Abstract
The plant hormone abscisic acid (ABA) has been suggested to play a role in fruit development, but supporting genetic evidence has been lacking. Here, we report that ABA promotes strawberry (Fragaria ananassa) fruit ripening. Using a newly established Tobacco rattle virus-induced gene silencing technique in strawberry fruit, the expression of a 9-cis-epoxycarotenoid dioxygenase gene (FaNCED1), which is key to ABA biosynthesis, was down-regulated, resulting in a significant decrease in ABA levels and uncolored fruits. Interestingly, a similar uncolored phenotype was observed in the transgenic RNA interference (RNAi) fruits, in which the expression of a putative ABA receptor gene encoding the magnesium chelatase H subunit (FaCHLH/ABAR) was down-regulated by virus-induced gene silencing. More importantly, the uncolored phenotype of the FaNCED1-down-regulated RNAi fruits could be rescued by exogenous ABA, but the ABA treatment could not reverse the uncolored phenotype of the FaCHLH/ABAR-down-regulated RNAi fruits. We observed that down-regulation of the FaCHLH/ABAR gene in the RNAi fruit altered both ABA levels and sugar content as well as a set of ABA- and/or sugar-responsive genes. Additionally, we showed that exogenous sugars, particularly sucrose, can significantly promote ripening while stimulating ABA accumulation. These data provide evidence that ABA is a signal molecule that promotes strawberry ripening and that the putative ABA receptor, FaCHLH/ABAR, is a positive regulator of ripening in response to ABA.
Fleshy fruits are classically defined as either climacteric or nonclimacteric, with the former being defined as those that show an increase in both respiration and ethylene production at the onset of ripening (Giovannoni, 2001). Much progress has been made toward understanding the molecular mechanisms of ethylene action in climacteric fruit ripening (Alexander and Grierson, 2002; Adams-Phillips et al., 2004); however, the identity of the signal or signals triggering ripening in nonclimacteric fruit remains largely unknown.
Previous reports suggested that the phytohormone abscisic acid (ABA) may be associated with the regulation of nonclimacteric fruit ripening (Coombe, 1992; Davies et al., 1997; Giovannoni, 2001; Rodrigo et al., 2003; Zhang et al., 2009a). In addition to its well-known roles in various aspects of plant growth and development and responses to environmental stresses (Leung and Giraudat, 1998; Finkelstein et al., 2002; Himmelbach et al., 2003; Hirayama and Shinozaki, 2007), ABA was shown to promote sugar metabolism and accumulation in fleshy fruits (Yamaki and Asakura, 1991; Kobashi et al., 1999; Richings et al., 2000; Pan et al., 2005). It was previously observed that 9-cis-epoxycarotenoid dioxygenase (NCED), a key enzyme involved in ABA biosynthesis (Qin and Zeevaart, 1999), is implicated in the ripening of several fruits, including avocado (Persea americana; Chernys and Zeevaart, 2007), orange (Citrus sinensis; Rodrigo et al., 2006), grape (Vitis vinifera), peach (Prunus persica; Zhang et al., 2009a), and tomato (Solanum lycopersicum; Zhang et al., 2009b). Strawberry (Fragaria ananassa), which is widely used as a research model to study nonclimacteric fruits, has also been associated with ABA as an inducer of ripening (Kano and Asahira, 1981; Manning, 1994; Perkins-Veazie, 1995; Jiang and Joyce, 2003). However, direct genetic/molecular evidence has not yet been reported.
Significant progress has recently been made in characterizing the molecular mechanisms of ABA signaling in the reference plant Arabidopsis (Arabidopsis thaliana), which can potentially shed light on ABA-associated signaling and regulatory processes in ripening fruit. For example, over the past decades, numerous ABA signaling components have been identified in Arabidopsis, including the following: G proteins and phospholipases C/D; various protein kinases such as receptor-like kinases, SNF1-related protein kinases (SnRKs), calcium-dependent protein kinases, calcineurin B-like protein kinases, and mitogen-activated protein kinases; protein phosphatases consisting of type-2C/A protein phosphatases (PP2C/A); and various classes of transcription factors, such as members of the MYB/MYC, B3, APETALA2, bZIP, and WRKY families (for review, see Nambara and Marion-Poll, 2005; Hirayama and Shinozaki, 2007; Verslues and Zhu, 2007; Wang and Zhang, 2008; Cutler et al., 2010). A plasma membrane ABA receptor, GTG1/GTG2 (Pandey et al., 2009), a class of cytosolic ABA receptors, PYR/PYL/RCAR (Ma et al., 2009; Park et al., 2009), and a plastid/chloroplast ABA receptor, magnesium chelatase H subunit, ABAR/CHLH (Shen et al., 2006; Wu et al., 2009; Shang et al., 2010), have also been identified, resulting in the suggestion that there are two ABA signaling pathways in Arabidopsis. One model is the PYR1-PP2C-SnRK2 core signaling network, in which ABA promotes the interaction of PYR1 and PP2C, resulting in PP2C inhibition and SnRK2 activation, which transduces ABA signals through the phosphorylation of downstream factors such as AREB/ABF (Fujii et al., 2009; Ma et al., 2009; Park et al., 2009. The other model is the ABAR-WRKY40-ABI5 signaling network, in which ABA stimulates the ABAR-WRKY interaction and relieves the ABI5 gene of inhibition by repressing WRKY40 expression (Shang et al., 2010).
We used these significant advances in ABA signal transduction research as a platform on which to assess the action and molecular mechanisms of ABA in strawberry fruit ripening. In this study, we established a new Tobacco rattle virus (TRV)-induced gene silencing (VIGS) technique, which provides an opportunity to regulate gene expression levels in strawberry fruit. This strategy was then used to alter the expression of both the FaNCED1 gene controlling ABA levels and a putative ABA receptor gene, FaCHLH/ABAR, which potentially modulates ABA signaling. We report that ABA plays a crucial role in the regulation of strawberry fruit ripening and that this is mediated through FaCHLH/ABAR.
RESULTS
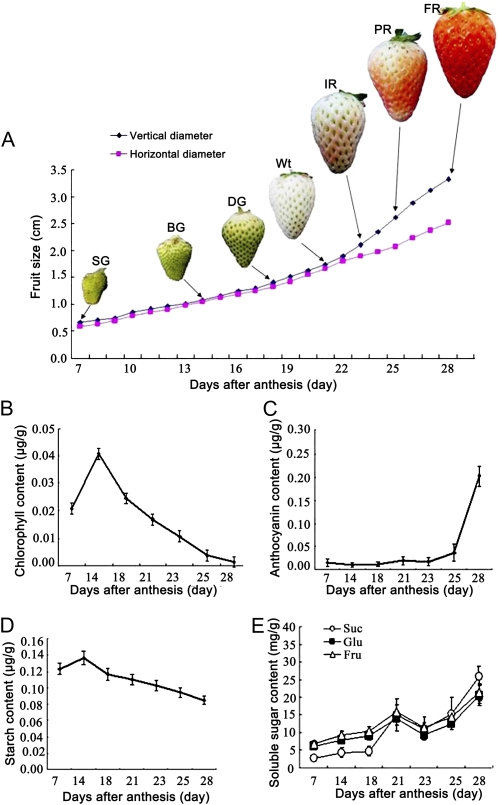
Morphological and Physiological Changes during Strawberry Fruit Development
We modified a previous report of a six-stage division of strawberry fruit development (Fait et al., 2008) and defined seven developmental visual stages of the strawberry cv Fugilia: small green (SG), big green (BG), degreening (DG), white (Wt), initial red (IR), partial red (PR), and full red (FR), at about 7, 14, 18, 21, 23, 25, and 28 d after anthesis, respectively (Fig. 1A). We observed that Fugilia strawberry fruit developed rapidly under our growth conditions (about 30 d from anthesis to ripeness), and distinct morphological and physiological changes, including fruit size and color, as well as levels of chlorophyll, anthocyanins, soluble sugars, and starch occurred in the receptacles (Fig. 1). Notably, the levels of chlorophyll and starch declined continually after the BG stage (Fig. 1, B and D), whereas the anthocyanin content increased rapidly after the Wt stage (Fig. 1C). Levels of soluble sugars (Suc, Glc, and Fru), particularly Suc, showed two phases of rapid increase concomitant with fruit degreening and red coloring (Fig. 1E).
Figure 1.
Morphological and physiological changes in the receptacle of strawberry fruit during developmental processes divided into the following seven stages: SG, BG, DG, Wt, IR, PR, and FR. A, Changes in fruit size and color. B, Changes in chlorophyll content. C, Changes in anthocyanin content. D, Changes in starch content. E, Changes in soluble sugar contents (Suc, Glc, and Fru). Error bars represent se (n = 3).
Establishment of a Posttranscriptional VIGS Technique in Strawberry Fruit
The difficulties in transgenic manipulation of fruit trees, including the requirement of a long incubation time from transformant screening to bearing fruit, represent a constraint to the development of fleshy fruit molecular biology. Therefore, developing an efficient system for gene manipulation is of particular significance in the study of fleshy fruits (Jia et al., 2010). A previous report demonstrated that TRV-mediated VIGS is a promising tool in the study of tomato fruit ripening (Fu et al., 2005). However, for our studies, it was not known whether TRV can infect strawberry fruit and can thus be used for functional genetic studies. We first tested this technique on strawberry fruit via the silencing of a chalcone synthase (CHS) gene, which is responsible for anthocyanin biosynthesis and has a clear phenotype in transgenic fruit (Hoffmann et al., 2006).
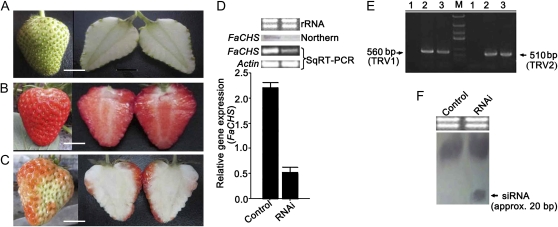
To establish VIGS in strawberry fruit, we first cloned a 630-bp cDNA fragment of the CHS homologous gene (from 31 to 660 bp; accession no. AY997297). The cDNA sequence (FaCHS) was then inserted into the SacI and XbaI restriction enzyme sites within the multiple cloning site of the TRV2 virus vector (Supplemental Fig. S1). To silence FaCHS in strawberry fruit, a mixture of Agrobacterium tumefaciens strain GV3101 cultures containing pTRV1 and pTRV2 or pTRV2 carrying a 630-bp fragment of the FaCHS gene (pTRV2-FaCHS) in a 1:1 ratio was syringe inoculated into 2-week-old BG fruit (Fig. 2A). Control fruits were inoculated with TRV alone (empty vector). Two weeks after injection, the surface of the control fruit turned a full red color (Fig. 2B), but the inoculated portion on the surface of the RNA interference (RNAi) fruit remained white, showing no red coloring (Fig. 2C).
Figure 2.
VIGS for the FaCHS reporter gene in strawberry fruit. A, Two-week-old BG fruits still attached to a plant were used for inoculation. B, The control fruit phenotype inoculated with Agrobacterium containing the TRV only. C, The RNAi fruit phenotype inoculated with Agrobacterium containing TRV carrying a FaCHS fragment. D, FaCHS transcription levels in the control and RNAi fruits by semiquantitative (Sq) RT-PCR, real-time PCR, and northern-blot analyses. E, Analysis of the transcripts of the 560-bp TRV1 and 510-bp TRV2 vectors by RT-PCR in fruits inoculated with Agrobacterium alone (lane 1), control fruits (lane 2), and RNAi fruits (lane 3). F, Detection of siRNA (approximately 20 bp) specific to the FaCHS gene in the control and RNAi fruits. rRNA was the loading control for the RNA samples stained with ethidium bromide. Actin mRNA was used as the internal control. Error bars represent se (n = 3). Bars = 0.4 cm.
We performed a series of analyses to confirm the suppression of FaCHS gene transcription at the molecular level, which resulted from RNAi by TRV-mediated posttranscriptional gene silencing. First, the FaCHS transcription levels were down-regulated in RNAi fruit, as evidenced by semiquantitative reverse transcription (RT)-PCR, real-time PCR, and northern-blot analysis (Fig. 2D). Second, FaCHS-related small interfering RNA (siRNA), which is a key component of RNA silencing, was detected in RNAi fruit but not in control fruit (Fig. 2F). Third, 560- and 510-bp amplification products specific to TRV-RNA1 and TRV-RNA2, respectively, were both detected in Agrobacterium-mediated TRV-inoculated fruits but not in the fruit inoculated with Agrobacterium alone (Fig. 2E). Finally, of the 20 TRV-inoculated RNAi fruits, two showed no obvious phenotype, and FaCHS transcription levels were not markedly down-regulated in these fruits (decreased only by 5%; Supplemental Fig. S2). These results demonstrated that TRV-mediated VIGS is a viable strategy to silence gene expression in strawberry fruit.
Down-Regulation of ABA Biosynthesis with an ABA Synthesis Inhibitor or FaNCED1-RNAi Inhibits Fruit Ripening, and the FaNCED1-RNAi-Induced Phenotype Is Rescued by Exogenous ABA
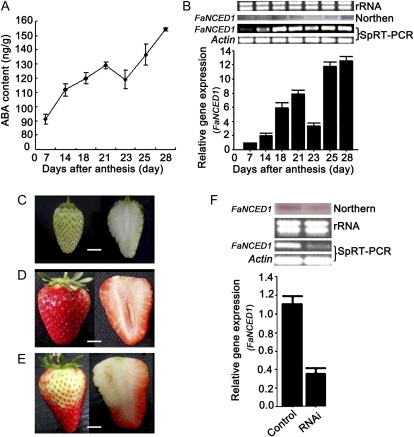
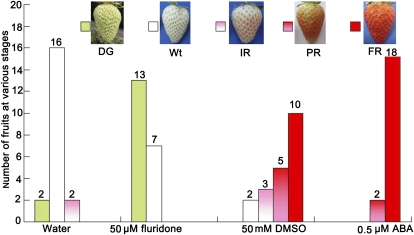
There are several reports suggesting that strawberry fruit ripening may be promoted by ABA (Kano and Asahira, 1981; Manning, 1994; Perkins-Veazie, 1995; Jiang and Joyce, 2003). To confirm this, we first quantified ABA levels in the receptacles of Fugilia strawberry fruit and detected an increase during development (Fig. 3A). In addition, we injected ABA (0.5 μm), the ABA biosynthesis inhibitor fluridone (50 μm), or the ABA accelerator dimethyl sulfoxide (DMSO [Bray and Zeevaart, 1986]; 50 mm) into separate 2-week-old BG fruit that were still attached to a plant. One week after injection, we found that ABA and DMSO significantly promoted fruit development, whereas fluridone markedly inhibited fruit development compared with controls injected with distilled water (Fig. 4). These data suggest that ABA accelerates strawberry fruit ripening.
Figure 3.
ABA positively regulates strawberry fruit ripening. A, Changes in ABA levels in the receptacles of the fruits during developmental stages. B, Semiquantitative (Sq) RT-PCR, real-time PCR, and northern-blot analyses of the FaNCED1 mRNA levels in the receptacles of fruits during developmental stages. C to F, VIGS for FaNCED1 in strawberry fruit: 2-week-old fruit (C) still attached to the plant were inoculated with Agrobacterium containing TRV alone (control) or TRV carrying an NCED1 fragment (RNAi); 2 weeks after inoculation, phenotypes were investigated for the control fruit (D) and RNAi fruit (E); semiquantitative RT-PCR, real-time PCR, and northern-blot analyses of the FaNCED1 transcripts in receptacles of the control or RNAi fruit (F) were conducted. rRNA indicates the loading control of the RNA samples stained with ethidium bromide. Actin was used as the internal control. Error bars represent se (n = 3). Bars = 0.4 cm.
Figure 4.
Effects of ABA, DMSO, and fluridone on strawberry fruit development. ABA at 0.5 μm, fluridone at 50 μm, or DMSO at 50 mm was injected into 2-week-old BG fruits on alternate days for 6 d (three times) using a 0.2-mL syringe. Control fruits were injected with distilled water, and phenotypes were investigated 1 week after treatment. Twenty fruits still attached to plants were selected for each treatment (n = 20).
To provide direct genetic evidence for the role of ABA in the regulation of strawberry fruit ripening, we cloned a strawberry homolog of the key gene for ABA biosynthesis in Arabidopsis, AtNCED1, which was named FaNCED1 (GenBank accession no. HQ290318). Transcription levels of FaNCED1 were congruent with ABA content, as evidenced by semiquantitative RT-PCR, real-time PCR, and northern-blot analyses (Fig. 3B). This suggests that ABA accumulation in developing fruit may be due to an increase in FaNCED1 expression and that FaNCED1 might be involved in the regulation of strawberry fruit development. To confirm this hypothesis by down-regulating the ABA biosynthesis pathway in fruit, we generated transgenic FaNCED1 RNAi fruits using TRV-mediated VIGS in 2-week-old fruits (Fig. 3C), as described above (Fig. 2; Supplemental Fig. S1). Two weeks after inoculation, the surfaces of control fruits turned fully red (Fig. 3D), whereas the inoculated portions on the surfaces of the RNAi fruits remained white (Fig. 3E). Semiquantitative RT-PCR, real-time PCR, and northern-blot analyses showed that the mRNA level of the FaNCED1 gene was reduced in RNAi fruits compared with that in control fruits (Fig. 3F).
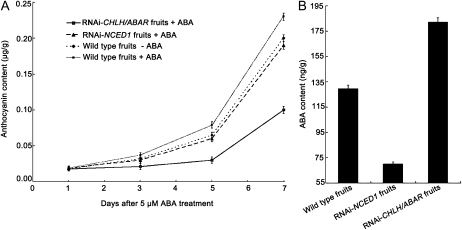
To further confirm the role of ABA in strawberry fruit ripening, we investigated the effects of exogenous ABA on anthocyanin content in FaNCED1-RNAi fruits. We found that anthocyanin levels in the ABA-treated FaNCED1-RNAi fruits were comparable to those in the wild-type control fruits and slightly lower than those in the ABA-treated wild-type control fruits (Fig. 5A), demonstrating that ABA could rescue the phenotype of the FaNCED1-RNAi fruits. Notably, prior to the treatment, the mean ABA contents in the wild-type control fruits and the transgenic portion (white sector) of the NCED1-RNAi fruits were 129.5 ± 3.0 and 69.9 ± 1.8 ng g–1, respectively (Fig. 5B), indicating that disruption of the FaNCED1 gene markedly inhibited ABA biosynthesis. These results demonstrated that the loss of red color in the FaNCED1-RNAi fruits resulted from a decreased ABA level. Taken together, this represents strong evidence that ABA is a central, positive regulator of strawberry fruit ripening.
Figure 5.
Effects of the ABA treatments on anthocyanin contents of the FaNCED1- and FaCHLH/ABAR-RNAi fruits during development. A, ABA at 0.5 μm was injected into FaNCED1- or FaCHLH/ABAR-RNAi fruits still attached to strawberry plants (n = 20). Control fruits were injected with distilled water. Anthocyanin content was detected after the first treatment. B, Prior to the treatments, ABA contents in the control fruits, FaNCED1-RNAi fruits, and FaCHLH/ABAR-RNAi fruits were assayed. Error bars represent se (n = 20).
Down-Regulation of FaCHLH/ABAR Expression Inhibits Strawberry Ripening, Which Cannot Be Rescued by Exogenous ABA
Given that ABA action is initiated by ABA perception that triggers downstream signaling cascades to induce physiological effects, we explored the molecular mechanism of the ABA regulation of strawberry fruit ripening. For this purpose, a 4,352-bp cDNA gene homologous to the Arabidopsis ABA receptor gene ABAR, namely the H subunit of magnesium chelatase CHLH (Shen et al., 2006; Wu et al., 2009; Shang et al., 2010), was isolated from strawberry and named FaCHLH/ABAR. This gene encodes a predicted 1,381-amino acid protein (GenBank accession no. GQ201451; Supplemental Fig. S3) with a high degree of similarity to homologs in a broad range of plants, with the closest orthologs being sequences from peach and castor bean (Ricinus communis; Supplemental Fig. S4). Genomic Southern-blot analysis of FaCHLH/ABAR indicated that the strawberry genome contains a single copy of the CHLH/ABAR-related gene (Supplemental Fig. S5D).
To explore whether the putative ABA receptor FaCHLH/ABAR is involved in ABA signaling during strawberry fruit development, we first analyzed the FaCHLH/ABAR expression pattern and showed that the gene is expressed ubiquitously in the strawberry plant (Supplemental Fig. S5, A–C), suggesting that it functions throughout the plant. The FaCHLH/ABAR transcript and protein levels in the developing receptacle suggest that FaCHLH/ABAR expression was higher in green fruit than in nongreen fruit (Supplemental Fig. S6) and increased further with the concomitant onset of red coloration (Supplemental Fig. S6), suggesting that FaCHLH/ABAR might be involved in regulating strawberry fruit ripening.
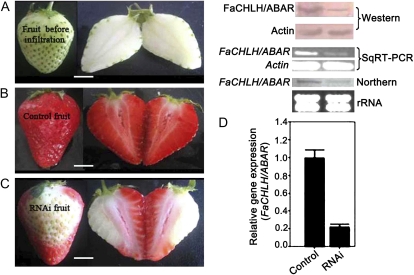
To assess the role of FaCHLH/ABAR in strawberry fruit ripening, we generated FaCHLH/ABAR RNAi fruits (Supplemental Fig. S1) using 2-week-old BG fruits (Fig. 6A). Two weeks after the inoculation, the surface of the wild-type control fruits turned fully red (Fig. 6B), whereas the inoculated sections on the surface of the RNAi fruits remained white (Fig. 6C). Semiquantitative RT-PCR, real-time PCR, and northern-blot and western-blot analyses were conducted to confirm FaCHLH/ABAR gene suppression at both the levels of transcript and protein abundance. This revealed that the expression of FaCHLH/ABAR was significantly down-regulated at both transcript and protein levels in the RNAi fruits compared with the wild-type control fruits (Fig. 6D).
Figure 6.
Down-regulation of FaCHLH/ABAR expression inhibits strawberry fruit ripening. A, Two-week-old fruits attached to strawberry plants were inoculated with Agrobacterium containing TRV alone (control fruit) or TRV carrying a FaCHLH fragment (RNAi fruit) 2 weeks after anthesis. B, Phenotypes of the control fruit 2 weeks after inoculation. C, Phenotypes of the FaCHLH/ABAR-RNAi fruit 2 weeks after inoculation. D, Semiquantitative (Sq) RT-PCR, real-time PCR (bottom columns), and northern-blot and western-blot analyses of the FaCHLH/ABAR transcription and translation levels in the receptacles of the control (left) and RNAi (right) fruit. rRNA was the loading control for the RNA samples stained with ethidium bromide. Both the Actin protein and Actin mRNA were used as internal controls. Error bars represent se (n = 3). Bars = 0.4 cm.
Furthermore, we assayed the effects of exogenous ABA on the anthocyanin content of the FaCHLH/ABAR-RNAi fruits and observed that the anthocyanin content in the ABA-treated FaCHLH/ABAR-RNAi fruits was significantly lower than that in either the ABA-free or the ABA-treated wild-type control fruits (Fig. 5A), suggesting that ABA does not rescue the FaCHLH/ABAR-RNAi fruit uncolored phenotype. Notably, prior to the treatment, the average ABA concentration in the wild-type control fruits or the transgenic portions (white sector) of the FaCHLH/ABAR-RNAi fruits was 129.5 ± 3.00 or 182.2 ± 3.6 ng g–1, respectively (Fig. 5B). The significant increase in ABA levels caused by down-regulation of FaCHLH/ABAR may be attributed to a feedback effect on the number of available ABA signal molecules when the ABA signaling pathway is repressed. These data showed that the FaCHLH/ABAR-RNAi-induced uncolored phenotype is not caused by alterations in the internal ABA levels in the transgenic RNAi fruits but rather by a partial disruption in ABA signaling. Taken together, this represents molecular/genetic evidence that FaCHLH/ABAR is an important component of the ABA signaling pathway in ripening strawberry fruit.
Sugar Promotes Fruit Ripening While Stimulating ABA Levels
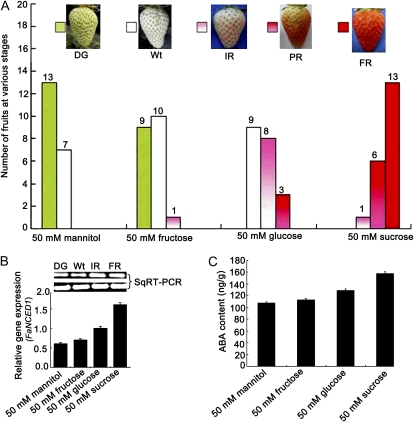
We investigated the effect of soluble sugars on strawberry fruit development. Suc, Glc, Fru, or mannitol (as a control sugar) was injected at a concentration of 50 mm into 2-week-old fruits attached to the plant. One week after injection, all Suc-injected fruits turned red, with most turning completely red (13 of 20); nearly half of the Glc-injected fruits turned red (11 of 20), and some turned initially red (eight of 20), whereas a minority turned only partially red (three of 20); almost half of the Fru-injected fruits turned white (10 of 20) or remained degreened (nine of 20), and only one turned initially red. In contrast, most of the mannitol-injected control fruits remained degreened (13 of 20), and only a small group turned white (seven of 20; Fig. 7A). These results showed that, of the tested sugars, Suc most significantly promoted ripening, followed by Glc, whereas Fru did not promote fruit ripening and was comparable to the mannitol control.
Figure 7.
Sugars promote strawberry fruit ripening while stimulating ABA accumulation. A, Effects of the sugar treatments on fruit ripening. Suc, Glc, Fru, or mannitol (as a control sugar) was injected into 2-week-old BG fruits. Phenotypes were investigated 1 week after treatment. Twenty fruits still attached to strawberry plants were used for each treatment. B, Semiquantitative (Sq) RT-PCR and real-time PCR (columns) analyses of FaNCED1 mRNA levels in the receptacles of mannitol-treated DG, Fru-treated Wt, Glc-treated IR, and Suc-treated FR fruits 1 week after the treatments. C, The corresponding ABA contents in the sugar-treated fruits from B. Error bars represent se (n = 3).
Next, we investigated whether Suc- and Glc-induced fruit-ripening acceleration was possibly associated with ABA accumulation in the fruits. The ABA content and FaNCED1 transcripts were detected in sugar-treated fruits (Fig. 7, B and C). The FaNCED1 mRNA transcript level was the highest in the Suc-treated fruits, followed by that in Glc-treated fruits, but it was not significantly changed in Fru-treated fruits compared with the mannitol-treated control fruits, as evidenced by real-time PCR and semiquantitative RT-PCR (Fig. 7B). Consistent with the FaNCED1 mRNA transcript levels, the average ABA contents in mannitol-treated DG, Fru-treated Wt, Glc-treated IR, and Suc-treated FR fruits were 107.3 ± 1.9, 119.1 ± 2.0, 129.0 ± 2.4, and 157.3 ± 3.2 ng g–1, respectively (Fig. 7C), displaying an increasing pattern with the highest ABA content in the Suc-treated fruits. These data support the idea that ABA positively regulates strawberry fruit ripening and suggest that sugars, especially Suc, may promote ripening partly by regulating ABA levels.
Down-Regulation of the FaCHLH/ABAR Gene Decreases Sugar Content and Alters the Expression of a Set of ABA/Sugar-Responsive Genes
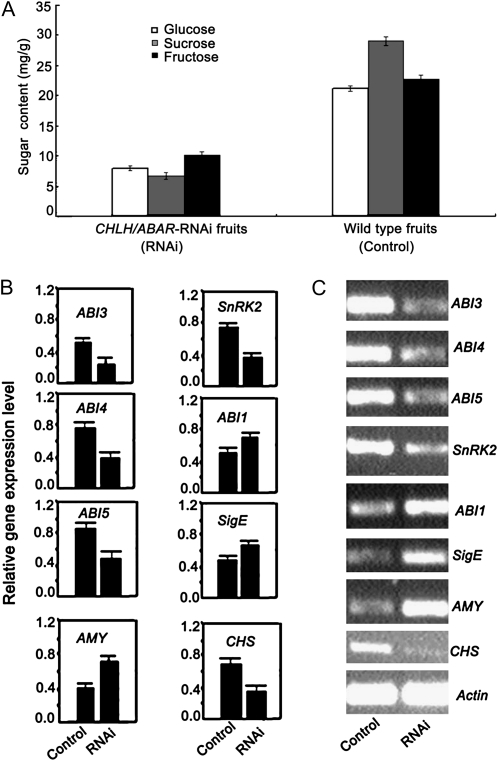
We investigated whether the down-regulation of the FaCHLH/ABAR gene affects sugar levels and observed that the concentrations of Suc, Glc, and Fru in the FaCHLH/ABAR-RNAi fruits significantly decreased compared with those in the wild-type control fruits (Fig. 8A). These data are consistent with FaCHLH/ABAR acting as a positive regulator of fruit ripening.
Figure 8.
Changes in both sugar contents and mRNA expression levels of ABA-responsive genes in the FaCHLH/ABAR-RNAi fruits. A, Sugar contents. B and C, Real-time PCR (B) and semiquantitative RT-PCR (C) analyses of the expression levels of ABA-responsive genes in the control fruit and RNAi fruit.
We tested the expression levels of ABA-responsive genes and sugar metabolism- and pigment biosynthesis-related genes, such as ABI1 (Gosti et al., 1999), ABI3 (Mönke et al., 2004), ABI4 (Finkelstein et al., 1998), ABI5 (Finkelstein and Lynch, 2000), SnRK2.2 (Fujii and Zhu, 2009; Zheng et al., 2010), SigE (Osanai et al., 2009), AMY (Hoecker et al., 1999), and CHS (Peters et al., 1986), in the transgenic fruit. Semiquantitative RT-PCR and real-time PCR analyses showed that the expression levels of these genes were significantly altered in the FaCHLH/ABAR-RNAi fruits compared with those in the control fruits. Notably, ABI1, SigE, and AMY were up-regulated, whereas most of the genes tested, including ABI3, ABI4, ABI5, SnRK2, and CHS, were down-regulated in the RNAi fruits (Fig. 8, B and C). Up-regulation of a negative ABA signaling regulator (ABI1) and down-regulation of the positive ABA signaling regulators (ABI3, ABI4, ABI5, and SnRK2) and sugar metabolism-/pigment biosynthesis-related genes (SigE, AMY, and CHS) are consistent with the positive role for FaCHLH/ABAR in ABA-mediated signaling in ripening strawberry fruit.
DISCUSSION
Establishment of the VIGS Technique in Strawberry Fruit
VIGS is a powerful tool to study gene functions in plants. When the virus infects plant tissues and spreads systemically throughout the organism, the endogenous gene transcripts specific to the insert fragment in the viral vector are degraded (Baulcombe, 1999). TRV, which disseminates more vigorously in meristem tissue than in other tissues (Ratcliff et al., 2001), has potential as a tool in tomato fruit ripening research (Fu et al., 2005). However, due to its restricted host range, TRV-based VIGS has only been studied in tobacco (Nicotiana tabacum; Ratcliff et al., 2001), tomato (Liu et al., 2002; Fu et al., 2005), pepper (Capsicum annuum; Chung et al., 2004), petunia (Petunia hybrida; Chen et al., 2004), and peach (Jia et al., 2010). In this study, we successfully adapted the TRV-based VIGS to a new horticultural plant species, strawberry fruit, providing a novel technical system for the study of this fleshy fruit.
A previous report showed that when an Agrobacterium strain carrying an RNAi construct specific to a CHS gene was injected into receptacles of growing fruits still attached to the plant, a white-red chimeric phenotype was observed on fruit surfaces (Hoffmann et al., 2006). In this study, a mixture of Agrobacterium strain GV3101 cultures that contain pTRV1 and pTRV2 carrying a 630-bp fragment of the FaCHS gene (pTRV2-FaCHS) in a 1:1 ratio was syringe infiltrated into 2-week-old DG fruits, resulting in RNAi fruits with chimeric symptoms that were similar to the results reported by Hoffmann et al. (2006). A TRV-infiltration efficiency of 90% with obvious phenotype was achieved, and the remaining 10% of fruit that did not show a phenotype, most likely resulting from nonsignificant alterations in the transcript abundance of the FaCHS gene (Supplemental Fig. S2). Similar TRV-infiltration efficiencies were also observed in the FaCHLH/ABAR and FaNCED1 RNAi fruits (data not shown).
Taken together, we provide multiple lines of evidence for the successful establishment of the TRV-based VIGS technique in strawberry fruit using the reporter gene FaCHS. First, TRV-inoculated fruit showed the same phenotype as that described in a previous report using a different technique with the same reporter gene in strawberry (Hoffmann et al., 2006). Second, the TRV vectors (TRV1 and TRV2) were detected in the TRV-inoculated fruits. Third, various RNAi phenotypes were detected that were consistent with corresponding decreases in the levels of the FaCHS gene transcripts. Finally, siRNA specific to the FaCHS gene was also detected in the TRV-inoculated fruits (Fig. 2).
ABA Is a Central, Positive Regulator of Strawberry Fruit Ripening
Strawberries are defined as nonclimacteric because they do not exhibit a peak in respiration and ethylene production during ripening, and the application of ethylene to green strawberries does not affect the rate of ripening (Knee et al., 1977; Given et al., 1988; Abeles and Takeda, 1990). In contrast, ABA levels gradually increase concomitant with a decline in indole-3-acetic acid during the late stage of strawberry fruit development. It has been hypothesized that the ABA-auxin ratio could be part of the signal that triggers fruit ripening (Archbold and Dennis, 1984; Perkins-Veazie, 1995). Auxin stimulates receptacle expansion during early fruit development and later inhibits fruit ripening (Given et al., 1988), whereas exogenous ABA markedly promotes fruit maturation (Kano and Asahira, 1981; Manning, 1994; Perkins-Veazie, 1995; Jiang and Joyce, 2003). This model was supported by studies of another nonclimacteric fruit, the grape berry (Coombe, 1992; Davies et al., 1997; Giovannoni, 2001; Rodrigo et al., 2003; Yu et al., 2006). Based on such data, previous reports suggested that ABA is a key regulator of nonclimacteric fruit ripening; however, supporting molecular evidence has been lacking to date.
In this study, using a combination of several pharmaceutical treatments and a molecular approach of gene silencing by a newly established VIGS system, we provide evidence that ABA plays a crucial role in the regulation of strawberry fruit ripening. We confirmed that exogenous ABA promoted strawberry ripening while repressing ABA levels by the administration of an ABA biosynthesis inhibitor that resulted in a delay in ripening (Figs. 3 and 4). Most importantly, genetic evidence showed that a reduction in endogenous ABA levels as a consequence of silencing the FaNCED1 gene, which encodes a protein that is important in ABA biosynthesis, inhibited ripening. Moreover, exogenous ABA rescued the uncolored phenotype of these FaNCED1-RNAi fruits. These data suggest that ABA indeed promotes the ripening of strawberry fruits (Fig. 3).
FaCHLH/ABAR Positively Regulates ABA Signaling in Strawberry Fruit Ripening
The identification of ABA signaling regulators is essential to understanding the molecular mechanisms underlying ABA-mediated ripening. We showed that down-regulation of a putative ABA receptor gene, FaCHLH/ABAR, which may potentially trigger early ABA signaling events, inhibits fruit ripening (Fig. 6). The FaCHLH/ABAR down-regulation-induced uncolored phenotype of the strawberry fruits was similar to the FaNCED1 down-regulation-induced uncolored phenotype (Figs. 3 and 6). However, whereas exogenous ABA rescued the uncolored phenotype of the FaNCED1-RNAi fruits, it did not do so for the FaCHLH/ABAR-RNAi fruits (Fig. 5). The ABA-insensitive phenotype of the FaCHLH/ABAR-RNAi fruits demonstrated that the ABA signaling process is reduced as a result of the FaCHLH/ABAR down-regulation, providing evidence for a substantial link between ABA signaling and the FaCHLH/ABAR protein. These data clearly indicate that fruit cell signaling associated with ripening in response to ABA is mediated by a FaCHLH/ABAR-regulated signaling pathway. This notion was further supported by expression analysis of a set of ABA-responsive genes (Fig. 8), which suggested that a negative ABA signaling regulator (ABI1) and several positive ABA signaling regulators (ABI3, ABI4, ABI5, and SnRK2) and the sugar-mediated/pigment biosynthesis-related genes (SigE, AMY, and CHS) may be involved in the FaCHLH/ABAR-mediated ABA signaling pathway in ripening fruit.
Promotion of Strawberry Fruit Ripening by Suc May Be Associated with ABA
In recent years, much progress has been made toward understanding the molecular mechanism of sugar partitioning and accumulation in the reference plant Arabidopsis. Sugars, such as Suc and Glc, may act as signal molecules and play pivotal roles in plant development and stress responses (León and Sheen, 2003; Price et al., 2003; Rook et al., 2006; Wu et al., 2007). The interaction of the Glc signal with ABA is responsible for the induction of senescence (Wingler and Roitsch, 2008), starch synthesis (Baguma et al., 2008), and pigment biosynthesis (Gollop et al., 2001, 2002; Loreti et al., 2008), and Glc modulates the transcription of genes involved in ABA biosynthesis and signaling during Arabidopsis germination (León and Sheen, 2003; Price et al., 2003).
In this study, we observed that the soluble sugars present at the early stages of fruit development were mainly Glc and Fru, but Suc was a relatively minor component. However, after the onset of fruit ripening, Suc accumulated rapidly in two phases, and the final amount of Suc was 1.25 times that of Glc and Fru (Fig. 1). Two obvious developmental events that occurred concomitant with the rapid increases in Suc content were related to fruit degreening and red coloring, suggesting that Suc might play a role in the regulation of fruit ripening. We asked whether, in addition to serving as an energy source and structural component, Suc could act as a signal in the fruit-ripening process and if this putative Suc signal could be involved in cross talk with the ABA signal. We showed that treatments with Glc and particularly Suc both increased FaNCED1 mRNA abundance and the levels of ABA (Fig. 7), suggesting that Suc may function as a signal to promote fruit ripening by stimulating ABA biosynthesis. In contrast, down-regulation of the FaCHLH/ABAR gene decreased the Suc, Glc, and Fru levels (Fig. 8), suggesting that repression of the FaCHLH/ABAR-mediated ABA signaling pathway may affect ABA-induced Suc accumulation. These data suggest that the two signaling pathways may cooperatively interact to regulate strawberry fruit ripening. Future studies aimed at identifying novel ABA signaling regulators involved in the FaCHLH/ABAR-mediated signaling pathway will help to elucidate the molecular mechanism of ABA signal transduction in nonclimacteric fruit.
MATERIALS AND METHODS
Plant Material
Octaploid strawberry (Fragaria ananassa ‘Fugilia’) fruit was used. Strawberry plants were grown in a greenhouse (20°C–25°C, relative humidity of 70%–85%, 14-h/10-h light/dark cycles) during spring seasons from 2000 to 2010. Three hundred flowers on 40 strawberry plants were tagged during anthesis. Seven stages of fruit (SG, BG, FG, Wt, IR, PR, and FR) were collected at 7, 14, 18, 21, 23, 25, and 28 d after anthesis, respectively. Twenty uniformly sized fruits were sampled at every stage (one replicate). After removing the achenes (seeds), the receptacle (pulp) was cut into 0.5- to 0.8-cm3 cubes and was quickly stored at –80°C after being snap frozen in liquid nitrogen.
RNA Isolation and RT-PCR Analyses
Total RNA was extracted from 10 g of fresh or treated strawberry fruit using an RNA extraction kit (SV Total RNA Isolation System; Promega). Genomic DNA was eliminated with a 15-min incubation at 37°C with RNase-Free DNase (TaKaRa Bio), followed by the use of an RNA Clean Purification Kit (BioTeke). The purity and integrity of the RNA were determined by agarose gel electrophoresis and the A260-A230 and A260-A280 ratios. To generate first-strand cDNA, 3 μg of total RNA was reverse transcribed using a Clontech kit (TaKaRa Bio) according to the manufacturer’s protocol. Random primers were used to reverse the first-strand cDNA of inoculated strawberry fruit to detect the TRV vector. The semiquantitative RT-PCR and real-time PCR primers for genes were designed as follows: CHLH (sense, 5′-TCCTCATGGAATGATGAGAAGC-3′; antisense, 5′-GTGGTGGTGTCAGCAATGTAAG-3′); AMY (sense, 5′-CAGGAGGACCACCTTTCG-3′; antisense, 5′-TACAACCAGGACAACCATCG-3′); ABI1 (sense, 5′-GTCGTGGCAAACAACCTG-3′; antisense, 5′-TTCCACTGTATGACCTTCCCT-3′); NCED1 (sense, 5′-ACGACTTCGCCATTACCG-3′; antisense, 5′-AGCATCGCTCGCATTCT3′); CHS (sense, 5′-GAGCAAACAACGAGAACACG-3′; antisense, 5′-GCTGTCAAGGCCATTAAGGA-3′); ABI3 (sense, 5′-CGGCGCCTGTATTAGTCCC-3′; antisense, 5′-TGCAGTCTCCAGCGTTTGAT-3′); ABI4 (sense, 5′-TCCTCATCACCACCGTCTT-3′; antisense, 5′-ACTCTGGCTCGTTTGCTCT-3′); ABI5 (sense, 5′-GGAGCTGGCAATGGTCG-3′; antisense, 5′-AGGCCCGCCTTTCCTT-3′); SnRK2 (sense, 5′-GCACTTCCGTCCAAGAGTG-3′; antisense, 5′-AGGATATGTAGTGCTGGTAGATT-3′); SigE (sense, 5′-TGAGAATGAGATGATGGAGGAG-3′; antisense, 5′-AGGAACTAAGAACAGGAGGAAT-3′); and Actin (sense, 5′-TGGGTTTGCTGGAGATGAT-3′; antisense, 5′-CAGTAGGAGAACTGGGTGC-3′).
RNA Blots
Digoxigenin (DIG)-labeled probes were synthesized using a PCR-DIG Probe Synthesis Kit (Roche Diagnostics) according to the manufacturer’s protocol. RNA (15 μg) was prepared as described above to determine the mRNA level. RNA blots were hybridized with a 572-bp FaCHLH/ABAR probe that was amplified using the primers 5′-CTGAACTAGATGAGCCACTTGA-3′ (sense) and 5′-GCACTCCACCCGACTGTATT-3′ (antisense; GenBank accession no. GQ201451) with a 646-bp FaNCED1 probe that was amplified using the primers 5′-ACCGAGACGTACCGTATGGTCC-3′ (sense) and 5′-AGAAGCAGTCGGGAGCCTCGACCC-3′ (antisense; GenBank accession no. HQ290318) or with a 534-bp FaCHS probe that was amplified using the primers 5′-GTCCGTCAAGCGTCTCAT-3′ (sense) and 5′-ATGTTTCCATACTCGGATAGG-3′ (antisense; GenBank accession no. AY997297).
Construction of the Viral Vector and Agroinoculation
The pTRV1 and pTRV2 VIGS vectors (described by Liu et al., 2002) were kindly provided by Dr. Yu-Le Liu. A 675-bp cDNA fragment of FaCHLH/ABAR was amplified using the primers 5′-ATCCACAATCTATACCCACCAC-3′ (sense) and 5′-GTGAGATTTCTGATGAGTCCAG-3′ (antisense). A 679-bp FaNCED1 cDNA fragment was amplified using the primers 5′-CTACGCCTGCCGGTTCACCGAGAC-3′ (sense) and 5′-GTTCCAGAGATGGAAGCAGAAGCA-3′ (antisense). A 630-bp FaCHS cDNA fragment was amplified using the primers 5′-GAGCAAACAACGAGAACACG-3′ (sense) and 5′-GCTGTCAAGGCCATTAAGGA-3′ (antisense). The three amplified fragments were inserted into the pMD19-T vector (TaKaRa Bio), digested with SacІ and XbaI, and then inserted into the SacІ-XbaI-cut site on the pTRV2 viral vector. The Agrobacterium tumefaciens strain GV3101 containing pTRV1, pTRV2, and the pTRV2 derivative pTRV2-FaCHLH/ABAR675 or pTRV2-FaNCED1679 was used for RNAi. Agroinoculation and syringe inoculation were performed as described by Fu et al. (2005).
Determination of Soluble Sugar and Anthocyanin Contents
Samples (25 g) were removed from –80°C storage and ground to a powder with liquid nitrogen. The powder (0.5 g) and 10 mL of 80% ethanol were mixed, incubated in a water bath for 3 min at 80°C, and then centrifuged at 10,000g for 10 min; the supernatant was collected in a 100-mL triangular flask. The residues were mixed with 10 mL of 80% ethanol, incubated in a water bath at 80°C for 20 min, and then centrifuged at 10,000g for 20 min. This entire process was repeated twice, after which the supernatants were combined. The remaining residues were washed and filtered with 1 mL of 80% ethanol; the filtrate was moved to a 10-mL test tube, and two drops of 5% α-naphthol were added and mixed. Concentrated sulfuric acid was added slowly along the tube wall until no purple ring appeared at the junction of two layers, indicating that the sugar was completely extracted from the sample. The supernatant was evaporated in boiling water and washed twice with 20 mL of distilled water; the volume was then increased to 50 mL, 2 mL of which was taken for LC-18 solid-phase extraction. Of this 2 mL, 1 mL was extracted and discarded, and then the remaining 1 mL was collected and passed through a 0.45-μm membrane to determine the soluble sugar content.
HPLC was then performed with the following components and parameters: an Agilent Technologies 1200 Series, 6.5- × 300-mm Sugar-Pak TM-1 column (Waters); Chemical Workstation version B.02.01-SR2; ultrapure water as a mobile phase, at a flow rate of 0.4 mL min–1; a column temperature of 60°C; a refractive index detector temperature of 50°C; and an injection volume of 20 μL. The standard samples used were d-(+)Glc, d-(–)Fru, and Suc (Sigma-Aldrich). The entire process was repeated three times. The recovery rates were 90.61% for Glc, 86.30% for Fru, and 97.61% for Suc. Anthocyanin measurements were performed as described by Fuleki and Francis (1968a, 1968b).
Treatment of Fruits with Sugars
Suc, Glc, or Fru (200 μL; a total of 50 mm of each sugar) was injected with a 0.5-mL syringe (n = 20) into 20 receptacles of 2-week-old fruits attached to plants. Mannitol (50 mm) was used as a control.
Determination of ABA Content
ABA content was determined by gas chromatography-mass spectroscopy. Fruit samples were removed from –80°C storage, and 1 g of fruit was weighed and mixed with an antioxidant copper reagent, quartz sand, a small amount of 80% cold methanol, and D3-ABA as an internal standard; the mixture was then ground to homogeneity at 4°C. The fruit mixture was mixed with 80% methanol and soaked overnight at 4°C. On the second day, the mixture was filtered, the filtrate was collected, and the residues were extracted with 80% methanol for 1 h and leached. The filtrate was combined with a drop of ammonia water and then was concentrated to an aqueous phase with a vacuum at 35°C. The filtrate was transferred to a 10-mL centrifuge tube in a freezer and freeze thawed three times. Finally, the tubes were placed in the refrigerator overnight to melt and were centrifuged the following day; the supernatant was collected by centrifugation after adding polyvinylpolypyrrolidone stirring paste to remove the pigment. The supernatant was adjusted to pH 2.5 to 3.0 and extracted three times with equal volumes of ethyl acetate. The organic phase was combined and frozen to remove water, and one drop of ammonia water was added to the concentrate to dry at 35°C. The extracts were separated on a small C18 column after dissolving with 0.1 mm acetic acid. The eluate was collected, two drops of ammonia water were added, and the mixture was dried at 40°C. Finally, diazomethane was added for esterification. The sample was dissolved with acetic acid ethyl ester, transferred to a capillary tube, and concentrated to determine the ABA content by gas chromatography-mass spectroscopy. This entire process was repeated three times.
Preparation of Crude Fruit Protein and Western Blotting
The seven-stage strawberry fruits, control fruits, or RNAi fruits were sampled and ground into powders after being snap frozen in liquid nitrogen. The samples were transferred to an Eppendorf tube containing ice-cold extraction buffer (1 mL g–1 sample) including 100 mm Tris-HCl (pH 7.5), 250 mm Suc, 5 mm EDTA, 3.5% polyvinylpolypyrrolidone, 10% glycerol, 5 mm vitamin C, 0.2% mercaptoethanol, and 1 × protease inhibitor cocktail (Roche). The samples were extracted for 3 h on ice. The extracts were centrifuged for 20 min at 16,000g, and the supernatant was transferred to a new tube and centrifuged again at 16,000g for 20 min. The supernatant concentration was detected by Coomassie Brilliant Blue G-250 (Amresco). The samples were either kept at 0°C for immediate use or stored at –80°C until needed.
Antibody against Arabidopsis (Arabidopsis thaliana) AtABAR/CHLH was described previously (Shen et al., 2006). FaCHLH/ABAR protein was detected by immunoblotting with the anti-AtABAR/CHLH serum according to our previously described procedures (Shen et al., 2006). Our preliminary experiments showed that the anti-AtABAR/CHLH serum recognized the FaCHLH/ABAR protein well because plant CHLH is a highly conserved protein (Supplemental Fig. S2).
siRNA Test
Total RNA was prepared as described above, fractionated on a polyacrylamide-urea gel, and blotted onto a nylon membrane (Nytran SPC; 0.45 μm). The membrane was then hybridized with a DIG-labeled FaCHS cDNA fragment as a probe (Roche) and visualized using the Hybridization Digoxigenin Detection Kit (Roche). All reagents and bottles were washed with diethylpyrocarbonate-treated water. The primers used to synthesize the DIG-labeled probe (482–1,016 bp) were 5′-GTCCGTCAAGCGTCTCAT-3′ (sense) and 5′-ATGTTTCCATACTCGGATAGG-3′ (antisense).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers CHS, AY997297; CHLH, GQ201451; NCED1, HQ290318; Actin, AB116565; ABI1, AY142623; ABI3, AK220879; ABI4, DQ446612; ABI5, BT026517; SigE, NM_122317; and SnRK2, NM_001203118.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. TRV VIGS vectors and construction.
Supplemental Figure S2. Correlation between various TRV-induced transcripts of the strawberry FaCHS gene and their corresponding fruit phenotypes.
Supplemental Figure S3. cDNA and deduced amino acid sequence of the putative strawberry ABA receptor gene FaCHLH/ABAR.
Supplemental Figure S4. A phylogenetic tree based on the putative strawberry ABA receptor protein FaCHLH/ABAR and its homologs from other plant species.
Supplemental Figure S5. Detection of both mRNA expression pattern and copy numbers of the putative strawberry ABA receptor gene FaCHLH/ABAR.
Supplemental Figure S6. Variations in the expression level of the putative strawberry ABA receptor FaCHLH/ABAR gene in fruit.
Acknowledgments
We thank Dr. Yu-Le Liu (Qinghua University) for the pTRV vectors.
References
- Abeles FB, Takeda F. (1990) Cellulase activity and ethylene in ripening strawberry and apple fruits. Sci Hortic (Amsterdam) 42: 269–275 [Google Scholar]
- Adams-Phillips L, Barry C, Giovannoni J. (2004) Signal transduction systems regulating fruit ripening. Trends Plant Sci 9: 331–338 [DOI] [PubMed] [Google Scholar]
- Alexander L, Grierson D. (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53: 2039–2055 [DOI] [PubMed] [Google Scholar]
- Archbold DD, Dennis FG. (1984) Quantification of free ABA and free and conjugated IAA in strawberry achene and receptacle tissue during fruit development. J Am Soc Hortic Sci 109: 330–335 [Google Scholar]
- Baguma Y, Sun C, Borén M, Olsson H, Rosenqvist S, Mutisya J, Rubaihayo PR, Jansson C. (2008) Sugar-mediated semidian oscillation of gene expression in the cassava storage root regulates starch synthesis. Plant Signal Behav 3: 439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe DC. (1999) Fast forward genetics based on virus-induced gene silencing. Curr Opin Plant Biol 2: 109–113 [DOI] [PubMed] [Google Scholar]
- Bray EA, Zeevaart JA. (1986) Compartmentation and equilibration of abscisic acid in isolated xanthium cells. Plant Physiol 80: 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Jiang CZ, Gookin TE, Hunter DA, Clark DG, Reid MS. (2004) Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol Biol 55: 521–530 [DOI] [PubMed] [Google Scholar]
- Chernys JT, Zeevaart JAD. (2007) Characterization of the 9-cis- epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol 124: 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Seong E, Kim YC, Chung EJ, Oh SK, Lee S, Park JM, Joung YH, Choi D. (2004) A method of high frequency virus-induced gene silencing in chili pepper (Capsicum annuum L. cv. Bukang). Mol Cells 17: 377–380 [PubMed] [Google Scholar]
- Coombe BG. (1992) Research on development and ripening of the grape berry. Am J Enol Vitic 43: 101–111 [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Davies C, Boss PK, Robinson SP. (1997) Treatment of grape berries, a non-climacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol 115: 1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fait A, Hanhineva K, Beleggia R, Dai N, Rogachev I, Nikiforova VJ, Fernie AR, Aharoni A. (2008) Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol 148: 730–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ. (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB. (2005) Virus-induced gene silencing in tomato fruit. Plant J 43: 299–308 [DOI] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Zhu JK. (2009) Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA 106: 8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuleki T, Francis FJ. (1968a) Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J Food Sci 33: 72–78 [Google Scholar]
- Fuleki T, Francis FJ. (1968b) Quantitative methods for anthocyanins. 2. Determination of total anthocyanin and degradation index for cranberry juice. J Food Sci 33: 78–82 [Google Scholar]
- Giovannoni J. (2001) Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 52: 725–749 [DOI] [PubMed] [Google Scholar]
- Given NK, Venis MA, Grierson D. (1988) Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta 174: 402–406 [DOI] [PubMed] [Google Scholar]
- Gollop R, Even S, Colova-Tsolova V, Perl A. (2002) Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. J Exp Bot 53: 1397–1409 [PubMed] [Google Scholar]
- Gollop R, Farhi S, Perl A. (2001) Regulation of the leucoanthocyanidin dioxygenase gene expression in Vitis vinifera. Plant Sci 161: 579–588 [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AAR, Vartanian N, Giraudat J. (1999) ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11: 1897–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach A, Yang Y, Grill E. (2003) Relay and control of abscisic acid signaling. Curr Opin Plant Biol 6: 470–479 [DOI] [PubMed] [Google Scholar]
- Hirayama T, Shinozaki K. (2007) Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci 12: 343–351 [DOI] [PubMed] [Google Scholar]
- Hoecker U, Vasil IK, McCarty DR. (1999) Signaling from the embryo conditions Vp1-mediated repression of alpha-amylase genes in the aleurone of developing maize seeds. Plant J 19: 371–377 [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Kalinowski G, Schwab W. (2006) RNAi-induced silencing of gene expression in strawberry fruit (Fragaria x ananassa) by agroinfiltration: a rapid assay for gene function analysis. Plant J 48: 818–826 [DOI] [PubMed] [Google Scholar]
- Jia HF, Guo JX, Qin L, Shen YY. (2010) Virus-induced PpCHLH gene silencing in peach leaves (Prunus persica). J Hortic Sci Biotechnol 85: 528–532 [Google Scholar]
- Jiang Y, Joyce DC. (2003) ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul 39: 171–174 [Google Scholar]
- Kano Y, Asahira T. (1981) Roles of cytokinin and abscisic acid in the maturing of strawberry fruits. J Jpn Soc Hortic Sci 50: 31–36 [Google Scholar]
- Knee M, Sargent JA, Osborne DJ. (1977) Cell wall metabolism in developing strawberry fruits. J Exp Bot 28: 377–396 [Google Scholar]
- Kobashi K, Gemma H, Iwahori S. (1999) Sugar accumulation in peach fruit as affected by abscisic acid treatment in relation to some sugar metabolizing enzymes. J Jpn Soc Hortic Sci 68: 465–470 [Google Scholar]
- León P, Sheen J. (2003) Sugar and hormone connections. Trends Plant Sci 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Leung J, Giraudat J. (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49: 199–222 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. (2008) Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol 179: 1004–1016 [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Manning K. (1994) Changes in gene expression during strawberry fruit ripening and their regulation by auxin. Planta 94: 62–68 [Google Scholar]
- Mönke G, Altschmied L, Tewes A, Reidt W, Mock HP, Bäumlein H, Conrad U. (2004) Seed-specific transcription factors ABI3 and FUS3: molecular interaction with DNA. Planta 219: 158–166 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Osanai T, Imashimizu M, Seki A, Sato S, Tabata S, Imamura S, Asayama M, Ikeuchi M, Tanaka K. (2009) ChlH, the H subunit of the Mg-chelatase, is an anti-sigma factor for SigE in Synechocystis sp. PCC 6803. Proc Natl Acad Sci USA 106: 6860–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan QH, Li MJ, Peng CC, Zhang N, Zou X, Zou KQ, Wang XL, Yu XC, Wang XF, Zhang DP. (2005) Abscisic acid activates acid invertases in developing grape berry. Physiol Plant 125: 157–170 [Google Scholar]
- Pandey S, Nelson DC, Assmann SM. (2009) Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell 136: 136–148 [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins-Veazie P. (1995) Growth and ripening of strawberry fruit. Hortic Rev (Am Soc Hortic Sci) 17: 267–297 [Google Scholar]
- Peters NK, Frost JW, Long SR. (1986) A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233: 977–980 [DOI] [PubMed] [Google Scholar]
- Price J, Li TC, Kang SG, Na JK, Jang JC. (2003) Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol 132: 1424–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Zeevaart JAD. (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA 96: 15354–15361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC. (2001) Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25: 237–245 [DOI] [PubMed] [Google Scholar]
- Richings EW, Cripps RF, Cowan AK. (2000) Factors affecting ‘Hass’ avocado fruit size: carbohydrate, abscisic acid and soprenoid metabolism in normal and phenotypically small fruit. Physiol Plant 109: 81–89 [Google Scholar]
- Rodrigo MJ, Alquezar B, Zacarías L. (2006) Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J Exp Bot 57: 633–643 [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Marcos JF, Alférez F, Mallent MD, Zacarías L. (2003) Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J Exp Bot 54: 727–738 [DOI] [PubMed] [Google Scholar]
- Rook F, Hadingham SA, Li Y, Bevan MW. (2006) Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ 29: 426–434 [DOI] [PubMed] [Google Scholar]
- Shang Y, Yan L, Liu ZQ, Cao Z, Mei C, Xin Q, Wu FQ, Wang XF, Du SY, Jiang T, et al. (2010) The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 22: 1909–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YY, Wang XF, Wu FQ, Du SY, Cao Z, Shang Y, Wang XL, Peng CC, Yu XC, Zhu SY, et al. (2006) The Mg-chelatase H subunit is an abscisic acid receptor. Nature 443: 823–826 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Zhu JK. (2007) New developments in abscisic acid perception and metabolism. Curr Opin Plant Biol 10: 447–452 [DOI] [PubMed] [Google Scholar]
- Wang XF, Zhang DP. (2008) Abscisic acid receptors: multiple signal-perception sites. Ann Bot (Lond) 101: 311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Roitsch T. (2008) Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol (Stuttg) (Suppl 1) 10: 50–62 [DOI] [PubMed] [Google Scholar]
- Wu FQ, Xin Q, Cao Z, Liu ZQ, Du SY, Mei C, Zhao CX, Wang XF, Shang Y, Jiang T, et al. (2009) The magnesium-chelatase H subunit binds abscisic acid and functions in abscisic acid signaling: new evidence in Arabidopsis. Plant Physiol 150: 1940–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Mo X, Wu P. (2007) AtCYT-INV1 in Arabidopsis sugar signaling. Plant Signal Behav 2: 496–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki S, Asakura T. (1991) Stimulation of the uptake of sorbitol into vacuoles from apple fruit flesh by abscisic acid and into protoplasts by indoleacetic acid. Plant Cell Physiol 32: 315–318 [Google Scholar]
- Yu XC, Li MJ, Gao GF, Feng HZ, Geng XQ, Peng CC, Zhu SY, Wang XJ, Shen YY, Zhang DP. (2006) Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiol 140: 558–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Leng P, Zhang G, Li X. (2009a) Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol 166: 1241–1252 [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan B, Leng P. (2009b) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60: 1579–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B, Wang L, Ni W, Sopko MS, Yao C, et al. (2010) The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol 153: 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]