Abstract
Differentiation of human cytotrophoblasts to syncytiotrophoblast and the associated induction of aromatase/hCYP19 gene expression are dependent upon a critical O2 tension; however, the underlying molecular mechanisms remain undefined. In this study, we provide compelling evidence that expression of the orphan nuclear receptor, estrogen-related receptor γ (ERRγ), is also O2 dependent, induced during human syncytiotrophoblast differentiation, and plays an obligatory role in the induction of placenta-specific hCYP19I.1 gene expression. Treatment with the selective ERRγ agonist, DY131, or overexpression of ERRγ, stimulated hCYP19 expression in syncytiotrophoblast. Overexpression of ERRγ prevented effects of hypoxia to repress hCYP19 gene expression in cultured trophoblasts. Conversely, small interfering RNA-mediated knockdown of endogenous ERRγ in primary trophoblasts markedly inhibited hCYP19 expression. Promoter and site-directed mutagenesis studies in transfected placental cells identified a nuclear receptor element within placenta-specific hCYP19 promoter I.1 required for ERRγ-stimulated activity. Recruitment of endogenous ERRγ to the nuclear receptor element region in hCYP19 promoter during trophoblast differentiation, assessed by chromatin immunoprecipitation, was prevented by hypoxia. Deferoxamine-induced hypoxia-inducible factor-1α (HIF-1α) levels decreased ERRγ expression, whereas knockdown of endogenous HIF-1α prevented ERRγ suppression by hypoxia. Chromatin immunoprecipitation analysis of trophoblasts cultured in hypoxia revealed recruitment of HIF-1α to one of two putative hypoxia response elements in the ERRγ promoter, providing in vivo evidence of a direct HIF-1α involvement in ERRγ expression. Collectively, these novel findings identify ERRγ as an O2-dependent transcription factor and HIF-1α target gene that serves a critical role in the induction of hCYP19 expression during human trophoblast differentiation.
The ability of the human placenta to synthesize estrogens is vastly increased after the ninth week of gestation (1) in association with cytotrophoblast invasion and enlargement of the uterine arterioles, increased blood flow, and O2 availability to the floating chorionic villi (2, 3). Estrogens are synthesized from C19-steroid precursors via the action of aromatase P450, encoded by CYP19A1 (heretofore referred to as hCYP19) gene. The trophoblast stem cells and the cytotrophoblasts do not express hCYP19 ; however, when cytotrophoblasts fuse to form multinucleated syncytiotrophoblast, aromatase is markedly induced (4, 5). The extraordinarily high levels of human placental aromatase (4, 6) likely serve two major functions: 1) to metabolize vast amounts of C19-steroids produced by the human fetal adrenals (e.g. dehydroepiandrosterone), thus preventing conversion of these steroids to active androgens, which can masculinize the fetus; 2) to generate biologically active estrogens and estrogen metabolites, which enhance angiogenesis and uteroplacental blood flow and reduce systemic vascular resistance (7–9). The genetic programs that are initiated during cytotrophoblast differentiation into syncytiotrophoblast and the transcriptional mechanisms that regulate these events are incompletely defined.
The hCYP19 gene contains unique tissue-specific promoters that lie upstream of tissue-specific first exons, which are alternatively spliced onto a common site just upstream of the translation initiation codon in exon II. The hCYP19 placenta-specific first exon (exon I.1) and promoter lie approximately 95,000 bp upstream of the start site of translation in exon II (10). In previous studies using transgenic mice, we observed that as little as 246 bp of hCYP19 exon I.1 5′-flanking DNA were sufficient to mediate placenta-specific transgene expression that was developmentally regulated and expressed in the labyrinth (11, 12), a region that contains syncytial cells and is analogous to human syncytiotrophoblast. Expression of this transgene was induced in mouse labyrinthine trophoblast as early as embryonic d 10.5, a time when the embryonic vasculature invades and branches extensively to facilitate efficient transport of nutrients and O2 to the embryo (13, 14). This suggested that increased O2 tension might play a permissive role in the regulation of hCYP19 promoter I.1 activity.
In studies using cultured human trophoblasts, we previously observed that syncytiotrophoblast differentiation and induction of hCYP19 expression were prevented when the cells were cultured under hypoxic (2% O2) conditions (3), suggesting a role of hypoxia/O2-regulated transcription factors. Notably, we observed that hypoxia induced expression of the basic-helix-loop-helix transcription factor mammalian achaete scute homologous protein-2 (Mash-2/ASCL2), which promoted cytotrophoblast proliferation and blocked syncytiotrophoblast differentiation and induction of hCYP19 expression (3). The action of Mash-2 to inhibit hCYP19 expression was mediated by up-regulation of upstream stimulatory factors (USF) 1 and 2, which bound as heterodimers to E-boxes within the hCYP19 exon I.1 5′-flanking region and first exon (15, 16).
Although these findings suggest the importance of a decline in inhibitory transcription factors during trophoblast differentiation, our understanding of the response elements, activating transcription factors, and mechanisms that mediate induction of hCYP19 expression during syncytiotrophoblast differentiation remain incomplete.
There is evidence to suggest that placentally derived estrogen may play an autocrine role in trophoblast differentiation (17, 18). In recent studies, we observed that estrogen receptor (ER)α is expressed in human trophoblasts, and up-regulated during differentiation in culture. Further, estrogen acting via ERα up-regulates hCYP19 expression (19). Importantly, we identified an estrogen-response element-like sequence (ERE-LS) at −208 bp upstream of exon I.1 that is required for the inductive effect of estrogen on hCYP19 promoter I.1 activity. Proximal to the ERE-LS at −183 bp, we identified a sequence with similarity to a nuclear receptor element (NRE) that we previously found to be required for increased promoter I.1 activity in transfected human syncytiotrophoblast (5). In consideration of the established role of estrogen-related receptor β (ERRβ/NR3B2) in placental development in mice (20) and the finding that ERRγ is expressed at very high levels in human placenta (21, 22), we considered the possibility that this NRE-like sequence may serve as a binding site for a member of the ERR subfamily of nuclear receptors. We report that ERRγ is selectively up-regulated in human trophoblast during syncytiotrophoblast differentiation and induction of hCYP19 gene expression. Moreover, ERRγ, which is essential for O2-mediated induction of hCYP19 expression in human trophoblast cells, is inhibited by hypoxia via a hypoxia-inducible factor 1α (HIF-1α)-dependent pathway.
Results
ERRγ mRNA and protein are expressed in human trophoblasts and induced with trophoblast differentiation
To determine whether ERRα, ERRβ, and/or ERRγ are expressed in midgestation human trophoblasts and whether protein and mRNA expression levels change with differentiation, immunoblotting of nuclear proteins, and quantitative RT-PCR (qRT-PCR) of RNA isolated from cells before and after 24–72 h of culture were performed. We observed that both ERRα and ERRγ mRNA were expressed in the human trophoblasts during culture, whereas ERRβ expression was undetectable (Fig. 1A). To validate these results, RT-PCR analysis also was performed. Using this method, again only transcripts for ERRα and ERRγ were detectable in differentiated placental cells (data not shown). Moreover, only ERRγ mRNA levels were dynamically up-regulated during syncytiotrophoblast differentiation and with induction of hCYP19I.1 mRNA expression (Fig. 1A). Furthermore, ERRγ mRNA expression was much higher in the differentiated syncytiotrophoblast than in the choriocarcinoma cell lines BeWo and JEG3, which are cytotrophoblast like and manifest low levels of hCYP19I.1 mRNA expression (Fig. 1B). Western blot analysis indicated that ERRγ protein also was present at low levels in cytotrophoblasts and was up-regulated during syncytiotrophoblast differentiation (Fig. 1C).
Fig. 1.
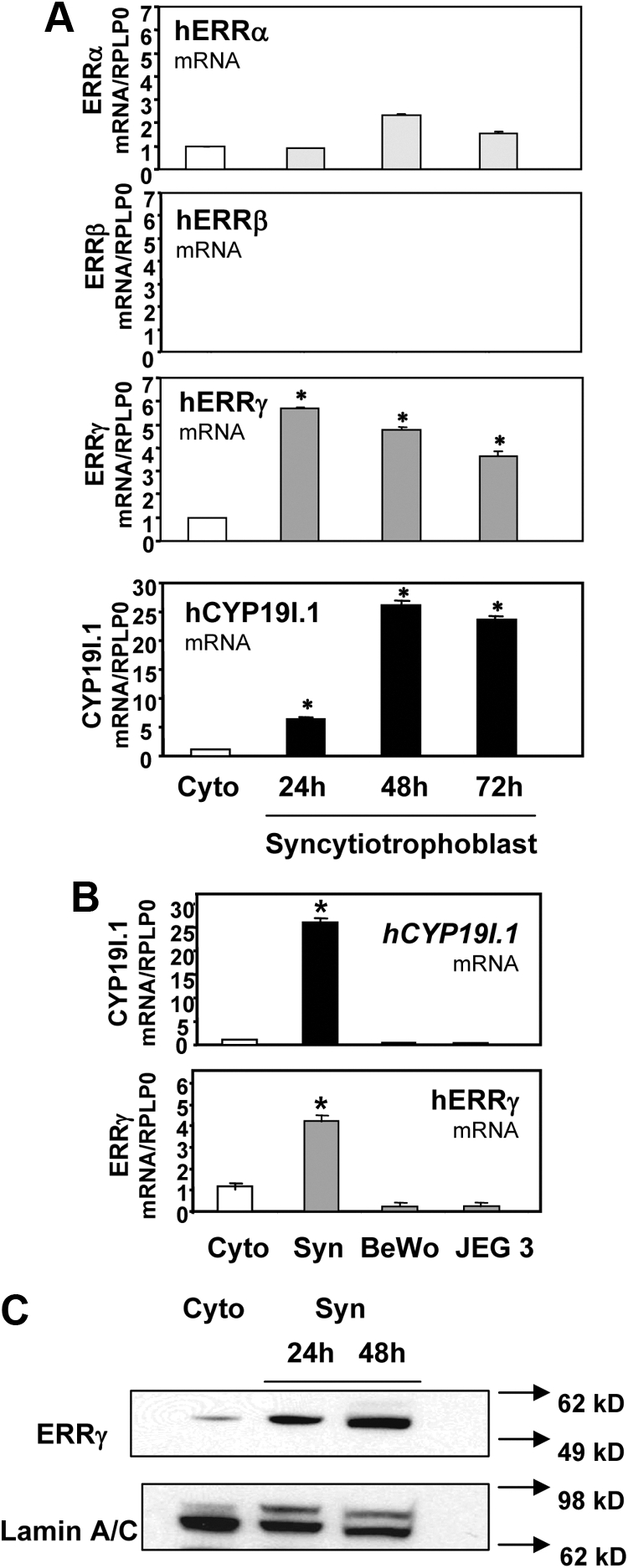
ERRγ mRNA and protein are expressed in human trophoblasts and induced with trophoblast differentiation. A, Freshly isolated human placental cytotrophoblasts were suspended in phenol red-containing DMEM with 2% FBS (DMEM) and plated at a density of 2 × 106 cells per dish. RNA was isolated from freshly isolated cytotrophoblasts (Cyto) before or after 24 h, 48 h, or 72 h [syncytiotrophoblast (Syn)] of culture in DMEM, and the expression of mRNA transcripts for ERRα, ERRβ, and ERRγ was analyzed by qRT-PCR; RPLP0 was used as the reference. *, Significantly different (P < 0.05) from values in freshly isolated cytotrophoblasts. B, RNA was isolated from freshly isolated cytotrophoblasts (Cyto) before or after 48 h of culture (Syn) and from BeWo and JEG-3 cells; hCYP19I.1 and ERRγ mRNA levels were analyzed by qRT-PCR; RPLP0 was used as the reference. C, Nuclear proteins (30 μg) extracted from freshly isolated cytotrophoblasts before (Cyto) or after 72 h of culture (Syn) were analyzed by immunoblotting using antisera to ERRγ or lamin A/C, as loading control. Data are the mean ± sem of values from three independent experiments; each conducted in triplicate and are expressed relative to expression levels in cytotrophoblasts before culture. *, Significantly different (P < 0.05) from values in cytotrophoblast.
ERRγ stimulates hCYP19I.1 mRNA expression in human trophoblasts and acts synergistically with ERα to increase hCYP19I.1 promoter activity in transfected placental cells
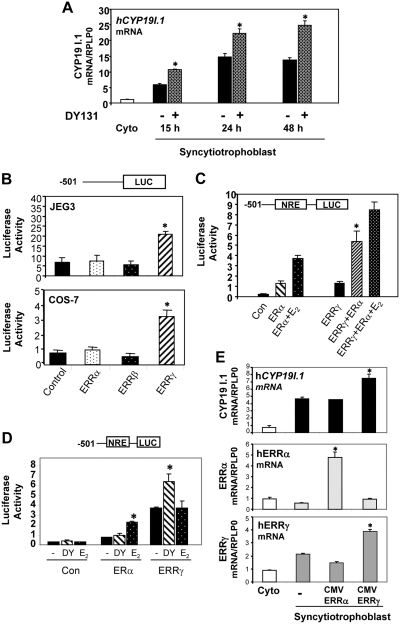
To begin to assess the role of ERRγ in hCYP19I.1 gene expression in human trophoblasts, we examined the effect of the ERRγ synthetic agonist DY131 on hCYP19I.1 mRNA expression. DY131 was demonstrated to effectively and selectively activate ERRβ/γ in reporter gene assays (23). Treatment of freshly isolated cytotrophoblasts with 10 μm DY131 enhanced hCYP19I.1 mRNA expression at 15 h, 24 h, and 48 h as measured by qRT-PCR (Fig. 2A). To evaluate a direct inductive role of ERRγ on hCYP19I.1 promoter activity, we carried out reporter assays in JEG3 and COS-7 cells with hCYP19I.1 reporter constructs containing 501 bp of DNA flanking the 5′-end of hCYP19 exon I.1 fused to the luciferase (LUC) gene, as reporter, and cotransfected with either ERRα, ERRβ, or ERRγ expression vectors or an empty expression vector, as control. Luciferase activity was increased in JEG3 and COS-7 cells cotransfected with the ERRγ expression plasmid (Fig. 2B); by contrast, expression vectors for ERRα or ERRβ had no inductive effect.
Fig. 2.
Activation or overexpression of ERRγ induces hCYP19I.1 mRNA expression in human trophoblast cells. A, Freshly isolated human cytotrophoblasts were suspended in DMEM with 2% FBS, plated at a density of 2 × 106 cells per dish, and treated with 10 μm of the ERRγ-selective agonist, DY131, or vehicle. RNA was isolated from freshly isolated cytotrophoblasts before (Cyto) or after 15 h, 24 h, or 48 h of culture in DMEM; hCYP19I.1 mRNA was analyzed by qRT-PCR. *, Significantly different (P < 0.05) from vehicle-treated cells. B, COS-7 or JEG-3 cells were transiently cotransfected with a luciferase reporter plasmid containing 501 bp of hCYP19I.1 5′-flanking DNA (−501 hCYP19I.1/Luc) and either ERRα-, ERRβ-, or ERRγ-CMV expression plasmids or an empty expression plasmid as control (Con). Cells also were cotransfected with β-gal expression plasmid to monitor transfection efficiency. The cells were lysed 72 h after transfection and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed as luciferase activity corrected for transfection efficiency with β-gal activity. *, Significantly different (P < 0.05) from cells transfected with empty expression vector. C, COS-7 cells were transiently cotransfected with a luciferase reporter plasmid containing 501 bp of hCYP19I.1 5′-flanking DNA (−501 hCYP19I.1/Luc) and either ERα-, or ERRγ-CMV expression plasmids or an empty expression plasmid, as control (Con), and cotransfected with β-gal expression plasmid to monitor transfection efficiency. The cells were treated with 10 nm E2 or vehicle. The cells were lysed 72 h after transfection and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments each conducted in triplicate and are expressed as luciferase activity corrected for transfection efficiency by analysis of β-gal activity. *, Significantly different (P < 0.05) from cells transfected with either ERα or ERRγ. D, COS-7 cells were transiently cotransfected with a luciferase reporter plasmid containing 501 bp of hCYP19I.1 5′-flanking DNA (−501 hCYP19I.1/Luc) and either ERα-, or ERRγ-CMV expression plasmids or an empty expression plasmid, as control (Con), and cotransfected with β-gal expression plasmid to monitor transfection efficiency. The cells were treated with 10 μm of the ERRγ-selective agonist, DY131, or 10 nm E2 or vehicle. The cells were lysed 72 h after transfection and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed as luciferase activity corrected for transfection efficiency by analysis of β-gal activity. *, Significantly different (P < 0.05) from cells treated with vehicle. E, Freshly isolated human placental cytotrophoblasts plated at a density of 2 × 106 cells per dish were infected with recombinant adenoviruses containing CMV-ERRα or CMV-ERRγ and cultured for 48 h; RNA was isolated and analyzed for expression of hCYP19I.1, ERRα, and ERRγ mRNA by qRT-PCR; RPLP0 was used as the reference. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are compared with expression levels in cytotrophoblasts before culture. *, Significantly different (P < 0.05) from values in cytotrophoblast.
In previous studies, we identified an ERE-LS at −208 bp upstream of exon I.1 that is required for the inductive effect of estrogen and ERα on hCYP19 promoter I.1 activity (19). In the present study, we analyzed the effects on hCYP19I.1-501-Luc promoter activity of ERRγ and ERα expression vectors cotransfected into COS-7 cells alone and in combination, in the absence or presence of estradiol (10−8 m) for 72 h. As observed previously (19), hCYP19I.1 promoter activity was increased in cells cotransfected with ERα and further up-regulated by E2. Interestingly, promoter activity was synergistically induced by cotransfection of both ERRγ and ERα, as compared with activity in cells cotransfected with either construct alone (Fig. 2C). In parallel cotransfection assays, we observed that the ERRγ- and ERα-specific ligands, DY131 and E2, respectively, acted as selected agonists of their respective receptors and correspondingly increased hCYP19I.1 promoter activity (Fig. 2D).
To determine whether increased ERRγ expression could further induce hCYP19I.1 expression in primary human trophoblast cultures, freshly isolated cytotrophoblasts were infected with recombinant adenoviruses expressing ERRγ or ERRα and cultured for 48 h, after which ERRα, ERRγ, and hCYP19I.1 expression was analyzed by qRT-PCR. Transduction of ERRγ expression vector significantly increased endogenous hCYP19I.1 expression in the cultured human trophoblast cells, whereas overexpression of ERRα had no effect (Fig. 2E). The somewhat modest effect of ERRγ on endogenous hCYP19I.1 mRNA is likely due to relatively high levels of endogenous ERRγ in the cells after syncytiotrophoblast differentiation.
ERRγ is required for hCYP19I.1 expression in human trophoblast
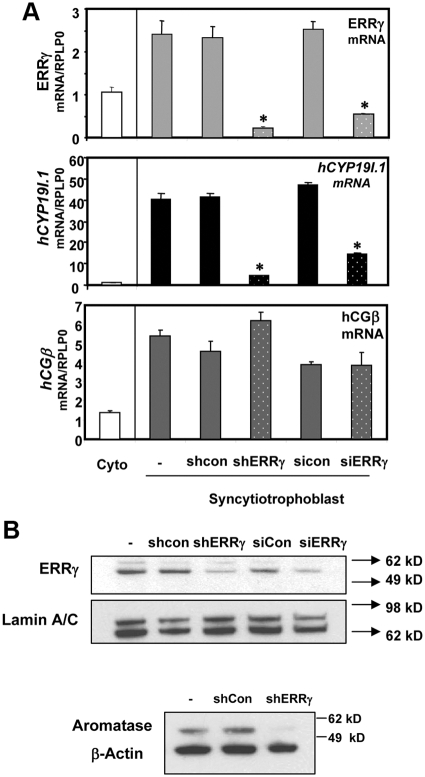
To further establish a functional role of endogenous ERRγ in hCYP19I.1 expression in human syncytiotrophoblast, we used an RNA interference approach. Freshly isolated cytotrophoblasts from human placenta were infected with two different lentiviral constructs carrying ERRγ-targeting short hairpin (ERRγ shRNA), or a control, non-silencing shRNA at a multiplicity of infection (moi) of 0.5 for each. In parallel experiments, we cultured cytotrophoblasts with chemically synthesized double-stranded small interfering RNA (siRNA) for ERRγ to silence endogenous ERRγ expression. After 72 h of culture there was a pronounced knockdown of ERRγ mRNA levels (Fig. 3A) using either the lentiviral-based shRNA technique or the siRNA method. Importantly, this was associated with a corresponding marked decrease in hCYP19I.1 expression whereas expression of human chorionic gonadotropin-β, a syncytiotrophoblast marker, was unaltered in the placental cells (Fig. 3A). Western blot analysis also revealed a comparable knockdown of ERRγ protein using both RNA interference systems and a decrease in aromatase protein expression in syncytiotrophoblast infected with shRNA targeting ERRγ (Fig. 3B). Thus, reduced ERRγ expression was associated with down-regulation of hCYP19I.1 gene expression in syncytiotrophoblast. Collectively, these findings suggest an essential role for ERRγ in regulating hCYP19I.1 expression during human trophoblast differentiation.
Fig. 3.
Knockdown of endogenous ERRγ inhibits hCYP19I.1 expression in human placental cells. A, Freshly isolated human cytotrophoblasts were plated at a density of 2 × 106 cells per dish and infected with lentiviral vectors carrying ERRγ-targeting shRNA (shERRγ) or control shRNA (shcon). Other dishes of cells were untreated (−). In parallel experiments, the cells were transfected with chemically synthesized double-stranded siRNAs for ERRγ (siERRγ) or control (sicon) si-oligonucleotides as described in Materials and Methods. RNA was isolated 72 h later, and expression of hCYP19I.1, ERRγ, and human chorionic gonadotropin-β mRNA was analyzed by qRT-PCR; RPLP0 was used as the reference. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are compared with expression levels in cytotrophoblasts before culture. *, Significantly different (P < 0.05) from values in cells transduced with control shRNA or control siRNA. B, Nuclear proteins (30 μg) extracted from syncytiotrophoblast after 72 h of culture were analyzed by immunoblotting using antisera to ERRγ or lamin A/C. Cytoplasmic proteins (30 μg) were analyzed for aromatase, using β-actin as loading control.
ERRγ increases hCYP19I.1 promoter activity through a nuclear receptor response element at −183 bp
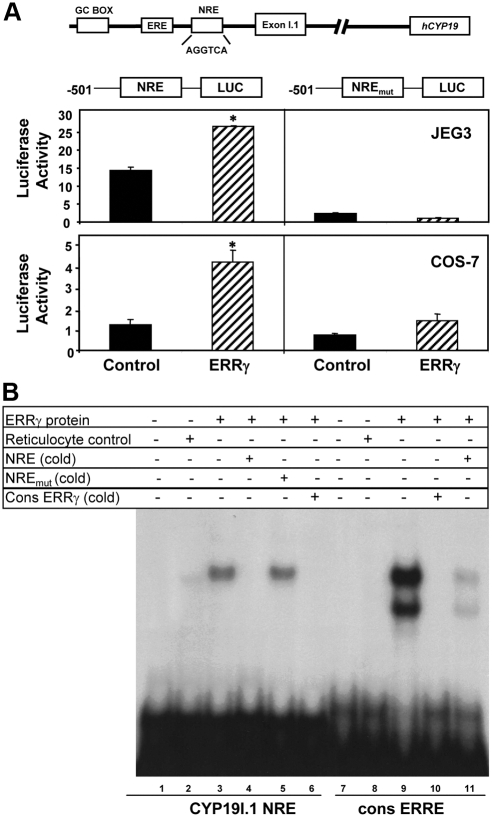
To identify genomic sequences that mediate the inductive effect of ERRγ on aromatase expression, we considered the possibility that a hexameric NRE-like sequence (AGGAGGTCA) at −183 bp might serve as an ERR response element (ERRE). This sequence differs from a consensus ERRE (T/C / G/AC/GAAGGTCA) motif (24) by one nucleotide. We previously observed in primary human trophoblasts infected with recombinant adenoviruses containing hCYP19I.1-501 reporter constructs that mutation of this element caused a marked decrease in promoter activity (5). In the present study, COS-7 and JEG3 cells were cotransfected with luciferase reporter constructs containing 501 bp of hCYP19 exon I.1 5′-flanking DNA with or without mutation in the NRE-like sequence. As was shown above (Fig. 2B), cotransfection of ERRγ had an inductive effect on hCYP19I.1 promoter activity in COS-7 and JEG3 cells (Fig. 4A). Mutation of the NRE at −183 bp resulted in a reduction in basal and profound loss of ERRγ-stimulated hCYP19I.1-501/Luc activity both in COS-7 and JEG3 cells (Fig. 4A). These findings suggest that the hexameric NRE element may be critical for hCYP19I.1 basal promoter activity in human trophoblast cells and functionally serves as an ERRE. Although JEG3 cells express very low levels of ERRγ (Fig. 1B), we suggest that binding of ERRγ or a related endogenous transcription factor to the NRE is required for basal and stimulated promoter activity. Interestingly, in preliminary transfection studies in COS-7 cells, we observed that cotransfection of ERα in the presence of E2 failed to induce expression of the 501-bp NREmut-LUC construct (data not shown). Thus, the NRE likely plays a critical role in both ERα and ERRγ induction of hCYP19I.1 promoter activity.
Fig. 4.
A NRE at −183 bp in hCYP19 placenta-specific promoter I.1 mediates responsiveness to ERRγ. A, COS-7 or JEG-3 cells were transiently cotransfected with hCYP19I.1-501/Luc reporter plasmid without or with a mutation of the NRE at −183 bp, along with either an ERRγ expression plasmid or an empty control plasmid. All cells were cotransfected with β-gal expression plasmid as a control for transfection efficiency. The cells were lysed 72 h after transfection and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed as luciferase activity corrected for transfection efficiency using β-gal activity as a standard. *, Significantly different (P < 0.05) from cells cotransfected with empty expression vector. B, In vitro transcribed and translated ERRγ protein (lanes 3–6 and lanes 9–11) or nonprogrammed reticulocyte lysate (lanes 2 and 8) were incubated with a radiolabeled probe containing the hCYP19I.1 NRE region (lanes 1–6) or a radiolabeled consensus ERRE (cons ERRE) probe (lanes 7–11) in the absence or presence of a 200-fold excess of nonradiolabeled double-stranded wild-type or mutated NRE probes or the consensus ERRE probe. Protein was omitted from incubations with probe in lanes 1 and 7.
ERRγ protein binds to the −192/−169 region of hCYP19I.1 promoter
To determine whether ERRγ can recognize and bind directly to the NRE sequence at −183 bp, EMSA was performed using a radiolabeled NRE oligonucleotide and in vitro-transcribed-translated ERRγ protein. Expressed ERRγ protein bound to the radiolabeled NRE probe (Fig. 4B, lane 3) and was effectively competed by excess nonradiolabeled NRE probe (Fig. 4B, lane 4) or a consensus ERRE-binding sequence (Fig. 4B, lane 6) but not by the mutated NRE sequence (Fig. 4B, lane 5). The expressed ERRγ protein bound to a radiolabeled consensus ERRE sequence as two mobility complexes (Fig. 4B, lane 9), which were effectively competed by ERRE consensus oligonucleotide (Fig. 4B, lane 10) and greatly reduced by the hCYP19I.1 NRE probe (Fig. 4B, lane 11). Binding of expressed ERRγ to a consensus ERRE as two different mobility complexes was previously observed and suggested to be due to alternatively initiated transcripts or to interaction of ERRγ molecules bound to probe forming higher order structures (21). Notably, only one binding complex was observed with the NRE within the hCYP19I.1 promoter. Although binding of expressed ERRγ to the hCYP19I.1 NRE appeared weaker than to the consensus ERRE, these gel shift assays indicate that ERRγ can specifically recognize and bind to the NRE sequence at −183 bp.
ERRγ and hCYP19I.1 expression levels coordinately decline in human trophoblasts cultured in a hypoxic environment; overexpression of ERRγ rescues hCYP19I.1 expression
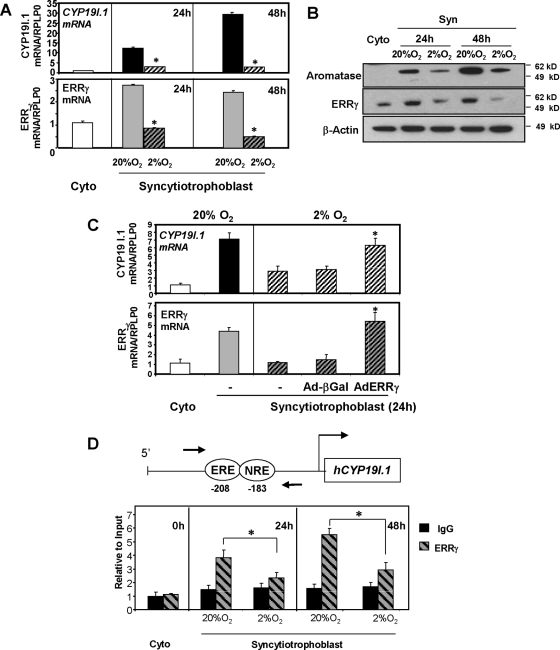
Trophoblast differentiation and induction of hCYP19I.1 expression are inhibited when freshly isolated cytotrophoblasts are cultured in a 2% O2 environment (3). To determine whether these effects of hypoxia could be mediated by associated effects on ERRγ, cytotrophoblasts were cultured in 20% O2 or 2% O2 for 24 h and 48 h; ERRγ and hCYP19I.1 mRNA expression levels were analyzed by qRT-PCR. The induction of both ERRγ and hCYP19I.1 expression, observed in trophoblast cells cultured in 20% O2 after 24 h or 48 h, was blocked when cells were maintained in a hypoxic (2% O2) environment (Fig. 5A). This hypoxia-mediated inhibition of mRNA accumulation was paralleled by a decrease in both ERRγ and aromatase protein expression (Fig. 5B). To further evaluate whether the effect of hypoxia to inhibit the induction of hCYP19I.1 expression was mediated by the associated decline in ERRγ expression, freshly isolated cytotrophoblasts were infected with recombinant adenoviruses containing expression vectors for ERRγ or β-gal and cultured in 2% O2 for 24 h; ERRγ and hCYP19I.1 mRNA levels were then assessed by qRT-PCR. As can be seen in Fig. 5C, overexpression of ERRγ restored hCYP19I.1 mRNA to levels comparable to those of trophoblasts cultured in 20% O2.
Fig. 5.
ERRγ expression and recruitment to the hCYP19I.1 promoter in human trophoblast cells are inhibited by hypoxia. A, Freshly isolated human placental cytotrophoblasts were plated at a density of 2 × 106 cells per dish and cultured in a 20% O2 or 2% O2 environment for 24 h or 48 h. RNA was isolated from cells either before (Cyto) or after 24 h or 48 h of culture, and the expression of hCYP19I.1 and ERRγ mRNA was analyzed by qRT-PCR; RPLP0 was used as the reference. *, Significantly different (P < 0.05) from values in cells cultured in 20% O2. B, Nuclear proteins (30 μg) extracted from cells before culture (Cyto) or after 24 h or 48 h of culture in 20% O2 or 2% O2 were analyzed by immunoblotting using antisera to ERRγ or lamin A/C as loading control. Cytoplasmic proteins (30 μg) were immunoblotted for aromatase or β-actin, as loading control for cytoplasmic proteins. C, Freshly isolated human placental cytotrophoblasts were plated at a density of 2 × 106 cells per dish, cultured in 20% O2 or 2% O2, and infected with recombinant adenoviruses expressing CMV-ERRγ (AdERRγ) or CMV-βgal (Ad-βGal) as control. After 24 h, RNA was isolated and the expression of hCYP19I.1 and ERRγ was analyzed by qRT-PCR; RPLP0 was used as the reference. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are compared with expression levels in cytotrophoblasts before culture. *, Significantly different (P < 0.05) from values of cells infected with β-gal-expressing adenovirus. D, Freshly isolated human placental cytotrophoblasts (0 h) were cultured in 20% O2 or 2% O2 for 24 h or 48 h. The freshly isolated and cultured trophoblasts were treated with 1% formaldehyde and subjected to ChIP analysis using ERRγ IgG (ERRγ) or nonimmune IgG (IgG), as control. The immunoprecipitated ERRγ complexes bound to an approximately 100-bp region (−255 to −155 bp, indicated by the arrows) surrounding the CYP19I.1 NRE site were quantified by qPCR using specific primers. The ChIP data were normalized to ‘input control’ data and expressed relative to binding at the 0 h time point. The data shown are the mean ± sem of values from three independent experiments. Complexes immunoprecipitated with nonspecific IgG in the same experiment were quantified as a negative control. *, Significantly different (P < 0.05) from values of cells cultured in 20% O2.
Hypoxia decreases binding of endogenous ERRγ to the hCYP19I.1 promoter in differentiating human trophoblast cells
To determine whether hypoxia blocks the recruitment of endogenous ERRγ to the genomic region containing the ERRE, chromatin immunoprecipitation (ChIP) assays were performed using formaldehyde-cross-linked chromatin from freshly isolated human cytotrophoblast cells (0 h) or from cells cultured either for 24 h or 48 h in 2% vs. 20% O2. After immunoprecipitation of the chromatin complexes with ERRγ or control IgG, quantitative PCR (qPCR) was carried out using primers to amplify an approximately 100-bp region between −255 and −155 bp containing the NRE. Increased recruitment of endogenous ERRγ to the genomic region containing the NRE was observed between 0 h and 24 h and 48 h of culture in 20% O2; however, ERRγ recruitment was significantly reduced in trophoblasts cultured in hypoxia at both 24 h and 48 h (Fig. 5D). This hypoxia-mediated decline in ERRγ recruitment was associated with an inhibitory effect of hypoxia on ERRγ expression levels (Fig. 5, A and B).
HIF-1α mediates repression of ERRγ in human trophoblasts cultured in a hypoxic environment via binding to a hypoxia-responsive element (HRE) in the ESRRG promoter
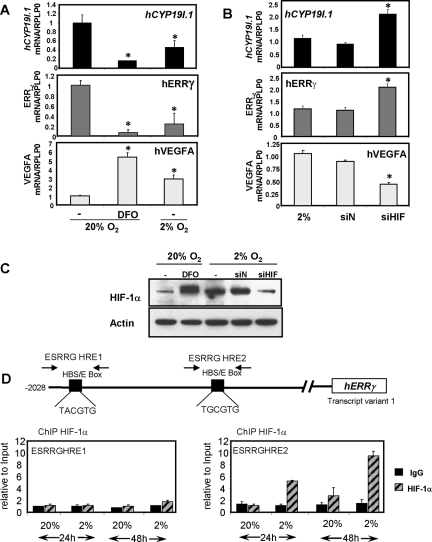
To determine whether inhibition of ERRγ expression by hypoxia is mediated by HIF-1α, cytotrophoblasts were cultured in 20% O2 in the absence or presence of 100 μm deferoxamine mesylate (DFO) or in 2% O2 for 48 h. DFO has been reported to increase HIF-1α stability and transactivation through sequestration of iron, a necessary cofactor for several enzymes involved in HIF-1α degradation (25). Quantitative RT-PCR revealed a marked inhibition of ERRγ and hCYP19I.1 mRNA expression levels in cells maintained in a hypoxic (2% O2) environment or when cultured in the presence of DFO in 20% O2 (Fig. 6A). Conversely, siRNA-mediated knockdown of endogenous HIF-1α in trophoblasts cultured in a hypoxic (2% O2) environment significantly increased ERRγ and hCYP19I.1 mRNA expression levels, as compared with cells transfected with a control nontargeting siRNA (Fig. 6B). Expression of vascular endothelial growth factor (VEGF), a HIF-1α target gene (26), was used as a positive control. Western blot analysis confirmed increased HIF-1α protein expression levels with DFO treatment and by hypoxia (2% O2) and its down-regulation with siRNA transfection (Fig. 6C). These data provide evidence supporting a functional role for HIF-1α in the repression of ERRγ and hCYP19 gene expression in trophoblasts cultured in a hypoxic environment.
Fig. 6.
HIF-1α, which mediates repression of ERRγ in human trophoblasts cultured in a hypoxic environment, is recruited to the ERRγ promoter in cultured human trophoblasts. A, Freshly isolated human cytotrophoblasts were plated at a density of 2 × 106 cells per dish and cultured in a 2% O2 environment or a 20% O2 environment in the absence or presence of 100 μm DFO for 48 h. B, For siRNA experiments, the cells were transfected with chemically synthesized double-stranded siRNAs for HIF-1α (siHIF) or control (siN) si-oligonucleotides as described in Materials and Methods. RNA was isolated from cells after 48 h of culture and hCYP19I.1, ERRγ, and VEGFA mRNA levels were analyzed by qRT-PCR; RPLP0 was used as the reference. *, Significantly different (P < 0.05) from values in cells cultured in 20% O2. C, Nuclear proteins (30 μg) extracted from syncytiotrophoblast after 48 h of culture were analyzed by immunoblotting using antisera to HIF-1α and anti-β-actin. D, Freshly isolated human cytotrophoblasts (0 h) were cultured in 20% O2 or 2% O2 for 24 h or 48 h. The cultured trophoblasts were treated with 1% formaldehyde and subjected to ChIP analysis using HIF-1α IgG or nonimmune IgG (IgG), as control. The immunoprecipitated HIF-1α complexes bound to an approximately 100-bp region surrounding the two putative HREs in the hERRγ promoter were quantified by qPCR using specific primers. The ChIP data were normalized to input control data and expressed relative to binding at the 24-h time point in 20% O2. The results shown are the mean ± sem of values from two independent experiments. *, Significantly different (P < 0.05) from values of cells cultured in 20% O2.
Using the Genomatix MatInspector program (Genomatix Software GmbH, Munich, Germany), we identified two putative response elements within the ERRγ promoter that are similar to the core consensus HRE (5′-RCGTG-3′). To assess in vivo binding of HIF-1α to these potential HREs within the ERRγ promoter, we performed ChIP assays using formaldehyde-cross-linked chromatin from trophoblast cells cultured either for 24 h or 48 h in 2% vs. 20% O2. After immunoprecipitation of the chromatin complexes with HIF-1α or control IgG, qPCR was carried out using primers to amplify an approximately 100-bp region flanking each of the two HREs within the ERRγ promoter. Increased recruitment of endogenous HIF-1α to HRE2, but not HRE1, was observed at 24 h and 48 h of culture in 2% O2 as compared with 20% O2. (Fig. 6D), suggesting the functional role of HRE2 in the hypoxia-mediated down-regulation of ERRγ.
Discussion
Differentiation of human cytotrophoblasts to syncytiotrophoblast during culture in a 20% O2 environment is associated with a marked induction of hCYP19 gene expression (5). By contrast, when cytotrophoblasts are cultured in a hypoxic (2% O2) environment, syncytiotrophoblast differentiation and induction of hCYP19 gene expression are prevented (3). In the present study, we obtained compelling evidence to suggest that ERRγ, which is markedly induced during differentiation of cultured human trophoblasts (Fig. 1) in an O2-dependent manner (Fig. 5), serves a critical role in the induction of hCYP19 expression. Accordingly, RNA interference-mediated knockdown of ERRγ mRNA and protein caused a coordinate, pronounced suppression of hCYP19I.1 mRNA (Fig. 3). ERRγ was found to bind both in vitro (Fig. 4) and in vivo (Fig. 5) to a response element (NRE) at −183 bp upstream of hCYP19 placenta-specific exon I.1. Moreover, upon mutagenesis, the NRE was observed to be essential for basal and ERRγ stimulation of hCYP19I.1 promoter activity in transfected placental cells (Fig. 4).
ERRγ is a member of the ERR/NR3 subfamily of orphan nuclear receptors, made up of three closely related proteins, ERRα/NR3B1, ERRβ/NR3B2, and ERRγ/NR3B3, which are capable of binding to the same response elements in DNA (27). Although ERRs are structurally similar to ERα, they are not activated by estrogens. Whereas ERRα plays an important role in lipid homeostasis and oxidative energy metabolism (28), mice homozygous null for ERRβ/Nr3b2 die at 10.5 d post coitum because of severe defects in chorion formation and trophoblast differentiation (20). On the other hand, mice that are ERRγ null die at birth from defects in ion homeostasis in heart, kidney, and gastric tissues (29). Interestingly, in mice in which the ERRβ/Nr3b2 gene was conditionally disrupted in embryonic but not extraembryonic tissues, there was markedly reduced expression of ion channel and transporter genes in the inner ear, resulting in auditory and vestibular defects (30). These findings suggest that although ERRβ and ERRγ may manifest tissue-specific regulation of expression in the mouse, they both have the capacity to regulate related target genes encoding ion transport proteins.
Based on the important role played by ERRβ in mouse placental development (20), we speculated that ERRβ may be critical for human syncytiotrophoblast differentiation and the associated up-regulation of hCYP19 expression. However, to our surprise, ERRβ mRNA was undetectable in midgestation human trophoblast and was not altered during syncytiotrophoblast differentiation. Because various splice variants of ERRβ have been detected in human endometrium (31), it is possible that one of these transcripts may be present in human placenta and not detected by the real-time PCR primers used in the present study. However, in JEG3 and COS-7 cells cotransfected with a hCYP19I.1:Luc reporter construct and ERRα, β, and γ expression vectors, only ERRγ had the capacity to stimulate hCYP19I.1 promoter activity (Fig. 2B). This suggests a unique, species-specific role for ERRγ in the regulation of hCYP19 gene expression in human placenta. In this regard, ERRβ was previously reported to be expressed abundantly in mouse embryonic stem cells but was undetectable in human embryonic stem cells even after early stages of differentiation (32). It was suggested that these differences in ERRβ expression may underlie fundamental differences in early embryogenesis and placental development between mice and humans (32).
In contrast to ERRα, which was only modestly increased during human trophoblast differentiation in culture, we observed that ERRγ was rapidly and dramatically up-regulated, and this temporally proceeded the induction in hCYP19 expression. Moreover, as we observed for hCYP19, ERRγ expression was barely detectable in BeWo and JEG 3 choriocarcinoma cell lines, which are cytotrophoblast like and do not express aromatase (Fig. 1). Notably, ERRγ was reported to be expressed at very high levels in the human placenta (21, 22). Because the syncytiotrophoblast serves a critical role in nutrient and ion transport between mother and fetus, it is of interest that ERRγ null mice manifest marked decreases in expression of potassium channel subunits in cardiac, gastric, and renal tissues (29), as mentioned above. Conversely, transgenic mice overexpressing ERRγ in a muscle-specific manner demonstrated increased expression of several genes involved in angiogenic and calcium-handling pathways (33). In this regard, it will be of interest to determine whether ERRγ also regulates ion transport across the maternal-fetal interface.
In previous studies using transgenic mice, we observed that a 246-bp region immediately upstream of hCYP19 exon I.1 mediates placenta-specific transgene expression, which is localized to labyrinth and giant cells (12). We recently found that an ERE-LS within this region (−208 bp) mediated positive feedback regulation by estradiol/ERα of hCYP19I.1 promoter activity, associated with permissive changes in histone H3 modification (19). Our findings further suggested that ERα may not bind to DNA directly, but may interact with other transcription factors bound to the −255 to −155-bp region to stimulate hCYP19 promoter I.1 activity (19). Within this 100-bp region, we previously identified a G/C-rich sequence, which binds transcription factor Sp1 and is important for hCYP19I.1 promoter activity. Although ERα and Sp1 interactions at this site may play a role, our findings, that both ERRγ (Fig. 6A) and ERα (19) interact within the −255 to −155 bp region of hCYP19 promoter I.1 to synergistically increase hCYP19 promoter I.1 activity (Fig. 2B), suggest that these structurally related transcription factors may functionally interact to promote hCYP19 gene expression in human placenta; however, we have not obtained evidence thus far to support their direct physical interaction.
Gestational changes in placental perfusion and O2 availability serve a critical role in trophoblast invasion, differentiation, and function. During the first trimester of gestation, the relatively hypoxic environment favors cytotrophoblast proliferation (34), impairs cell fusion (35) and differentiation (36), decreases placental polypeptide hormone production (37), and mimics the placental defect associated with preeclampsia (2, 36). After the ninth week of human gestation, increased cytotrophoblast invasion of the decidua and myometrium causes remodeling and enlargement of the uterine arterioles, resulting in enhanced placental perfusion and increased O2 tension. This coincides with a marked increase in placental estrogen production. The present findings suggest that ERRγ is an O2-regulated transcription factor that serves a critical role in the activation of hCYP19 gene expression in human placenta. The finding that ERRγ mRNA and protein levels were coordinately regulated suggests that alterations in ERRγ expression during trophoblast differentiation and with changes in O2 tension are mediated primarily at the level of gene transcription. To our knowledge, ERRγ is the first member of the nuclear receptor family shown to be regulated by O2 tension at the level of gene expression. On the other hand, the mammalian orphan nuclear receptors, Rev-erbα and Rev-erbβ (38, 39), which regulate circadian rhythm and energy metabolism, and Drosophila Rev-erb homolog, ecdysone-induced protein 75 (E75), a developmental regulator (40), are heme-binding redox sensors that bind the diatomic gases, carbon monoxide and nitric oxide (NO) (41). Notably, transcriptional repression by heme-bound Rev-erbα and β was found to be selectively reversed by NO (42).
In this study, we observed that hypoxia-dependent down-regulation of ERRγ expression was mediated, in part, by HIF-1α, a master regulator of O2 homeostasis (43). Notably, components of the HIF-1α pathway have been demonstrated to serve a critical role in normal placental development. Mice that are null for von Hippel-Lindau, which targets HIF-1α for proteosomal degradation in normoxia, or for HIF-1β/ARNT, die in utero due to defective placentation with reduced labyrinthine development and markedly fewer fetal blood vessels (44, 45). These placental defects are likely due to the absence of direct actions of HIF-1α/β to stimulate target genes that promote angiogenesis, cell proliferation, and invasion (46). Although the mechanisms whereby hypoxia induction of HIF-1α results in up-regulation of genes involved in erythropoiesis, glycolysis and angiogenesis are well understood (47); those for HIF-1α-mediated gene repression are generally not well defined (48–50). However, HIF-1α has been reported to inhibit expression of DNA repair genes by blocking the binding of the transcriptional activator, c-Myc (46). In consideration of the ChIP results presented in Fig. 6C, we suggest that hypoxia-mediated induction of HIF-1α binding to response elements in the ESRRG promoter may possibly antagonize the actions of transcription factors that mediate ERRγ induction.
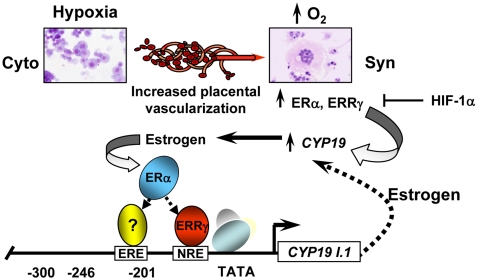
In conclusion, our findings suggest that ERRγ serves as a novel O2-responsive transcription factor that directly regulates hCYP19 gene expression during human trophoblast differentiation. Based on our present and previous findings, we propose a mechanism whereby increased placental perfusion and O2 tension after the ninth week of human gestation promote decreased expression of inhibitory transcription factors (e.g. USF, Mash-2/ASCL2, HIF-1α) and enhanced expression and recruitment of ERRγ to the hCYP19I.1 promoter, resulting in increased hCYP19 gene expression. The estrogens produced, in turn, activate ERα, which may functionally interact with ERRγ and other activating transcription factors and coregulators, resulting in further up-regulation of hCYP19 expression (Fig. 7). In consideration of the suggested role of placental hypoxia in the pathogenesis of preeclampsia and the influence of estrogen on vasculogenesis and vasomotor tone during pregnancy, our findings may provide novel insight into the molecular events that underlie this devastating disease.
Fig. 7.
Model of hCYP19 regulation in human trophoblasts. Early in gestation, the placenta is relatively hypoxic; the elevated levels of HIF-1α inhibit ERRγ transcription via interaction with a response element (HRE) in the ERRγ promoter. This, together with hypoxia-mediated induction of basic helix loop helix factors ASCL2 and USF1/2, blocks syncytiotrophoblast differentiation and hCYP19 gene expression. After the ninth week of gestation, increased placental vascularization results in increased oxygen availability to the trophoblast cells. The consequent decrease in HIF-1α levels and expression of other inhibitory transcription factors causes up-regulation of ERRγ and induction of hCYP19I.1 transcription. The increased estrogens produced, in turn, activate ERα, which may functionally interact with ERRγ and other activating transcription factors to further up-regulate hCYP19 expression.
Materials and Methods
Primary culture of human trophoblast cells
Midtrimester human placental tissues were obtained from Advanced Bioscience Resources (Alameda, CA) in accordance with the Donors Anatomical Gift Act of the State of Texas. Protocols were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. A placental primary culture system (51) was modified (5) for isolation and culture of cytotrophoblasts from midgestation human placenta. Briefly, the placental tissues were washed with Hank's balanced salt solution, pH 7.4 (Life Technologies, Inc., Gaithersburg, MD), and then finely minced and digested with 0.125% trypsin in Hank's balanced salt solution at 37 C for 20 min. This procedure was repeated three times. At the end of each digestion step, the supernatant was collected, layered over 10 ml of serum, and then briefly centrifuged. The pellet was suspended in DMEM (Life Technologies, Inc.), filtered and layered over a Percoll gradient (70–5%). The gradients were centrifuged at 1200 × g for 20 min at room temperature, and cells in the middle layer (density, 1.045–1.062 g/ml) were collected, washed, and counted. The cells were then resuspended in DMEM supplemented with 10% fetal bovine serum (FBS) and 1.2% antibiotic/antimycotic solution (Life Technologies, Inc.) and plated at a density of 2 × 106 cells per 35-mm culture dish or 15 × 106 cells per 100-mm dish. The cells were cultured overnight, after which the medium was changed to DMEM containing 2% FBS. To determine the effects of hypoxia, the cells were placed in an incubator chamber (Heraeus; Thermo Scientific, Rockford, IL) with an atmosphere of 2% O2, 93% N2, and 5% CO2. DFO (no. D9533, Sigma, St Louis, MO) was added at indicated concentrations to mimic hypoxia.
Quantitative RT-PCR
Total RNA from trophoblast cells cultured for 24, 48, or 72 h, was extracted by the one-step method of Chomczynski and Sacchi (52) (TRIzol; Invitrogen, Carlsbad, CA). RNA was treated with deoxyribonuclease to remove any contaminating DNA, and 2 μg were reversed transcribed using random primers and Superscript II RNase H-reverse transcriptase (Invitrogen). Primer sets were generated utilizing Primer Express software (PE Applied Biosystems, Boston, MA).
Primers for hCYP19I.1 are as follows: forward, 5′-ACG GAA GGT CCT GTG CTC G-3′; reverse, 5′-GTA TCG GGT TCA GCA TTT CCA-3′.
Primers for ERRγ are as follows: forward, 5′-CTG ACG GAC AGC GTC AAC C-3′; reverse, 5′-GGC GAG TCA AGT CCG TTC TG-3′.
Primers for VEGFA are: forward, 5′-CAT GCA GAT TAT GCG GAT CAA; reverse, 5′-TTT GTT GTG CTG TAG GAA GCT CAT.
Primers for RPLP0 are: forward, 5′-TGC ATC AGT ACC CCA TTC TAT CA-3′; reverse, 5′-AAG GTG TAA TCC GTC TCC ACA GA-3′.
The relative abundance of each transcript was determined by qRT-PCR using a modification of previously published methods. For the quantitative analysis of mRNA expression, the ABI Prism 7700 Detection System (Applied Biosystems, Foster City, CA) was employed using the DNA binding dye SYBR Green (PE Applied Biosystems) for the detection of PCR products. The cycling conditions were 50 C for 2 min, 95 C for 10 min, followed by 40 cycles of 95 C for 15 sec, and 60 C for 1 min. The cycle threshold was set at a level at which the exponential increase in PCR amplification was approximately parallel between all samples. All primer sets produced amplicons of the expected size and sequence. The relative fold changes were calculated using the comparative cycle times (Ct) method with RPLP0 as the reference guide.
EMSA
Double-stranded oligonucleotides used were as follows (mutated nucleotides are lowercase and underlined).
CYP19I.1 NRE: forward, 5′-TTC CAG AGG AGG TCA TGC CCC ATA-3′; reverse, 5′-TAT GGG GCA TGA CCT CCT CTG GAA-3′
CYP19I.1 NREmut: forward, 5′-TTC CAG tcg acc cca TGC CCC ATA-3′; reverse, 5′-TAT GGG gca tgg ggt CGA CTG GAA-3′.
Consensus ERRE: forward, 5′-CCG GGG CTT TCA AGG TCA TAT GCA GAT C-3′; reverse, 5′-GAT CTG CAT ATG ACC TTG AAA GCC CCG G-3′
For EMSA, the double-stranded oligonucleotides were end labeled with [γ-32P]ATP using T4 kinase (Invitrogen) and incubated with in vitro transcribed/translated ERRγ (10 μl) for 30 min at room temperature in binding buffer [20 mm HEPES (pH 7.4), 12% glycerol, 84 mm KCl, 1 mm EDTA, 1 mm dithiothreitol] and 1 μg of poly(dI-dC)-poly(dI-dC) (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) as nonspecific competitor. The DNA-protein complexes were resolved on 5% nondenaturing polyacrylamide gels and visualized by autoradiography. In vitro transcribed/translated ERRγ was synthesized using the TNT Coupled Transcription/Translation System (Promega Corp., Madison, WI). To determine the specificity of the DNA-protein complexes, competition assays were performed with a 200-fold excess of unlabeled double-stranded oligonucleotides.
ChIP assay
Human trophoblasts were cultured overnight in DMEM with 2% FBS, in 20% or 2% O2 (hypoxia). Utilizing the ChIP Kit (Millipore Corp., Bedford, MA), a ChIP assay was performed according to an earlier described protocol (53). Precleared cross-linked chromatin was immunoprecipitated using either ERRγ IgG (PA1–316; Affinity Bioreagents, Golden, CO), HIF-1α IgG (NB100–105, Novus Biologicals, Littleton, CO), or normal rabbit IgG (sc-2027; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), as control. Quantitative RT-PCR was used to assess the fold enrichment of the immunoprecipitated protein-DNA complex for ERRγ or HIF-1α. Primers were designed to amplify an approximately 100-bp region surrounding the CYP19I.1 ERRE or putative hypoxia-responsive elements (HRE1 and HRE2) of the human ERRγ (ESRRG) promoter.
Primers to amplify putative ERRE in hCYP19 promoter I.1are as follows: forward, 5′-TGC CCT CCT TTC ATC CAC C-3′; reverse, 5′-TCC TTC CTC CAG GGT ATG GG-3′.
Primers to amplify putative HREs in the ESRRG promoter are as follows. ESRRG HRE1: forward, 5′-ATC ATG TTT CTT TTG CAA AAT GCC TGT TT-3′; reverse, 5′-AAT CCA AGC ATT CTG CAG ACA CTG-3′; ESRRG HRE2: forward, 5′-AAC ACA GAC AAC GGC AGG ACA AAC-3′; reverse, 5′-AAG CAC TGG AGG GAT TTG CCT TTG-3′.
Quantitative RT-PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) on an ABI Prism 7700 Detection System. Signals were normalized to input samples. Samples from 2–3 independent ChIP experiments were analyzed.
Western blot analysis
Nuclear and cytoplasmic extracts were prepared from human trophoblast, as described previously (15). Equivalent amounts of protein determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA) were resolved by 4–12% Bis-Tris gel (Invitrogen) electrophoresis and blotted to Hybond-P membranes (Amersham Biosciences, Piscataway, NJ). Rabbit polyclonal ERRγ antibody (PA1-316) was obtained from Affinity Bioreagents (Golden, CO). Antiaromatase (A7981) was obtained from Sigma, antilamin A/C (catalog no. 05-714) was from Upstate Biotechnology, Inc. (Lake Placid, NY), anti-HIF-1α (catalog no. 610958) was from BD Biosciences (Palo Alto, CA) and anti-β-actin (ab8227) was obtained from Abcam (Cambridge, MA). Horseradish peroxidase-conjugated antirabbit or antimouse IgG from GE Healthcare were used as secondary antibodies. The membranes were developed by enhanced Supersignal West Pico Chemiluminescent substrate (Thermo Scientific, Rockford, IL).
Cell culture and transient transfection assays
COS-7 cells [American Type Culture Collection (ATCC), Manassas, VA] grown in DMEM (Life Technologies) supplemented with 10% FBS, or JEG3 cells (ATCC HTB-36) maintained in RPMI-1640 (ATCC) supplemented with 10% FBS, were transfected with hCYP19I.1:luciferase (LUC) reporter constructs containing 501 bp of the placenta-specific hCYP19 promoter I.1 containing the wild-type or mutated NRE site, β-gal expression vector, and human ERRγ or ERRα expression vector or empty vector, as a control, using LipofectAMINE Plus (Invitrogen). The cells were lysed 48 h after transfection in reporter lysis buffer (Promega, Madison, WI) and assayed for β-gal and luciferase (Promega). The mutation of the NRE site in placenta-specific hCYP19 promoter I.1 was generated using QuikChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). All constructs were confirmed by DNA sequencing.
Adenoviral overexpression and RNA interference
Recombinant adenoviruses containing ERRγ or ERRα expression vectors were generated as described previously (54). The recombinant adenoviruses were titrated in human embryonic kidney 293 cells to quantify the number of infectious viral particles (plaque-forming units/ml). Cytomegalovirus (CMV)-β-gal adenovirus was kindly provided by Dr. Joseph Alcorn (University of Texas Medical School, Houston, TX). Recombinant adenoviruses were used to infect freshly isolated cytotrophoblasts at a moi of 2.0. Lentiviral vectors containing shRNA targeting ERRγ in pGIPZ vectors were obtained from a lentivirus shRNA library (Open Biosystems, Huntsville, AL). Lentiviral supernatants were produced in human embryonic kidney 293T cells by calcium phosphate transfection of plasmids, pMD2.G, psPAX2, and human GIPZ lentiviral shRNA for ERRγ. Lentiviral supernatants were collected after 24, 48, and 72 h of culture. The viral particles were concentrated by ultracentrifugation, and the titer of the viral stock (moi or number of transducing units per cell) was determined according to the protocol recommended by Open Biosystems. For control experiments, cells were infected with nonsilencing GIPZ lentiviral shRNA control. Two different shRNA vectors were used for silencing of ERRγ: Open Biosystems catalog no. RHS4430-98514497 and catalog no. RHS4430-98912788. ERRγ gene silencing also was performed using custom ON-TARGET-Specificity Enhanced ESRRG duplex; siGLO cyclophilin B siRNA (Dharmacon, Lafayette, CO; Thermo Scientific) was used as control. HIF-1α gene silencing was performed using Silencer select siRNA (catalog no. 4390824, Invitrogen). Freshly isolated trophoblast cells from midgestation placenta were transfected in six-well plates using 25 nm siRNA with Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol.
Data analysis
Data are expressed as mean ± sem. Differences between groups were analyzed by Student's t test. Statistical significance was set as P < 0.05; each experiment was performed at least three times in triplicate.
Acknowledgments
We thank Ms. Jo Smith, University of Texas Southwestern Medical Center, for her expert assistance in preparing the primary human trophoblast cultures for these studies.
This work was supported by National Institutes of Health Grant 5 R01 DK031206 (to C.R.M.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:†:
Ligands: 17β-estradiol
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- ChIP
- Chromatin immunoprecipitation
- CMV
- cytomegalovirus
- DFO
- deferoxamine mesylate
- ER
- estrogen receptor
- ERE-LS
- estrogen-response element-like sequence
- ERR
- estrogen-related receptor
- ERRE
- ERR response element
- FBS
- fetal bovine serum
- HIF-1α
- hypoxia-inducible factor-1α
- HRE
- hypoxia-responsive element
- Mash-2
- mammalian achaete scute homologous protein-2
- moi
- multiplicity of infection
- NRE
- nuclear receptor element
- qPCR
- quantitative PCR
- qRT-PCR
- quantitative RT-PCR
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- USF
- upstream stimulatory factor
- VEGF
- vascular endothelial growth factor.
References
- 1. Everett RB, MacDonald PC. 1979. Endocrinology of the placenta. Annu Rev Med 30:473–488 [DOI] [PubMed] [Google Scholar]
- 2. Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. 1996. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 97:540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang B, Kamat A, Mendelson CR. 2000. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol Endocrinol 14:1661–1673 [DOI] [PubMed] [Google Scholar]
- 4. Fournet-Dulguerov N, MacLusky NJ, Leranth CZ, Todd R, Mendelson CR, Simpson ER, Naftolin F. 1987. Immunohistochemical localization of aromatase cytochrome P-450 and estradiol dehydrogenase in the syncytiotrophoblast of the human placenta. J Clin Endocrinol Metab 65:757–764 [DOI] [PubMed] [Google Scholar]
- 5. Kamat A, Alcorn JL, Kunczt C, Mendelson CR. 1998. Characterization of the regulatory regions of the human aromatase (P450arom) gene involved in placenta-specific expression. Mol Endocrinol 12:1764–1777 [DOI] [PubMed] [Google Scholar]
- 6. Thompson EA, Jr, Siiteri PK. 1974. The involvement of human placental microsomal cytochrome P-450 in aromatization. J Biol Chem 249:5373–5378 [PubMed] [Google Scholar]
- 7. Rosenfeld CR, Morriss FH, Jr, Battaglia FC, Makowski EL, Meschia G. 1976. Effect of estradiol-17β on blood flow to reproductive and nonreproductive tissues in pregnant ewes. Am J Obstet Gynecol 124:618–629 [DOI] [PubMed] [Google Scholar]
- 8. Magness RR, Phernetton TM, Gibson TC, Chen DB. 2005. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17β-treated, intact follicular and pregnant sheep. J Physiol 565:71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gargett CE, Zaitseva M, Bucak K, Chu S, Fuller PJ, Rogers PA. 2002. 17β-estradiol up-regulates vascular endothelial growth factor receptor-2 expression in human myometrial microvascular endothelial cells: role of estrogen receptor-α and -β. J Clin Endocrinol Metab 87:4341–4349 [DOI] [PubMed] [Google Scholar]
- 10. Kamat A, Hinshelwood MM, Murry BA, Mendelson CR. 2002. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab 13:122–128 [DOI] [PubMed] [Google Scholar]
- 11. Kamat A, Graves KH, Smith ME, Richardson JA, Mendelson CR. 1999. A 500-bp region, approximately 40 kb upstream of the human CYP19 (aromatase) gene, mediates placenta-specific expression in transgenic mice. Proc Natl Acad Sci USA 96:4575–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamat A, Smith ME, Shelton JM, Richardson JA, Mendelson CR. 2005. Genomic regions that mediate placental cell-specific and developmental regulation of human CYP19 (aromatase) gene expression in transgenic mice. Endocrinology 146:2481–2488 [DOI] [PubMed] [Google Scholar]
- 13. Hemberger M, Cross JC. 2001. Genes governing placental development. Trends Endocrinol Metab 12:162–168 [DOI] [PubMed] [Google Scholar]
- 14. Rossant J, Cross JC. 2001. Placental development: lessons from mouse mutants. Nat Rev Genet 2:538–548 [DOI] [PubMed] [Google Scholar]
- 15. Jiang B, Mendelson CR. 2003. USF1 and USF2 mediate inhibition of human trophoblast differentiation and CYP19 gene expression by Mash-2 and hypoxia. Mol Cell Biol 23:6117–6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang B, Mendelson CR. 2005. O2 enhancement of human trophoblast differentiation and hCYP19 (aromatase) gene expression are mediated by proteasomal degradation of USF1 and USF2. Mol Cell Biol 25:8824–8833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petraglia F, Florio P, Nappi C, Genazzani AR. 1996. Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev 17:156–186 [DOI] [PubMed] [Google Scholar]
- 18. Cronier L, Guibourdenche J, Niger C, Malassiné A. 1999. Oestradiol stimulates morphological and functional differentiation of human villous cytotrophoblast. Placenta 20:669–676 [DOI] [PubMed] [Google Scholar]
- 19. Kumar P, Kamat A, Mendelson CR. 2009. Estrogen receptor α (ERα) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta. Mol Endocrinol 23:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguère V. 1997. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-β. Nature 388:778–782 [DOI] [PubMed] [Google Scholar]
- 21. Heard DJ, Norby PL, Holloway J, Vissing H. 2000. Human ERRγ, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol 14:382–392 [DOI] [PubMed] [Google Scholar]
- 22. Takeda Y, Liu X, Sumiyoshi M, Matsushima A, Shimohigashi M, Shimohigashi Y. 2009. Placenta expressing the greatest quantity of bisphenol A receptor ERRγ among the human reproductive tissues: predominant expression of type-1 ERRγ isoform. J Biochem 146:113–122 [DOI] [PubMed] [Google Scholar]
- 23. Yu DD, Forman BM. 2005. Identification of an agonist ligand for estrogen-related receptors ERRβ/γ. Bioorg Med Chem Lett 15:1311–1313 [DOI] [PubMed] [Google Scholar]
- 24. Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguère V. 2007. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab 5:345–356 [DOI] [PubMed] [Google Scholar]
- 25. Hirsilä M, Koivunen P, Xu L, Seeley T, Kivirikko KI, Myllyharju J. 2005. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J 19:1308–1310 [DOI] [PubMed] [Google Scholar]
- 26. Semenza GL, Agani F, Iyer N, Kotch L, Laughner E, Leung S, Yu A. 1999. Regulation of cardiovascular development and physiology by hypoxia-inducible factor 1. Ann NY Acad Sci 874:262–268 [DOI] [PubMed] [Google Scholar]
- 27. Giguère V, Yang N, Segui P, Evans RM. 1988. Identification of a new class of steroid hormone receptors. Nature 331:91–94 [DOI] [PubMed] [Google Scholar]
- 28. Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguère V. 2003. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol 23:7947–7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alaynick WA, Way JM, Wilson SA, Benson WG, Pei L, Downes M, Yu R, Jonker JW, Holt JA, Rajpal DK, Li H, Stuart J, McPherson R, Remlinger KS, Chang CY, McDonnell DP, Evans RM, Billin AN. 2010. ERRγ regulates cardiac, gastric, and renal potassium homeostasis. Mol Endocrinol 24:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J, Nathans J. 2007. Estrogen-related receptor β/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell 13:325–337 [DOI] [PubMed] [Google Scholar]
- 31. Bombail V, MacPherson S, Critchley HO, Saunders PT. 2008. Estrogen receptor related β is expressed in human endometrium throughout the normal menstrual cycle. Hum Reprod 23:2782–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie CQ, Jeong Y, Fu M, Bookout AL, Garcia-Barrio MT, Sun T, Kim BH, Xie Y, Root S, Zhang J, Xu RH, Chen YE, Mangelsdorf DJ. 2009. Expression profiling of nuclear receptors in human and mouse embryonic stem cells. Mol Endocrinol 23:724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rangwala SM, Wang X, Calvo JA, Lindsley L, Zhang Y, Deyneko G, Beaulieu V, Gao J, Turner G, Markovits J. 2010. Estrogen-related receptor γ is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem 285:22619–22629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fox H. 1970. Effect of hypoxia on trophoblast in organ culture. A morphologic and autoradiographic study. Am J Obstet Gynecol 107:1058–1064 [DOI] [PubMed] [Google Scholar]
- 35. Alsat E, Wyplosz P, Malassiné A, Guibourdenche J, Porquet D, Nessmann C, Evain-Brion D. 1996. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol 168:346–353 [DOI] [PubMed] [Google Scholar]
- 36. Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. 1997. Regulation of human placental development by oxygen tension. Science 277:1669–1672 [DOI] [PubMed] [Google Scholar]
- 37. Huot RI, Foidart JM, Stromberg K. 1979. Effects of culture conditions on the synthesis of human chorionic gonadotropin by placental organ cultures. In Vitro 15:497–502 [DOI] [PubMed] [Google Scholar]
- 38. Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. 2007. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 318:1786–1789 [DOI] [PubMed] [Google Scholar]
- 39. Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. 2007. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol 14:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reinking J, Lam MM, Pardee K, Sampson HM, Liu S, Yang P, Williams S, White W, Lajoie G, Edwards A, Krause HM. 2005. The Drosophila nuclear receptor e75 contains heme and is gas responsive. Cell 122:195–207 [DOI] [PubMed] [Google Scholar]
- 41. Marvin KA, Reinking JL, Lee AJ, Pardee K, Krause HM, Burstyn JN. 2009. Nuclear receptors homo sapiens Rev-erbβ and Drosophila melanogaster E75 are thiolate-ligated heme proteins which undergo redox-mediated ligand switching and bind CO and NO. Biochemistry 48:7056–7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pardee KI, Xu X, Reinking J, Schuetz A, Dong A, Liu S, Zhang R, Tiefenbach J, Lajoie G, Plotnikov AN, Botchkarev A, Krause HM, Edwards A. 2009. The structural basis of gas-responsive transcription by the human nuclear hormone receptor REV-ERBβ. PLoS Biol 7:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Semenza GL. 2001. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1–3 [DOI] [PubMed] [Google Scholar]
- 44. Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. 1997. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA 94:9102–9107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. 2000. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev 14:3191–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoo YG, Hayashi M, Christensen J, Huang LE. 2009. An essential role of the HIF-1α-c-Myc axis in malignant progression. Ann NY Acad Sci 1177:198–204 [DOI] [PubMed] [Google Scholar]
- 47. Ke Q, Costa M. 2006. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70:1469–1480 [DOI] [PubMed] [Google Scholar]
- 48. Seifeddine R, Dreiem A, Blanc E, Fulchignoni-Lataud MC, Frère Belda MA, Lecuru F, Mayi TH, Mazure N, Favaudon V, Massaad C, Barouki R, Massaad-Massade L. 2008. Hypoxia down-regulates CCAAT/enhancer binding protein-α expression in breast cancer cells. Cancer Res 68:2158–2165 [DOI] [PubMed] [Google Scholar]
- 49. Narravula S, Colgan SP. 2001. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor α expression during hypoxia. J Immunol 166:7543–7548 [DOI] [PubMed] [Google Scholar]
- 50. Chen KF, Lai YY, Sun HS, Tsai SJ. 2005. Transcriptional repression of human cad gene by hypoxia inducible factor-1α. Nucleic Acids Res 33:5190–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III 1986. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118:1567–1582 [DOI] [PubMed] [Google Scholar]
- 52. Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- 53. Chakrabarti SK, James JC, Mirmira RG. 2002. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem 277:13286–13293 [DOI] [PubMed] [Google Scholar]
- 54. Liu D, Benlhabib H, Mendelson CR. 2009. cAMP enhances estrogen-related receptor α (ERRα) transcriptional activity at the SP-A promoter by increasing its interaction with protein kinase A and steroid receptor coactivator 2 (SRC-2). Mol Endocrinol 23:772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]