Abstract
Phosphate (Pi) deficiency is one of the leading causes of loss in crop productivity. Plants respond to Pi deficiency by increasing Pi acquisition and remobilization involving organic and inorganic Pi transporters. Here, we report the functional characterization of a putative organic Pi transporter, Glycerol-3-phosphate permease (G3Pp) family, comprising five members (AtG3Pp1 to -5) in Arabidopsis (Arabidopsis thaliana). AtG3Pp1 and AtG3Pp2 showed 24-and 3-fold induction, respectively, in the roots of Pi-deprived seedlings, whereas Pi deficiency-mediated induction of AtG3Pp3 and -4 was evident in both roots and shoots. Furthermore, promoter-β-glucuronidase (GUS) fusion transgenics were generated for AtG3Pp2 to -5 for elucidation of their in planta role in Pi homeostasis. During Pi starvation, there was a strong expression of the reporter gene driven by AtG3Pp4 promoter in the roots, shoots, anthers, and siliques, whereas GUS expression was specific either to the roots (AtG3Pp3) or to stamens and siliques (AtG3Pp5) in other promoter-GUS fusion transgenics. Quantification of reporter gene activities further substantiated differential responses of AtG3Pp family members to Pi deprivation. A distinct pattern of reporter gene expression exhibited by AtG3Pp3 and AtG3Pp5 during early stages of germination also substantiated their potential roles during seedling ontogeny. Furthermore, an AtG3Pp4 knockdown mutant exhibited accentuated total lateral root lengths under +phosphorus and −phosphorus conditions compared with the wild type. Several Pi starvation-induced genes involved in root development and/or Pi homeostasis were up-regulated in the mutant. A 9-fold induction of AtG3Pp3 in the mutant provided some evidence for a lack of functional redundancy in the gene family. These results thus reflect differential roles of members of the G3Pp family in the maintenance of Pi homeostasis.
Phosphorus (P) plays a central role in metabolic processes like photosynthesis, respiration, glycolysis, maintenance of redox balance, and energy synthesis and is an indispensable building block for the biosynthesis of nucleic acids and phospholipids (Jain et al., 2007b; Schachtman and Shin, 2007). Phosphate (Pi) is the only anion capable of forming charged diesters at physiological pH and impacts the phosphorylation and dephosphorylation of proteins, thus making it a crucial component of signal transduction (Vance et al., 2003; Epstein and Bloom, 2004; Ticconi and Abel, 2004).
Pi forms organic complexes and undergoes inorganic fixation with cations in soil solution, thus rendering it as the least available macronutrient (2–10 μm) in many agroecosystems (Marschner, 1995). An array of morphophysiological, biochemical, and molecular adaptations help the plant to cope with Pi deficiency (Raghothama, 1999; Raghothama and Karthikeyan, 2005; Schachtman and Shin, 2007). These adaptations include reduced plant growth, anthocyanin accumulation, modulation of root system architecture (RSA), increased secretion of phosphatases and organic acids, activation of alternate nonphosphorylating glycolytic pathways, and differential expression of a large number of Pi-responsive genes (López-Bucio et al., 2002; Rausch and Bucher, 2002; Vance et al., 2003; Hammond et al., 2004; Misson et al., 2005).
Among the molecular responses, Pi starvation-induced high-affinity Pi transporters play a pivotal role in the acquisition and mobilization of Pi in plants (Karthikeyan et al., 2002; Mudge et al., 2002; Rausch and Bucher 2002; Shin et al., 2004). Promoter-reporter gene fusions of Pht1;4, Pht1;5, Pht1;6, and Pht1;7 have indicated their potential involvement in the remobilization of Pi from older leaves to developing organs like younger leaves, flowers, and siliques (Karthikeyan et al., 2002; Mudge et al., 2002). Nearly 80% of Pi in senescing leaves of Arabidopsis (Arabidopsis thaliana), pine (Pinus sp.), pea (Pisum sativum), soybean (Glycine max), and wheat (Triticum aestivum) appears to be remobilized, and low-affinity transporters may play a major role in the movement of Pi along the concentration gradient (Himmelblau and Amasino, 2001). The organellar Pi homeostasis is maintained by a host of transporters responsible for mobilizing organic and inorganic Pi. In addition, transporters facilitating the movement of phosphorylated C3 and C6 compounds play a role in the metabolic adaptations during Pi stress. These include the triose-phosphate, phosphoenolpyruvate phosphate, Glc-6-P, and xylulose-5-phosphate translocators (Stitt, 1997; Streatfield et al., 1999; Flügge, 2001; Eicks et al., 2002).
Glycerol-3-phosphate transporter (GlPt), a sugar-phosphate/anion antiporter, has also been implicated in Pi mobilization (Elvin et al., 1985; Huang et al., 2003). GlPt has been characterized in Escherichia coli, and it mediates not only the exchange of glycerol-3-phosphate (G3P) and Pi across the periplasmic membrane (Silhavy et al., 1976; Fann et al., 2003; Lemieux et al., 2004) but also transports glycerol-2-phosphate, arsenate, and an antibiotic named fosmidomycin (Elvin et al., 1985). The presence of homologs of this gene in Mus musculus, Haemophilus influenzae, Bacillus subtilis, spirochetes, Drosophila melanogaster, and plants (rice [Oryza sativa], potato [Solanum tuberosum], tomato [Solanum lycopersicum], soybean, and maize [Zea mays]) and the conserved nature of certain residues (Bartoloni et al., 2000) suggest that members of the GlPt family are likely conserved functionally across the plant and animal kingdoms. An earlier study of global gene expression using an Affymetrix gene chip revealed differential regulation of Glycerol-3-phosphate permease (G3Pp) during Pi deficiency in Arabidopsis (Misson et al., 2005). Therefore, the involvement of G3Pp in the mobilization of G3P generated by the hydrolysis of diacylglycerol, a product of phospholipid breakdown during Pi starvation, could be postulated.
In this study, using transcript analysis and promoter-reporter fusion lines, we show Pi deficiency-mediated differential spatiotemporal regulation across members of the Arabidopsis G3Pp family. LePS3, a G3Pp from tomato, was also found to be responsive to Pi deprivation. The potential involvement of G3Pps in seedling ontogeny was also demonstrated. Analysis of the mutant atg3pp4 exhibited compensation by other members within the gene family. In addition, we show that the mutant revealed an altered root phenotype, and several Pi starvation-induced genes involved in root development and/or Pi homeostasis were up-regulated.
RESULTS
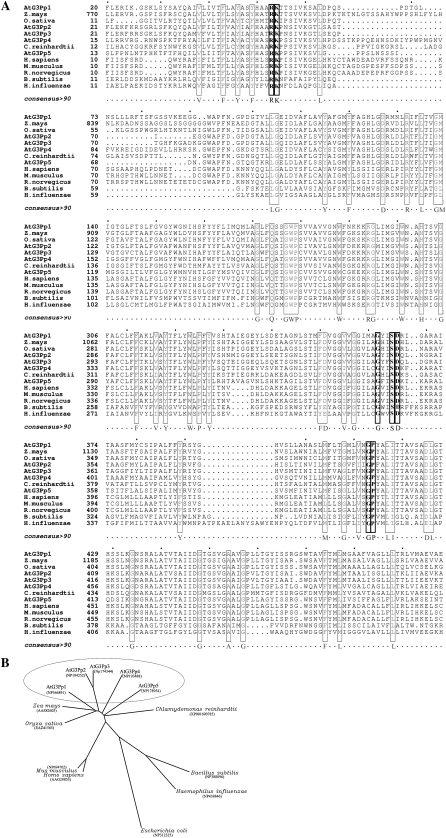
Bioinformatic analysis revealed five putative G3Pps in Arabidopsis homologous to GlPt in E. coli. The nomenclature of the members of the G3Pp family, the lengths of their open reading frames, the sizes of their putative proteins, and their predicted subcellular localizations are presented (Supplemental Tables S1 and S2). An alignment of putative amino acid sequences of G3Pps from various organisms revealed the presence of conserved residues (Fig. 1A). The amino acids contributing to the permease activity of G3Pps (Bartoloni et al., 2000) were found within these conserved regions. Furthermore, ClustalW alignment was used to derive the phylogenetic relationship of Arabidopsis G3Pps with those from lower and higher organisms at the amino acid level (Fig. 1B). The percentage amino acid identity across the members of the Arabidopsis G3Pp family varied from 51% to 81%. High percentage similarity (81%) was revealed between AtG3Pp2 and AtG3Pp3. In addition, Arabidopsis G3Pps also shared homology with the G3Pps from diverse plant species. For instance AtG3Pp1 shared about 65% identity with the G3Pps from rice and maize, whereas 52% similarity was detected between AtG3Pp5 and G3Pp from a lower organism (Chlamydomonas reinhardtii). However, only a limited identity was exhibited between Arabidopsis G3Pps and those from animal species and prokaryotes, despite their having many conserved residues considered essential for transport activity.
Figure 1.
Comparative analysis of G3Pps. A, Alignment of the putative amino acid sequences of G3Pps from various organisms. Highly conserved residues are enclosed by boxes. Residues in boldface are considered essential for transport function. B, Unrooted tree of the G3Pps from various plant and animal species derived by ClustalW alignment.
Pi Deficiency-Mediated Differential Spatial Expression of Members of the G3Pp Family
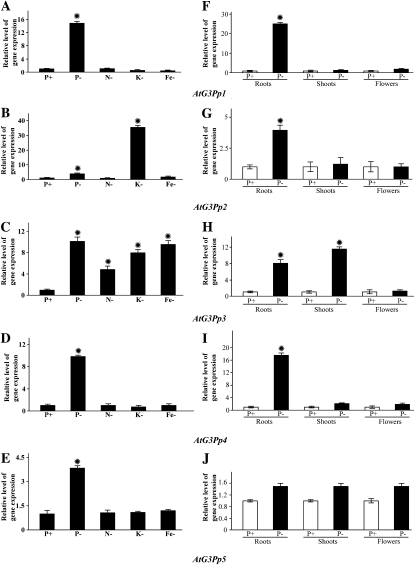
Real-time PCR analyses with gene-specific primers were carried out for evaluating the effects of different nutrient deficiencies on the whole seedlings and of Pi deprivation on the spatial expression profiles of different members of the G3Pp family (Fig. 2). For determining the effects of various nutrient deficiencies, seedlings were grown under nutrient-rich conditions (P+) and deprived of one of the nutrients (nitrogen [N], potassium [K], and iron [Fe]) for 7 d (Fig. 2, A–E). There was a distinct differential effect of Pi deprivation on the relative expression profiles of members of the G3Pp family, with induction ranging from as high as 12-fold in AtG3Pp1 to only 2-fold in AtG3Pp5. Several studies have also shown a prevalence of cross talk between P and the macroelement K and the microelements (zinc and Fe) in Arabidopsis and other crop species (Huang et al., 2000; Wang et al., 2002; Sánchez-Calderón et al., 2005; Svistoonoff et al., 2007; Ward et al., 2008). Therefore, the effects of other nutrient deficiencies (N−, K−, and Fe−) were also evaluated on the relative expression profiles of all members of the AtG3Pp family. Similar to the effect of Pi deprivation, the differential effects of other nutrient deficiencies were also evident in the AtG3Pp family. For instance, AtG3Pp3 showed significant induction in response to both N and Fe deficiencies. K deficiency also triggered 8- and 35-fold inductions of AtG3Pp3 and AtG3Pp2, respectively, compared with P+ seedlings. These data showed the differential effects of various nutrient deficiencies on the relative expression profiles of different members of the AtG3Pp family. Although the relative expression profiles to various nutrient deficiencies revealed identical patterns in AtG3Pp1, AtG3Pp4, and AtG3Pp5, there were significant differences in their magnitude, and AtG3Pp3 was induced significantly (about 4- to 9-fold) when the seedlings were grown in the medium deprived of any one of these nutrients. This suggested a complex cross talk across different nutrient signaling pathways resulting in differential regulation of this gene family. Relatively, the effect of Pi deprivation was more specific and pronounced on the expression of AtG3Pp1 and AtG3Pp4.
Figure 2.
Relative expression analyses of G3Pps during Pi and other nutrient deficiencies. Real-time PCR analyses are shown for whole seedlings grown under full nutrient solution (P+) and those subjected to different nutrient deficiencies (Pi, N, K, and Fe) for 7 d (A–E) and different tissues (roots, shoots, and flowers) of hydroponically grown 4-week-old wild-type seedlings that were subjected to P+ (250 μm Pi) and P− (0 μm Pi) treatments for 7 d (F–J). AtACT2 was used as an internal control, and P+ values were normalized to 1. Data presented are means of six technical replicates ± se. An asterisk indicates a significant (>2.0-fold) increase in the relative level of gene expression compared with P+.
Misson et al. (2005) carried out a detailed global spatiotemporal expression analysis of Pi-deprived genes in Arabidopsis by using a whole genome Affymetrix gene chip. The analysis revealed early and sustained induction of AtG3Pp1, whereas the expression of AtG3Pp3 was detected only in the roots during long-term Pi deprivation. Other members (AtG3Pp2, AtG3Pp4, and AtG3Pp5) did not show any significant induction in response to Pi deprivation. To further validate these observations under our growth conditions, the spatial expression of all members of the AtG3Pp family was evaluated in seedlings grown under different Pi regimes (Fig. 2, F–J). The relative abundance of AtG3Pp1 and AtG3Pp4 transcripts was significantly higher in the roots compared with other members of this family, whereas Pi deficiency resulted in significant induction of AtG3Pp3 and AtG3Pp4 in the shoots. None of the members of this family showed any significant induction in the flowers during Pi deprivation. Induction of AtG3Pp1 and AtG3Pp2 specifically in the roots suggested their putative involvement in the acquisition of organic P. On the other hand, the role of AtG3Pp3 and AtG3Pp4 appeared to be broader due to their induction in both Pi-deprived roots and shoots. In an earlier study, differential screening of a tomato subtraction library was used for isolating a cDNA clone encoding G3Pp, designated as LePS3 (Baldwin et al., 1999). In Pi-deprived tomato plants, LePS3 exhibited higher abundance of the transcripts in the roots than in the leaves (Supplemental Fig. S1). These results highlighted the potential role of G3Pp in the maintenance of Pi homeostasis in taxonomically diverse plant species.
Differential Responsiveness of G3Pp Promoter-GUS Fusion Transgenics to Pi Deprivation during Vegetative and Reproductive Growth Phases
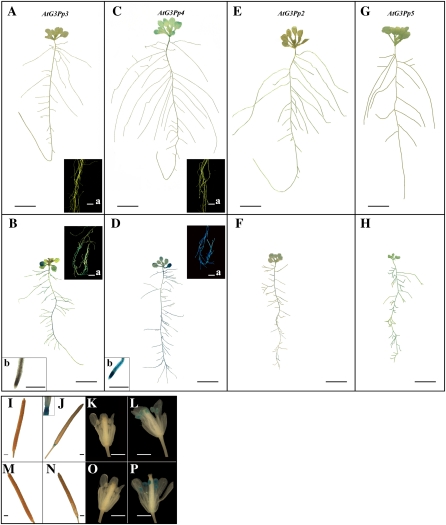
To characterize the in planta spatial responses of members of the G3Pp family to Pi deprivation, promoter-reporter gene fusions were generated. The promoter-GUS fusion transgenics were developed by amplifying the promoter region of each gene and fusing it to the coding region of the GUS reporter. The length of the promoter sequence used for the transcription-fusion constructs is presented in Supplemental Table S1. Bioinformatic analysis of the promoter sequences of the G3Pps in Arabidopsis revealed the presence of several potential binding sites for transcription factors (Supplemental Table S3). The fusion constructs were introduced into Arabidopsis by Agrobacterium tumefaciens-mediated transformation (Clough and Bent, 1998). The transgenics were screened for kanamycin resistance, and a minimum of 10 independent lines were recovered for each of the promoter-GUS fusions. Multiple lines for each of the promoter-GUS fusions were tested for reporter gene expression in response to Pi stress during vegetative (petri plate culture) and reproductive (hydroponics) growth phases. One line was chosen as a representative for detailed characterization. Despite repeated attempts, promoter-GUS fusion transgenics for AtG3Pp1 could not be generated. Therefore, subsequent studies were carried out with promoter-GUS fusion transgenics generated for AtG3Pp2 to -5. To elucidate the effects of Pi deprivation on the responses of the promoter-GUS fusion transgenics during the early vegetative growth phase, 7-d-old seedlings (grown on 0.5× Murashige and Skoog medium) were transferred to vertically oriented petri plates containing P+ and P− media (Fig. 3). Under Pi-replete conditions, the expression of the reporter gene driven by the AtG3Pp3 promoter was neither evident in the cotyledonary leaves (Fig. 3A) nor in the root system (Fig. 3A, inset a). However, Pi starvation resulted in a pronounced expression of the reporter gene driven by this promoter both in the cotyledonary leaves (Fig. 3B) and in the root system (Fig. 3B, inset a), but with a notable lack of activity in the primary root tip (Fig. 3B, inset b). Pi deprivation augmented the expression of the reporter gene driven by the AtG3Pp4 promoter by many fold in both the rosette leaves (Fig. 3D) and the root system (Fig. 3D, inset a), including the primary root tip (Fig. 3D, inset b). On the contrary, only faint expression of the reporter gene was observed in Pi-deprived AtG3Pp2-GUS and AtG3Pp5-GUS transgenics (Fig. 3, F and H, respectively). These data thus highlighted the differential spatial regulation of the reporter gene activity driven by different members of the G3Pp family.
Figure 3.
Differential Pi deficiency-mediated GUS-reporter gene expression driven by the promoters of the different members of G3Pp family. A to H, Transgenic plants (2 weeks old) grown on vertically oriented agar petri plates containing P+ (A, C, E, and G) and P− (B, D, F, and H) nutrient media revealed differential in planta expression of the GUS-reporter gene driven by AtG3Pp3 (A and B), AtG3Pp4 (C and D), AtG3Pp2 (E and F), and AtG3Pp5 (G and H) promoters during the vegetative growth phase. The insets (a) in A, C and B, D show magnified images of the reporter gene expression in the root system of P+ and P− transgenics, respectively. The insets (b) in B and D show reporter gene expression in the primary root tip. I to P, Four-week-old transgenics (AtG3Pp4-GUS and AtG3Pp5-GUS) were grown hydroponically under P+ (I, K, M, and O) and P− (J, L, N, and P) conditions for 7 d. Expression of the reporter gene is shown in siliques (I and J) and flowers (K and L) driven by the AtG3Pp4 promoter and in siliques (M and N) and flowers (O and P) driven by the AtG3Pp5 promoter. Photographs are representative of eight to 10 GUS-stained transgenic seedlings for each treatment. Bars = 1 mm for I to P and insets b, 5 mm for insets a, and 1 cm for A to H.
To further determine whether such differential regulation is evident during the reproductive phase, 3-week-old hydroponically grown transgenic plants were subjected to P+ and P− treatments for 7 d and the expression of the reporter gene was assayed in the flowers and siliques (Fig. 3, I–P). Transgenic plants (AtG3Pp4-GUS and AtG3Pp5-GUS) grown under Pi-replete conditions did not show expression of the reporter gene in siliques (Fig. 3, I and M) and flowers (Fig. 3, K and O). However, Pi deficiency brought about the expression of the reporter gene driven by the AtG3Pp4 promoter at the junction of the silique and the pedicel (Fig. 3J), in the stamens (Fig. 3L), and in the root system and the rosette leaves (Supplemental Fig. S2A). A similar trend of Pi deficiency-induced reporter gene expression driven by the AtG3Pp5 promoter was also observed at the junction of the silique and the pedicel (Fig. 3N) and in the stamens (Fig. 3P) of hydroponically grown plants. However, reporter gene expression driven by this promoter was not detected in the leaves or the root system of the hydroponically raised seedlings (Supplemental Fig. S2B). On the contrary, reporter gene driven either by the AtG3Pp3 or AtG3Pp2 promoter did not show any expression in the reproductive organs of Pi-deprived transgenic seedlings (data not shown). In case of AtG3Pp3, reporter gene expression was largely confined to the root system (Supplemental Fig. S2C). The least Pi-responsive member of the G3Pp family was AtG3Pp2, as indicated by a rather faint expression in the petioles of the leaf (Supplemental Fig. S2D). These results thus demonstrated the effect of Pi deficiency on spatial responses of different members of the G3Pp family both during vegetative and reproductive growth phases. However, there was an apparent incongruity in the relative expression profiles of AtG3Pp4 and AtG3Pp5 and their respective GUS expression analyses of promoter-reporter fusion transgenics (Figs. 2 and 3). To resolve this discrepancy, GUS activity was quantified in the transgenic seedlings (AtG3Pp2-5-GUS) that were grown under P+ and P− conditions for 7 d (Supplemental Fig. S3). Although GUS quantification was largely consistent with the data generated from real-time PCR, there was an apparent inconsistency between these two assays for AtG3Pp4 and AtG3Pp5 in Pi-deprived flowers.
G3Pps Have a Role during Seedling Growth in Arabidopsis
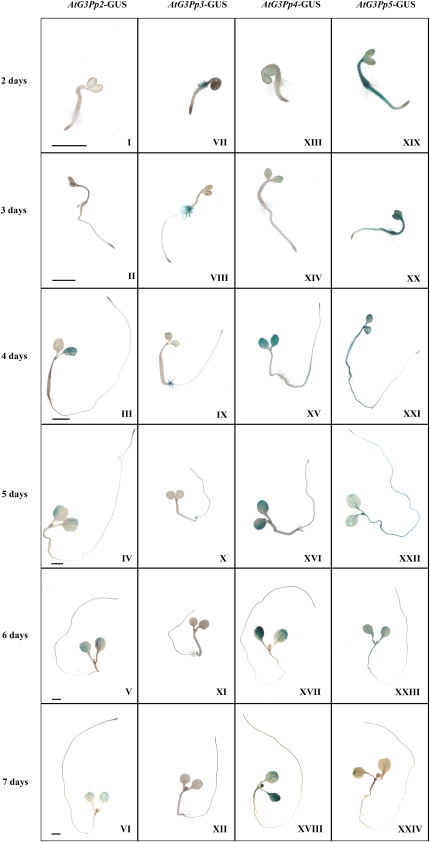
An earlier study has demonstrated the breakdown of stored lipids and the generation of G3P during seed germination (Baud et al., 2002). Therefore, to decipher whether there is a role for the G3Pp family during early stages (days 1–7) of seedling growth, reporter gene expression driven by G3Pps was evaluated (Fig. 4). Although AtG3Pp2 promoter-driven GUS expression was not seen in the cotyledonary leaves on days 2 and 3 after seed germination (Fig. 4, I and II), it became evident on day 4 and persisted until day 7 (Fig. 4, III–VI). However, the expression of the reporter gene driven by this promoter was not evident in the roots of the seedling at any of the developmental stages observed (Fig. 4, I–VI). On the contrary, the expression of the reporter gene driven by the AtG3Pp3 promoter was clearly evident in the root hairs at the collet region (hypocotyl-root junction) on day 2 after germination, which persisted until day 5 (Fig. 4, VII–IX). However, on days 6 and 7, there was a significant reduction in the expression of the reporter gene in this region (Fig. 4, X–XII). This expression pattern in the root hairs during early stages of seedling growth was unique to AtG3Pp3. The expression of the reporter gene driven by the AtG3Pp4 promoter was not evident on day 2 after seed germination (Fig. 4, XIII). However, the expression became prominent in the cotyledonary leaves and relatively weaker in primary root close to the hypocotyl-root junction from day 3 onward and persisted until day 7 (Fig. 4, XIV–XVIII). Interestingly, the AtG3Pp5 promoter fused to the GUS reporter gene showed a strong expression in the whole seedling, with a notable lack of expression in the root tip and the root hairs at the collet region 2 d after germination (Fig. 4, XIX). However, the expression of the reporter gene driven by this promoter declined conspicuously during development of the seedling and was barely noticeable at 7 d after germination (Fig. 4, XX–XXIV). These results thus highlighted the differential spatiotemporal role of different members of the G3Pp family during seedling ontogeny.
Figure 4.
Differential expression patterns of the promoter-GUS fusion transgenics for the G3Pp gene family during seedling growth. Localization of GUS expression in the transgenic seedlings AtG3Pp2-GUS (I–VI), AtG3Pp3-GUS (VII–XII), AtG3Pp4-GUS (XIII–XVIII), and AtG3Pp5-GUS (XIX–XXIV) is shown at 2 to 7 d after germination. The photographs are representative of 10 to 15 GUS-stained transgenic seedlings for each developmental stage. Panels in the same row have similar scales. Bars = 1 mm.
Characterization of the AtG3Pp4 Knockdown Mutant
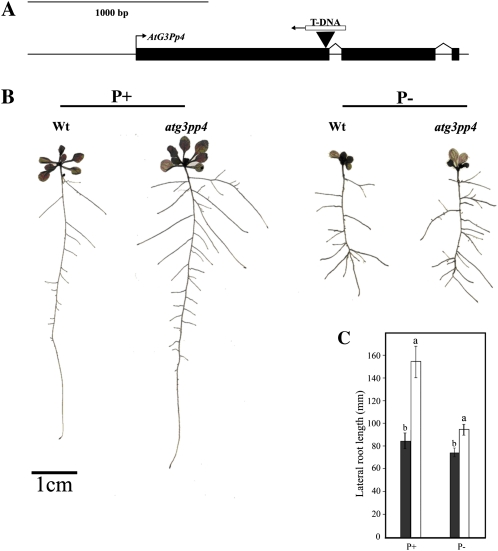
To further understand the in planta role of G3Pps in Arabidopsis, we screened T-DNA insertion mutants for all members using gene-specific primers (Supplemental Table S4). Among the T-DNA insertion lines, SALK071338.34.95.x (AtG3Pp4) displayed reduced expression of the transcripts, thereby confirming it to be a knockdown mutant, whereas other T-DNA insertion lines showed transcript accumulation similar to that of wild-type plants. The reason for this could be due to the presence of insertions either in the promoter region (AtG3Pp1, AtG3Pp3, AtG3Pp5) or in the 3′ untranslated region (AtG3Pp2). Since Pi deficiency induced a many-fold increase in the expression of the reporter gene driven by the AtG3Pp4 promoter in both vegetative and reproductive tissues (Fig. 3), we further evaluated the effects of knockdown mutation of this gene on Pi deficiency-related morphological (Fig. 5) and molecular (Fig. 6) traits. The T-DNA insertion in the SALK mutant for AtG3Pp4 was confirmed by PCR, and sequencing showed the insertion in the first exon, between 1,030 and 1,051 nucleotides, leading to the loss of 21 nucleotides (Fig. 5A). Real-time PCR analysis revealed a significant reduction in the expression of this gene in Pi-deprived seedlings, confirming it to be a knockdown mutant (Fig. 6A). Several studies have shown a modulation of RSA in response to Pi deprivation (Williamson et al., 2001; López-Bucio et al., 2002, 2005; Nacry et al., 2005; Jain et al., 2007a). Therefore, to assess the effect of mutation in AtG3Pp4, if any, on the different traits of RSA, seedlings were raised on vertically oriented petri plates containing P+ and P− media. Although Pi deprivation resulted in a significant (P < 0.05) reduction of primary root length in the wild type and atg3pp4, no significant (P < 0.05) differences were observed between them under both P+ and P− conditions (data not shown). However, the mutant seedlings revealed a significant increase in the total lateral root length under both P+ and P− conditions as compared with the wild-type seedlings (Fig. 5, B and C). These data suggested a potential role of AtG3Pp4 in root development under both high- and low-Pi environments. Despite significant differences in lateral root development between the wild type and atg3pp4, the total P concentration was comparable between them (data not shown).
Figure 5.
Phenotypic characterization of atg3pp4. A, Diagrammatic illustration, drawn to scale, showing the insertion of the T-DNA in AtG3Pp4. Boxes indicate the exons, and lines between them represent the introns. The arrow at the end of the T-DNA indicates the orientation of the insertion in the gene. B, RSA is representative of 20 seedlings each of the wild type (Wt) and atg3pp4 grown on vertically oriented petri plates containing P+ and P− media. Lateral roots of the wild type and mutant were spread under a stereomicroscope and scanned at 600 dots per inch to document their architectural details. C, Data are presented for total lateral root length of wild-type (black bars) and atg3pp4 (white bars) seedlings grown under P+ and P− conditions. Values are means ± se; n = 20. Different letters on the histograms indicate that the means differ significantly (P ≤ 0.05). [See online article for color version of this figure.]
Figure 6.
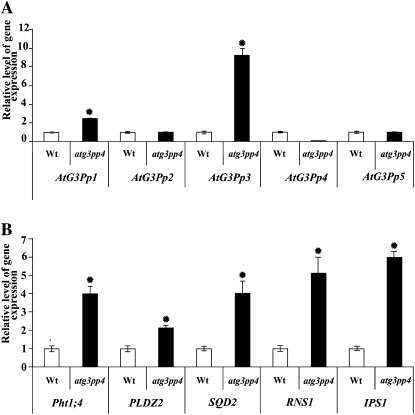
Real-time PCR analyses of the relative expression levels of various G3Pps and PSI genes in atg3pp4. Wild-type (Wt) and atg3pp4 seedlings were grown on vertically oriented agar petri plates containing P+ medium for 14 d. Data are shown for the relative expression levels of G3Pps (A) and PSI (B) genes in the wild type and the mutant. The Arabidopsis housekeeping gene AtACT2 (At3g18780) was used as an internal control. The expression values for the G3Pps and PSI genes in the wild type were set at 1. The values presented are means of six technical replicates ± se. An asterisk indicates significant (>2.0 fold) increase in the relative level of gene expression compared with wild type.
To determine if there is any compensation of the mutation in AtG3Pp4 by other members of the G3Pp family, transcript abundance was determined by real-time PCR analysis (Fig. 6A). Although atg3pp4 seedlings grown under P+ condition revealed only a marginal increase in the expression of AtG3Pp1, there was a 9-fold induction of AtG3Pp3 in the mutant. These data indicated that some members of the family could potentially compensate for the lack of expression of AtG3Pp4. Since there was a significant modulation of lateral root development in the mutant (Fig. 5, B and C), we further hypothesized the possible effects of the mutation on some of the Pi starvation-induced genes potentially implicated in root development and maintenance of Pi homeostasis. An earlier study has shown the involvement of a Pi-responsive phospholipase D (PLDZ2; At3g05630) in root development (Cruz-Ramírez et al., 2006). PLDZ2 along with many other Pi-responsive genes involved in Pi acquisition (Pht1;4 [At2g38940]; Shin et al., 2004), mobilization (RNS1 [At2g02990]; Bariola et al., 1994), and phospholipid substitution (SQD2 [At5g01220]; Yu et al., 2002) show early and sustained induction during Pi starvation (Misson et al., 2005). Although Pi starvation-induced IPSI (At3g09922) was not detected in an earlier microarray study (Misson et al., 2005), real- time PCR analysis of Pi-starved roots of hydroponically grown seedlings showed about 70-fold induction (Jain et al., 2009). Therefore, this gene was also included for real-time PCR analysis of the mutant (Fig. 6B). Interestingly, among the PSI genes, IPS1 and RNS1 showed 5- to 6-fold induction in the mutant. Relatively, there was only 4-fold induction of Pht1;4 and SQD2 and about 2-fold induction of PLDZ2. These results suggest the effect of mutation in AtG3Pp4 on the PSI genes involved in Pi mobilization, phospholipid substitution, and Pi acquisition, thereby indicating a global effect of the mutation on Pi homeostasis.
DISCUSSION
The Members of the G3Pp Family Show Pi Deficiency-Mediated Differential Spatial Regulation
Several high-affinity Pi transporters involved in Pi acquisition and mobilization are known to be induced as an adaptive response to Pi starvation (Mudge et al., 2002; Shin et al., 2004). Although a putative organic Pi transporter, G3Pp, has been implicated in the mobilization of G3P and the maintenance of Pi homeostasis in bacteria, its function in plants is not known. Therefore, the role of G3Pps, a gene family of five members in Arabidopsis, was investigated. The relative conservation of the G3Pps at the amino acid level in plants, animals, and bacteria (Fig. 1A) suggests the potential involvement of G3P transport in maintaining Pi homeostasis and redox potential across living organisms. This view could be corroborated by the Pi responsiveness of the AtG3Pp family (Fig. 2). Interestingly, real-time PCR analysis revealed Pi deficiency-mediated differential spatial responses of different members of the G3Pp family (Fig. 2, F–J).
The reporter gene activity in transgenic plants has traditionally been used in earlier studies to elucidate the spatiotemporal expression pattern of Pi-responsive genes (Karthikeyan et al., 2002; Mudge et al., 2002). The results of this study provided an insight into their potential involvement during seedling growth and development under Pi starvation condition (Fig. 3; Supplemental Fig. S2). For instance, reporter gene expression driven by the promoters of AtG3Pp3 and AtG3Pp4 was induced strongly in the roots (Fig. 3, B and D). These results suggested the potential involvement of G3Pps in the plant’s adaptation to Pi deficiency. Under Pi stress, the amount of galactolipids increase with a concomitant decrease in phospholipids, and this change is more drastic in the roots compared with the rosette leaves (Li et al., 2006). G3P is released by the breakdown of phospholipids, which are replaced by digalactosyldiacylglycerol in the nonplastidial membranes during Pi starvation (Härtel et al., 2000). A similar finding has been reported in oat (Avena sativa), where nearly 70% of the plasma membrane phospholipids were replaced by galactolipids when grown under severe Pi stress (Andersson et al., 2003). G3P is also generated by a glyceryl-phosphodiester phosphodiesterase induced intracellularly and extracellularly by Pi stress in Arabidopsis, carrot (Daucus carota), and sycamore (Acer pseudoplatanus) cell cultures (Van der Rest et al., 2002, 2004). Therefore, it is not surprising that Pi deficiency-mediated release of G3P and induction of G3Pps are coordinated. Although there were no significant increases in the relative expression levels of any members of the AtG3Pp family in Pi-deprived flowers, there was distinct GUS expression in the anthers and siliques of the transgenics (Fig. 3, I–P). There was evidence of incongruity in the data between real-time PCR and GUS histochemical analysis. At present, we do not have any empirical evidence to explain this. However, a few studies have attributed a lack of consistency in the steady-state mRNA levels and the activity of transcriptional GUS fusions to the involvement of some of the enhancer elements located upstream of the promoter and/or introns in the coding region exerting an influence on the expression of the gene (Koyoma et al., 2005; Jeong et al., 2006; Weise et al., 2008). Whether these elements could also have any influence on the expression of AtG3Pps is a matter of conjecture and definitely warrants further detailed investigations. Nevertheless, the reporter gene expression in the reproductive organs of AtG3Pp4-GUS and AtG3Pp5-GUS transgenics suggested their likely involvement during the transition from vegetative to reproductive phase, leading to the creation of a new Pi sink. Earlier studies have also highlighted a differential spatial regulation of members of the Pht1 family of high-affinity Pi transporters in Arabidopsis (Karthikeyan et al., 2002; Mudge et al., 2002). The specificities in the expression profiles of different G3Pps could be due to the interaction of trans-factors with specific cis-elements located on their promoters. However, the role of intron-mediated enhancement in the differential spatiotemporal regulation of different members of the G3Pp family could not be ruled out. Earlier studies have also shown the role of intron-mediated enhancement in the accumulation of transcripts in plants (Callis et al., 1987; Mascarenhas et al., 1990; Simpson and Filipowicz, 1996).
AtG3Pps Have a Role during Early Seedling Growth
Four members (AtG3Pp2, AtG3Pp3, AtG3Pp4, and AtG3Pp5) of the Arabidopsis G3Pp family are expressed during the early stages of germination, implicating them in the remobilization of P resources during early seedling growth (Fig. 4). During seed germination, triacylglycerol, a preferred form of carbon reserve, is broken down to release fatty acids and glycerol, which is converted to G3P by glycerol kinase (Baud et al., 2002). The generated G3P is then remobilized to photosynthetic tissues (Penfield et al., 2005). Therefore, in our study, the observed differential expression of the G3Pps could be potentially correlated with the production of G3P during seed germination. A coordinated release of G3P and induction of its transporters would facilitate the plants to utilize released carbon and P. Furthermore, during germination, a significant reduction in stored fractions of organic P in the oat endosperm was correlated with a concomitant increase of P in roots and leaves (Hall and Hodges, 1966). This further suggests a role for putative organic Pi transporters such as G3Pp during the early growth phase of the seedling.
The expression pattern of the AtG3Pp5-GUS (Fig. 4, XIX–XXIV) transgenic was observed to be similar to that of a high-affinity Pi transporter (Pht1;5) during germination and seedling growth (Mudge et al., 2002). GUS expression driven by the Pht1;5 promoter was observed in the cotyledons and the hypocotyl on day 3 after germination, which is similar to AtG3Pp5. The similarity in the expression of Pht1;5 and AtG3Pp5 confirms that both organic and inorganic P is mobilized during seedling development. The mobilized G3P could possibly be utilized in membrane lipid biosynthesis in actively expanding roots and leaves. A distinct expression of the reporter gene driven by AtG3Pp3 was also observed in the root hairs at the collet region from day 2 after germination (Fig. 4, VIII). Therefore, it can be presumed that translocation of G3P by AtG3Pp3 may have a role in the expansion of the root hair cells. A similar expression has been reported for the potassium transporter TRH1, involved in the generation of a potassium gradient required for the growth of root hair tips in Arabidopsis (Rigas et al., 2001). Second, during root hair formation, there is evidence of phospholipid signaling involving PLD, namely AtPLDZ1 (Ohashi et al., 2003). Also, AtPLDZ2 is expressed during days 1 to 4 after germination, and PLDZs have been shown to generate phosphatidic acid, a second messenger involved in signaling.
The Mutation in AtG3Pp4 Is Compensated, and the Mutant Has Morphological and Molecular Phenotypes
Interestingly, there was an increase in the expression of AtG3Pp1 and AtG3Pp3 but not of AtG3Pp2 and AtG3Pp5 (Fig. 6A) in atg3pp4, pointing toward a compensation within the gene family. A similar phenomenon was observed in the case of the Pht1 gene family and ethylene receptors in tomato comprising structurally diverse members. In the Pht1;4 knockout mutant, there was a distinct increase in the expression of Pht1;1 leading to higher Pi uptake rates (Shin et al., 2004). Similarly, NR and LeETR4 are two of the five ethylene receptors characterized in tomato, and it has been found that NR antisense lines show an increase in the expression of LeETR4 only (Tieman et al., 2000). Compensation by the members of the gene family was also observed in the Ubiquitin-specific protease gene family in Arabidopsis (Liu et al., 2008).
In Arabidopsis, inhibition of primary root growth, increase in lateral root density, and increases in the number and length of root hairs are among the characteristic responses to Pi starvation (Williamson et al., 2001; López-Bucio et al., 2002, 2005; Jain et al., 2007a). In addition to this modulation in RSA, the expression of several genes is also altered spatiotemporally during Pi deficiency (Misson et al., 2005). Therefore, we investigated the effects of the mutation in AtG3Pp4 on some of the morphological and molecular traits associated with Pi deficiency. Both the mutant and the wild type showed inhibition of primary root growth under P− condition (Fig. 5B). Interestingly, there was a significant increase in the number of higher order laterals in the mutant. The differential responses observed by primary and lateral roots in the wild type and knockdown of atg3pp4 could be due to their different ontogeny. For instance, primary and lateral roots are embryonic and postembryonic in origin, respectively. Also, an earlier study (Jain et al., 2007a) has shown that Pi deficiency-mediated inhibition of primary root is more of a local sensing response. This possibly suggests that knockdown of AtG3Pp4 influenced the traits that are specifically governed by systemic Pi status. However, there was no significant difference in the total P concentration between the mutant and the wild type, and thus a likelihood of P reallocation could be presumed. Studies have indicated the involvement of Pi starvation-induced genes in the development of lateral roots. For instance, the double mutant of PLDZ1 and PLDZ2 exhibited a low phosphatidic acid-mediated increase in lateral root growth (Li et al., 2006). Analysis of the root system of the Arabidopsis PLDZ2 mutant also revealed an alteration in RSA (Cruz-Ramírez et al., 2006). These studies thus suggested the possible role of Pi starvation-induced genes in the observed altered lateral root phenotype in the mutant. We also found the induction of IPS1 (6-fold), RNS1 (5-fold), Pht1;4 (4-fold), SQD2 (4-fold), and PLDZ2 (2-fold) in atg3pp4 (Fig. 6B) under Pi-replete condition. A similar pattern of induction of Pi starvation-induced genes such as IPS1, AtACP5, and Pht1;1 under P+ condition has been reported in the mutant for Phosphate transporter traffic facilitator1 (González et al., 2005). Further induction of At4, a gene belonging to the noncoding RNA class as IPS1, was observed in the Pht1;1 and Pht1;4 double mutant under Pi-replete conditions (Shin et al., 2004).
In conclusion, our study showed that the AtG3Pps are a gene family of putative organic Pi transporters induced by Pi starvation and that they exhibit differential spatiotemporal expression. They also seem to play a role during early seedling growth and display compensation within the gene family. The knockdown mutant atg3pp4 exhibited a modulation in root growth and induction of several Pi starvation-induced genes. Therefore, this study assigns a role for the AtG3Pp gene family in the maintenance of Pi homeostasis and seedling ontogeny.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia was used for this study. Surface-sterilized seeds (wild type and transgenics) were stratified at 4°C for 2 d and germinated (20–30 seeds per plate) initially on petri plates (150 mm × 15 mm) containing 0.5× Murashige and Skoog medium, supplemented with 1.5% (w/v) Suc and 1.2% (w/v) agar (Sigma A1296, lot no. 110K0195). Agar used in this study was analyzed for elemental composition by inductively coupled plasma-mass spectrometry, revealing an impurity of 15 μm Pi in 1.2% agar (Jain et al., 2009). Petri plates were placed vertically oriented under controlled conditions in a tissue culture room (16-h-day/8-h-night cycle at 22°C and average photosynthetically active radiation of 60–70 μmol m−2 s−1 provided by florescent tubes for 7 d). Subsequently, seedlings were transferred to P+ (1,250 μm Pi) and P− (0 μm Pi) medium (López-Bucio et al., 2002) for 7 d. KH2PO4 in Murashige and Skoog medium was replaced with K2SO4 for P− medium. For growing Arabidopsis in hydroponic culture, wild-type and transgenic plants were germinated on profile and grown in a greenhouse under a 16-h-light/8-h-dark cycle. Root systems of the 3-week-old plants (four- to six-leaf stage) were washed to get rid of the soil medium and subsequently transferred to hydroponics. The plants were allowed to acclimatize to these conditions in 0.5× modified Hoagland solution for 7 d and transferred to P+ (250 μm Pi) and P− (0 μm Pi) for 7 d. The different parts (roots, rosette leaves, and flowers) of wild-type plants were harvested for transcript analysis by real-time PCR.
Construction of Promoter-GUS Fusion Lines for G3Pps
Transcriptional fusions of the G3Pps with the GUS reporter gene were generated by cloning the promoters in the binary vector pGREEN-GUS. The GUS coding sequence from pGTV-BAR was excised using the XbaI and SacI enzyme sites and cloned into pGREEN vector. The promoters were amplified from genomic DNA, and restriction sites were included in the primers designed to amplify the length of the promoters mentioned in Supplemental Table S1.
Plant Transformation
Binary vectors containing the promoter-GUS fusions were introduced into Agrobacterium tumefaciens by the freeze-thaw method of transformation. The floral dip method was used to transform wild-type Arabidopsis plants (Clough and Bent, 1998). The transgenic seedlings were selected on 0.5× Murashige and Skoog medium containing 50 μg L−1 kanamycin. At least 10 independent transgenic lines were obtained for each construct and were confirmed by PCR analysis.
Identification of Insertion Mutants
Homozygous lines of atg3pp4 were isolated from the SALK T-DNA insertion line SALK_071338.34.95.x, available from the Arabidopsis Biological Resource Center at Ohio State University (Alonso et al., 2003). Individual homozygous lines were isolated by PCR using T-DNA left-border primer and gene-specific primers for AtG3Pp4. The actual site of insertion was identified by PCR and sequencing. The primers used for isolation of the homozygous lines are provided in Supplemental Table S5.
RSA Analysis
atg3pp4 seedlings grown on P+ and P− media were spread out on petri plates and scanned at 600 dots per inch using a desktop scanner (UMAX Powelook 2100 XL). The lengths of the primary and lateral roots were measured using the ImageJ program (http://rsb.info.nih.gov/ij).
Histochemical Analysis and GUS Quantification
GUS reaction buffer supplemented with 5 mm each of K3Fe(CN)6 and K4Fe(CN)6.3H2O was used for the histochemical analysis of reporter gene activity in the AtG3Pp promoter-GUS fusion lines (Malamy and Ryan, 2001). The seedlings were cleared with ethanol after staining for GUS, and about eight to 10 seedlings were analyzed for the expression profiles. GUS quantification was carried out as described (Karthikeyan et al., 2002).
RNA Extraction and Northern-Blot Analysis
Total RNA was extracted by using Trizol Reagent according to the manufacturer’s instructions (Life Technologies/Gibco BRL). Ten micrograms of total RNA was electrophoretically separated on a 1.2% (w/v) denaturing agarose/formaldehyde gel containing 1× MOPS buffer. The gel was blotted onto a 0.45-μm MAGNA nylon transfer membrane (Osmonics) following a standard protocol and UV cross-linked. Gene-specific primers were used for PCR amplification to generate the probes for LePS3. Probes were labeled with [32P]dCTP using the DECA prime II DNA-labeling kit (Ambion). For hybridization, the nylon membrane with transferred RNA was prehybridized at 42°C for 2 h in a solution containing 50% (v/v) formamide, 5× Denhardt’s solution, 6× SSPE, 0.1% (w/v) SDS, and 100 μg mL−1 denatured salmon sperm DNA (Sambrook et al., 1989). Membrane was washed twice with 2× SSC and 0.2% (w/v) SDS, at the temperature used for hybridization, for 5 min before autoradiography. Additional washes with 0.5× SSC or 0.2× SSC and 0.1% (w/v) SDS for 5 min were used, if required.
Real-Time PCR
Total RNA was isolated from the whole plants by using the RNeasy plant mini kit (Qiagen; http://www.qiagen.com/). Total RNA was treated with RQ1 RNase-free DNase (Promega; http://www.promega.com/) before reverse transcription. The DNase-treated RNA (1 μg) was reverse transcribed by using the SuperScript III first-strand synthesis kit (Invitrogen; http://www.invitrogen.com/). Real-time PCR was performed on an Applied Biosystems 7500 real-time PCR system using SYBR Green detection chemistry (Applied Biosystems; http://www.appliedbiosystems.com/) and gene-specific primers listed in Supplemental Table S6. The Arabidopsis housekeeping gene AtACT2 (At3g18780) was used as an internal control. The relative expression levels of the genes were computed by the 2–ΔΔCt method of relative quantification (Livak and Schmittgen, 2001).
Statistical Analysis
The data were subjected to Student’s t test, and different letters on the histograms were used to indicate means that were statistically different at P ≤ 0.05.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_001161041.1, NM_001161042.1, AF360170, NM_117861.2, NM_118654.3, and NM_102793.1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Sequence of LePS3 and its expression in Pi-deprived tomato.
Supplemental Figure S2. GUS expression driven by members of the G3Pp gene family in hydroponically raised Pi-deprived transgenics.
Supplemental Figure S3. Pi deficiency-mediated differential GUS activity in the promoter-reporter gene fusions for AtG3Pp family members.
Supplemental Table S1. Nomenclature, locus identifiers, length of open reading frames, predicted number of amino acids, and size of the promoters used for the promoter-GUS fusion constructs for each of the Arabidopsis G3Pp gene family members.
Supplemental Table S2. Putative subcellular localization of the Arabidopsis G3Pp family members as predicted by the pSORT program.
Supplemental Table S3. Number and sequence of the binding motifs present in the promoters of Arabidopsis G3Pps.
Supplemental Table S4. Details of the SALK mutants for the AtG3Pp family members.
Supplemental Table S5. Sequences of the primers used for the isolation of homozygous T-DNA insertion lines for AtG3Pp4.
Supplemental Table S6. Primers for real-time PCR analysis of AtG3Pps.
Acknowledgments
We thank Mike Poling and Vinay Nagarajan (Department of Horticulture and Landscape Architecture, Purdue University) for their valuable help in preparing the manuscript. We acknowledge Professor Richard B. Meagher (Department of Genetics, University of Georgia) for assistance with real-time PCR analyses.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Andersson MX, Stridh MH, Larsson KE, Liljenberg C, Sandelius AS. (2003) Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett 537: 128–132 [DOI] [PubMed] [Google Scholar]
- Baldwin JC, Karthikeyan AS, Raghothama KG. (1999) LePS3, a novel phosphate starvation induced gene in tomato (abstract no. 937). Annual American Society of Plant Physiologists Meeting, July 24–28, Baltimore. American Society of Plant Physiologists, Rockville, MD, p 190 [Google Scholar]
- Bariola PA, Howard CJ, Taylor CB, Verburg MT, Jaglan VD, Green PJ. (1994) The Arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. Plant J 6: 673–685 [DOI] [PubMed] [Google Scholar]
- Bartoloni L, Wattenhofer M, Kudoh J, Berry A, Shibuya K, Kawasaki K, Wang J, Asakawa S, Talior I, Bonne-Tamir B, et al. (2000) Cloning and characterization of a putative human glycerol 3-phosphate permease gene (SLC37A1 or G3PP) on 21q22.3: mutation analysis in two candidate phenotypes, DFNB10 and a glycerol kinase deficiency. Genomics 70: 190–200 [DOI] [PubMed] [Google Scholar]
- Baud S, Boutin JP, Miquel M, Lepiniec L, Rochat C. (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 40: 151–160 [Google Scholar]
- Callis J, Fromm M, Walbot V. (1987) Introns increase gene expression in cultured maize cells. Genes Dev 1: 1183–1200 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cruz-Ramírez A, Oropeza-Aburto A, Razo-Hernández F, Ramírez-Chávez E, Herrera-Estrella L. (2006) Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc Natl Acad Sci USA 103: 6765–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicks M, Maurino V, Knappe S, Flügge UI, Fischer K. (2002) The plastidic pentose phosphate translocator represents a link between the cytosolic and the plastidic pentose phosphate pathways in plants. Plant Physiol 128: 512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin CM, Hardy CM, Rosenberg H. (1985) Pi exchange mediated by the GlpT-dependent sn-glycerol-3-phosphate transport system in Escherichia coli. J Bacteriol 161: 1054–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Bloom AJ. (2004) Mineral Nutrition of Plants: Principles and Perspectives. Sinauer Associates, Sunderland, MA [Google Scholar]
- Fann MC, Busch A, Maloney PC. (2003) Functional characterization of cysteine residues in GlpT, the glycerol 3-phosphate transporter of Escherichia coli. J Bacteriol 185: 3863–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge UI. (2001) Plant chloroplasts and other plastids. Encyclopedia of Life Sciences. Macmillan Publishers, Nature Publishing Group, London [Google Scholar]
- González E, Solano R, Rubio V, Leyva A, Paz-Ares J. (2005) PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17: 3500–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JR, Hodges TK. (1966) Phosphorus metabolism of germinating oat seeds. Plant Physiol 41: 1459–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, Broadley MR, White PJ. (2004) Genetic responses to phosphorus deficiency. Ann Bot (Lond) 94: 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Dormann P, Benning C. (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA 97: 10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelblau E, Amasino MR. (2001) Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J Plant Physiol 158: 1317–1323 [Google Scholar]
- Huang C, Barker SJ, Langridge P, Smith FW, Graham RD. (2000) Zinc deficiency up-regulates expression of high-affinity phosphate transporter genes in both phosphate-sufficient and -deficient barley roots. Plant Physiol 124: 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. (2003) Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301: 616–620 [DOI] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG. (2007a) Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol 144: 232–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Poling MD, Smith AP, Nagarajan VK, Lahner B, Meagher RB, Raghothama KG. (2009) Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiol 150: 1033–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Vasconcelos MJ, Sahi SV, Raghothama KG. (2007b) Molecular mechanisms of plant adaptation to phosphate deficiency. Janick J, , Plant Breeding Reviews, Vol 29. John Wiley & Sons, Hoboken, NJ, pp 359–419 [Google Scholar]
- Jeong YM, Mun JH, Lee I, Woo JC, Hong CB, Kim SG. (2006) Distinct roles of the first introns on the expression of Arabidopsis profilin gene family members. Plant Physiol 140: 196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D’Urzo MP, Damsz B, Raghothama KG. (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Ono T, Shimizu M, Jinbo T, Mizuno R, Mitsukawa N, Kawazu T, Kimura T, Ohmiya K, Sakka K. (2005) Promoter of Arabidopsis thaliana phosphate transporter gene drives root specific expression of transgene in rice. J Biosci Bioeng 99: 38–42 [DOI] [PubMed] [Google Scholar]
- Lemieux MJ, Huang Y, Wang DN. (2004) Glycerol-3-phosphate transporter of Escherichia coli: structure, function and regulation. Res Microbiol 155: 623–629 [DOI] [PubMed] [Google Scholar]
- Li M, Welti R, Wang X. (2006) Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation: roles of phospholipases Dζ1 and Dζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol 142: 750–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang F, Zhang H, He H, Ma L, Deng XW. (2008) Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. Plant J 55: 844–856 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129: 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Pérez-Torres A, Rampey RA, Bartel B, Herrera-Estrella L. (2005) An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis: identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol 137: 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS. (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127: 899–909 [PMC free article] [PubMed] [Google Scholar]
- Marschner H. (1995) Mineral Nutrition of Higher Plants. Academic Press, London [Google Scholar]
- Mascarenhas D, Mettler IJ, Pierce DA, Lowe HW. (1990) Intron-mediated enhancement of heterologous gene expression in maize. Plant Mol Biol 15: 913–920 [DOI] [PubMed] [Google Scholar]
- Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, et al. (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102: 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW. (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31: 341–353 [DOI] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, Rossignol M, Doumas P. (2005) A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138: 2061–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, Ruberti I, Morelli G, Aoyama T. (2003) Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 300: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Penfield S, Graham S, Graham IA. (2005) Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem Soc Trans 33: 380–383 [DOI] [PubMed] [Google Scholar]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Raghothama KG, Karthikeyan AS. (2005) Phosphate acquisition. Plant Soil 274: 37–49 [Google Scholar]
- Rausch C, Bucher M. (2002) Molecular mechanisms of phosphate transport in plants. Planta 216: 23–37 [DOI] [PubMed] [Google Scholar]
- Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann KA, Grabov A, Dolan L, Hatzopoulos P. (2001) TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 13: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L. (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46: 174–184 [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Shin R. (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58: 47–69 [DOI] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ. (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39: 629–642 [DOI] [PubMed] [Google Scholar]
- Silhavy TJ, Hartig-Beecken I, Boos W. (1976) Periplasmic protein related to the sn-glycerol-3-phosphate transport system of Escherichia coli. J Bacteriol 126: 951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Filipowicz W. (1996) Splicing of precursors to mRNA in higher plants: mechanism, regulation and sub-nuclear organisation of the spliceosomal machinery. Plant Mol Biol 32: 1–41 [DOI] [PubMed] [Google Scholar]
- Stitt M. (1997) The flux of carbon between the chloroplast and cytoplasm. Plant Physiology, Biochemistry and Molecular Biology. Longman Scientific & Technical, Harlow, UK, pp 319–338 [Google Scholar]
- Streatfield SJ, Weber A, Kinsman EA, Häusler RE, Li J, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flügge UI, Chory J. (1999) The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell 11: 1609–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T. (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39: 792–796 [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Abel S. (2004) Short on phosphate: plant surveillance and countermeasures. Trends Plant Sci 9: 548–555 [DOI] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ. (2000) The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA 97: 5663–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- Van der Rest B, Boisson AM, Gout E, Bligny R, Douce R. (2002) Glycerophosphocholine metabolism in higher plant cells: evidence of a new glyceryl-phosphodiester phosphodiesterase. Plant Physiol 130: 244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Rest B, Rolland N, Boisson AM, Ferro M, Bligny R, Douce R. (2004) Identification and characterization of plant glycerophosphodiester phosphodiesterase. Biochem J 379: 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Garvin DF, Kochian LV. (2002) Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots: evidence for cross talk and root/rhizosphere-mediated signals. Plant Physiol 130: 1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG. (2008) The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol 147: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise A, Lalonde S, Kühn C, Frommer WB, Ward JM. (2008) Introns control expression of sucrose transporter LeSUT1 in trichomes, companion cells and in guard cells. Plant Mol Biol 68: 251–262 [DOI] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO. (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126: 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Xu C, Benning C. (2002) Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc Natl Acad Sci USA 99: 5732–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]