Abstract
Reactive oxygen species (ROS) are generated continuously during aerobic metabolism. ROS are highly reactive molecules and in excessive amounts, can lead to protein and DNA oxidation, protein cross-linking, and cell death. Cell-culture models provide a valuable tool in understanding the mechanisms that lead to cell death. Accumulation of ROS within cells and/or their release into the culture media are highly cell type-specific. The ability to estimate ROS levels in the culture media is an important step in understanding the mechanisms contributing to disease processes. In this paper, we describe the optimization of a simple method to estimate ROS levels in the culture media using the Acridan Lumigen PS-3 reagent provided in the Amersham ECL Plus kit (GE Healthcare, UK). We have shown that the Acridan Lumigen PS-3 assay generates ROS-specific chemiluminescence in fresh as well as media stored at −20°C, in as little as 10–20 μl of samples. The method was able to detect the dose (of stimulants)- and time (acute and chronic)-dependent changes in ROS levels in media collected from various cell types. Our results suggest that the kit reagents, PBS buffer, and various media did not contribute significantly to the overall chemiluminescence generated in the assay; however, we suggest that the unused medium specific for each cell type should be used as blanks and final readings of test samples normalized against these readings. As this method uses commonly available laboratory equipment and commercially available reagents, we believe this assay is convenient, economical, and specific in estimating ROS released extracellularly into the culture media.
Keywords: ROS, free radicals, chemiluminescent assay
INTRODUCTION
In mammalian cells, free radical species, including ROS and reactive nitrogen species (RNS), are continuously generated during aerobic metabolism.1–3 These reactive species include hydroxyl radicals (OH·), hydroperoxyl radicals (HOO·), peroxy nitrite (ONOO−), nitric oxide radicals (NO·), oxygen radicals, and superoxide radical anions (O2·−).1–5 Superoxide is generated as a by-product of the respiratory chain, and toxic OH· are generated from hydrogen peroxide (H2O2) and superoxide through the Fenton reaction and the Haber-Weiss cycle. Superoxide may also spontaneously break down into oxygen and H2O2. In addition, several oxidases generate H2O2 as a by-product.1 H2O2 is a very mild and a less-reactive, non-radical ROS; however, it gets converted into highly reactive OH· within cells.1,6 Depending on the cell type, the ROS produced can be accumulated within cells or released into the extracellular environment.7,8 In the central nervous system (CNS), microglia are the main immune effectors; these cells protect the CNS from invading microorganisms and clear debris generated as a result of cell damage.9,10 Once activated, microglia produce a cocktail of cytokines and enzymes (such as proteases) and release ROS.11–13 As ROS are highly reactive molecules, their accumulation can lead to damage, such as protein and lipid oxidation, protein cross-linking leading to protein inactivation, and breakage of DNA strands.14,15 Oxidative stress-mediated damage, as a result of excessive accumulation of ROS/RNS, contributes to the pathogenesis of several chronic diseases involving the loss of specific cells. The effect of ROS-mediated cell loss also depends on the ability of certain cell types to produce, accumulate, and/or release ROS into the extracellular environment and the susceptibility of other cell types to ROS-induced damage.
In vitro culture models are important tools to understand the pathways involved in cell loss during disease processes. It is therefore critical to study ROS-mediated damage resulting from ROS generated and retained within cells, as well as ROS that are secreted extracellularly into the culture environment. There are a number of well-established methods available to measure intracellular ROS. Most widely used methods involve oxidizable fluorescent dyes, such as dihydroethidium (DHE), chemically reduced and acetylated forms of 2′-7′-dichlorofluorescein (DCF), carboxyl derivatives, and other derivatives of fluorescein such as 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFH-DA) and H2DCF-DA. DHE is oxidized specifically by superoxide anions, and the oxidized dye intercalates with nuclear DNA and fluoresces red; the intensity and number of red dots in the nucleus represent the amount of superoxide present in the cell.14,16,17 By contrast, DCF and its derivatives are oxidized by a wide range of ROS, including H2O2, hydroxyl radicals (OH·), hydroperoxy, and ONOO−, producing red fluorescence.16,18 Some studies have also used NBT for detecting the presence of intracellular ROS; NBT can be oxidized into purple-blue formazan compounds, detected as a purple-colored precipitate in the cells, which can be quantified by lysing cells in acetic acid and reading absorbance at an appropriate wavelength.19,20 In addition, there are several chemiluminescent substances that are very sensitive and have been used in measuring intracellular ROS.21–23
Current methods used to estimate extracellular ROS released into the culture media are complex, and most of the methods need to be performed in the presence of live cells and require special equipment. These include the use of compounds such as luminol and lucigenin and techniques such as electron spin resonance spectroscopy and liquid scintillation counting.22–25 Recently, Stratagene (La Jolla, CA, USA) developed a luminol-based method (LumiMax kit) to detect ROS released extracellularly in the presence of live cells.26 As ROS are very unstable, and current methods require the presence of live cells and/or sophisticated equipment, measurement of ROS in the culture media becomes inconvenient for researchers. We believe that there is a clear need for a simple method that can measure extracellular ROS in the media. In this study, we have developed a simple, quick, and reproducible method of measuring extracellular ROS in culture media using the Acridan Lumigen PS-3 reagents provided in the Amersham ECL Plus kit (GE Healthcare, UK), which is commonly used for Western blotting. Acridan compounds (or acridinium derivatives) can be oxidized enzymatically or electrochemically; in both cases, oxidation products generate chemiluminescence.21,27 Acridan Lumigen PS-3 reacts with H2O2 and forms acridinium ester, which can exchange electrons with free radicals, generating acridanyl radical intermediate. Acridanyl radical decarboxylates spontaneously to give a singlet-excited state of N-methylacridone, which emits light at 430 nm (Fig. 1).28 This method has been described briefly in our recently published paper.7 Here, we present the methods in full, as well as details relating to assay specificity and effects of certain components. We then go on to compare the assay with a well-known in vitro assay to measure ROS, generated by a chemical reaction, followed by validating the assay under various oxidative stress conditions in a range of different cell types.
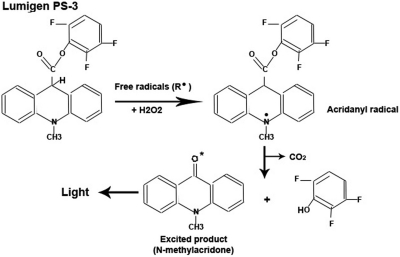
FIGURE 1.
Schematic diagram showing the ROS excitation of the Acridan Lumigen PS-3 by ROS. Diagram showing the exchange of electrons between acridan Lumigen PS-3 and reactive species (ROS/RNS) in the presence of H2O2. The intermediate acridanyl radical spontaneously decarboxylates into CO2 and excited N-methyl acridone, which emits light at 430 nm in the form of chemiluminescence. This diagram was modified from Wilson et al.28 and the Amersham ECL Plus Western blotting detection kits (GE Healthcare), product booklet (codes: RPN2132 and RPN2133), http://gehealthcare.com/lifesciences.
MATERIALS AND METHODS
Cell Culture and Treatment of Different Cell Types with Rotenone
Three different cell lines of human origin and rat primary blood monocytes were used to demonstrate that different levels of ROS/RNS are produced in response to an identical stimulus. Transformed human microglial cell line CHME-5 [a generous gift from Professor Pierre Talbot, Laboratory of Neuroimmunovirology, Institut Nationale de Recherches Scientifiques (INRS)-Institute, Armand-Frappier, Quebec, Canada], human SH-SY5Y neuroblastoma (American Type Culture Collection, Manassas, VA, USA) cell line, and a human acute monocytic leukemia cell line THP-1 (a gift from Dr. Fiona Radcliff, Department of Molecular Medicine and Pathology, University of Auckland, New Zealand) were used. The majority of experiments was carried out using the CHME-5 microglial cell line; CHME-5 is obtained from embryonic human microglia through transformation with simian virus-40.29,30 This cell line has been well-characterized by Professor Talbot's group and has been used successfully by us7 and others29,31 as a model of microglial activation. SH-SY5Y is a human neuroblastoma cell line. These cells share several properties with dopaminergic neurons.32 The THP-1 cell line was derived from human acute monocytic leukemia. This cell line exhibits properties similar to the human monocyte-derived macrophages, maintains distinct monocytic characteristics in culture,33 and shares the same lineage with microglia.33,34 For routine cultures, all cell types were cultured at 37°C/5% CO2. CHME-5 (9×103 cells/25 cm2) and SH-SY5Y (5×105/25 cm2) cells were cultured in DMEM-F12 (Invitrogen, Carlsbad, CA, USA) medium with 10% heat-inactivated FBS (Invitrogen), 2 mM glutamine, and 1 mM sodium pyruvate. To carry out time-dependent chronic treatments, the cell number of each cell type was adjusted to achieve 90–95% confluency within the required treatment period. THP-1 cells were grown in RPMI-1640 medium, supplemented with 10% FBS and 0.05 mM beta-mercaptoethanol (β-ME). Cells were split before reaching 1 × 106 cells/ml. Rat peripheral blood mononuclear cells (BMNCs) were used as primary cells; the procedure of isolation of BMNCs is given below. For performing the acridan Lumigen PS-3 ROS assay and assessing other parameters, cells were treated as described in each section.
Isolation of rat BMNCs
Rat blood was provided by the Vernon Jensen Unit (the animal housing facility; University of Auckland). Blood sample (10 ml) was collected from Wistar rats by cardiac puncture, according to the standard protocol approved by the Animal Ethics Committee of the University of Auckland (Ethics No. R763); EDTA was used as an anticoagulant. The blood sample was subjected to gradient fractionation using Histopaque 1083 solution (Sigma-Aldrich, St. Louis, MO, USA). Mononuclear cells at the interface of Histopaque and plasma were removed and washed twice with Ca2+- and Mg2+-free Dulbecco's PBS (Gibco, Invitrogen). Cells were then suspended in complete RPMI-1640 medium (with 10% FBS) and counted using a hemocytometer. Cells (6.53×105 cells/ml) were cultured in a 24-well format in RPMI-1640 medium (with 10% FBS) for 1 h at 37°C under standard humidity and CO2 conditions. After 1 h, floating lymphocytes were removed by replacing the medium with the fresh medium.
Acridan Lumigen PS-3 assay
CHME-5 cells were cultured in triplicate using complete DMEM-F12 medium in the presence of 5 nM rotenone (mitochondrial complex I enzyme inhibitor, Sigma-Aldrich) or DMSO (solvent for rotenone; same volume that was used for rotenone, Sigma-Aldrich) for 1 week in triplicate. At the end of Week 1, culture media were collected and stored at −20°C. To measure ROS released into the medium by the cells, the Acridan Lumigen PS-3 assay was developed in a 96-well plate format. To develop this 96-well plate format assay, we used the Acridan Lumigen PS-3 reagent, provided as a chemiluminescent substrate for HRP (in the Amersham ECL Plus kit, GE Healthcare). For the purpose of measuring ROS in the culture media, we mixed Reagent A (H2O2 in Tris buffer, Amersham ECL Plus kit, GE Healthcare) and Reagent B (acridan solution in dioxane and ethanol, Amersham ECL Plus kit, GE Healthcare) in a 40:1 ratio; this mixture was named the ALPS-3 substrate. The detection of ROS in the media was done in the presence of heat-inactivated serum.
Volume of the Media Required (Linear Curve) and Effect of Media Storage Temperature
To determine the appropriate volume of the medium required for the assay, 10, 20, 30, 40, and 50 μl of the culture media were used. These media were collected at the end of Week 1 from the rotenone-treated and untreated (control) CHME-5 cells (cultured in three replicates). Each sample was placed in an individual well of a 96-well plate. The final volume of each sample was adjusted to 100 μl with PBS (pH 7.4), followed by 50 μl of the ALPS-3 substrate. The plate was incubated in the dark for 5 min at room temperature. Chemiluminescence was recorded digitally using a Fujifilm luminescent image analyzer (LAS-3000, Version 2.2). Light intensity (photons) was also measured using a BioTek Synergy 2 plate reader and Gen5 software. Readings recorded by the BioTek plate reader were used for analysis. Fresh (collected on ice) or stored (−20°C) DMEM-F12 media collected after 1 week's treatment with 5 nM rotenone were processed for the Acridan Lumigen PS-3 assay to assess the effect of freezing on the ROS-mediated chemiluminescence.
Treatment with Antioxidants to Confirm ROS-Specific Chemiluminescence
The specificity of the Acridan Lumigen PS-3 assay in generating ROS-specific chemiluminescence was confirmed by using two different antioxidants. The culture media collected from rotenone-treated CHME-5 cells at the end of Week 1 were incubated with different doses (10, 30, and 100 μM) of ascorbic acid (vitamin C) or different dilutions (1:500, 1:750, 1:1000) of NuPAGE antioxidant (Invitrogen, Cat No. NP0005), which contains sodium bisulfite. The incubation of the media with these antioxidants was carried for 5 min at room temperature before the addition of the ALPS-3 substrate. Chemiluminescence was recorded as described above.
Contribution of H2O2 to the Chemiluminescence Generated in the Assay
H2O2 is an important component of Reagent A of the ECL Plus kit, which is also part of the acridan Lumigen PS-3 assay. As H2O2 is a mild oxidant and a nonradical ROS, we studied the effect of H2O2 in the generation of chemiluminescence. H2O2 (441 mM) in PBS was incubated with/without HRP enzyme (0.3 unit/μl) for 5 min at room temperature, followed by incubation with ALPS-3 substrate and recording of chemiluminescence as described above. Controls included H2O2 (441 mM in PBS) only and HRP only (0.3 unit/μl), treated similarly in the presence of PBS buffer.
Comparison of NBT and ALPS-3 Substrates in Measuring ROS Generated in an In Vitro (Cell-Free) Xanthine-Xanthine Oxidase (XO) Reaction
Oxidation of xanthine by XO is a well-known method to generate superoxide in a cell-free in vitro reaction.35,36 Nitroblue tetrazolium (NBT) assay is generally used to estimate superoxide produced in this reaction through the generation of purple-colored formazan compounds.35,36 We first generated a standard plot of formazan, produced by incubating increasing concentrations of xanthine (0, 100, 200, and up to 1000 μM, made in PBS) with 1 U/ml XO. The reaction showed a linear increase in the generation of formazan crystals (formed as a result of reaction of superoxide with NBT), with increasing concentrations of xanthine (data not shown). We then compared the NBT and ALPS-3 assays using four concentrations (0, 500, 700, and 1000 μM) of xanthine. For the NBT assay, the four selected concentrations of xanthine were incubated with XO (1 U/ml, in PBS) and NBT (0.33 mg/ml, diluted in PBS) in a 96-well format with 100 μl reaction mixture in each well. The plate was incubated at room temperature, in the dark for 5 min, followed by addition of 50 μl DMSO in each well to dissolve the purple-colored formazan crystals. The absorbance was recorded at 630 nm using the BioTek Synergy 2 plate reader. The highest concentration of xanthine (1000 μM) + XO was also incubated with the 1:500-diluted NuPAGE antioxidant to confirm that the specificity of the color development was a result of the generation of superoxide by xanthine + XO.
For Acridan Lumigen PS-3 assay, xanthine + XO was mixed in similar concentrations as described above, followed by the addition of 50 μl ALPS-3 substrate. The plate was incubated in the dark for 5 min, and chemiluminescence was measured at 430 nm using the BioTek plate reader. For this experiment, the highest concentration of xanthine (1000 μM) + XO was also incubated with a 1:500-diluted NuPAGE antioxidant.
Effect of Different Media and PBS Buffer on ROS-Mediated Chemiluminescence
The media used to culture the four different cells types (SH-SY5Y, CHME-5, THP-1, and BMNCs) used in this study were DMEM-F12, RPMI-1640, or DMEM high-glucose medium. PBS buffer was used to wash cells and dilute media before the assay. We therefore tested whether these media and PBS contributed to the chemiluminescence in the assay. Media collected from rotenone-treated CHME-5 cells (5 nM, 1 week) were used as a positive control in conjunction with unused/fresh RPMI 1640, DMEM-F12, DMEM high glucose, and PBS. In addition, all solutions were incubated with and without NuPAGE antioxidant (1:500, Invitrogen), followed by incubation with the ALPS-3 substrate in the dark for 5 min and measurement of chemiluminescence as described above.
Rotenone Dose-Dependent Release of ROS
CHME-5 cells are activated in response to rotenone and produce ROS.7 To determine the sensitivity of the acridan Lumigen PS-3 assay in measuring the dose-related response of microglia in terms of ROS production, CHME-5 cells (4×105 cells/cm2) were cultured in DMEM-F12 for 48 h. Media were supplemented with DMSO (control) or 5, 10, or 20 nM rotenone. After 48 h, media were collected and stored immediately at −20°C until required. ROS released into the media were measured by the acridan Lumigen PS-3 assay.
Acute Response of Cells (of the Same Lineage) to Various Stimulants
CHME-5 cells were plated in complete DMEM-F12 medium supplemented with 10% FBS, and the cell number was adjusted to achieve 90–95% confluency within 24 h. After allowing cells to adhere to the flask overnight, the medium was replaced by media supplemented with 10 μM advanced glycation end-products (AGEs; known to activate microglia7), 20 nM rotenone, 100 EU/ml LPS, or 100 nM phorbol myristate acetate (PMA) for 1 h at 37°C in 5% CO2. Media from three independent replicates of each treatment group were processed for the acridan assay as described above. We also tested the ability of the acridan assay to detect ROS-mediated chemiluminescence in the human monocytic THP-1 cells and rat primary blood monocytes (BMNCs). THP-1 cells were pelleted and resuspended at 1 × 106 cells/ml in RPMI-1640 media, supplemented with 10% FBS and 100 nM PMA or 100 EU/ml LPS for 1 and 6 h. At each time-point, cell suspensions were pelleted by centrifugation at 4°C and media collected for detection of chemiluminescence using the Acridan Lumigen PS-3 assay. To detect very acute release of ROS from primary cells, rat blood monocytes were stimulated with PMA for 15 min. Adherent monocytes were supplemented with RPMI medium containing 100 nM PMA or DMSO (amount equivalent to 100 nM PMA) and incubated at 37°C for 15 min. All media from each cell type and treatment groups were collected on ice and processed immediately or stored at −20°C. Controls included untreated cells. Chemiluminescence (photon) counts of each group were normalized with corresponding blank (medium-only) readings.
Acute and Chronic Response of Cells to Rotenone
To determine the efficacy of the Acridan Lumigen PS-3 assay, we used two types of cells from different lineages; microglial CHME-5 and neuronal SH-SY5Y cells. Our previous work suggested that these two cell types demonstrate differences in their ability to respond to the same stimulus in terms of ROS production.7 Both cell types were cultured in DMEM-F12 (supplemented with 10% heat-inactivated FBS) in the presence of DMSO (control) or with 5 nM rotenone for 1 h (acute) and 1 week (chronic). The number of each cell type was adjusted to achieve 90–95% confluency at the end of 1 week. Culture media were collected at the end of each time-point and processed for the measurement of ROS using the Acridan Lumigen PS-3 assay in the presence and absence of 100 μM ascorbic acid or NuPAGE antioxidant (1:500). Photon counts in each group were normalized with the respective “medium-only” readings.
Statistical Analysis
Data from three independent experiments were used for statistical analysis. Means of various treatment groups were compared using one-way ANOVA (Prism software, version 3.02, GrapPad Software, San Diego, CA, USA), and Tukey's post hoc test (for multiple comparisons) was carried out to determine if the differences between means were statistically significant. Student's t test was also used where appropriate; P < 0.05 was considered to be statistically significant. Data were expressed as the mean ± sd.
RESULTS
Standardization of the Acridan Lumigen PS-3 Assay
Volume of the media and the effect of freezing
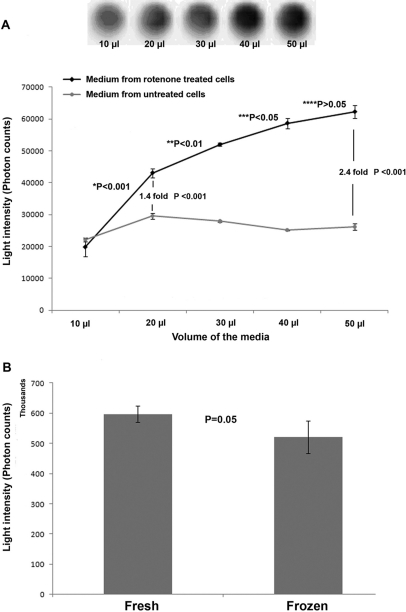
Microglia have been shown to be activated upon rotenone treatment, and activated microglia are known to produce ROS.7 We therefore used microglial cells (CHME-5) and rotenone treatment to standardize the Acridan Lumigen PS-3 assay. Media from untreated cells were used as controls. The media collected from controls showed a slight increase in photon counts between 10 and 20 μl of sample, however no further change in the levels observed with increasing volume of the media from untreated cells (Fig. 2A). The media collected from 1-week rotenone-treated CHME-5 cells showed a continuous increase in the chemiluminescence produced with an increasing volume (from 10 to 50 μl) of the culture medium (Fig. 2A). The assay detected chemiluminescence (20,000 photon units) in 10 μl medium, however the values observed in 10 μl medium from rotenone-treated and control cells were not different. The significant difference (1.46-fold, P<0.001) in the photon counts between the two groups was noticeable in as little as 20 μl media, and this difference kept escalating with increasing volume (Fig. 2A). The maximum difference in the photon counts between rotenone-treated and untreated media was observed at 50 μl (2.4-fold); this may, however, depend on the type of cell and the treatment protocol. We used 50 μl medium for checking all other parameters.
FIGURE 2.
Determination of appropriate media volume and the effect of −20°C storage on ROS-mediated chemiluminescence. (A) Graph showing the increase in the ROS-mediated chemiluminescence with increase in the volume of the media collected from CHME-5 cells treated with 5 nM rotenone for 1 week. The media collected from untreated control cells remained unchanged after a small increase in the photon counts between 10 and 20 μl. The light intensity (photons) was recorded using the BioTek Synergy 2 chemiluminescent plate reader. The upper panel shows the chemiluminescence recorded digitally using a Fujifilm luminescent image analyzer (LAS-3000, Version 2.2) in various volumes of media from rotenone-treated cells. Results indicated that the Acridan Lumigen PS-3 assay can detect the ROS released in the media from volumes as small as 10 and 20 μl media. Results shown are representative of three independent experiments, and each point represents the mean and sd. *P < 0.001: significance between 10 and 20 μl rotenone-treated samples; **P < 0.01: significance between 20 and 30 μl rotenone-treated samples; ***P < 0.05: significance between 30 and 40 μl rotenone-treated samples; ****P > 0.05: significance between 40 and 50 μl samples. (B) Histogram showing the effect of storage of media at −20°C on chemiluminescence in the Acridan Lumigen PS-3 assay. Media collected from rotenone-treated CHME-5 cells (1 week) were processed immediately (Fresh) or stored at −20°C (Frozen) and analyzed later by the Acridan Lumigen PS-3 assay. The results indicate that there was no significant difference in the photon counts (chemiluminescence) in the fresh or frozen media. Results shown are representative of three independent experiments, and each column represents the mean and sd. P = 0.05 indicates no significant difference between the two groups.
Media collected freshly or stored at −20°C from CHME-5 cells after 1-week treatment with 5 nM rotenone did not show significant difference in the ROS-mediated chemiluminescence when processed using the Acridan Lumigen PS-3 assay (Fig. 2B), although the values were slightly reduced in frozen media (Fig. 2B).
Specificity of the Acridan Lumigen PS-3 assay
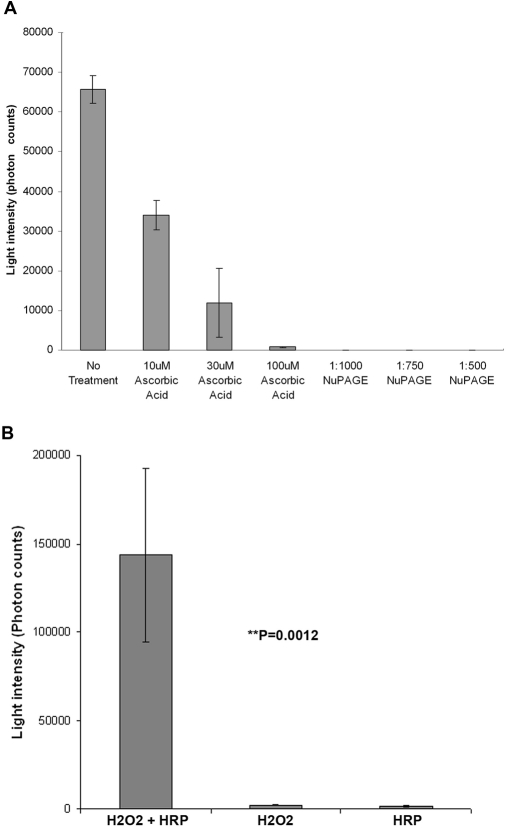
To test if the chemiluminescence generated in our Acridan Lumigen PS-3 assay was specifically a result of the presence of ROS and that the intensity of chemiluminescence was directly proportional to the amount of ROS present in the media, the Acridan Lumigen PS-3 assay was performed in the presence and absence of different concentrations of two well-known, strong antioxidants. Results showed a dramatic dose-dependent reduction in chemiluminescence upon incubation with different concentrations of ascorbic acid (10, 30, and 100 μM). Medium incubated with 100 μM ascorbic acid showed a 98.74% decrease in chemiluminescence (Fig. 3A). In addition, media incubated with different dilutions (1:500, 1:750, and 1:1000) of NuPAGE antioxidant showed complete inhibition of chemiluminescence at all dilutions (Fig. 3A).
FIGURE 3.
Specificity of acridan Lumigen PS-3 assay. (A) Histogram showing the dose-dependent decrease in the chemiluminescence in culture media collected from CHME-5 cells treated with 5 nM rotenone for 1 week in the presence and absence of ascorbic acid (10, 30, and 100 μM) and NuPAGE antioxidant, which contains sodium bisulfite (Invitrogen; 1:1000, 1:750, and 1:500 diluted). The results showed a 98% reduction in the chemiluminescence in the presence of 100 μM ascorbic acid and complete inhibition of the chemiluminescence in the presence of NuPAGE antioxidant. Results shown are representative of three independent experiments, and each column represents the mean and sd. (B) Histogram showing the dramatically high levels of photon counts in H2O2 (441 mM in PBS) samples incubated with HRP (0.3 unit/μl). The chemiluminescence observed in H2O2 and/or HRP alone was negligible. **P = 0.0012 indicates a highly significant difference in the values observed between samples of H2O2 + HRP and H2O2 or HRP only.
An important component in the Amersham ECL Plus kit (GE Healthcare) is Reagent A, which contains H2O2. As H2O2 is a nonradical ROS and mild oxidant, we determined whether H2O2 contributed to the overall chemiluminescence observed in the Acridan Lumigen PS-3 assay by incubating H2O2 with HRP enzyme prior to performing the Acridan Lumigen PS-3 assay. The reaction between HRP and H2O2 resulted in high photon counts (143,458.7 photons) compared with the almost-negligible counts when only H2O2 or HRP was processed in a similar way (Fig. 3B), indicating that H2O2, on its own, does not contribute to the chemiluminescence in the acridan Lumigen PS-3 assay.
Comparison between Acridan Lumigen PS-3 and NBT assays in measuring the ROS generated in the xanthine-XO in vitro reaction
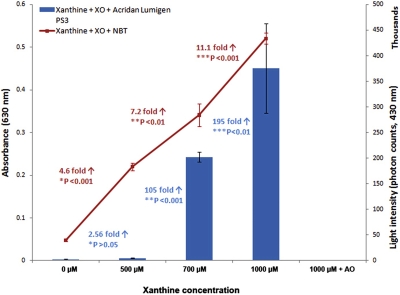
The NBT assay showed a linear increase in absorbance with increasing concentration of xanthine as a result of the superoxide-mediated generation of formazan. The absorbance increased 4.6-, 7.2-, and 11.1-fold when XO was mixed with 500, 700, and 1000 μM xanthine compared with 0 μM (Fig. 4). Similar to the NBT assay, the Acridan Lumigen PS-3 assay also showed an increase in photon counts with increasing concentration of xanthine in the reaction. Interestingly, the photon counts in the Acridan Lumigen PS-3 assay were only 2.56-fold between 0 and 500 μM; however, there was a dramatic increase of 105- and 195-fold in 700 μM and 1000 μM xanthine, respectively, when compared with 0 μM (Fig. 4).
FIGURE 4.
Measurement of ROS using the Acridan Lumigen PS-3 and NBT assays in the xanthine-XO in vitro reaction. Diagram representing comparison of levels of ROS measured by NBT (line graph presenting absorbance values, primary y-axis) and Acridan Lumigen PS-3 (bar graph presenting photon counts, secondary y-axis). Both assays showed an increase in ROS levels with increasing concentrations of xanthine. The NBT assay showed a linear increase of 4.6-, 7.2-, and 11.1-fold in absorbance in 500, 700, and 1000 μM xanthine, respectively, compared with 0 μM, whereas the Acridan Lumigen PS-3 assay showed a dramatic 105- and 195-fold increase in photon counts in 700 and 1000 μM xanthine, respectively, compared with 0 μM. Results shown are representative of three independent experiments, and each column represents the mean and sd. *P: significance between 0 and 500 μM; **P: significance between 0 and 700 μM; ***P: significance between 0 and 1000 μM.
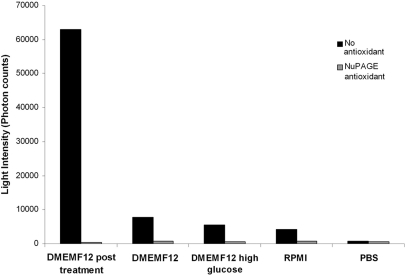
Effect of media and PBS buffer on the chemiluminescence generated in the Acridan Lumigen PS-3 assay
To assess the influence of different media and PBS on the overall chemiluminescence generated in the Acridan Lumigen PS-3 assay, DMEM-F12 medium collected from the rotenone-treated CHME-5 cells (5 nM, 1 week) and fresh, unused RPMI-1640, DMEM-F12, DMEM high-glucose media, and PBS were assessed (Fig. 5). All media used in the study were supplemented with 10% heat-inactivated FBS. Results showed that the chemiluminescence generated by DMEM-F12 was 12.4% of the chemiluminescence recorded in the medium collected from the rotenone-treated cells, DMEM high glucose generated 9.7% of chemiluminescence, RPMI generated only 6.5%, whereas the chemiluminescence generated by PBS was a negligible 1.6% of the chemiluminescence generated in the medium from rotenone-treated CHME-5 cells. The chemiluminescence in these media was inhibited by the antioxidant (1:500 NuPAGE). The results indicate that the unused/fresh media must be used as controls/blanks and final readings normalized or adjusted against the chemiluminescence intensity generated in the respective medium used. The negligible chemiluminescence produced by PBS indicates that the components of the Amersham ECL Plus kit (GE Healthcare) did not contribute significantly to the overall chemiluminescence detected.
FIGURE 5.
Effect of different media and PBS buffer on the generation of chemiluminescence in the Acridan Lumigen PS-3 assay. DMEM-F12 medium collected from the rotenone (5 nM for 1 week)-treated CHME-5 cells, fresh/unused DMEM-F12, DMEM high-glucose, and RPMI-1640 media, and PBS buffer were processed for the Acridan Lumigen PS-3 assay in the presence and absence of the NuPAGE antioxidant (1:500). The results indicated that PBS had a negligible effect on the chemiluminescence generated in the assay. The influence of DMEM-F12, DMEM high-glucose, and RPMI media was only 12.5%, 9.3%, and 6.5%, respectively, of the chemiluminescence observed in the media from rotenone-treated cells.
Dose-Dependent Generation of Chemiluminescence
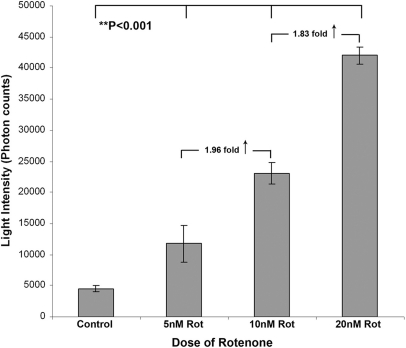
The Acridan Lumigen PS-3 assay was performed on media collected from the microglial (CHME-5) cells treated with 5, 10, and 20 nM rotenone for 48 h. The assay showed an increase in the levels of ROS released with increasing concentrations of rotenone (Fig. 6). Media from 10 nM rotenone-treated cells showed a significant 1.96-fold increase in ROS-mediated chemiluminescence into the medium compared with cells treated with 5 nM rotenone (P<0.001). Whereas the media from the cells treated with 20 nM rotenone showed a further 1.83-fold increase (P<0.001) in the chemiluminescence generated compared with the media collected from the cells treated with 10 nM rotenone. These results indicate a linear, dose-dependent relationship between the amount of ROS/chemiluminescence generated by CHME-5 cells in response to the dose of rotenone.
FIGURE 6.
Dose (rotenone)-dependent release of ROS in culture media by CHME-5 cells. Histogram showing a linear dose-dependent increase in the chemiluminescence (light intensity, photon units) in the culture media collected from the untreated controls and CHME-5 cells treated with 5, 10, and 20 nM rotenone for 48 h. Results shown are representative of three independent experiments, and each column represents the mean and sd. *P < 0.001 indicates significant difference between untreated control and three treatment groups.
Acute Response of Different Types of Cells to Various Stimulants
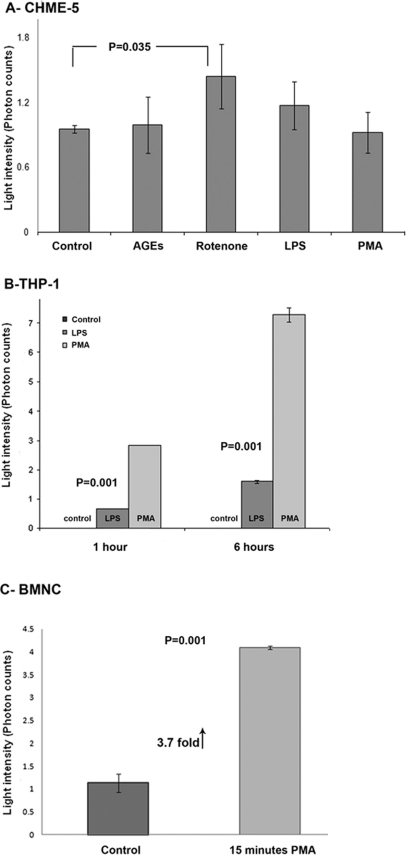
Three cell types of the macrophage/monocyte lineage (CHME-5, THP-1, and rat primary blood monocytes) were used to test the ability of the Acridan Lumigen PS-3 assay to measure the ROS released in the culture media after acute stimulation (Fig. 7). Microglial CHME-5 cells showed a maximum (P=0.035) amount of chemiluminescence when stimulated with 20 nM rotenone for 1 h, followed by the treatment with LPS. The treatment of CHME-5 cells with PMA and AGEs for 1 h did not result in any significant changes in ROS levels compared with untreated controls (Fig. 7A). THP-1 monocytic cells showed a dramatic increase in the photon counts compared with untreated controls in response to LPS and PMA. THP-1 response to produce ROS was greater in PMA-treated cells compared to LPS-treated at both 1- and 6-h time-points (4.2- and 4.6-fold, respectively; Fig. 7B). Rat primary blood monocytes showed a 3.7-fold increase in the ROS-mediated chemiluminescence in the culture media within 15 min of stimulation with PMA compared with untreated controls (Fig. 7C).
FIGURE 7.
Acute response of cells of the same lineage to various stimulants. (A) Histogram showing the amount of ROS-mediated photon counts in untreated CHME-5 cells and CHME-5 cells treated with AGEs (10 μM), rotenone (20 nM), LPS (100 EU/ml), or PMA (100 nM). Acute rotenone treatment for 1 h (at 37°C) induced a maximum increase in the photon counts, followed by LPS. AGEs and PMA did not show any change in the number of photon counts compared with untreated controls. (B) Histogram representing the amount of photon counts (light intensity) in media from THP-1 cells upon stimulation with LPS and PMA for 1 and 6 h. The response of THP-1 to generate ROS-mediated chemiluminescence was greater when activated with PMA compared with LPS at both time-points. Media collected from untreated cells were used as controls. (C) Histogram representing the amount of photon counts (light intensity) in media from rat primary blood monocytes upon stimulation with 100 nM PMA for 15 min. The ROS-mediated chemiluminescence was 3.7-fold higher in primary monocytes upon activation with PMA within 15 min. Results shown are representative of three independent experiments, and each column represents the mean and sd. P = 0.035 shows the significant difference between control and rotenone-treated cells in A, whereas P = 0.001 shows the significant difference between controls and treatment groups in B and C.
Acute and Chronic Response of Cells in Releasing ROS upon Low-Dose Rotenone Treatment
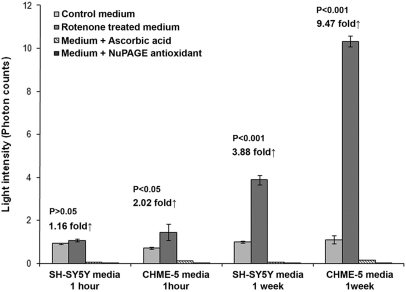
We tested the ability of two cell types of different lineages to release ROS in culture media upon acute and chronic rotenone (low dose) treatment. After 1-h treatment with 5 nM rotenone, SH-SY5Y showed a negligible 1.16-fold increase in the ROS levels released in the culture medium, whereas CHME-5 cells showed a twofold increase compared with controls (Fig. 8). After a 1-week chronic treatment, SH-SY5Y cells showed a 3.88-fold increase in the ROS levels, whereas CHME-5 cells showed a dramatic 9.47-fold increase compared with controls (Fig. 8), indicating that microglial CHME-5 cells have a greater tendency to release ROS into the extracellular environment, and this ability of CHME-5 cells is enhanced (2.66-fold) upon chronic treatment with rotenone. The chemiluminescence generated in the assay was ROS-specific, as indicated by the inhibition of the chemiluminescence in the presence of the two different antioxidants (Fig. 8).
FIGURE 8.
Acute and chronic response of cells to rotenone and ROS-mediated chemiluminescence in culture media. Histogram showing release of ROS from microglial CHME-5 and neuronal SH-SY5Y cells treated with 5 nM rotenone. Media collected at the end of 1 h and 1 week were processed for the Acridan Lumigen PS-3 assay in the presence and absence of 100 μM ascorbic acid or 1:500 NuPAGE antioxidant. Results showed that microglial cells had greater capacity to release ROS into the culture media compared with SH-SY5Y cells at both time-points. One-week chronic treatment enhanced the ROS-mediated chemiluminescence in the culture media in both cell types. The chemiluminescence was inhibited in the presence of antioxidants. Results shown are representative of three independent experiments, and each column represents the mean and sd.
DISCUSSION
Cell culture models are very important tools in understanding the basic physiological and biological processes and pathways involved in cell death. Culture media collected during experiments can be stored immediately following collection and analyzed for various metabolites such as cytokines, growth factors, and reactive molecules (ROS/RNS). There are several methods available to estimate intracellular ROS but very few estimate extracellular ROS in the culture medium. Therefore, this study aimed to develop and optimize a simple, reproducible, sensitive, and economical method to measure ROS in culture media.
Methods to measure extracellular ROS to date require the use of specialized equipment, or the assay must be carried out in the presence of live cells.6,21–23 In addition, there are now several sensitive chemiluminescent substrates commercially available that can be oxidized electrochemically or by certain enzymes.25,27 Chemiluminescence is defined as the emission of light when an electronically excited compound returns to the ground state after a chemical reaction.28,37 This property of chemiluminescence has been used in analytical chemistry to conduct sensitive assays without the need for expensive equipment. The most commonly used chemiluminescent compound in biological assays is Luminol, which is used as a substrate for the enzyme hydrogen peroxidase in immunoassays.22,23,26,37 ROS are unstable and have a short life, and Luminol detects global levels of ROS released from live cells, including spermatozoa. In the presence of H2O2, Luminol undergoes a deoxygenation reaction mediated by a heterogeneous group of peroxidases, present in cellular membranes. Luminol can measure a wide range of oxyradicals under physiological conditions28,37; however, the use of luminol to measure ROS in the culture media is not documented.
Other commonly used chemiluminescent probes are acridinium compounds. Acridinium esters are routinely used as a chemiluminescent substrate for the enzyme HRP to detect the targets of interests in Western blotting. One of the acridinium compounds, lucigenin, which is specifically oxidized by superoxide extracellularly, has been used successfully to measure the release of ROS in the presence of live cells.23,27,28,37 One major drawback of acridinium compounds, however, is that in conditions suitable for immunoassays, acridinium compounds have the tendency to react with nucleophiles and are converted to an inactive pseudobase upon reaction with hydroxide ions.23,27,37,38 This pseudobase has to be reconverted into an acridinium compound through the addition of acidic H2O2, followed by an alkaline solution to trigger light emission at 430 nm. Formation of a pseudobase also affects the intensity of the light emitted. Advances with acridinium compounds include synthesis of acridan esters. These esters are synthesized by reducing the corresponding acridinium compound with ammonium chloride and zinc.28 These reduced compounds do not react with nucleophiles, and hence, the probability of formation of a pseudobase is minimized.28 Several acridan esters have been developed as substrates for HRP. One such compound, Acridan Lumigen PS-3, is used as a chemiluminescent substrate in an Amersham ECL Plus kit (available from GE Healthcare). Some authors28,38 suggest that acridan compounds can be reconverted into corresponding acridinium esters in the presence of H2O2, which can then form acridanyl radicals in the presence of free radicals by electrochemical reactions. These acridanyl radicals spontaneously decarboxylate into N-methylacridone and CO2, which then emit light on reaching ground state (Fig. 1). We used this property of Acridan Lumigen PS-3 (Amersham ECL Plus kit, GE Healthcare) in this study to develop a simple and reliable assay to measure extracellular ROS present in culture media. We propose that the ROS, present in the media, are able to initiate oxidative (electrochemical) reactions and exchange electrons with Acridan Lumigen PS-3 in the presence of H2O2, resulting in the generation of the excited N-methylacridone and light. This assay can therefore only detect reactive species that are able to exchange electrons with the acridan molecule and therefore, cannot detect all of the forms of ROS/RNS (especially non-radical ROS/RNS).
Our results suggested that the Acridan Lumigen PS-3 can detect ROS in as little as 10–20 μl culture medium, which was stored at −20°C. We repeated the experiments with freshly collected media samples from rotenone-treated cells. The readings in frozen media were slightly lower than freshly collected media; however, the difference was not significant. The frozen media we used were stored at −20°C for a couple of months until used, and they were thawed once or twice. We did not study the specific effects of frequent freezing/thawing of media on the levels of ROS-mediated chemiluminescence. Our results suggest that media stored immediately in appropriate conditions after collection can be used later to estimate the levels of ROS. Frequent freeze/thaw of the media may, however, affect the levels of detectable ROS.
As rotenone is known to generate ROS by inhibiting mitochondrial complex I enzyme activity,16,39,40 we used rotenone in this study as an agent to induce oxidative stress. We found that the Acridan Lumigen PS-3 method was sensitive enough to detect time- and dose (of stimulant)-dependent changes in ROS, as well as the ability of various cell types in producing different levels of ROS. Although the increased levels of ROS could be a result of an increased number of cells with time, and we did not normalize the amount of ROS for cell numbers; the cells were consistently 90–95% confluent each time the medium was collected.
The generation of chemiluminescence can be influenced by factors present in the media, such as pH, dissolved oxygen, and other trace elements, which can react with acridans. We therefore confirmed that the chemiluminescence generated in the assay was specifically a result of the presence of ROS in the culture media by using various doses of two different antioxidants; ascorbic acid and the potent antioxidant sodium bisulfite NuPAGE antioxidant (Invitrogen).41–45 As H2O2, a mild, nonradical ROS, is the main component of Reagent A, we, therefore, checked if H2O2 contributed to the overall chemiluminescence generated in the assay. When we individually incubated H2O2 or the peroxidase (HRP) enzyme with the ALPS-3 substrate, the chemiluminescence observed was negligible. However, when H2O2 was incubated with HRP, it resulted in a dramatic increase in the number of photon counts within 5 min. This increase could be a result of the formation of highly reactive enzyme intermediate complex-I upon reaction of HRP with H2O2. This complex can carry out a single electron oxidation of various organic substrates (usually phenols and anilines), including chemiluminescent substrates, such as acridan and luminol.46–47 Given this reaction, we suggest that only reactive species that are able to exchange electrons with acridan compounds contribute to the chemiluminescence in the assay. These may include ROS/RNS radicals, such as peroxy radicals (ROO·, HOO·), ONOO−, NO·, oxygen radicals, and O2·−.
We also compared the ALPS–3 assay with the well-known NBT assay using an in vitro reaction between xanthine and XO.19,20,35,36 ALPS-3 and NBT assays showed an increase in chemiluminescence and absorbance with increasing concentration of xanthine, respectively. Interestingly, NBT showed a linear increase in the absorbance of 4.6-, 7.2-, and 11.1-fold in 500, 700, and 1000 μM xanthine, respectively, compared with 0 μM xanthine, whereas the increase in the chemiluminescence observed using ALPS-3 was dramatic (105- and 195-fold in 700 and 1000 μM compared with 0 μM). This is most likely because XO + xanthine reaction generates superoxide and H2O2 in a 1:3 molar ratio, and superoxide radicals can react with H2O2 to generate OH·.48 NBT reacts specifically with superoxide,19,20,35,36 whereas ALPS-3 can react with superoxide radicals and OH·, thus explaining the dramatic (several-fold) increase in chemiluminescence in the Acridan Lumigen PS-3 assay compared with the NBT assay. These data also suggest that superoxide radicals that are lost as a result of reaction with H2O2 cannot be measured by the NBT assay, whereas in the Acridan lumigen PS-3 assay, the OH· generated by reaction of superoxide with H2O2 can also be detected. As ALPS-3 can react with a wide range of ROS, this assay may be suitable for measuring global levels of ROS.
We also found that the influence of different media and PBS buffer used in the assay had an insignificant effect on the overall chemiluminescence. In particular, the negligible amount of chemiluminescence in the “PBS-only” sample also confirmed that none of the reagents in the Amersham ECL Plus kit interfered and/or contributed to the overall chemiluminescence generated in the assay. However, we do suggest that the fresh media should be used as blanks, and readings should be normalized against these blanks before making any comparisons between cell types or treatment groups. One interesting observation was that the chemiluminescence generated in the “medium-only” blanks was also inhibited completely by the antioxidants. This observation cannot be explained completely. One of the important points to note is that we stimulated our cells in the presence of 10% FBS (heat-inactivated serum). The percentage of serum in the culture media was selected based on the work of others19,49–52 and our previous work,7,14 where there is no interference of FBS in the detection of ROS. To support this, we also performed experiments to estimate ROS in media with 10% and 3% serum and did not find any difference in the levels of photon counts between these two groups (data not shown). In addition, depriving cells with serum (especially in chronic, long-term treatments) can activate pathways (including increased oxidative stress), which may not be associated with the pathways directly induced by the stimulants and/or mechanisms of interest.
Within the CNS, excessive amounts of cytokines and/or ROS can be released by microglia as a result of acute cellular interaction with a variety of stimuli, such as infection, trauma, or cell death and the pathway induced, and hence, the type of cytokine and/or ROS released is often stimuli-dependent.53 We tested the acute response of CHME-5 cells to four stimuli that are known to induce ROS production in a chronic environment. These were AGEs, rotenone, LPS, and PMA. In CHME-5 cells, we observed maximum (statistically significant) ROS-mediated chemiluminescence as an acute response to rotenone within 1 h, whereas AGEs and PMA did not activate microglia within the same time period. It was surprising that microglia did not respond to PMA, given that microglia belong to a macrophage lineage, and PMA has been shown to activate leukocytes and macrophages, resulting in the generation of free oxyradicals.54 Given the lack of response to PMA, we then tested activation of human monocytic THP-1 cells and primary rat blood monocytes. As expected, in the presence of PMA, THP-1 cells showed a dramatic increase in chemiluminescence after 1 and 6 h compared with untreated controls. Furthermore, the response of THP-1 cells to produce ROS was greater upon stimulation with PMA compared with LPS, irrespective of the time-point. This indicates that although CHME-5 and THP-1 belong to macrophage lineage, their acute response to various stimuli is different. As it is well-established that primary leukocytes respond quickly and acutely upon PMA stimulation, we treated rat primary blood monocytes (primary cells of a macrophage lineage) with PMA, and these cells showed a 3.7-fold increase in ROS-mediated chemiluminescence within 15 min of stimulation. By using this assay, we were also able to test for the ability of two cell types of different lineage to produce different levels of ROS in response to acute and chronic rotenone treatment. Although rotenone is a robust inhibitor of the mitochondrial complex I enzyme in various cell types, some cells are more vulnerable to rotenone-mediated oxidative stress than others.7,39,55 There is currently insufficient evidence in the literature to suggest that THP-1 cells and primary rat monocytes can be used for chronic treatment protocols33,34,56, therefore, these cells were not used for chronic comparison. In agreement with our previous study,7,14 CHME-5 cells released higher amounts of ROS into culture media compared with neuronal SH-SY5Y cells. The ability of CHME-5 cells to produce ROS was enhanced upon chronic treatment with rotenone. This suggests that there is a difference in the capacity of various cell types to produce and/or release ROS in response to the same stimulus. Although cells that produce and accumulate large amount of ROS within themselves can have detrimental effects on their own survival, the cells that are able to release a large amount of ROS extracellularly can cause damage to other cells, as is the case with microglial-mediated damage to neuronal cells.
Although the Acridan Lumigen PS-3 assay is a simple, economical, less labor-intensive, and quick assay to estimate ROS levels released in the culture media, there are some important considerations. This assay can only estimate reactive species (ROS/RNS) that are able to exchange electrons with acridan molecules. As ROS are very unstable molecules, extreme care should be taken to collect media on ice and process immediately or store appropriately at −20°C; freeze/thaw of media can affect the ROS level. This study also did not examine the specific type of reactive species (ROS/RNS), which can be detected by this method, and we believe this is an avenue for further investigation. Although we observed that media components and PBS buffer do not significantly influence the overall chemiluminescence generated in the assay, the contribution of each component of the various media has not been tested. There may be some unknown biological compounds in complex media, which may interfere and/or influence the chemiluminescence in the assay by reacting with H2O2 (present in Reagent A used in the assay) or with reactive species (ROS/RNS), thereby yielding a false increase or decrease in the chemiluminescence, respectively. This can be addressed, to some extent, by using appropriate controls.
Conclusion
We have developed a simple, reliable, and reproducible assay to measure the amount of reactive species (ROS/RNS) released by cells into the culture media, collected freshly or stored at −20°C, using reagents supplied in the Amersham ECL Plus kit (GE Healthcare). The assay is specific for reactive species present in the culture media and sensitive enough to detect reactive species in small volumes of culture media. The specific types of individual ROS and/or RNS, which can be measured by this assay, need to be investigated further. Although there is a scope to develop this method further, using the Acridan Lumigen PS-3 reagents (Amersham ECL Plus kit, GE Healthcare) makes this assay economical, as there is no need to buy additional reagents or equipment to perform this assay.
ACKNOWLEDGMENT
This work was supported by the Faculty of Medical and Health Science Research Development Fund (University of Auckland), The School of Medical Foundation Fund (University of Auckland), and Maurice and Phyllis Paykel Trust. We thank Professor Pierre Talbot (Laboratory of Neuroimmunovirology, INRS-Institute, Armand-Frappier, Canada) for the generous gift of the human microglial cell line (CHME-5) and Dr. Fiona Radcliff (Department of Molecular Medicine and Pathology, University of Auckland) for the gift of the THP-1 cell line.
REFERENCES
- 1. Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal 2005;7:1223–1233 [DOI] [PubMed] [Google Scholar]
- 2. Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 2001;18:685–716 [DOI] [PubMed] [Google Scholar]
- 3. Halliwell B, Gutteridge J. Free Radicals in Biology and Medicine. New York, NY, USA: Oxford University Press, 1999 [Google Scholar]
- 4. Hendry DG, Schuetzle D. Reactions of hydroperoxy radicals. Comparison of reactivity with organic peroxy radicals. J Org Chem 1976;41:3179–3182 [Google Scholar]
- 5. Choe E, Min DB. Chemistry and reactions of reactive oxygen species in foods. Crit Rev Food Sci Nutr 2006;46:1–22 [DOI] [PubMed] [Google Scholar]
- 6. Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells 2010;29:539–549 [DOI] [PubMed] [Google Scholar]
- 7. Shaikh SB, Nicholson LF. Effects of chronic low dose rotenone treatment on human microglial cells. Mol Neurodegener 2009; 4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheret C, Gervais A, Lelli A, et al. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci 2008;28:12039–12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318 [DOI] [PubMed] [Google Scholar]
- 10. Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 2004;173:3916–3924 [DOI] [PubMed] [Google Scholar]
- 11. Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci Lett 2003;341:87–90 [DOI] [PubMed] [Google Scholar]
- 12. McGeer EG, McGeer PL. The role of the immune system in neurodegenerative disorders. Mov Disord 1997;12:855–858 [DOI] [PubMed] [Google Scholar]
- 13. Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys 1998;353:312–321 [DOI] [PubMed] [Google Scholar]
- 14. Shaikh S, Nicholson LF. Advanced glycation end products induce in vitro cross-linking of α-synuclein and accelerate the process of intracellular inclusion body formation. J Neurosci Res 2008;86:2071–2082 [DOI] [PubMed] [Google Scholar]
- 15. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 1996;313:17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radad K, Rausch WD, Gille G. Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochem Int 2006;49:379–386 [DOI] [PubMed] [Google Scholar]
- 17. Shimoni Y, Hunt D, Chuang M, et al. Modulation of potassium currents by angiotensin and oxidative stress in cardiac cells from the diabetic rat. J Physiol 2005;567:177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Rad Biol Med 1999;27:612–616 [DOI] [PubMed] [Google Scholar]
- 19. Miller DS, Sen M. Potential role of WISP3 (CCN6) in regulating the accumulation of reactive oxygen species. Biochem Biophys Res Commun 2007;355:156–161 [DOI] [PubMed] [Google Scholar]
- 20. Nitti M, Furfaro AL, Traverso N, et al. PKC δ and NADPH oxidase in AGE-induced neuronal death. Neurosci Lett 2007;416:261–265 [DOI] [PubMed] [Google Scholar]
- 21. Gasbarrini A, Pasini P, Nardo B, et al. Chemiluminescent real time imaging of post-ischemic oxygen free radicals formation in livers isolated from young and old rats. Free Radic Biol Med 1998;24:211–216 [DOI] [PubMed] [Google Scholar]
- 22. Binder CJ, Weiher H, Exner M, Kerjaschki D. Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot process flattening and proteinuria: a model of steroid-resistant nephrosis sensitive to radical scavenger therapy. Am J Pathol 1999;154:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobayashi H, Gil-Guzman E, Mahran AM, et al. Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. J Androl 2001;22:568–574 [PubMed] [Google Scholar]
- 24. Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology 1996;48:835–850 [DOI] [PubMed] [Google Scholar]
- 25. Sekiya M, Umezawa K, Sato A, Citterio D, Suzuki K. A novel luciferin-based bright chemiluminescent probe for the detection of reactive oxygen species. Chem Commun (Camb) 2009;7:3047–3049 [DOI] [PubMed] [Google Scholar]
- 26. Hoang D, Pfefferkorn L. Luminol-Enhanced Assay for Superoxide Anion (O2·−). Palo Alto, CA, USA: Agilent Technologies, 2009, http://www.stratagene.com/Newsletter/vol11_1/p1-2htm [Google Scholar]
- 27. Dodeigne C, Thunus L, Lejeune R. Chemiluminescence as diagnostic tool. A review. Talanta 2000;51:415–439 [DOI] [PubMed] [Google Scholar]
- 28. Wilson R, Akhavan-Tafti H, DeSilva R, Schaap AP. Comparison between acridan ester, luminol, and ruthenium chelate electrochemiluminescence. Electroanalysis 2001;13:1083–1092 [Google Scholar]
- 29. Atanassov CL, Muller CD, Dumont S, et al. Effect of ammonia on endocytosis and cytokine production by immortalized human microglia and astroglia cells. Neurochem Int 1995;27:417–424 [DOI] [PubMed] [Google Scholar]
- 30. Peudenier S, Hery C, Montagnier L, Tardieu M. Human microglial cells: characterization in cerebral tissue and in primary culture, and study of their susceptibility to HIV-1 infection. Ann Neurol 1991;29:152–161 [DOI] [PubMed] [Google Scholar]
- 31. de Gannes FM, Merle M, Canioni P, Voisin P-J. Metabolic and cellular characterization of immortalized human microglial cells under heat stress. Neurochem Int 1998;33:61–73 [PubMed] [Google Scholar]
- 32. Xie HR, Hu LS, Li GY. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson's disease. Chin Med J (Engl) 2010;123:1086–1092 [PubMed] [Google Scholar]
- 33. Tsuchiya S, Yamabe M, Yamaguchi Y, et al. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 1980;26:171–176 [DOI] [PubMed] [Google Scholar]
- 34. Kim SJ. Leptin potentiates Prevotella intermedia lipopolysaccharide-induced production of TNF-α in monocyte-derived macrophages. J Periodontal Implant Sci 2010;40:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tunc O, Thompson J, Tremellen K. Development of the NBT assay as a marker of sperm oxidative stress. Int J Androl 2010;33:13–21 [DOI] [PubMed] [Google Scholar]
- 36. Weng M, Zhang M-H, Shen T. Electron transfer interaction between hypocrellin A and biological substrates and quantitative analysis of superoxide anion radicals. J Chem Soc, Perkin Trans 1997;2:2393–2398 [Google Scholar]
- 37. Wilson R, Akhavan-Tafti H, DeSilva R, Schaap AP. Electrochemiluminescence determination of 2′,6′-difluorophenyl 10-methylacridan-9-carboxylate. Anal Chem 2001;73:763–767 [DOI] [PubMed] [Google Scholar]
- 38. Wilson R, Akhavan-Tafti H, DeSilva R, Schaap AP. Electrochemiluminescence of 2,6-difluorophenyl 10-methyl-9,10-dihydroacridine-9-carboxylate. Chem Commun (Camb) 2000;2067–2068 [Google Scholar]
- 39. Cannon JR, Tapias V, Na HM, et al. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis 2009;34:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson's disease: genes, environment and mitochondria. Parkinsonism Relat Disord 2003;9:59–64 [DOI] [PubMed] [Google Scholar]
- 41. Geng H, Meng Z. Inhibition of superoxide dismutase, vitamin C and glutathione on chemiluminescence produced by luminol and the mixture of sulfite and bisulfite. Spectrochim Acta A Mol Biomol Spectrosc 2006;64:87–92 [DOI] [PubMed] [Google Scholar]
- 42. Shi Y, Zhan X, Ma L, Li L, Li C. Evaluation of antioxidants using oxidation reaction rate constants. Front Chem China 2007;2:140–145 [Google Scholar]
- 43. Akiyuki M, Naoko M, Yoshifumi I, et al. Acidic conditions enhance bactericidal effects of sodium bisulfite on Helicobacter pylori. Helicobacter 2005;10:132–135 [DOI] [PubMed] [Google Scholar]
- 44. Rocch M, Kearse C. Modulation of sodium bisulfite effects on the corneal endothelium by antioxidant. J Toxicol Cut Ocular Toxicol 1995;14:169–178 [Google Scholar]
- 45. Kropec A, Huebner J, Frank U, et al. In vitro activity of sodium bisulfite and heparin against Staphylococci: new strategies in the treatment of catheter-related infection. J Infect Dis 1993;168:235–237 [DOI] [PubMed] [Google Scholar]
- 46. Rodríguez-López JN, Lowe DJ, Hernández-Ruiz J, et al. Mechanism of reaction of hydrogen peroxide with horseradish peroxidase: identification of intermediates in the catalytic cycle. J Am Chem Soc 2001;123:11838–11847 [DOI] [PubMed] [Google Scholar]
- 47. Hernández-Ruiz J, Arnao MB, Hiner AN, García-Cánovas F, Acosta M. Catalase-like activity of horseradish peroxidase: relationship to enzyme inactivation by H2O2. Biochem J 2001;354:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kellogg E, III, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem 1975;250:8812–8817 [PubMed] [Google Scholar]
- 49. Shavali S, Combs C, Ebadi M. Reactive macrophages increase oxidative stress and α-synuclein nitration during death of dopaminergic neuronal cells in co-culture: relevance to Parkinson's disease. Neurochem Res 2006;31:85–94 [DOI] [PubMed] [Google Scholar]
- 50. Korystov YN, Shaposhnikova VV, Korystova AF, Emel'yanov MO. Detection of reactive oxygen species induced by radiation in cells using the dichlorofluorescein assay. Radiat Res 2007;168:226–232 [DOI] [PubMed] [Google Scholar]
- 51. Munch G, Gasic-Milenkovic J, Dukic-Stefanovic S, et al. Microglial activation induces cell death, inhibits neurite outgrowth and causes neurite retraction of differentiated neuroblastoma cells. Exp Brain Res 2003;150:1–8 [DOI] [PubMed] [Google Scholar]
- 52. Park JS, Woo MS, Kim DH, et al. Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J Pharmacol Exp Ther 2007;320:1237–1245 [DOI] [PubMed] [Google Scholar]
- 53. Hanisch UK. Microglia as a source and target of cytokines. Glia 2002;40:140–155 [DOI] [PubMed] [Google Scholar]
- 54. Johnson KJ, Ward PA. Acute and progressive lung injury after contact with phorbol myristate acetate. Am J Pathol 1982;107:29–35 [PMC free article] [PubMed] [Google Scholar]
- 55. Betarbet R, Sherer TB, MacKenzie G, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 2000;3:1301–1306 [DOI] [PubMed] [Google Scholar]
- 56. Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia 1991;47:22–31 [DOI] [PubMed] [Google Scholar]