Abstract
Homeobox A10 (HOXA10), a transcription factor required for uterine development and embryo receptivity, functions downstream of estrogen and progesterone in uterine endometrium. HOXA10 represses endometrial expression of empty spiracles homeobox 2 (EMX2), the human ortholog of Drosophila empty spiracles. The ATPases associated with various cellular activities (AAA) ATPase spastin has a well-characterized role in neurotransmitter trafficking. In this study, we characterize a novel role of spastin in transcriptional regulation. We identified spastin as a novel component of the HOXA10 transcriptional complex in Ishikawa nuclear extracts by immunoprecipitation and mass spectrophotometry. Using EMX2 as a model endometrial HOXA10 target gene, we show that the HOXA10-spastin corepressor complex bound the EMX2 promoter in chromatin immunoprecipitation assays. HOXA10 has been previously shown to repress endometrial EMX2 expression. We further observed that, although cotransfection of HOXA10 and spastin continued to repress endometrial EMX2-luciferase expression, the repression was reversed when spastin small interfering RNA was cotransfected with HOXA10. Mutations in the nuclear localization signal sequences of spastin abrogated not only its nuclear translocation but also its colocalization with HOXA10 as well as reversed EMX2-luciferase repression. Here, we describe a novel role for the AAA ATPase spastin in Ishikawa cells as a HOXA10 corepressor of EMX2. Uterine EMX2 levels are inversely related to embryo implantation rates. HOXA10 acts downstream of progesterone and has been shown to facilitate embryo implantation through regulation of endometrial EMX2 expression. Endometrial spastin, therefore, likely has a novel function downstream of estrogen and progesterone in implantation biology as a cofactor of HOXA10.
The homeobox genes, first described in Drosophila melanogaster, are transcription factors that provide developmental identity to various body segments (1–4). There are 39 human/vertebrate orthologs of the eight Drosophila homeobox genes. In the mouse and human, they occur in four separate clusters A, B, C, and D, each of which is located on a separate chromosome (1, 5–7). Genes at the 3′ end of each cluster are expressed earlier during embryogenesis and in the more anterior body segments compared with genes at the 5′ end, which are expressed later and in posterior body segments (8). We have previously shown that the vertebrate Abd-B orthologs homeobox A10 (Hoxa10)/HOXA10 and Hoxa11/HOXA11 are necessary for uterine development (9). Interestingly, both Hoxa10/HOXA10 and Hoxa11/HOXA11 are also expressed in adult uterine endometrium, where their expression is driven directly by the sex steroids estrogen and progesterone via their respective receptors (10, 11). Endometrial HOXA10 expression demonstrates a cyclic expression pattern in the endometrium, with high levels in the progesterone dominant secretory phase, coinciding with the time of embryo implantation (11, 12). Targeted mutation of Hoxa10 results in failure of embryo implantation (13, 14). HOXA10 and HOXA11 are necessary not only for embryonic uterine development but additionally also in the adult, where they mediate sex steroid-controlled endometrial functional development and maturation that is imperative for embryo implantation.
We have previously identified EMX2 as a uterine target of HOXA10 transcriptional regulation (15, 16). EMX2/Emx2, the vertebrate ortholog of Drosophila empty spiracles (ems) is a divergent homeobox gene that is necessary for urogenital tract development. Targeted Emx2 mutations result in embryonic lethality from agenesis of urogenital organs (17–19). We have previously demonstrated in Drosophila that abdominal-B (Abd-B) activates ems expression, whereas in human uterine epithelial cells, the corresponding Abd-B ortholog, HOXA10, represses EMX2 expression (16, 20). These findings demonstrate that although there is evolutionary conservation of the transcription factor-target gene relationship, the direction of regulation is reversed. Differential coactivator/corepressor recruitment by a transcriptional factor could reverse the direction regulation of the target gene. Therefore, we initially investigated the role of two well-characterized HOX gene cofactors pre-B-cell leukemia homeobox and Meis homeobox but were unable to demonstrate a role for these coregulators in HOXA10-mediated empty spiracles homeobox (EMX2) repression (21–26). Our findings therefore could not explain why, despite the evolutionary conservation of a specific transcription factor (Abd-b/HOXA10)-target gene (ems/EMX2) relationship between Drosophila and humans, the direction of regulation was reversed. We therefore investigated for the possibility of a novel cofactor that could participate in and thereby alter the dynamics of this conserved interaction. In this study, we identify and characterize a novel role for the AAA ATPase spastin, as a transcriptional corepressor; specifically in conjunction with HOXA10 in the regulation of uterine EMX2 expression.
Spastin is a 67.2-kDa protein derived from the SPG4 locus (27). Spastin belongs to the ATPases associated with various cellular activities (AAA) ATPase family. Like other ATPase family members, spastin contains a conserved C-terminal AAA cassette. ATPase activity resides in conserved motifs that comprise this cassette. In addition, spastin also contains a N-terminal microtubule-interacting domain enabling interaction with cytoskeletal microtubules. Mammalian spastin also contains unique nuclear localization sequences that may enable nuclear-cytoplasmic trafficking and a potential role in the nucleus (27). Spastin C-terminal mutations are associated with the autosomal dominant form of hereditary spastic paraplegia, a disease characterized by progressive weakness and spasticity of the legs due to degeneration of the corticospinal tracts (27–29). Here, we further characterize the N terminus and nuclear translocation of spastin as well as describe a novel role for nuclear spastin as a transcriptional corepressor of the homeobox gene HOXA10 in Ishikawa cells, a model cell line for endometrial epithelium. Spastin has not previously been implicated in transcriptional regulation.
Results
Spastin is a member of the HOXA10 transcription complex
To determine novel cofactors of HOXA10 in uterine epithelial transcriptional regulation, Ishikawa cells were transfected with a pcDNA3.1HOXA10/FLAG construct. Ishikawa, a well-differentiated uterine epithelial adenocarcinoma cell line has been well characterized as a model of uterine epithelium (30–32). Ishikawa cells express HOXA10 and maintain sex steroid responsiveness via expression of estrogen and progesterone receptors (11, 33). Nuclear extracts were obtained 48 h after transfection. The HOXA10 transcription complex was isolated by immunoprecipitation with anti-FLAG. Spastin was identified as a member of the HOXA10 transcription complex by matrix-assisted laser desorption ionization (MALDI)-mass spectrophotometry (MS) and sequencing.
Spastin is expressed in the adult human reproductive tract
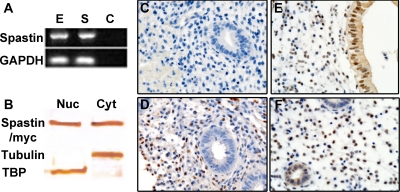
To confirm and localize spastin expression in human endometrial cells, we performed RT-PCR to determine mRNA expression in Ishikawa and human endometrial stromal cells (HESC) (Fig. 1A). We also performed Western blot analysis and immunohistochemistry to determine spastin protein expression in these cells as well as in uterine endometrial tissue (Fig. 1, B–F).
Fig. 1.
Spastin is expressed in the adult human reproductive tract. A, Total RNA was obtained from Ishikawa cells as well as primary HESC and expression of spastin was evaluated by RT-PCR and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Spastin was expressed in both Ishikawa endometrial epithelial cells (E) as well as HESC (S). Lane C represents the negative control (water). B, Spastin protein expression was evaluated in Ishikawa endometrial epithelial cells transfected with pMT21/spastin-myc by Western blot analysis. For Western blot analysis, nuclear as well as cytoplasmic extracts were derived from Ishikawa cells and evaluated separately using antispastin. Spastin (65 kDa) was expressed in both Ishikawa nuclear (Nuc) as well as cytoplasmic extracts (Cyt). Anti-TBP and anti-α-tubulin were used as nuclear and cytoplasmic-compartment-specific markers. C–F, Endogenous uterine spastin expression was evaluated in intact human endometrium using antispastin, by immunohistochemistry. Spastin expression was evaluated on d 12 (D), 17 (E), and 24 (F) of the human menstrual cycle. C, Negative control using mouse IgG. D–F, Spastin was expressed in both the nucleus and cytoplasm of endometrial epithelial cells as well as stromal cells (D–F). Stromal spastin remained unchanged from the proliferative phase of the menstrual cycle (D) to the secretory phase (E and F). In contrast, epithelial spastin expression was observed only in the progesterone driven secretory phase of the menstrual cycle (E and F). All magnifications, ×100 (C–F).
Total RNA was obtained from Ishikawa cells and HESC. Results of RT-PCR are shown in Fig. 1A. Both Ishikawa cells and HESC demonstrated spastin expression. To determine spastin protein expression in Ishikawa endometrial epithelial cells, we performed Western blot analysis using antispastin on nuclear as well as cytoplasmic extracts derived from these cells (Fig. 1B). Anti-TATA-binding protein (TBP) and anti-α-tubulin were used as nuclear and cytoplasm compartment-specific markers. Spastin was expressed in both cytological fractions. Although cytoplasmic spastin associates with microtubules and has a well-characterized role in neurons, there may be an additional role for nuclear spastin that we observed in uterine epithelial cells.
Uterine spastin expression was evaluated in intact human endometrium by immunohistochemistry (Fig. 1, C–F). Spastin expression was evaluated on d 12, 17, and 24 of the human menstrual cycle to determine whether there was a change in the expression pattern of spastin from the estrogen-regulated first half to the progesterone-mediated second half of the cycle. Representative photomicrographs are shown in Fig. 1, C–F. Immunohistochemistry to detect endogenous spastin was performed using antispastin (Fig. 1, C—F). Spastin was expressed in both endometrial epithelial cells and stromal cells throughout the menstrual cycle (Fig. 1, D–F). Spastin was expressed in both the nucleus and cytoplasm of endometrial epithelial (Fig. 1, E and F) as well as stromal cells (Fig. 1, D–F). Although stromal spastin remained unchanged from the estrogen-regulated first half of the menstrual cycle (Fig. 1D) to the progesterone-regulated second half (Fig. 1, E and F), epithelial spastin expression was only observed in the progesterone-regulated second half of the cycle (Fig. 1, E and F).
Spastin is expressed in endometrial epithelial cell nuclei
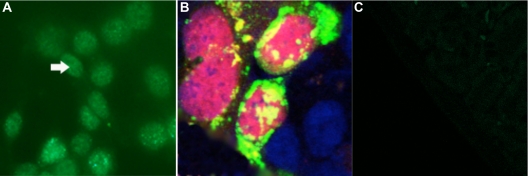
Although Drosophila spastin is exclusively expressed in the cytoplasm, in mammalian neurons, spastin expression has been previously observed in both cytoplasmic and perinuclear regions (29, 34). Mammalian spastin has several candidate nuclear localization signal (NLS) sequences, and some of these exhibit nuclear translocation in experiments using artificial spastin-NLS-green fluorescent protein (GFP) reporter constructs (35). We used antispastin to detect endogenous spastin in Ishikawa cells by immunofluorescence with a GFP-tagged secondary antibody (Fig. 2A).
Fig. 2.
Spastin is expressed in endometrial epithelial cell nuclei. Spastin expression was expressed in the cytoplasm, perinuclear region, and in nuclei of Ishikawa endometrial epithelial cells. A, Endogenous spastin was expressed predominantly in Ishikawa cell nuclei. Antispastin and a GFP-tagged antimouse secondary antibody were used to detect endogenous spastin expression in Ishikawa cells. B and C, Nuclear spastin colocalized with HOXA10 in Ishikawa cells. For colocalization experiments, Ishikawa cells were transfected with pcDNA3.1/FLAGHOXA10 and pMT21/spastin-myc. Anti-FLAG and anti-myc antibodies were used to detect FLAG-HOXA10 and spastin-myc expression, respectively. Colocalization was determined by immunofluorescence using RFP- and GFP-tagged secondary antibodies to detect HOXA10 and spastin expression, respectively. Additionally DAPI staining was used to detect DNA. HOXA10 expression was confined to the nuclei, whereas spastin expression was observed in cytoplasmic, perinuclear, and nuclear domains in Ishikawa cells (B). Only nuclear spastin was found to colocalize with FLAG-HOXA10. C, Negative control: tagged secondary antibodies were used in the absence of primary antibodies. All magnifications, ×40. Arrow points to nucleus.
To detect colocalization of spastin and HOXA10 in Ishikawa cells, we transfected cells with pcDNA3.1/FLAGHOXA10 and pMT21/spastin-myc. Anti-FLAG and anti-myc antibodies were used to detect FLAG-HOXA10 and spastin-myc expression, respectively (Fig. 2, B and C). Colocalization was subsequently determined by immunofluorescence using red fluorescent protein (RFP)- and GFP-tagged secondary antibodies to detect FLAG-HOXA10 and spastin expression, respectively. HOXA10 expression was confined to the nuclei, whereas spastin expression was observed in cytoplasmic, perinuclear, and nuclear domains in endometrial epithelial cells. Only nuclear spastin was found to colocalize with FLAG-HOXA10 (Fig. 2B).
Spastin and HOXA10 bind together in a complex at the EMX2 5′ regulatory region
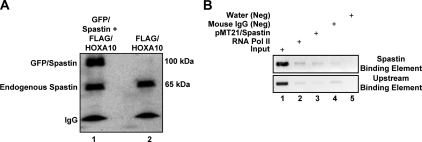
To confirm that spastin is a binding partner of HOXA10, we performed coimmunoprecipitation and chromatin immunoprecipitation (ChIP). To detect the spastin-HOXA10 interaction by coimmunoprecipitation, Ishikawa cells were transfected with either pEGFP-spastin and pcDNA3.1/HOXA10-FLAG or pcDNA3.1/HOXA10-FLAG only. Controls were transfected with pEGFP-spastin only. Protein extracts were obtained 48 h later. Anti-Flag M1-coated beads were used for immunoprecipitation and antispastin antibody for Western blot analysis. Results of coimmunoprecipitation are shown in Fig. 3A. In cells transfected with both pEGFP-spastin and pcDNA3.1/HOXA10-FLAG, bands corresponding to the molecular masses of both GFP-spastin (95 kDa) and endogenous spastin (65 kDa) were observed on Western blot analysis. Although this data suggests that HOXA10 and spastin bind each other in a complex, the possibility of an intermediary peptide mediating the interaction between HOXA10 and spastin cannot be ruled out. In cells transfected with pcDNA3.1/HOXA10-FLAG alone, a band corresponding to endogenous spastin only was observed. Results of coimmunoprecipitation therefore demonstrated that both GFP-spastin as well as endogenous spastin bound in a complex with HOXA10 in Ishikawa cells.
Fig. 3.
Spastin and HOXA10 directly bind together in a complex at the EMX2 5′ regulatory region. A, Endogenous spastin as well as transfected GFP-spastin bound HOXA10 in Ishikawa endometrial epithelial cells. Spastin-HOXA10 interaction was evaluated by coimmunoprecipitation in Ishikawa cells transfected for 48 h with either pEGFP-spastin and pcDNA3.1/HOXA10-FLAG or pcDNA3.1/HOXA10-FLAG only (endogenous spastin). Anti-Flag M1 antibody was used for immunoprecipitation and antispastin antibody for Western blot analysis. In cells transfected with pEGFP-spastin and pcDNA3.1/HOXA10-FLAG, discrete bands corresponding to the molecular weights of both GFP-spastin (95 kDa) and endogenous spastin (65 kDa) were observed on Western blot analysis (lane 1). In cells transfected with pcDNA3.1/HOXA10-FLAG alone, a band corresponding endogenous spastin only was observed (lane 2). B, Spastin directly bound the EMX2 promoter in the vicinity of the HOXA10-binding site. To detect the interaction of spastin with the EMX2 promoter, ChIP was performed on Ishikawa cells transfected with pMT21/spastin-myc for 48 h. Immunoprecipitation was performed using antispastin followed by PCR to detect amplification of a previously characterized EMX2 promoter element that has been shown to bind HOXA10. Spastin specifically bound the EMX2 promoter element (lane 3, upper panel) but not to an adjacent upstream EMX2 promoter element (lane 3, lower panel). Also shown in B are input, positive control (anti-RNA polymerase II), negative control for antispastin (mouse IgG), and negative control for PCR (no DNA template) (lanes 1, 2, 4, and 5, respectively).
To determine whether nuclear spastin also directly bound the EMX2 promoter in the vicinity of the HOXA10-binding site, we performed ChIP in Ishikawa cells transfected with pMT21/spastin-myc using antispastin. Spastin binding to the previously characterized HOXA10-binding EMX2 promoter element (Fig. 3B, upper panel, lane 3), and not to another promoter element located 150 bp upstream (Fig. 3B, lower panel, lane 3), was detected and confirmed by PCR (Fig. 3B). Also shown in Fig. 3B are input, positive control (anti-RNA polymerase II), negative control for antispastin (mouse IgG), and negative control for PCR (no DNA template) (Fig. 3B, lanes 1, 2, 4, and 5, respectively).
Spastin alters endometrial HOXA10-mediated EMX2 repression
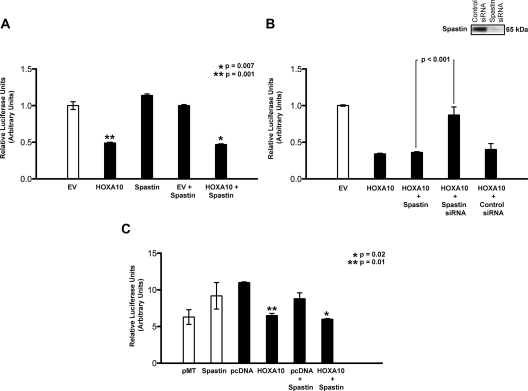
To determine whether spastin influenced endometrial HOXA10 transcriptional regulation, we interrogated a previously well-characterized EMX2 5′ regulatory element-luciferase reporter in Ishikawa cells (Fig. 4A). The EMX2-luciferase reporter construct was cotransfected with either a HOXA10 expression vector (pcDNA3.1/HOXA10), spastin expression vector (pMT21/spastin), or both. Additionally, the cells were transfected with Renilla Luciferase vector as a control for transfection efficiency. Luciferase and Renilla activity were measured in the cellular lysate. Luciferase activity was normalized to Renilla activity. Cotransfection of pcDNA3.1/HOXA10 with pGL3/EMX2-luciferase resulted in a significant more than 60% reduction (**, P = 0.001) in luciferase activity compared with cotransfection of pGL3/EMX2-luciferase with the parent vector pcDNA3.1 alone, in keeping with previous observations (Fig. 4A). Cotransfection of pGL3/EMX2-luciferase with pMT21/spastin alone did not alter luciferase expression levels compared with expression levels observed on cotransfection with the corresponding parent vector pMT21. EMX2 Luciferase levels were similarly repressed (*, P = 0.007) when Ishikawa cells were cotransfected with pMT21/spastin and pcDNA3.1/HOXA10 compared with pMT21/ spastin and the empty vector pCDNA3.1. Repression of EMX2-luciferase with HOXA10 and spastin was comparable with that observed with HOXA10 alone. Overexpression of spastin therefore did not affect EMX2- luciferase expression levels either alone or in combination with HOXA10.
Fig. 4.
Spastin altered endometrial HOXA10-mediated EMX2 repression. A, Overexpression of spastin did not affect EMX2-luciferase expression levels either alone or in combination with HOXA10. Ishikawa cells were cotransfected for 48 h with an EMX2-luciferase reporter construct and either a HOXA10 expression vector (pcDNA3.1/HOXA10), spastin expression vector (pMT21/spastin-myc), or both. The cells were additionally also transfected with a Renilla Luciferase vector as a control for transfection efficiency. Each experiment was done in triplicate and repeated three times. Luciferase and Renilla activity were measured in the cellular lysate. Luciferase activity normalized to Renilla activity shows that compared with empty vector (EV), HOXA10 decreased luciferase expression by more than 60% (**, P = 0.001), in keeping with previous observations. In contrast, cotransfection of pGL3/EMX2-luciferase with pMT21/spastin alone did not alter luciferase expression levels compared with the corresponding parent vector pMT21. EMX2-luciferase levels were also repressed (*, P = 0.007) when Ishikawa cells were cotransfected with both pMT21/spastin and pcDNA3.1/HOXA10 compared with pMT21/spastin and empty vector pcDNA3.1; the repression was comparable with that observed with pcDNA3.1/HOXA10 alone. B, Repression of endometrial spastin, reversed repression of EMX2-Luciferase reporter expression. Ishikawa cells were cotransfected with EMX2-Luciferase reporter and either pCDNA3.1/HOXA10 or pMT21/spastin-myc or were treated with spastin siRNA. The cells were additionally also transfected with a Renilla Luciferase vector as a control for transfection efficiency. Each experiment was done in triplicate and repeated three times. Luciferase and Renilla activity were measured in the cellular lysate. Luciferase activity normalized to Renilla activity showed that cotransfection of either HOXA10 alone or spastin and HOXA10 resulted in diminished EMX2-luciferase expression. However, in the presence of spastin siRNA, EMX2 luciferase expression was significantly increased (P < 0.001), and transcriptional repression was reversed. Transfection of Ishikawa cells with spastin siRNA resulted in a 80-fold reduction in spastin mRNA (real-time RT-PCR) levels. Spastin protein levels were also diminished in Ishikawa cells transfected with spastin siRNA compared with scrambled siRNA (inset). C, Spastin repressed EMX2 only in the presence of HOXA10. BT-20, a breast adenocarcinoma cell line that does not endogenously express HOXA10, was cotransfected with either pMT21 (empty vector) or pMT21/spastin-myc (white bars) and EMX2-luciferase. The cells were additionally also transfected with a Renilla Luciferase vector as a control for transfection efficiency. Each experiment was done in triplicate and repeated three times. Luciferase and Renilla activity were measured in the cellular lysate. In the absence of HOXA10, spastin failed to repress EMX2-luciferase expression compared with corresponding empty vector (white bars) (P = 0.07). As characterized previously, EMX2-luciferase levels were repressed when BT-20 cells were transfected with pcDNA3.1/HOXA10 compared with empty vector pcDNA3.1 (**, P = 0.01). When BT-20 cells were cotransfected with both pMT21/spastin and pcDNA3.1/HOXA10, EMX2-luciferase activity was also significantly repressed (*, P = 0.02) compared with spastin and empty vector pcDNA3.1. As seen in Ishikawa cells (A), repression of EMX2 luciferase activity in BT-20 was comparable when either HOXA10 or HOXA10 and spastin was cotransfected with EMX2-luciferase.
Despite binding spastin in nuclear extracts, our results showed that spastin overexpression did not further alter HOXA10-mediated transcriptional repression of EMX2. To determine whether transcriptional repression of EMX2 by HOXA10 was due to endogenous spastin binding, we determined the effect of diminishing spastin expression levels on HOXA10-mediated transcriptional regulation of EMX2 luciferase in Ishikawa cells (Fig. 4B). To diminish spastin expression levels, we used spastin small interfering RNA (siRNA). Transfection of Ishikawa cells with spastin siRNA resulted in an 80-fold reduction in spastin mRNA (real-time RT-PCR) levels. Spastin protein levels were also diminished in Ishikawa cells transfected with spastin siRNA compared with scrambled siRNA (Fig. 4B, inset; Western blot analysis using antispastin). We observed that although cotransfection of spastin and HOXA10 resulted in diminished EMX2-luciferase expression, in the presence of spastin siRNA, EMX2 luciferase expression was significantly increased (P < 0.001), and transcriptional repression, previously reported to occur with HOXA10, was reversed (Fig. 4B). It is therefore likely that spastin functioned a corepressor with HOXA10 in Ishikawa cells.
To further clarify the role of spastin-HOXA10 interaction in transcriptional regulation of EMX2, we transfected another cell line BT-20, with pGL3/EMX2-luciferase and pMT21/spastin (Fig. 4C). BT-20 is a poorly differentiated breast adenocarcinoma cell line that does not endogenously express HOXA10. In the absence of HOXA10, spastin failed to repress EMX2-luciferase and in fact marginally elevated EMX2-luciferase levels (P = 0.07). To determine the role of HOXA10 in BT-20 cells, we transfected them with a constitutive expression construct (pcDNA3.1/HOXA10). As expected, HOXA10 repressed EMX2/luciferase expression (**, P = 0.01). Although in BT-20 cells, in the absence of HOXA10, spastin did not repress EMX2/luciferase expression compared with control parent vector (Fig. 4C, white bars), when spastin was cotransfected with HOXA10, EMX2-luciferase levels were repressed (*, P = 0.02), thereby recapitulating the results observed in Ishikawa cells. It is therefore likely that spastin binds HOXA10 and acts as a corepressor on the EMX2 promoter.
NLS sequences in mammalian spastin are necessary for its role as a HOXA10 corepressor
Drosophila spastin is an exclusively cytoplasmic protein (29). Mammalian spastin has several predicted NLS, and these have been shown to be capable of directing nuclear translocation of artificial NLS-GFP constructs to variable extents (35). We identified spastin as a member of the HOXA10 transcription complex from nuclear extracts in Ishikawa cells. To clarify the role of spastin in HOXA10 transcriptional regulation, we determined the effect of deletion/mutation of the NLS sequences and any consequent effect on pGL3/EMX2-luciferase expression.
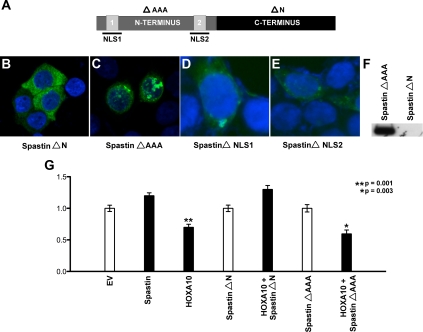
The N terminus of spastin contains two candidate NLS sequences (Fig. 5A). We initially determined the localization of GFP-spastin chimeric deletion constructs: ΔN-spastin and ΔAAA-spastin (corresponding to C-terminal and N-terminal deletion constructs, respectively) in Ishikawa cells (Fig. 5, A–C). Although ΔN-spastin-GFP comprised only of the spastin C terminus and lacking either of the putative NLS sequences displayed no nuclear localization (Fig. 5B), in contrast, ΔAAA-spastin-GFP comprised of the entire spastin N terminus containing both putative NLS sequences localized the nucleus (Fig. 5C).
Fig. 5.
NLS sequences in mammalian spastin are necessary for its role as a HOXA10 corepressor. A, Spastin contains two predicted NLS sequences NLS1 and NLS2 in its N-terminal domain. Also indicated are the deletion constructs: ΔAAA-spastin (C-terminal deletion) indicated in bold typeface and ΔN-spastin (N-terminal deletion) indicated in regular typeface. NLS residues deleted by in vitro mutagenesis are highlighted. B and C, ΔN-spastin-GFP comprised only of the spastin C terminus displayed no nuclear localization (B), whereas ΔAAA-spastin-GFP comprised of the entire spastin N terminus containing both putative NLS sequences localized the nucleus (C). Ishikawa cells were transfected with 4 μg of either spastin deletion construct, DAPI was used to stain nuclear DNA. Images were obtained by confocal microscopy at ×40 magnification. D and E, Mutagenesis of either NLS sequence outlined in A abrogated nuclear localization of full-length spastin-GFP. Ishikawa cells were transfected with 4 μg of in vitro mutagenized full-length spastin-GFP, DAPI was used to stain nuclear DNA. Images were obtained at ×40 magnification using confocal microscopy. F, Coimmunoprecipitation assays in Ishikawa cells cotransfected with pcDNAHOXA10-FLAG and either ΔN-spastin-GFP or ΔAAA-spastin-GFP confirmed that spastin bound HOXA10 via its N terminus (ΔAAA-spastin bound HOXA10). Anti-FLAG was used for immunoprecipitation on nuclear extracts from these cells, and anti-GFP was used for Western blot analysis. G, Ishikawa cells were cotransfected with 4 μg pGL3/EMX2-luciferase, pcDNA3.1/HOXA10, and the spastin deletion constructs ΔN-spastin and ΔAAA-spastin. The cells were additionally also transfected with a Renilla Luciferase vector as a control for transfection efficiency. Luciferase and Renilla activity was measured in the cellular lysate. Each experiment was done in triplicate and repeated three times. Luciferase activity normalized to Renilla activity showed that compared with cotransfection with empty vector pcDNA3.1 (white bars), significant repression of EMX2-luciferase levels was observed (*, P = 0.001) only when nuclear localizing ΔAAA-spastin (C) and not ΔN-spastin (B) was cotransfected with pcDNA3.1/HOXA10 (black bars).
To further determine the role of the N-terminal NLS sequences in nuclear localization of spastin, we performed in vitro mutagenesis of each NLS by deletion (Fig. 5A, highlighted amino acid residues) individually and observed that both NLS1 as well as NLS2 effectively mediated nuclear localization of spastin (Fig. 5, D and E). To further confirm that spastin bound HOXA10 via its N terminus, we performed coimmunoprecipitation assays in Ishikawa cells cotransfected with pcDNAHOXA10-FLAG and either ΔN-spastin-GFP or ΔAAA-spastin-GFP. Immunoprecipitation on nuclear extracts obtained from these cells was done using anti-FLAG and Western blot analysis using anti-GFP. As shown in Fig. 5F, ΔAAA-spastin (N terminus) but not ΔN-spastin (C terminus) bound HOXA10. Our data therefore suggest that spastin displays N-terminal binding with HOXA10. To determine the role of nuclear spastin on HOXA10-mediated EMX2 regulation, Ishikawa cells were cotransfected with pGL3/EMX2-luciferase, pcDNA3.1/HOXA10, and the spastin deletion/mutation constructs (Fig. 5G). We observed repression of EMX2-luciferase levels only when ΔAAA-spastin, but not when ΔN-spastin, was cotransfected with pcDNA3.1/HOXA10 in Ishikawa cells (*, P = 0.003). From our data, we therefore infer that spastin localizes to nuclei of Ishikawa cells via N-terminal NLS sequences. In turn, nuclear spastin binds with HOXA10 on the EMX2 promoter acting as a corepressor; spastin siRNA reverses this transcriptional repression.
Discussion
The homeobox transcription factor HOXA10 is necessary for uterine development during embryogenesis as well as for functional differentiation of the endometrium during the adult menstrual cycle (11, 14, 36). In support of these observations, mice with a congenital absence of Hoxa10 (Hoxa10−/−) display failed embryo implantation. Similarly, in wild-type (WT) mice, diminution of adult endometrial Hoxa10 levels decreases litter size (14, 37). Both embryonic as well as adult endometrial Hoxa10 expression is necessary for embryo implantation. The human ortholog HOXA10 is expressed both in the endometrial epithelium as well as in the stroma. Estrogen and progesterone drive HOXA10 expression in endometrial cells (11). Although HOXA10 functions downstream of the sex steroids, its role in uterine physiology is not well defined.
Our laboratory has focused on characterization of the regulation and function of HOXA10 in human endometrium and have previously described the regulation of several target genes by HOXA10, including EMX2, β3-integrin, Krueppel-like factor 9, and 3-phosphoglycerate dehydrogenase, all of which have been implicated in endometrial physiology (15, 16, 38, 39). EMX2 is a divergent homeobox gene that is necessary for reproductive tract development (19). Here, we describe transcriptional regulation by HOXA10 in the endometrium using EMX2 as a model target gene.
We have previously shown that in Drosophila, the HOXA10 ortholog Abd-B regulates the EMX2 ortholog ems (20). In Drosophila, Abd-B activates ems, in contrast to our findings in humans, where HOXA10 represses EMX2. Although the transcription factor-target gene relationship appears to be evolutionarily conserved, their regulatory relationship has been reversed (16). Because differential cofactor recruitment could explain the divergence in transcriptional regulation, we initially evaluated the role of previously well-characterized HOX gene cofactors pre-B-cell leukemia homeobox 1 and Meis homeobox 1. Because neither of these additionally influenced the regulation of EMX2 by HOXA10, we looked for novel endometrial HOXA10-binding partners that could possibly have a role in transcriptional regulation by HOXA10 in endometrial cells. In these studies, we identify and characterize the role of spastin as a novel HOXA10-binding partner and corepressor in Ishikawa cells. Although the role of spastin as an AAA-ATPase involved in neuromuscular trafficking is well characterized, this is the first study to describe its role in transcription. We have also characterized for the first time the nuclear localization of WT-spastin and its association with HOXA10. Finally, we report that spastin acts as a corepressor of HOXA10 in transcriptional regulation of EMX2. We found that diminution of spastin expression resulted in activation of EMX2 by HOXA10, which is analogous to our findings in Drosophila (20). Interestingly, Drosophila spastin lacks the N terminus, which is present in mammalian spastin (27, 29). Consequently, Drosophila spastin is exclusively cytoplasmic and therefore unlikely to bind Abd-B or participate in transcriptional regulation. We plan to further elucidate the mechanism of HOXA10 and spastin cooperation by mapping mutually interacting domains as well as determining the mechanism of transcriptional repression on chromatin through recruitment of other repressive cofactors.
In several human diseases where embryos fail to implant in the uterus, endometrial HOXA10 levels are diminished (40, 41). In contrast, endometrial EMX2 expression levels are elevated (42). It is therefore likely that diminished endometrial HOXA10 expression in disease relieves EMX2 repression. The effect of EMX2 on embryo implantation is evident when adult uterine EMX2 levels are altered; murine litter size is consistently diminished when EMX2 is overexpressed and increased when EMX2 expression was decreased (43). EMX2 therefore appears to function downstream of HOXA10, which itself functions downstream of estrogen and progesterone in endometrial cells. EMX2 may also have a role in modulating the implantation capability of human endometrium. It may be speculated that by limiting implantation ability, EMX2 has a role in the prevention of multiple or frequent pregnancies in humans, with beneficial health and survival implications for both mother and fetus. Therefore, spastin's function as a corepressor may have significant health implications.
Despite the structural and functional diversity of the Drosophila and human reproductive systems, underlying developmental pathways appear to be conserved. Nevertheless, these conserved pathways are able to complete divergent developmental programs in Drosophila and mammalian reproductive tracts through evolutionary acquisition of novel interaction partners.
Materials and Methods
MALDI-MS and sequencing
For these experiments, Ishikawa cell nuclear extracts were obtained from three independent experiments using published protocols (44). Nuclear extracts were immunoprecipitated using anti-FLAG, and resultant protein complexes were analyzed by MALDI-MS and sequencing. MALDI-MS and sequencing were performed at Keck Peptide Synthesis Resources at Yale University (http://keck.med.yale.edu). An online HPLC system was used for MALDI-MS, and N-terminal (Edman) sequencing was performed using the Applied Biosystems protein sequencer (Applied Biosystems, Foster City, CA).
Cell culture
All cell lines were of human origin. Ishikawa cells were maintained in phenol-red free MEM (Invitrogen, Carlsbad, CA) containing 2.0 mm l-glutamine and Earl's salts, supplemented with 10% fetal bovine serum (FBS), 1% sodium pyruvate, and 1% penicillin/streptomycin. BT-20 cells were maintained in phenol-red-free RPMI 1640 (Invitrogen) containing 2.0 mm l-glutamine and Earl's salts supplemented with 10% FBS, 1% sodium pyruvate, and 1% penicillin/streptomycin. HESC were a kind gift from Graciella Krikun at Yale University. HESC were maintained in phenol-red-free DMEM (Invitrogen), supplemented with 10% FBS.
Tissue collection
Endometrium (uterine epithelium) was collected from normal cycling human subjects by Pipelle endometrial biopsy under an approved human investigations committee protocol. The tissue was immediately placed in liquid nitrogen and stored at −72 C. Endometrial dating was determined from cycle history and confirmed histologically using Noyes criteria (45).
Reverse transcription-polymerase chain reaction
Ishikawa cells, a well-differentiated human endometrial adenocarcinoma cell line, were grown to 80% confluence as described above. Total RNA was extracted from whole cells using the QIAGEN RNeasy Mini kit (QIAGEN, Inc., Valencia, CA); 0.5 μg total RNA was used for cDNA synthesis using the Invitrogen first strand kit. To detect spastin expression, SPA_Ca/SPA_Cm primer sets were used as described by Hazan et al. (27) and available at http://www.genoscope.cns.fr. The expected PCR product size was 766 bp.
Western blot analysis
Ishikawa cells were lysed in lysis buffer (50 mm HEPES, 150 mm NaCl, 10% glycerol, 1% Triton X-100, 1.5 mm MgCl2, 1 mm EGTA, 100 mm NaF, 10 mm sodium pyrophosphate, 1 mm NaSO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride), centrifuged at 12,000 × g for 2 min at 4 C and supernatant collected. The protein content was quantified by the Bradford method using a protein assay kit (Bio-Rad Laboratories, Richmond, VA); 30 μg aliquots were loaded on to a 6% sodium dodecyl sulfate polyacrylamide gel, size fractioned, and transferred to a nitrocellulose membrane using a transblot apparatus (Bio-Rad Laboratories) at 100 V for 2 h at 4 C. The membrane was immersed in a 3% gelatin-Tris-buffered saline (TBS) (20 mm Tris and 500 mm NaCl) blocking solution for 30 min at room temperature, washed in TBS [20 mm Tris, 500 mm NaCl, and 0.05% Tween 20 (pH 7.5)], and then incubated for 1 h with a 1:500 antispastin monoclonal antibody (Abcam, Cambridge, MA). For loading controls, anti-TBP or antitubulin (Abcam) was used. The membrane was washed with TBS for 5 min at room temperature and incubated for 1 h with a 1:2000 dilution of goat antimouse IgG-HRP (Bio-Rad Laboratories, Hercules, CA). The membrane was then washed twice in TBS at room temperature and immersed in a horseradish peroxidase color developer buffer (Bio-Rad Laboratories) for 30 min. Photographs were taken immediately after color development.
Immunofluorescence and immunohistochemistry
Cells grown on coverslips were fixed in ice-cold methanol for 10 min. The cells were washed twice in cold PBS and then permeabilized with 0.2% Triton X-100 for 10 min. The cells were then washed three times in PBS. To reduce nonspecific staining, the cells were blocked with 10% normal goat serum (NGS) in phosphate buffered saline Tween (PBST) for 1 h. The coverslips were then incubated overnight at 4 C in a 1:500 dilution of antispastin (ab31850; Abcam) in PBST containing 1% NGS. A biotinylated antimouse secondary antibody was used (Vector Laboratories, Burlingame, CA), followed by ABC-DAB reagent and hematoxylin. For immunofluorescence, the cells were washed in PBS three times and then incubated in a 1:600 dilution of the fluorescein tagged secondary antibody in PBST containing 1% NGS. The coverslips were subsequently incubated in the dark, washed three times in PBS, and then 4′,6-diamidino-2-phenylindole (DAPI) staining was added for 1 min. The coverslips were finally rinsed with 1× PBS and then mounted and sealed with clear nail polish. For immunohistochemistry, timed endometrial biopsy paraffin-embedded specimens were handled as previously described (46). For this study, we selected timed endometrial biopsies obtained on d 12, 17, and 24 (three individual patient samples for each day) of the menstrual cycle for analysis. Briefly, the samples were deparaffinized in xylene and ethanol. Endogenous peroxidase was blocked with 0.6% H2O2. After a 45-min incubation with 1.5% normal goat blocking serum, the sections were incubated overnight at 4 C in a 1:500 dilution of antispastin (ab31850; Abcam). The sections were then incubated with biotinylated mouse secondary IgM antisera for 30 min, avidin and biotinylated peroxidase (Vectastain; Vector Laboratories) for 45 min, and diaminobenzidene (400 mg/ml) for 5 min. Hematoxylin and Li2CO3 were used for counterstaining.
Reporter constructs and expression plasmids and transfection
For EMX-luciferase reporter constructs, we used a previously characterized HOXA10 responsive 450-bp EMX regulatory element (GenBank accession no. AY 116142) inserted into the SacI/SmaI sites of pGL3 promoter to create a pGL3/EMX-luciferase reporter construct (16). Expression vectors for HOXA10 were generated by cloning full-length HOXA10 cDNA into the expression vectors pCDNA3.1 and pcDNA3/RFP to generate pcDNA3.1/HOXA10 and pcDNA3/HOXA10RFP, respectively. pMT21/spastin-myc containing full-length spastin cDNA was a gift from Elena Rugarli. We additionally generated pEGFP3-spastin and the deletion constructs pEGFP3-spastinΔN and pEGFP3-spastinΔAAA lacking the N terminus and C terminus of spastin, respectively. We also generated the following constructs by in vitro mutagenesis: EGFP3spastin NLSDel1/1a and EGFP3spastin NLSDel2. These constructs contained mutations in the NLS of spastin. All transfections were performed using 4 μg of plasmid as previously described (15, 16). Briefly, preconfluent (75–80%) Ishikawa or BT-20 cells, in 25-cm2 flasks, were transfected using Lipofectamine (Invitrogen) in serum-free OptiMEM medium (Invitrogen). Transfectants were incubated for 5 h at 37 C, 5% CO2, washed with 1× PBS replenished with regular medium, and grown to confluence for an additional 48 h.
Coimmunoprecipitation
For coimmunoprecipitation, Ishikawa cells were transiently cotransfected (using the protocol described above in Materials and Methods under transfection) with either pEGFP-spastin, pEGFP3-spastinΔN, or pEGFP3-spastinΔAAA and pcDNA3.1/HOXA10-FLAG. Controls were transfected with pcDNA3.1/HOXA10-FLAG and empty vector pEGFP or pEGFP-spastin alone. Nuclear extracts were obtained 48 h later using a nuclear extract kit (ActiveMotif, Carlsbad, CA). Anti-Flag M1: 1:500 (Sigma, St. Louis, MO) was added to precleared samples and incubated at 4 C overnight. Protein G slurry (Sigma) 25 μl was added to the samples. The precipitation was allowed to continue for 2 h at 4 C. The samples were centrifuged at 3000 rpm for 1 min to pellet the beads, the supernatant was discarded, and the beads washed five times in 1× lysis buffer. The precipitated proteins were removed from the beads by heating to 95 C in 2× Laemmle loading buffer for 4 min and analyzed by Western blotting using antispastin antibody (1:200; Abcam). Anti-GFP (1:500) was used for Western blot analysis in experiments where Ishikawa cells had been transfected with the spastin deletion constructs pEGFP3-spastinΔN or pEGFP3-spastinΔAAA.
Chromatin immunoprecipitation
Preconfluent (80%) Ishikawa cells were transfected with pMT21/spastin-myc. ChIP was performed using the EZ-ChIP kit (Millipore, Bedford, MA) per protocol. Briefly, the cells were fixed with 1% formaldehyde 48 h after transfection, harvested, and lysed in sodium dodecyl sulfate-lysis buffer. Total cellular DNA was then sheared by sonication, precleared with protein A-agarose beads, and incubated overnight with antispastin (Abcam). An aliquot of input DNA was obtained from the precleared supernatant before addition of antispastin. The antibody-DNA complexes were then collected on protein A-agarose beads. DNA obtained after elution was subjected to PCR using the following set of primers: 5′-TCCGCCACCTTTCTCTCCAATC-3′ (forward) and 5′-AAGTCCCCCAGCAACTCACCTGA-3′ (reverse) designed to amplify the EMX2 promoter element. A 155-bp PCR product representing the EMX2 promoter containing the previously characterized HOXA10-binding site was examined on a 2% agarose gel in pMT/spastin transfected cells.
In vitro mutagenesis
EGFP/spastin plasmid containing WT human spastin cDNA was mutated using the QuikChange Site-Directed Mutagenesis kit protocol (Stratagene, La Jolla, CA). Primers for mutagenesis were designed using the QuikChange Primer Design Program. To create plasmid NLS1 mutant-spastin-GFP, nucleotides 27–35 corresponding to three lysine residues (9–12) were deleted. To create plasmid NLS2 mutant-spastin-GFP, nucleotides 615–626 corresponding to amino acids PKRK (205–208) were deleted. Mutagenesis was verified by ABI 377 Fluorescent DNA sequencing.
Transfection and luciferase assays
Preconfluent (75–80%) Ishikawa or BT-20 cells, in 25-cm2 flasks, were transfected with 4 μg per plasmid using Lipofectamine (Invitrogen). Transfectants were incubated for 5 h at 37 C, 5% CO2, washed with 1× PBS, and grown to confluence for an additional 48 h. At the end of 48 h, the cells were washed with cold PBS, lysed with 1× Reporter Lysis Buffer (Promega, Madison, WI) and lysate collected. The lysate was snap frozen in a dry ice/ethanol bath and centrifuged at maximum speed for 2 min. Luciferase activity was assayed by using the luciferase assay kit (Promega) and a luminometer. A Renilla luciferase reporter construct was cotransfected to correct for variations in transfection efficiency. Reporter activity was normalized to Renilla expression. For siRNA experiments, Ishikawa cells were divided into six-well tissue culture plates and cultured overnight. When cells reached 50–70% confluence, the cells were cotransfected in triplicate with plasmids and siRNA using Lipofectamine 2000 (Invitrogen) with OptiMEM (Invitrogen), a serum-free medium. Cells received an EMX2 promoter-luciferase reporter plasmid (1 μg/well), a Renilla reporter plasmid (1 μg/well), a HOXA10 plasmid or empty vector control (1 μg/well), a spastin plasmid or empty vector control (1 μg/well), and either spastin siRNA (sc-61603; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or nonspecific control siRNA (sc-37007; Santa Cruz Biotechnology, Inc.) (60 pmol/well). For each well, 5 μl of Lipofectamine 2000 were diluted in 250 μl of OptiMEM and incubated for 5 min to allow vesicles to form. The plasmids and siRNA were diluted in 250 μl of OptiMEM per well and then combined with the Lipofectamine 2000 mixture. Cells were washed once with warmed PBS, and then 0.5 ml of the Lipofectamine-plasmid-siRNA-OptiMEM mixture were added to each well. The cells were cultured for 6 h, and then 1.5 ml of OptiMEM were added to each well. Twenty-four hours after transfection, cells were washed once with cold PBS, harvested, and lysed using a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's protocol; 20 μl of each cell lysate were transferred to a 96-well plate and assayed immediately for luciferase and Renilla activity using a luminometer. Luciferase activity was normalized to Renilla activity. All experiments were done in triplicate and repeated three times.
Statistical analysis
Results are expressed as means with sem. Each experiment was repeated in triplicate at least three times. Two-tailed Student's t test was used. Statistical analysis was performed using SAS software (version 6.12; SAS Institute, Cary, NC). All statistical tests were two sided.
Acknowledgments
This work was supported by funding the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54 HD052668 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and by National Institutes of Health Grant HD0388679.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAA
- ATPases associated with various cellular activities
- Abd-B
- abdominal-B
- ChIP
- chromatin immunoprecipitation
- DAPI
- 4′,6-diamidino-2-phenylindole
- ems
- Drosophila empty spiracles
- EMX2
- empty spiracles homeobox
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- HESC
- human endometrial stromal cell
- Hoxa10
- homeobox A10
- MALDI
- matrix-assisted laser desorption ionization
- NGS
- normal goat serum
- NLS
- nuclear localization signal
- PBST
- phosphate buffered saline Tween
- RFP
- red fluorescent protein
- siRNA
- small interfering RNA
- TBP
- TATA-binding protein
- TBS
- Tris-buffered saline
- WT
- wild type.
References
- 1. Akam M. 1989. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell 57:347–349 [DOI] [PubMed] [Google Scholar]
- 2. Favier B, Dollé P. 1997. Developmental functions of mammalian Hox genes. Mol Hum Reprod 3:115–131 [DOI] [PubMed] [Google Scholar]
- 3. McGinnis W, Krumlauf R. 1992. Homeobox genes and axial patterning. Cell 68:283–302 [DOI] [PubMed] [Google Scholar]
- 4. Morata G, Sánchez-Herrero E. 1999. Patterning mechanisms in the body trunk and the appendages of Drosophila. Development 126:2823–2828 [DOI] [PubMed] [Google Scholar]
- 5. Boncinelli E, Somma R, Acampora D, Pannese M, D'Esposito M, Faiella A, Simeone A. 1988. Organization of human homeobox genes. Hum Reprod 3:880–886 [DOI] [PubMed] [Google Scholar]
- 6. Krumlauf R. 1994. Hox genes in vertebrate development. Cell 78:191–201 [DOI] [PubMed] [Google Scholar]
- 7. Schughart K, Kappen C, Ruddle FH. 1988. Mammalian homeobox-containing genes: genome organization, structure, expression and evolution. Br J Cancer Suppl 9:9–13 [PMC free article] [PubMed] [Google Scholar]
- 8. Lewis EB. 1978. A gene complex controlling segmentation in Drosophila. Nature 276:565–570 [DOI] [PubMed] [Google Scholar]
- 9. Taylor HS, Vanden Heuvel GB, Igarashi P. 1997. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod 57:1338–1345 [DOI] [PubMed] [Google Scholar]
- 10. Daftary GS, Taylor HS. 2006. Endocrine regulation of HOX genes. Endocr Rev 27:331–355 [DOI] [PubMed] [Google Scholar]
- 11. Taylor HS, Arici A, Olive D, Igarashi P. 1998. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 101:1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor HS, Igarashi P, Olive DL, Arici A. 1999. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab 84:1129–1135 [DOI] [PubMed] [Google Scholar]
- 13. Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. 1996. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 122:2687–2696 [DOI] [PubMed] [Google Scholar]
- 14. Satokata I, Benson G, Maas R. 1995. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 374:460–463 [DOI] [PubMed] [Google Scholar]
- 15. Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. 2002. Direct regulation of β3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol 16:571–579 [DOI] [PubMed] [Google Scholar]
- 16. Troy PJ, Daftary GS, Bagot CN, Taylor HS. 2003. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol 23:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dalton D, Chadwick R, McGinnis W. 1989. Expression and embryonic function of empty spiracles: a Drosophila homeo box gene with two patterning functions on the anterior-posterior axis of the embryo. Genes Dev 3:1940–1956 [DOI] [PubMed] [Google Scholar]
- 18. Grossniklaus U, Cadigan KM, Gehring WJ. 1994. Three maternal coordinate systems cooperate in the patterning of the Drosophila head. Development 120:3155–3171 [DOI] [PubMed] [Google Scholar]
- 19. Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. 1997. Defects of urogenital development in mice lacking Emx2. Development 124:1653–1664 [DOI] [PubMed] [Google Scholar]
- 20. Taylor HS. 1998. A regulatory element of the empty spiracles homeobox gene is composed of three distinct conserved regions that bind regulatory proteins. Mol Reprod Dev 49:246–253 [DOI] [PubMed] [Google Scholar]
- 21. Chang CP, Brocchieri L, Shen WF, Largman C, Cleary ML. 1996. Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol Cell Biol 16:1734–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang CP, Jacobs Y, Nakamura T, Jenkins NA, Copeland NG, Cleary ML. 1997. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol Cell Biol 17:5679–5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knoepfler PS, Kamps MP. 1995. The pentapeptide motif of Hox proteins is required for cooperative DNA binding with Pbx1, physically contacts Pbx1, and enhances DNA binding by Pbx1. Mol Cell Biol 15:5811–5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu Q, Kamps MP. 1996. Structural determinants within Pbx1 that mediate cooperative DNA binding with pentapeptide-containing Hox proteins: proposal for a model of a Pbx1-Hox-DNA complex. Mol Cell Biol 16:1632–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen WF, Montgomery JC, Rozenfeld S, Moskow JJ, Lawrence HJ, Buchberg AM, Largman C. 1997. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol 17:6448–6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen WF, Rozenfeld S, Kwong A, Köm ves LG, Lawrence HJ, Largman C. 1999. HOXA9 forms triple complexes with PBX2 and MEIS1 in myeloid cells. Mol Cell Biol 19:3051–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hazan J, Fonknechten N, Mavel D, Paternotte C, Samson D, Artiguenave F, Davoine CS, Cruaud C, Dürr A, Wincker P, Brottier P, Cattolico L, Barbe V, Burgunder JM, Prud'homme JF, Brice A, Fontaine B, Heilig B, Weissenbach J. 1999. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat Genet 23:296–303 [DOI] [PubMed] [Google Scholar]
- 28. Trotta N, Orso G, Rossetto MG, Daga A, Broadie K. 2004. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr Biol 14:1135–1147 [DOI] [PubMed] [Google Scholar]
- 29. Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. 2004. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol 2:e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hata H, Kuramoto H. 1992. Immunocytochemical determination of estrogen and progesterone receptors in human endometrial adenocarcinoma cells (Ishikawa cells). J Steroid Biochem Mol Biol 42:201–210 [DOI] [PubMed] [Google Scholar]
- 31. Lessey BA, Ilesanmi AO, Castelbaum AJ, Yuan L, Somkuti SG, Chwalisz K, Satyaswaroop PG. 1996. Characterization of the functional progesterone receptor in an endometrial adenocarcinoma cell line (Ishikawa): progesterone-induced expression of the α1 integrin. J Steroid Biochem Mol Biol [Erratum (1997) 60:161] 59:31–39 [DOI] [PubMed] [Google Scholar]
- 32. Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. 1985. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nippon Sanka Fujinka Gakkai Zasshi 37:1103–1111 [PubMed] [Google Scholar]
- 33. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr 1988. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 67:334–340 [DOI] [PubMed] [Google Scholar]
- 34. Errico A, Ballabio A, Rugarli EI. 2002. Spastin, the protein mutated in autosomal dominant hereditary spastic paraplegia, is involved in microtubule dynamics. Hum Mol Genet 11:153–163 [DOI] [PubMed] [Google Scholar]
- 35. Beetz C, Brodhun M, Moutzouris K, Kiehntopf M, Berndt A, Lehnert D, Deufel T, Bastmeyer M, Schickel J. 2004. Identification of nuclear localisation sequences in spastin (SPG4) using a novel Tetra-GFP reporter system. Biochem Biophys Res Commun 318:1079–1084 [DOI] [PubMed] [Google Scholar]
- 36. Daftary GS, Taylor HS. 2004. Pleiotropic effects of Hoxa10 on the functional development of peri-implantation endometrium. Mol Reprod Dev 67:8–14 [DOI] [PubMed] [Google Scholar]
- 37. Bagot CN, Troy PJ, Taylor HS. 2000. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther 7:1378–1384 [DOI] [PubMed] [Google Scholar]
- 38. Du H, Sarno J, Taylor HS. 2010. HOXA10 inhibits Kruppel-like factor 9 expression in the human endometrial epithelium. Biol Reprod 83:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du H, Vitiello D, Sarno JL, Taylor HS. 2010. 3-Phosphoglycerate dehydrogenase expression is regulated by HOXA10 in murine endometrium and human endometrial cells. Reproduction 139:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Daftary GS, Taylor HS. 2002. Hydrosalpinx fluid diminishes endometrial cell HOXA10 expression. Fertil Steril 78:577–580 [DOI] [PubMed] [Google Scholar]
- 41. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. 1999. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 14:1328–1331 [DOI] [PubMed] [Google Scholar]
- 42. Daftary GS, Taylor HS. 2004. EMX2 gene expression in the female reproductive tract and aberrant expression in the endometrium of patients with endometriosis. J Clin Endocrinol Metab 89:2390–2396 [DOI] [PubMed] [Google Scholar]
- 43. Taylor HS, Fei X. 2005. Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol Endocrinol 19:2839–2846 [DOI] [PubMed] [Google Scholar]
- 44. Dignam JD, Lebovitz RM, Roeder RG. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noyes RW, Hertig AT, Rock J. 1950. Dating the endometrial biopsy. Fertil Steril 1:3. [DOI] [PubMed] [Google Scholar]
- 46. Daftary GS, Taylor HS. 2001. Efficient liposome-mediated gene transfection and expression in the intact human uterus. Hum Gene Ther 12:2121–2127 [DOI] [PubMed] [Google Scholar]