Abstract
We investigated responses of growth, leaf gas exchange, carbon-isotope discrimination, and whole-plant water-use efficiency (WP) to elevated CO2 concentration ([CO2]) in seedlings of five leguminous and five nonleguminous tropical tree species. Plants were grown at CO2 partial pressures of 40 and 70 Pa. As a group, legumes did not differ from nonlegumes in growth response to elevated [CO2]. The mean ratio of final plant dry mass at elevated to ambient [CO2] (ME/MA) was 1.32 and 1.24 for legumes and nonlegumes, respectively. However, there was large variation in ME/MA among legume species (0.92–2.35), whereas nonlegumes varied much less (1.21–1.29). Variation among legume species in ME/MA was closely correlated with their capacity for nodule formation, as expressed by nodule mass ratio, the dry mass of nodules for a given plant dry mass. WP increased markedly in response to elevated [CO2] in all species. The ratio of intercellular to ambient CO2 partial pressures during photosynthesis remained approximately constant at ambient and elevated [CO2], as did carbon isotope discrimination, suggesting that WP should increase proportionally for a given increase in atmospheric [CO2]. These results suggest that tree legumes with a strong capacity for nodule formation could have a competitive advantage in tropical forests as atmospheric [CO2] rises and that the water-use efficiency of tropical tree species will increase under elevated [CO2].
Tropical forests play an important role in the global carbon cycle, accounting for about one-third of global primary productivity (Saugier et al., 2001; Beer et al., 2010). Thus, responses of tropical tree growth and water use to steadily increasing atmospheric [CO2] over the next century will partly control the response of the global carbon cycle to continuing human industrialization. In general, physiological responses of tropical trees to elevated [CO2] have been poorly studied compared with the responses of temperate trees (Stork et al., 2007; Körner, 2009), and there is debate about how tropical tree physiology will respond to continuing increases in atmospheric [CO2] (Clark, 2004a, 2004b; Wright, 2005; Lloyd and Farquhar, 2008; Körner, 2009; Lewis et al., 2009; Holtum and Winter, 2010).
Leguminous tree species, in the family Fabaceae, are both abundant and diverse in tropical forests, especially in the Neotropics and Africa (Gentry, 1988; Losos and Leigh, 2004). Some, but not all, leguminous tree species have the ability to form N2-fixing nodules on their roots, in symbiosis with rhizobial bacteria (de Faria et al., 1989; Moreira et al., 1992; Sprent, 2009). Because nodule-forming legumes have the ability to acquire N2 from the atmosphere, they may be able to respond more strongly to elevated [CO2] than species that lack this capacity, particularly in nitrogen-poor soils. Thus, N2-fixing legumes may be able to overcome nutritional constraints that could otherwise limit growth responses to elevated [CO2] (Tissue et al., 1997; Schortemeyer et al., 2002; Reich et al., 2006; Rogers et al., 2009). An ability to up-regulate N2 fixation may also be beneficial for phosphorus (P) acquisition, because N-rich phosphatase enzymes can be deployed on root surfaces and released into the soil to hydrolyze organically bound P, making it available for plant uptake (Richardson et al., 2005; Houlton et al., 2008; Turner, 2008).
Changes in plant water use may additionally play an important role in controlling the responses of tropical forest trees to rising atmospheric [CO2] (Holtum and Winter, 2010). In general, water-use efficiency is expected to increase as atmospheric [CO2] increases (Drake et al., 1997). If pi/pa, the ratio of intercellular to ambient CO2 partial pressures, remains constant, leaf-level water-use efficiency (WL) is expected to increase linearly as atmospheric [CO2] increases, all else being equal (Farquhar and Richards, 1984). Under such conditions, a doubling of pa would cause a doubling of WL. Therefore, it is important to determine whether pi/pa varies in response to growth at elevated [CO2] in tropical forest trees. For C3 plants, an integrated estimate of pi/pa can further be obtained from the carbon isotope discrimination of plant tissues (Farquhar et al., 1982).
Our principle objective in this study was to investigate whether growth responses to elevated [CO2] would be stronger in leguminous than in nonleguminous tropical tree seedlings. A second objective was to investigate responses of water-use efficiency to elevated [CO2] in tropical tree seedlings, both in the presence and absence of constraints on growth caused by low nutrient availability and/or drought. We aimed to test the following two hypotheses: (1) growth responds more strongly to elevated [CO2] in legumes than in nonlegumes for tropical tree seedlings grown in unfertilized soil; and (2) pi/pa remains approximately constant in tropical tree seedlings grown at ambient and elevated [CO2], even under conditions of variable nutrient and water supply, such that elevated [CO2] causes proportional increases in whole-plant water-use efficiency (WP). To test these hypotheses, we conducted two experiments. In the first experiment, one leguminous (Ormosia macrocalyx) and one nonleguminous (Swietenia macrophylla) tree species were grown under drought or well-watered conditions, with or without added fertilizer, at both ambient and elevated [CO2]. In the second experiment, four leguminous (Albizia adinocephala, Dalbergia retusa, Inga punctata, and Schizolobium parahyba) and four nonleguminous (Chrysophyllum cainito, Coccoloba uvifera, Hieronyma alchorneoides, and Pachira quinata) tree species were grown at ambient and elevated [CO2] in unfertilized soil under well-watered conditions.
RESULTS
Growth, Transpiration, and Water-Use Efficiency
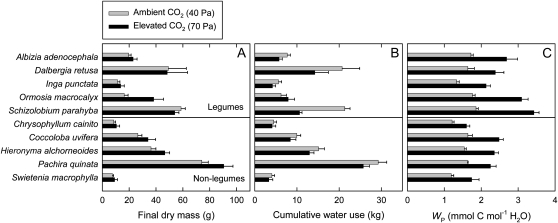
Results for plants grown in unfertilized soil at high water supply are summarized in Figure 1 for final plant dry mass (MF), cumulative transpiration over the course of the experiments (ET), and WP. The MF increased at elevated (70 Pa) compared with ambient (40 Pa) CO2 partial pressure (Fig. 1A; Table I), with mean values of 30.8 and 36.8 g, respectively. On the other hand, the ET decreased in response to elevated [CO2] (Fig. 1B; Table I). Mean ET at ambient and elevated [CO2] was 12.5 and 9.7 kg, respectively. As a result, the WP showed a marked increase in response to elevated [CO2] (Fig. 1C). Mean WP at ambient and elevated [CO2] was 1.6 and 2.4 mmol carbon mol−1 water, respectively. Thus, WP increased by 54% in response to elevated [CO2]. The MF, ET, and WP all varied among species (Fig. 1; Table I). In addition, WP was higher in legumes as a group than in nonlegumes. Mean WP under ambient [CO2] was 1.7 mmol carbon mol−1 water for legumes and 1.4 mmol carbon mol−1 water for nonlegumes. The WP also increased more under elevated [CO2] in legumes than in nonlegumes, resulting in a significant [CO2] × legume interaction in the ANOVA (Table I). Mean WP under elevated [CO2] was 2.7 mmol carbon mol−1 water for legumes and 2.1 mmol carbon mol−1 water for nonlegumes.
Figure 1.
Final plant dry mass (A), cumulative plant water use (B), and WP (C) of seedlings of 10 tropical tree species grown at either ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure. Plants were grown in unfertilized soil at high water supply. The top five species are legumes and the bottom five species are nonlegumes. Each bar represents the average of five or six plants. Error bars represent se.
Table I. Results of ANOVAs for 10 tropical tree species grown at either ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure.
Plants analyzed here were grown in unfertilized soil at high water supply. Species were nested into legumes and nonlegumes to test for differences between these two groups. Five to six individuals of each species were grown at each CO2 partial pressure. Statistically significant results are shown in boldface.
| Parameter | [CO2] | Legume | Species | [CO2] × Legume | [CO2] × Species |
| Log10MF (g dry mass) | F = 7.6P < 0.01 | F = 3.7 P = 0.06 | F = 56.1P < 0.001 | F = 0.1 P = 0.82 | F = 1.3 P = 0.24 |
| Log10ET (kg water) | F = 11.7P < 0.001 | F = 0.0 P = 1.00 | F = 46.8P < 0.001 | F = 0.5 P = 0.49 | F = 1.4 P = 0.21 |
| WP (mmol carbon mol−1 water) | F = 217.5P < 0.001 | F = 56.5P < 0.001 | F = 13.3P < 0.001 | F = 14.0P < 0.001 | F = 2.4P < 0.05 |
| Log10NF (mg N) | F = 0.0 P = 0.97 | F = 116.8P < 0.001 | F = 26.5P < 0.001 | F = 0.6 P = 0.43 | F = 1.0 P = 0.45 |
| [N] (mg N g−1 dry mass) | F = 69.0P < 0.001 | F = 703.8P < 0.001 | F = 85.5P < 0.001 | F = 2.0 P = 0.16 | F = 2.1P < 0.05 |
| δ15N (‰) | F = 6.5P < 0.05 | F = 265.9P < 0.001 | F = 51.5P < 0.001 | F = 1.2 P = 0.28 | F = 0.3 P = 0.97 |
| gs (mol water m−2 s−1) | F = 35.0P < 0.001 | F = 41.6P < 0.001 | F = 8.1P < 0.001 | F = 1.0 P = 0.33 | F = 3.3P < 0.01 |
| A (μmol CO2 m−2 s−1) | F = 21.3P < 0.001 | F = 2.8 P = 0.10 | F = 6.4P < 0.001 | F = 0.0 P = 0.96 | F = 0.5 P = 0.86 |
| pi/pa (Pa Pa−1) | F = 0.6 P = 0.44 | F = 69.8P < 0.001 | F = 3.3P < 0.01 | F = 0.1 P = 0.70 | F = 1.0 P = 0.42 |
| Δ13C (‰) | F = 2.8 P = 0.10 | F = 134.9P < 0.001 | F = 18.2P < 0.001 | F = 12.6P < 0.001 | F = 3.0P < 0.01 |
| SD (mm−2) | F = 2.4 P = 0.12 | F = 111.9P < 0.001 | F = 80.6P < 0.001 | F = 2.0 P = 0.17 | F = 1.2 P = 0.32 |
| SI (%) | F = 4.5P < 0.05 | F = 6.9P < 0.05 | F = 70.2P < 0.001 | F = 0.6 P = 0.43 | F = 1.8 P = 0.13 |
| δ18O (‰) | F = 17.7P < 0.001 | F = 28.4P < 0.001 | F = 146.0P < 0.001 | F = 7.0P < 0.01 | F = 5.7P < 0.001 |
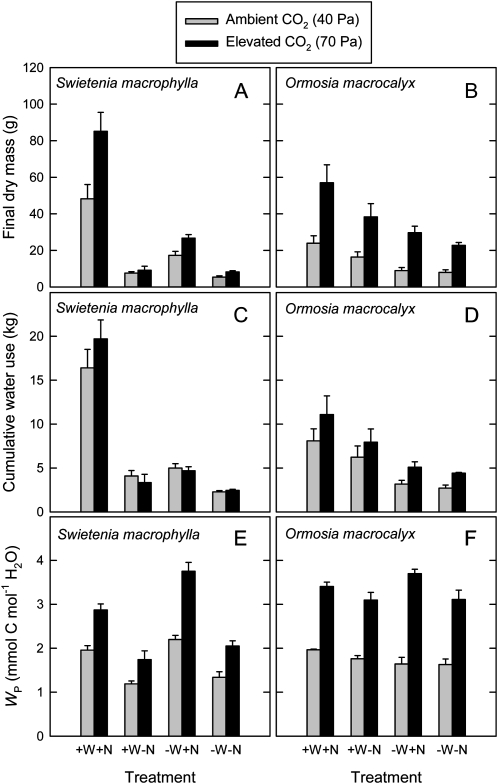
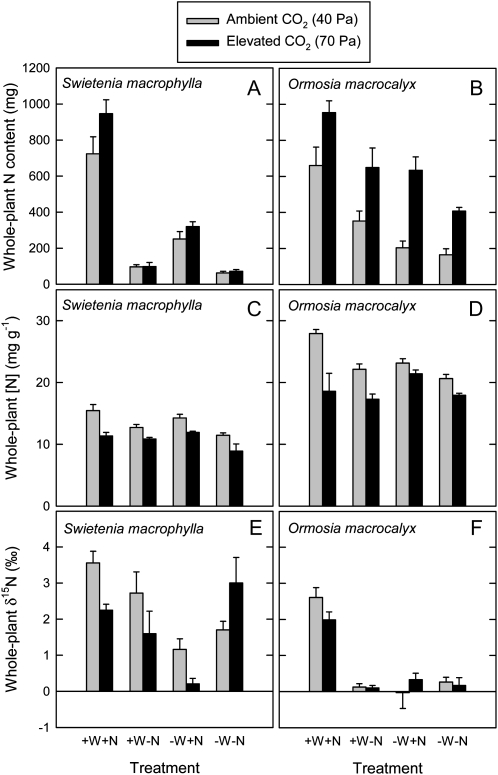
Results for MF, ET, and WP of S. macrophylla grown in fertilized and unfertilized soil, and at high and low water supply, are summarized in Figure 2. The MF of S. macrophylla increased in response to elevated [CO2] and in response to high water supply and fertilizer addition (Fig. 2A; Table II); there was also a significant water × nutrient interaction (Table II). The ET for S. macrophylla did not vary significantly in response to elevated [CO2]. The ET was higher in S. macrophylla at high compared with low water supply and in fertilized compared with unfertilized plants, and there was a significant water × nutrient interaction (Fig. 2C; Table II). The WP of S. macrophylla increased in response to elevated [CO2] for all combinations of water and nutrient treatments (Fig. 2E). Across all water and nutrient treatments, mean WP of S. macrophylla was 1.7 mmol carbon mol−1 water under ambient [CO2] and 2.6 mmol carbon mol−1 water under elevated [CO2]. Thus, WP showed a mean increase of 54% in response to elevated [CO2] for this species.
Figure 2.
Final plant dry mass (A and B), cumulative plant water use (C and D), and WP (E and F) of seedlings of two tropical tree species grown at either ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure and in four soil treatments. Soil treatments were as follows: high water supply, fertilizer addition (+W+N); high water supply, no fertilizer addition (+W−N); low water supply, fertilizer addition (−W+N); and low water supply, no fertilizer addition (−W−N). O. macrocalyx is a legume and S. macrophylla is a nonlegume. Each bar represents an average of five plants. Error bars represent se.
Table II. Results of ANOVAs for S. macrophylla grown at ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure, in fertilized or unfertilized soil, and at high or low water supply.
For each treatment combination, n = 5. Significance tests for the three-way interaction terms are not shown. Statistically significant results are shown in boldface. Units are as in Table I.
| Parameter | [CO2] | Water | Nutrient | [CO2] × Water | [CO2] × Nutrient | Water × Nutrient |
| Log10MF | F = 15.4P < 0.001 | F = 37.1P < 0.001 | F = 249.6P < 0.001 | F = 0.3 P = 0.61 | F = 1.6 P = 0.22 | F = 19.5P < 0.001 |
| Log10ET | F = 0.0 P = 0.83 | F = 77.1P < 0.001 | F = 152.0P < 0.001 | F = 0.1 P = 0.76 | F = 0.9 P = 0.36 | F = 24.4P < 0.001 |
| WP | F = 88.3P < 0.001 | F = 16.0P < 0.001 | F = 126.9P < 0.001 | F = 4.0 P = 0.05 | F = 9.1P < 0.01 | F = 2.8 P = 0.11 |
| Log10NF | F = 2.8 P = 0.11 | F = 46.8P < 0.001 | F = 304.2P < 0.001 | F = 0.3 P = 0.59 | F = 1.2 P = 0.27 | F = 13.2P < 0.01 |
| [N] | F = 38.1P < 0.001 | F = 4.6P < 0.05 | F = 26.3P < 0.001 | F = 0.4 P = 0.55 | F = 1.3 P = 0.26 | F = 2.1 P= 0.16 |
| δ15N | F = 3.1 P = 0.09 | F = 11.6P < 0.01 | F = 2.4 P = 0.13 | F = 5.5P < 0.05 | F = 4.2P < 0.05 | F = 16.5P < 0.001 |
| gs | F = 20.4P < 0.001 | F = 103.0P < 0.001 | F = 0.3 P = 0.61 | F = 16.2P < 0.001 | F = 0.2 P = 0.63 | F = 0.7 P = 0.42 |
| A | F = 37.2P < 0.001 | F = 84.3P < 0.001 | F = 14.4P < 0.001 | F = 1.3 P = 0.26 | F = 1.5 P = 0.23 | F = 2.5 P = 0.12 |
| pi/pa | F = 3.7 P = 0.06 | F = 105.3P < 0.001 | F = 18.9P < 0.001 | F = 4.7P < 0.05 | F = 2.7 P = 0.11 | F = 0.7 P = 0.41 |
| Δ13C | F = 10.3P < 0.01 | F = 113.1P < 0.001 | F = 164.4P < 0.001 | F = 1.4 P =0.25 | F = 1.4 P = 0.25 | F = 6.3P < 0.05 |
The MF, ET, and WP of O. macrocalyx in fertilized and unfertilized soil, and at high and low water supply, are also summarized in Figure 2. The MF of O. macrocalyx increased strongly in response to elevated [CO2] (Fig. 2B; Table III). The ET of O. macrocalyx showed modest increases in response to elevated [CO2] and in response to high water supply (Fig. 2D; Table III). Marked increases in WP were recorded in response to elevated [CO2] across all water and nutrient treatments (Fig. 2F; Table III). At ambient [CO2], mean WP of O. macrocalyx was 1.7 mmol carbon mol−1 water, whereas at elevated [CO2], mean WP was 3.3 mmol carbon mol−1 water, for a mean increase of 91% in response to elevated [CO2]. Overall, the dependence of MF and WP on fertilizer addition was much more pronounced in the nonleguminous S. macrophylla compared with the nodule-forming legume O. macrocalyx (Fig. 2; Tables II and III).
Table III. Results of ANOVAs for O. macrocalyx grown at ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure, in fertilized or unfertilized soil, and at high or low water supply.
For each treatment combination, n = 5. Significance tests for the three-way interaction terms are not shown. Statistically significant results are shown in boldface. Units are as in Table I.
| Parameter | [CO2] | Water | Nutrient | [CO2] × Water | [CO2] × Nutrient | Water × Nutrient |
| Log10MF | F = 62.7P < 0.001 | F = 28.7P < 0.001 | F = 5.0P = 0.03 | F = 1.5 P = 0.23 | F = 0.1 P = 0.78 | F = 0.9 P = 0.36 |
| Log10ET | F = 10.0P < 0.01 | F = 32.5P < 0.001 | F = 3.4 P = 0.08 | F = 0.7 P = 0.42 | F = 0.0 P = 0.96 | F = 0.7 P = 0.42 |
| WP | F = 285.0P < 0.001 | F = 0.1 P = 0.71 | F = 8.8P < 0.01 | F = 4.2P < 0.05 | F = 3.3 P = 0.08 | F = 0.0 P = 0.83 |
| Log10NF | F = 50.3P < 0.001 | F = 39.2P < 0.001 | F = 14.7P < 0.001 | F = 6.3P < 0.05 | F = 0.0 P = 1.0 | F = 1.0 P = 0.32 |
| [N] | F = 29.7P < 0.001 | F = 0.7 P = 0.41 | F = 14.5P < 0.001 | F = 8.1P < 0.01 | F = 1.1 P = 0.31 | F = 0.1 P = 0.75 |
| δ15N | F = 0.3 P = 0.56 | F = 39.0P < 0.001 | F = 41.8P < 0.001 | F = 1.9 P= 0.18 | F = 0.0 P = 0.83 | F = 47.2P < 0.001 |
| gs | F = 7.5P < 0.01 | F = 31.0P < 0.001 | F = 0.5 P = 0.49 | F = 8.6P < 0.01 | F = 1.2 P = 0.27 | F = 0.3 P = 0.60 |
| A | F = 23.0P < 0.001 | F = 15.3P < 0.001 | F = 0.0 P = 0.83 | F = 1.0 P = 0.32 | F = 0.4 P = 0.54 | F = 0.1 P = 0.77 |
| pi/pa | F = 7.8P < 0.01 | F = 33.3P < 0.001 | F = 2.0 P = 0.17 | F = 2.3 P = 0.14 | F = 8.8P < 0.01 | F = 0.1 P = 0.79 |
| Δ13C | F = 49.2P < 0.001 | F = 68.0P < 0.001 | F = 16.7P < 0.001 | F = 2.9 P = 0.10 | F = 2.4 P = 0.13 | F = 0.6 P = 0.45 |
Nodulation Patterns among Legumes
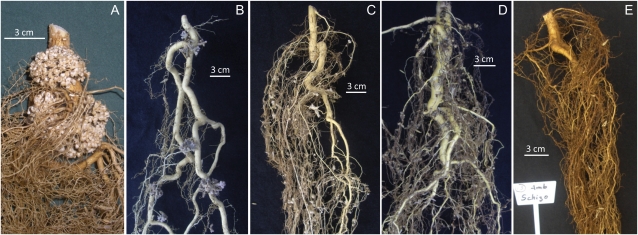
Four of the legume species included in the study formed nodules on their roots. These species were O. macrocalyx (Fig. 3A), I. punctata (Fig. 3B), A. adinocephala (Fig. 3C), and D. retusa (Fig. 3D). The only legume species that did not form nodules was S. parahyba (Fig. 3E). Nodule mass ratio did not vary in response to elevated [CO2] for the nodulated species (P = 0.32, n = 35). The nodule mass ratio of O. macrocalyx decreased sharply in fertilized compared with unfertilized plants (P < 0.001, n = 40). Mean nodule mass ratio for unfertilized O. macrocalyx plants was 48.9 mg g−1, whereas that for fertilized plants was 2.8 mg g−1.
Figure 3.
Examples of root systems for plants growing in unfertilized soil showing different nodulation patterns among the legume species O. macrocalyx (A), I. punctata (B), A. adinocephala (C), D. retusa (D), and S. parahyba (E). Species are ordered from left to right from highest to lowest nodule mass ratio. S. parahyba on the far right did not form nodules.
Nitrogen Uptake, Nitrogen Concentration, and δ15N
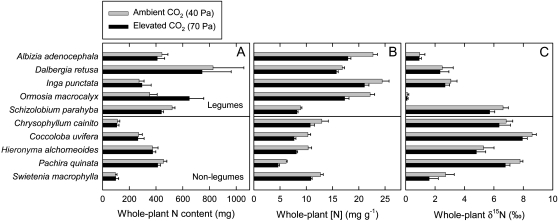
For unfertilized plants at high water supply, final plant nitrogen (N) mass (NF) did not vary in response to elevated [CO2], on average (Fig. 4A; Table I). However, NF varied among species and was higher in legumes as a group than in nonlegumes. On the other hand, whole-plant [N] decreased significantly in response to elevated [CO2] across all species (Fig. 4B; Table I). The [N] was also higher in legumes than in nonlegumes and varied among species within the two groups. Whole-plant δ15N decreased in response to elevated [CO2]. Average δ15N for plants grown at ambient [CO2] was 4.6‰ and that for plants grown at elevated [CO2] was 4.1‰. The δ15N was lower in legumes than in nonlegumes and varied among species within the two groups (Fig. 4C; Table I).
Figure 4.
Final plant N mass (A), final plant [N] (B), and final plant δ15N (C) of seedlings of 10 tropical tree species grown at either ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure. Plants were grown in unfertilized soil at high water supply. The top five species are legumes and the bottom five species are nonlegumes. Each bar represents the average of five or six plants. Error bars represent se.
The nonlegume S. macrophylla and the legume O. macrocalyx differed in responses of NF to elevated [CO2] (Fig. 5, A and B; Tables II and III). The NF of S. macrophylla did not increase significantly in response to elevated [CO2], whereas that of O. macrocalyx showed a pronounced increase in response to elevated [CO2]. The NF of both species increased in response to fertilizer addition and at high compared with low water supply. However, interactions between fertilizer addition and water supply differed between species (Tables II and III). The [N] of both S. macrophylla and O. macrocalyx decreased in response to elevated [CO2] (Fig. 5, C and D; Tables II and III). The [N] was also higher in fertilized compared with unfertilized plants for both species. Responses of whole-plant δ15N to elevated [CO2] in S. macrophylla and O. macrocalyx were variable, depending on the nutrient and water treatments (Fig. 5, E and F; Tables II and III). The δ15N of O. macrocalyx was near 0‰, except in fertilized plants at high water supply.
Figure 5.
Final plant N mass (A and B), final plant [N] (C and D), and final plant δ15N (E and F) of seedlings of two tropical tree species grown at either ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure and in four soil treatments. Soil treatments were as follows: high water supply, fertilizer addition (+W+N); high water supply, no fertilizer addition (+W−N); low water supply, fertilizer addition (−W+N); and low water supply, no fertilizer addition (−W−N). O. macrocalyx is a legume and S. macrophylla is a nonlegume. Each bar represents an average of five plants. Error bars represent se.
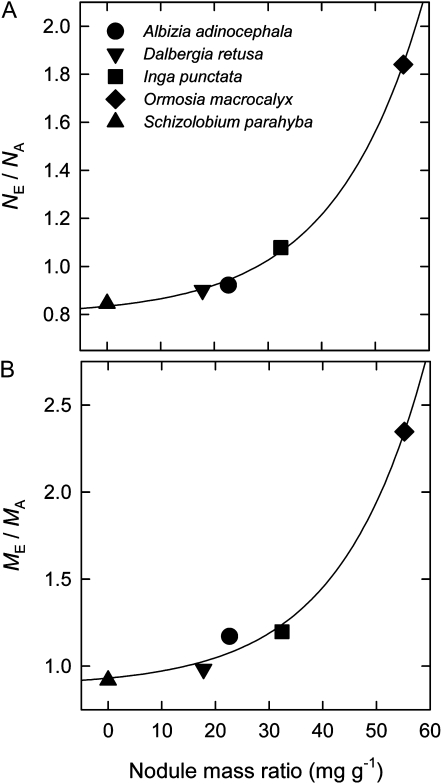
Among legume species, variation in nodule mass ratio explained much of the variation in responses of both N uptake and growth to elevated [CO2]. The ratio of NF at elevated to ambient [CO2] (NE/NA) is plotted against nodule mass ratio in Figure 6A. The NE/NA was less than unity in the non-nodule-forming species S. parahyba. It then increased exponentially as nodule mass ratio increased across the nodule-forming species. A very similar relationship existed between the ratio of final dry mass at elevated to ambient [CO2] (ME/MA) and nodule mass ratio (Fig. 6B). The ME/MA was 0.92 in S. parahyba and increased exponentially as a function of nodule mass ratio, reaching a maximum of 2.35 in O. macrocalyx. In contrast, nonlegumes showed a much narrower range of ME/MA, with values ranging from 1.21 for S. macrophylla to 1.29 for C. uvifera.
Figure 6.
The ratio of final plant N mass at elevated (70 Pa) to that at ambient (40 Pa) CO2 partial pressure (A), and the ratio of final plant dry mass at elevated to that at ambient CO2 partial pressure (B) plotted against the nodule mass ratio at elevated CO2 partial pressure for the five leguminous tree species included in the study. Plants were grown in unfertilized soil at high water supply. The line in A represents a nonlinear regression equation: NE/NA = 0.80 + 0.038exp(0.060NMR), where NMR is nodule mass ratio. The line in B represents a similar equation: ME/MA = 0.88 + 0.047exp(0.062NMR).
Leaf Gas Exchange and Whole-Plant Carbon-Isotope Discrimination
The drawdown in CO2 partial pressure from air outside the leaf to the leaf intercellular air spaces (pa-pi), as assessed by instantaneous gas-exchange measurements, was a good predictor of variation in WP determined over the full course of the experiment (r2 = 0.60, P < 0.001, n = 171). All data from both experiments were included in the analysis. The WP normalized for variation in growth CO2 partial pressure (WP/pa) showed a strong correlation with whole-plant carbon-isotope discrimination (Δ13C; r2 = 0.54, P < 0.001, n = 171). Finally, the Δ13C was closely correlated with the instantaneous pi/pa (r2 = 0.62, P < 0.001, n = 171). Together, these analyses demonstrate consistency between instantaneous gas-exchange measurements and Δ13C as useful predictors of variation in the experiment-long WP.
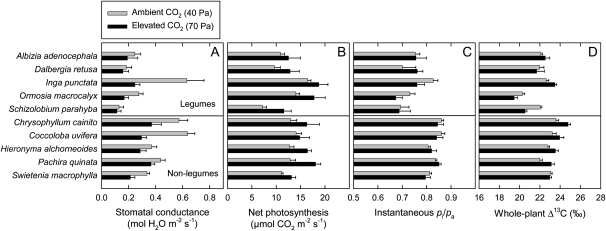
Patterns of variation in leaf-level gas exchange supported observations at the whole-plant level for plants grown in unfertilized soil at high water supply. Stomatal conductance (gs) decreased and net photosynthesis (A) increased in response to elevated [CO2] (Fig. 7, A and B; Table I). The mean gs decreased from 0.382 to 0.241 mol m−2 s−1 in response to elevated [CO2], and the mean A increased from 12.2 to 15.2 μmol m−2 s−1. The pi/pa did not vary significantly in response to elevated [CO2] (Fig. 7C; Table I), whereas the mean pa-pi increased from 8.0 to 15.6 Pa in response to elevated [CO2]. Whole-plant Δ13C also did not vary significantly between ambient and elevated [CO2] (Fig. 7D; Table I), consistent with instantaneous assessments of pi/pa. Overall, legumes displayed lower gs compared with nonlegumes, whereas A was similar between the two groups. As a result, pi/pa and Δ13C were both lower in legumes than in nonlegumes (Fig. 7; Table I).
Figure 7.
Stomatal conductance (A), net photosynthesis (B), pi/pa (C), and Δ13C (D) of seedlings of 10 tropical tree species grown at either ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure. Gas-exchange measurements were made at the growth CO2 partial pressure for each treatment. Plants were grown in unfertilized soil at high water supply. The top five species are legumes and the bottom five species are nonlegumes. Each bar is the average of measurements on one leaf from each of five or six plants. Error bars represent se.
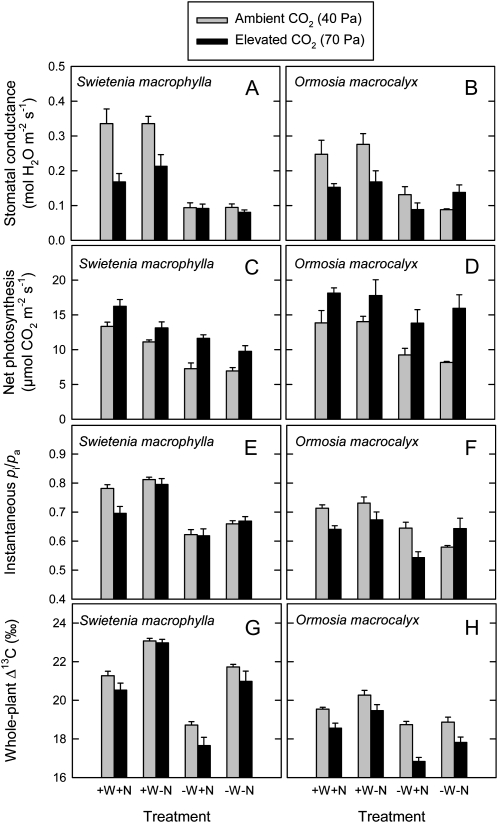
The gs decreased in S. macrophylla and in O. macrocalyx in response to elevated [CO2] in plants under high water supply but not in plants under low water supply (Fig. 8, A and B). Thus, there was a significant [CO2] × water interaction for gs in both species (Tables II and III). On the other hand, the A increased in response to elevated [CO2] across all water and nutrient treatments for both species (Fig. 8, C and D). For S. macrophylla, the A was also higher at high compared with low water supply and in fertilized compared with unfertilized plants (Table II). For O. macrocalyx, the A increased at high compared with low water supply but did not vary in response to fertilizer addition (Table III). The pi/pa did not vary in response to elevated [CO2] in S. macrophylla (Fig. 8E; Table II) and decreased in response to elevated [CO2] in O. macrocalyx (Fig. 8F; Table III). This indicated large increases in WL in both species in response to elevated [CO2] caused by large increases in pa-pi. In S. macrophylla, the pi/pa also decreased at low compared with high water supply and decreased in fertilized compared with unfertilized soil (Fig. 8E; Table II). In O. macrocalyx, the pi/pa decreased at low compared with high water supply but did not vary in response to fertilizer addition (Fig. 8F; Table III). Patterns of Δ13C were generally consistent with patterns of pi/pa for the two species. The Δ13C decreased in response to elevated [CO2], decreased at low compared with high water supply, and decreased in response to fertilizer addition (Fig. 8, G and H; Tables II and III).
Figure 8.
Stomatal conductance (A and B), net photosynthesis (C and D), pi/pa (E and F), and Δ13C (G and H) of seedlings of two tropical tree species grown at either ambient (40 Pa) or elevated (70 Pa) CO2 partial pressure and in four soil treatments. Soil treatments were as follows: high water supply, fertilizer addition (+W+N); high water supply, no fertilizer addition (+W−N); low water supply, fertilizer addition (−W+N); and low water supply, no fertilizer addition (−W−N). O. macrocalyx is a legume and S. macrophylla is a nonlegume. Each bar represents an average of five plants. Error bars represent se.
Stomatal Density, Stomatal Index, and δ18O
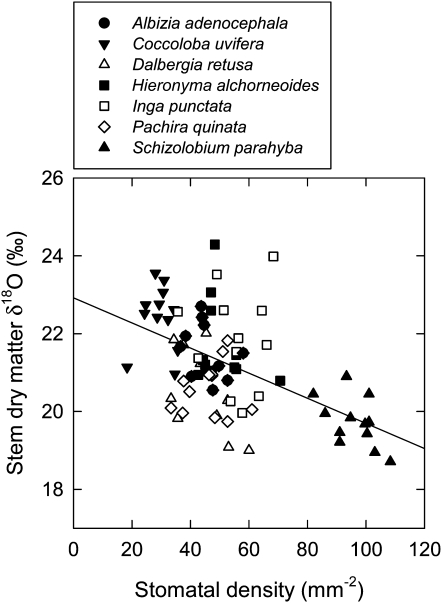
Stomatal density (SD) did not show a significant response to elevated [CO2], but stomatal index (SI) decreased significantly at elevated compared with ambient [CO2] (Table I). Mean values for SD were 54.0 and 51.0 mm−2 at ambient and elevated [CO2], respectively, and those for SI were 19.9% and 19.0%. Both SD and SI varied between legumes and nonlegumes and among species within these groups (Table I). The δ18O of stem dry matter showed a small but significant increase in response to elevated [CO2] (Table I). Mean values were 22.2‰ and 22.7‰ at ambient and elevated [CO2], respectively. Stem δ18O also varied between legumes and nonlegumes and among species within these groups (Table I). Across the full data set, SD explained 29% of variation in stem dry matter δ18O (Fig. 9), whereas gs and δ18O were not correlated in these plants (P = 0.08, n = 80).
Figure 9.
Stem dry matter δ18O plotted against SD for seedlings of seven tropical tree species. The figure includes plants grown at both ambient (40 Pa) and elevated (70 Pa) CO2 partial pressure. SD was measured on abaxial leaf surfaces. The line is a least-squares linear regression: δ18O = −0.03SD + 23 (r2 = 0.29, P < 0.001, n = 80).
DISCUSSION
Legume-Versus-Nonlegume Responses to Elevated [CO2]
As a group, leguminous tropical tree seedlings did not show a stronger growth response to elevated [CO2] than nonleguminous seedlings for plants grown in unfertilized soil at high water supply (Fig. 1A; Table I). However, within legumes, there was a large range of variation among species in growth response to elevated [CO2]. This was closely related to their capacity for nodule formation, as quantified by nodule mass ratio (Fig. 6B). These results suggest that predictions of legume responses to elevated [CO2] in tropical forests could benefit by taking into account interspecific patterns in nodulation potential. Such patterns have been reasonably well documented (de Faria et al., 1989; Moreira et al., 1992; Sprent, 2009). Our results for tropical tree legumes are consistent with observations in herbaceous legumes, where wide-ranging capacities for N2 fixation among species and among genotypes within species influenced growth responses to elevated [CO2] (Ainsworth et al., 2004; Niklaus and Körner, 2004; Reich et al., 2006; Rogers et al., 2009). A similar pattern was also observed for several temperate Acacia species (Schortemeyer et al., 2002).
We assumed that the nodule mass ratio provided an index in our study of the capacity of the leguminous tree species to fix atmospheric N2. This assumption was supported by measurements of the δ15N of plant biomass. Of the legume species, O. macrocalyx had the highest nodule mass ratio and also had whole-plant δ15N nearest to 0‰ (Fig. 4C), which is the δ15N of atmospheric N2. In addition, the nonnodulating S. parahyba had whole-plant δ15N furthest from 0‰ of the legume species. The other three legume species were intermediate between these two for both nodule mass ratio and whole-plant δ15N. Thus, δ15N patterns among legume species provided evidence for a positive relationship between N2 fixation and nodule mass ratio. Taking the nonnodulating legume S. parahyba as a reference plant (Pons et al., 2007) and assuming a discrimination of 1‰ during N2 fixation (Yoneyama et al., 1986), we estimated the proportion of plant N acquired by N2 fixation to be 84% for O. macrocalyx, 73% for A. adinocephala, 52% for D. retusa, 46% for I. punctata, and 0% for S. parahyba. However, these values should be considered as indicative only, because the non-N2-fixing species included in the study showed a relatively large range of plant δ15N, similar to previous observations for non-N2-fixing tropical tree species (Guehl et al., 1998; Cernusak et al., 2007a, 2009c; Pons et al., 2007). This introduces uncertainty into the estimate of δ15N for soil-derived plant N (Shearer and Kohl, 1986).
The interspecific nodulation pattern observed in our experiment was entirely consistent with observations of field-grown trees. Thus, S. parahyba did not nodulate in our experiment (Fig. 3E) and does not nodulate in the field (Allen and Allen, 1981; Moreira et al., 1992; Aguilar et al., 1994); A. adinocephala nodulated in our experiment (Fig. 3C) and in the field (Allen and Allen, 1981); D. retusa nodulated in our experiment (Fig. 3D) and in the field (Parker, 2004); I. punctata nodulated in our experiment (Fig. 3B) and in the field (Powell, 1995; Barron et al., 2011); and O. macrocalyx nodulated in our experiment (Fig. 3A) and in the field (Moreira et al., 1992). This pattern is also consistent with observations under natural conditions at the genus level (Allen and Allen, 1981; de Faria et al., 1989, 2010; Sprent, 2009). Moreover, we inferred that O. macrocalyx had the highest rate of N2 fixation relative to other legume species in our study based on observations of nodule mass ratio and plant δ15N. This is consistent with observations for other Ormosia species under natural field conditions. Out of 12 nodulating legume genera surveyed in natural forest stands in Guyana, Ormosia had the highest estimate for the proportion of leaf N derived from symbiotic N2 fixation (Pons et al., 2007). These comparisons demonstrate that our experiment effectively replicated field-based patterns of nodulation and N2 fixation among the legume species included in the study. This result was achieved without artificially inoculating the seedlings with rhizobia, indicating that compatible N2-fixing bacteria were sufficiently abundant in the forest topsoil used for the experiment.
Nodulation of leguminous tree species in a lowland tropical forest in Panama was observed to be most pronounced at disturbed sites and in forest gaps (Barron et al., 2011). Nodule formation was promoted when N availability in the soil was comparatively low (Barron et al., 2011) and when plant demand for N was high due to the stimulation of plant growth by sunlight. Thus, nodule formation can be suppressed by increasing the availability of N in the soil, as demonstrated for fertilized O. macrocalyx in this study, and by growth under low irradiance (McHargue, 1999). The unfertilized treatments in our experiments replicated a low-N gap environment, insofar as there was high irradiance (approximately 80% of full sunlight) and low soil N availability, caused by the addition of rice husks to the soil mixture (Cernusak et al., 2007b). Therefore, these experimental conditions should have led to maximal expression of nodule mass ratio and N2 fixation, when compared with the range of conditions likely to be encountered by these species in their native environments.
The increasing productivity response to elevated [CO2] with increasing nodule mass ratio among the legume species resulted from two components. The first component was a stimulation of N acquisition by elevated [CO2] at high nodule mass ratio, as can be clearly seen in Figure 6A. Moreover, the NE/NA correlated negatively with plant δ15N (r2 = 0.81, P = 0.04, n = 5), indicating that species with high NE/NA acquired a larger proportion of their N from N2 fixation than species with low NE/NA. Therefore, legume species with large nodule mass ratio had a greater capacity to up-regulate N2 fixation under elevated [CO2] than legume species with little or no nodule mass ratio. The second component was a greater stimulation of whole-plant N-use efficiency by elevated [CO2] (Drake et al., 1997) in species with large nodule mass ratio compared with those with little or no nodule mass ratio. This was demonstrated by the fact that the slope of the relationship between ME/MA and NE/NA was greater than unity (slope = 1.4; r2 = 0.98, P < 0.001, n = 5), which can also be seen by comparing the y axes in Figure 6. Thus, biomass production per unit of N taken up was stimulated by elevated [CO2], and the extent of stimulation increased with increasing nodule mass ratio. This was likely caused by reduced sink limitation to photosynthesis under elevated [CO2] in plants with large nodule mass ratio compared with those with little or no nodule mass ratio (Ainsworth et al., 2004). This second component of the productivity response is further evidenced by a negative correlation among legume species between the ratio of plant carbon to N at elevated to ambient [CO2] and plant δ15N (r2 = 0.77, P < 0.05, n = 5). This indicates that greater increases in plant carbon-to-N ratio under elevated [CO2] were achieved in plants with high N2 fixation rates compared with those with little or no N2 fixation.
The growth response of S. macrophylla to elevated [CO2] was larger in fertilized soil than in unfertilized soil at high water supply (Fig. 2A). For this species, the ME/MA was 1.76 in fertilized soil and 1.21 in unfertilized soil. Other nonleguminous tropical tree seedlings also showed little growth stimulation under elevated [CO2] in unfertilized soil but large responses in fertilized soil (Lovelock et al., 1998; Winter et al., 2000, 2001a, 2001b; Körner, 2009). In contrast, the legume O. macrocalyx showed a similar proportional increase in biomass under elevated [CO2] in both fertilized and unfertilized soil at high water supply. Here, the ME/MA was 2.35 in fertilized soil and 2.33 in unfertilized soil. At low water supply, on the other hand, S. macrophylla enjoyed a similar proportional increase in biomass under elevated [CO2] in both fertilized and unfertilized soil, with ME/MA of 1.55 and 1.53, respectively, whereas O. macrocalyx had ME/MA of 3.33 and 2.87 in fertilized and unfertilized soil at low water supply. These data suggest that tropical tree seedlings may experience growth stimulation in response to elevated [CO2] during dry spells even when nutrient availability is relatively low. This could have important consequences for distribution patterns of tropical tree species, because drought sensitivity of seedlings has been shown to play an important role in structuring tropical tree communities (Engelbrecht et al., 2007).
We observed that legumes as a group had higher WP than nonlegumes when grown in unfertilized soil at high water supply (Fig. 1C; Table I). Consistent with this, legumes also had lower pi/pa and lower Δ13C (Fig. 7, C and D; Table I). This agrees with previous observations for a smaller number of species (Cernusak et al., 2007a, 2008, 2009b). When plants were grown in fertilized soil, the legume species appeared to lose their advantage in WP, as shown for the comparison between O. macrocalyx and S. macrophylla in this study (Fig. 2, E and F) and for comparisons between the legume species Platymiscsium pinnatum and the nonlegume species Tectona grandis and S. macrophylla in previous studies (Cernusak et al., 2009a, 2009b). Interestingly, the higher WP of legumes than nonlegumes in unfertilized soil in this study did not appear to be associated exclusively with N2 fixation, because the nonnodulating legume S. parahyba had the highest WP of any of the studied species (Fig. 1C). These results suggest that high water-use efficiency may be a general trait of tropical legume trees, consistent with generally high foliar N concentrations and high photosynthetic capacity (McKey, 1994).
Water-Use Efficiency Responses to Elevated [CO2]
We observed that pi/pa either remained constant or tended to decrease in tropical tree seedlings grown at elevated compared with ambient CO2 partial pressure (Figs. 7 and 8). Constant or decreasing pi/pa should lead to a marked increase in WP under increasing pa. This is consistent with our observations (Fig. 1C). If other determinants of WP, in addition to pi/pa, also remained constant at elevated compared with ambient [CO2], an increase in WP of 75% would be predicted for an increase in pa from 40 to 70 Pa. For the 10 species in our study grown in unfertilized soil at high water supply, we observed an average increase in WP of 54% for growth at pa of 70 compared with 40 Pa. This discrepancy between prediction and observation is most likely explained by an increase in the leaf-to-air vapor pressure difference (v) under elevated [CO2]. We observed a decrease in gs at elevated compared with ambient [CO2] (Figs. 7 and 8), as has been commonly observed in other studies (Long et al., 2004; Ainsworth and Rogers, 2007; Leakey et al., 2009). A decrease in gs would cause a decrease in transpiration, and as a result, an increase in leaf temperature during photosynthesis, which would in turn cause an increase in v. Although an increase in v appeared to reduce the response of WP to elevated pa, it certainly did not cancel it, and large increases in WP were recorded across all species and all fertilizer and water treatments (Figs. 1C and 2, E and F).
We observed approximately constant pi/pa in response to an increase in pa from 40 to 70 Pa. The pi/pa of tropical trees also appears to have remained approximately constant in response to variation in pa from preindustrial times to the present, as inferred from tree-ring Δ13C (Hietz et al., 2005; Nock et al., 2010; Brienen et al., 2011). A similar pattern was inferred for subtropical vegetation from preindustrial times to the present, based on changes in SD and morphology (de Boer et al., 2011; Lammertsma et al., 2011). Together, these observations suggest that WP of tropical trees has increased in response to increasing pa over the past 200 years and will continue to increase in the future as pa continues to increase. If increases in tree growth in response to increasing pa are smaller than increases in WP, as was the case in our study (Fig. 1), canopy water use will decrease. This will likely have important consequences for the hydrological cycle and the biogeochemistry of tropical forests, although the implications of the manifold feedbacks between water, carbon, and nutrient cycles are complex and difficult to predict (Galbraith et al., 2010). Nevertheless, the apparently conservative nature of pi/pa in tropical trees in response to variable pa could provide an effective set point for modeling responses of tropical tree growth and water use to variations in atmospheric [CO2] (Buckley, 2008).
We observed a 37% mean reduction in gs in response to elevated [CO2] for plants grown in unfertilized soil at high water supply (Fig. 7A). For the most part, this reduction in gs was not caused by reduced SD in response to elevated [CO2]. SD showed a mean reduction of only 6% in response to elevated [CO2], and SI showed a similar mean reduction of only 5%. These results are similar to observations in temperate species, where growth at [CO2] above ambient did not lead to significant reductions in SD or SI (Estiarte et al., 1994; Reid et al., 2003; Marchi et al., 2004; Tricker et al., 2005; Ainsworth and Rogers, 2007).
The δ18O of leaf water, and consequently organic material, is expected to increase with decreasing gs, all else being equal (Farquhar and Lloyd, 1993; Barbour and Farquhar, 2000; Cernusak et al., 2004; Farquhar et al., 2007). We observed a mean increase in stem dry matter δ18O of 0.5‰ in response to elevated [CO2], qualitatively consistent with this prediction. However, we also observed that some of the variation among species in stem dry matter δ18O was explained by SD (Fig. 9), whereas the δ18O did not correlate with gs across the full data set. This suggests that leaf anatomy may play an important role in determining variations among species in 18O enrichment. The relationship shown here between stem dry matter δ18O and SD may provide a promising avenue for future research into variation in 18O enrichment among terrestrial plant species.
CONCLUSION
The legume species with the highest nodulation capacity, O. macrocalyx, was by far the strongest responder to elevated [CO2] in this study (Figs. 1 and 6). We conclude that legume species with capacity to achieve large nodule mass ratios will respond more strongly to elevated [CO2] than other species; they will likely be among the winners in tropical forests as atmospheric [CO2] rises. Across all species, pi/pa was approximately constant in response to elevated [CO2], and this was supported by measurements of Δ13C (Figs. 7 and 8). As a result, WP increased markedly in response to elevated [CO2] (Fig. 1). Similar increases were recorded in fertilized and unfertilized soil and at both high and low water supply (Fig. 2). We conclude that tropical forest trees will likely experience large increases in WP as atmospheric [CO2] continues to rise over the coming century.
MATERIALS AND METHODS
Plant Material and Experimental Treatments
Experiments took place at the Santa Cruz Experimental Field Facility, Smithsonian Tropical Research Institute, Gamboa, Panama (9°07′N, 79°42′W). The study site is located at an altitude of approximately 28 m above sea level. Plants were grown in two glasshouses, one of which had CO2 partial pressure similar to ambient (40 Pa) and the other of which had an elevated CO2 partial pressure (70 Pa), maintained by releasing CO2 gas from a high-pressure cylinder when the CO2 partial pressure in the glasshouse declined below 69 Pa. The glasshouses were air conditioned, with the air conditioners programmed to turn on when the air temperature exceeded 30°C. Air temperature and relative humidity were recorded in the two glasshouses every 15 min during experiments with a data logger (CR10X; Campbell Scientific). Average daytime air temperature was 30.4°C in the ambient [CO2] glasshouse and 30.3°C in the elevated [CO2] glasshouse, with average nighttime air temperatures of 27.1°C and 27.2°C in the two glasshouses, respectively. Average daytime relative humidity was 71% in the ambient [CO2] glasshouse and 72% in the elevated [CO2] glasshouse, with average nighttime relative humidity of 86% and 90% in the two glasshouses, respectively. The average CO2 partial pressure recorded in the elevated [CO2] glasshouse was 69 ± 1 Pa (mean ± sd); that recorded in the ambient [CO2] glasshouse was 39 ± 3 Pa.
Two experiments were conducted. In the first experiment, 20 seedlings each of Swietenia macrophylla (Meliaceae) and Ormosia macrocalyx (Fabaceae-Papilionoideae) were grown in each of the two glasshouses. Seeds were collected from trees growing in the Panama Canal watershed. Seedlings were grown individually in 19-L pots. Each pot contained 13.5 kg of dry homogenized soil mixture. The soil mixture comprised 60% by volume dark, air-dried forest topsoil and 40% by volume air-dried rice husks. The rice husks were added to improve soil structure and drainage. The pots were saturated with water and drained overnight to establish the pot water content at field capacity, which was determined to be 5.0 kg. The soil surface of each pot was then covered with 1.5 kg of gravel to reduce evaporation. Ten control pots with no plants were placed in each glasshouse along with the 40 pots containing plants to estimate pot water loss due to evaporation from the soil surface.
Within each glasshouse, two fertilizer treatments and two water supply treatments were deployed. At the beginning of the experiment, 10 of the 20 pots for each species were randomly chosen to receive approximately 12 g of Osmocote-Plus controlled-release fertilizer (Scotts-Sierra). The fertilizer contained by weight 15% N, 9% P, and 12% potassium and had an estimated release time of 5 to 6 months. Five fertilized and five unfertilized pots from each species were then randomly allocated to receive reduced water supply. All pots started the experiment watered to field capacity. Those receiving high water supply were weighed each week and rewatered to near field capacity. Later in the experiment, pots were weighed and rewatered at shorter intervals, depending on water loss rates. Pots receiving low water supply were allowed to dry down to pot water contents of less than 1.5 kg, or approximately 30% of field capacity, over several weeks. Thereafter, they were weighed and rewatered to this pot water content each week, or at shorter intervals, as necessary. Pots were weighed to the nearest 5 g with a 64-kg-capacity balance (Sartorius QS64B; Thomas). Drain holes at the bases of the pots were sealed for the duration of the experiment.
Pots were placed in the ambient and elevated [CO2] glasshouses on January 8, 2007, approximately 1 month after seedlings had germinated. Within each glasshouse, the pots were placed on plastic tables, such that they were elevated approximately 1 m above the glasshouse floor. Initial plant dry mass was estimated by harvesting five representative individuals of each species. Mean values were 0.3 g and 0.2 g for O. macrocalyx and S. macrophylla, respectively. Plants were harvested on April 26, 2007, after more than 3 months of growth at either ambient or elevated [CO2].
The second experiment was similar to the first, except that six individuals of each of eight tree species were grown in each of the two glasshouses. The eight species were Albizia adinocephala (Fabaceae-Mimosoideae), Chrysophyllum cainito (Sapotaceae), Coccoloba uvifera (Polygonaceae), Dalbergia retusa (Fabaceae-Papilionoideae), Hieronyma alchorneoides (Phyllanthaceae), Inga punctata (Fabaceae-Mimosoideae), Pachira quinata (Malvaceae), and Schizolobium parahyba (Fabaceae-Caesalpinioideae). This experiment involved only one soil treatment for each species, corresponding to the high-water-supply, unfertilized treatment in the first experiment. Preparation of the pots followed the same method as in the first experiment. Seedlings of the eight species were obtained from the nursery of the Native Species Reforestation Project, based at the Smithsonian Tropical Research Institute. They were approximately 2 months old when the experiment began. The seedlings were transplanted to the experimental pots on June 20, 2007, with one seedling in each pot. They were allowed 2 weeks to recover from transplanting before being placed in the ambient and elevated [CO2] glasshouses on July 3, 2007. Five control pots with no plants were placed in each glasshouse to estimate evaporation from the soil surface. Initial dry mass at the beginning of the experiment for each species was 0.2, 0.6, 0.6, 0.3, 0.5, 0.2, 0.2, and 1.0 g, given in the same order as the species names are given above. Plants were harvested on October 1, 2007, after 3 months of growth under ambient or elevated [CO2].
The forest topsoil used in the experiments had a relatively high P availability. Soil obtained from the same forest area and used in a recent pot experiment had a mean extractable P concentration of 15 μg P g−1 dry soil, determined by extraction in Bray solution (30 mm NH4F and 25 mm HCl). This extractable P concentration is in the high range of values typically observed for neotropical rainforest soils (Clinebell et al., 1995; Turner and Engelbrecht, 2011). We previously observed that the addition of rice husks to the soil mixture differentially reduced N availability relative to P availability, presumably due to microbial immobilization of available N (Cernusak et al., 2007b). Based on these considerations, the unfertilized soil treatments in our experiments likely had relatively low N availability compared with P availability. The mean leaf N-P ratio of all plants grown in unfertilized soil at high water supply in the two experiments was 10.8 g g−1, consistent with this assessment.
We did not add an artificial source of rhizobia to the experimental soil mixture but rather relied on bacteria already present in the forest topsoil to induce nodulation in the leguminous seedlings.
Water Use and Biomass Measurements
Cumulative plant water use over the course of the experiment was calculated as the sum of pot water loss minus the average sum of water loss from the control pots. Pot water loss was determined for each weekly or subweekly interval in the experiment from the gravimetric measurements described above. Half the control pots in the first experiment were allowed to dry down to a pot water content of 1.5 kg to estimate soil evaporation for the low-water-supply treatment. The other half were returned to a pot water content of 5.0 kg each week, as done for the high-water-supply treatment. Immediately following the harvest in each experiment, total leaf area of each plant was measured with a Li-3100 leaf area meter (Li-Cor). Harvested plants were dried to constant mass at 70°C, and dry mass of leaves, stems, and roots was determined separately for each plant to the nearest 0.02 g. For the nodulated legumes, nodules were removed from dried roots, and the nodule dry mass was determined. Nodule mass ratio was then calculated as nodule dry mass divided by total plant dry mass. For D. retusa, nodules were removed from roots of only one plant per [CO2] treatment, because the small size and disaggregated distribution of nodules in this species made them very difficult to separate from roots.
The water-use efficiency of each plant was calculated as WP = (MC2 − MC1 + LC)/ET, where MC1 and MC2 are the plant carbon mass at the beginning and end of the experiment, LC is the carbon mass of leaves abscised over the course of the experiment, and ET is the sum of water transpired over the course of the experiment.
Leaf Gas-Exchange Measurements
The A, gs, and pi/pa were measured on the youngest fully expanded leaf on each plant using a Li-6400 portable photosynthesis system (Li-Cor). Measurements were made at a photon flux of 1,500 μmol m−2 s−1, which was supplied by an artificial light source (Li-Cor). Mean v during measurements was 2.0 ± 0.4 kPa (mean ± sd), and mean leaf temperature was 34°C ± 1°C. The [CO2] in the gas-exchange cuvette during measurements was similar to the growth [CO2]: the mean value for measurements in the ambient [CO2] glasshouse was 381 ± 11 μmol mol−1; that for measurements in the elevated [CO2] glasshouse was 701 ± 12 μmol mol−1. Measurements in the first experiment took place on April 18 and 20, 2007, and the two sets of measurements were averaged for each plant. Measurements for the second experiment took place on September 22, 2007. Thus, in each experiment, leaf gas-exchange measurements took place following approximately 3 months of growth in the respective glasshouses, and the leaves that were measured had expanded under the conditions imposed by the different experimental treatments.
Stable Isotope and Elemental Analyses
Leaves, stems, and roots of each plant were ground separately to a fine, homogenous powder. Samples of approximately 2 mg were analyzed for δ13C, δ15N, [C], and [N] with an elemental analyzer (CE Instruments) coupled to an isotope ratio mass spectrometer (Delta V; Thermo Fisher Scientific) in the Stable Isotope Laboratory of the Smithsonian Tropical Research Institute. Approximately 1 mg of stem dry matter of each plant was analyzed for δ18O on an isotope ratio mass spectrometer (Delta XP; Finnigan MAT) following pyrolysis in a high-temperature furnace (Thermoquest TC/EA; Finnigan MAT) at the Stable Isotope Core Laboratory, Washington State University. The δ13C, δ18O, and δ15N values were expressed relative to standards of PeeDee Belemnite, Vienna Standard Mean Ocean Water, and air, respectively. The Δ13C of plant material was calculated as Δ13C = (δa − δp)/(1 + δp), where δa is δ13C of CO2 in air in each glasshouse and δp is δ13C of plant carbon. The δa was estimated by growing two species of C4 plant in each glasshouse and outside in the open air. The C4 plant species were Saccharum spontaneum and Portulaca oleracea. The δ13C of source CO2 for plants grown outside was assumed to be –8‰. This value of δa was used to calculate the Δ13C for each C4 species. The Δ13C for the C4 species was assumed to be the same in the glasshouses and outside and was then used to estimate δa in each glasshouse. Using this method, we estimated that δa in the ambient [CO2] glasshouse was –9‰ and that δa in the elevated [CO2] glasshouse was –7‰. The supplier of the tank CO2 used to fumigate the elevated [CO2] glasshouse (Aceti-Oxigeno) indicated that the CO2 originated from a CO2 spring, and not from fossil fuel combustion, consistent with our estimation of δa in the elevated [CO2] glasshouse.
SD and SI
In the second experiment, leaves were sampled from plants grown in each glasshouse for measurements of SD and SI. Leaves were collected on September 25, 2007, approximately 1 week before the plants were harvested. The sampled leaves were the same as those that had been measured for leaf gas exchange (i.e. the youngest fully expanded leaf on each plant). An impression of each leaf was taken in the interveinal area of the lamina using clear fingernail polish. Impressions were taken only from the abaxial surface of the leaves. The impressions were mounted on slides and observed with a light microscope (Nikon Eclipse 200) at 40× magnification. Three fields were observed for each leaf. The total number of stomatal and epidermal cells was counted in each field. The three fields together covered an area appropriate for calculation of the SI. The SI was defined as SI (%) = [SD/(SD + ED)] × 100, where SD is the SD (mm−2) and ED is the epidermal cell density (mm−2). The species C. cainito was excluded from these analyses, because the pubescence on the abaxial surface of its leaves obscured the epidermal impressions.
Statistical Analyses
Relationships between continuous variables were analyzed by least-squares linear regression. A nested ANOVA was used to test for effects of elevated [CO2] on growth, leaf gas exchange, stable isotope composition, water-use efficiency, and other response variables under conditions of high water supply in unfertilized soil. The eight species from the second experiment and the two species from the first experiment with the corresponding soil treatment were analyzed together. Species were nested into legumes and nonlegumes to test whether the two groups differed in their responses to elevated [CO2]. Separate ANOVAs were conducted to test for effects of the different soil treatments and elevated [CO2] on response variables in S. macrophylla and O. macrocalyx in the first experiment. Where necessary, data were log transformed prior to analyses to meet assumptions of normality and homogeneity of variance. Results were considered statistically significant at P < 0.05. Statistical analyses were carried out in Systat 12 (Systat Software).
Acknowledgments
We thank Dayana Agudo and Ben Harlow for assistance with isotopic and elemental analyses.
References
- Aguilar NO, Pitargue FC, Cajano MO. (1994) Nodulation of legumes in the Philippines. Sprent JI, McKey D, , Advances in Legume Systematics. Part 5. The Nitrogen Factor. Royal Botanic Gardens, Kew, UK, pp 25–31 [Google Scholar]
- Ainsworth EA, Rogers A. (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Nelson R, Long SP. (2004) Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric For Meteorol 122: 85–94 [Google Scholar]
- Allen ON, Allen EK. (1981) The Leguminosae: A Source Book of Characteristics, Uses, and Nodulation. University of Wisconsin Press, Madison, WI [Google Scholar]
- Barbour MM, Farquhar GD. (2000) Relative humidity- and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant Cell Environ 23: 473–485 [Google Scholar]
- Barron AR, Purves DW, Hedin LO. (2011) Facultative nitrogen fixation by canopy legumes in a lowland tropical forest. Oecologia 165: 511–520 [DOI] [PubMed] [Google Scholar]
- Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N, Rödenbeck C, Arain MA, Baldocchi D, Bonan GB, et al. (2010) Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329: 834–838 [DOI] [PubMed] [Google Scholar]
- Brienen RJW, Wanek W, Hietz P. (2011) Stable carbon isotopes in tree rings indicate improved water use efficiency and drought responses of a tropical dry forest tree species. Trees (Berl) 25: 103–113 [Google Scholar]
- Buckley TN. (2008) The role of stomatal acclimation in modelling tree adaptation to high CO2. J Exp Bot 59: 1951–1961 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Aranda J, Marshall JD, Winter K. (2007a) Large variation in whole-plant water-use efficiency among tropical tree species. New Phytol 173: 294–305 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Pate JS, Farquhar GD. (2004) Oxygen and carbon isotope composition of parasitic plants and their hosts in southwestern Australia. Oecologia 139: 199–213 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Aranda J, Turner BL. (2008) Conifers, angiosperm trees, and lianas: growth, whole-plant water and nitrogen use efficiency, and stable isotope composition (δ13C and δ18O) of seedlings grown in a tropical environment. Plant Physiol 148: 642–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD. (2007b) Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. J Exp Bot 58: 3549–3566 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Aranda J, Virgo A, Garcia M. (2009a) Transpiration efficiency over an annual cycle, leaf gas exchange and wood carbon isotope ratio of three tropical tree species. Tree Physiol 29: 1153–1161 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Turner BL. (2009b) Physiological and isotopic (δ13C and δ18O) responses of three tropical tree species to water and nutrient availability. Plant Cell Environ 32: 1441–1455 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Turner BL. (2009c) Plant δ 15N correlates with the transpiration efficiency of nitrogen acquisition in tropical trees. Plant Physiol 151: 1667–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA. (2004a) Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Philos Trans R Soc Lond B Biol Sci 359: 477–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA. (2004b) Tropical forests and global warming: slowing it down or speeding it up? Front Ecol Environ 2: 73–80 [Google Scholar]
- Clinebell RR, Phillips OL, Gentry AH, Stark N, Zuuring H. (1995) Prediction of neotropical tree and liana species richness from soil and climatic data. Biodivers Conserv 4: 56–90 [Google Scholar]
- de Boer HJ, Lammertsma EI, Wagner-Cremer F, Dilcher DL, Wassen MJ, Dekker SC. (2011) Climate forcing due to optimization of maximal leaf conductance in subtropical vegetation under rising CO2. Proc Natl Acad Sci USA 108: 4041–4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Faria SM, Diedhiou AG, de Lima HC, Ribeiro RD, Galiana A, Castilho AF, Henriques JC. (2010) Evaluating the nodulation status of leguminous species from the Amazonian forest of Brazil. J Exp Bot 61: 3119–3127 [DOI] [PubMed] [Google Scholar]
- de Faria SM, Lewis GP, Sprent JI, Sutherland JM. (1989) Occurrence of nodulation in the Leguminosae. New Phytol 111: 607–619 [DOI] [PubMed] [Google Scholar]
- Drake BG, Gonzalez-Meler MA, Long SP. (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48: 609–639 [DOI] [PubMed] [Google Scholar]
- Engelbrecht BMJ, Comita LS, Condit R, Kursar TA, Tyree MT, Turner BL, Hubbell SP. (2007) Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447: 80–82 [DOI] [PubMed] [Google Scholar]
- Estiarte M, Penuelas J, Kimball BA, Idso SB, Lamorte RL, Pinter PJ, Wall GW, Garcia RL. (1994) Elevated CO2 effects on stomatal density of wheat and sour orange trees. J Exp Bot 45: 1665–1668 [Google Scholar]
- Farquhar GD, Cernusak LA, Barnes B. (2007) Heavy water fractionation during transpiration. Plant Physiol 143: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Lloyd J. (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. Ehleringer JR, Hall AE, Farquhar GD, , Stable Isotopes and Plant Carbon-Water Relations. Academic Press, San Diego, pp 47–70 [Google Scholar]
- Farquhar GD, O’Leary MH, Berry JA. (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9: 121–137 [Google Scholar]
- Farquhar GD, Richards RA. (1984) Isotopic composition of plant carbon correlates with water-use efficiency in wheat genotypes. Aust J Plant Physiol 11: 539–552 [Google Scholar]
- Galbraith D, Levy PE, Sitch S, Huntingford C, Cox P, Williams M, Meir P. (2010) Multiple mechanisms of Amazonian forest biomass losses in three dynamic global vegetation models under climate change. New Phytol 187: 647–665 [DOI] [PubMed] [Google Scholar]
- Gentry AH. (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Mo Bot Gard 75: 1–34 [Google Scholar]
- Guehl JM, Domenach AM, Bereau M, Barigah TS, Casabianca H, Ferhi A, Garbaye J. (1998) Functional diversity in an Amazonian rainforest of French Guyana: a dual isotope approach (δ15N and δ13C). Oecologia 116: 316–330 [DOI] [PubMed] [Google Scholar]
- Hietz P, Wanek W, Dünisch O. (2005) Long-term trends in cellulose δ13C and water-use efficiency of tropical Cedrela and Swietenia from Brazil. Tree Physiol 25: 745–752 [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Winter K. (2010) Elevated [CO2] and forest vegetation: more a water issue than a carbon issue? Funct Plant Biol 37: 694–702 [Google Scholar]
- Houlton BZ, Wang YP, Vitousek PM, Field CB. (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454: 327–330 [DOI] [PubMed] [Google Scholar]
- Körner C. (2009) Responses of humid tropical trees to rising CO2. Annu Rev Ecol Evol Syst 40: 61–79 [Google Scholar]
- Lammertsma EI, de Boer HJ, Dekker SC, Dilcher DL, Lotter AF, Wagner-Cremer F. (2011) Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc Natl Acad Sci USA 108: 4035–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60: 2859–2876 [DOI] [PubMed] [Google Scholar]
- Lewis SL, Lloyd J, Sitch S, Mitchard ETA, Laurance WF. (2009) Changing ecology of tropical forests: evidence and drivers. Annu Rev Ecol Evol Syst 40: 529–549 [Google Scholar]
- Lloyd J, Farquhar GD. (2008) Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos Trans R Soc Lond B Biol Sci 363: 1811–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591–628 [DOI] [PubMed] [Google Scholar]
- Losos E, Leigh E, (2004) Tropical Forest Diversity and Dynamism: Findings from a Large-Scale Plot Network. University of Chicago Press, Chicago [Google Scholar]
- Lovelock CE, Winter K, Mersits R, Popp M. (1998) Responses of communities of tropical tree species to elevated CO2 in a forest clearing. Oecologia 116: 207–218 [DOI] [PubMed] [Google Scholar]
- Marchi S, Tognetti R, Vaccari FP, Lanini M, Kaligaric M, Miglietta F, Raschi A. (2004) Physiological and morphological responses of grassland species to elevated atmospheric CO2 concentrations in FACE-systems and natural CO2 springs. Funct Plant Biol 31: 181–194 [DOI] [PubMed] [Google Scholar]
- McHargue LA. (1999) Factors affecting the nodulation and growth of tropical woody legume seedlings. PhD thesis. Florida International University, Miami [Google Scholar]
- McKey D. (1994) Legumes and nitrogen: the evolutionary ecology of a nitrogen-demanding lifestyle. Sprent JI, McKey D, , Advances in Legume Systematics. Part 5. The Nitrogen Factor. Royal Botanic Gardens, Kew, UK, pp 211–228 [Google Scholar]
- Moreira FMS, da Silva MF, de Faria SM. (1992) Occurrence of nodulation in legume species in the Amazon region of Brazil. New Phytol 121: 563–570 [Google Scholar]
- Niklaus PA, Körner C. (2004) Synthesis of a six-year study of calcareous grassland responses to in situ CO2 enrichment. Ecol Monogr 74: 491–511 [Google Scholar]
- Nock CA, Baker PJ, Wanek W, Leis A, Grabner M, Bunyavejchewin S, Hietz P. (2010) Long-term increases in intrinsic water-use efficiency do not lead to increased stem growth in a tropical monsoon forest in western Thailand. Glob Change Biol 17: 1049–1063 [Google Scholar]
- Parker MA. (2004) RRNA and dnaK relationships of Bradyrhizobium sp. nodule bacteria from four papilionoid legume trees in Costa Rica. Syst Appl Microbiol 27: 334–342 [DOI] [PubMed] [Google Scholar]
- Pons TL, Perreijn K, van Kessel C, Werger MJA. (2007) Symbiotic nitrogen fixation in a tropical rainforest: 15N natural abundance measurements supported by experimental isotopic enrichment. New Phytol 173: 154–167 [DOI] [PubMed] [Google Scholar]
- Powell MH. (1995) Nitrogen fixing trees and shrubs for acid soils: an overview. Evans DO, Szotte LT, , Nitrogen Fixing Trees for Acid Soils. Winrock International Institute for Agricultural Development, Turrialba, Costa Rica, pp 185–195 [Google Scholar]
- Reich PB, Hungate BA, Luo YQ. (2006) Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annu Rev Ecol Evol Syst 37: 611–636 [Google Scholar]
- Reid CD, Maherali H, Johnson HB, Smith SD, Wullschleger SD, Jackson RB. (2003) On the relationship between stomatal characters and atmospheric CO2. Geophys Res Lett 30: 1983 [Google Scholar]
- Richardson AE, George TS, Hens M, Simpson RJ. (2005) Utilization of soil organic phosphorus by higher plants. Turner BL, Frossard E, Baldwin DS, , Organic Phosphorus in the Environment. CABI Publishing, Wallingford, UK, pp 165–184 [Google Scholar]
- Rogers A, Ainsworth EA, Leakey ADB. (2009) Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol 151: 1009–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugier B, Roy J, Mooney HA. (2001) Estimations of global terrestrial productivity: converging toward a single number? Roy J, Saugier B, Mooney HA, , Terrestrial Global Productivity. Academic Press, San Diego, pp 543–557 [Google Scholar]
- Schortemeyer M, Atkin OK, McFarlane N, Evans JR. (2002) N2 fixation by Acacia species increases under elevated atmospheric CO2. Plant Cell Environ 25: 567–579 [Google Scholar]
- Shearer G, Kohl DH. (1986) N2-fixation in field settings: estimations based on natural 15N abundance. Aust J Plant Physiol 13: 699–756 [Google Scholar]
- Sprent JI. (2009) Legume Nodulation: A Global Perspective. Wiley-Blackwell, Oxford [Google Scholar]
- Stork NE, Balston J, Farquhar GD, Franks PJ, Holtum JAM, Liddell MJ. (2007) Tropical rainforest canopies and climate change. Austral Ecol 32: 105–112 [Google Scholar]
- Tissue DT, Megonigal JP, Thomas RB. (1997) Nitrogenase activity and N2 fixation are stimulated by elevated CO2 in a tropical N2-fixing tree. Oecologia 109: 28–33 [DOI] [PubMed] [Google Scholar]
- Tricker PJ, Trewin H, Kull O, Clarkson GJJ, Eensalu E, Tallis MJ, Colella A, Doncaster CP, Sabatti M, Taylor G. (2005) Stomatal conductance and not stomatal density determines the long-term reduction in leaf transpiration of poplar in elevated CO2. Oecologia 143: 652–660 [DOI] [PubMed] [Google Scholar]
- Turner BL. (2008) Resource partitioning for soil phosphorus: a hypothesis. J Ecol 96: 698–702 [Google Scholar]
- Turner BL, Engelbrecht BMJ. (2011) Soil organic phosphorus in tropical rainforests. Biogeochemistry 103: 297–315 [Google Scholar]
- Winter K, Aranda J, Garcia M, Virgo A, Paton SR. (2001a) Effect of elevated CO2 and soil fertilization on whole-plant growth and water use in seedlings of a tropical pioneer tree, Ficus insipida Willd. Flora 196: 458–464 [Google Scholar]
- Winter K, Garcia M, Gottsberger R, Popp M. (2001b) Marked growth response of communities of two tropical tree species to elevated CO2 when soil nutrient limitation is removed. Flora 196: 47–58 [Google Scholar]
- Winter K, Garcia M, Lovelock CE, Gottsberger R, Popp M. (2000) Responses of model communities of two tropical tree species to elevated atmospheric CO2: growth on unfertilized soil. Flora 195: 289–302 [Google Scholar]
- Wright SJ. (2005) Tropical forests in a changing environment. Trends Ecol Evol 20: 553–560 [DOI] [PubMed] [Google Scholar]
- Yoneyama T, Fujita K, Yoshida T, Matsumoto T, Kambayashi I, Yazaki J. (1986) Variation in natural abundance of 15N among plant parts and in 15N/14N fractionation during N2 fixation in the legume-rhizobia symbiotic system. Plant Cell Physiol 27: 791–799 [Google Scholar]