Abstract
Chronic wasting disease (CWD) is a prion disease of cervids that causes neurodegeneration and death. Susceptibility to prion infections, including CWD, can be dependent on the amino acid sequence of the host prion protein (PrP). Here, CWD agent obtained from a deer expressing the 96SS genotype, associated with partial resistance to CWD, was used to infect transgenic (tg) mice expressing either 96GG or 96SS deer PrP. Transgenic mice expressing 96GG deer PrP succumbed to this agent, but tg mice expressing 96SS deer PrP did not. Additional studies using inocula from 96GG deer showed no transmission to 96SS PrP mice and delayed disease in 96GS mice. Thus, 96S PrP played an inhibitory role in disease progression in tg mice.
TEXT
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) or prion disease of North American cervids that causes progressive neurodegeneration and death (24, 26). Disease occurs when the normal (sensitive) host prion protein (PrPsen) is converted to an abnormal, disease-associated, protease-resistant form (PrPres). The incidence of CWD continues to increase in wild and captive cervids of North America, most likely via direct animal-to-animal contact or exposure to CWD agent-contaminated environments. It is known that the sequence of the host prion protein gene (PRNP) can affect susceptibility to TSE diseases, including scrapie in sheep (4) and laboratory mice (2), sporadic Creutzfeldt-Jakob disease (sCJD) (1, 6, 17), variant CJD (25) and kuru (11) in humans, and CWD in cervids (23). Susceptibility to the CWD agent has been shown to correlate with specific amino acids at defined locations within the PRNP gene, including codon 96 in white-tailed deer (9, 12, 15, 27), codons 20 and 225 in mule deer (7, 27), and codon 132 in elk (5). Approximately 55% of white-tailed deer are homozygous for glycine at position 96 (96GG), ∼35% have glycine/serine at this position (96GS), and ≤10% are serine homozygotes (96SS), depending on geographic location (9, 15, 27). Strong evidence exists to show that both the 96GS and 96SS genotypes are underrepresented among populations of CWD agent-infected white-tailed deer (8, 9, 15, 27). It is unclear if the low incidence of disease in 96GS and 96SS deer reflects only resistance to a particular CWD agent strain or is in fact a protective factor provided by cervid 96S prion protein.
In some situations in which TSE occurs in an animal with a previously resistant genotype or a new species, agent adaptation and selection can occur with greater efficiency in the subsequent spread to additional individuals with similar genotypes (14, 19). By analogy, in cervids, PrPres from a few CWD agent-positive 96SS individuals might adapt to become more infectious for additional 96SS deer. To test this possibility, we developed transgenic (tg) mice that express various PRNP genotypes at codon 96 to model the situation in deer. Transgenic mice that express deer PrP with glycine at position 96 (96GG) (described previously as tg33 [12, 18]) expressed PrPsen at levels comparable to the physiologic levels found in deer (12, 18). A second tg mouse line which expressed serine at position 96 (96SS) (described previously as tg60 [12]) expressed PrPsen at 70% of the level seen in 96GG mice. In our current study, we tested whether the CWD agent from a 96SS deer could infect tg mice expressing either the 96SS or the 96GG genotype. All mice were housed at the Rocky Mountain Laboratories (RML) in an AAALAC International-accredited facility, and experimentation followed NIH RML Animal Care and Use Committee-approved protocols.
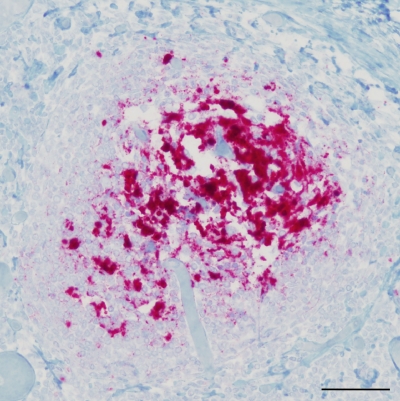
Transgenic mice were inoculated intracerebrally with the CWD agent from a 96SS genotype white-tailed deer (W104). Deer W104 was infected orally with a pool of seven 96GG white-tailed deer brains (WTD-1) containing the CWD agent(s) (20). At 477 days postinoculation, a tonsil biopsy specimen was collected from deer W104 and tested PrPres positive based on immunohistochemistry (IHC) (28). Deer W104 was later euthanized at 993 days postinoculation due to illness unrelated to CWD. The retropharyngeal lymph node (RPLN) collected at necropsy from deer W104 was positive for PrPres by IHC (Fig. 1) (13) but negative by Western blotting. The obex was PrPres negative by both IHC and Western blot analysis (data not shown). 96SS and 96GG tg mice were inoculated intracerebrally with 50 μl of either 1% or 0.01% tissue homogenates from either the obex or RPLN of deer W104.
Fig. 1.
Immunohistochemistry of retropharyngeal lymph node from 96SS white-tailed deer W104. Monoclonal antibody F99/97.6.1 (13) was used to detect PrPres deposition (red). Scale bar, 50 μm.
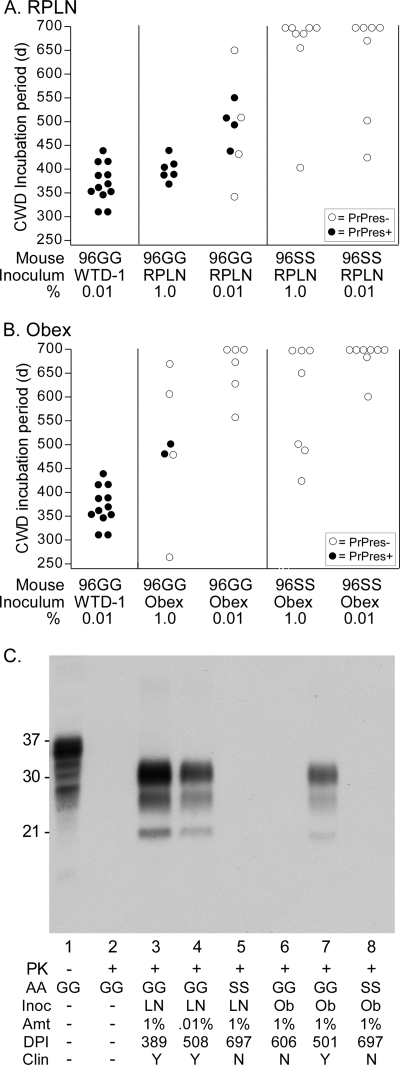
Interestingly, RPLN and the obex from deer W104 did not cause disease in any 96SS mice but did cause disease in 96GG mice (Fig. 2A and B). RPLN from W104 caused clinical disease in 100% of 96GG mice receiving a 1% tissue homogenate but had only a 50% attack rate when a 0.01% tissue homogenate was inoculated (Fig. 2A). Compared to the incubation period for our 0.01% 96GG positive-control inoculum (WTD-1) containing 2.0 × 108 50% lethal doses (LD50) per ml of 100% homogenate (18), the incubation period for 0.01% RPLN was much longer, likely due to a low titer of infectivity. The W104 obex had even lower levels of infectivity than W104 RPLN, as indicated only by a partial attack rate and longer incubation periods in 96GG mice inoculated with a 1% tissue homogenate (Fig. 2B). No infectivity could be detected in the 0.01% obex inoculum by 700 days postinoculation in 96SS tg mice (Fig. 2B). Confirmation of CWD agent infection and screening for subclinical infections was done by Western blot detection of PrPres in brains (12) using anti-PrP antibody 6H4 (Fig. 2C). Each lane was loaded with 1 mg tissue equivalents. As expected, 96GG tg mice showing clinical disease had detectable PrPres. PrPres was not detected in any 96SS tg mice at the conclusion of the study. These data suggested that the CWD agent from an infected 96SS deer retained the ability to infect 96GG tg mice but did not adapt during replication in a 96SS deer to more easily infect tg mice with a 96SS genotype.
Fig. 2.
Column graphs indicating parameters and test results for 96GG and 96SS mice inoculated with deer W104 RPLN (A) or W104 obex (B). Inoculum dilutions and recipient tg mice are shown beneath the x axis in each graph. WTD-1 was used as a positive control and is shown in both panels A and B. Solid symbols indicate tg mice that were confirmed positive for PrPres by Western blotting, and open symbols indicate mice that did not develop CWD and were euthanized for other reasons or at the termination of the experiment. Each symbol represents an individual mouse. All inoculations were intracerebral in a volume of 50 μl. Note that none of the 96SS mice were susceptible to either 96SS inoculum. (C) Western blot detection of PrPres found in tg mice inoculated with W104 tissue homogenates. Lane 1 was loaded with 0.4 mg wet-tissue equivalents, and lanes 2 to 8 were loaded with 1.0 mg tissue equivalents. All samples were proteinase K (PK) digested, except that in lane 1. AA, amino acid genotype of recipient mice at residue 96; Inoc, inocula, which were either retropharyngeal lymph node (LN) or obex (Ob); Amt, the percentage of tissue homogenate inoculated (wt/vol); DPI, number of days postinoculation of the recipient mouse shown in the blot; Clin, mice that showed (Y) or did not show (N) clinical signs of CWD. The blot was developed using anti-PrP antibody 6H4 (Prionics) at a 1:10,000 dilution and enhanced-chemiluminescence detection with a 1-min film exposure.
To evaluate the effect of heterogeneity at position 96 on CWD susceptibility, we challenged 96SS, 96GS, 96GG, and 96G− tg mice with high-titer 96GG CWD agents. Transgenic mice with the 96G− genotype are hemizygous for the 96G transgene and express approximately half the level of PrPsen as 96GG mice. Transgenic 96GS mice were created by breeding 96GG mice with 96SS mice. All tg mice were inoculated intracerebrally with one of three different 1% CWD agent brain homogenate pools containing the following lethal doses per inoculum, as measured previously by endpoint titration in 96GG tg mice: WTD-1, 100,000; MD-2, 6,500; and Elk-1, 65,000 (18). Mice were monitored for disease onset, and observation of clinical CWD was confirmed by Western blotting for PrPres in the brains of sick mice. All three inocula caused disease in 96GS mice, but disease was delayed in onset compared to that in both 96GG and 96G− mice (Fig. 3). In contrast, none of the inocula caused disease in 96SS mice during 600 days of observation (data not shown). The presence of the S allele in 96GS mice delayed disease by 40 to 50 days compared to that of 96GG mice. If the S allele did not inhibit prion infection, we would have expected the GS mice to have incubation periods similar to those of G− mice. However, the 96GS mice were in fact slower to develop disease than 96G− mice, despite having higher overall PrPsen levels. These data suggested that the 96S allele confers resistance when it is expressed alone and provides an inhibitory effect when it is coexpressed with 96G PrP. These results were similar to results of CWD in 96GS deer, where extended incubation periods have been observed (9, 10, 28). We did not test to see if our 96SS inocula could infect 96GS tg mice since our 96SS inocula appeared to have low levels of infectivity. We hypothesize that the 96GS mice are susceptible, although likely with extended incubation periods due to the inhibitory nature of the S allele and the apparent failure of 96SS inocula to adapt to 96SS mice.
Fig. 3.
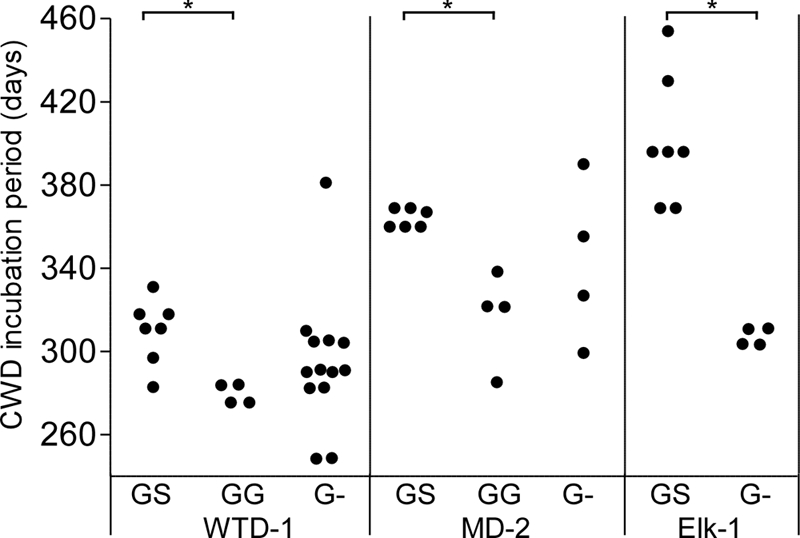
Inhibitory effects of the 96S allele on CWD incubation periods. tg mice expressing 96SS, 96GS, 96GG, or 96G− were inoculated intracerebrally with CWD agent brain homogenates (WTD-1, MD-2, or Elk-1) and euthanized when they developed clinical signs of CWD. Each solid circle indicates one PrPres-positive mouse. Statistical analysis was done using a Kruskal-Wallis test with a Dunn's multiple-comparison posttest on the groups inoculated with WTD-1 or MD-2. Mann-Whitney analysis was performed on the group inoculated with Elk-1. Bars with an asterisk indicate a P value of <0.05. Group differences with P values of >0.05 are not shown. None of the 96SS mice inoculated with the three inocula used in this experiment developed disease, and they are not shown in the figure.
The reason for the lack of clinical disease in CWD agent-inoculated 96SS mice is not clear. This result is in contrast to field data showing that 96SS deer are in fact susceptible to CWD, albeit with a much lower incidence than that in 96GG and 96GS deer (9, 15, 27). Possibly, 96SS mice are infected with CWD agents, but disease progression is so slow that neither clinical disease nor detection of PrPres is observed during the life span of the mouse (Fig. 2A and B). Data from deer W104 and others in the same study cohort suggest that CWD progresses slowly in 96SS white-tailed deer. Deer W104 was PrPres positive by tonsil biopsy specimen at 477 days postinoculation (dpi) but had no clinical CWD signs at 993 dpi or detectable PrPres in the obex following euthanasia. Two additional 96SS deer in the study cohort with W104 also had extended incubation periods (28).
The low titer of the 96SS inoculum used in our experiments might also explain the lack of disease in 96SS mice. In our experiments, we used tissues from a preclinical 96SS deer, and PrPres could not be detected by Western blot analysis in the samples used for inoculation. The combination of preclinical status, negative Western blot results, and extended incubation periods in 96GG tg mice suggest that the 96SS tissues had a low titer of infectivity and may not have been sufficient to cause disease in 96SS tg mice. Unfortunately, higher-titer tissues from terminal CWD agent-infected 96SS deer were not available at the time that we initiated the study.
Alternatively, some studies have shown that there may be many strains of CWD agents (16, 21, 22). It is possible that a specific strain of prion disease may be more virulent or specific to a particular PrP genotype (3). In our current study, we inoculated 96SS tg mice with CWD agent(s) from four sources, including two brain homogenate pools representing 7 white-tailed deer (WTD-1), 4 mule deer (MD-2), and two individual brain homogenates (Elk-1 and W104). If different strains of CWD agents were present in these inocula, they did not appear to affect the susceptibility of our 96SS tg mice. This was somewhat surprising given the fact that deer W104 had a 96SS genotype. While we cannot rule out the possibility that a 96SS-tropic CWD agent exists, our current data suggest that 96SS provides a protective effect against most CWD agents.
Expression levels of PrPsen in mammals can affect incubation periods and susceptibility to TSE infections and might also be a factor in our experiments. The 96SS tg mice inoculated in our experiments expressed 30%-lower PrPsen levels than the susceptible 96GG tg mice (12). However, additional lines of 96GG tg mice and hemizygous (G−) mice expressed lower levels of PrP than the 96SS mice used here, and all these lines were susceptible to CWD agent infection (12). Therefore, we believe that it is unlikely that the slightly lower levels of PrPsen expression in our 96SS tg mice can explain the lack of observed disease or detectable PrPres.
The fact that the 96SS PrP genotype provides some level of resistance to CWD agents may be useful in situations where white-tailed deer are raised in captivity. Recently, the incidence of sheep scrapie has been markedly reduced by careful selection of replacement stock that carried resistant genotypes. In a similar manner, deer farmers could retain and breed individuals that were serine homozygotes at codon 96. Unfortunately, the 96SS genotype is not 100% resistant to CWD, and the high density of animals on some game farms may overcome the resistance effect of the 96SS genotype. This could result in CWD agent-infected 96SS deer that become subclinical, long-term carriers and could possibly expose additional deer over a long period of time. In addition, long incubation periods in 96SS deer may provide sufficient time for adaptation to a 96SS tropism in some individuals.
Acknowledgments
We thank Byron Caughey, Mikael Klingeborn, Andrew Timmes, and James Striebel for critical review of the manuscript; Ed Schreckendgust for animal husbandry; and the Colorado Division of Wildlife for providing CWD-infected tissues.
Funding for this work was provided by the National Institute of Allergy and Infectious Diseases, Division of Intramural Research.
Footnotes
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Bishop M. T., Will R. G., Manson J. C. 2010. Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc. Natl. Acad. Sci. U. S. A. 107:12005–12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carlson G. A., et al. 1986. Linkage of prion protein and scrapie incubation time genes. Cell 46:503–511 [DOI] [PubMed] [Google Scholar]
- 3. Dickinson A. G., Meikle V. M. 1969. A comparison of some biological characteristics of the mouse-passaged scrapie agents, 22A and ME7. Genet. Res. 13:213–225 [DOI] [PubMed] [Google Scholar]
- 4. Goldmann W., Hunter N., Smith G., Foster J., Hope J. 1994. PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie. J. Gen. Virol. 75:989–995 [DOI] [PubMed] [Google Scholar]
- 5. Green K. M., et al. 2008. The elk PRNP codon 132 polymorphism controls cervid and scrapie prion propagation. J. Gen. Virol. 89:598–608 [DOI] [PubMed] [Google Scholar]
- 6. Head M. W., et al. 2004. Prion protein heterogeneity in sporadic but not variant Creutzfeldt-Jakob disease: UK cases 1991-2002. Ann. Neurol. 55:851–859 [DOI] [PubMed] [Google Scholar]
- 7. Jewell J. E., Conner M. M., Wolfe L. L., Miller M. W., Williams E. S. 2005. Low frequency of PrP genotype 225SF among free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. J. Gen. Virol. 86:2127–2134 [DOI] [PubMed] [Google Scholar]
- 8. Johnson C., Johnson J., Clayton M., McKenzie D., Aiken J. 2003. Prion protein gene heterogeneity in free-ranging white-tailed deer within the chronic wasting disease affected region of Wisconsin. J. Wildl. Dis. 39:576–581 [DOI] [PubMed] [Google Scholar]
- 9. Johnson C., et al. 2006. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J. Gen. Virol. 87:2109–2114 [DOI] [PubMed] [Google Scholar]
- 10. Johnson C. J., et al. 2011. Prion protein polymorphisms affect chronic wasting disease progression. PLoS One 6:e17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H. S., et al. 2001. Increased susceptibility to Kuru of carriers of the PRNP 129 methionine/methionine genotype. J. Infect. Dis. 183:192–196 [DOI] [PubMed] [Google Scholar]
- 12. Meade-White K., et al. 2007. Resistance to chronic wasting disease in transgenic mice expressing a naturally occurring allelic variant of deer prion protein. J. Virol. 81:4533–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller M. W., Williams E. S. 2002. Detection of PrP(CWD) in mule deer by immunohistochemistry of lymphoid tissues. Vet. Rec. 151:610–612 [DOI] [PubMed] [Google Scholar]
- 14. Moore R. A., Vorberg I., Priola S. A. 2005. Species barriers in prion diseases—brief review. Arch. Virol. Suppl. 19:187–202 [DOI] [PubMed] [Google Scholar]
- 15. O'Rourke K. I., et al. 2004. Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white-tailed deer. J. Gen. Virol. 85:1339–1346 [DOI] [PubMed] [Google Scholar]
- 16. O'Rourke K. I., et al. 2007. Elk with a long incubation prion disease phenotype have a unique PrPd profile. Neuroreport 18:1935–1938 [DOI] [PubMed] [Google Scholar]
- 17. Palmer M. S., Dryden A. J., Hughes J. T., Collinge J. 1991. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature 352:340–342 [DOI] [PubMed] [Google Scholar]
- 18. Race B., et al. 2009. Susceptibilities of nonhuman primates to chronic wasting disease. Emerg. Infect. Dis. 15:1366–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Race R., Raines A., Raymond G. J., Caughey B., Chesebro B. 2001. Long-term subclinical carrier state precedes scrapie replication and adaptation in a resistant species: analogies to bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease in humans. J. Virol. 75:10106–10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raymond G. J., et al. 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 19:4425–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raymond G. J., et al. 2007. Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. J. Virol. 81:4305–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Safar J. G., et al. 2002. Measuring prions causing bovine spongiform encephalopathy or chronic wasting disease by immunoassays and transgenic mice. Nat. Biotechnol. 20:1147–1150 [DOI] [PubMed] [Google Scholar]
- 23. Sigurdson C. J. 2008. A prion disease of cervids: chronic wasting disease. Vet. Res. 39:41. [DOI] [PubMed] [Google Scholar]
- 24. Sohn H. J., et al. 2002. A case of chronic wasting disease in an elk imported to Korea from Canada. J. Vet. Med. Sci. 64:855–858 [DOI] [PubMed] [Google Scholar]
- 25. Wadsworth J. D., et al. 2004. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science 306:1793–1796 [DOI] [PubMed] [Google Scholar]
- 26. Williams E. S., Miller M. W. 2002. Chronic wasting disease in deer and elk in North America. Rev. Sci. Tech. 21:305–316 [DOI] [PubMed] [Google Scholar]
- 27. Wilson G. A., et al. 2009. Polymorphisms at the PRNP gene influence susceptibility to chronic wasting disease in two species of deer (Odocoileus spp.) in western Canada. J. Toxicol. Environ. Health A 72:1025–1029 [DOI] [PubMed] [Google Scholar]
- 28. Wolfe L. L., et al. 2007. PrPCWD in rectal lymphoid tissue of deer (Odocoileus spp.). J. Gen. Virol. 88:2078–2082 [DOI] [PubMed] [Google Scholar]