Abstract
Serial transmission electron microscopy of human megakaryocytes (MKs) revealed their polyploidization and gradual maturation through consecutive transition in characteristics of various organelles and others. At the beginning of differentiation, MK with ploidy 32N, e.g., has 16 centrosomes in the cell center surrounded by 32N nucleus. Each bundle of microtubules (MTs) emanated from the respective centrosome supports and organizes 16 equally volumed cytoplasmic compartments which together compose one single 32N MK. During the differentiation, single centriole separated from the centriole pair, i.e., centrosome, migrates to the most periphery of the cell through MT bundle, corresponding to a half of the interphase array originated from one centrosome, supporting one “putative cytoplasmic compartment” (PCC). Platelet demarcation membrane (DM) is constructed on the boundary surface between neighbouring PCCs. Matured PCC, composing of a tandem array of platelet territories covered by a sheet of DM is designated as protoplatelet. Eventually, the rupture of MK results in release of platelets from protoplatelets.
Keywords: platelet production, megakaryocyte, proplatelet, protoplatelet, cytoplasmic fragmentation model
Introduction
In 1906, Wright1) confirmed that blood platelets originate from the cytoplasm of matured megakaryocytes (MKs). This proposal was widely accepted by many investigators, as many studies using light microscopy were published to support the proposal. In 1956, Bessis2) classified bone marrow derived MKs into 4 groups, representing sequential stages in their differentiation namely, the megakaryoblast, the basophilic MK, the granular MK, and the thrombocytogenic MK.
In an early transmission electron microscopic (TEM) study in 1957, Yamada3) stated that pairs of sheets constituting walls are called “platelet demarcation membranes” (DM), which partition the MK cytoplasm into small fractions, each of which develops into a platelet. The entire process of MK differentiation leading to the release of platelets was analyzed in 1973 by Kosaki et al.4–6) by serial transmission electron microscopy of 212 human MKs. Detailed TEM studies by Behnke7,8) clarified the origin of DM system (DMS) and then discussed the liberation of platelets via pieces of cytoplasm released from the cytoplasmic extension(s). However, these studies have an intrinsic weak point that the specimen lacked MKs in some specified differentiation stage, probably due to the perfusion of the bone marrow with a fixative.
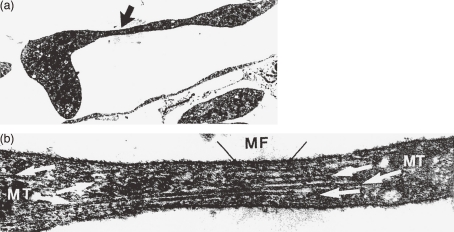
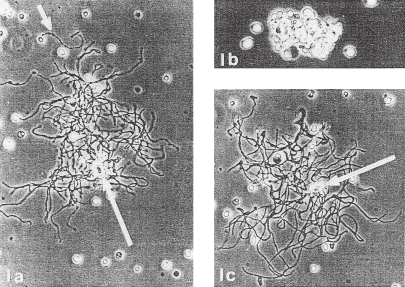
In 1976, scanning electron microscopic (SEM) studies of the perfusion-fixed bone marrow by Becker and De Bruyn9) and by Muto10) independently demonstrated the proplatelet process formation (PPF) from MKs situated in the extravascular compartment. They clearly suggested the possible liberation of platelets by fragmentation at the constricted portions of MK processes. Similar cytoplasmic process formation in vitro from MK was already observed in 1956 by phase contrast microcinematography by Thiéry and Bessis11) and Albrecht.12) However, at that time it was generally accepted that this phenomenon of process formation might be an artifact, because the actual release of platelets from the processes was not observed. In a TEM study, Radley and Scurfield13) observed that the proplatelet that have extended into sinusoid had specialized microtubule (MT) bundle similar to that of circulating platelets, instead of the original MT of MK. This specialized MT, present in the proplatelet processes, oriented longitudinally passing through all of the putative platelets, and became concentrated in the constrictions of processes between putative platelets as shown in Fig. 1. And they proposed that “Rupture at sites of constriction is thought to release platelets”. This was then named as the “proplatelet model” of platelet production. Many contradictions in this model and the modification made by Italiano et al.14) will be discussed in the last section.
Figure 1.
Transmission electron micrographs of proplatelet process in bone marrow sinus of mouse perfused with a fixative. (a) Section through megakaryocyte cytoplasmic process in a sinus, showing attenuated arm with developing constriction (arrow) (original ×5700). (b) Enlargement of the area beneath the arrow in (a). Microtubules (MT) run lengthwise and splay out at the ends of the constriction. Note presence of microfilament (arrow) (original ×60,000). (Reproduced from Radley and Scurfield13) Figs. 2,3 with permission from the publisher)
The last step in the process of platelet production is the release of nascent platelets from matured MKs. Wright15) speculated that platelets are pinched off from the MK cytoplasm. He described that the portion of the cytoplasm which is to form the platelet is separated from the rest by a zone of hyaline cytoplasm and arranged in a more or less sharply outlined, rounded or oval mass. This mass was later described as platelet territory or platelet field by many investigators. A half century had elapsed since the actual release of platelets by cytoplasmic fragmentation was observed in vivo by Kinosita and Ohno16) and ex vivo by Albrecht12,17) both by phase contrast microcinematography. These observations are the basis of “cytoplasmic fragmentation model”. Recently, Junt et al.18) succeeded in observing dynamic visualization of platelet generation within the bone marrow. The process reported in this article is considered as a prototype of cytoplasmic fragmentation model. But they designated the released portion of cytoplasm as proplatelet, without providing a clear mechanism by which the conversion from proplatelet into platelet occurs. Richardson et al.19) stated that there is no direct observation of platelet release from proplatelet. In addition, both the light and electron micrographs of MKs suspended in liquid cultures20–22) strongly suggest that platelet shedding takes place without the proplatelet formation.
Three years ago, one of the authors published a review article on the mechanism of platelet production and release by MK.23) Recently, we further analyzed the mechanism of platelet production utilizing a serial TEM study published earlier.4–6) This study captured almost the entire process of maturation of MK and release of platelets from it. We confirmed that many studies reported so far did not contradict the earlier TEM findings and also with the protoplatelet hypothesis,24,25) proposed in 2003 for the interpretation of the cytoplasmic fragmentation model. Since then, a large number of additional studies on platelet production have been published employing modern methodologies but there remain some unexplained phenomenon. In this review an attempt has been made to clarify further the fundamental mechanism of platelet production based upon large volume of related literature including the recent and authors own studies.
1. Process of the polyploidization and maturation of megakaryocytes and release of platelets observed by electron microscopy
Kosaki et al.4–6) adopted an immersion fixation of specimen in glutaraldehyde in their TEM study. Small pieces of human bone marrow carefully removed from the rib resected by thoracotomy procedure, were used for the study. After post fixation with osmium, the specimen was stained with uranyl acetate and lead citrate. Some specimens were obtained by marrow aspiration. One hundred and twenty six MKs from marrow tissue and 86 MKs from marrow aspirate were analyzed. Observed MKs were classified by their differentiation grade judged by the following criteria: starting from a relatively small cell with high N/C ratio having many large mitochondria and abundant ribosomes showing active synthesis, probably belonging to endomitosis stage (as shown in Fig. 2) to a large cell with senescent nuclei and cytoplasm partitioned into many platelet territories, having mitochondria of small size and clusters of glycogen granules. The process of differentiation proceeded gradually through consecutive transition in characteristics of various organella. These criteria are; 1: nucleus (fine structure, N/C ratio), 2: cell size, 3: mitochondria (size, electron translucency of matrix), 4: free ribosome, 5: rough endoplasmic reticulum, 6: platelet specific granules, 7: Golgi body, 8: glycogen granules, and 9: platelet demarcation membrane system (DMS).
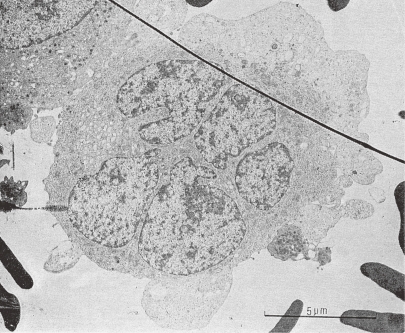
Figure 2.
Transmission electron micrograph of a human megakaryocyte in Stage I. The specimen was obtained by marrow aspiration. A MK, probably in endomitosis stage, has a multilobulated large nucleus and abundant free ribosomes, mitochondria of large size with electron translucent matrix, and prominent Golgi body. Upper left side: a part of a MK with platelet specific granules, right side: a circulating platelet attached to the MK is seen. (Reproduced from Kosaki and Fujimoto6) Fig. 1.50 with permission from the publisher)
Two hundred and twelve MKs observed were classified into the following 5 groups. In stage I (stage of active synthesis, as shown in Fig. 2) 15 MKs (7.1%); in stage II (stage of platelet specific granule production, as shown in Fig. 3) 33 MKs (15.6%); in stage III (stage of DMS construction, as shown in Fig. 4) 87 MKs (41%); in stage IV (stage of platelet release, as shown in Fig. 5) 61 MKs (29%) were classified. Sixteen (7.5%) MKs were divided into unclassified group. However, no MK with proplatelet process was identified. Additionally, serial 261 human MKs obtained from bone marrow aspirate were classified by light microscopy into 4 groups according to Bessis.26) The respective distribution of the differentiation stages in light microscopic study and in TEM study above mentioned was in agreement with the study reported by Bessis.26)
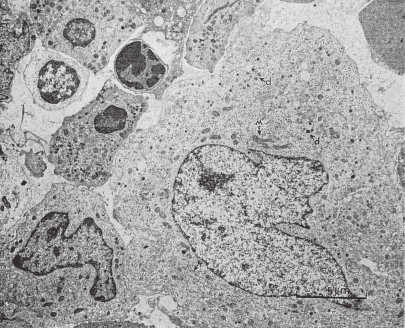
Figure 3.
TEM picture of a human MK in stage II. A megakaryocyte, probably situated in the extravascular compartment of the bone marrow, has some platelet specific granules (marked as d). This cell has mitochondria (M) of large size and many rough endoplasmic reticulum (r) and free ribosomes. (Reproduced from Kosaki and Fujimoto6) Fig. 1.51 with permission from the publisher)
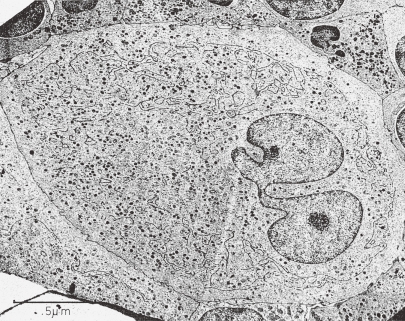
Figure 4.
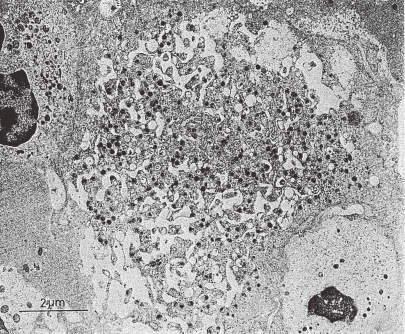
TEM picture of a human MK in stage III. This MK is tightly packed with the adjacent cells in the extravascular compartment of the bone marrow. Black line or dotted black line of DMS is partitioning the MK cytoplasm except peripheral zone into platelet territories. Many platelet specific granules are present. Size of mitochondria is small, but some are large. Clusters of glycogen granules are also seen. (Reproduced from Kosaki and Fujimoto6) Fig. 1.52 with permission from the publisher)
Figure 5.
TEM picture of a human MK in stage IV. Cytoplasm of a fully matured MK in extravascular compartment of the bone marrow shows global fragmentation into individual platelets. Fragmentation process seems to proceed from cell periphery to the cell center, excepting peripheral zone. (Reproduced from Kosaki and Fujimoto6) Fig. 1.56 with permission from the publisher)
2. A polyploid megakaryocyte may be composed of multiple putative cell units
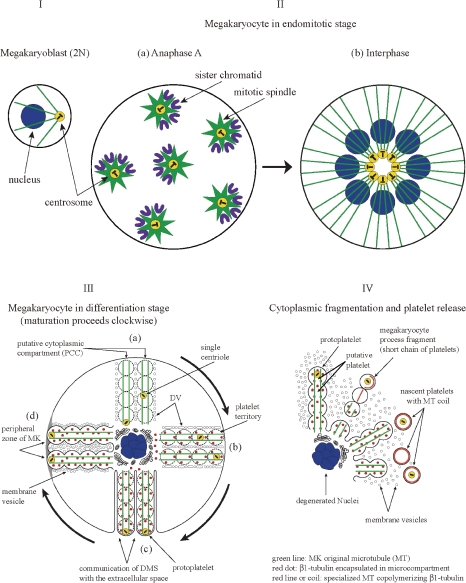
In 1961, Kinosita and Ohno16) reported in situ observation of MK maturation in bone marrow of living rabbit by phase contrast microscopy through a set of windows created in the femur. According to this report, at the time of mitosis and cell division of 2N megakaryoblast, the daughter cells suddenly fuse together into one 4N cell in about half the cases. Then, this 4N cell, which consists of two diploid cell units, develops into 8N cell containing two tetraploid cell units through S phase and enters into mitotic phase, and two sets of bipolar mitosis occur simultaneously in the cell. Configuration of 4 mitotic spindles and 4 sets of chromatids in such cell in anaphase A was demonstrated by Nagata et al.27) by immunostaining of mouse MKs cultured in the presence of TPO but cell division does not seem to occur (vid. Fig. 4E of ref. 27). This 8N cell develops further into 16N through S phase, and eventually 4 sets of bipolar mitosis proceed simultaneously to anaphase A (vid. Fig. 4F of ref. 27) as illustrated in Fig. 6IIa. At the time of reassembly of nuclear envelope which was broken down during the prophase, one nuclear envelop may enclose all the sister chromatids in a single nucleus.27–29) Yet another possibility may exist: one nuclear envelope may enclose respective one set of sister chromatids. Thus, polyploid MKs enter into the next interphase, possessing multilobular nucleus or multiple nuclei and centrosomes. From the above discussion, one can envisage that such a polyploid MK may be an assemblage of multiple diploid “putative cell units”. One centrosome and a MT array emanated from it, 2N nucleus and the cytoplasmic area supported by the MT array compose the basic unit of cellular structure which may be termed as “putative cell unit”. For instance, the 16N polyploid MK is supposed to be composed of 8 such putative cell units. At the beginning of interphase, 8 centrosomes, although continuing to exist in each putative cell unit as in Fig. 6IIa, gather towards the center of MK as shown in Fig. 6IIb. Meanwhile, the communication and the transportation between the cell center and the periphery are principally carried out through the channel of MTs restricted within respective one putative cell unit.
Figure 6.
Schematic interpretation of megakaryocyte development, maturation and fragmentation leading to the release of platelets. I. Megakaryoblast (2N cell); II. Megakaryocyte (MK) in endomitotic stage: (a) Anaphase A. MK with ploidy 16N has 8 mitotic spindles including centrosomes, towards which each one set of sister chromatids is pulled presumably in individual putative cell unit. (b) Interphase. 16N MK in interphase. In the cell center 8 centrosomes, although existing in individual putative cell unit, are clustered. From which 8 bundles of microtubules (MT: green line) radiate to the cell periphery; III. MK in differentiation stage: Maturation of each putative cell unit, composed of two “putative cytoplasmic compartment” (PCCs), proceeds in a clockwise fashion as shown in the illustration. (a) Migration of a single centriole from the cell center to the cell periphery, presumably along the MK original MT, in a PCC. Intrinsic organelles of platelet increase in each future platelet. Platelet demarcation vesicles (DV) are arranged at the boundary surface between neighboring PCC. (b) Fusion and fission of DV proceed at the interface of PCCs. β1-tubulin is synthesized and encapsulated in microcompartment (marked as red dot), and delivered into each platelet territory. Platelet territories in a tandem array are under elaboration inside of each PCC. (c) Demarcation membrane system (DMS) is constructed, protoplatelets are delineated by one sheet of DMS. Single centriole is located in the most peripheral platelet territory. (d) Excessively produced membrane vesicles intervene between respective protoplatelets; IV. Cytoplasmic fragmentation and platelet release: In liberated nascent platelets, MK original MTs are disassembled and β1-tubulin is liberated from encapsulated microcompartments. Respective MTOC in each nascent platelet nucleates specialized MT, leading to the formation of MT coils. In some cases, nascent platelets are released as short chain of platelets which fragment further into individual platelets in the pulmonary circulation. (Reproduced with some modification from Fig. 6 in Kosaki’s review article23) with permission from the publisher)
Polyploid MK in interphase contains multiple centrosomes in the central zone of its huge cytoplasm surrounded by polyploid nuclei as seen in a TEM picture (e.g. Fig. 2a of ref. 22). Each centrosome acts as microtubule organizing center (MTOC) which is composed of a centriole pair surrounded by pericentriolar material including γ tubulin30) and pericentrin.31) In the MTOC, γ tubulin nucleates new MT through polymerization of tubulin subunits on its ring complex (γ-TuRC). MT elongates towards the cell periphery in respective putative cell unit. Patel et al.32) succeeded in visualizing the tip of MT in premature MKs by utilizing a specific antibody against end-binding protein 3 (EB3) which binds specifically to the plus-end of MT. They demonstrated that centrosomes are located centrally and that the tips of MTs are extended to the periphery in a radial fashion (vid. Figs. 4E and F of ref. 32).
3. Translocation of the centriole during differentiation of the megakaryocyte
The centriole does migrate in a cell under certain conditions. Mori et al.33) demonstrated the ciliogenesis in cultured cells LI10, an established cell line derived from rat liver. They observed that the centrioles migrated from the center of Golgi complex to the apex of the cells at the postconfluent stage. Then one of the centrioles changed to a basal body to produce a cilium. On the other hand, according to McNiven et al.,34) teleost melanophore possesses several long cytoplasmic arms, and in the arms MT extends from the cell body to the arms, but there is no centrosome. They performed an experiment to sever one arm and observed some type of centriole-free MTOC after 2–4 hours of incubation in the arm surgically removed from cell body. Presumable existence of γ-tubulin along the MT bundle may be suspected.
Moskwin-Tarkhanov and Onishchenko35) had reported that at the conclusion of endomitotic phase, the centriolar complex of the MK disappears, and centrioles separate while retaining a juxtanuclear position. In a maturing and thrombopoietic MK, individual centrioles lie separately in the cytoplasm. Though it is rare, Behnke observed a platelet with a single centriole.36) Radley and Scurfield13) confirmed that centrioles were located singly close to the surface of MK at the late stage of its differentiation. They reported that centrioles were never found in the remnants of cytoplasm retained around senescent MK nuclei in the extravascular compartment, although random sections of well over 100 have been examined. In addition, they observed existence of a single centriole in each of 8 cytoplasmic processes examined, protruding into sinusoid by serial sectioning. The latter observation is very important to support the concept of “putative cytoplasmic compartment”, which will be discussed herein. It is assumed that during MK differentiation, the migration of a single centriole to the cell periphery takes place inside of a MT bundle along with pericentriolar material. Furthermore, deduced from the observations,13,35) it may be reasonable to speculate that the two centrioles migrate inside of the two different MT bundles as illustrated in Fig. 6IIIa,b,c. The putative cytoplasmic compartment (PCC) mentioned above is composed of one centriole within one MT bundle corresponding to the half of the MT array which supports one putative cell unit, and cytoplasm supported by one MT bundle. Thus, a MK with ploidy 16N contains 16 PCCs and in each PCC translocation of a single centriole takes place.
According to Italiano et al.,14) when MKs derived from mouse fetal liver are incubated in the presence of cytochalasin B, an inhibitor of actin assembly, protrusion of some dozen long slender proplatelet-like projections are observed instead of prototypical PPF as shown in Fig. 7. Strikingly, it looks like that platelet like bulges are identified around the tip of only some but not all protrusions. To explain this peculiar observation the following 3 assumptions are supposed to be necessary. First, it may be assumed that ligation of integrin αvβ3 expressed on the MK plasma membrane37) may not be affected by the presence of cytochalasin B. This ligation triggers PPF; in other words it evokes localized cytoplasmic derangement leading to morphological changes, such as assembly of specialized MT. The mechanism of PPF will be discussed in detail in Section 8. Secondly, one may assume that cytoplasmic derangement evoked in the most distal putative platelet in respective PCC may not extend to the next one, probably due to the presence of cytochalasin B. So, MTOC only in the most distal putative platelet nucleates new specialized MT containing β1-tubulin. Thirdly, it may be assumed that in about half of the cases MTs elongate towards the cell center, others towards opposite direction. The former may exhibit a long slender projection with platelet-like bulge at its tip.
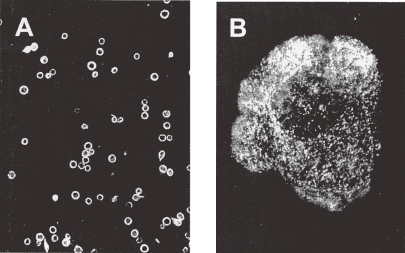
Figure 7.
Effect of cytoskeletal disrupting agent on proplatelet growth. Gallery of phase contrast micrographs of mouse megakaryocytes treated with 2 µM cytochalasin B. Extensions from the megakaryocyte surface show no beading and absence of apparent branching, but some show platelet-like bulge at the tip. (Reproduced from Italiano et al.14) Fig. 7a, with permission from the publisher)
Recently, Patel et al.32) proved that one of the above assumptions is true in their experiments. They demonstrated, using EB3-GFP (green fluorescent protein) expressing MKs that elongation of MTs in one proplatelet process takes place in both directions almost equally, towards the cell body from the tip of proplatelet and from the cell body towards the proplatelet tip. This observation may indicate that in one proplatelet process there exist several (at least 2 or more; presumably localizing in every putative platelet) MTOCs, and from one of them a bundle of MTs elongates towards the cell center and from another one towards the tip of proplatelet. And the number of the former MTOC is same as that of the latter. Contrary to the case of MK with proplatelet, premature MK have several centrosomes in the cell center, and MT bundles emanate from each of them towards the cell periphery as already mentioned in Section 2 (vid. Figs. 4E and F of ref. 32). On the other hand, they observed many but scattered spots indicating existence of MT plus-end in the cell body of another MK (vid. Fig. 2D of ref. 32). In this fully matured MK, all single centrioles should have already migrated from the cell center to the periphery inside of each PCC. However, that picture shows that MTOC still existed in the cell body, more precisely in the PCC. Of course, the origin of some spots is most likely from a single centriole migrated to the cell periphery but origins of others are apparently different. Viewing from a different standpoint, the MTs elongating to the tip of the proplatelet originate most likely from centriole free MTOC remaining in the cell body, naturally located in a putative platelet in the PCC.
Thus, the authors speculate that centriole free MTOC in the PCC of matured MK may be a portion of pericentriolar material left behind at a certain site, possibly in each putative platelet, when single centriole surrounded by pericentriolar material migrates to the cell periphery in respective PCC. Recently, Patel-Hett et al.38) demonstrated the existence of γ-tubulin in every platelet, operating as MTOC. As to the mechanism of distribution of γ-tubulin, deduced from the McNiven’s experiment,34) some possibility of constant γ-tubulin transit along the MK original MT bundle may be left.
4. Elaboration of platelet territories in respective PCC
After the conclusion of endomitosis phase, polyploid MK composed of certain number of putative cell units or PCCs, as already discussed in Sections 2 and 3, begins to differentiate. In the process of differentiation, a vast amount of organelles including platelet specific granules and mitochondria and substances required for the construction of plasma membrane, cytoskeleton, etc., are produced mainly in the central area of each putative cell unit. These organelles and substances are distributed evenly in respective PCC via transportation along MT bundle. Differentiation proceed from MK shown in Fig. 3 to MK in Fig. 4 and finally many platelet territories in a tandem array in each PCC are constructed as future platelets. Structure of respective platelet territory is identical to the circulating platelet as judged by TEM, except that the territory lacks MT coils. β1-tubulin, an essential component of MT coils, is synthesized specifically in the late stage of MK maturation and is immediately isolated in a myriad of microcompartment without assembling into MT39) and is allocated evenly in each platelet territory. This isolation may be quite efficient in keeping the β1-tubulin for the newly produced platelets. β1-tubulin isolation will be discussed in detail in paragraph 3 of Section 9.
As stated in Section 3, an approximate thickness of PCC is determined by the size of the cytoplasm supported by the half of MT bundle originating from one centrosome. Determination of three dimensional size of future platelets in relation to some conditions may decide the virtual length of platelet territories. During translocation of the centrioles to the cell periphery small portions of pericentriolar material may be separated and left behind presumably in the respective platelet territory, as described in the preceding section.
Generally, elongation of MTs by polymerization of new tubulin subunits at its plus-end occurs naturally in the original direction. Bifurcation of MT cannot take place. Branching of proplatelet processes is a peculiar phenomenon and reported to occur only occasionally according to Radley and Haller40) or Haller and Radley.41) There is no report of proplatelet branching in vivo, in the sinusoid. Italiano et al.14) insisted that the branching of proplatelet processes is necessary to increase the numbers of proplatelet ends because they firmly believed that platelets are assembled principally at the ends of proplatelet processes. As to be discussed later in Section 8, in the process of PPF cytoplasmic derangement triggers the new assembly of specialized MT containing β1-tubulin in one putative platelet after another in respective proplatelet. For new assembly of MT, MTOC is already prepared in every putative platelet as discussed above. With the participation of actin filaments as suggested by Italiano et al.,14) the direction of elongation of new MT may possibly be different from that of the preexisting proplatelet and thus the branching of proplatelet process occurs. Execution of proplatelet branching which occurs actually only under certain in vitro conditions indicates the existence of MTOC in relevant putative platelet corresponding to the platelet territory.
5. Platelet demarcation membrane
In parallel to the translocation of single centriole and elaboration of platelet territories, platelet demarcation membrane system (DMS) is constructed during the maturation of MK. Behnke7) studied TEM of MK in the bone marrow treated with perfusion fixation and concluded that the DMS is a derivative of the plasma membrane of the MK. He also suggested that the cavities of the DMS are continuous with and are part of the extracellular space at all stages of the DMS development. He further stated that DMS formation is initiated by tubular invaginations at multiple sites of the plasma membrane of the MK. Based upon the observations utilizing freeze fracture technique, Shaklai and Tavassoli42) and Tavassoli43) proposed a model for the formation of paired membranes from tubules by both fusion and fission. According to Radley and Haller40) DMS derives from the invaginated plasma membrane stored randomly in the cell body (in flow). When proplatelet extends outward, the DMS evaginates and covers the surface of the proplatelet (out flow). This proposal is called as “flow model” and has been widely supported.14) Recently, Mahaut-Smith et al.44) demonstrated that the DMS is electrophysiologically contiguous with the peripheral plasma membrane, and this was confirmed using membrane-impermeant fluorescent indicators. Schulze et al.45) also confirmed that invaginated DMS possesses similar characteristics as that of plasma membrane covering MK or proplatelets using biochemical methods.
On the other hand concomitant with the enlargement of MK, from a megakaryoblast to a matured MK, vast area of plasma membrane is synthesized in the cell to cover its surface. Generally phospholipids, the main lipid components of the plasma membrane, are synthesized on the surface of smooth endoplasmic reticulum and transported to the Golgi body. There, actual small membranes as vesicles are assembled by incorporating proteins and sugars that were initially synthesized at other sites. Eventually the membrane vesicles (MVs) are delivered to the proper site which necessitates them via the MT railway. Yamada3) who first described DMS stated that initially platelet demarcation vesicles (DVs) are arranged at the site of future DMS and those then become tubular by fusion eventually yielding DMS. It is now postulated that DVs formed at Golgi body are transported to be arranged to form a paired membrane on the boundary between neighboring PCCs delivered from both sides. According to the invagination theory, DVs must be transported initially to the cell surface and then invaginated towards the cell center. The proposed process takes much longer way than direct transportation mentioned above, and their theory seems quite unreasonable. When the cavity of the paired membrane system communicates with the extracellular space DMS formation is completed. Thus, PCC is completely covered by one continuous sheet of DMS except at its attachment to the core of the cell. The sheet may include the portion of preexisting MK plasma membrane and may have some fenestrations. The unit of PCC covered by one sheet of DMS is the protoplatelet from which platelets are released.24,25) These processes are schematically shown in Fig. 6IIIa–c.
Whole cytoplasm except peripheral zone of a maturing MK, which is tightly packed with adjacent cells in the extravascular compartment of the bone marrow, shows active partitioning into platelet territories with black line or dotted black line of DMS, as seen in Fig. 4. In magnified micrograph (vid. Fig. 5 of ref. 23) electron-dense materials are contained between the paired membranes of the transection of DV, tubule or DM. The authors observed very similar findings in most of stage III MK, which accounts for 41% of all the MK observed by TEM.4–6) Following the completion of DMS, in stage IV MKs, which accounts for 29% of all MK observed, the electron-dense material contained in DMS disappears completely, leaving empty space between the paired membranes. This disappearance may be facilitated by cell movement. Because some free migrating MK in the bone marrow were observed without dense materials between the paired membranes, these were identified as “unclassified” as judged from coexistence of premature organelles. Also, connection of DMS with the external milieu can possibly occur before the complete construction of DMS. In MKs cultured in a liquid suspension, it is more natural to observe DMS without electron-dense material. Later, the gap between the paired DM enlarges and the parallel position of the paired membrane is lost, probably leading to the liberation of platelets. The nature of the electron-dense material, which is stained by uranyl acetate or lead citrate, has not yet been elucidated. The compatibility of this observation with the invagination theory proposed by Behnke is not completely clear. Most TEM pictures of DMS by Behnke7) showed the existence of staining material (e.g. ruthenium red) or substance to be stained (e.g. horse radish peroxidase) in the gap of the paired membranes, which had been taken from the external milieu. This fact indicates that his DMS has been connected with the extracellular space at the time of experimentation. He did not demonstrate the stage III MK as shown in Fig. 4, in which DMS did not connect to the external milieu.
6. Further maturation of MK with protoplatelets
Wright15) observed a large number of platelet territories surrounded by a hyaline cytoplasm in the matured MKs as already described. In their TEM study, Zucker-Franklin and Petursson20) also observed intervening vesicular or tubular structures between platelet territories (Figs. 2b,c of ref. 20). The issue, how these structures are formed, should be discussed here. It is presumed that when the MVs are continuously produced in the cell center of MK, budding of MVs at a certain site of plasma membrane of PCC (protoplatelet) and their liberation may produce these vesicular or tubular structures between protoplatelets. It is assumed that the budding sites may possibly correspond to the openings of the surface-connected canalicular system of future platelet. Reconstruction study6) of serial ultra-thin sections of 10 whole platelets revealed that each platelet has several number of surface-connected canalicular system composed of a group of canaliculi connecting with each other and having one or two openings on the cell surface. But the exact mechanism of such transportation of MVs through the plasma membrane of the protoplatelet is quite incomprehensible at present. However, the phenomena described above actually take place.
Meanwhile, it is also assumed that the cylindrical cytoplasm, named as PCC and covered by a sheet of DM, connecting the cell center and the most periphery of the cell, may go along not straight but winding and twisting as suggested by Becker and De Bruyn.9) This cylindrical cytoplasm is supported by a longitudinally running bundle of MK original MTs emanated from one MTOC. So that, ultra-thin sections of matured MK cytoplasm probably reveal many platelet-like fields of cytoplasm delineated by plasma membrane, in other words platelet territories. As MK matures, the respective platelet territory becomes visible even by light microscopy. At this stage, the original MT bundle of MK, extending from the center of the cell to the periphery is still functioning: because a mature MK with multiple protoplatelets is still capable of operating integrated cellular motility as a single cell. TEM captured such MKs migration into sinusoid.4–6) As for the functional aspect, putative platelet as a bulge in proplatelet, corresponding structure to platelet territory undergoes quite similar morphological changes to that of a circulating platelet (centralization of secretory granules, contraction of microfilaments) in response to thrombin as described by Radley et al.46)
7. Birth of platelets
All matured MKs are composed of a number of protoplatelets in radial direction from the center to the periphery, sharing a common central cytoplasmic area including degenerating nuclei as already mentioned. Also, “excess membrane vesicles” exist between the protoplatelets. Triggered by an unknown mechanism, the peripheral zone or the distal portion of MK loses its integrity and breakup of the whole cell proceeds from periphery to the center explosively into individual protoplatelets, which remain united at the cell center, and into numerous vesicular structures. Almost simultaneously, platelet territories, namely new platelets are released generally from the periphery of protoplatelets as seen in Fig. 5. It takes several hours to complete the release of platelets from the core of MK composed of degenerated nuclei.12,16) In those new platelets just released, original MT of MK disassembles and β1-tubulin becomes free from the insulation in microcompartment. Centriole free pericentriolar material or single centriole surrounded by pericentriolar material functions as MTOC as described previously. In this new assembly of MT, β1-tubulin will be copolymerized and MT coil will be formed. In the MT coil, β1-tubulin is the most abundant (more than 90%) among the isotypes of β-tubulin.39) The site of cytoplasmic fragmentation of MK leading to the platelet release may primarily be the bone marrow, in the extravascular compartment (as in Fig. 5) or in sinusoid.6,16)
Further, Eldor et al.47) demonstrated that the adhesion of MK to the extracellular matrix and some additional shear force triggers the complete fragmentation of MKs into platelet-like particles in vitro, without the formation of proplatelet. They also suggested a possible contribution of attachment of MK to the extracellular matrix to induce platelet release from MKs in vivo. Junt et al.18) also suggested some role of shear force in the production of proplatelets and release from MK which proceeds in the bone marrow.
Maximow48) revealed by light microscopic observation that the cytoplasmic fragmentation of MK begins at its periphery and extends towards the cell center. This was substantiated further by Kinosita and Ohno16) through microcinematographic in situ observation of living rabbit bone marrow. TEM observation by one of the authors suggested a similar process (Fig. 5). Additionally, a TEM study by Cramer et al.49) revealed similar events as shown in Fig. 8, but they interpreted that the platelet release was via proplatelet formation. However, the details of the TEM do not support that it is prototypical for proplatelet but rather typical for protoplatelets. Because, specialized MT bundle running straight through several platelet territories in a cytoplasmic compartment delineated by plasma membrane as shown in Fig. 1 is not seen in the picture. The existence of such specialized MT bundle is the necessary and sufficient condition of proplatelet, as to be discussed in the next section, On the other hand, Cramer et al.49) demonstrated that platelet releasing MKs were surrounded by numerous microparticles ranging from 0.1 to 0.3 µm in diameter, and these particles strongly expressed αIIbβ3. It would be of interest to investigate the relationship between the microparticles and “excess membrane vesicles”. Recently, Flaumenhaft et al.50) reported microparticles shedding from the surface of MK. The size and features of surface marker of these microparticles are almost similar to that of Cramer’s microparticles. Flaumenhaft et al.50) did not clarify the origin of these microparticles but showed clearly that these particles were different from the platelet-derived microparticles. However, there has been no report on the shedding of large numbers of microparticles during the process of PPF. Therefore, the authors hypothesize that the “excess membrane vesicles” will form the membrane covering a long process of proplatelet by means of fusion. Thus, it would be interesting to study further the fate of the “excess membrane vesicles” during PPF.
Figure 8.
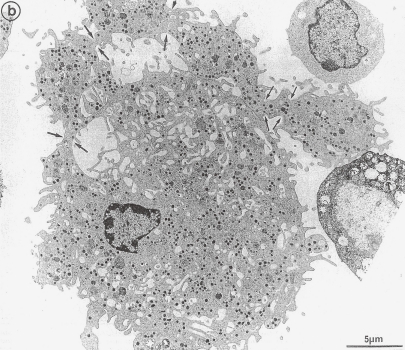
TEM picture of a cultured megakaryocyte grown for 7 days from CD34+ CD38+ progenitors in the presence of Mpl-1, presenting signs of platelet formation. Protoplatelet-like peripheral sheet of cytoplasm has unfolded from the cell core. Specialized microtubule bundle running straight through the sheet of cytoplasm is not seen. Constriction zones are regularly disposed along this cytoplasmic extension (arrows). (Reproduced from Cramer et al.49) Fig. 3b, with permission from the publisher)
Radley and Hartshorn51) suggested that the release of platelets from MK processes does not necessarily take place from the distal ends. They observed by phase contrast microscopy the liberation of MK process fragments in addition to many single platelets from bone marrow explants. They interpreted that these events had no relation to PPF and the liberation has already initiated prior to the marrow explantation. These MK process fragments composed of a chain of putative platelets, 2–10 or more in number. Similar structure, which may be called short chain of platelets has been recovered from the central vein and considered to be fragments of proplatelets by Handagama et al.52) and by Tong et al.53) Besides, it would seem that Radley and Hartshorn51) inferred the phenomenon which they observed as the ex vivo liberation of platelets and short chains of platelets from proplatelet-like processes extended from MK in vivo conditions. However, TEM by Tong et al.53) has revealed a short chain of platelets to be composed of three platelets elongated in a straight line which are attached to each other at the tip of the elongated cell body (vid. Fig. 5B of ref. 53). This picture is definitely different from the typical TEM of proplatelet in sinusoid; proplatelet is a cytoplasmic compartment including several putative platelets delineated by a plasma membrane as shown in Fig. 1. The appearance of these short chains of platelets increases by accelerated thrombocytopoiesis due to the administration of anti-tumor drug,51) platelet antiserum53) and acute blood loss.52) Considering these reports from three different institutions, it would be reasonable to speculate that the emergence of short chains of platelets is due to the release into circulation prior to the completion of separation into single platelets from protoplatelets. Thus, the above mentioned observations indicate that in matured MKs putative platelets are present in a tandem array forming protoplatelet, in accordance with the TEM picture (Fig. 8) observed by Cramer et al.49)
It has been reported that the short chains of platelets may be fragmented into individual platelets in the pulmonary circulation.52) Also, even under normal conditions, small number of short chains of platelets are released in the process of platelet birth from matured MKs in the bone marrow, which become independent platelets in the pulmonary circulation.51,52) Especially in case of accelerated thrombocytopoiesis, the opportunity of the matured MKs protruding into sinusoid is enhanced and as a result, the number of matured MKs found and of released platelets in the pulmonary capillary beds increases.54) This explains the presence of short chains of platelets in peripheral blood (vid. Fig. 2 of ref. 25; a short chain of platelets from a patient with bleeding remains connected to a cytoplasmic core of MK, showing resemblance to Fig. 8 of this review). The steps from megakaryoblast to the platelet release thus far proposed are summarized in Fig. 6.
8. One proplatelet process can develop from one protoplatelet
Mechanism of platelet production and its release from MK described above is based on protoplatelet hypothesis. If this hypothesis is established on the basis of true state of MK in its differentiation stage, then other phenomenon occurring in this stage, e.g. proplatelet process formation, should also be explained clearly by this hypothesis without any contradictions. Although PPF is an artifact, it has been shown to occur under certain conditions.
In matured MKs, in which DMS and protoplatelets are already structured, the respective protoplatelet can elongate as a proplatelet process, in response to appropriate stimuli in vitro, though platelet release does not occur from it. In other words, ligation of integrins located in the plasma membrane (tip of the protoplatelet) results in the activation of integrin-generated signaling pathway. Upon its crosstalk with PKC generated signaling pathway, the signal is further amplified leading to the strong activation of ERK1/ERK2,37) that is the state of cytoplasmic derangement. The integrin responsible for the initiation of PPF in vitro is considered to be αvβ3.37,55) A recent report56) suggests the involvement of αIIbβ3 as well but this needs to be reconfirmed.
The cytoplasmic derangement that occurs at the tip of one protoplatelet is recognized also by the following two morphological changes. One is pseudopod formation11) probably expressed by actin reorganization57) which possibly play some important role in the propagation of derangement towards the cell center,14) as discussed in Section 3. The other is the assembly of new specialized MT, an analogous structure to MT coils of circulating platelets. In this assembly, tubulin, except β1-tubulin, and some microtubule-associated proteins are supplied through disassembly of MK original MT probably triggered by cytoplasmic derangement. This derangement may also release β1-tubulin from the isolation in punctate spots.39) Thus, new specialized MTs containing abundant β1-tubulin58) are assembled. The MTOC concerning with this MT assembly and its localization in respective platelet territory has already been mentioned in Sections 3 and 4. The direction of microtubule elongation from MTOC is dependent on the condition in respective platelet territory (putative platelet) as also discussed above. Originally the cytoplasmic derangement is initiated at the tip of protoplatelet and it propagates towards the center of MK within cylindrical cytoplasm covered with the plasma membrane through tandem array of platelet territories. As a result, a single protoplatelet becomes a single elongated proplatelet. During this process, there are no major structural changes in the platelet territories but the formation of new long specialized MT from the original MT of MK would be the most outstanding change. The vast area of plasma membrane covering the elongated proplatelet will be prepared from the fusion of excess membrane vesicles as hypothesized in Section 7. Thus, the elongated beads-like proplatelet process is formed, which contains many putative platelets as bulges along the shaft. A bundle of the specialized MTs as the backbone of proplatelet process, half-and-half mixture of MTs elongating from the tip of the process to the center of MK or in opposite direction,32) runs usually straight, through the putative platelets and the shaft of proplatelet in a cytoplasmic compartment delineated by the plasma membrane.
As stated in Section 5, the plasma membrane covering protoplatelet is not perfect and the cytoplasmic derangement in one protoplatelet may be transmitted to the neighboring ones through fenestra present in the plasma membrane. Eventually, the cytoplasmic derangement may be propagated throughout the entire protoplatelets, namely whole cytoplasm, resulting into many elongated proplatelets emanating from the core of MK as shown in Fig. 9a. The initiation of cytoplasmic derangement by integrin ligation may be multifocal. It has been reported that it takes several hours or so to complete PPF.11,41) A careful review of Fig. 1 of Italiano’s publication14) would support the above explanation on the morphological changes of MK in relation to protoplatelet and PPF. Additionally, author’s view does not contradict the observations made by Thiéry and Bessis11) (time dependent 12 micrographs) and Haller and Radley41) (Figs. 1a–f, Figs. 7,8,9).
Figure 9.
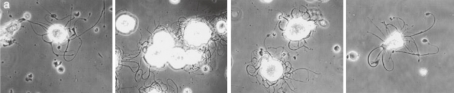
Mouse megakaryocyte in culture, photographed using phase-contrast. a. Four hours after setting up culture and immediately before exposure to 4 ℃. Note extensive development of attenuated processes, which have a “beaded” appearance. Long arrow points to the nucleus of the cell. A detached process is visible above the megakaryocyte (short arrow) (×200). b. Same cell after 2-hr exposure to cold. Processes are retracted around the nucleus (×300). c. Same cell after having been re-exposed at 37 ℃ for 1 hr. Processes again extended but not yet “beaded” (×200). (Reproduced from Radley and Haller40) Figs. 1a,b,c with permission from the publisher)
Contrary to the above description on the initiation of proplatelet formation in the matured MK, Hartwig and Italiano59) described as follows; “In summary, platelet assembly begins when cytoplasmic microtubules aggregate into bundles at the megakaryocyte cortex. Sliding of microtubules and new assembly provide the propulsive force that elongate the proplatelet.” It appears that they do not recognize the essential difference of MK original MTs from specialized MTs, which aggregate in the MK cortex. The latter consists of abundant β1-tubulin, which is synthesized only in the late stage of MK maturation.58) Moreover it exists as a dense array of punctate spots.39,60) Accordingly β1-tubulin cannot assemble into MTs in normally matured MK as to be discussed in Section 9. Implications of the above mentioned aggregation of MTs into bundles at the MK cortex and the mechanism by which this occurs must be elucidated. Meanwhile, Italiano et al.14) concluded that platelets are assembled principally at the ends of proplatelet processes based on the observation that the destined formation of marginal coils of microtubules is limited only at the ends of proplatelet processes. However, marginal coils of MTs are originally formed de novo in respective nascent platelet, as already described23) by one of the authors after protoplatelet hypothesis. Also, Patel-Hett et al.38) demonstrated the existence of γ-tubulin and the dynamic assembly of marginal coils both in circulating platelets. Thus, the Italiano’s thesis that platelets are assembled principally at the ends of proplatelet processes needs to be reconsidered. Cardinal problem is how individual platelet separate from MK cytoplasm. But no one witnessed the separation of platelets from proplatelets as stated by Richardson et al.19) And the cytoplasmic fragmentation model would solve the above problem.
Protruding of a few elongated proplatelets into bone marrow sinusoid through the fenestra of the endothelial cell body has been observed in the perfusion-fixed matured MKs,9,10) which are located on the abluminal surface of the sinus endothelial cell.61) The underlying mechanism of the protruding proplatelet may be different from what has been observed in vitro. However, also in such case, the one to one conversion of protoplatelet to proplatelet takes place. This may be supported by the observations by Radley and Scurfield.13) In case of immersion fixation of bone marrow instead of perfusion fixation, such formation of proplatelet could not be observed at all.4–6,62) The proplatelet formation of MK has not been observed in the spleen which was fixed by perfusion and the difference may be due to the different microvascular architecture of the spleen.
The findings of the unique study by Radley and Haller40) will be discussed herein. They tried to observe continuously the same MK by phase contrast microscopy in serial different thermal conditions, i.e. 37 ℃ for 4 hours, 4 ℃ for 2 hours and then 37 ℃ for 1 hour. First they observed protruding of proplatelet processes outward from the cell core by incubating at 37 ℃ and PPF was completed within 4 hours as seen in Fig. 9a. Then the same MK was cooled at 4 ℃ for two hours, depolymerization of tubulin occurred. Eventually the protruded processes were retracted towards the core of the cell as shown in Fig. 9b. Close observation of the phase contrast micrograph revealed that the entire cell is composed of multiple tubular structures with similar thickness, which bear a remarkable resemblance to the proposed protoplatelet. According to Radley and Haller40) other MKs with retracted processes appear like MKs with DMS as seen in TEM pictures (vid. Figs. 4,5 of ref. 40). But it seems that the true nature of the DMS they called may be gaps between tubular protoplatelet-like structures. It was further observed that the same MK, which was cooled to 4 ℃ underwent the protruding of proplatelet processes when rewarmed to 37 ℃ for one hour as shown in Fig. 9c, and with additional incubation it even regained the bulges. After reviewing the phase contrast micrographs, the general morphology seems essentially similar between the two images (Figs. 9a and 9c): before cooling and after re-warming. This unique phenomenon may be interpreted from the view point of protoplatelet hypothesis as follows: The respective protoplatelet in the matured MK is transformed into elongated proplatelet process by the mechanism mentioned earlier in this section. When the cell is exposed to cold environment, the proplatelet processes lose their backbone support because of the depolymerization of tubulin, leading to the retraction towards the core of the cell. When the cell is re-warmed, specialized MTs reassemble inside respective protoplatelet-like structure nucleated by MTOCs located probably in respective putative platelet, leading to re-protruding. These series of events may be better explained by protoplatelet hypothesis rather than the flow model proposed by Radley and Haller.40) Especially, as shown in Fig. 9b, surface of the MK after retraction of proplatelet processes looks like an assemblage of similar tubular structures and the flow model does not provide an explanation for this observation. According to the flow model a MK after process retraction should appear as a cell with smooth surface containing many reinvaginated membrane system in the cell body.
9. Contradictions and illogical aspects in the proplatelet model
The proplatelet model predicts that platelets are released from MKs in a process that remodels the entire MK cytoplasm into long, beaded extensions termed proplatelets. This model also stipulates that platelets assemble and package their organelles de novo within proplatelets.14,19) For this proposal to hold water, already elaborated putative platelets in the body of fully maturated MK, as observed by TEM, must undergo 1) disassembly, even if partially, and 2) packets of platelet material must be delivered to the ends of proplatelet processes to reassemble again into platelets. It seems unlikely that such an energy-expensive futile process has evolved. In addition there is no provision in this model explaining how numerous platelets of almost similar size are produced from matured MKs.
Schwer et al.63) generated β1-tubulin−/− mice, and demonstrated that matured MK from these mice almost never elaborated proplatelet in vitro (vid. Fig. 2c of ref. 63). Regardless of the absence of proplatelet process formation (PPF), these β1-tubulin−/− mice produced platelets almost normally, but they were spherocytic because of the lack of specialized microtubule coils. However, these platelets had almost normal hemostatic function.64) Although PPF depends on the presence of β1-tubulin, as suggested by many investigators, β1-tubulin itself contributes nothing actively to the differentiation of MK cytoplasm including construction of platelet territories. As already mentioned in Section 4, β1-tubulin is insulated in punctate spots immediately after its synthesis in MK until the birth of a new platelet. Original MT of normal MKs, lacking β1-tubulin, plays the role as cytoskeleton and also in transportation of organelles as in other cells. Therefore, the above observation in β1-tubulin−/− mice is not entirely unreasonable but clearly contradicts to the proplatelet model, which predicts the remodeling of entire cytoplasm into proplatelet for platelet release. In addition, another ex vivo experiment reported by Albrecht17) indicates that the liberation of platelet from matured MK was not disturbed in the presence of colchicine or vinca alkaloid at a concentration which abolishes completely the mitosis through specific binding to tubulin. This observation also clearly suggests that platelet liberation from MK does not require preexisting proplatelet. As reported by Stenberg et al.,65) 4–24 h following intravenous administration of vincristine in Wistar rats, partitioning of platelet territories changed dramatically, presumably related to the disassembly of MT. Under these circumstances release of platelets from MKs takes place. Although proper construction of platelet territories depends upon the presence of MT, release of platelets from MK does not depend on the presence of MT or proplatelet.
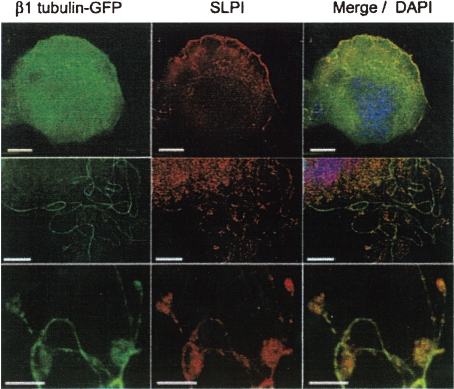
The most important observation by which cytoplasmic fragmentation model is criticized by the proplatelet model supporters14,40) is the lack of the marginal bundle of specialized MTs in the platelet territories, which is normally observed in circulating platelets. As an essential component of specialized MTs, β1-tubulin is synthesized specifically in MK at the late stage of its differentiation.58) Lewis et al.39) observed that the β1-tubulin exists as a dense array of punctate spots that fills the cytoplasm as shown in Fig. 10. They interpreted their observations in terms of myriad compartmentalized cytoplasmic pools of unassembled β1-tubulin that are destined to polymerize into platelet marginal bands upon disintegration of parental cells. This immediate isolation of β1-tubulin in myriad pools may be the best way which enables to allocate and keep β1-tubulin evenly in respective new born platelets, as discussed above repeatedly. However, mysterious mechanism by which an immediate isolation of β1-tubulin molecule after its synthesis occurs has not been elucidated yet. Interestingly, this isolation phenomenon observed by Lewis et al.39) was widely and persistently ignored by the supporters of proplatelet model.14,60) Nevertheless, an observation in the report of Schulze et al.60) verified the authenticity of Lewis’ observation by a new technique. They demonstrated the expression of secretory leucocyte protease inhibitor SLPI, which binds specifically to β1-tubulin, in MK utilizing immunostaining with specific antibody. By immunofluorescence analysis of matured MK expressing a β1-tubulin–GFP fusion protein they revealed that β1-tubulin existed in the cytoplasm as punctate structures, and SLPI co-localized on these punctate structures as shown in Fig. 11. Upper row of Fig. 11 shows a matured MK without proplatelet, middle shows a MK in which PPF developed partially, bottom shows developed PPF. These pictures indicate the transition of β1-tubulin from cytoplasmic punctate structures to MT fiber in the process of proplatelet process formation. According to Lewis et al.39) β1-tubulin whether normally expressed in fetal erythroblasts or transfected into HeLa cells, assembles into both interphase cytoskeletal and mitotic spindle microtubules. On the other hand, β1-tubulin in differentiated MK, either normally expressed39) or transfected60) exists in cytoplasm as punctate spots without assembling into microtubule. This may strongly suggest the existence of some unknown conditions related to the isolation of the β1-tubulin into punctate spots in differentiating MK. In conclusion, in the cytoplasm of matured MK without PPF β1-tubulin exists as punctate structures without assembling into MTs, so that MT coils cannot be formed; platelet territory is not a mere vesicle as described in a report18) but a true future platelet.
Figure 10.
Immunofluorescence analysis of mouse platelets and megakaryocytes with an antibody specific to mouse β1-tubulin. (A) Mouse platelets show the marginal bands. (B) Mouse MK shows a dense array of punctate spots that fills the cytoplasm. Magnification of A and B is the same. (Reproduced from Lewis et al.39) Figs. 5 A and C with permission from the publisher)
Figure 11.
Immunofluorescence (IF) analysis of primary MKs expressing a β1-tubulin-GFP fusion protein, SLPI (secretory leukocyte protease inhibitor) is expressed naturally in MKs. Cellular SLPI is detected by a specific antibody and Texas red-labelled secondary antibody. First column; IF analysis, second column; immunostaining of SLPI, third column; merge, including nuclear DAPI staining. Each row represents different cells. Scale bars, 10 µm (Reproduced from Schulze et al.60) Fig. 1B, with permission from the publisher)
In vitro proplatelet process formation (PPF) triggered by integrin ligation37,57) seems clearly to be an artifact as already mentioned in Introduction and Section 8. Exact mechanism of formation of new specialized MT with β1-tubulin in proplatelet processes must be elucidated more precisely. Also, the mechanism of PPF in vivo must be elucidated. If the proplatelet process in vivo is a true and natural structure, why PPF cannot be observed in the bone marrow specimen treated by immersion fixation. In case of spleen, arterial blood containing fixative directly flows into the red pulp where MKs reside. In a SEM study of perfusion-fixed mouse spleen by Ihzumi et al.66) proplatelet process formation from MK was not recognized clearly. On the other hand, almost simultaneously performed study10) by the same group demonstrated typical PPF of MK in perfusion-fixed bone marrow. Finally, Radley et al.67) reported in their long-term marrow culture study, that the MKs which extended proplatelet processes retracted their processes within 24–48 hours, usually without displaying the evidence of fragmentation. They stated that “the subsequent fate of such cells was presumably degeneration, but this aspect was not pursued further”. This observation by Radley implies that the logics of the proplatelet model may be negated.
Choi et al.68) demonstrated the transition from MK progenitors to functional platelets in an in vitro culture system. In culture vessel, some MKs attached to the vessel wall developed proplatelets, while other MKs in suspension released platelets probably via cytoplasmic fragmentation. Because in the latter MKs, ligation of integrin αvβ3 leading to proplatelet formation did not occur. They stated that platelet formation appears to occur via proplatelet intermediate structures. However, they observed MKs with proplatelets and released functional platelets separately. Continuous observation of platelet liberation from proplatelets or of conversion of proplatelets into platelets was not described in their report. Italiano et al.14) reported serial video-enhanced light micrographs of a MK showing developing process of proplatelet processes in Fig. 1. In these micrographs covering the full time course (10 hours) of PPF, almost nobody could witness the apparent release of platelets through proplatelet intermediates. Junt et al.18) succeeded in observing liberation of proplatelets from the matured MK in living bone marrow. But as described by them, the velocity of elongation or extension of proplatelets from MK was totally different: much faster than that usually observed in vitro.32) It seems that there is not enough time to transport and to package their organelle within platelets or proplatelets de novo. Additionally, they suggested a possibility of conversion from proplatelets into platelets deduced from the observation by Handagama et al.52) They observed that the proplatelet counts are higher in pre-pulmonary vessels than in post-pulmonary vessels, whereas platelet counts are higher in the latter as observed by Howell and Donahue.69) But above mentioned proplatelet observed by Handagama in the central vein was demonstrated not the proplatelet but the short chain of platelets by TEM study,53) as described in Section 7. The short chain of platelets may be separated into individual platelets by passing through the pulmonary vessels. But nobody could observe the conversion of proplatelets into individual platelets during pulmonary circulation. It seems that proplatelet is an artifact, and cannot exist in circulating blood.
Recently, Dunois-Lardé et al.70) reported the accelerated platelet production from human MK at high shear rates. They demonstrated in Fig. 5F a TEM picture of a MK on a von Willebrand factor-coated coverslip after exposure to shear, showing additional 2 platelet-sized fragments located close to the adhering MK. They claimed that adhered MK released platelets by shear exposure through proplatelet formation. They also suggested similar mechanism in platelet release from matured MK in the pulmonary capillary beds. The structure described by them as surface-connected canalicular system (SCCS) in MK and platelet-sized fragments in Fig. 5F rather seems to be DMS, because the width of tubular structure of DMS is almost uniform and usually wider than that of SCCS. SCCS in platelet has variable width and random distribution.6) In addition, this MK does not show any evidence of PPF, and platelet-sized fragments seem to be fragments of MK cytoplasm including 2 or 3 future platelets. Thus, they clearly showed accelerated direct fragmentation of MK cytoplasm into platelets or platelet-sized pieces at high shear rate by TEM. On the other hand, Zucker-Franklin and Philipp54) showed platelet release from MK through cytoplasmic fragmentation in pulmonary vessels without proplatelet formation by TEM.
Conclusion
At present, proplatelet theory is widely advocated as the mechanism of platelet production from MKs. However, liberation of platelets from proplatelet processes has not been witnessed despite of so many efforts by investigators for the last one third of a century. Thus, the proplatelet formation may be an artifactual phenomenon observed under certain experimental conditions. Based upon our own published data and a careful review of related scientific publications, we have proposed the protoplatelet hypothesis, which justifies cytoplasmic fragmentation model. Now we look forward to seeing more solid scientific data, without preconception of proplatelet model.
Acknowledgement
The authors would like to thank Dr. Narendra Tandon (Otsuka Maryland Medicinal Laboratories Inc, Rockville, MD USA) for his generous support in the preparation of this manuscript.
Non-standard abbreviations
- MK
megakaryocyte
- PPF
proplatelet process formation
- DMS
platelet demarcation membrane system
- DM
platelet demarcation membrane
- DVs
platelet demarcation vesicles
- MVs
membrane vesicles
- MT
microtubule
- TEM
transmission electron microscopy
- SEM
scanning electron microscopy
Biographies
Profile
Goro Kosaki was born in Chiba Prefecture in 1919. He graduated from Osaka University Medical School in 1943, and started his career as a surgeon in the field of gastroenterology and oncology in the Department of Surgery II, Osaka University. During World War II, he initiated his research on hemostasis and blood platelets. In 1961 he moved to the Osaka Medical Center for Cancer and Cardiovascular Diseases as head of the Department of Surgery. In 1975, he was elected as Chairman and Professor of Department of Surgery II, Osaka University. After retirement from the Osaka University in 1983, he worked at the Tokyo Metropolitan Komagome Hospital as vice-President, President and Advisor till 1992. In parallel with his clinical practice and associated research, he engaged himself in basic research on the birth of blood platelets from megakaryocyte utilizing electron microscopy (1961–1975), and also on calpain, calpastatin and calmodulin in platelets (1975–1983). In the field of cancer research, in collaboration with H. Nakazato and S. Oikawa of Suntory Institute for Biomedical Research, he elucidated primary structure of CEA, a tumor marker, firstly in 1987 and later contributed to the establishment of the concept of CEA gene families. He served as President to various prestigious societies: the Japan Surgical Society (1982–83), the Japanese Cancer Association (1982), the Japanese Society of Thrombosis and Hemostasis (1981). At the age of 92, he is still active in searching for the latest information in various life science journals.
Junichi Kambayashi was born in 1948 and graduated from Osaka University Medical School in 1972 with the goal to become physician/scientist in surgery. His bitter experience relating to the loss of a patient due to postoperative bleeding motivated him to study thrombosis and hemostasis as his life’s work. Between 1974 and 1978, he studied heparin and platelets at Mt. Sinai Medical Center, WI and underwent clinical training as a surgical resident at Buffalo General Hospital, NY. When Professor Goro Kosaki became the chairman of the 2nd department surgery at Osaka University, he was asked to return to Osaka to establish a research lab on hemostasis. Thus, from 1978 to 1996, he engaged in teaching, treating patients (especially vascular surgery), and conducting/mentoring research on hemostasis and platelets at Osaka University. In 1996, he went back to the US to work in the pharmaceutical industry (Otsuka America) with dual appointments; clinical studies of new chemical entities for various diseases and conducting research on advanced pharmacology and drug discovery targeting platelets. He continues to contribute to the scientific/medical community as an expert physician/scientist in the area of hemostasis, vascular surgery, and international clinical studies. As Professor Kosaki has been a superb mentor for him for many years, he is extremely honored to serve as a co-author on this review.
References
- 1).Wright J.H. (1906) Die Entstehung der Blutplättchen. Virchows Arch. 186, 55–63 [Google Scholar]
- 2).Bessis, M. (1956) Cytology of the Blood and Blood-forming Organs. Grune & Stratton, New York. [Google Scholar]
- 3).Yamada E. (1957) The fine structure of the megakaryocyte in the mouse spleen. Acta Anat. (Basel) 28, 267–290 [DOI] [PubMed] [Google Scholar]
- 4).Kosaki G., Tanaka K., Inoshita K. (1973) Electron microscopic observations on megakaryocyte differentiation and platelet production. Acta Haematol. Jpn. 36, 383–384(in Japanese) [Google Scholar]
- 5).Kosaki, G., Inoshita, K. and Okuma, H. (1974) Electron microscopic studies on human thrombocytogenesis. In Platelets, Thrombosis and Inhibitors (eds. Didisheim, P., Shimamoto, T. and Yamazaki, H.). Schattauer, Stuttgart, pp. 97–102. [Google Scholar]
- 6).Kosaki, G. and Fujimoto, T. (1979) Morphology of blood platelets, megakaryocytes differentiation and platelet release. In Nippon-Ketsuekigaku-Zensho (New Edition), vol. 11 Hemorrhagic diathesis (ed. The Publication Committee). Maruzen, Tokyo, pp. 1–55 (in Japanese). [Google Scholar]
- 7).Behnke O. (1968) An electron microscope study of the megacaryocyte of the rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. J. Ultrastruct. Res. 24, 412–433 [DOI] [PubMed] [Google Scholar]
- 8).Behnke O. (1969) An electron microscope study of the rat megacaryocyte. II Some aspects of platelet release and microtubules. J. Ultrastruct. Res. 26, 111–129 [DOI] [PubMed] [Google Scholar]
- 9).Becker R.P., De Bruyn P.P.H. (1976) The transmural passage of blood cells into myeloid sinusoids and the entry of platelets into the sinusoidal circulation; a scanning electron microscopic investigation. Am. J. Anat. 145, 183–206 [DOI] [PubMed] [Google Scholar]
- 10).Muto M. (1976) A scanning and transmission electron microscopic study on rat bone marrow sinuses and transmural migration of blood cells. Arch. Histol. Jpn. 39, 51–66 [DOI] [PubMed] [Google Scholar]
- 11).Thiéry J.P., Bessis M. (1956) Mecanisme de la plaquettogenese. Etude “in vitro” par la microcinematographie. Rev. Hematol. (Paris) 11, 162–174 [PubMed] [Google Scholar]
- 12).Albrecht M. (1957) Studien zur Thrombocytenbildung an Megakaryocyten in menschlichen Knochenmarkkulturen. Acta Haematol. 17, 160–168 [DOI] [PubMed] [Google Scholar]
- 13).Radley J.M., Scurfield G. (1980) The mechanism of platelet release. Blood 56, 996–999 [PubMed] [Google Scholar]
- 14).Italiano J.E., Jr., Lecine P., Shivdasani R.A., Hartwig J.H. (1999) Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J. Cell Biol. 147, 1299–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Wright J.H. (1910) The histogenesis of the blood platelets. J. Morphol. 21, 263–278 [Google Scholar]
- 16).Kinosita, R. and Ohno, S. (1961) Biodynamics of thrombopoiesis. In Blood Platelets (eds. Johnson, S.A., Monto, R.W., Rebuck, J.W. and Horn, R.C.). Little Brown, Boston, pp. 611–616. [Google Scholar]
- 17).Albrecht, M. (1969) Untersuchungen zur Thrombozytenbildung an Megakaryozyten in vitro. In Der Thrombozyt (ed. Marx, R.). Lehmanns, München, pp. 44–51. [PubMed] [Google Scholar]
- 18).Junt T., Schulze H., Chen Z., Massburg S., George T., Krueger A., Wagner D.D., Graf T., Italiano J.E. Jr., Shivdasani R.A., von Andrian U.H. (2007) Dynamic visualization of thrombopoiesis within bone marrow. Science 317 (5845), 1767–1770 [DOI] [PubMed] [Google Scholar]
- 19).Richardson J.L., Shivdasani R.A., Boers C., Hartwig J.H., Italiano J.E., Jr. (2005) Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood 106, 4066–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Zucker-Franklin D., Petursson S. (1984) Thrombocytopoiesis: analysis by membrane tracer and freeze-fracture studies on fresh human and cultured mouse megakaryocytes in vitro. J. Cell Biol. 99, 390–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Straneva J.E., Goheen M.P., Hui S.L., Bruno E., Hoffman R. (1986) Terminal cytoplasmic maturation of human megakaryocytes in vitro. Exp. Hematol. 14, 919–929 [PubMed] [Google Scholar]
- 22).Falcieri E., Bassini A., Pierpaoli S., Luchetti F., Zamai L., Vitale M., Guidotti L., Zauli G. (2000) Ultrastructural characterization of maturation, platelet release, and senescence of human cultured megakaryocytes. Anat. Rec. 258, 90–99 [DOI] [PubMed] [Google Scholar]
- 23).Kosaki G. (2008) Platelet production by megakaryocytes: protoplatelet theory justifies cytoplasmic fragmentation model. Int. J. Hematol. 88, 255–267 [DOI] [PubMed] [Google Scholar]
- 24).Kosaki G. (2003) Ultrastructural characterization of in vivo platelet release from megakaryocytes. Does it occur via proplatelets? Proposal of a new concept “protoplatelet”. Jpn. J. Thromb. Hemost. 14, 495–506(in Japanese) [Google Scholar]
- 25).Kosaki G. (2005) In vivo platelet production from mature megakaryocytes: does platelet release occur via proplatelets? Int. J. Hematol. 81, 208–219 [DOI] [PubMed] [Google Scholar]
- 26).Bessis, M. (1973) The thrombocytic series. In Living Cells, Their Ultrastructure (ed. Bessis M.). Springer, Berlin, p. 370. [Google Scholar]
- 27).Nagata Y., Muro Y., Todokoro K. (1997) Thrombopoietin-induced polyploidization of bone marrow megakaryocytes is due to a unique regulatory mechanism in late mitosis. J. Cell Biol. 139, 449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Vitrat N., Cohen-Solal K., Pique C., Le Couedic J.P., Norol F., Larsen A.K., Katz A., Vainchenker W., Debili N. (1998) Endomitosis of human megakaryocytes are due to abortive mitosis. Blood 91, 3711–3723 [PubMed] [Google Scholar]
- 29).Bassini A., Pierpaoli S., Falcieri E., Vitale M., Guidotti L., Capitani S., Zauli G. (1999) Selective modulation of the cyclin B/CDK1 and cyclin D/CDK4 complexes during in vitro human megakaryocyte development. Br. J. Haematol. 104, 820–828 [DOI] [PubMed] [Google Scholar]
- 30).Oakley C.E., Oakley B.R. (1989) Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature 338, 662–664 [DOI] [PubMed] [Google Scholar]
- 31).Dictenberg J.B., Zimmerman W., Sparks C.A., Young A., Vidair C., Zheng Y., Carrington W., Fay F.S., Doxsey S.J. (1998) Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Patel S.R., Richardson J.L., Schulze H., Kahle E., Galjart N., Drabek K., Shivdasani R.A., Hartwig J.H., Italiano J.E. Jr. (2005) Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood 106, 4076–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Mori Y., Akedo H., Tanigaki Y., Tanaka K., Okada M. (1979) Ciliogenesis in tissue-cultured cells by the increased density of cell population. Exp. Cell Res. 120, 435–439 [DOI] [PubMed] [Google Scholar]
- 34).McNiven M.A., Wang M., Porter K.R. (1984) Microtubule polarity and the direction of pigment transport reverse simultaneously in surgically severed melanophore arms. Cell 37, 753–765 [DOI] [PubMed] [Google Scholar]
- 35).Moskwin-Tarkhanov M.I., Onishchenko G.E. (1978) Centrioles in megakaryocytes of mouse bone marrow. Tsitologiia 20, 1436–1437 [PubMed] [Google Scholar]
- 36).Behnke O. (1970) Microtubules in disk-shaped blood cells. Int. Rev. Exp. Pathol. 9, 1–92 [PubMed] [Google Scholar]
- 37).Jiang F., Jia Y., Cohen I. (2002) Fibronectin- and protein kinase C-mediated activation of ERK/MAPK are essential for proplatelet-like formation. Blood 99, 3579–3584 [DOI] [PubMed] [Google Scholar]
- 38).Patel-Hett S., Richardson J.L., Schulze H., Drabek K., Isaac N.A., Hoffmeister K., Shivdasani R.A., Bulinski J.C., Galjart N., Hartwig J.H., Italiano J.E. Jr. (2008) Visualization of microtubule growth in living platelets reveals a dynamic marginal band with multiple microtubules. Blood 111, 4605–4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Lewis S.A., Gu W., Cowan N.J. (1987) Free intermingling of mammalian β-tubulin isotypes among functionally distinct microtubules. Cell 49, 539–548 [DOI] [PubMed] [Google Scholar]
- 40).Radley J.M., Haller C.J. (1982) The demarcation membrane system of the megakaryocyte: a misnomer? Blood 60, 213–219 [PubMed] [Google Scholar]
- 41).Haller C.J., Radley J.M. (1983) Time-lapse cinemicrography and scanning electron microscopy of platelet formation by megakaryocytes. Blood Cells 9, 407–418 [PubMed] [Google Scholar]
- 42).Shaklai M., Tavassoli M. (1978) Demarcation membrane system in rat megakaryocyte and the mechanism of platelet formation: a membrane reorganization process. J. Ultrastruct. Res. 62, 270–285 [DOI] [PubMed] [Google Scholar]
- 43).Tavassoli M. (1979) Fusion-fission reorganization of membrane: a developing membrane model for thrombocytogenesis in megakaryocytes. Blood Cells 5, 89–99 [PubMed] [Google Scholar]
- 44).Mahaut-Smith M.P., Thomas D., Higham A.B., Usher-Smith J.A., Hussain J.F., Martinez-Pinna J., Skepper J.N., Mason M.J. (2003) Properties of the demarcation membrane system in living rat megakaryocytes. Biophys. J. 84, 2646–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Schulze H., Korpal M., Hurov J., Kim S.W., Zhang J., Cantley L.C., Graf T., Shivdasani R.A. (2006) Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood 107, 3868–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Radley J.M., Hartshorn M.A., Green S.L. (1987) The response of megakaryocytes with processes to thrombin. Thromb. Haemost. 58, 732–736 [PubMed] [Google Scholar]
- 47).Eldor A., Levine R.F., Caine Y.G., HyAm E., Vlodavsky I. (1986) Megakaryocyte interaction with the subendothelial extracellular matrix. Prog. Clin. Biol. Res. 215, 399–404 [PubMed] [Google Scholar]
- 48).Maximow, A. (1927) Megakaryocyten. In Handbuch der mikroskopischen Anatomie des Menschen. 2ter Band, 1terTeil (ed. Moellendorff, W.). Julius Springer, Berlin, pp. 405–409. [Google Scholar]
- 49).Cramer E.M., Norol F., Guichard J., Breton-Goruis J., Vainchenker W. (1997) Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood 89, 2336–2346 [PubMed] [Google Scholar]
- 50).Flaumenhaft R., Dilks J.R., Richardson J., Alden E., Patel-Hett S.R., Battinelli E., Klement G.L., Sola-Visner M., Italiano J.E. Jr. (2009) Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood 113, 1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Radley J.M., Hartshorn M.A. (1987) Megakaryocyte fragments and the microtubule coil. Blood Cells 12, 603–610 [PubMed] [Google Scholar]
- 52).Handagama P.J., Feldman B.F., Jain N.C., Farver T.B., Kono C.S. (1987) Circulating proplatelets: isolation and quantitation in healthy rats and in rats with induced acute blood loss. Am. J. Vet. Res. 48, 962–965 [PubMed] [Google Scholar]
- 53).Tong M., Seth P., Penington D.G. (1987) Proplatelets and stress platelets. Blood 69, 522–528 [PubMed] [Google Scholar]
- 54).Zucker-Franklin D., Philipp C.S. (2000) Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am. J. Pathol. 157, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Leven R.M., Tablin F. (1992) Extracellular matrix stimulation of guinea pig megakaryocyte proplatelet formation in vitro is mediated through the vitronectin receptor. Exp. Hematol. 20, 1316–1322 [PubMed] [Google Scholar]
- 56).Larson M.K., Watson S.P. (2006) Regulation of proplatelet formation and platelet release by integrin αIIbβ3. Blood 108, 1509–1514 [DOI] [PubMed] [Google Scholar]
- 57).Rojnuckarin P., Kaushansky K. (2001) Actin reorganization and proplatelet formation in murine megakaryocytes: the role of protein kinase Cα. Blood 97, 154–161 [DOI] [PubMed] [Google Scholar]
- 58).Lecine P., Italiano J.E., Jr., Kim S.W., Villeval J.L., Shivdasani R.A. (2000) Hematopoietic-specific β1-tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2. Blood 96, 1366–1373 [PubMed] [Google Scholar]
- 59).Hartwig J., Italiano J., Jr. (2003) The birth of the platelet. J. Thromb. Haemost. 1, 1580–1586 [DOI] [PubMed] [Google Scholar]
- 60).Schulze H., Korpal M., Bergmeier W., Italiano J.E., Jr., Wahl S.M., Shivdasani R.A. (2004) Interactions between the megakaryocyte/platelet-specific β1 tubulin and the secretory leucocyte protease inhibitor SLPI suggest a role for regulated proteolysis in platelet functions. Blood 104, 3949–3957 [DOI] [PubMed] [Google Scholar]
- 61).Lichtman M.A., Chamberlain J.K., Simon W., Santillo P.A. (1978) Parasinusoidal location of megakaryocytes in marrow: a determinant of platelet release. Am. J. Hematol. 4, 303–312 [DOI] [PubMed] [Google Scholar]
- 62).Kojima H., Kozuma Y., Nagasawa T. (2006) Mechanism of platelet production from megakaryocytes—role of proplatelet formation—. Jpn. J. Thromb. Hemost. 17, 261–271(in Japanese) [Google Scholar]
- 63).Schwer H.D., Lecine P., Tiwari S., Italiano J.E., Jr., Hartwig J.H., Shivdasani R.A. (2001) A lineage-restricted and divergent β-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr. Biol. 11, 579–586 [DOI] [PubMed] [Google Scholar]
- 64).Italiano J.E., Jr., Bergmeier W., Tiwari S., Falet H., Hartwig J.H., Hoffmeister K.M., André P., Wagner D.D., Shivdasani R.A. (2003) Mechanisms and implications of platelet discoid shape. Blood 101, 4789–4796 [DOI] [PubMed] [Google Scholar]
- 65).Stenberg P.E., McDonald T.P., Jackson C.W. (1995) Disruption of microtubules in vivo by vincristine induces large membrane complexes and other cytoplasmic abnormalities in megakaryocytes and platelets of normal rats like those in human and Wistar Furth rat hereditary macrothrombocytopenias. J. Cell. Physiol. 162, 86–102 [DOI] [PubMed] [Google Scholar]
- 66).Ihzumi T., Hattori A., Sanada M., Muto M. (1977) Megakaryocyte and platelet formation: A scanning electron microscope study in mouse spleen. Arch. Histol. Jpn. 40, 305–320 [DOI] [PubMed] [Google Scholar]
- 67).Radley J.M., Rogerson J., Ellis S.L., Hasthorpe S. (1991) Megakaryocyte maturation in long-term marrow culture. Exp. Hematol. 19, 1075–1078 [PubMed] [Google Scholar]
- 68).Choi E.S., Nichol J.L., Hokom M.M., Hornkohl A.C., Hunt P. (1995) Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood 85, 402–413 [PubMed] [Google Scholar]
- 69).Howell W.H., Donahue D.D. (1937) The production of blood platelets in the lungs. J. Exp. Med. 65, 177–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Dunois-Lardé E., Capron C., Fichelson S., Bauer T., Cramer-Bordé E., Baruch D. (2009) Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood 114, 1875–1883 [DOI] [PubMed] [Google Scholar]