Abstract
The adaptation of mRNA stability to environmental changes is a means of cells to adjust the level of gene expression. The Escherichia coli ompA mRNA has served as one of the paradigms for regulated mRNA decay in prokaryotes. The stability of the transcript is known to be correlated inversely with the bacterial growth rate. Thus, the regulation of ompA mRNA stability meets the physiological needs to adjust the level of ompA expression to the rate of cell division. Recently, host factor I (Hfq/HF1) was shown to be involved in the regulation of ompA mRNA stability under slow growth conditions. Here, we present the first direct demonstration that 30S ribosomes bound to the ompA 5′-UTR protect the transcript from RNase E cleavage in vitro. However, the 30S protection was found to be abrogated in the presence of Hfq. Toeprinting and in vitro translation assays revealed that translation of ompA is repressed in the presence of Hfq. These in vitro studies are corroborated by in vivo expression studies demonstrating that the reduced synthesis rate of OmpA effected by Hfq results in functional inactivation of the ompA mRNA. The data are discussed in terms of a model wherein Hfq regulates the stability of ompA mRNA by competing with 30S ribosomes for binding to the ompA 5′-UTR.
Keywords: Hfq, mRNA stability, ompA, translation initiation
Several lines of evidence suggest that the rate of formation of the ternary complex between mRNA, the 30S ribosomal subunit and the initiator tRNA affects the stability of different mRNAs (Baumeister et al. 1991; Wagner et al. 1994; Arnold et al. 1998). Accordingly, antibiotics that affect translation initiation (Flache et al. 1992) have destabilizing effects on mRNA. Moreover, inhibition or reduction of translation brought about by translational repressors, mutations in the ribosome binding site (RBS) or by premature translation termination, have been shown to reduce the stability of different mRNAs (Cole and Nomura 1986; Nilsson et al. 1987; Cho and Yanofsky 1988; Yarchuk et al. 1991, 1992; Jain and Kleckner 1993; Rapaport and Mackie 1994; Comer et al. 1996). Desynchronization of transcription and translation can also result in mRNA instability by unmasking RNase E cleavage sites normally shielded by elongating ribosomes (Iost and Dreyfus 1995; Makarova et al. 1995).
The decay of the long-lived ompA mRNA encoding the outer membrane protein A of Escherichia coli has been examined extensively, and previous studies have revealed that endonuclease cleavages in the 5′-untranslated region (UTR) provide the initial steps for its degradation in slow growing cells (Fig.1; Melefors and von Gabain 1988; Nilsson et al. 1988; Georgellis et al. 1992). The initial cleavage in the 5′-UTR of the ompA transcript during slow growth has been attributed to RNase E (Lundberg et al. 1990), which is part of the degradosome (Carpousis et al. 1994; Py et al. 1994; Miczak et al. 1996). Recently, host factor I (Hfq) was shown to bind to the ompA 5′-UTR. Hfq binding to ompA mRNA was found to be increased at a reduced growth rate when the half-life of the mRNA is decreased. Vice versa, in hfq strains the half-life of ompA mRNA was found to be increased and the growth rate-dependent regulation of ompA stability was abolished (Vytvytska et al. 1998).
Initially, Hfq has been identified as a bacterial host factor required for replication of phage Qβ RNA (Fernandez et al. 1968; Su et al. 1997). More recent studies revealed that Hfq mediates the access of Qβ replicase to phage plus strand RNA presumably by melting out its 3′ end (Miranda et al. 1997; Schuppli et al. 1997). In addition, Hfq has been reported to act as a positive regulator of the rpoS gene encoding the ςS subunit of RNA polymerase (Muffler et al. 1996; Brown and Elliott 1997). It has been suggested that Hfq activates translation of the rpoS mRNA by altering the secondary structure sequestering its RBS (Muffler et al. 1996; Brown and Elliott 1996; Cunning et al. 1998). In contrast, but similar to its apparent function on ompA mRNA, Hfq has been shown to affect the stability of mutS, miaA, and hfq mRNA, respectively (Tsui et al. 1997).
Recent work by Arnold et al. (1998) suggested that ribosome binding at the ompA RBS rather than the translational initiation frequency stabilizes the mRNA. Here, we demonstrate for the first time that 30S ribosomes protect the ompA 5′-UTR from endonucleolytic cleavage by RNase E in vitro. We further show that Hfq regulates ompA mRNA stability by interfering with ribosome binding. Thus, the Hfq-mediated ompA mRNA decay (Vytvytska et al. 1998) results from a lack of translation.
Results
30S ribosomal subunits protect the ompA mRNA from RNase E cleavage
Recently, we have shown that the stability of ompA mRNA is inversely correlated with the intracellular concentration of Hfq. The stability of the mRNA was found to be increased and decreased at low and high levels of Hfq, respectively (Vytvytska et al. 1998). However, in vitro degradation assays with 32P-5′-end-labeled ompA180 mRNA (Fig. 1) performed with an E. coli degradosome preparation (Miczak et al. 1996) did not reveal a stimulating effect of Hfq on RNase E cleavage at sites C and D (Fig. 1) in the ompA 5′-UTR (data not shown). On the basis of genetic experiments, Arnold et al. (1998) have proposed recently that ribosomes bound at the ompA RBS stabilize the full-length transcript. Because Hfq per se did not stimulate RNase E cleavage in the 5′-UTR, it was feasible that it interferes with ribosome binding, and thereby effects destabilization of the transcript. To obtain direct biochemical evidence for the stabilizing function of the ribosome, we first tested in vitro whether the RNase E induced cleavage in the ompA 5′-UTR is prevented in the presence of 30S subunits. The experiment shown in Figure 2A, lanes 3–5, demonstrated that a ternary complex between tRNAfMet, 30S subunits and ompA180 mRNA inhibits RNase E cleavage. When compared to the reaction in which ribosomes were omitted (Fig. 2A, lane 2), at a 5:1 molar ratio of 30S subunits to mRNA cleavage at the major in vivo cleavage site D (Melefors and von Gabain 1988; Fig. 1) was ∼75% inhibited (Fig. 2A, lane 3). A molar excess of 25:1 of 30S subunits to mRNA resulted in an almost complete inhibition of cleavage at site D (Fig. 2A, lane 4), and a 50:1 molar ratio of ribosomes to mRNA also protected from cleavage at site C (Fig. 2A, lane 5).
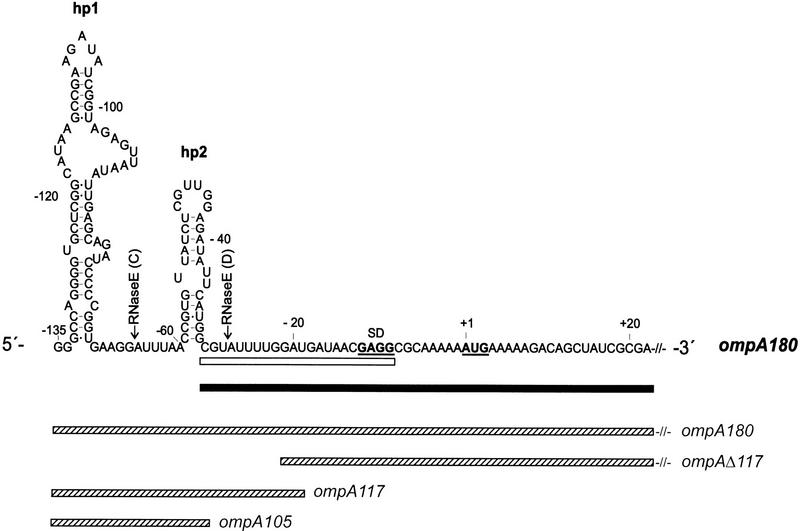
Figure 1.
Primary structure of the ompA 5′-UTR. The Shine and Dalgarno sequence as well as the start codon are in bold and underlined. The positions of the RNase E cleavage sites C and D are indicated. ompA180 mRNA (hatched bar; nucleotide −135 to +45; nucleotides +23 to +44 are not shown) comprising the 5′-UTR of ompA with the terminal stem–loop structures (hp1 and hp2) and the 5′–initial coding region was used in the RNase E degradation assays (see Fig. 2) and in the gel mobility-shift assays (see Fig. 3). The ompA117, ompA105, and ompAΔ117(nucleotides 23 to +111 of ompAΔ117 mRNA are not shown) mRNAs used in the gel mobility-shift assays (see Fig. 3) are depicted by hatched bars. The positions of oligonucleotides used in the mobility-shift assay (open bar; see Fig. 3) and for the determination of the ompA mRNA half-life (solid bar; see Fig. 6A, B) are also shown.
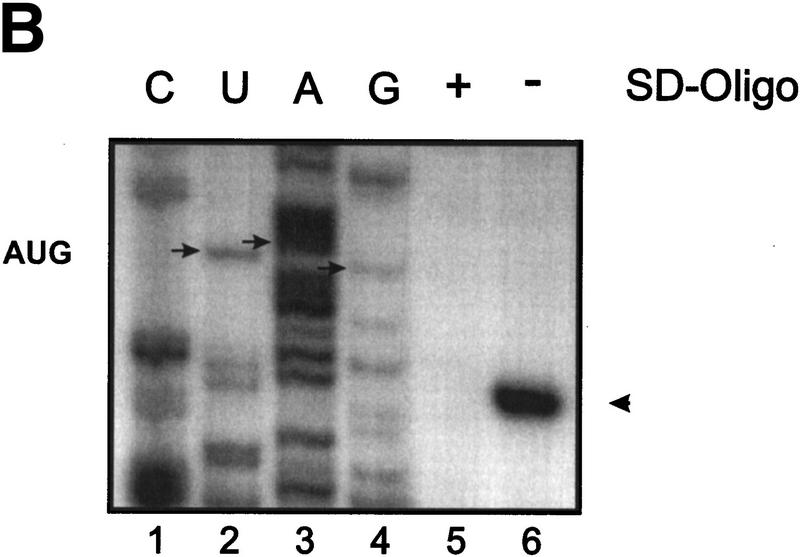
Figure 2.


30S ribosomal subunits protect from RNase E cleavage in the ompA 5′-UTR. (A) In vitro cleavage of ompA180 in the presence and absence of ribosomes. 32P-5′-end-labeled ompA180 mRNA (see Fig. 1) was incubated in RNase E cleavage buffer for 1 hr at 30°C without (lane 1) or with an equimolar concentration of a degradosome preparation (lane 2). Cleavage at sites D and C (see Fig. 1) by the degradosome preparation is indicated at the right. (Lanes 3–5), 30S ribosomes were added before the addition of the degradosome preparation in a 5:1, 25:1, and 50:1 molar ratio to mRNA, respectively. Initiator tRNAfMet was included in the reactions in a 5:1 molar ratio with ribosomes. (Lane 6) Hfq was added in fivefold molar excess over mRNA before the addition of 30S subunits (30S: mRNA ratio was 5:1; tRNAfMet: 30S ratio was 5:1) followed by the addition of the degradosome preparation. (B) Inhibition of 30S binding to the ompA RBS by a Shine and Dalgarno RNA oligonucleotide. (Lanes 1–4) Sequencing reactions. The position of the ompA start codon is indicated by small arrows. The toeprint signal at position +15 relative to the A (lane 6) of the start codon of ompA mRNA obtained with 30S ribosomes is marked by anarrowhead.(Lane 5) “blocked ribosomes” do not bind to the ompA RBS, which results in a lack of a toeprint signal. In all reactions, the molar ratio of mRNA-to-30S subunits was 1:100, and the tRNAfMet was in fivefold molar excess over ribosomes. (C) RNase E cleavage in the 5′ ompA UTR in the presence of blocked ribosomes. (Lane 1) ompA180 mRNA; (lane 2) 5′ end-labeled ompA180 mRNA (see Fig. 1) was cleaved with RNase E; (lane 3) RNase E cleavage in the presence of a 25:1 molar ratio of 30S ribosomes to mRNA; (lane 4) RNase E cleavage in the presence of a 25:1 molar ratio of blocked 30S ribosomes to mRNA. Initiator tRNAfMet was included in the reaction in a fivefold molar excess over ribosomes. The RNase E cleavage conditions were as described in A, except that the cleavage reactions were performed in toeprinting buffer [60 mm NH4Cl, 10 mm MgOAc, 6 mm mercaptoethanol, 10 mm Tris-HCl (pH 7.3)].
To ensure that 30S inhibition of RNase E cleavage requires 30S translation initiation complex formation at the ompA mRNA RBS, the following control experiment was performed. The RNA oligonucleotide 5′-UAAGGAGGUG-3′ complementary to the anti-Shine Dalgarno sequence of the 16S rRNA was annealed to 30S subunits as described in Materials and Methods. The inability of the “blocked ribosome” to bind to the ompA RBS was verified by a toeprinting assay (Hartz et al. 1988). Briefly, in the presence of initiator tRNA, 30S subunits bind at the translation initiation region and block reverse transcriptase primed downstream. The inhibition of primer extension at the 3′ edge of the 30S subunit generates a stop signal (toeprint), the strength of which provides a measure for translation initiation. As shown in Figure 2B (lane 5) the blocked 30S ribosome did not form a ternary complex on ompA RNA and therefore, no toeprint signal was obtained. Using the same buffer conditions as for toeprinting, 30S and blocked 30S ribosomes, respectively, were included together with tRNAfMet in the RNase E-dependent cleavage reaction of ompA180 mRNA (Fig. 2C). When compared to the equivalent reaction performed in RNase E cleavage buffer (see Fig. 2A, lane 4; Materials and Methods), under these conditions, 30S protection at site D was 100% (Fig. 2C, lane 3), and 30S protection at site C was increased further (Fig. 2C, lane 3). However, when the blocked ribosomes were included in the RNase E cleavage reaction, cleavage at sites C and D occurred (Fig 2C, lane 4) with the same efficiency as in the absence of ribosomes (Fig. 2C, lane 2). Given that the 30S subunit covers 35 (±2) nucleotides upstream of the A of the start codon (Hüttenhofer and Noller 1994), we concluded that the 30S subunit confers protection at site D and to a somewhat lower extend to site C when bound at the ompA RBS. Protection at site C is apparently achieved through linear binding of the 30S subunit without destabilization of hp2 (see Fig. 1), which is reminiscent of the situation in phage T4 gene 38 (Hartz et al. 1991).
Next, Hfq was incubated with ompA mRNA in RNase E cleavage buffer before the addition of initiator tRNA and 30S subunits, the latter of which were added equimolar with Hfq. When compared to the same assay performed in the absence of Hfq (Fig. 2A, lane 3), the addition of Hfq resulted in an approximately threefold increase of RNase E cleavage at site D (Fig. 2A, lane 6). The abrogation of 30S protection conferred to site D by Hfq did also occur when the reaction was performed in toeprinting buffer (not shown). Taken together, these experiments suggested that Hfq interferes with ribosome binding, which in turn counteracts RNase E cleavage.
Hfq binding requires elements of the ompA translation initiation region
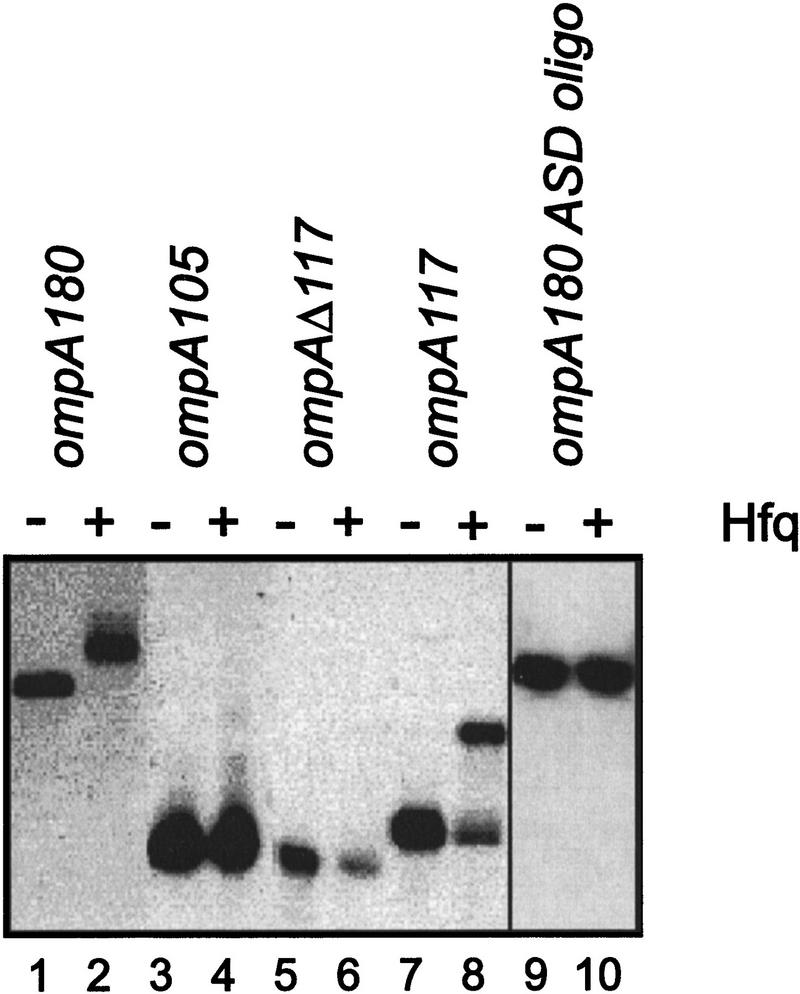
Because Hfq interfered with ribosome binding, we anticipated that Hfq would bind either within or in the vicinity of the ompA RBS. Therefore, we tested the affinity of Hfq for different ompA 5′-UTR deletion constructs using gel mobility-shift assays. As recently shown (Vytvytska et al. 1998), preincubation of 32P 5′ end-labeled ompA180 mRNA with a 10-fold molar excess of purified Hfq resulted in a mobility shift (Fig. 3, lane 2). Using the same conditions, the segment of the 5′-UTR containing the two stem loop structures (hp1 and hp2) up to the 3′ base of hp2 (ompA105 mRNA; see Fig. 1) and ompAΔ117 mRNA encompassing the translational initiation signals and the initial coding region of the ompA mRNA (see Fig. 1), respectively, were not sufficient to be shifted by Hfq (Fig. 3, lanes 4,6). However, ompA117 mRNA (see Fig. 1), which in addition to ompA105 mRNA contains 12 nucleotides downstream of hp2, was sufficient to be shifted by Hfq (Fig. 3, lane 8) albeit with a reduced efficiency when compared to ompA180 mRNA (Fig. 3, lane 2). To further verify whether Hfq binding requires elements of the ompA translation initiation region (TIR), in particular the segment between hp2 and the SD sequence, an oligonucleotide complementary to bases −30 to −9 of the ompA 5′-UTR (see Fig. 1) was annealed to ompA180 mRNA. As shown in Figure 3, lane 10, the annealed oligonucleotide inhibited the Hfq-mediated band shift suggesting that Hfq contacts or binds to the TIR.
Figure 3.
Binding of Hfq to the ompA 5′-UTR. The 5′ end-labeled ompA180, ompA105, ompAΔ117, and ompA117 mRNAs were incubated with purified Hfq protein to allow formation of the RNA–protein complex as specified in Materials and Methods and then resolved on a 4% native polyacrylamide gel. (Lanes 1, 3, 5, 7) Electrophoretic mobility of ompA180, ompA105, ompAΔ117, and ompA117 mRNAs, respectively. (Lanes 2, 4, 6, 8) Electrophoretic mobility of ompA180, ompA105, ompAΔ117, and ompA117 mRNAs in the presence of a 10-fold molar excess of Hfq. (Lanes 9, 10) The oligonucleotide complementary to −30 to −9 of the ompA 5′-UTR (see Fig. 1) was annealed to ompA180 mRNA and the annealing mix was purified by chromatography as described in Materials and Methods. Hfq was added to the purified annealing mix (lane 10) in a molar ratio of 10:1 of Hfq to the annealing mix.
In vitro repression of ompA mRNA translation by Hfq
To test directly whether Hfq prevents ribosome binding at the ompA RBS, a toeprinting assay was performed. Toeprinting provides a suitable method to assess the effect of cis- and trans-acting factors on formation of the ternary complex (Winter et al. 1987; Bläsi et al. 1989; Philippe et al. 1993). Hfq has been found to be associated with ribosomes (Kajitani et al. 1994). Thus, the formal possibility existed that Hfq associated with ribosomes per se prevents ribosome binding to the ompA RBS. Therefore, 30S ribosomes were added in molar excess over Hfq, which in turn was added in excess over mRNA (see legend to Fig. 4A). The addition of Hfq at different molar ratios to ompA mRNA before the addition of 30S subunits and tRNAfMet resulted in either a diminished toeprint signal (Fig. 4A, lane 6) or abolished ternary complex formation (Fig. 4A, lanes 7,8). In contrast, when 30S subunits and tRNAfMet were added before (Fig. 4A, lanes 13,14) or concomitantly with (Fig. 4A, lanes 10,11) the addition of Hfq, the protein did not interfere with ternary complex formation. Taken together with the in vitro RNase E cleavage assay (see Fig. 2), the toeprint experiments showed that Hfq inhibits 30S binding to the ompA RBS. It has been shown recently that the half-life of E. coli lpp mRNA is not affected by Hfq (Vytvytska et al. 1998). Therefore, the lpp mRNA was used as a control in the toeprint experiments. As expected, the addition of Hfq in molar excess over lpp mRNA before the addition of 30S subunits did not prevent ternary complex formation (Fig. 4B, lanes 7, 8). Furthermore, this result confirmed the view that Hfq acts only on certain mRNAs and that Hfq does not simply prevent binding of the ribosome to the RBS by virtue of its capability to bind to 30S subunits.
Figure 4.
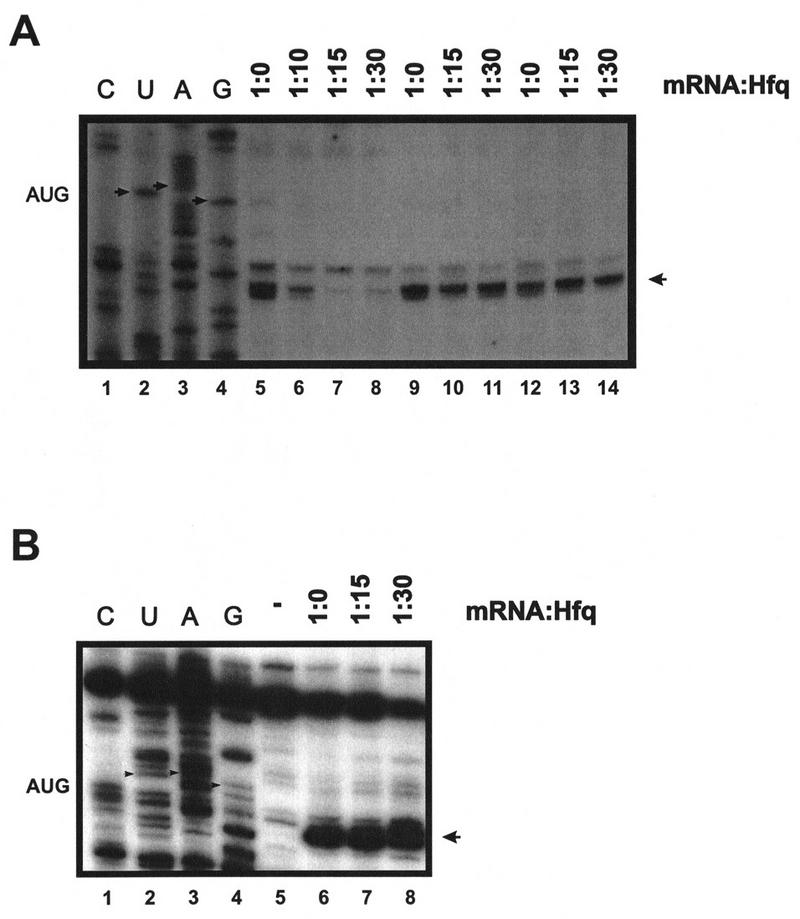
Hfq inhibits ternary complex formation on ompA mRNA. (A) Ternary complex formation on ompA mRNA. (Lanes 1–4) Sequencing reactions. The position of the ompA start codon is indicated by small arrows. The toeprint signal at position +15 relative to the A of the start codon is marked by an arrowhead. In all reactions, the molar ratio of mRNA-to-30S subunits was 1:100 and the tRNAfMet was in fivefold molar excess over ribosomes. Hfq was added at the molar ratios to mRNA as indicated on top. 30S subunits were in 10:1 (lane 6), 5:1 (lane 7), 4:1 (lane 8) molar excess to Hfq. (Lanes 5,9,12) Toeprint analysis in the presence of 30S subunits and tRNAfMet. (Lanes 6–8) Hfq was added prior to the addition of 30S subunits and tRNAfMet; (lanes 10, 11) 30S subunits and tRNAfMet were added concomitantly with the addition of Hfq; (lanes 13, 14) 30S subunits and tRNAfMet were added before the addition of Hfq. (B) Ternary complex formation on lpp mRNA. The position of the lpp start codon is indicated by small arrows. The toeprint signal at position +15 relative to the A of the start codon is marked by an arrowhead. (Lanes 1–4) Sequencing reactions; (lane 5) primer extension in the absence of 30S subunits and tRNAfMet; (lane 6) toeprint analysis in the presence of 30S subunits and tRNAfMet; (lanes 7,8) Hfq was added before the addition of 30S subunits and tRNAfMet. Hfq was added at the molar ratios to mRNA as indicated on top. 30S subunits were in 5:1 (lane 7) and 4:1 (lane 8) molar excess to Hfq.
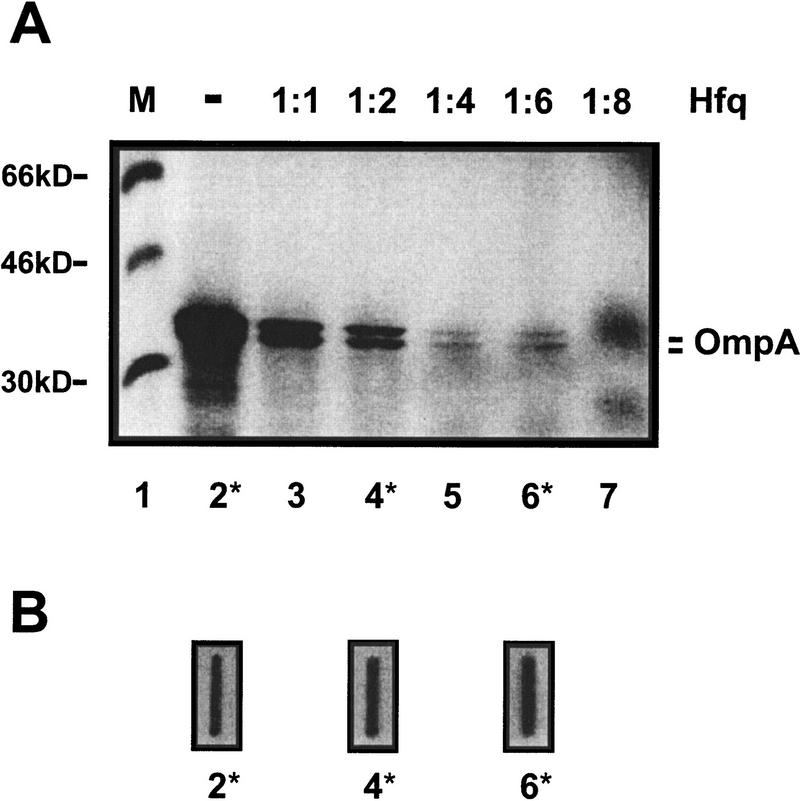
Next, in vitro transcribed full-length ompA mRNA was translated in an in vitro translation system in the presence of purified Hfq protein. As shown in Figure 5A, a 1:1 and 1:2 molar ratio of mRNA to Hfq reduced the translation rate of ompA mRNA by a factor of 3, whereas a four- and sixfold molar excess reduced it by a factor of 20. Finally, translation was abolished when Hfq was added in eightfold molar excess to mRNA. The determination of the ompA mRNA concentration after completion of the in vitro translation assays in three reactions (Fig. 5B) revealed that under the in vitro conditions the ompA mRNA was not degraded in the presence of Hfq. Taken together, the in vitro experiments demonstrated that Hfq represses ompA translation.
Figure 5.
In vitro translation of ompA mRNA in the presence of Hfq. (A) The positions and molecular masses (in kD) of marker proteins (M; lane 1) and of the two OmpA-specific bands are given at the left and at the right, respectively. (Lane 2) In vitro translation of ompA mRNA in the absence of Hfq; (lanes 3–7) In vitro translation of ompA mRNA in the presence of Hfq. Hfq was added to mRNA at different molar ratios as shown on top of the autoradiograph of the 12.5% SDS–polyacrylamide gel. (B) Slot–blot hybridizations showing that the ompA mRNA was not degraded in the S30 extracts upon addition of Hfq. The mRNA concentrations in the slot–blots marked with 2*, 4*, and 6* were directly determined after completion of the translation reactions shown in lanes 2*, 4*, and 6* of A as described in Materials and Methods.
Hfq decreases the functional half-life of ompA mRNA
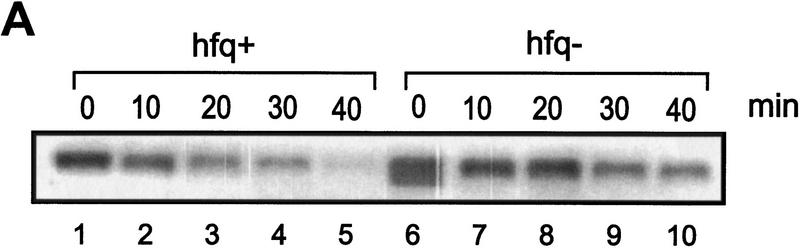
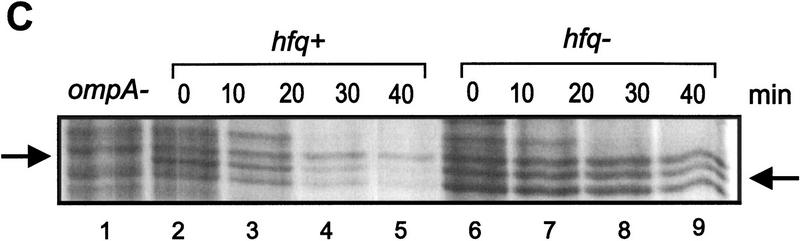
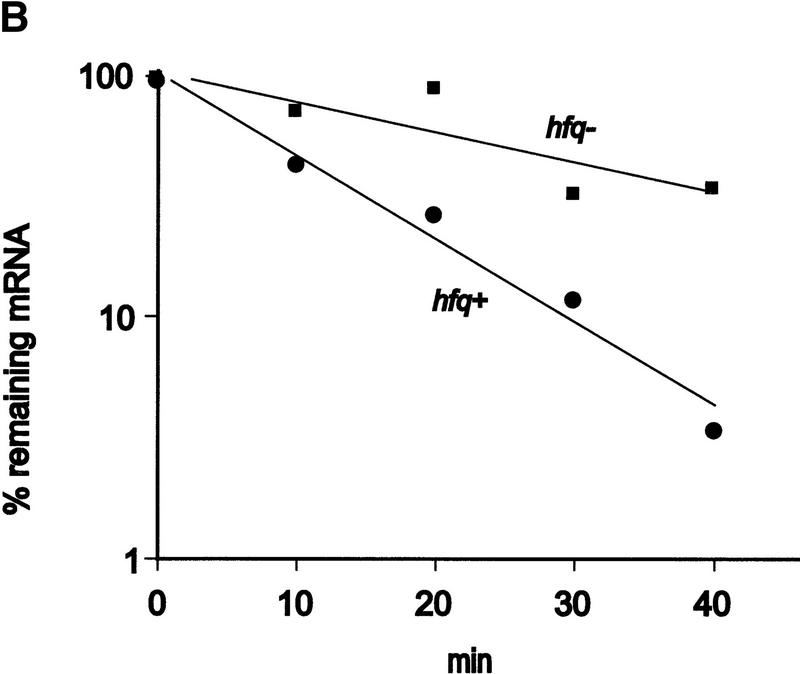
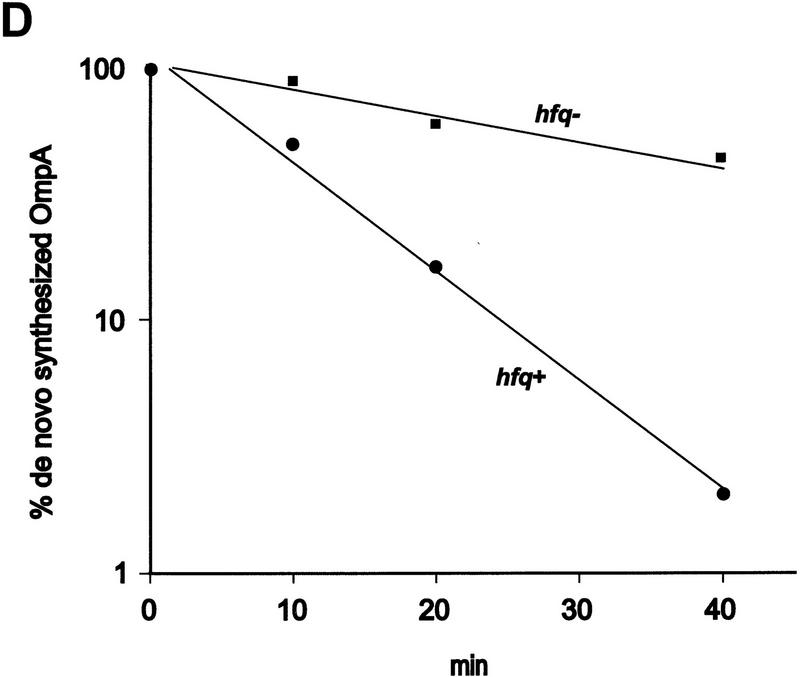
Finally, we attempted to verify in vivo the effect of Hfq on translation of ompA mRNA observed in vitro. The rate of OmpA protein synthesis was related to the steady state concentration of ompA mRNA in both an hfq+ and an hfq− strain. Both strains were cultivated in MOPS acetate medium until they reached an OD600 of 0.5. Then, rifampicin was added, and aliquots were withdrawn at different time intervals thereafter. The total cellular RNA was extracted and probed in a Northern blot with the 32P-labeled oligonucleotide complementary to bases −31 to +22 of ompA mRNA (see Fig. 1). Because the oligonucleotide comprises the region covered by the ribosome (Hüttenhofer and Noller 1994), the assay should determine functional ompA mRNA. As shown in Figure 6A and B the half-life of the ompA mRNA was found to be approximately threefold decreased in the hfq+ strain when compared to the hfq− strain. Basically the same observation has been made in recent studies (Vytvytska et al. 1998) in which a probe complementary to the central part of the ompA mRNA was used. Therefore, these results agree with the notion that the initial regulatory events in the ompA 5′-UTR are sufficient to induce degradation of the downstream part of the mRNA (Melefors and von Gabain 1988). To test whether the rate of the ompA mRNA decay is reflected in the de novo OmpA synthesis rate, OmpA synthesis was determined in the same cultures as used for the determinations of the steady state levels of ompA mRNA. Aliquots of the cultures were withdrawn 1 min before the time values shown on top of Figure 6C and pulse labeled for 1 min with [35S]methionine. As shown in Figure 6C,D, the rate of OmpA synthesis directly mirrored the rate of ompA mRNA decay in the hfq+ and hfq− strains, that is, the inhibition of ompA translation exerted by Hfq results in functional inactivation of the mRNA.
Figure 6.
Hfq affects the functional half life of ompA mRNA in vivo. (A) ompA mRNA decay in an hfq+ (lanes 1–5) and an hfq− strain (lanes 6–10). The Northern blot analysis was carried out as described in Materials and Methods using the 5′ end-labeled oligonucleotide complementary to nucleotide −31 to +22 of the ompA mRNA (see Fig. 1). (B) The signals obtained from the autoradiographs were evaluated by densitometry using a Molecular Dynamics PhosphorImager. The signal strength obtained at time 0 was set as 100%. The graph shows the percentage of remaining ompA mRNA at the different times after addition of rifampicin in the hfq+ (●) and hfq− (█) strain. The half-life for ompA mRNA was 11 ± 0.8 min and 30 ± 1.6 min in the hfq+ and hfq− strain, respectively. (C) Synthesis of OmpA protein at different times after addition of rifampicin in the hfq+ (lanes 2–5) and in the hfq− (lanes 6–9) strain. The position of the ompA band in the autoradiograph of the 12% SDS–polyacrylamide gel is marked at the left and at the right. (lane 1), Pulse labeling of the ompA− strain UH203 for unambiguous identification of the ompA protein. Only the relevant section of the gel is shown. (D) Graphic representation of the de novo synthesis of OmpA protein at different times after the block of transcription (represents functional half-life of ompA mRNA) in the hfq+ (●) and hfq− (█) strain. The values are normalized to the synthesis rate at time 0.
Discussion
Hfq and growth rate-dependent stability of ompA mRNA
Recently, we have shown that Hfq protein binds to the 5′-UTR of ompA mRNA and negatively affects its longevity under conditions of slow growth (Vytvytska et al. 1998). The intracellular concentration of Hfq increases with decreasing growth rate or upon entry into stationary phase (Tsui et al. 1997; O. Vytvytska, unpubl.). These observations implied that Hfq destabilizes ompA mRNA and thereby down-regulates its expression, as previously speculated for other mRNAs including mutS, miaA, and hfq itself (Tsui et al. 1997). It was conceivable that Hfq directly facilitates ompA mRNA degradation by increasing the susceptibility of the substrate for the endonuclease. However, the presence of Hfq did not accelerate the rate of RNase E cleavage in the ompA 5′ UTR (O. Vytvytska, unpubl.). Therefore, we favored the idea that Hfq acts in an indirect manner by interfering with ribosome binding at the ompA RBS, the occupancy of which by ribosomes has been suggested to retard the decay of the ompA transcript (Arnold et al. 1998). Our studies showed that 30S subunits bound to the ompA RBS protect the transcript from RNase E cleavage at the major in vivo cleavage site D (Melefors and von Gabain 1988), that Hfq interferes with this protection (Fig. 2), that elements of the TIR are required for Hfq binding (Fig. 3), and finally that Hfq inhibits ompA translation (Figs. 4–6). We conclude that Hfq exerts its destabilizing effect on ompA mRNA by preventing ribosomes from their role to block the decay-initiating RNase E cleavages in the 5′-UTR. To our knowledge this study provides the first direct evidence that a 30S subunit bound to the 5′ end of a mRNA protects it from endonucleolytic cleavage by RNase E. Indirect evidence for this has been provided by Wagner et al. (1994) and Arnold et al. (1998), who showed that mRNA stability depends on efficient ribosome loading at the RBS. In contrast to ribosomal protection at the 5′ end, Braun et al. (1998) have recently shown that ribosomes lingering at the termination codon of rpsO mRNA protect from RNase E cleavage 10 nucleotides downstream of the rpsO coding sequence. It is intriguing that ribosomes can act as mRNA stabilizers when arriving as well as when they depart from mRNA. However, regardless of whether mRNA degradation ensues at the 5′ or the 3′ end continuous translation appears to prevent the initial steps mediated by RNase E.
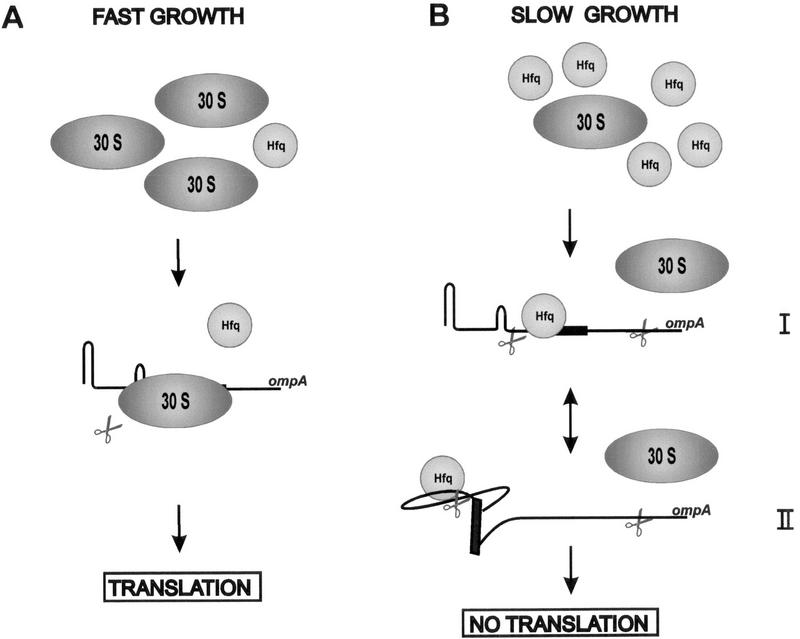
The data presented in this study suggest the following model for the growth rate-dependent degradation of ompA mRNA. Under conditions of fast growth and moderate intracellular levels of Hfq, an excess of 30S subunits can compete efficiently with Hfq for binding to the ompA 5′-UTR. This results in suppression of the RNase E cleavages in the 5′-UTR by the ribosome bound at the RBS (Fig. 7A). When the growth rate decreases, the intracellular level of Hfq increases (Tsui et al. 1997; O. Vytvytska, unpubl.). At the same time the concentration of free 30S subunits required for translation initiation drops with a concomitant increase of competition of mRNAs for 30S subunits (Bremer and Dennis 1996). The competition of Hfq with 30S subunits for ompA mRNA prevents translation and renders the mRNA vulnerable for rate-limiting cleavages in the 5′-UTR, and taken together with our recent results (Vytvytska et al. 1998) also in the ompA coding region (Fig. 7B). Moreover, the study provides a molecular basis for the finding that the translational efficiency of ompA mRNA does not significantly change at different growth rates, whereas its stability does (Lundberg et al. 1988). The determination of the translational efficiencies of ompA deduced from the data shown in Figure 6 revealed that they were approximately the same in both the hfq+ and hfq− strain. The fact that Hfq action prevents ompA translation, which in turn results in immediate functional as well as chemical inactivation of the mRNA does explain why the translational efficiency is not affected at different growth rates. The decreased synthesis rate of OmpA protein at slow growth rate is simply the result of the reduced concentration of functional ompA mRNA.
Figure 7.
Model for growth rate-regulated ompA mRNA translation and stability by Hfq. (A) Under conditions of fast growth 30S ribosomes are in excess over Hfq, which results in frequent translation initiation of ompA mRNA, which, in turn, prevents RNase E cleavages in the 5′-UTR and leads to an enhanced stability of the transcript. (B) Under conditions of slow growth, Hfq outcompetes 30S ribosomes and binds to the ompA 5′-UTR. Ribosome binding is either sterically hindered (I) or inhibited by a structural change imposed by Hfq (II). The lack of translation then facilitates RNase E cleavages in the 5′-UTR as well as in the coding region, and results in rapid functional decay of the transcript. Endonucleolytic cleavage is illustrated by scissors.
Hfq as a negative regulator of translation
Most of the translational repressors described in the past decade bind to the TIR of a given mRNA and sterically inhibit ribosome binding (Winter et al. 1987; McPheeters et al. 1988; Tuerk et al. 1990). There are some exceptions including ribosomal protein S15 that induces a pseudoknot structure upon binding to its operator. The pseudoknot that affects the codon–anticodon interaction does not obstruct ribosome binding but prevents stable ternary complex formation (Philippe et al. 1993).
Hfq in molar excess to ompA mRNA but not to 30S subunits abolished ternary complex formation (Fig. 4A). In contrast, when 30S ribosomes and initiator tRNA were added before or concomitantly with the addition of Hfq the ternary complex prevailed (Fig. 4A). These results showed that Hfq interferes with ternary complex formation. Likewise, when Hfq and ompA mRNA were preincubated before the addition of 30S ribosomes and the degradosome preparation, the ribosomes did not protect from RNase E cleavage (Fig. 2A), which indicates that binding of Hfq and 30S ribosomes is mutually exclusive. However, although Hfq apparently binds to or contacts the ompA TIR we can at present not distinguish as to whether Hfq prevents binding of the ribosome by shielding part of the RBS (Fig. 7BI) or whether it causes conformational changes in the mRNA that are incompatible with ribosome binding (Fig. 7BII). Preincubation of Hfq with ompA mRNA at different molar ratios did not result in a stop signal in toeprint assays. This shows that binding of Hfq is at least not strong enough to block by itself the elongating reverse transcriptase, which agrees with similar studies performed with the T4 regA translational repressor (Winter et al. 1987). We attempted to map the Hfq-binding site by enzymatic RNA footprinting. However, the analysis did not reveal a contiguous segment of the ompA 5′-UTR protected by Hfq from cleavage by different riboendonucleases, which is in contrast to studies performed with canonical translational repressors (Winter et al. 1987; Philippe et al. 1993). Our experiments indicated that Hfq exposes certain areas in the ompA 5′-UTR to nuclease attack (I. Moll, unpub.), which seems indicative for structural changes. It has been suggested that Hfq promotes structural rearrangements in phage Qβ RNA (Schuppli et al. 1997; Klovins et al. 1998), and structural changes in the mRNA have been proposed to be the consequence of Hfq binding to rpoS mRNA (Muffler et al. 1996; Brown and Elliot 1997; Cunning et al. 1998). Cunning et al. (1998) have suggested that Hfq binds to upstream elements in the rpoS mRNA and from there interacts with downstream elements, thereby relieving an inhibitory secondary structure at the RBS.
The inactivation of the hfq gene in E. coli causes pleiotropic phenotypes (Tsui et al. 1994). Moreover, in an E. coli hfq mutant a number of proteins were shown to be overproduced (Muffler et al. 1997). The data presented here support the hypothesis that Hfq regulates negatively a subset of genes at the level of translation, which in turn results in accelerated degradation of the corresponding mRNAs. The genes encoding products that are not required under conditions of slow growth or after cessation of growth such as ompA would be candidates for negative regulation by Hfq. In light of our results it is tempting to speculate that Hfq acts as a general translational regulator. In this scenario Hfq would provide a mediator of the interdependence between translation (initiation) and mRNA stability. A question that clearly needs to be addressed in further studies is the molecular interaction with and the recognition of different target mRNAs by Hfq.
Materials and methods
Bacterial strains and growth conditions
E. coli strain MC4100 [F′ Δ(argF lac) U169 araD139 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR] (Casadaban 1976) and its derivative AM111(hfq1) [hfq::Ω, BclI] (Tsui et al. 1994) were used in this study. Cells were grown in MOPS medium supplemented with 0.2% (wt/vol) sodium acetate at 30°C, as described (Vytvytska et al. 1998). The E. coli ompA− strain UH203 [(F′ lacIQ lacZΔ M15 proAB) lac supF ompA recA proA rpsL] was used as a control for identification of the OmpA protein in the pulse-labeling experiments (see below).
mRNAs used in this study and gel mobility-shift assay
Preparation of Hfq and the mobility-shift assays were performed as recently described (Vytvytska et al. 1998). Plasmid pT7OMPA+5* (Chen et al. 1991) linearized with HindIII served as a source for ompA180 mRNA (see Fig. 1) comprising nucleotides −135 to + 45 of wild-type ompA mRNA. The ompA117 mRNA (nucleotides −135 to −18) was obtained after linearization of plasmid pT7OMPA+5* with MnlI. ompA105 mRNA (nucleotides −135 to −30) was prepared after MnlI cleavage of plasmid p132, which is a derivative of the pT7OMPA+5 plasmid (Emory et al. 1992). The ompAΔ117 RNA comprising nucleotides −21 to +111 of wild-type ompA mRNA was prepared as described earlier (Tedin et al. 1997). The mRNAs were synthesized in vitro using T7 RNA polymerase and a Stratagene transcription kit. Purified Hfq was added to the 32P-5′-end-labeled ompA mRNAs at a molar ratio of 10:1, incubated for 10 min on ice, and then the RNA–protein complexes were resolved on 4% native polyacrylamide gels.
For the oligonucleotide-inhibition assay, the oligonucleotide complementary to bases −30 to −9 of the ompA 5′ UTR was annealed to 32P-labeled ompA180 mRNA using a 10-fold molar excess of the oligonucleotide to mRNA. After annealing, the excess of oligonucleotides was removed by Sephadex G-25 gel filtration. Quantitative hybridization of the oligonucleotide was confirmed by treatment of an aliquot of the annealing mix with RNase H (not shown). The purified annealing mix was used in the band shift assay, which was performed exactly as described above.
In vitro RNase E assay
The 32P-5′-end-labeled ompA180 mRNA served as substrate for in vitro RNase E cleavage. RNA cleavage was carried out as described previously (Kaberdin et al. 1996) using the E. coli degradosome as a source for RNase E. The degradosome was purified according to Miczak et al. (1996) including micrococcal nuclease treatment that prevents the copurification of proteins dependent on the presence of RNA. These preparations did not contain Hfq as judged by Western blot analysis. The 32P-5′-end-labeled ompA180 mRNA was incubated with an equimolar amount of the degradosome in RNase E cleavage buffer that did not contain Mg2+ ions (Kaberdin et al. 1996). For cleavage protection 30S ribosomal subunits were added in the molar ratios as specified in Figure 2A. Initiator tRNAfMet was added in fivefold molar excess to 30S subunits. The cleavage reaction was started by addition of MgSO4 (final concentration was 5 mm) and carried out for 1 hr at 30°C. The reaction was stopped by addition of EDTA to a final concentration of 10 mm. The samples were extracted with phenol and analyzed on a 6% polyacrylamide-urea gel.
Toeprinting of ompA and lpp mRNA in the presence of Hfq
Plasmid pUH100 (Lundberg et al. 1990) served as a source for wild-type ompA mRNA. The run-off transcript of HindII-digested pUH100 was used for toeprinting. The 32P-5′-end-labeled AvaII primer (Lundberg et al. 1990) complementary to the region +110 to +87 downstream of the start codon of ompA mRNA was used in the toeprinting reactions as previously described (Tedin et al. 1997). Blocked 30S ribosomes were prepared by incubation of a twofold molar excess of the Shine-Dalgarno RNA oligonucleotide 5′-UAAGGAGGUG-3′ with purified 30S subunits at 37°C for 10 min.
PCR templates containing the phage T7φ10 promoter for generation of lpp mRNA were obtained using chromosomal DNA of strain MC4100. The oligonucleotide used were 5′-GGGTAATACGACTCACTAGTGCGCTACATGGAGATTAAC-3′, which contained the T7 promoter and nucleotides −21 to −38 upstream of the lpp gene and oligonucleotide 5′-GTTGTCCAGACGCTGG-3′ complementary to nucleotides +143 to +128 in the lpp gene. The template was generated by a standard PCR protocol and used for transcription with T7 RNA polymerase as described before (Resch et al. 1996). The 32P-5′-end-labeled oligonucleotide complementary to nucleotides +143 to +128 of the lpp gene was used as the primer for cDNA synthesis in the toeprinting reactions. Primer purification, primer labeling, and the toeprinting assays were carried out with 30S ribosomal subunits as previously described (Hartz et al. 1988). The M-MLV reverse transcriptase was premixed with the dNTPs before addition to the toeprint assays. Hfq protein was added either before, concomitantly with, or after the addition of 30S subunits to the toeprinting reactions at the molar ratios to mRNA as specified in the legend to Figure 4.
In vitro translation
For synthesis of full-length ompA mRNA, the plasmid pUH101 (Lundberg et al. 1990) was digested with HindIII. The linearized plasmid served as template for in vitro transcription with T7 RNA polymerase. Translation reactions with E. coli ribosomes were performed as outlined in Tedin et al. (1997). After addition of Hfq protein at different molar ratios to the ompA mRNA, the translation reactions were incubated at 37°C for 10 min. Reactions were stopped by addition of three volumes of icecold 10% TCA and placed on ice for 15 min followed by centrifugation at 6000g, 4°C for 15 min. The resulting pellets were washed once with 90% acetone and dried under vacuum for 5 min. The pellets were resuspended in 25 μl of Laemmli buffer (Laemmli 1970) and boiled for 3 min before layering onto a 12% SDS-polyacrylamide gel. The gels were dried and exposed to an X-ray film. The mRNA concentrations were directly determined in three different reactions (see Fig. 5B) after completion of the translation reactions by hybridization with the 32P 5′ end-labeled AvaII primer (Lundberg et al. 1990). Filter binding was assayed in a Schleicher Schuell SRC 072/0 Minifold II Slot Blot apparatus.
Determination of the steady state concentration of ompA mRNA and de novo OmpA synthesis upon block of transcription in a hfq+ and a hfq− strain
E. coli MC4100 and AM111 (hfq−) were grown in MOPS acetate medium to an OD600 of 0.5. Then, rifampicin (0.2 mg/ml) was added and 10-ml aliquots were withdrawn for isolation of total RNA (Georgellis et al. 1992) at time 0, 10, 20, 30, and 40 min thereafter. Five micrograms of total RNA from each sample were gel fractionated and Northern blot analysis was performed as previously described (Georgellis et al. 1992) using the 32P 5′ end-labeled oligonucleotide complementary to nucleotides −31 to +22 of ompA mRNA.
De novo synthesis of OmpA protein at the different times after addition of rifampicin was determined using the same cultures as used for the mRNA determinations. One hundred-microliter aliquots were removed 1 min before the times given above for the RNA isolation. One microliter of [35S]methionine (10 mCi/ml) was added and pulse labeling was carried out for 1 min at 37°C. The reactions were stopped by addition of 10% TCA and centrifugation, and the pellets were processed as described above. The same amount of cpm for each sample were loaded on a 12% SDS–polyacrylamide gel. The proteins were separated and the rate of OmpA synthesis at the various times was determined by quantitation of the incorporated radioactivity in the OmpA bands using a Molecular Dynamics PhosphorImager. A mid-log. phase culture of the ompA− strain UH203 grown in MOPS acetate medium was pulse labeled with l μl of [35S]methionine (10 mCi/ml), and processed as described above. The protein pattern of the labeled UH203 cells served for unambiguous identification of the ompA protein in the autoradiograph shown in Figure 6C.
Acknowledgments
We thank Dr. R. Hengge-Aronis for providing the hfq mutant strain and Drs. J.G. Belasco and M. Birnstiel for suggestions and critical reading of the manuscript. This work was supported by grants P11841-MOB (to A.v.G.) and P12065-MOB (to U.B.) from the Austrian Science Foundation and by INTERCELL Biotechnologies, Vienna, Austria.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL UDO@GEM.UNIVIE.AC.AT; FAX 43-1-4277-9546.
E-MAIL ALEX@GEM.UNIVIE.AC.AT; FAX 43-1-4277-9546.
References
- Arnold TE, Yu J, Belasco JG. mRNA stabilization by the ompA 5′ untranslated region: Two protective elements hinder distinct pathway for mRNA degradation. RNA. 1998;4:319–330. [PMC free article] [PubMed] [Google Scholar]
- Baumeister R, Flache P, Melefors O, von Gabain A, Hillen W. Lack of 5′ non-coding region in Tn721 encoded tetR mRNA is associated with a low efficiency of translation and a short half-life in Escherichia coli. Nucleic Acids Res. 1991;19:4595–4600. doi: 10.1093/nar/19.17.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsi U, Nam K, Hartz D, Gold L, Young R. Dual translational start sites control function of the lambda S gene. EMBO J. 1989;8:3501–3510. doi: 10.1002/j.1460-2075.1989.tb08515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun F, Derout J, Regnier P. Ribosomes inhibit an RNase E cleavage which induces the decay of the rpsO mRNA of Escherichia coli. EMBO J. 1998;17:4790–4797. doi: 10.1093/emboj/17.16.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H, Dennis PP. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Washington, D.C.: ASM Press; 1996. pp. 1553–1569. [Google Scholar]
- Brown L, Elliott T. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J Bacteriol. 1996;178:3763–3770. doi: 10.1128/jb.178.13.3763-3770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Mutations that increase expression of the rpoS gene and decrease its dependence on hfq function in Salmonella typhimurium. J Bacteriol. 1997;179:656–662. doi: 10.1128/jb.179.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpousis AJ, vanHouwe G, Ehretsman C, Krisch HM. Copurification of E. coli RNAse E and PNPase: Evidence for a specific association between two enzymes important in RNA processing and degradation. Cell. 1994;76:889–900. doi: 10.1016/0092-8674(94)90363-8. [DOI] [PubMed] [Google Scholar]
- Casadaban MJ. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chen LH, Emory SA, Bricker AL, Bouvet P, Belasco JG. Structure and function of a bacterial mRNA stabilizer: Analysis of the 5′ untranslated region of ompA mRNA. J Bacteriol. 1991;173:4578–4586. doi: 10.1128/jb.173.15.4578-4586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Yanofsky C. Sequence changes preceding a Shine-Dalgarno region influence trpE mRNA translation and decay. J Mol Biol. 1988;204:51–60. doi: 10.1016/0022-2836(88)90598-0. [DOI] [PubMed] [Google Scholar]
- Cole JR, Nomura M. Changes in the half-life of ribosomal protein messenger RNA caused by translational repression. J Mol Biol. 1986;188:383–392. doi: 10.1016/0022-2836(86)90162-2. [DOI] [PubMed] [Google Scholar]
- Comer MM, Dondon J, Graffe M, Yarchuk O, Springer M. Growth-rate dependent control, feedback regulation and steady-state mRNA levels of the threonyl-tRNA synthetase gene of Escherichia coli. J Mol Biol. 1996;261:108–124. doi: 10.1006/jmbi.1996.0445. [DOI] [PubMed] [Google Scholar]
- Cunning C, Brown L, Elliot T. Promoter substitution and deletion analysis of upstream region required for rpoS translation regulation. J Bacteriol. 1998;180:4564–4570. doi: 10.1128/jb.180.17.4564-4570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emory SA, Bouvet P, Belasco J. A 5′-terminal stem–loop structure can stabilize mRNA in Escherichia coli. Genes & Dev. 1992;6:135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- Fernandez FM, Eoyang L, August JT. Factor fraction required for the synthesis of bacteriophage Qβ RNA. Nature. 1968;219:588–590. doi: 10.1038/219588a0. [DOI] [PubMed] [Google Scholar]
- Flache P, Baumeister R, Hillen W. The Tn10 encoded tetracycline resistance mRNA contains a translational silencer in the 5′ non translated region. J Bacteriol. 1992;174:2478–2484. doi: 10.1128/jb.174.8.2478-2484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgellis D, Arvidson S, von Gabain A. Decay of ompA mRNA and processing of 9S RNA are immediately affected by shifts in growth rate, but in opposite manners. J Bacteriol. 1992;174:5382–5390. doi: 10.1128/jb.174.16.5382-5390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz D, McPheeters DS, Traut R, Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- Hartz D, McPheeters DS, Green L, Gold L. Detection of Escherichia coli ribosome binding at translation initiation sites in the absence of tRNA. J Mol Biol. 1991;218:99–105. doi: 10.1016/0022-2836(91)90876-8. [DOI] [PubMed] [Google Scholar]
- Hüttenhofer A, Noller HF. Footprinting mRNA-ribosome complexes with chemical probes. EMBO J. 1994;13:3892–3901. doi: 10.1002/j.1460-2075.1994.tb06700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iost I, Dreyfus M. The stability of the E. coli lacZ mRNA depends upon the simultaneity of its synthesis and translation. EMBO J. 1995;14:3252–3261. doi: 10.1002/j.1460-2075.1995.tb07328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain C, Kleckner N. IS10 mRNA stability and steady-state levels in Escherichia coli: Indirect effects of translation and role of rne function. Mol Microbiol. 1993;9:233–247. doi: 10.1111/j.1365-2958.1993.tb01686.x. [DOI] [PubMed] [Google Scholar]
- Kaberdin VR, Chao YH, Lin-Chao S. RNase E cleaves at multiple sites in bubble regions of RNAI stem loops yielding products that dissociate differentially from the enzyme. J Biol Chem. 1996;271:13103–13109. doi: 10.1074/jbc.271.22.13103. [DOI] [PubMed] [Google Scholar]
- Kajitani M, Kato A, Wada A, Inokuchi Y, Ashihama A. Regulation of the Escherichia coli hfq gene encoding the host factor for phage Qβ. J Bacteriol. 1994;176:531–534. doi: 10.1128/jb.176.2.531-534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klovins J, Berzins V, van Duin J. A long-range interaction in Qβ RNA that bridges the thousand nucleotides between the M-site and the 3′ end is required for replication. RNA. 1998;4:948–957. doi: 10.1017/s1355838298980177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assembly of the head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Nilsson G, von Gabain A. The different stability of the Escherichia coli ompA and bla mRNA at various growth rates is not correlated to the efficiency of translation. Gene. 1988;72:141–149. doi: 10.1016/0378-1119(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Lundberg U, von Gabain A, Melefors Ö. Cleavages in the 5′ region of the ompA and the bla mRNA control stability: Studies with an E. coli mutant altering mRNA stability and a novel endoribonuclease. EMBO J. 1990;9:2731–2741. doi: 10.1002/j.1460-2075.1990.tb07460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova OV, Makarov EM, Sousa R, Dreyfus M. Transcribing Escherichia coli genes with mutant T7 RNA polymerase: Stability of lacZ mRNA inversely correlated with polymerase speed. Proc Natl Acad Sci. 1995;92:12250–12254. doi: 10.1073/pnas.92.26.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters DS, Stormo G, Gold L. Autogenous regulatory sites on the bacteriophage T4 gene 32 messenger RNA. J Mol Biol. 1988;201:517–535. doi: 10.1016/0022-2836(88)90634-1. [DOI] [PubMed] [Google Scholar]
- Melefors Ö, von Gabain A. Site-specific endoribonucleolytic cleavages and the regulation of stability of E. coli ompA mRNA. Cell. 1988;52:893–901. doi: 10.1016/0092-8674(88)90431-x. [DOI] [PubMed] [Google Scholar]
- Miczak A, Kaberdin V, Wei CL, Lin-Chao S. Proteins associated with RNase E in a multicomponent ribonucleolytic complex. Proc Natl Acad Sci. 1996;93:3865–3869. doi: 10.1073/pnas.93.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda G, Schuppli D, Barrera I, Hausherr C, Sogo JM, Weber H. Recognition of bacteriophage Qβ plus strand RNA as a template by Qβ replicase: Role of RNA interactions mediated by ribosomal proteins S1 and host factor. J Mol Biol. 1997;267:1089–1103. doi: 10.1006/jmbi.1997.0939. [DOI] [PubMed] [Google Scholar]
- Muffler A, Fischer D, Hengge-Aronis R. The RNA-binding protein HF-I, known as a host factor for phage Qβ RNA replication, is essential for rpoS translation in Escherichia coli. Genes & Dev. 1996;10:1143–1151. doi: 10.1101/gad.10.9.1143. [DOI] [PubMed] [Google Scholar]
- Muffler A, Traulsen DD, Fischer D, Lange R, Hengge-Aronis R. The RNA-binding protein HF-1 plays a global regulatory role which is largely but not exclusively, due to its role in expression of the ςs subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1997;179:297–300. doi: 10.1128/jb.179.1.297-300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G, Belasco JG, Cohen SN, von Gabain A. Effect of premature termination of translation on mRNA stability depends on the site of ribosome release. Proc Natl Acad Sci. 1987;84:4890–4894. doi: 10.1073/pnas.84.14.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G, Lundberg U, von Gabain A. In vivo and in vitro identity of site specific cleavages in the 5′ non-coding region of ompA and bla mRNA in Escherichia coli. EMBO J. 1988;7:2269–2275. doi: 10.1002/j.1460-2075.1988.tb03067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe C, Eyermann F, Benard L, Portier C, Ehresmann B, Ehresmann C. Ribosomal protein S15 from Escherichia coli modulates its own translation by trapping the ribosome on the mRNA loading site. Proc Natl Acad Sci. 1993;90:4394–4398. doi: 10.1073/pnas.90.10.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py B, Causton H, Mudd EA, Higgins CF. A protein complex mediating mRNA degradation in Escherichia coli. Mol Microbiol. 1994;14:717–729. doi: 10.1111/j.1365-2958.1994.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Rapaport LR, Mackie GA. Influence of translation efficiency on the stability of the mRNA for ribosomal protein S20 in Escherichia coli. J Bacteriol. 1994;176:992–998. doi: 10.1128/jb.176.4.992-998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch A, Tedin K, Gründling A, Mündlein A, Bläsi U. Downstream box-anti downstream box interactions are dispensable for translation initiation of leaderless mRNA. EMBO J. 1996;15:4740–4748. [PMC free article] [PubMed] [Google Scholar]
- Schuppli D, Miranda G, Tsui HCT, Winkler ME, Sogo JM, Weber H. Altered 3′-terminal RNA structure in phage Qβ adapted to host factor-less Escherichia coli. Proc Natl Acad Sci. 1997;94:10239–10242. doi: 10.1073/pnas.94.19.10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q, Schuppli D, Tsui HCT, Winkler ME, Weber H. Strongly reduced phage Qβ replication, but normal phage MS2 replication in an Escherichia coli K12 mutant with inactivated Qβ host factor (hfq) gene. Virology. 1997;227:211–214. doi: 10.1006/viro.1996.8302. [DOI] [PubMed] [Google Scholar]
- Tedin K, Resch A, Bläsi U. Requirements for ribosomal protein S1 for translation initiation of mRNAs with and without a 5′ leader sequence. Mol Microbiol. 1997;25:189–199. doi: 10.1046/j.1365-2958.1997.4421810.x. [DOI] [PubMed] [Google Scholar]
- Tsui HCT, Leung HCE, Winkler ME. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol Microbiol. 1994;13:35–49. doi: 10.1111/j.1365-2958.1994.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Tsui HCT, Feng G, Winkler ME. Negative regulation of mutS and mutH repair gene expression by the Hfq and RpoS global regulation of Escherichia coli K-12. J Bacteriol. 1997;179:7476–7487. doi: 10.1128/jb.179.23.7476-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C, Eddy S, Parma D, Gold L. Autogenous translational operator recognized by bacteriophage T4 DNA polymerase. J Mol Biol. 1990;213:749–761. doi: 10.1016/S0022-2836(05)80261-X. [DOI] [PubMed] [Google Scholar]
- Vytvytska O, Jacobsen JS, Balcunaite G, Andersen JS, Baccarini M, von Gabain A. Host factor I, Hfq, binds to Escherichia coli ompA mRNA in a growth-rate dependent fashion and regulates its stability. Proc Natl Acad Sci. 1998;95:14118–14123. doi: 10.1073/pnas.95.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner LA, Gesteland RF, Dayhuff TJ, Weiss RB. An efficient Shine-Dalgarno sequence but not translation is necessary for lacZ mRNA stability in Escherichia coli. J Bacteriol. 1994;176:1683–1688. doi: 10.1128/jb.176.6.1683-1688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter RB, Morrissey L, Gauss P, Gold L, Hsu T, Karam J. Bacteriophage T4 regA protein binds mRNAs and prevents translation initiation. Proc Natl Acad Sci. 1987;84:1822–1826. doi: 10.1073/pnas.84.22.7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchuk O, Iost I, Dreyfus M. The relation between translation and mRNA degradation in the lacZ gene. Biochimie. 1991;73:1533–1541. doi: 10.1016/0300-9084(91)90188-7. [DOI] [PubMed] [Google Scholar]
- Yarchuk O, Jacques N, Guillerez J, Dreyfus M. Interdependence of translation, transcription and mRNA degradation in the lacZ gene. J Mol Biol. 1992;226:581–596. doi: 10.1016/0022-2836(92)90617-s. [DOI] [PubMed] [Google Scholar]