Abstract
Carbonyl sulfide (COS) and C18OO exchange by leaves provide potentially powerful tracers of biosphere-atmosphere CO2 exchange, and both are assumed to depend on carbonic anhydrase (CA) activity and conductance along the diffusive pathway in leaves. We investigated these links using C3 and C4 plants, hypothesizing that the rates of COS and C18OO exchange by leaves respond in parallel to environmental and biological drivers. Using CA-deficient antisense lines of C4 and C3 plants, COS uptake was essentially eliminated and discrimination against C18OO exchange (18Δ) greatly reduced, demonstrating CA’s key role in both processes. 18Δ showed a positive linear correlation with leaf relative uptake (LRU; ratio of COS to CO2 assimilation rates, As/Ac, normalized to their respective ambient concentrations), which reflected the effects of stomatal conductance on both COS and C18OO exchange. Unexpectedly, a decoupling between As and 18Δ was observed in comparing C4 and C3 plants, with a large decrease in 18Δ but no parallel reduction in As in the former. This could be explained by C4 plants having higher COS concentrations at the CA site (maintaining high As with reduced CA) and a high phosphoenolpyruvate carboxylase/CA activity ratio (reducing 18O exchange efficiency between CO2 and water, but not As). Similar As but higher Ac in C4 versus C3 plants resulted in lower LRU values in the former (1.16 ± 0.20 and 1.82 ± 0.18 for C4 and C3, respectively). LRU was, however, relatively constant in both plant types across a wide range of conditions, except low light (<191 μmol photon m−2 s−1).
The seasonal cycling of CO2 concentration measured in the background atmosphere is often taken as evidence of the breathing of Earth and corresponds to about 10 Pg carbon. This is less than 10% of what we estimate to be the total cycling of CO2 due to respiration and photosynthesis of the terrestrial biosphere (Beer et al., 2010). The discrepancy stems from the fact that most of the cycling CO2 mixes in the atmosphere near the surface, and we only see the net sum of respiration and photosynthesis, which are often more or less balanced over the seasonal cycle. Modeling and measurements of CO2 exchange by ecosystems over diurnal cycles have been used to deconvolve the primary biological processes (e.g. Desai et al., 2008). However, our confidence in these approaches disintegrates as we move to larger scales because of the increase in the internal mixing of CO2 fluxes. Other approaches are needed to quantify the basic physiological processes at these scales.
Recent studies have identified another atmospheric trace gas that may be helpful in this regard—carbonyl sulfide (COS)—a chemical analog of CO2 that is routinely measured by the National Oceanic and Atmospheric Administration atmospheric sampling program (Montzka et al., 2007). However, its cycling differs from that of CO2. The major source of COS is the ocean either by direct emission or via CS2 oxidation (Kettle et al., 2002), and its major sink is the leaves of the terrestrial biosphere, where it is taken up in parallel with photosynthesis. There is no significant source of COS from terrestrial ecosystems; hence, the mixing between sources and sinks occurs on a much larger scale in the atmosphere. Several authors have suggested that COS might be a good tracer for terrestrial gross primary productivity (GPP; Montzka et al., 2007; Blake et al., 2008; Campbell et al., 2008; Suntharalingam et al., 2008; Seibt et al., 2010). The initial development of this tracer was built on theoretical insight into the likely mechanism of COS uptake and a few empirical studies that compared the rates of GPP and COS uptake at the leaf scale (Sandoval-Soto et al., 2005). However, if the goal is to use COS exchange estimates as a proxy for gross photosynthesis (or GPP) on larger scales, then it becomes important to understand the physiological basis for linking these fluxes.
At the leaf scale, correlations of both COS and CO2 fluxes with photosynthetically active radiation have been interpreted as evidence of stomata-dominated control of COS uptake rates, an interpretation that is supported by the daily pattern of this flux (Bartell et al., 1993; Hofman, 1993; Kuhn et al., 1999; Xu et al., 2002). Stimler et al. (2010a) also recently confirmed that there is no leaf-scale compensation point for COS, supporting the notion of no COS emission on this scale. The deposition velocity (flux normalized to concentration) for COS has been shown to be greater than that for CO2 in most species, with a mean ratio of the deposition velocities of COS to CO2 at the leaf level of about 3 (Sandoval-Soto et al., 2005). Recently, Campbell et al. (2008) expressed these ratios as leaf-scale relative uptake (LRU):
where A is the leaf uptake rate, Ca is the ambient concentration, and superscript s and c denote COS and CO2, respectively. As clearly revealed by Campbell et al. (2008), characterizing LRU and its response to environmental and physiological drivers is key to incorporating COS measurements into flux-partitioning studies.
In vitro studies have established that COS, which is meta stable in water, can be hydrolyzed to H2S and CO2 by the enzyme carbonic anhydrase (CA; Kluczewski et al., 1985; Lorimer and Pierce, 1989; Miller et al., 1989; Tiwari et al., 2005; see also Yakir, 2002). This enzyme is ubiquitous in plant leaves and is thought to be the biochemical sink for COS, which is further enhanced as this is an exergonic reaction, making the reverse reaction unfavorable (Liu et al., 2010):
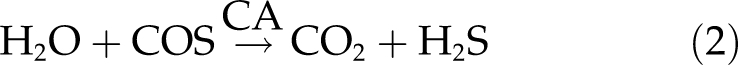 |
This enzyme is also known to catalyze the exchange of stable isotopes of oxygen between CO2 and leaf water. This isotopic exchange can be monitored by measuring the change in the 18O content of CO2 in gas exchange as well as in large-scale flux studies (Farquhar et al., 1993; Yakir and Wang, 1996; Gillon and Yakir, 2001). For CO2, CA facilitates the reversible hydration (Lindskog and Coleman, 1973):
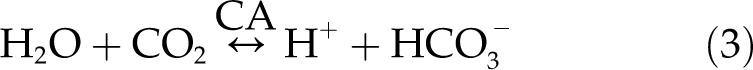 |
This reversible hydration/dehydration of CO2 provides the opportunity for efficient 18O exchange between leaf water, normally enriched in 18O, and CO2 inside the leaf (Francey and Tans, 1987; Farquhar and Lloyd, 1993; Farquhar et al., 1993; Yakir and Wang, 1996; Gillon and Yakir, 2000, 2001; Seibt et al., 2007).
C18O16O isotopic exchange and COS flux can be expected to respond to the same variables. Sufficient CA activity is needed to both bring about full isotopic equilibrium and maintain near-zero COS concentration at the site of CA activity. Furthermore, the same diffusion resistances limit both the inward diffusion of COS flux into leaves and the retrodiffusion of 18O-labeled CO2 back into the atmosphere. Note, however, that the diffusion gradient of CO2 is driven by the photosynthetic flux, while that of COS is independent of it.
Recognizing the potential importance of incorporating COS into gas exchange and flux measurements, we developed a laser-based instrument for the continuous measurement of COS concentration at normal atmospheric levels (approximately 400 parts per trillion, or one-millionth the concentration of CO2). This instrument enables conducting gas exchange studies analogous to those typically conducted with nondispersive infrared gas analyzers (Stimler et al., 2010a). A subsequent study (Stimler et al., 2010b) examined the stoichiometry of COS and CO2 fluxes in leaves of C3 and C4 species with particular emphasis on the control of these processes by stomatal conductance (gs). In this article, we focus on the mechanism of COS uptake. We also hypothesize that COS uptake will be closely linked to 18O labeling of CO2 during leaf gas exchange, and we take advantage of natural and genetically engineered variations in CA to examine this hypothesis.
RESULTS AND DISCUSSION
Relative COS/CO2 Uptake in C3 versus C4 Plants
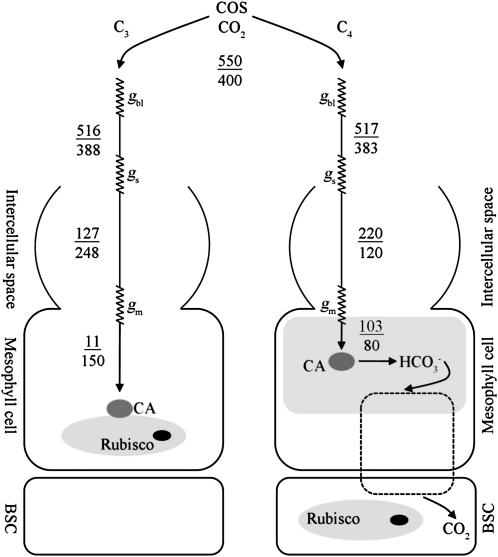
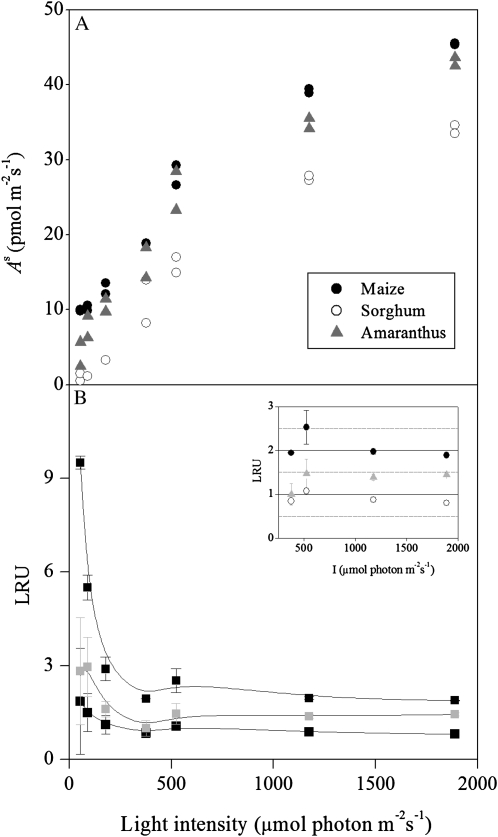
The mean rates of net CO2 assimilation (Ac) and COS uptake (As) and the fluxes involved are summarized in Table I and Figure 1, respectively, and are consistent with previous studies (Kesselmeier and Merk, 1993; Sandoval-Soto et al., 2005; Stimler et al., 2010a). Due to the higher Ac but similar As values for C4 compared to C3 plants, the LRU values were higher in the latter (Table I; Fig. 2; see Kesselmeier and Merk, 1993; Sandoval-Soto et al., 2005; Seibt et al., 2010; Stimler et al., 2010a). Irrespective of these differences, the results showed that, as noted previously for C3 plants (Stimler et al., 2010a), LRU is relatively constant across a wide range of light intensities in both C3 and C4 plants. Large deviations were only observed under low light (<191 μmol photon m−2 s−1), when the ratio could increase to up to 9.6 in both C3 and C4 plants (Fig. 2B). These results support the assumption that both Ac and As are influenced to a similar extent by stomatal conductance. Indeed, As was linearly correlated to gs (As = 322.0gs + 11.2; r2 = 0.78, P < 0.0001 for C3, and As = 109.0gs + 3.4; r2 = 0.86, P < 0.0001 for C4). The deviations from the relatively constant LRU under low light probably reflect the light dependency of Rubisco activation and of ribulose 1,5-bisP supply, whereas CA activity is presumably light insensitive (Sage and Sharkey, 1987).
Table I. COS versus CO2 uptake fluxes; simple (As/Ac) or normalized (LRU = As/Ac × ([CO2]/[COS]) ratio of COS and CO2 uptake rates, and COS total conductance under maximum-intensity light, for C4 and C3 plants.
Gas exchange conditions during the light response experiments were T approximately 24°C, RH approximately 70%, [COS] approximately 550 pmol mol−1, and [CO2] = 400 μmol mol−1; two to five replicates per species; sd is indicated in parentheses.
| Plant Species | As/Ac | LRU | As | Ac | gts |
| μmol mol−1 | pmol m−2 s−1 | μmol m−2 s−1 | mol m−2 s−1 | ||
| C4 | |||||
| Maize | 1.97 (0.50) | 1.05 (0.76) | 42.33 (3.65) | 16.23 (5.04) | 0.17 (0.18) |
| Sorghum | 1.91 (0.39) | 1.05 (0.44) | 34.10 (13.91) | 20.20 (4.18) | 0.09 (0.06) |
| Amaranthus | 2.51 (0.85) | 1.37 (0.79) | 41.54 (10.38) | 17.48 (3.72) | 0.13 (0.04) |
| Average | 2.13 (0.33) | 1.16 (0.18) | 39.32 (4.54) | 17.97 (2.03) | 0.13 (0.04) |
| C3 | |||||
| Tobacco | 2.61 (0.96) | 1.63 (0.36) | 27.01 (4.89) | 9.41 (2.08) | 0.1 (0.03) |
| Sage | 2.67 (1.11) | 1.82 (0.89) | 40.80 (24.38) | 13.50 (5.71) | 0.05 (0.03) |
| Hibiscus | 2.87 (1.28) | 2.02 (0.29) | 40.23 (12.68) | 9.91 (1.25) | 0.07 (0.02) |
| Average | 2.72 (0.14) | 1.82 (0.20) | 36.01 (13.98) | 10.94 (3.01) | 0.07 (0.03) |
Figure 1.
Comparison of the diffusion pathways of COS and CO2 from the atmosphere to the site of biochemical reactions in C3 and C4 leaves. Concentrations of COS (pmol mol−1) and CO2 (μmol mol−1) are indicated above and below the lines, respectively. Conductance through resistance steps associated with the boundary layer, stomata, and mesophyll (mol m−2 s−1) are indicated by gbl, gs, and gm, respectively, based on leaves with net CO2 flux of 14 or 20 μmol m−2 s−1 (C3 and C4, respectively), and COS uptake flux of approximately 35 pmol m−2 s−1 (for both plant types). Values of gm (0.3 mol m−2 s−1 for both C4 and C3 leaves, respectively) were estimated by Stimler et al. (2010a) and incorporate dissolution, liquid-phase diffusion, and the biochemical step (assuming first-order reaction); gm estimates for CO2 were based on CO2 concentration at the hydration site derived from isotopic measurements (Gillon and Yakir, 2001).
Figure 2.
COS uptake rates (pmol m−2 s−1; A) and LRU values (B) during light response measurements in C4 plants. Species used were: maize, sorghum, and amaranthus. Conditions during the experiments were: T approximately 24°C, RH approximately 70%, [COS] approximately 550 pmol mol−1, and [CO2] = 400 μmol mol−1. Symbols without error bars have sd < 2% (in section B only). Inset: LRU (the normalized ratio of COS/CO2 uptake rates) under high light intensity.
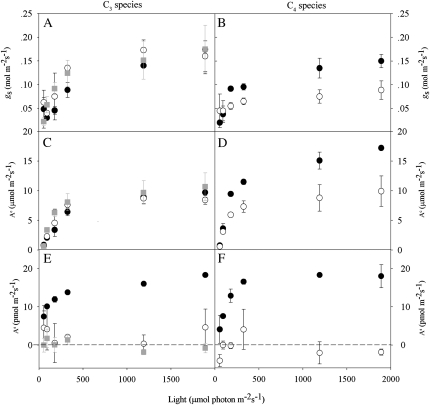
Stomatal conductance is only one of the two major factors regulating leaf COS exchange. Ultimately, COS uptake depends on the reaction rate of COS with CA and water to produce CO2 and H2S (Eq. 2; Liu et al., 2010; Stimler et al., 2010a). Here, we confirmed the critical role of CA by carrying out light response experiments with CA-deficient antisense lines of C3 and C4 species (Price et al., 1994; von Caemmerer et al., 2004). COS uptake was essentially eliminated in both of these antisense lines (Fig. 3). In the C3 plants, this was not associated with significant changes in gs or Ac (Fig. 3, A, C, and E). In C4 plants, however, antisense lines also showed reductions in Ac and gs (Fig. 3, B, D, and F). This presumably reflects the C4 pathway’s dependence on bicarbonate, produced by the CA-facilitated hydration of CO2.
Figure 3.
Stomatal conductance (gs; mol m−2 s−1; A and B), CO2 uptake (Ac; μmol m−2 s−1; C and D), and COS uptake (As; pmol m−2 s−1; E and F) in tobacco (C3) and F. bidentis (C4) wild-type and antisense lines with different levels of CA activity during the light response experiments. Conditions during the experiments were as in Figure 2. Symbols (black circles, white circles, and gray squares) indicate wild type, moderate (10%, C3 and C4 plants), and low (2%, C3 only) CA activities of the antisense lines, respectively.
Note that in this study, the use of CA-deficient antisense lines did not address the entire range of CA types or enzyme locations in the plants’ leaves (Fabre et al., 2007). Nevertheless, the CA that was affected in these lines clearly dominated the COS and 18O response.
COS Exchange and 18Δ
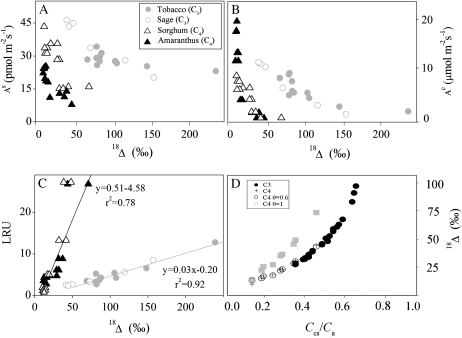
Consistent with previous studies (Farquhar and Lloyd, 1993; Gillon and Yakir, 2001), leaf-scale discrimination against C18OO (18Δ) was correlated with Ccs/Ca in all plants (Fig. 4D; where Ccs refers to CO2 concentration at the chloroplast surface). Moreover, the C4 plants showed markedly lower 18Δ than the C3 plants. This most likely reflects incomplete isotopic equilibrium between CO2 and water in the C4 leaves, and accounting for this effect (i.e. θeq < 1; Gillon and Yakir, 2000, 2001) revealed consistent relationships between 18Δ and Ccs/Ca in both C4 and C3 plants (Fig. 4).
Figure 4.
Relationships between C18OO (18Δ; ‰) and rates of COS uptake (pmol m−2 s−1; A), CO2 uptake (μmol m−2 s−1; B), and LRU (C), observed in C3 (circles) and C4 (triangles) plants. Each curve represents the full set of data from a complete light response experiment. In C, r2 values for the linear best-fit lines for C4 and C3 plants are indicated (P < 0.0001). D, The relationship between 18Δ and CO2 concentrations at the hydration site (Ccs) from different light response experiments and plants. Conditions during gas exchange measurements were as in Figure 2. Black circles and crosses represent observed discrimination values; white symbols represent predicted values based on the indicated parameters and as described in Table II, following Gillon and Yakir (2001).
As expected (see above; see also Stimler et al., 2010a), a clear negative correlation was observed between As and 18Δ. This correlation was apparent in all leaves during the light response experiments, for both C4 and C3 species (Fig. 4A, showing representative species). Increasing light intensity was generally associated with a decrease in 18Δ values and increases in As. For example, As increased from 15 to 43 pmol m−2 s−1 and from 8 to 24 pmol m−2 s−1, whereas 18Δ decreased from 84‰ to 13‰ and from 48‰ to 12‰ in amaranthus (Amaranthus cruentus) and sorghum (Sorghum halepense), respectively. In C3 species, As increased from 20 to 46 pmol m−2 s−1 and from 20 to 35 pmol m−2 s−1, while 18Δ values decreased from 157‰ to 40‰ and from 239‰ to 81‰, for sage (Salvia longispicata × Salvia farinacea) and tobacco (Nicotiana tabacum), respectively. This inverse correlation between As and 18Δ is readily explained by the effects of gs and Ccs: Increasing gs with light intensity results in increasing uptake rates of COS (as well as CO2). At the same time, the increase in photosynthetic capacity with increasing light intensity leads to a decrease in Ccs, resulting in decreased retroflux of 18O-labeled CO2 out of the leaf and, consequently, in the observed reduction in18Δ (see Farquhar and Lloyd, 1993; Gillon and Yakir, 2001).
On average, a reduction of 75‰ in 18Δ was associated with a decrease in Ccs/Ca, from 0.7 to 0.4, in C3 plants and from 0.5 to 0.2 in C4 plants. As the decrease in Ccs reflects a proportionately greater increase in photosynthetic capacity (influencing only Ac and not As) relative to stomatal conductance (but gs influences both Ac and As), it can be expected that the LRU will also change under these circumstances. Indeed, linear relationships between LRU and 18Δ were observed (r2 = 0.76, 0.87, and 0.99) for the C4 species sugarcane (Saccharum officinarum), sorghum, and maize (Zea mays), and r2 = 0.89, 0.98, and 0.79 for the C3 species hibiscus (Rosa sinensis), tobacco, and sage, respectively. Grouping the results for the C4 and C3 plants yielded best-fit lines of y = 0.51x − 4.58 with r2 = 0.78 (P < 0.0001) for C4 plants and y = 0.03x − 0.20 with r2 = 0.92 (P < 0.0001) for C3 plants.
The COS-18Δ Link in C4 versus C3 Plants
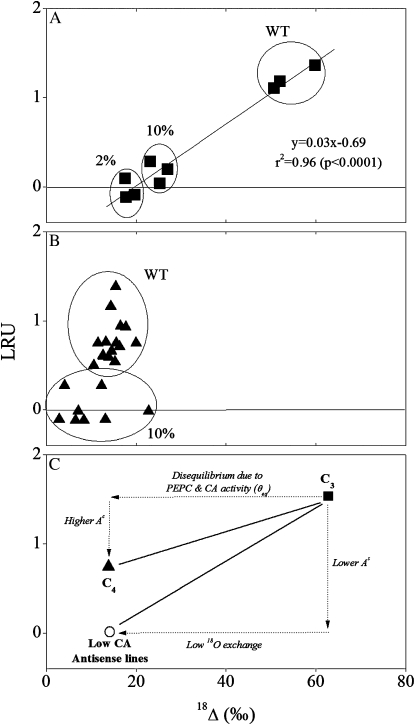
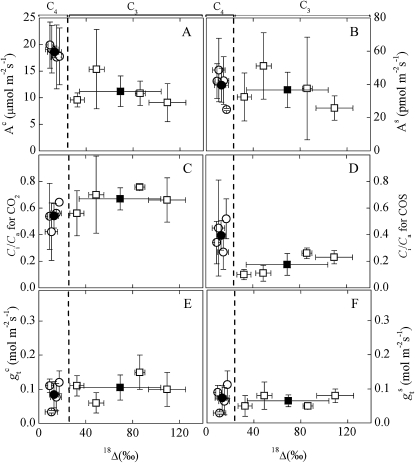
The correlation between LRU and 18Δ covered the entire range of both wild-type and CA-deficient antisense lines of C3 plants (Fig. 5A), but not of C4 plants (Fig. 5B), or in comparing C3 to C4 plants (Fig. 6). Unexpectedly, while 18Δ was lower in C4 than in C3 plants, consistent with previous studies (Gillon and Yakir, 2000, 2001), there was essentially no difference in the rate of COS uptake between the two photosynthetic groups (mean As values of 36.0 ± 13.9 and 39.32 ± 4.54 pmol m−2 s−1 for C3 and C4, respectively). Other variables generally showed the expected differences between C3 and C4 leaves: Ac was markedly higher, Ci/Ca (CO2) lower, and gs (CO2) similar in C4 compared to C3 leaves (Fig. 6; Table I). Cis/Cas (for COS) was <0.2, as observed previously in C3 plants (Stimler et al., 2010a), but twice as high in C4 plants (Fig. 6D), which may reflect differences in internal resistances, as well as the fact that CA is located higher up (toward the atmosphere) in the diffusive pathway in C4 versus C3 leaves (see Fig. 1).
Figure 5.
Relationship between LRU and 18O discrimination (18Δ) in plants with different levels of CA activity (antisense lines). A, Mean values in C3 species (tobacco) with moderate and low levels of CA activity (percent of wild type). B, Data of C4 species (F. bidentis) with moderate CA activity. Values are based on light response experiments under high light intensity (1,889 μmol photon m−2 s−1) and all other conditions as in Figure 2. C, Schematic summary of the relationships between LRU and 18Δ values, indicating the possible factors underlying the observed changes based on data from A and B. CA deficiency eliminates COS uptake (LRU approaches zero) and reduces 18O discrimination (18Δ) in both C3 and C4 plants. Compared to C3 wild-type plants, the C4 wild-type (WT) species show a small decrease in LRU (lower As/Ac due to high Ac values in these plants) but a large decrease in 18Δ (PEPC in these plants limits 18O exchange and CO2-water isotopic equilibrium).
Figure 6.
Relationships between gas exchange parameters and 18O discrimination (18Δ) in C3 and C4 plants. A and B, Rates of CO2 uptake (Ac; A) or COS uptake (As; B); C and D, ratio of intercellular to ambient concentrations of CO2 (Cic/Cac; C) and COS (Cis/Cas; D); E and F, total leaf conductance of CO2 (gtc; E) or COS (gts; F). Data are compiled from different light response experiments with C4 (circles) and C3 (squares) species. Each data point represents four to six measurements (sd is indicated). Black symbols indicate mean values. C4 species used were maize, sorghum, and amaranthus, and C3 species were sage, tobacco, and hibiscus.
Table II. Isotopic and gas exchange values for a C4 plant (amaranthus) based on light response measurements (compare, Fig. 4D).
18Δm and 18Δp are measured and predicted 18O discrimination (‰) when θeq = 1 and θeq = 0.6 (full or partial isotopic equilibrium, respectively), ε = Ccs/(Ca − Ccs) where Ca and Ccs indicate ambient and chloroplast surface CO2 concentrations, Δea is isotopic discrimination between atmospheric CO2, δa, and that in equilibrium with leaf water, δe: Δea = 1,000 × [(δe/1,000 + 1)/( δa/1,000 + 1) − 1]. Irradiance values are in μmol photon m−2 s−1. Analysis and definitions are based on Gillon and Yakir (2001).
| Irradiance | 18Δm | 18Δp (θeq = 0.6) | 18Δp (θeq = 1) | ε | Δea |
| 171 | 30.2 | 30.35 | 48.87 | 0.95 | 35.55 |
| 352 | 31.1 | 31.58 | 51.10 | 1.00 | 31.25 |
| 524 | 23.35 | 23.62 | 36.93 | 0.72 | 31.30 |
| 1,179 | 16.95 | 17.26 | 25.54 | 0.45 | 32.78 |
| 1,889 | 3.77 | 10.20 | 14.20 | 0.18 | 34.59 |
The explanation for the unexpected discrepancy between changes in 18Δ and As in going from C3 to C4 plants can be based on two mechanisms. These mechanisms are outlined below, but at this stage we do not have sufficient information to accurately quantify their relative contributions.
First, note that as indicated above, low 18Δ values for C4 plants result from incomplete isotopic equilibrium between CO2 and water (Gillon and Yakir, 2000; Eq. 3), which is assumed to be due to reduced CA activity (observed in whole-leaf extracts of C4 leaves; Gillon and Yakir, 2000). No parallel reduction in COS flux was observed in the C4 plants examined (Fig. 6B), indicating sufficient CA activity. Note that in going from C3 to C4 plants, gs remains similar but Ci/Ca (CO2) decreases and Cis/Cas (COS) increases. This is likely because a reduction in Cic (CO2) in C4 leaves is associated with higher Ac, whereas an increase in Cis (COS) is associated with lower CA activity. The increase in Cis (COS), however, helps restore As to levels similar to those in C3 plants. Therefore, given that As is a function of both CA and the COS concentration at the enzyme site, the higher Cis/Cas observed in the C4 plants (Fig. 6D) could accommodate proportionately lower CA activity while maintaining relatively high As values. This could explain, at least in part, the observed discrepancy in As versus 18Δ. Accordingly, estimating total internal conductance to COS, gsm (combining dissolution and the biochemical steps, assuming a first-order reaction, as: gms=1/[(Cas/As) − (1/(1.94/gsw) + 1/(1.56/gblw))]; see Stimler et al., 2010a), indicated a mean gms value of approximately 0.3 mol m−2 s−1 similar to our earlier study and, considering the observed variations (about ±0.5), similar for both C3 and C4 plants. This upholds the high internal COS concentration near the biochemical reaction site (Fig. 1).
The second mechanism that could help explain the As versus 18Δ discrepancy (required when, for example, the reduced CA activity accommodated by the first mechanism remains too high to produce previously reported θeq values) relies on the fact that in C4 plants, the primary photosynthetic substrate is bicarbonate consumed by phosphoenolpyruvate carboxylase (PEPC; Cousins et al., 2007). A high ratio of PEPC to CA activity, ρ, must result in reduced bicarbonate concentrations which, in turn, reduce the efficiency of the isotopic exchange between CO2 and water (the ratio of Rubisco to CA activities is assumed to be low and is neglected here; see Farquhar and Lloyd, 1993). Since PEPC activity directly influences the hydration rate, the apparent CO2 hydration rate constant, KH, should not be identical to the CA rate constant, CAleaf, originally used in estimating θeq (Gillon and Yakir, 2001). To account for this effect, we revised the disequilibrium term introduced in Gillon and Yakir (2001), substituting KH for CAleaf, where KH = CAleaf × (1 − ρ), to obtain:
where CAleaf is the hydration rate scaled to leaf conditions (CAleaf = k/Fin, k is CA rate constant, Fin is the gross CO2 influx rate, μmol m−2 s−1), and 3 reflects the slower isotopic exchange associated with the three oxygens in bicarbonate.
Limited information is available on the variations in PEPC and CA activities among plant species. Based on our gas exchange data, supplemented with values in the literature (e.g. Cousins et al., 2007), we can assume for a typical C4 leaf, with As of approximately 40 pmol m−2 s−1 and Cis/Cas of approximately 0.4, midrange CA activity of approximately 600 μmol m−2 s−1 and PEPC activity of approximately 100 μmol m−2 s−1, which yields ρ = 0.17. This, in turn, results in a disequilibrium, θeq, value of approximately 0.7, consistent with the observed mean value of approximately 0.6 (Fig. 4D) for our C4 plants. As expected, θeq values approach zero and 1 when ρ values approach 1 and zero, respectively. Note that the above estimates of the ρ effects incorporate both the effect of the relatively high PEPC activities and the high Cis in C4 leaves.
Similar effects of PEPC on 18Δ have been demonstrated previously using amaranthus (C4) plants with different levels of PEPC (Cousins et al., 2007). In that study, suppressing the enzyme activity by a factor of approximately 40 enhanced 18Δ from 16.8‰ to 207‰, consistent with our results, but this was ascribed to errors in estimating CA activities. Note that in C4 plants, there was no change in 18Δ between the wild-type and antisense lines (Fig. 5B), which may indicate similar CA/PEPC in both plants or an effect on 18Δ of CA/PEPC in the wild type similar to the reduced CA in the antisense plants.
Our current, preliminary, perspective of the links between COS uptake rates and 18Δ, summarized in Figure 5C, highlights three points. First, 18Δ in C4 plants is markedly reduced compared to C3 plants, as has been repeatedly reported. Second, CA activity is key to both 18O discrimination and COS uptake. Third, 18Δ and LRU are decoupled in C4 plants because of the high Cis and reduced bicarbonate concentrations associated with high PEPC activity.
CONCLUSION
In this study, we extend our previous results showing that LRU is relatively constant in both C3 and C4 plants. We show that the two processes, COS uptake and C18OO exchange, can be measured simultaneously and that their rates are closely correlated, whether modulated by changing environmental conditions or by manipulating the activity of the key enzyme CA. The unexpected decoupling of COS and C18OO exchange in comparing C3 and C4 plants demonstrated that our understanding of CA reaction and 18O discrimination in leaves is incomplete. Both COS and C18ΟΟ can serve as potentially powerful tracers of plant-atmosphere CO2 fluxes, and as a means to partition these fluxes to their gross components. Using the observed links between COS and 18Δ could greatly improve their use as tracers and constrain interpretations of the underlying processes.
MATERIALS AND METHODS
Plant Material
C3 species sage (Salvia longispicata × Salvia farinacea), tobacco (Nicotiana tabacum), and hibiscus (Rosa sinensis) were purchased from local nurseries. C4 species maize (Zea mays) and sorghum (Sorghum halepense) were grown from seeds in the greenhouse, while amaranthus (Amaranthus cruentus) and sugarcane (Saccharum officinarum) were purchased from nurseries. CA-deficient antisense line and wild-type seeds of tobacco (C3) and Flaveria bidentis (C4) were contributed by Prof. S. von Caemmerer (Australian National University) and grown in the greenhouse. Various levels of CA activity were achieved using the suppression methods described by Price et al. (1994) and von Caemmerer et al. (2004). Plants were kept under ambient light and temperature during the experimental period.
Gas Exchange Measurements
Uptake rates of CO2 and COS were measured in all C3 and C4 plants with attached leaves. The experimental system consisted of a flow-through leaf cuvette made of Teflon-coated stainless steel with a magnetically operated fan and a glass window at the top. A whole leaf or branch was sealed in the cuvette (O-ring seal except around the petiole, which was sealed with high-vacuum putty). Measurements were performed under a relative humidity (RH) of approximately 70% and an air temperature of approximately 24°C. Light intensity was 55 to 1,889 μmol photon m−2 s−1, regulated with layers of miracloth, and filtered through 5 cm of water. Continuous airflow coming out of the cuvette was split into two paths for COS and CO2 analysis. Following the CO2 analysis, the sampled air was passed through a magnesium perchlorate drying trap (Sigma-Aldrich) to remove water before collecting the air in 115-mL flasks for analysis of the isotopic composition of CO2. COS and CO2 mixing ratios in air entering the leaf cuvette were adjusted to the desired values by mixing purified synthetic air with known gas mixtures produced from a COS-permeation device (VICI Metronics), and a compressed gas mixture of 1% CO2 in air, followed by a three-stage dilution system. All flow rates were regulated and measured by mass-flow controllers (MKS).
CO2 and COS Analyses
CO2 and water-vapor concentrations in the air entering and leaving the leaf cuvette were measured by an infrared gas analyzer (Li-6262; Li-Cor) at a precision better than 0.5 μmol mol−1 for CO2 and 0.1 mmol mol−1 for water vapor.
COS concentrations were measured using a mid-IR dual quantum cascade laser spectrometer (Aerodyne Research Inc.) at a wavenumber of 2,056 cm−1 with a LN2-cooled HgCdTe (MCT) detector (Kolmar Technologies) as described by Stimler et al. (2010b). Briefly, direct detection of the absorption spectrum was followed by quantitative spectral fitting combined with the measured pressure, temperature, and path length of the absorption cell and the laser spectral line width using TDL WINTEL software, as described by Nelson et al. (2004). The concentrations of COS and the laser line widths were determined in real time from the spectra through a nonlinear least-squares fitting algorithm that uses spectral parameters from HITRAN (Rothman et al., 2003). The data analysis procedure included pulse normalization reduction of the sample signal and automatic background correction (N2). Pulse normalization corrects for variations in pulse-to-pulse amplitude in pulsed laser systems, by normalizing the signal pulse train to a reference pulse train. The automatic background correction divides the sample spectra by the spectrum of dry N2. Corrections were carried out every 300 s.
Isotopic Analysis of CO2
Oxygen isotope analysis of CO2 was based on sampling CO2 in the air entering and exiting the leaf cuvette by passing it through a 115-mL glass flask under atmospheric pressure. The CO2 in the flask was measured as described by Klein et al. (2005). Briefly, a 1.5-mL aliquot was removed from each flask into a sampling loop and the CO2 was cryogenically trapped using helium as the carrier gas. It was then passed through a Carbosieve G-packed column at 70°C to separate N2O, and the eluted CO2 was analyzed in a Europa 20-20 continuous-flow isotope ratio mass spectrometer (Crewe). Batches of 15 flasks at a time were measured from an automated manifold system, with five flasks of a standard gas being measured for every 10 samples at a precision of 0.2‰. Results are expressed in the small δ notation (δ‰) versus VPDB-CO2 for 18O, where δ‰ = (Rsample/Rstandard − 1) × 1,000 and Rsample and Rstandard are the isotopic ratios of the sample and the appropriate standard, respectively. Instantaneous leaf discriminations, Δ, were calculated as described by Evans et al. (1986) for 13C and extended to 18O (Gillon and Yakir, 2001). The isotopic composition of CO2 in air supplied to the leaves during the experiments was around δ18O = −27‰. The δ18O values of water supplied to the plants was −5‰, and that of leaf water at the evaporating surfaces was estimated using the modified Craig-Gordon model (Craig and Gordon, 1965; Farquhar and Lloyd, 1993) to be 10‰ ± 2‰ (depending on conditions and species).
Leaf discrimination against C18OO, 18Δ, and the extent of CO2-water isotopic equilibrium, θeq, were calculated as detailed by Farquhar and Lloyd (1993) and Gillon and Yakir (2000). Calculation of CO2 concentration at the hydration site (Ccs) was based on the difference between the measured and predicted discrimination against 13C (13Δ) as described by Gillon and Yakir (2000), based on the approach of Evans et al. (1986). For 18Δ estimation in C4 plants, incomplete isotopic equilibrium (θeq) was assumed, as measured by Gillon and Yakir (2001).
References
- Bartell U, Hoffman U, Hoffman R, Kreuzburg B, Andreae MO. (1993) OCS and H2S fluxes over a wet meadow in relation to photosynthetic activity: an analysis of measurements made on 6th September 1990. Atmos Environ 27A: 1851–1864 [Google Scholar]
- Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N, Rödenbeck C, Arain MA, Baldocchi D, Bonan GB, et al. (2010) Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329: 834–838 [DOI] [PubMed] [Google Scholar]
- Blake NJ, Campbell JE, Vay SA, Fuelberg HE, Huey LG, Sachse G, Meinardi S, Rowland FS, Blake DR. (2008) Carbonyl sulfide (OCS): large scale distributions over North America during INTEX-NA and relationship to CO2. J Geophys Res 113: D09S90 [Google Scholar]
- Campbell JE, Carmichael GR, Chai T, Mena-Carrasco M, Tang Y, Blake DR, Blake NJ, Vay SA, Collatz GJ, Baker I, et al. (2008) Photosynthetic control of atmospheric carbonyl sulfide during the growing season. Science 322: 1085–1088 [DOI] [PubMed] [Google Scholar]
- Cousins AB, Baroli I, Badger MR, Ivakov A, Lea PJ, Leegood RC, von Caemmerer S. (2007) The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiol 145: 1006–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig H, Gordon HI. (1965) Deuterium and oxygen-18 variations in the ocean and marine atmosphere. Tongiorgi E, , Proceedings of a Conference on Stable Isotopes in Oceanographic Studies and Palaeotemperatures. Laboratory of Geology and Nuclear Science, Pisa, Italy, pp 9–130 [Google Scholar]
- Desai AR, Richardson AD, Moffat AM, Kattge J, Hollinger DY, Barr A, Falge E, Noormets A, Papale D, Reichstein M, et al. (2008) Cross-site evaluation of eddy covariance GPP and RE decomposition techniques. Agric Forest Meteorol 148: 821–838 [Google Scholar]
- Evans JR, Sharkey TD, Berry AJ, Farquhar GD. (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Fabre N, Reiter IM, Becuwe-Linka N, Genty B, Rumeau D. (2007) Characterization and expression analysis of genes encoding alpha and beta carbonic anhydrases in Arabidopsis. Plant Cell Environ 30: 617–629 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Lloyd J. (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. Ehleringer JR, Hall AE, Farquhar GD, , Stable Isotopes and Plant Carbon-Water Relations. Academic Press, San Diego, pp 47–70 [Google Scholar]
- Farquhar GD, Lloyd J, Taylor JA, Flanagan AB, Syverts JP, Hubick KT, Wong SC, Ehleringer JR. (1993) Vegetation effects on the oxygen isotopic composition of atmospheric CO2. Nature 363: 439–443 [Google Scholar]
- Francey RJ, Tans PP. (1987) Latitudinal variation in oxygen-18 of atmospheric CO2. Nature 327: 495–497 [Google Scholar]
- Gillon JS, Yakir D. (2001) Influence of carbonic anhydrase activity in terrestrial vegetation on the 18O content of atmospheric CO2. Science 291: 2584–2587 [DOI] [PubMed] [Google Scholar]
- Gillon JS, Yakir D. (2000) Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant Cell Environ 23: 903–915 [Google Scholar]
- Hofman DJ. (1993) 20 years of balloon-borne trophospheric aerosol measurements at Laramie, Wyoming. J Geophys Res-Atmos 98: 12753–12766 [Google Scholar]
- Kesselmeier J, Merk L. (1993) Exchange of carbonyl sulfide (COS) between agricultural plants and the atmosphere: studies on the deposition of COS to peas, corn and rapeseeds. Biogeochemistry 23: 47–59 [Google Scholar]
- Kettle AJ, Kuhn U, von Hobe M, Kesselmeier J, Andreae MO. (2002) Global budget of atmospheric carbonyl sulfide: temporal and spatial variations of the dominant sources and sinks. J Geophys Res 107: 4658 [Google Scholar]
- Klein T, Hemming D, Lin T, Grünzweig JM, Maseyk K, Rotenberg E, Yakir D. (2005) Association between tree-ring and needle δ13C and leaf gas exchange in Pinus halepensis under semi-arid conditions. Oecologia 144: 45–54 [DOI] [PubMed] [Google Scholar]
- Kluczewski SM, Brown KW, Bell JNB. (1985) Deposition of [35S]-carbonyl sulphide to vegetable crops. Radiat Prot Dosimetry 11: 173–177 [Google Scholar]
- Kuhn U, Ammann C, Wolf A, Meixner FX, Andreae MO, Kesselmeier J. (1999) OCS exchange on an ecosystemscale: soil represents a dominant sink for atmospheric OCS. Atmos Environ 33: 995–1008 [Google Scholar]
- Lindskog S, Coleman JE. (1973) Catalytic mechanism of carbonic anhydrase. Proc Natl Acad Sci USA 70: 2505–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ma J, He H. (2010) Heterogeneous reactions of carbonyl sulfide on mineral oxides: mechanism and kinetics study. Atmos Chem Phys 10: 10335–10344 [Google Scholar]
- Lorimer GH, Pierce J. (1989) Carbonyl sulfide: an alternate substrate for but not an activator of ribulose-1,5-bisphosphate carboxylase. J Biol Chem 264: 2764–2772 [PubMed] [Google Scholar]
- Miller AG, Espie GS, Canvin DT. (1989) Use of carbon oxysulfide, a structural analog of CO2, to study active CO2, transport in the Cyanobacterium synechococcus UTEX 625. Plant Physiol 90: 1221–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montzka S, Calvert P, Hall BD, Elkins JW, Conway TJ, Tans PP, Sweeny C. (2007) On the global distribution, seasonality and budget of atmospheric carbonyl sulfide (COS) and some similarities to CO2. J Geophys Res 112: D09302 [Google Scholar]
- Nelson DD, McManus B, Urbanski S, Herndon S, Zahniser MS. (2004) High precision measurements of atmospheric nitrous oxide and methane using thermoelectrically cooled mid-infrared quantum cascade lasers and detectors. Spectrochim Acta A Mol Biomol Spectrosc 60: 3325–3335 [DOI] [PubMed] [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Yu JW, Lloyd J, Oja V, Kell P, Harrison K, Gallagher A, Badger MR. (1994) Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation. Planta 193: 331–340 [Google Scholar]
- Rothman LS, Barbe A, Benner DC, Brown LR, Camy-Peyret C, Carleer MR, Chance K, Clerbaux C, Dana V, Devi VM, et al. (2003) The HITRAN molecular spectroscopic database: edition of 2000 including updates through 2001. J Quant Spectrosc Radiat Transfer 82: 5–44 [Google Scholar]
- Sage RF, Sharkey TD. (1987) The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiol 84: 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Soto L, Stanimirov M, von Hobe M, Schmitt V, Valdes J, Wild A, Kesselmeier J. (2005) Global uptake of carbonyl sulfide (COS) by terrestrial vegetation: estimates corrected by deposition velocities normalized to the uptake of carbon dioxide (CO2). Biogeosciences 2: 125–132 [Google Scholar]
- Seibt U, Kesselmeier J, Sandoval-Soto L, Kuhn U, Berry JA. (2010) A kinetic analysis of leaf uptake of COS and its relation to transpiration, photosynthesis and carbon isotope fractionation. Biogeosciences 7: 333–341 [Google Scholar]
- Seibt U, Wingate L, Berry JA. (2007) Nocturnal stomatal conductance effects on the delta(18)O signatures of foliage gas exchange observed in two forest ecosystems. Tree Physiol 27: 585–595 [DOI] [PubMed] [Google Scholar]
- Stimler K, Montzka SA, Berry JA, Rudich Y, Yakir D. (2010a) Relationships between carbonyl sulfide (COS) and CO2 during leaf gas exchange. New Phytol 186: 869–878 [DOI] [PubMed] [Google Scholar]
- Stimler K, Nelson D, Yakir D. (2010b) High precision measurements of atmospheric concentrations and plant exchange rates of carbonyl sulfide (COS) using mid-IR Quantum Cascade Laser. Glob Change Biol 16: 2496–2503 [Google Scholar]
- Suntharalingam P, Kettle AJ, Montzka S, Jacob DJ. (2008) Global 3-D model analysis of the seasonal cycle of atmospheric carbonyl sulfide: implications for terrestrial vegetation uptake. Geophys Res Lett 35: L19801 [Google Scholar]
- Tiwari A, Kumar P, Singh S, Ansari SA. (2005) Carbonic anhydrase in relation to higher plants. Photosynthetica 43: 1–11 [Google Scholar]
- von Caemmerer S, Quinn V, Hancock NC, Price GD, Furbank RT, Ludwig M. (2004) Carbonic anhydrase and C4 photosynthesis: a transgenic analysis. Plant Cell Environ 27: 697–703 [Google Scholar]
- Xu X, Bingemer HG, Schmidt U. (2002) The flux of carbonyl sulfide and carbon disulfide between the atmosphere and a spruce forest. Atmos Chem Phys 2: 171–181 [Google Scholar]
- Yakir D. (2002) Global enzymes: sphere of influence. Nature 416: 795. [DOI] [PubMed] [Google Scholar]
- Yakir D, Wang XF. (1996) Fluxes of CO2 and water fluxes between terrestrial vegetation and the atmosphere estimated from isotope measurements. Nature 380: 515–517 [Google Scholar]