Abstract
We previously showed that leucine deprivation decreases abdominal fat mass largely by increasing energy expenditure, as demonstrated by increased lipolysis in white adipose tissue (WAT) and uncoupling protein 1 (UCP1) expression in brown adipose tissue (BAT). The goal of the present study was to investigate the possible involvement of central nervous system (CNS) in this regulation and elucidate underlying molecular mechanisms. For this purpose, levels of genes and proteins related to lipolysis in WAT and UCP1 expression in BAT were analyzed in wild-type mice after intracerebroventricular administration of leucine or corticotrophin-releasing hormone antibodies, or in mice deleted for three β-adrenergic receptors, after being maintained on a leucine-deficient diet for 7 d. Here, we show that intracerebroventricular administration of leucine significantly attenuates abdominal fat loss and blocks activation of hormone sensitive lipase in WAT and induction of UCP1 in BAT in leucine-deprived mice. Furthermore, we provide evidence that leucine deprivation stimulates fat loss by increasing expression of corticotrophin-releasing hormone in the hypothalamus via activation of stimulatory G protein/cAMP/protein kinase A/cAMP response element-binding protein pathway. Finally, we show that the effect of leucine deprivation on fat loss is mediated by activation of the sympathetic nervous system. These results suggest that CNS plays an important role in regulating fat loss under leucine deprivation and thereby provide novel and important insights concerning the importance of CNS leucine in the regulation of energy homeostasis.
Energy homeostasis is maintained by a balance between calorie intake and energy expenditure. A disruption of energy homeostasis involving excess caloric intake and/or decreased energy expenditure often results in obesity and associated metabolic disorders, such as insulin resistance. The central nervous system (CNS) has been shown to be critical in the regulation of energy homeostasis, among which hypothalamus is one of the most extensively studied areas (1). The hypothalamus integrates nutritional and hormonal signals from peripheral tissues through membrane receptors expressed in arcuate nucleus, paraventricular nucleus of the hypothalamus (PVN), and other hypothalamic nuclei (2, 3). Based upon these signals from the periphery, the hypothalamus regulates food intake by modulating the activity of orexigenic and anorexigenic neurons (4). By contrast, the hypothalamus regulates energy expenditure, including thermogenesis, by increasing secretion of norepinephrine (NE) from sympathetic nerves and expression of uncoupling protein 1 (UCP1) in brown adipose tissue (BAT) (5–7).
Corticotropin-releasing hormone (CRH) is a 41-amino acid peptide, produced mainly in the PVN and other sites of the brain and peripheral tissues (8). It is well established that CRH expression is positively regulated by stimulatory G protein (Gs) and cAMP-dependent activation of protein kinase A (PKA) and phosphorylation of cAMP response element (CRE)-binding protein (CREB) (9–12) and negatively regulated by increased serum levels of glucocorticoids via binding to glucocorticoid receptors expressed in CRH neurons in the hypothalamus (13). Studies have shown that intracerebroventricular (icv) administration of CRH decreases food intake (14, 15) and increases energy expenditure (16). Furthermore, CRH has also been shown to be important in the regulation of thermogenesis in BAT (16, 17) and lipolysis in white adipose tissue (WAT) (18). The above effects are mediated by activation of the sympathetic nervous system (SNS), because it has been reported that icv administration of CRH increases NE release (19) and stimulates sympathetic activity (20, 21). The role of CRH in the regulation of energy homeostasis under different nutritional conditions, however, needs to be further investigated.
We previously showed that leucine deprivation for 7 d decreases abdominal fat mass largely by increasing energy expenditure (22, 23). Consistent with increased energy expenditure, we observed increased lipolysis in WAT and UCP1 expression in BAT in leucine-deprived mice (22, 23). Because leucine deficiency has previously been shown to be detected in the CNS (24), we hypothesized that CNS leucine may play a role in the regulation of fat loss under leucine deprivation. The goal of our current study was to investigate this possibility and elucidate the underlying molecular and cellular mechanisms.
As described below, we show that icv administration of leucine decreases levels of activated hormone sensitive lipase (HSL) in WAT and UCP1 expression in BAT and significantly attenuates fat loss in leucine-deprived mice. Furthermore, we provide evidence that leucine deprivation stimulates fat loss via increasing expression of CRH in the hypothalamus and activating the SNS. Again, these effects are blocked by icv leucine. We also show that CRH expression in the hypothalamus is stimulated by activation of Gs/cAMP/PKA/CREB pathway in leucine-deprived mice. Taken together, these results suggest that CNS leucine plays an important role in leucine deprivation-induced fat loss.
Results
Intracerebroventricular administration of leucine significantly attenuates abdominal fat loss under leucine deprivation
To investigate the possibility that CNS leucine may play a role in the regulation of fat loss under leucine deprivation, leucine (1.1 μg of leucine in 1.0 μl of PBS) or PBS was administered by icv injection once a day for 7 d to mice maintained on a leucine-deficient [(−) leu] diet, a protocol based on one previously described by Cota et al. (25). We found that leucine levels in the hypothalamus 1 h after injection increased only to levels detected in mice maintained on a control diet and that this level of leucine was largely maintained at 12 and 20 h after injection (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). By contrast, plasma leucine concentrations remained unchanged after icv injection of leucine (data not shown). These results suggest that pulsed icv administration of leucine can produce long-lasting and stable increases in leucine in the hypothalamus to normal levels, without affecting plasma leucine concentrations.
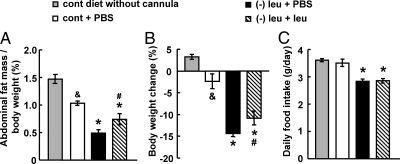
To control for the effect of cannula implantation, PBS was administered by icv injection to mice maintained on a control diet for 7 d. We found that cannula implantation and PBS administration caused a reduction in fat mass and body weight (Fig. 1, A and B), which is most likely a consequence of the surgery and anesthesia required for the daily icv injections. These are the levels of fat mass and body weight to which the effect of icv administration of leucine in leucine-deprived mice should be compared. Based on these controls, leucine deprivation reduced fat mass (∼50%) and body weight (∼14%) to similar levels as shown previously (22, 23). By contrast, icv administration of leucine significantly attenuates the fat loss reduction (∼50%) and modestly blocked body weight reduction (∼30%) in leucine-deprived mice (Fig. 1, A and B). Food intake, however, was not affected by icv administration of leucine compared with those receiving PBS (Fig. 1C). Consistent with the results of Cota et al. (25), we found that food intake decreased after increases in CNS leucine concentration, which may have contributed to decreased body weight in these mice (data not shown). Fat mass, however, was not affected by icv leucine in mice maintained on a control diet (data not shown).
Fig. 1.
Intracerebroventricular administration of leucine significantly attenuates abdominal fat loss in leucine-deprived mice. A–C, Mice received icv administration of leucine (1.1 μg of leucine in 1.0 μl of PBS) or PBS once a day for 7 d under a control (cont) or (−) leu diet. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 8–11 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effect of control diet with icv PBS vs. without cannula implantation (&, P < 0.05), (−) leu diet with icv PBS or leucine vs. control diet with icv PBS (*, P < 0.01), or (−) leu diet with icv leucine vs. (−) leu diet with icv PBS (#, P < 0.05). A, Adipose tissue mass in proportion to body weight. B, Body weight change. C, Daily food intake.
Intracerebroventricular administration of leucine prevents changes in WAT and BAT in leucine-deprived mice
We have previously shown that leucine deprivation increases lipolysis in WAT and UCP1 expression in BAT (23). Therefore, we examined whether icv administration of leucine has an effect on changes of these genes and proteins in WAT and BAT in leucine-deprived mice.
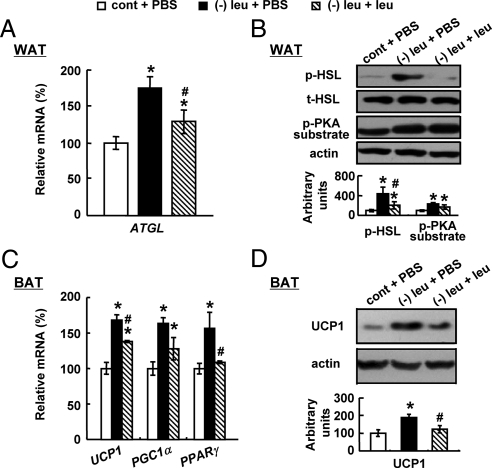
Consistent with changes in fat mass and body weight, icv administration of leucine significantly decreased levels of adipose triglyceride lipase (Atgl) mRNA and phosphorylated (p)-HSL in WAT (Fig. 2, A and B). Levels of p-PKA substrate, however, were not decreased in WAT (Fig. 2B). In addition, icv administration of leucine significantly decreased UCP1 expression (Fig. 2, C and D). Levels of mRNA encoding UCP1 upstream regulators peroxisome proliferator-activated receptor (Ppar)γ and PPARγ coactivator 1 (Pgc1)α (26, 27) were also examined in BAT. Intracerebroventricular leucine significantly decreased Pparγ expression. Levels of Pgc1α mRNA also exhibited decreased tendency (Fig. 2, C and D). In contrast to what we observed in leucine-deprived mice, icv injection of leucine into mice fed a control diet had no effect on UCP1 expression in BAT or the expression of genes and proteins related to lipolysis in WAT, compared with mice receiving icv PBS (data not shown).
Fig. 2.
Intracerebroventricular administration of leucine prevents changes in WAT and BAT in leucine-deprived mice. Mice received icv administration of leucine (1.1 μg of leucine in 1.0 μl of PBS) or PBS once a day for 7 d under a control or (−) leu diet. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 8–11 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effect of (−) leu diet with icv PBS or leucine vs. control diet with icv PBS (*, P < 0.01), or (−) leu diet with icv leucine vs. (−) leu diet with icv PBS (#, P < 0.05). A, Atgl mRNA expression in WAT. B, p-HSL and p-PKA substrate protein in WAT (upper, Western blotting; lower, quantitative measurements of p-HSL and p-PKA substrate protein relative to total (t)-HSL and actin, respectively). C, Ucp1, Pparγ, and Pgc1α mRNA expression in BAT. D, UCP1 protein in BAT.
Intracerebroventricular administration of CRH antibodies significantly attenuates abdominal fat loss under leucine deprivation
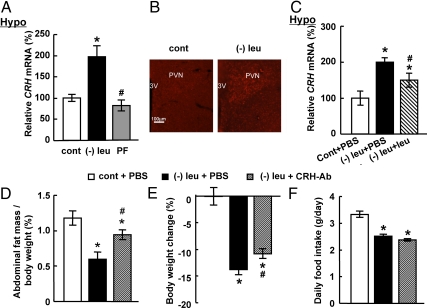
CRH has been shown to be important in the regulation of energy homeostasis (28), suggesting that CRH may mediate the effect of CNS leucine on fat loss in leucine-deprived mice. To investigate this possibility, we first examined the expression levels of CRH in the hypothalamus of mice under different treatments. Levels of Crh mRNA were increased significantly in the hypothalamus of leucine-deprived mice compared with pair-fed or control diet-fed groups (Fig. 3A). Consistent with changes in mRNA levels, CRH protein levels were also increased in the PVN, where CRH mainly produced (28), of leucine-deprived mice (Fig. 3B). The possible involvement of CRH in the effect of CNS leucine was further examined by RT-PCR analysis of Crh expression after icv administration of leucine. As predicted, levels of Crh mRNA in the hypothalamus were decreased significantly by icv leucine (Fig. 3C). Again, icv injection of leucine into mice fed a control diet had no effect on Crh expression in the hypothalamus, compared with those receiving icv PBS (data not shown).
Fig. 3.
Intracerebroventricular administration of CRH antibodies significantly attenuates abdominal fat loss under leucine deprivation. A and B, Mice were fed a control (cont), (−) leu, or pair-fed diet for 7 d. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 8–9 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effects of (−) leu or pair-fed diet vs. control diet (*, P < 0.01), or (−) leu diet vs. pair-fed diet (#, P < 0.01). C–F, Mice received icv administration of leucine (1.1 μg of leucine in 1.0 μl of PBS), CRH antibodies (0.5 μg in 1 μl of PBS), or PBS once a day for 7 d under a control or (−) leu diet. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 8–9 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effect of (−) leu diet with icv PBS, leucine, or CRH antibodies vs. control diet with icv PBS (*, P < 0.01), or (−) leu diet with icv leuicne or CRH antibodies vs. (−) leu diet with icv PBS (#, P < 0.05). A and C, Crh mRNA expression in the hypothalamus (Hypo). B, Immunofluorescence of CRH in the PVN. 3v, Third ventricle. Original magnification, ×100. D, Adipose tissue mass in proportion to body weight. E, Body weight change. F, Daily food intake.
To assess the contribution of hypothalamic CRH in leucine deprivation-induced fat loss, we blocked CRH function by icv administration of CRH antibodies (0.5 μg in 1 μl of PBS) as shown previously (29) once a day for 7 d while maintaining the mice on a (−) leu diet. We found that icv administration of CRH antibodies significantly decreased leucine deprivation-induced fat loss and body weight reduction, compared with PBS administration (Fig. 3, D and E). Food intake, however, was not affected by icv administration of CRH antibodies (Fig. 3F).
Intracerebroventricular administration of CRH antibodies prevents changes in WAT and BAT in leucine-deprived mice
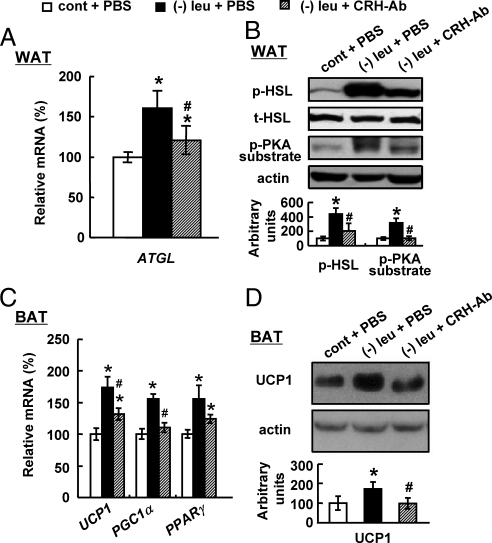
Consistent with changes of fat mass and body weight, icv administration of CRH antibodies significantly decreased levels of f p-HSL, p-PKA substrate in WAT compared with PBS control in leucine-deprived mice (Fig. 4, A and B). UCP1 expression, as well as mRNA encoding its upstream regulator Pgc1α, was also decreased in BAT of these mice (Fig. 4, C and D). Pparγ expression in BAT show decreased tendency by icv leucine (Fig. 4C).
Fig. 4.
Intracerebroventricular administration of CRH antibodies (CRH-AB) prevents changes in WAT and BAT in leucine-deprived mice. Mice received icv administration of CRH antibodies (0.5 μg in 1 μl of PBS) or PBS once a day for 7 d under a control (cont) or leucien-deficient diet. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 8–9 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effect of (−) leu diet with icv PBS or CRH antibodies vs. control diet with icv PBS (*, P < 0.01), or (−) leu diet with icv CRH antibodies vs. (−) leu diet with icv PBS (#, P < 0.05). A, Atgl mRNA expression in WAT. B, p-HSL and p-PKA substrate protein in WAT (upper, Western blotting; lower, quantitative measurements of p-HSL and p-PKA substrate protein relative to total (t)-HSL and actin, respectively). C, Ucp1, Pparγ, and Pgc1α mRNA expression in BAT. D, UCP1 protein in BAT.
Leucine deprivation induces CRH expression in the hypothalamus by activation of Gs/cAMP/PKA/CREB pathway
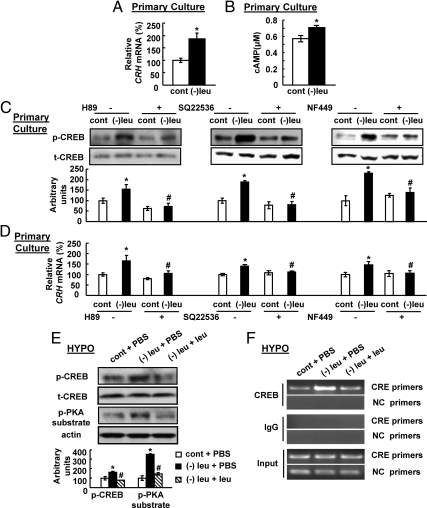
It is well established that Gs protein, which couples to adenylyl cyclase to stimulate cAMP production, regulates CRH expression through activation of PKA and recruitment of p-CREB to the CRE site of the CRH promoter (9–12), suggesting the possibility that increased CRH expression may be regulated by activation of PKA and phosphorylation of CREB during leucine deprivation.
To test this possibility, we examined Crh expression in vitro by incubating primary cultured hypothalamic neurons with (−) leu or control medium for 3 h. We found that Crh expression and cAMP levels were significantly increased by incubation with (−) leu medium (Fig. 5, A and B). A role of PKA/CREB pathway on CRH expression was further investigated in primary cultured hypothalamic neurons treated with the 10 μm PKA inhibitor H89. As expected, leucine deprivation-increased phosphorylation of CREB, and Crh expression was blocked by H89 in primary cultured hypothalamic neurons (Fig. 5, C and D). The role of cAMP in regulating CRH expression was investigated in primary cultured hypothalamic neurons treated with 10 μm SQ22536, an adenylyl cyclase inhibitor (30). Consistent with a role for cAMP in the regulation of CRH expression, leucine deprivation-induced CRH expression and CREB phosphorylation was blocked by SQ22536 (Fig. 5, C and D). Similar results were obtained when treated with 10 μm NF449, a Gs protein inhibitor (Fig. 5, C and D) (31).
Fig. 5.
Leucine deprivation induces CRH expression in the hypothalamus by activation of Gs/cAMP/PKA/CREB pathway. A–D, Primary hypothalamic neurons were incubated in control (cont) or (−) leu medium for 3 h in the presence or absence of treatment with 10 μm PKA inhibitor H89, adenylyl cyclase inhibitor SQ22536, or Gs protein inhibitor NF449. Data are mean ± sem for at least two independent experiments. Statistical significance was determined by either two-tailed Student's t test or ANOVA followed by the SNK test for the effect of (−) leu vs. control treatment (*, P < 0.05), or (−) leu with inhibitor vs. (−) leu without inhibitor (#, P < 0.05). E and F, Mice received icv administration of leucine (1.1 μg of leucine in 1.0 μl of PBS) or PBS once a day for 7 d under a control or (−) leu diet. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 8–11 for each group). Primers designed to amplify the region that is not involved in CREB response serve as a negative control (NC). Statistical significance was determined by ANOVA followed by the SNK test for the effect of (−) leu diet with icv PBS or leucine vs. control diet with icv PBS (*, P < 0.05), or (−) leu diet with icv leucine vs. (−) leu diet with icv PBS (#, P < 0.05). A and D, Crh expression in primary hypothalamic neurons. B, cAMP measurement in primary hypothalamic neurons. C and E, p-CREB protein in primary hypothalamic neurons or hypothalamus (HYPO) (upper, Western blotting; bottom, quantitative measurements of p-CREB protein relative to their total (t)-CREB protein). F, ChIP assay in the hypothalamus.
Similar changes of phosphorylation of CREB and PKA substrate in the hypothalamus were also observed under leucine deprivation in vivo (Fig. 5E). The increased phosphorylation of these proteins, however, was decreased by icv leucine (Fig. 5E). The importance for p-CREB in mediating leucine deprivation-induced CRH expression in vivo was further evaluated by chromatin immunoprecipitation (ChIP) assays that examine the ability of CREB binding to the CRE site of CRH promoter. After precipitation of cross-linked DNA with a CREB antibody, PCR on regions containing CRE site of the promoter was performed. Consistent with an increase in Crh expression in the hypothalamus by leucine deprivation (Fig. 3A), the amounts of CRH promoter were significantly increased by immunoprecipitation with anti-CREB antibodies in leucine-deprived mice compared with those maintained on a control diet (Fig. 5F). Again, consistent with a decrease in Crh expression after icv leucine (Fig. 3C), the leucine deprivation-increased CREB binding to CRH promoter was also decreased in mice after icv leucine (Fig. 5F). By contrast, in the absence of a specific antibody, the CRE-containing promoter is not precipitated in any case (Fig. 5F). To ensure that the CRE-containing promoter was being specifically precipitated, we also amplified, from the same immunoprecipitate, the upstream region that is not involved in the CREB response. No band, however, was detected in the hypothalamus of mice under any treatments when either anti-CREB antibody or control IgG was used for immunoprecipitation (Fig. 5F). The input chromatin showed same levels of bands amplifying either region under any treatments (Fig. 5F).
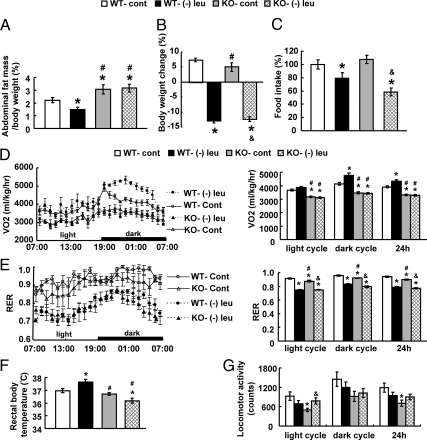
Leucine deprivation-induced fat loss and increased energy expenditure are blocked in three β-adrenergic receptors knockout (β-less) mice
We previously showed that leucine deprivation increases expression of β-3 adrenergic receptors (Adrb3) mRNA in WAT and BAT and levels of serum NE (23), suggesting that the SNS is involved in the regulation of fat loss. This possibility was further investigated by examining changes of abdominal fat mass and body weight in control and β-less mice fed a (−) leu diet for 7 d. In contrast to the 50% reduction of fat mass induced by leucine deprivation in wild-type mice, fat loss was completely blocked in β-less mice (Fig. 6A). The extent of leucine deprivation-induced reduction in body weight, however, was similar between wild-type and β-less mice (Fig. 6B). Food intake, however, decreased much more in β-less mice (∼40%) compared with wild-type mice maintained on a (−) leu diet (Fig. 6C).
Fig. 6.
Leucine deprivation-induced fat loss is blocked in β-less (KO) mice. Wild-type and β-less mice were fed a control (cont) or (−) leu diet for 7 d. The energy expenditure was measured by indirect calorimetry in these mice over 24–48 h after 6 h of acclimation to the metabolic chamber. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 6–10 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effects of either group vs. control diet-fed wild-type (WT) mice (*, P < 0.05), vs. (−) leu diet-fed wild-type mice (#, P < 0.05), or vs. control diet-fed β-less mice (&, P < 0.05). A, Adipose tissue mass in proportion to body weight. B, Body weight change. C, Daily food intake. D, Twenty-four-hour oxygen consumption. E, RER. F, Rectal temperature. G, Physical activity.
We previously have shown that the rapid fat loss by leucine deprivation is caused by an increase in energy expenditure (23). We therefore measured energy expenditure by indirect calorimetry, rectal temperature and physical activity in wild-type and β-less mice. As shown previously (23), the total energy expenditure (24-h O2 consumption, normalized to lean body mass) was markedly increased in wild-type mice by leucine deprivation. By contrast, not only basal energy expenditure levels were much lower, but also leucine deprivation-increased energy expenditure was blocked in β-less mice compared with wild-type mice (Fig. 6D). The respiratory exchange ratio (RER) (VCO2/VO2) was decreased by leucine deprivation in both wild-type and β-less mice during both dark and light phases. The basal levels of RER, however, were slightly lower in β-less mice during dark and light phase (Fig. 6E). Rectal temperatures measured at 1500 h (basal metabolic state) were significantly higher in leucine-deprived wild-type mice but not in β-less mice (Fig. 6F). We did not, however, see significant differences in rectal temperature between wild-type and β-less mice at other time point examined. We also did not see increased physical activity in either strain of mice, despite the slightly increased levels in β-less mice by leucine deprivation (Fig. 6G).
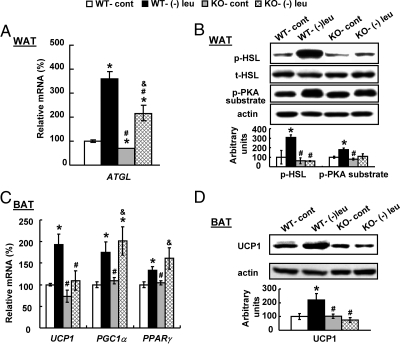
Leucine deprivation-induced changes in WAT and BAT are blocked in β-less mice
We further examined leucine deprivation-induced changes in WAT and BAT in wild-type and β-less mice. In contrast to the significantly increased levels of Atgl mRNA and p-HSL, p-PKA substrate in WAT of wild-type mice, these changes were either partially or completely blocked in β-less mice (Fig. 7, A and B). Consistent with previous report (23), UCP1 expression, as well as mRNA encoding its upstream regulators Pparγ and Pgc1α, was increased by leucine deprivation in BAT of wild-type mice (Fig. 7, C and D). By contrast, UCP1 expression was not changed in BAT of β-less mice. Levels of Pparγ and Pgc1α mRNA, however, were increased in these mice by leucine deprivation (Fig. 7, C and D).
Fig. 7.
Leucine deprivation-induced changes in WAT and BAT are blocked in β-less mice. Wild-type (WT) and β-less (KO) mice were fed a control (cont) or (−) leu diet for 7 d. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 5–6 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effects of either group vs. control diet-fed wild-type mice (*, P < 0.05), vs. (−) leu diet-fed wild-type mice (#, P < 0.05), or vs. control diet-fed β-less mice (&, P < 0.05). A, Atgl mRNA expression in WAT. B, p-HSL and p-PKA substrate protein in WAT (upper, Western blotting; lower, quantitative measurements of p-HSL and p-PKA substrate protein relative to total (t)-HSL and actin, respectively). C, Ucp1, Ppar, and Pgc1α mRNA expression in BAT. D, UCP1 protein in BAT.
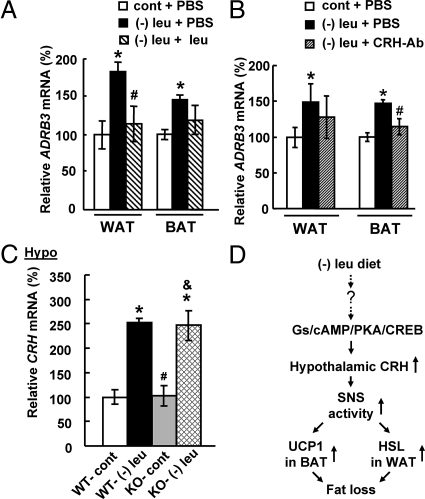
Intracerebroventricular leucine or CRH antibodies decreases SNS activity
To examine whether CNS leucine and CRH regulates fat loss in leucine-deprived mice via decreasing SNS activities, we examined changes of Adrb3 mRNA, as a measure for SNS activity (32), in WAT and BAT in mice after icv administration of leucine or CRH antibodies. As expected, leucine deprivation-increased levels of Adrb3 mRNA in WAT and BAT were decreased by icv administration of leucine (Fig. 8A). Similar observations were obtained in mice after icv administration of CRH antibodies (Fig. 8B). To further confirm the importance for SNS in mediating the effect of CRH, we examined levels of Crh expression in the hypothalamus in β-less mice. Levels of Crh expression in the hypothalamus, however, were also increased in β-less mice as in wild-type mice by leucine deprivation (Fig. 8C), despite the fact that fat loss was blocked in these mice (Fig. 6A).
Fig. 8.
Intracerebroventricular leucine or CRH antibodies decreases SNS activity. A and B, Mice received icv administration of leucine (1.1 μg of leucine in 1.0 μl of PBS), CRH antibodies (0.5 μg in 1 μl of PBS), or PBS once a day for 7 d under a control or (−) leu diet. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 8–11 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effect of (−) leu diet with icv PBS, leucine, or CRH antibodies vs. control diet with icv PBS (*, P < 0.01), or (−) leu diet with icv leucine or CRH antibodies vs. (−) leu diet with icv PBS (#, P < 0.05). C, Wild-type (WT) and β-less mice were fed a control or (−) leu diet for 7 d. Data are mean ± sem for at least two independent experiments with mice maintained on each diet for each experiment (n = 5–6 for each group). Statistical significance was determined by ANOVA followed by the SNK test for the effects of either group vs. control diet-fed WT mice (*, P < 0.01), vs. (−) leu diet-fed WT mice (#, P < 0.05), or vs. control diet-fed β-less mice (&, P < 0.05). A and B, Adrb3 mRNA expression in WAT. C, Crh mRNA expression in the hypothalamus. D, Working model.
Discussion
It has been shown that deficiency of essential amino acids, including leucine, is rapidly detected in the CNS (24, 33). As a result, mice develop an aversive response and stop eating food lacking leuicne or any other essential amino acids (23, 24, 33). Instead of rejecting food completely, mice eat 15% less compared with those fed a control diet during long-term leucine deficiency (22, 23). The reasons for the decreased food intake are still unclear. Our previous work show, however, that the decreased food intake makes only a minor contribution to leucine deprivation-induced fat loss, rather it is caused largely by increased energy expenditure (23). Therefore, the focus of our current study is to investigate the CNS regulation of energy expenditure during leucine deprivation.
Our hypothesis that CNS leucine plays a role in regulating fat loss under leucine deprivation was confirmed by the observation that icv administration of leucine significantly attenuates fat loss and body weight reduction in leucine-deprived mice. In contrast to the effect of icv administration of leucine, which decreased food intake and body weight, as shown in another study (25), we found that icv leucine did not have a similar effect on food intake in leucine-deprived mice. The source of this inconsistency is unclear but may reflect a difference in the times and dietary conditions under which food intake and body weights were measured. In our current study, we examined body weight 7 d after icv administration of leucine in leucine-deprived mice. By contrast, Cota et al. (25) examined the food intake and body weights after only 1-d icv administration of leucine after fasting for 24 h. We have not measured energy expenditure or the RER after icv leucine. It has previously been shown, however, that UCP1 expression in BAT is closely correlated with the status of energy expenditure, and many published studies have used UCP1 expression as an indicator for energy expenditure without carrying out oxygen consumption measurements (34–36). We therefore considered that the decrease in expression of UCP1 to be an accurate indicator of energy expenditure change after icv administration of leucine in leucine-deprived mice. Thus, our experiments suggest that decreased fat mass and body weight reduction after icv leucine are caused largely by a decrease in energy expenditure. In addition, our current study also demonstrates for the first time that CNS leucine regulates lipid metabolism in WAT and energy expenditure in BAT during leucine deprivation.
The hypothalamus is one of the major areas in the brain to regulate energy expenditure (4). Furthermore, CRH in the hypothalamus has been shown to be involved in the regulation of energy homeostasis (14–16, 20, 21). These observations motivated us to examine the possible involvement of CRH in mediating the effect of CNS leucine on fat loss in leucine-deprived mice. As described above, we showed that hypothalamic CRH expression is regulated by leucine availability, suggesting the possible involvement of CRH in mediating effects of leucine deprivation. The fact that fat loss is significantly decreased by blocking CRH function after icv administration of CRH antibodies strongly suggests that the hypothalamic CRH regulates fat loss under leucine deprivation. We observed that body weight and fat mass reduction were not completely prevented by icv leucine or CRH antibodies, suggesting the existence of additional signals from the CNS, cell-autonomous regulation in adipose tissue, or effects of hormones secreted by other tissues. One possible mediator is insulin, because insulin stimulates lipogenesis and inhibits lipolysis (37). Furthermore, levels of insulin decreased significantly after leucine deprivation (22), suggesting the possible involvement of insulin in the regulation of fat loss in these mice. Such possibilities will be investigated in future studies.
Previous studies have shown that icv CRH decreases food intake and increases energy expenditure (14–16). It has also been shown that CRH mediates the effects of leptin on food intake and energy expenditure (28). In our current study, we found that increased CRH expression regulates fat loss mainly by affecting energy expenditure in leucine-deprived mice. Our results are also consistent with a previous publication showing that energy expenditure is increased in mice after icv administration CRH (16). Consistent with this hypothesis, we found that blocking CRH function by icv administration of CRH antibodies inhibits levels of Atgl mRNA and p-HSL expression in WAT and UCP1 expression in BAT. The last result is also consistent with previous studies showing that CRH neurons in the PVN regulate UCP1 expression in BAT (16, 28). We did not see an effect of CRH antibodies on food intake. The reasons are unknown and will be studied in the future.
Consistent with others findings (9–12), we found that activation of Gs/cAMP/PKA/CREB pathway contributes to increased CRH expression in the hypothalamus during leucine deprivation as demonstrated by the fact that leucine deprivation-increased CRH expression is blocked by treatment with different inhibitors in this pathway in the primary cultured hypothalamic neurons. Furthermore, leucine deprivation increases phosphorylation of CREB and stimulates binding of CREB to CRE site at the promoter of CRH, during leucine deprivation. CRH expression, however, has also been shown to be negatively regulated by serum levels of glucocorticoids (13). Consistent with this possibility, we previously have shown that serum levels of glucocorticoid were decreased in leucine-deprived mice (23). Future studies will be required, however, to elucidate molecular mechanisms leading to increased cAMP levels and decreased serum glucocorticoid levels by leucine deprivation.
Lipolysis in WAT and UCP1 expression in BAT has previously been shown to be stimulated by activation of the SNS (38–41). The SNS-mediated physiological responses rely mostly on the release of NE from the sympathetic nerve endings, which then activates ADRB3 on the surfaces of cells in WAT and BAT. Activation of ADRB3 increases cAMP levels and PKA activity, which increases levels of genes encoding enzymes regulating the first-step lipolysis ATGL, and phosphorylated levels of HSL in WAT and UCP1 expression in BAT (39, 42). Our previous results (23) indicates that SNS is involved in the regulation of fat loss under leucine deprivation. Such a role is further confirmed in our current study, as demonstrated by the differential responses in wild-type and β-less mice maintained on a (−) leu diet. Leucine deprivation-increased expression levels of Atgl mRNA in WAT, however, are only partially blocked in β-less mice, suggesting the possible involvement of other receptors in regulation of lipolysis, as shown previously (43). Unexpectedly, food intake decreases by 40% in β-less mice under leucine deprivation. The reasons for such an effect are unclear. The significantly decreased food intake in β-less mice fed a (−) leu diet would normally be expected to cause a reduction in fat mass. Because fat use is blocked due to the deletion of β-receptors in these mice, however, fat loss did not occur. These results further indicate that activation of SNS is important for regulating lipolysis in WAT and UCP1 expression in BAT as demonstrated in other works (38–41).
In addition, we provide indirect evidence that SNS mediates effect of CNS leucine and CRH under leucine deprivation, because leucine deprivation-induced-increased Adrb3 expression in WAT and BAT is also decreased by icv leucine or CRH antibodies for 7 d. Furthermore, despite the fact that levels of Crh mRNA in the hypothalamus were increased, fat loss was blocked in β-less mice fed a (−) leu diet. In support of this, previous studies have shown that increased CRH expression stimulates activation of SNS (20, 21).
Our current data could not distinguish, however, whether leucine deprivation has a direct or indirect effect on UCP1 regulation in BAT. Because UCP1 expression is known to be regulated by the SNS (39), which is also activated by leucine deprivation, we speculate that leucine deprivation may increase UCP1 expression in this route. We could not, however, exclude the possibility that leucine deprivation increases UCP1 expression indirectly by increasing lipolysis in WAT, leading to increased serum free fatty acid (FFA). FFA in serum would be rapidly taken up by BAT, where they would stimulate expression of UCP1 to increase thermogenesis and energy expenditure as previously shown in other study (44). Consistent with this possibility, we found that serum levels of FFA and glycerol were decreased in leucine-deprived mice compared with pair-fed or control groups (23), suggesting that FFA released from WAT is going to other tissues, including BAT and muscle. Further investigation will be required to distinguish these models.
In summary, we show that CNS leucine plays a role in the regulation of fat loss under leucine deprivation. We also demonstrate for the first time that deficiency of leucine stimulates the expression of hypothalamic CRH via activation of Gs/cAMP/PKA/CREB pathway and provide evidence for a role for hypothalamic CRH in the regulation of fat loss under leucine deprivation. In our working model (Fig. 8D), leucine deprivation increases expression of CRH in the hypothalamus and activates the SNS. Activated SNS increases levels of Atgl and phosphorylation of HSL in WAT and expression of UCP1 in BAT, resulting in increases in lipolysis and energy expenditure, respectively. Further investigation will be required to elucidate mechanisms underlying the CNS leucine regulation of CRH expression in the hypothalamus. Another important issue that remains to be investigated is the specificity of our results for leucine deficiency as opposed to a general deficiency in essential amino acids. We plan to conduct a systematic analysis to examine the effects of dietary deprivation of other essential amino acids, and furthermore, if these changes are also under control of CNS.
Materials and Methods
Animals and diets
Wild-type (including C57BL/6J, Sprague Dawley rats and FVB) and three β-adrenergic receptors knockout (β-less) mice (45) were obtained from the Shanghai Laboratory Animals Co. Ltd. (Shanghai, China) and Brad Lowell (Harvard Medical School, Boston, MA), respectively. β-Less mice have been backcrossed to FVB for six generations, and FVB wild-type mice were included as controls. Eight- to 10-wk-old mice were maintained on a 12-h light, 12-h dark cycle at 25 C and were provided free access to commercial rodent chow and tap water before the experiments. Control (nutritionally complete amino acid) and (−) leu diets were obtained from Research Diets, Inc. (New Brunswick, NJ), and the diets compositions were provided in Supplemental Table 1. All diets were isocaloric and compositionally the same in terms of carbohydrate and lipid component. As previously described (22, 23), at the start of the feeding experiment, mice were acclimated to a control diet for 7–10 d and then randomly assigned to either control or (−) leu diet groups with free access to either diet for days as indicated. In addition, a pair-fed group was included to distinguish possible influences from a minor reduction in food intake previously observed in the (−) leu group (23). The pair-fed mice were provided with 15% less food compared with mice in the control diet group as previously described (23). Food intake and body weight were monitored at 1800 h everyday. At the end of the experiments, animals were killed by CO2 inhalation. WAT weight was recorded at the time of killing. WAT and BAT were isolated and stored at −80 C for future analysis. These experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
cAMP measurements
cAMP levels were determined using cAMP Direct Immunoassay kit from Biovision (Mountain View, CA), according to manufacturer's instructions.
Primary hypothalamic neurons isolation and treatments
Primary cultures of hypothalamic neurons were prepared from fetal Sprague Dawley rats, embryonic d 18, by collagenase dispersion and plated in 12-well plates as previously described (46). Cultures were maintained for eight additional days in B27 supplement (Life Technologies, Inc., Invitrogen, Carlsbad, CA) containing neurobasal medium (Invitrogen); 5 μm cytosine arabinoside (Sigma, St. Louis, MO) was added from d 4 to prevent glial proliferation. On d 10, cells were incubated in control (complete amino acid) or (−) leu medium prepared from amino acid-free DMEM (Invitrogen) by adding back all the amino acids contained in regular DMEM and without leucine only for 3 h; 10 μm H89 (Sigma), SQ22536 (Sigma), or NF449 (Tocris) was added when changed to incubation with (−) leu medium.
RNA isolation and relative quantitative RT-PCR
Total RNA was prepared from frozen tissues with TRIzol (Invitrogen) reagent following manufacturer's instruction as previously described (23). Quantitative amplification by PCR was carried out using SYBR Green I Master Mix reagent by ABI 7900 system (Applied Biosystems, Foster City, CA). PCR products were subjected to a melting curve analysis. Cycle numbers of both actin (as an internal control) and cDNA of interest at a specific threshold within the exponential amplification range were used to calculate relative expression levels of the genes of interest. The sequences of primers used in this study are available upon request.
Western blot analysis
Western blot analysis was performed as previously described (22, 23). Primary antibodies anti-p-HSL (1:1000, no. 4126; Cell Signaling Technology), anti-total-HSL (1:1000, no. 4107; Cell Signaling Technology), anti-p-PKA substrate antibodies (1:1000, no. 9621; Cell Signaling Technology), anti-UCP1 antibody (1:200, sc-6529; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-p-CREB antibodies (1:1000, no. 9198; Cell Signaling Technology), anti-total-CREB antibodies (1:1000, no. 9197; Cell Signaling Technology), and antiactin antibody (1:5000, A5316; Sigma) were incubated overnight at 4 C, and specific proteins were visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). Band intensities were measured using Quantity One (Bio-Rad Laboratories, Hercules, CA) and normalized to either relevant total protein or actin.
Immunofluorescence staining
Immunofluorescence staining was performed as described previously (47). Brain sections were incubated with anti-CRH (sc-1761; Santa Cruz Biotechnology, Inc.) overnight, followed by incubation with Alexa Fluor 555 conjugated antigoat antibody (Invitrogen) at room temperature for 1 h. Sections were visualized and pictures were taken by fluorescence microscope (BX61; Olympus, Tokyo, Japan) and Zeiss LSM 510 confocal microscope (Carl Zeiss Imaging, Oberkochen, Germany).
ChIP assay
ChIP assays were performed according to manufacturer's protocol (Millipore, Bedford, MA). Briefly, the DNA-protein complexes in the hypothalamus were cross-linked with 1% formaldehyde. Hypothalamus was lysed and sonicated before immunoprecipitation with anti-CREB antibody (1:50, no. 9197; Cell Signaling Technology) or normal rabbit IgG (1:50, no. C2210; Santa Cruz Biotechnology, Inc.) for negative control at 4 C overnight. DNA-protein immunocomplexes were collected using protein G magnetic beads. Immunoprecipitated CRH promoter was quantified using PCR with primers designed to amplify the region encompassing the 112 bp containing the CRE site (forward, 5′-TCAGTCTGTTTTCCACACTTGGAT-3′ and reverse, 5′-TTTATCGCCTCCTTGGTGAC-3′) or an upstream region encompassing 112 bp between −1920 and −1809 that is not involved in CREB response (forward, 5′-GTAAATTATGGTACTTC-3′ and reverse, 5′-GGCCCTCAAGATCCCTC-3′).
Intracerebroventricular administration experiments
Intracerebroventricular administration experiments were conducted as previously described (25, 28, 29). Briefly, mice were anesthetized by ip injection of ketamine-xylazine. A sterile stainless steel cannula was stereotaxically implanted into the right lateral brain ventricle (−0.5 mm anterior and 1.0 mm lateral relative to bregma and 2.5 mm below the surface of the skull). Normally, mice were allowed to recover for 7 d after cannulation surgery. At 1800 h in d 7, food was changed, and mice were infused with 1.0 μl of leucine (1.1 μg in 1.0 μl of PBS), anti-CRH antibodies (0.5 μg in 1 μl of PBS, C5348; Sigma), or PBS as a control (25) once daily for 7 d. In our current study, we found that one injection of leucine at this concentration is enough to maintain leucine levels in the hypothalamus to the levels as detected in mice maintained on a control diet, for up to 20 h (see Supplemental Data) but has no effect on plasma leucine levels (data not shown). Food intake and body weight were monitored at 1800 h everyday. At the end of experiments, mice were killed, and tissues were isolated for future analysis.
Statistical analysis
All values are presented as mean ± sem. Differences between groups were analyzed by two-tailed Student's t test or ANOVA followed by either two-tailed Student's t test or the Student-Newman-Keuls (SNK) test. Differences in which P < 0.05 were considered statistically significant.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (973 Program 2009CB919001 and 2010CB912502), National Natural Science Foundation (30871208 and 30890043), the Chief Scientist Program of Shanghai Institutes for Biological Sciences, the Chinese Academy of Sciences (SIBS2008006), and Key Program of Shanghai Scientific and Technological Innovation Action Plan (10JC1416900). Feifan Guo was also supported by the One Hundred Talents Program of the Chinese Academy of Sciences.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Adrb3
- β-3 Adrenergic receptor
- Atgl
- adipose triglyceride lipase
- BAT
- brown adipose tissue
- ChIP
- chromatin immunoprecipitation
- CNS
- central nervous system
- CRE
- cAMP response element
- CREB
- CRE-binding protein
- CRH
- corticotropin-releasing hormone
- FFA
- free fatty acid
- Gs
- stimulatory G protein
- HSL
- hormone sensitive lipase
- icv
- intracerebroventricular
- (−) leu
- leucine deficient
- NE
- norepinephrine
- p
- phosphorylated
- Pgc1
- PPARγ coactivator 1
- PKA
- protein kinase A
- Ppar
- peroxisome proliferator-activated receptor
- PVN
- paraventricular nucleus of the hypothalamus
- RER
- respiratory exchange ratio
- SNK
- Student-Newman-Keuls
- SNS
- sympathetic nervous system
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue.
References
- 1. Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. 1998. Signals that regulate food intake and energy homeostasis. Science 280:1378–1383 [DOI] [PubMed] [Google Scholar]
- 2. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- 3. Woods SC, Seeley RJ, Cota D. 2008. Regulation of food intake through hypothalamic signaling networks involving mTOR. Annu Rev Nutr 28:295–311 [DOI] [PubMed] [Google Scholar]
- 4. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- 5. Cao WH, Fan W, Morrison SF. 2004. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126:229–240 [DOI] [PubMed] [Google Scholar]
- 6. Morrison SF, Nakamura K, Madden CJ. 2008. Central control of thermogenesis in mammals. Exp Physiol 93:773–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359 [DOI] [PubMed] [Google Scholar]
- 8. Richard D, Rivest R, Naïmi N, Timofeeva E, Rivest S. 1996. Expression of corticotropin-releasing factor and its receptors in the brain of lean and obese Zucker rats. Endocrinology 137:4786–4795 [DOI] [PubMed] [Google Scholar]
- 9. Nikodemova M, Kasckow J, Liu H, Manganiello V, Aguilera G. 2003. Cyclic adenosine 3′,5′-monophosphate regulation of corticotropin-releasing hormone promoter activity in AtT-20 cells and in a transformed hypothalamic cell line. Endocrinology 144:1292–1300 [DOI] [PubMed] [Google Scholar]
- 10. Seasholtz AF, Thompson RC, Douglass JO. 1988. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol 2:1311–1319 [DOI] [PubMed] [Google Scholar]
- 11. Wölfl S, Martinez C, Majzoub JA. 1999. Inducible binding of cyclic adenosine 3′,5′-monophosphate (cAMP)-responsive element binding protein (CREB) to a cAMP-responsive promoter in vivo. Mol Endocrinol 13:659–669 [DOI] [PubMed] [Google Scholar]
- 12. Siegel GJ, Albers RW, Brady ST, Price DL. 2006. Basic neurochemistry: molecular, cellular, and medical aspects. 7th ed. Amsterdam: Elsevier Academic Press [Google Scholar]
- 13. Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB. 2007. Glucocorticoid receptor physiology. Rev Endocr Metab Disord 8:321–330 [DOI] [PubMed] [Google Scholar]
- 14. Buwalda B, de Boer SF, Van Kalkeren AA, Koolhaas JM. 1997. Physiological and behavioral effects of chronic intracerebroventricular infusion of corticotropin-releasing factor in the rat. Psychoneuroendocrinology 22:297–309 [DOI] [PubMed] [Google Scholar]
- 15. Momose K, Inui A, Asakawa A, Ueno N, Nakajima M, Fujimiya M, Kasuga M. 1999. Intracerebroventricularly administered corticotropin-releasing factor inhibits food intake and produces anxiety-like behaviour at very low doses in mice. Diabetes Obes Metab 1:281–284 [DOI] [PubMed] [Google Scholar]
- 16. LeFeuvre RA, Rothwell NJ, Stock MJ. 1987. Activation of brown fat thermogenesis in response to central injection of corticotropin releasing hormone in the rat. Neuropharmacology 26:1217–1221 [DOI] [PubMed] [Google Scholar]
- 17. Cerri M, Morrison SF. 2006. Corticotropin releasing factor increases in brown adipose tissue thermogenesis and heart rate through dorsomedial hypothalamus and medullary raphe pallidus. Neuroscience 140:711–721 [DOI] [PubMed] [Google Scholar]
- 18. Toriya M, Maekawa F, Maejima Y, Onaka T, Fujiwara K, Nakagawa T, Nakata M, Yada T. 2010. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotropin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 22:987–995 [DOI] [PubMed] [Google Scholar]
- 19. Brown MR, Fisher LA. 1985. Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc 44:243–248 [PubMed] [Google Scholar]
- 20. Egawa M, Yoshimatsu H, Bray GA. 1990. Effect of corticotropin releasing hormone and neuropeptide Y on electrophysiological activity of sympathetic nerves to interscapular brown adipose tissue. Neuroscience 34:771–775 [DOI] [PubMed] [Google Scholar]
- 21. Brown MR, Fisher LA, Spiess J, Rivier C, Rivier J, Vale W. 1982. Corticotropin-releasing factor: actions on the sympathetic nervous system and metabolism. Endocrinology 111:928–931 [DOI] [PubMed] [Google Scholar]
- 22. Guo F, Cavener DR. 2007. The GCN2 eIF2α kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 5:103–114 [DOI] [PubMed] [Google Scholar]
- 23. Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, Xiao F, Guo F. 2010. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 59:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW. 2005. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307:1776–1778 [DOI] [PubMed] [Google Scholar]
- 25. Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. 2006. Hypothalamic mTOR signaling regulates food intake. Science 312:927–930 [DOI] [PubMed] [Google Scholar]
- 26. Handschin C, Spiegelman BM. 2006. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27:728–735 [DOI] [PubMed] [Google Scholar]
- 27. Lowell BB, Spiegelman BM. 2000. Towards a molecular understanding of adaptive thermogenesis. Nature 404:652–660 [DOI] [PubMed] [Google Scholar]
- 28. Masaki T, Yoshimichi G, Chiba S, Yasuda T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H. 2003. Corticotropin-releasing hormone-mediated pathway of leptin to regulate feeding, adiposity, and uncoupling protein expression in mice. Endocrinology 144:3547–3554 [DOI] [PubMed] [Google Scholar]
- 29. Okamoto S, Kimura K, Saito M. 2001. Anorectic effect of leptin is mediated by hypothalamic corticotropin-releasing hormone, but not by urocortin, in rats. Neurosci Lett 307:179–182 [DOI] [PubMed] [Google Scholar]
- 30. Braas KM, May V. 1999. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem 274:27702–27710 [DOI] [PubMed] [Google Scholar]
- 31. Zhang M, Fei XW, He YL, Yang G, Mei YA. 2009. Bradykinin inhibits the transient outward K+ current in mouse Schwann cells via the cAMP/PKA pathway. Am J Physiol 296:C1364–C1372 [DOI] [PubMed] [Google Scholar]
- 32. Kawahara Y, Grimberg A, Teegarden S, Mombereau C, Liu S, Bale TL, Blendy JA, Nishikura K. 2008. Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J Neurosci 28:12834–12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leung PM, Rogers QR. 1971. Importance of prepyriform cortex in food-intake response of rats to amino acids. Am J Physiol 221:929–935 [DOI] [PubMed] [Google Scholar]
- 34. López M, Varela L, Vázquez MJ, Rodríguez-Cuenca S, González CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martínez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Diéguez C, Vidal-Puig A. 2010. Vidal-Puig A Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 16:1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen W, Zhang X, Birsoy K, Roeder RG. 2010. A muscle-specific knockout implicates nuclear receptor coactivator MED1 in the regulation of glucose and energy metabolism. Proc Natl Acad Sci USA 107:10196–10201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferron M, Hinoi E, Karsenty G, Ducy P. 2008. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105:5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thomas SH, Wisher MH, Brandenburg D, Sönksen PH. 1979. Insulin action on adipocytes. Evidence that the anti-lipolytic and lipogenic effects of insulin are mediated by the same receptor. Biochem J 184:355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collins S, Surwit RS. 2001. The β-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res 56:309–328 [DOI] [PubMed] [Google Scholar]
- 39. Lowell BB, Bachman ES. 2003. β-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem 278:29385–29388 [DOI] [PubMed] [Google Scholar]
- 40. Bianco AC, Sheng XY, Silva JE. 1988. Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J Biol Chem 263:18168–18175 [PubMed] [Google Scholar]
- 41. Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. 1998. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol 275:R291–R299 [DOI] [PubMed] [Google Scholar]
- 42. Thomas SA, Palmiter RD. 1997. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature 387:94–97 [DOI] [PubMed] [Google Scholar]
- 43. Tavernier G, Jimenez M, Giacobino JP, Hulo N, Lafontan M, Muzzin P, Langin D. 2005. Norepinephrine induces lipolysis in β1/β2/β3-adrenoceptor knockout mice. Mol Pharmacol 68:793–799 [DOI] [PubMed] [Google Scholar]
- 44. Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. 2000. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty acid-induced thermogenesis. J Biol Chem 275:25073–25081 [DOI] [PubMed] [Google Scholar]
- 45. Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. 2002. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297:843–845 [DOI] [PubMed] [Google Scholar]
- 46. Liu Y, Kamitakahara A, Kim AJ, Aguilera G. 2008. Cyclic adenosine 3′,5′-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology 149:3512–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mori H, Inoki K, Münzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers MG, Jr, Guan KL. 2009. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab 9:362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]