McKrae (58). Again, on both day 11 (Fig. 2A, left panel) and day 35 (Fig. 2A, right panel), the cells from 10 TG/group were pooled. The average number of CD8+ T cells per TG from five independent experiments was significantly higher with LAT+ virus than with LAT− virus (P < 0.05). These results indicate that the first 1.5 kb of the 8.3-kb LAT primary transcript is sufficient to directly or indirectly increase the CD8+ T-cell pool in TG. Similar to the results shown in Fig. 1, more CD3+ CD8+ T cells were detected on day 11 than during latency on day 35.
Fig. 2.
The presence of increased CD8+ T cells in the TG of LAT+- versus LAT−-infected mice is independent of mutant and HSV-1 strain. Groups of B6 mice (n = 5) were mock infected or infected ocularly with 2 × 105 PFU/eye of LAT+ or LAT− viruses derived from (A) HSV-1 strain McKrae (LAT+ LAT3.3A versus LAT− dLAT2903) or (B) HSV-1 strain 17 (LAT+ wt 17syn+ versus LAT− 17N/H. On days 11 and 35 postinfection in panel A and on day 35 postinfection in panel B, the 10 TG in each group were pooled and processed as in Fig. 1. Each bar represents the mean and SD of five independent experiments. nbr, number. *, P < 0.05.
To confirm that the above results were not specific to HSV-1 strain McKrae and its mutants, an additional set of experiments was conducted using HSV-1 17syn+ (LAT+) and its LAT− mutant, 17N/H (44) (Fig. 2B). As described above, two groups of five B6 mice were ocularly infected with 2 × 105 PFU/eye of either HSV-1 17syn+ (LAT+) or 17N/H (LAT−). The numbers of TG resident CD3+ CD8+ T cells were compared between the two groups during latency (35 days p.i.), using FACS as in Fig. 2A. Again, the average number of CD3+ CD8+ T cells/TG from five independent experiments was significantly higher in latently infected LAT+ TG compared to LAT− TG (Fig. 2B). These results indicate that the increase in the CD8+ T-cell pool in latently infected LAT+ TG was not virus strain dependent.
Increased PD-1-positive HSV-gB498–505-specific CD8+ T cells in TG of mice latently infected with LAT+ compared to LAT− virus.
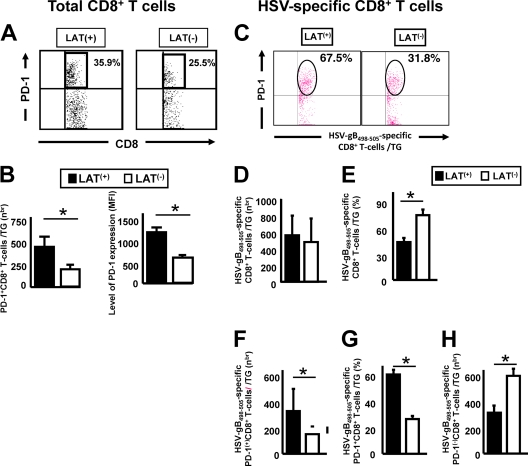
B6 mice were infected with wt McKrae (LAT+) or its LAT-null mutant, dLAT2903 (LAT−), as described above, and the percentage and the average number of total and HSV-gB498–505-specific CD8+ T cells expressing PD-1 were determined at 35 days postinfection (Fig. 3). Panel A shows a representative experiment of PD-1+/high CD8+ T cells from pools of 10 TG. The average number ± SD of CD8 T cells expressing high levels of PD-1 from five independent experiments is shown on the left side of Fig. 3B. As we previously showed (2), latently infected LAT+ TG had a significantly higher percentage (Fig. 3A) and number of PD-1+/high CD8+ T cells (Fig. 3B, left panel). In addition, the mean fluorescence intensity (MFI) of PD-1 on CD8+ T cells from LAT+ TG was higher than the MFI of PD-1 on CD8+ cells from LAT− TG (1,280 ± 5 versus 850 ± 5, respectively; P < 0.05) (Fig. 3B, right panel). These results indicate not only that a higher proportion of total CD8+ T cells in LAT+ TG express PD-1 but also that the expression level was significantly higher. On day 11 postinfection, no significant PD-1 was detected in TG resident CD8 T cells (data not shown).
Fig. 3.
More exhausted HSV-specific CD8+ T cells are detected in LAT+ TG versus LAT− TG. Groups of five B6 mice were ocularly infected as described above with wt McKrae (LAT+) or dLAT2903 (LAT−). Ten TG/group were harvested 35 days postinfection, pooled, and processed as described above. The cells were triple stained with FITC-labeled anti-PD-1, PerCP–anti-CD8 T cells, and PE-labeled HSV-gB498–505/H-2d tetramers. The numbers (nbr) and percentages of total CD8+ T cells and HSV-gB498–505 epitope-specific CD8+ T cells expressing PD-1 and the PD-1 expression levels were determined by FACS. (A) Representative dot plot of total PD-1+ CD8+ T cells in latently infected LAT+ versus LAT− TG. (B) Histograms of the average number of total PD-1+ CD8+ T cells (left) and the average mean fluorescence intensity (MFI) of PD-1 (right). (C) Representative dot plot of HSV-gB498–505 epitope-specific CD8+ T cells expressing PD-1 in latently infected LAT+ versus LAT− TG. (D) Average number/TG of HSV-gB498–505 epitope-specific CD8+ T cells. (E) Percentage of HSV-gB498–505 epitope-specific CD8+ T cells. (F) Average number of HSV-gB498–505 epitope-specific CD8+ T cells expressing PD-1. (G) Percentage of HSV-gB498–505 epitope-specific CD8+ T cells expressing PD-1. (H) Average number of HSV-gB498–505 epitope-specific CD8+ T cells not expressing PD-1. Each bar in panels B to G represents the mean and SD of five independent experiments. *, P < 0.05.
The numbers and percentages of CD8+ T cells specific to the immunodominant HSV-gB498–505 epitope were compared in B6 mice latently infected with LAT+ or LAT− virus using the HSV-gB498–505 H-2Kb tetramer (Fig. 3C to H). The HSV-gB498–505-epitope was used because in mice latently infected with HSV-1, the majority (over 50%) of CD8+ T cells in the periphery and TG are directed to this single immunodominant epitope (7, 9, 19). Single-cell suspensions of 10 pooled TG from latently infected mice were prepared as described above. A representative dot plot of HSV-gB498–505 epitope-specific CD8+ T cells expressing PD-1 in latently infected LAT+ versus LAT− TG is shown in Fig. 3C. Each bar in panels D to H shows the average ± SD of five independent experiments, each consisting of cells from 10 pooled TG. Figure 3D shows the average number of HSV-1-specific (i.e., HSV-gB498–505 H-2Kb tetramer-positive stained) CD8 T cells/TG. Similar numbers of HSV-gB498–505-specific CD8+ T cells were seen with LAT+ and LAT− virus. In contrast, the percentage of CD8 T cells in these TG that were HSV-gB498–505 specific was higher in LAT− TG than in LAT+ TG (Fig. 3E; P < 0.05). In contrast, the number of HSV-gB498–505-specific CD8+ T cells that highly expressed PD-1 was significantly higher in LAT+ TG compared to LAT− TG (Fig. 3F; P < 0.05). Figure 3G shows that ∼60% of the HSV-gB498–505-specific CD8+ T cells in LAT+ TG were PD-1+/high, compared to only 27% in LAT− TG (P < 0.05). These results suggest that although LAT+ and LAT− TG had similar numbers of HSV-gB498–505-specific CD8+ T cells, in LAT+ TG, a significantly higher proportion of the HSV-gB498–505-specific CD8+ T cells were PD-1+/high. Interestingly, the number of PD-1 negative HSV-gB498–505-specific CD8+ T cells appeared to be significantly higher in TG of mice latently infected with LAT− virus compared to LAT+ virus (Fig. 3H). If the HSV-1-specific PD-1− CD8+ T cells were indicative of functional HSV-specific CD8 T cells, it would suggest that these cells might play a role in the decreased viral reactivation seen with LAT− viruses (32, 55, 72).
The number of HSV-gB498–505 epitope-specific CD8+ T cells expressing PD-1 was also determined in TG of mice latently infected with McKrae-derived LAT3.3A (LAT+ virus expressing just the first 1,499 nt of LAT) compared to McKrae-derived LAT− virus (Fig. 4A) and in TG of mice latently infected with wt (LAT+) 17syn+ and its LAT− mutant (Fig. 4B). Each bar shows the mean and SD of three independent experiments. As with wt HSV-1 McKrae, LAT3.3A and wt 17syn+ each had significantly more HSV-gB498–505-specific CD8+ T cells expressing PD-1 than did their LAT− counterparts (P < 0.05). These results suggest that the findings shown in Fig. 3 were not an artifact of HSV-1 strain McKrae and that the LAT function involved mapped to the first 1.5 kb of LAT.
Fig. 4.
PD-1 expression in LAT3.3A and in LAT+ and LAT− HSV-1 strain 17syn+ viruses. Groups of five B6 mice were ocularly infected as described above with 17syn+ (LAT+), 17N/H (LAT−), LAT3.3A (LAT+), or dLAT2903 (LAT−). Ten TG/group were harvested 35 days postinfection, pooled, and processed as described above. The cells were triple stained with FITC-labeled anti-PD-1, PerCP–anti-CD8, and PE-labeled HSV-gB498–505/H-2d tetramers and analyzed by FACS as described above. The average numbers (nbr) of HSV-gB498–505 epitope-specific CD8+ T cells expressing PD-1 are shown. (A) McKrae-derived LAT+ LAT3.3A (expresses LAT nt 1 to 1499) versus McKrae-derived LAT− dLAT2903. (B) 17syn+-derived LAT+ wt versus 17syn+-derived LAT− 17N/H. Each bar shows the mean and SD of three independent experiments. *, P < 0.05.
Dramatically more functionally exhausted HSV-gB498–505-specific CD8+ T cells in TG of mice latently infected with LAT+ compared to LAT− virus.
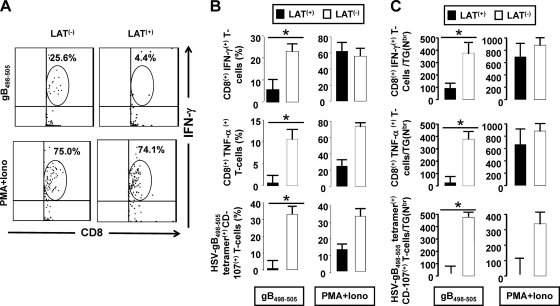
Without in vitro stimulation, no differences were seen in functional exhaustion as determined by the amounts of various cytokines produced by total TG resident CD8 T cells from mice latently infected with LAT+ versus LAT− viruses (2). In Fig. 5, we used in vitro stimulation to examine functional exhaustion of HSV-gB498–505-specific CD8+ T cells in LAT+ TG versus LAT− TG. Pools of cells from 10 latently infected TG/group were prepared as described above. In vitro stimulation was performed using the gB immunodominant peptide HSV-gB498–505, as described in Materials and Methods and in the legend to Fig. 5. Intracellular cytokine staining was then used to quantitate the frequency and number of HSV-specific CD8+ T cells expressing IFN-γ, TNF-α, or CD107. Because the cells were stimulated in vitro with HSV-gB498–505, the responding cells were HSV-1 specific. The nonspecific stimulant PMA + Iono, which should stimulate both HSV-gB498–505-specific and nonspecific CD8 T cells, was used as a positive control. Figure 5A shows a representative dot blot of HSV-gB498–505-specific CD8+ T cells that were IFN-γ positive. The top panels in Fig. 5B and C show the percentage and number ± SD from five independent experiments of HSV-gB498–505-specific CD8+ T cells that were IFN-γ positive. In LAT+ TG, compared to LAT− TG, a significantly lower percentage and number of CD8+ T cells that were stimulated with the HSV-gB498–505 epitope produced IFN-γ (P < 0.05). These results are consistent with our previous finding that LAT interferes with and delays interferon expression in productively infected neuroblastoma cells and TG of acutely infected mice (53).
Fig. 5.
HSV-gB498–505-specific CD8+ T cells from LAT+ TG have reduced cytokine production and cytolytic activity. Two groups of B6 mice (n = 5) were ocularly infected with wt McKrae (LAT+) or dLAT2903 (LAT−) as described above. At 35 days postinfection, the 10 TG in each group were pooled, and single-cell suspensions were prepared as described above. For intracellular cytokine staining, the cells were stimulated with gD498–505 peptide (10 μg/ml) or PMA + Iono (1 μg/ml) (positive control) or mock stimulated (negative control) at 37°C for 6 h in the presence of Golgi plug (BD Biosciences). The cells were then stained with PerCP-conjugated anti-mouse CD8 for 30 min, permeabilized, and stained with PE-labeled anti-mouse IFN-γ or TNF-α antibodies, as recommended by the manufacturer. For the CD107 degranulation assay, the cells were mock stimulated or stimulated with HSV-gB498–505 peptide or PMA + Iono (positive control) at 37°C for 6 h in the presence of 0.7 μl/ml of BD Golgi-Stop and 10 μl of CD107a/b-FITC, washed with FACS buffer, and stained with PerCP-conjugated anti-mouse CD8 MAb at 4°C. The percentages and average numbers (Nbr)/TG of HSV-gB498–505 epitope-specific CD8+ T cells producing IFN-γ, TNF-α, or expressing CD107a/b were determined by FACS. (A) Representative dot plots of intracellular IFN-γ+ CD8+T cells. (B) Percentage of HSV-gB498–505 epitope-specific CD8+ T cells producing IFN-γ, TNF-α, or CD107a/b per TG. (C) Average number/TG of HSV-gB498–505-epitope-specific CD8+ T cells producing IFN-γ, TNF-α, or CD107a/b. In panels B and C, each bar represents the average ± SD of five experiments. *, P < 0.05.
Figure 5B and C (middle panels) show the average ± SD of the percentage and number of CD8+ T cells simulated in vitro with gB498–505 peptide that produced TNF-α. The results shown are from five independent experiments. Similar to the results for IFN-γ, a significantly lower percentage and number of TNF-α-positive HSV-gB498–505+ CD8+ T cells were seen in LAT+ TG compared to LAT− TG. Finally, following in vitro stimulation with HSV-gB498–505, we also found a significantly decreased percentage and number of HSV-gB498–505-specific CD8+ T cells with cytotoxic activity (i.e., CD107a/b positive) in LAT+ TG compared to LAT− TG (Fig. 5B and C, bottom panels). Similar levels of proliferation of gB-specific CD4+ T cells were detected in LAT+ and LAT− TG, indicating that CD4+ T-cell function was not affected (data not shown) and was therefore unlikely to play a role in the observed reduction of CD8+ T-cell functions in LAT+ TG. Altogether, the results shown in Fig. 5 indicate that LAT, directly or indirectly, decreased the function of HSV-specific CD8+ T cells in latently infected TG. To our knowledge, this is the first demonstration that LAT appears to increase functional exhaustion of HSV-specific CD8+ T cells during latency. In addition, as seen in the left panels of Fig. 5C, the number of functional HSV-1-specific CD8+ T cells, as judged by IFN-γ, TNF-α, and cytotoxic activity, appeared to be higher in TG from mice latently infected with LAT− compared to LAT+ virus. This suggests that the higher number of functional HSV-1-specific CD8 T cells in TG of mice latently infected with LAT− virus may play an important role in the decreased viral reactivation seen with LAT− viruses (32, 55, 72).
Neuronally derived cells stably expressing LAT have elevated MHC-I and PD-L1.
Approximately 30% to over 50% of neurons in TG from mice latently infected with LAT+ (wt) virus harbor latent virus, as judged by the presence of viral DNA or LAT RNA (46, 63, 64). However, less than 1 neuron/TG has detectable viral Ag by immunostaining (2, 23, 24, 45, 80). The amount of latency and detectable viral Ag in TG from mice latently infected with LAT− virus is even smaller than with LAT+ virus (31). As expected, we obtained similar results in these studies (not shown). In addition, spontaneous reactivation in mice is extremely rare (28). Thus, HSV-1 latency in mice represents an almost completely dormant state. The very low levels of viral Ag detected during latency suggest that the continuous high-level exposure to Ag that is thought to be necessary to produce CD8 T-cell exhaustion may not occur during HSV-1 latency in mouse TG. It was therefore of interest to determine if LAT alone, in the absence of other viral genes, could contribute to the observed CD8+ T-cell exhaustion, so we examined two mouse neuroblastoma cell lines (Neuro2A cells and C1300 cells) that we had previously independently engineered to stably express LAT (12, 13, 37). JWLAT cells were derived from Neuro2A cells and express the first 1,499 nt of the 8.3-kb LAT transcript (37). DC-LAT6 cells were derived from C1300 cells and express the first 3,225 nt of the 8.3-kb LAT transcript (12, 13). Compared to their LAT− controls (and their parental lines [mock infected]), both LAT+ cell lines had significantly increased levels of MHC class I (Fig. 6A and B; P < 0.05). Thus, it is possible that in TG latently infected with LAT+ virus, LAT may increase viral Ag presentation to CD8+ T cells, and this may increase the likelihood of CD8 T-cell exhaustion. Note that in panels A and B, two completely independent clonal cell lines, each expressing a different segment of LAT, gave similar results. Nonetheless, to ensure that the LAT+ clonal cell lines had not somehow acquired a mutation that caused them to upregulate expression of MHC-1 and PD-L1, the remaining experiments in Fig. 6 were done using cells that were not cloned. As we previously described (37), Neuro2A cells were transfected with the same plasmids used to make the Neuro2A clonal cell lines (JWLAT and JW-ΔLAT), and antibiotic selection was initiated to eliminate cells that were not efficiently transfected. Instead of cell cloning, the total culture was then maintained with routine cell passage as needed. The resulting “bulk culture” was thus comprised of cells resistant to the antibiotic, the vast majority of which therefore contained the input plasmid. Using these bulk cultures, we analyzed the relative levels of PD-L1 mRNA by quantitative reverse transcription-PCR (qRT-PCR) (Fig. 6C). PD-L1 is the ligand for PD-1. We found significantly more PD-L1 mRNA in the LAT+ versus the LAT− cells (P < 0.05). In contrast, the LAT+ and LAT− cell lines had similar levels of PD-L2 mRNA (Fig. 6C). Thus, LAT appeared to increase the amount of PD-L1, but not PD-L2, mRNA in these neuroblastoma cell lines. To determine if the increased PD-L1 mRNA level in the LAT+ cells was reflected in the PD-L1 protein level, the relative PD-L1 protein levels were determined by Western blotting and FACS, using the anti-PD-L1 MAb. Figure 6D shows a representative Western blot result with whole-cell lysates of JWLAT LAT+ neuroblastoma cells compared to JW-ΔLAT (LAT−) control neuroblastoma cells. A representative FACS result from PD-L1 expression on JWLAT LAT+ neuroblastoma cells compared to JW-ΔLAT (LAT−) control neuroblastoma cells is shown in Fig. 6E. The mean ± SD of three independent FACS experiments, each done in triplicate, is shown in Fig. 6F. LAT+ and LAT− cells both had more PD-L1 than the normal control Neuro2A cells (mock). More importantly, there was significantly more PD-L1 expression on the LAT+ cells compared to the LAT− cells (Fig. 6F; P < 0.05). Thus, both PD-L1 mRNA and protein were elevated in the LAT+ compared to the LAT− Neuro2A cell lines. To our knowledge, this is the first report of LAT alone, in the absence of other viral genes or gene products, upregulating either MHC-I or PD-L1. PD-L1 protein also appeared to be upregulated in total cell extracts of TG from mice latently infected with LAT+ compared to LAT− virus (Fig. 6G; P < 0.05). The results shown in Fig. 6 suggest that LAT can upregulate both MHC-I and PD-L1 on neuronally derived cells (and possibly other LAT+ TG resident cells—for example, dendritic cells). This might increase CD8+ T-cell exhaustion by providing increased antigen presentation (via increased MHC-I) and/or by rendering them dysfunctional through the PD-L1/PD-1 negative regulatory pathway.
Fig. 6.
Upregulation of MHC-I and PD-L1 in neuroblastoma cell lines expressing LAT. (A) Parental Neuro2A cells, JWLAT (LAT+) cells, and JW-ΔLAT (LAT−) cells were stained with FITC-labeled anti-mouse MHC-I MAb (clone M1/42) (bold line) or an FITC-labeled isotype control (light line). The mean fluorescence intensities (MFI) of MHC-I are shown. (B) Parental C1300 cells or DC-LAT6 (LAT+) or DC-ΔLAT311 (LAT−) cells were treated as in panel A. The results are presented as overlaid histogram plots. (C) The histogram bars represent the mean and SD of the RNA fold increase compared to the parental control cell lines. (D) Western blot of PD-L1 in total cell lysates of JWLAT LAT+-expressing Neuro2A cells and JW-ΔLAT LAT− Neuro2A cells. (E) Relative PD-L1 expression in JWLAT LAT+-expressing Neuro2A cells and JW-ΔLAT LAT− Neuro2A cells as determined by FACS. (F) Mean fluorescent intensity of PD-L1 in the Neuro2A cell lines from panel E. Each bar is the mean ± SD of three independent experiments each done in triplicate. (G) PD-L1 from total TG cells from LAT+ versus LAT− latently infected mice. Each bar represents the mean ± SD of the fluorescence intensity from five experiments each done in triplicate. *, P < 0.05. The experiments shown in panels C to G were done using “bulk cultures” (rather than cloned cells) expressing LAT, as described in the text.
DISCUSSION
This report extends and expands recent findings regarding the role of HSV-1 LAT in CD8 T-cell exhaustion in TG of mice latently infected with HSV-1 (2). Using only HSV-1 strain McKrae-derived viruses, the previous report showed that, compared to LAT− virus, in mice latently infected with LAT+ virus, there is increased phenotypic exhaustion of total TG resident CD8 T cells. In this report, we have extended those findings to LAT+ and LAT− HSV-1 strain 17syn+ viruses, thus demonstrating that the previous findings were not an artifact of HSV-1 strain McKrae. We also showed that a mutant expressing just the first 1,499 nt of the 8.3-kb LAT was capable of producing phenotypic exhaustion of CD8 T cells. This mapped the LAT region involved to the beginning of the LAT transcript, the same region to which we previously mapped LAT's antiapoptosis activity (39) and LAT's ability to enhance spontaneous reactivation in rabbits (35, 58). In addition, we have now found that on day 11 postinfection (just after the acute infection has cleared, a time not previously examined), there were significantly more total CD8 T cells with LAT+ versus LAT− virus. This is similar to what was previously found during latency on day 30 postinfection (2) and what we found here on day 35 postinfection. However, in contrast to days 30 and 35, on day 11, we did not find significant CD8 T-cell exhaustion in either group (data not shown). Thus, the increase in CD8 T-cell numbers in LAT+ TG on day 11 was not the result of increased exhaustion, which had not yet occurred. More importantly, we now show that compared to LAT− virus, in mice latently infected with LAT+ virus, (i) HSV-1-specific CD8 T cells, as well as total CD8 T cells, were phenotypically exhausted; (ii) HSV-1-specific CD8 T cells were functionally exhausted; and (iii) LAT alone, in the absence of all other HSV-1 gene products, increased expression of both MHC-I and PD-L1 on neuronally derived cells. To our knowledge, this is the first report showing LAT-related functional exhaustion of HSV-1-specific CD8 T cells in TG of latently infected mice and the first report showing that LAT by itself can increase two different proteins (MHC-I and PD-L1), both of which could logically increase CD8 T-cell exhaustion.
During HSV-1 neuronal latency in TG of mice and humans, some neurons that may or may not be latently infected are surrounded by CD8+ T cells (34, 42, 68–70, 76). Since CD8+ T cells are presumably attracted to these neurons by viral Ags, it is assumed that the neurons surrounded by CD8+ T cells are those in which the virus has initiated the early stages of reactivation from latency. In mice, experimental reactivation of HSV-1 from latency is typically accomplished by explanting TG into tissue culture media for up to 14 days and testing for the appearance of infectious (i.e., reactivated) virus. In this TG explant-induced reactivation model, depletion of CD8+ T cells with a specific MAb leads to the detection of more reactivated virus (42, 47). Conversely, addition of exogenous CD8+ T cells reduces detection of reactivated virus (16, 33, 42). Thus, with wild-type HSV-1, CD8+ T cells in the TG are apparently able to reduce the detection of infectious reactivated virus.
Induced HSV-1 reactivation in mice and spontaneous HSV-1 reactivation in rabbits are both significantly reduced in animals latently infected with LAT− mutants compared to LAT+ viruses (32, 55, 72). Combining this fact with the above concept that increased numbers of CD8+ T cells in the TG result in decreased detection of HSV-1 reactivation, we hypothesized that TG from mice latently infected with LAT− viruses would have increased numbers of CD8+ T cells compared to TG from mice latently infected with LAT+ viruses. Unexpectedly, in the previous study (2) and here, we found the reverse. Both the absolute number of CD8+ T cells per mouse TG and the percentage of CD8+ T cells among all CD3+ T cells were significantly higher in mice infected with LAT+ compared to LAT− viruses. In addition, we now show that this was also the case on day 11 post-ocular infection (just after clearance of the acute infection) as well as when latency was well established (day 30 or 35).
Many persistent and/or chronic viral infections generate functionally impaired antigen-specific T-cell populations (4, 30, 38, 79). It should be noted that PD-1 expression by itself does not demonstrate exhaustion. However, it is well established that activated memory T cells and exhausted T cells have different levels of PD-1 expression, with exhausted CD8+ T cells expressing higher levels of PD-1 compared to activated memory T cells (4, 6, 79). With LCMV, PD-1 is markedly upregulated on exhausted T cells but only transiently expressed on effector T cells during acute infections and is not present on functionally competent memory T cells (4, 26, 36). These different levels of PD-1 can help distinguish between exhaustion and activation. In addition, in this study, exhausted (dysfunctional) CD8+ T cells were confirmed by impaired cytotoxicity activity and decreased IFN-γ and TNF-α production.
In the previous report (2), exhaustion was studied with total TG resident CD8 T cells. In this report, we studied exhaustion of HSV-specific CD8 T cells, since these are the CD8 T cells that would affect HSV-1 latency and reactivation. In the previous report, qRT-PCR of total TG RNA did not reveal any significant decreases in the amount of IL-2, IFN-γ, or TNF-α mRNAs in LAT+ versus LAT− latently infected TG (see Fig. 5 in reference 2). Thus, that experiment did not provide evidence of functional exhaustion in TG latently infected with LAT+ compared to LAT− virus. In addition, a FACS analysis in which the CD8 T cells were not stimulated in vitro did not reveal any obvious functional exhaustion of CD8+ T cells from LAT+ versus LAT− TG (see Table 2 in reference 2). In contrast, here we found that the percentage and number of IFN-γ HSV-specific CD8 T cells, TNF-α HSV-specific CD8 T cells, and CD107+ HSV-specific CD8 T cells were all significantly decreased in TG of mice latently infected with LAT+ compared to LAT− virus. This indicated functional exhaustion of both cytokine expression and cytotoxic activity of these CD8 T cells. To our knowledge, this is the first report to show significantly more phenotypically and functionally exhausted HSV-specific CD8+ T cells in TG from mice latently infected with LAT+ virus compared to LAT− virus. In addition, since we found here that most of the HSV-1-specific CD8+ T cells in the TG of mice latently infected with LAT+ virus appeared to be both phenotypically and functionally exhausted, there were actually significantly more functional (as judged by both cytotoxic activity and cytokine production [Fig. 5C]) CD8+ T cells in LAT− TG. Thus, our original hypothesis that one mechanism by which LAT enhances reactivation is by reducing the number of CD8+ T cells in the TG was correct, as long as we replace “CD8+ T cells” with “HSV-specific functional CD8+ T cells.”
In well-studied CD8+ T-cell exhaustion systems (6, 21, 49, 73), the exhaustion appears to results from overstimulation of CD8+ T cells by the presence of sustained high-level Ag. This seems unlikely to account for the increased CD8+ T-cell exhaustion in TG of mice latently infected with LAT+ virus compared mice latently infected with LAT− virus. Studies from several labs indicate that LAT suppresses lytic gene transcription during acute and latent infection (14, 27, 77). If this is correct, then there would be less viral Ag with LAT+ virus, and this could not account for the increased CD8+ T-cell exhaustion. In contrast, elegant reports from the Margolis lab (23, 24, 45, 80), using immunostaining to examine sufficient sections from each mouse TG to allow thorough examination of the entire TG for HSV-1 Ags, found more HSV-1 Ag in TG from mice latently infected with LAT+ compared to LAT− virus. This result could help explain our finding of more exhausted CD8+ T cells in LAT+ TG. However, the amount of viral Ag was quite low—less than 1 positive neuron/TG with LAT+ virus. We have also found less than 1 positive neuron/TG with LAT+ virus and less with LAT− virus, doing similar but less rigorous studies (2). Even when we extended the window for positive neurons to include even those with minimal staining, only 76 neurons in 60 LAT+ TG and only 21 neurons in 88 LAT− TG were scored as positive (not shown). In addition, if spontaneous reactivation occurs in mouse TG, it is minimal (28). Thus, even though CD8+ T cells are much more sensitive to Ag than the antibodies used for immunostaining, the very low unsustained Ag level that appears to be the situation in mouse TG is unlikely to result in exhaustion of CD8+ T cells, which in other systems requires high levels of continuous Ag exposure (6, 21, 49, 73). Thus, it seems unlikely that the CD8+ T-cell exhaustion detected was due to viral Ag load, unless additional factors contributed to immune stimulation. For example, TG resident HSV-specific CD8+ T cells could have a higher functional avidity (ability to respond to low epitope density) than their counterparts in the periphery (42). Alternatively, CD8+ T-cell exhaustion may suggest that there is a lot more viral Ag present in TG of mice latently infected with LAT+ virus than has previously been detected or that there is a lot of undetected viral reactivation in TG of mice latently infected with LAT+ viruses.
Various studies, including ours, have estimated that the amount of latency with LAT+ viruses is anywhere from not significantly different from that of LAT− virus to as high as 6-fold more than that of LAT− virus, with an ∼2-fold increase of LAT+ to LAT− being common (55, 61, 71). In an independent study done as part of this report, we found an approximately 2-fold increase in HSV-1 genomic DNA levels by qRT-PCR in TG of mice latently infected with LAT+ versus LAT− virus (data not shown). Thus, the above described exhaustion of TG resident HSV-specific CD8+ T cells in LAT+ TG was associated with an average ∼2-fold increase in the viral genome copy number. Note that this increased latent HSV-1 genome load does not imply increased viral Ag load. In addition, as discussed in the previous paragraph, the amount of viral Ag detectable by immunostaining in LAT+ and LAT− TG is probably too low, by itself, to account for the CD8 T-cell exhaustion.
We have previously shown that for acute infection with wt McKrae and dLAT2903 (the LAT+ and LAT− viruses used here), levels of virus replication in eyes and TG are identical both kinetically and for peak titers (55, 56). Since day 11 represents the end of the acute infection, the CD8+ T-cell response at this time is likely due to the acute infection and not the amount of latent viral DNA in the TG on day 11. The only difference between LAT− and LAT+ virus during the acute infection is the presence of LAT. This suggests that LAT+ neurons may play a role in modulating CD8+ T-cell responses in TG. This was supported by our in vitro finding that LAT+ neuroblastoma cells had elevated levels of PD-L1 and MHC-I.
Our finding that neuroblastoma cells expressing LAT had elevated MHC class I and elevated PD-L1 (the ligand for PD-1) may be relevant to why there was increased CD8+ T-cell exhaustion in TG of mice latently infected with LAT+ compared to LAT− viruses. Increased levels of MHC-I would be expected to increase presentation of viral Ag to CD8+ T cells. Thus, responding CD8+ T cells in LAT+ TG might be “seeing” more viral antigen because of an ability to interact with infected MHC-Ihigh neuronal cells. Even very low Ag levels might then be capable of leading to CD8+ T-cell exhaustion. In addition, elevated PD-L1 would result in increased binding to PD-1 on CD8+ T cells, which in turn could trigger exhaustion. Thus, it is possible that the continued presence of LAT during latency acts as just described and/or through some additional yet to be determined mechanism(s) to decrease the function of CD8+ T cells in the TG. This decreased CD8+ T-cell function would in turn help neurons in which virus is beginning to reactivate to avoid being killed by HSV-1-specific CD8+ T cells, resulting in increased HSV-1 reactivation. Such a scenario may be one mechanism by which the HSV-1 LAT gene works to increase successful viral reactivation from latency, thus allowing for recurrent ocular infection, recurrent ocular virus shedding in tears, and viral spread to new hosts. Consistent with this, we previously showed that neuronally derived cell lines expressing LAT were resistant to cytotoxic CD8 T-cell killing (37).
We examined several different LAT+ and LAT− mutants from two different strains of HSV-1. These results showed that our findings were not virus strain dependent. In addition, since one of the LAT+ viruses expresses just the first 1.5 kb of the primary 8.3-kb LAT transcript, our results indicate that the ability of LAT to directly or indirectly interfere with CD8+ T-cell function in latently infected TG resides within the first 20% of the LAT gene. This region of LAT is known to be responsible for LAT's antiapoptosis activity. Whether and how this may be involved in how LAT interferes with CD8+ T-cell function remains to be determined. Additionally, this region of LAT does not appear to encode an abundant protein, although low levels of one small protein appear to be expressed (31). Also note that CD4+ T cells appeared unaffected in LAT+ TG compared to LAT− TG, indicating that the effect of LAT was CD8+ T-cell specific.
The HSV-gB498–505-epitope and B6 mice were chosen to detect HSV-1 specific CD8+ T cells in this study because in this mouse strain the majority (over 50%) of CD8+ T cells in the periphery as well as in latently infected TG are directed to this single immunodominant epitope (7, 9, 19). Whether the functions of CD8+ T cells specific to other mouse HSV-1 epitopes or human CD8+ T-cell epitopes in humans are also affected by LAT expression remains to be determined. Using our HLA transgenic rabbit model (16, 20), we are currently in the process of assessing whether LAT affects the number and/or function of CD8+ T cells specific to the set of human CD8+ T-cell HSV-1 epitopes that we have identified as being strongly recognized by these animals.
HSV-1 has a broad tropism and high degree of infectivity, but during latency, sensory neurons are the main cell type harboring the virus. The number and type (i.e., neurons, dendritic cells, etc.) of TG cells that are infected will likely have a significant impact on the total antigenic burden seen by responding CD8+ T cells. In this study, we found an increase in MHC-I and PD-L1 expression on LAT-transfected neuroblastoma cells, which may reduce the function of CD8+ T cells. Furthermore, we observed a significant increase in the level of PD-L1 expressed in total TG from mice latently infected with LAT+ compared to LAT− viruses (Fig. 6). Following PD-L1/PD-L2 ligation, PD-1 inhibits activation and expansion of CD8+ T cells, rendering them dysfunctional (81). Under in vivo conditions, different neighboring cell types, such as stromal cells (50), microglial cells (3), and even dendritic cells (40), may also contribute to local concentrations of antigen-MHC complexes and influence the function of HSV-specific CD8+ T cells in latently infected TG. In addition, it is likely that other inhibitory signals, such as Galectin9-TIM3 ligand (29, 52) or IL-10 (4, 11, 49), or local concentrations of antigen-MHC complexes can influence CD8+ T-cell responses. This could explain why in these studies the amount of functional exhaustion appeared higher than the amount of phenotypic exhaustion based on PD-1 alone. In addition, since LAT is expressed in mouse TG during and throughout the acute infection and latency, it is possible that LAT could impact the immune response in the TG within a few days of ocular infection.
In summary, we show here for the first time that TG from mice latently infected with LAT+ viruses have more exhausted HSV-specific CD8+ T cells and fewer fully functional HSV-specific CD8+ T cells than do TG from mice latently infected with LAT− virus. Since functional HSV-specific CD8+ T cells appear to be important in decreasing reactivation from latency (42), the higher level of such cells during LAT− latency may help explain why LAT− viruses have reduced reactivation compared to wild-type LAT+ viruses. To our knowledge, the present study is also the first to report that LAT expression, in the absence of other virus genes, can produce elevated MHC-I and PD-L1, both of which could logically lead to generation of dysfunctional HSV-specific CD8+ T cells in latently infected TG. This would represent a novel immune evasion mechanism whereby LAT decreases HSV-specific CD8+ T-cell number and function in latently infected TG, thus allowing more reactivation. Blockade of the PD-1 pathway in combination with therapeutic vaccination may have great therapeutic promise.
ACKNOWLEDGMENTS
This work was supported by Public Health Service NIH grants EY14900, EY14017, and EY019896 to L.B.M., EY013191 and EY018171 to S.L.W., The Discovery Eye Foundation, The Henry L. Guenther Foundation, and a Research to Prevent Blindness Challenge grant. L.B.M. is an RPB Special Award Investigator. S.L.W. is an RPB Senior Scientific Investigator.
We thank Nigel W. Fraser for providing HSV-1 strain 17 and derived mutants and Alison Deckhut Augustine and Amy K. Stout from the NIH Tetramer Facility for providing tetramers used in this study.
Footnotes
Published ahead of print on 29 June 2011.
REFERENCES
- 1. Ahmed M., Lock M., Miller C. G., Fraser N. W. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76:717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen S. R., et al. 2011. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J. Virol. 85:4184–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aravalli R. N., Hu S., Rowen T. N., Gekker G., Lokensgard J. R. 2006. Differential apoptotic signaling in primary glial cells infected with herpes simplex virus 1. J. Neurovirol. 12:501–510 [DOI] [PubMed] [Google Scholar]
- 4. Barber D. L., et al. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 [DOI] [PubMed] [Google Scholar]
- 5. Bettahi I., et al. 2007. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest. Ophthalmol. Vis. Sci. 48:4643–4653 [DOI] [PubMed] [Google Scholar]
- 6. Blackburn S. D., et al. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blaney J. E., Jr., et al. 1998. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72:9567–9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Block T. M., et al. 1990. A herpes simplex virus type 1 latency-associated transcript mutant reactivates with normal kinetics from latent infection. J. Virol. 64:3417–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonneau R. H., Salvucci L. A., Johnson D. C., Tevethia S. S. 1993. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology 195:62–70 [DOI] [PubMed] [Google Scholar]
- 10. Branco F. J., Fraser N. W. 2005. Herpes simplex virus type 1 latency-associated transcript expression protects trigeminal ganglion neurons from apoptosis. J. Virol. 79:9019–9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brooks D. G., et al. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carpenter D., et al. 2008. Introducing point mutations into the ATGs of the putative open reading frames of the HSV-1 gene encoding the latency associated transcript (LAT) reduces its anti-apoptosis activity. Microb. Pathog. 44:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carpenter D., et al. 2007. Stable cell lines expressing high levels of the herpes simplex virus type 1 LAT are refractory to caspase 3 activation and DNA laddering following cold shock induced apoptosis. Virology 369:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen S. H., Kramer M. F., Schaffer P. A., Coen D. M. 1997. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 71:5878–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chentoufi A. A., BenMohamed L. 2010. Future viral vectors for the delivery of asymptomatic herpes epitopes-based immunotherapeutic vaccines. Future Virol. 5:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chentoufi A. A., et al. 2010. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J. Immunol. 184:2561–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chentoufi A. A., et al. 2010. Nasolacrimal duct closure modulates ocular mucosal and systemic CD4(+) T-cell responses induced following topical ocular or intranasal immunization. Clin. Vaccine Immunol. 17:342–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chentoufi A. A., et al. 2008. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J. Immunol. 180:426–437 [DOI] [PubMed] [Google Scholar]
- 19. Cose S. C., Kelly J. M., Carbone F. R. 1995. Characterization of diverse primary herpes simplex virus type 1 gB-specific cytotoxic T-cell response showing a preferential V beta bias. J. Virol. 69:5849–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dasgupta G., Chentoufi A. A., Nesburn A. B., Wechsler S. L., BenMohamed L. 2009. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev. Vaccines 8:1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Day C. L., et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354 [DOI] [PubMed] [Google Scholar]
- 22. Devi-Rao G. B., et al. 1997. Herpes simplex virus genome replication and transcription during induced reactivation in the rabbit eye. J. Virol. 71:7039–7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellison A. R., Yang L., Voytek C., Margolis T. P. 2000. Establishment of latent herpes simplex virus type 1 infection in resistant, sensitive, and immunodeficient mouse strains. Virology 268:17–28 [DOI] [PubMed] [Google Scholar]
- 24. Feldman L. T., et al. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. U. S. A. 99:978–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fourcade J., et al. 2010. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 207:2175–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freeman G. J., et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garber D. A., Schaffer P. A., Knipe D. M. 1997. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J. Virol. 71:5885–5893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gebhardt B. M., Halford W. P. 2005. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol. J. 2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golden-Mason L., et al. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 83:9122–9130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ha S. J., et al. 2008. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J. Exp. Med. 205:543–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henderson G., Jaber T., Carpenter D., Wechsler S. L., Jones C. 2009. Identification of herpes simplex virus type 1 proteins encoded within the first 1.5 kb of the latency-associated transcript. J. Neurovirol. 15:439–448 [DOI] [PubMed] [Google Scholar]
- 32. Hill J. M., Sedarati F., Javier R. T., Wagner E. K., Stevens J. G. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117–125 [DOI] [PubMed] [Google Scholar]
- 33. Hoshino Y., Pesnicak L., Cohen J. I., Straus S. E. 2007. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J. Virol. 81:8157–8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hufner K., et al. 2006. Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J. Neuropathol Exp. Neurol. 65:1022–1030 [DOI] [PubMed] [Google Scholar]
- 35. Inman M., et al. 2001. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 75:3636–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ishida Y., Agata Y., Shibahara K., Honjo T. 1992. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang X., et al. 2011. The herpes simplex virus type 1 latency associated transcript can protect neuronal derived C1300 and Neuro2A cells from granzyme B induced apoptosis and CD8 T-cell killing. J. Virol. 85:2325–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin H. T., et al. 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 107:14733–14738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jin L., et al. 2003. Identification of herpes simplex virus type 1 latency-associated transcript sequences that both inhibit apoptosis and enhance the spontaneous reactivation phenotype. J. Virol. 77:6556–6561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kather A., et al. 2010. Herpes simplex virus type 1 (HSV-1)-induced apoptosis in human dendritic cells as a result of downregulation of cellular FLICE-inhibitory protein and reduced expression of HSV-1 antiapoptotic latency-associated transcript sequences. J. Virol. 84:1034–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klenerman P., Hill A. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6:873–879 [DOI] [PubMed] [Google Scholar]
- 42. Knickelbein J. E., et al. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leib D. A., et al. 1989. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J. Virol. 63:2893–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maggioncalda J., Mehta A., Su Y. H., Fraser N. W., Block T. M. 1996. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology 225:72–81 [DOI] [PubMed] [Google Scholar]
- 45. Margolis T. P., et al. 2007. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J. Virol. 81:11069–11074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehta A., et al. 1995. In situ DNA PCR and RNA hybridization detection of herpes simplex virus sequences in trigeminal ganglia of latently infected mice. Virology 206:633–640 [DOI] [PubMed] [Google Scholar]
- 47. Miyajima K., Knickelbein M. B., Nakajima A. 2008. Stern-Gerlach study of multidecker lanthanide-cyclooctatetraene sandwich clusters. J. Phys. Chem. A 112:366–375 [DOI] [PubMed] [Google Scholar]
- 48. Mott K. R., et al. 2009. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J. Virol. 83:2246–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mueller S. N., Ahmed R. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 106:8623–8628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nagahara K., et al. 2008. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J. Immunol. 181:7660–7669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 51. Nesburn A. B., et al. 2007. Functional Foxp3+ CD4+ CD25(Bright+) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J. Virol. 81:7647–7661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oikawa T., et al. 2006. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J. Immunol. 177:4281–4287 [DOI] [PubMed] [Google Scholar]
- 53. Peng W., et al. 2005. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal ganglia of acutely infected mice. J. Virol. 79:6162–6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Perng G. C., et al. 1996. The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J. Virol. 70:282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perng G. C., et al. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045–8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perng G. C., et al. 2001. Three herpes simplex virus type 1 latency-associated transcript mutants with distinct and asymmetric effects on virulence in mice compared with rabbits. J. Virol. 75:9018–9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perng G. C., Ghiasi H., Kaiwar R., Nesburn A. B., Wechsler S. L. 1994. An improved method for cloning portions of the repeat regions of herpes simplex virus type 1. J. Virol. Methods 46:111–116 [DOI] [PubMed] [Google Scholar]
- 58. Perng G. C., Ghiasi H., Slanina S. M., Nesburn A. B., Wechsler S. L. 1996. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J. Virol. 70:976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perng G. C., et al. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287:1500–1503 [DOI] [PubMed] [Google Scholar]
- 60. Perng G. C., et al. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 76:1224–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perng G. C., et al. 2000. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J. Virol. 74:1885–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Petrovas C., et al. 2007. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 110:928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ramakrishnan R., Levine M., Fink D. J. 1994. PCR-based analysis of herpes simplex virus type 1 latency in the rat trigeminal ganglion established with a ribonucleotide reductase-deficient mutant. J. Virol. 68:7083–7091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ramakrishnan R., Poliani P. L., Levine M., Glorioso J. C., Fink D. J. 1996. Detection of herpes simplex virus type 1 latency-associated transcript expression in trigeminal ganglia by in situ reverse transcriptase PCR. J. Virol. 70:6519–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rodrigue-Gervais I. G., et al. 2010. Dendritic cell inhibition is connected to exhaustion of CD8+ T cell polyfunctionality during chronic hepatitis C virus infection. J. Immunol. 184:3134–3144 [DOI] [PubMed] [Google Scholar]
- 66. Sakuishi K., et al. 2010. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207:2187–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sawtell N. M., Thompson R. L. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 66:2150–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shimeld C., et al. 1995. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J. Neuroimmunol. 61:7–16 [DOI] [PubMed] [Google Scholar]
- 69. Simmons A., Tscharke D. C. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 175:1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Theil D., et al. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163:2179–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thompson R. L., Sawtell N. M. 1997. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 71:5432–5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Trousdale M. D., et al. 1991. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J. Virol. 65:6989–6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vali B., et al. 2010. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur. J. Immunol. 40:2493–2505 [DOI] [PubMed] [Google Scholar]
- 74. van Baarle D., et al. 2008. Progressive telomere shortening of Epstein-Barr virus-specific memory T cells during HIV infection: contributor to exhaustion? J. Infect. Dis. 198:1353–1357 [DOI] [PubMed] [Google Scholar]
- 75. van Lint A. L., et al. 2005. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J. Virol. 79:14843–14851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Verjans G. M., et al. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 104:3496–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang Q. Y., et al. 2005. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc. Natl. Acad. Sci. U. S. A. 102:16055–16059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wherry E. J., Blattman J. N., Murali-Krishna K., van der Most R., Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wherry E. J., et al. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27:670–684 [DOI] [PubMed] [Google Scholar]
- 80. Yang L., Voytek C. C., Margolis T. P. 2000. Immunohistochemical analysis of primary sensory neurons latently infected with herpes simplex virus type 1. J. Virol. 74:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yao S., Chen L. 2006. Reviving exhausted T lymphocytes during chronic virus infection by B7-H1 blockade. Trends Mol. Med. 12:244–246 [DOI] [PubMed] [Google Scholar]
- 82. Zhang X., et al. 2005. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nε-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J. Virol. 79:15289–15301 [DOI] [PMC free article] [PubMed] [Google Scholar]