Abstract
Most normal human diploid cells have no detectable telomerase; however, expression of the catalytic subunit of telomerase is sufficient to induce telomerase activity and, in many cases, will bypass normal senescence. We and others have previously demonstrated in vitro assembly of active telomerase by combining the purified RNA component with the reverse transcriptase catalytic component synthesized in rabbit reticulocyte extract. Here we show that assembly of active telomerase from in vitro-synthesized components requires the contribution of proteins present in reticulocyte extracts. We have identified the molecular chaperones p23 and Hsp90 as proteins that bind to the catalytic subunit of telomerase. Blockade of this interaction inhibits assembly of active telomerase in vitro. Also, a significant fraction of active telomerase from cell extracts is associated with p23 and Hsp90. Consistent with in vitro results, inhibition of Hsp90 function in cells blocks assembly of active telomerase. To our knowledge, p23 and Hsp90 are the first telomerase-associated proteins demonstrated to contribute to telomerase activity.
Keywords: Telomerase, telomeres, chaperones, senescence, cancer
Telomeres are specialized structures at the ends of linear eukaryotic chromosomes that, in conjunction with the enzyme telomerase, provide a mechanism for maintaining chromosome length. Additional critical functions include protecting chromosome ends from the double-strand break repair system and mediating chromosome alignment during meiosis (for review, see Blackburn 1995; Kipling 1995). Normal lagging-strand DNA replication fails to copy the ends of linear molecules, leaving a gap between the final RNA priming event and the terminus (Watson 1972; Olovnikov 1973; Wright et al. 1997). In the absence of a compensatory mechanism, this progressive shortening with each cell division ultimately causes proliferative failure. Telomerase provides the enzymatic activity that compensates for this shortening. Vertebrate telomeres are composed of many kilobases of TTAGGG repeats (Moyzis et al. 1988). Telomerase is a ribonucleoprotein that uses the complementary sequence in its associated RNA as a template for a reverse transcription reaction that adds telomeric repeats to the ends of chromosomes (Greider and Blackburn 1989; Feng et al. 1995; Lingner et al. 1997; Meyerson et al. 1997; Nakamura et al. 1997).
Telomerase is present in the germ line but is repressed in most human tissues during development (Wright et al. 1996). Although activity can be detected in the adult bone marrow and proliferating epithelial tissues such as skin and the intestine (e.g., Broccoli et al. 1995; Hiyama et al. 1996; Taylor et al. 1996), this activity is not sufficient to maintain telomere length in the progeny of these dividing cells, and telomere shortening is still observed as a function of donor age (Hastie et al. 1990; Lindsey et al. 1991; Vaziri et al. 1993; Hiyama et al. 1996). The telomere shortening observed in vivo correlates with decreased proliferative capacity of cells as a function of donor age (Martin et al. 1970; Schneider and Mitsui 1976), and telomere shortening is also observed as a function of population doublings of cultured cells (Harley et al. 1990; Allsopp et al. 1992), suggesting that telomere shortening might be the mechanism underlying in vitro cellular senescence (Harley 1991). This has now been confirmed by demonstrating that restoring telomerase activity through the exogenous expression of the telomerase catalytic subunit (hTERT) at levels sufficient to maintain or elongate telomeres is sufficient to prevent cellular senescence in a variety of cell types (Bodnar et al. 1998; Vaziri and Benchimol 1998).
The catalytic core of human telomerase has been identified and consists of a reverse transcriptase (hTERT) (Meyerson et al. 1997; Nakamura et al. 1997) complexed with the RNA template component (hTR) (Feng et al. 1995). Partially purified native telomerase migrates as a very high molecular weight complex on glycerol gradients, suggesting that it exists as a complex with a variety of other cellular proteins that may regulate assembly of the ribonucleoprotein (RNP) and/or the functional activity of telomerase at the telomere. A protein that interacts with human telomerase, TLP-1, has been identified (Harrington et al. 1997; Nakayama et al. 1997), but its role in the assembly or regulation of telomerase activity is not known and its absence does not influence telomerase activity in vitro (Beattie et al. 1998). To identify telomerase-associated proteins that contribute to the assembly and function of the holoenzyme, we have used the yeast two-hybrid system to isolate hTERT-interacting proteins and studied the requirement of these factors in the assembly of active telomerase in vitro and in vivo. We find that the molecular chaperones p23 and Hsp90 bind to hTERT and are associated with active telomerase in cells. Blocking association of p23 or Hsp90 with hTERT blocks assembly of functional telomerase in vitro and in vivo. Thus, the p23 and Hsp90 interaction with hTERT is required for production of active telomerase. These molecules are, to our knowledge, the first hTERT-associated proteins demonstrated to functionally contribute to telomerase activity.
Results
Factors present in rabbit reticulocyte lysate are required for assembly of active telomerase in vitro
We and others have demonstrated that in vitro-transcribed and -translated hTERT, together with purified hTR, is sufficient to assemble functional telomerase in vitro (Weinrich et al. 1997; Beattie et al. 1998). It is possible that unknown proteins present in rabbit reticulocyte lysates (RRLs) used to synthesize hTERT associate with hTERT and/or hTR and participate in the production of functional telomerase. To determine if such cofactors are present in RRLs, hTERT synthesized in the absence of hTR was diluted 20-fold in an assembly reaction containing purified hTR together with buffer or increasing amounts of RRL. Following incubation for 90 min, aliquots were removed and assayed for telomerase activity. Little activity was observed when hTERT and hTR were combined in the absence of RRLs; however, the presence of RRLs in the assembly reaction resulted in significant enhancement of detectable telomerase activity (Fig. 1). The telomerase activity in all samples was stable at 30°C for at least 6 hr, indicating that the reticulocyte lysate does not simply enhance enzyme stability (data not shown). A >100-fold increase in activity was observed when hTERT and hTR were assembled in the highest tested concentration of RRLs (Fig. 1). This stimulation was independent of additional protein synthesis, as the same effect was seen in the presence of cycloheximide (data not shown). Heat-denatured RRLs were incapable of enhancing assembly of telomerase activity, suggesting that the effects were mediated by proteins (data not shown).
Figure 1.
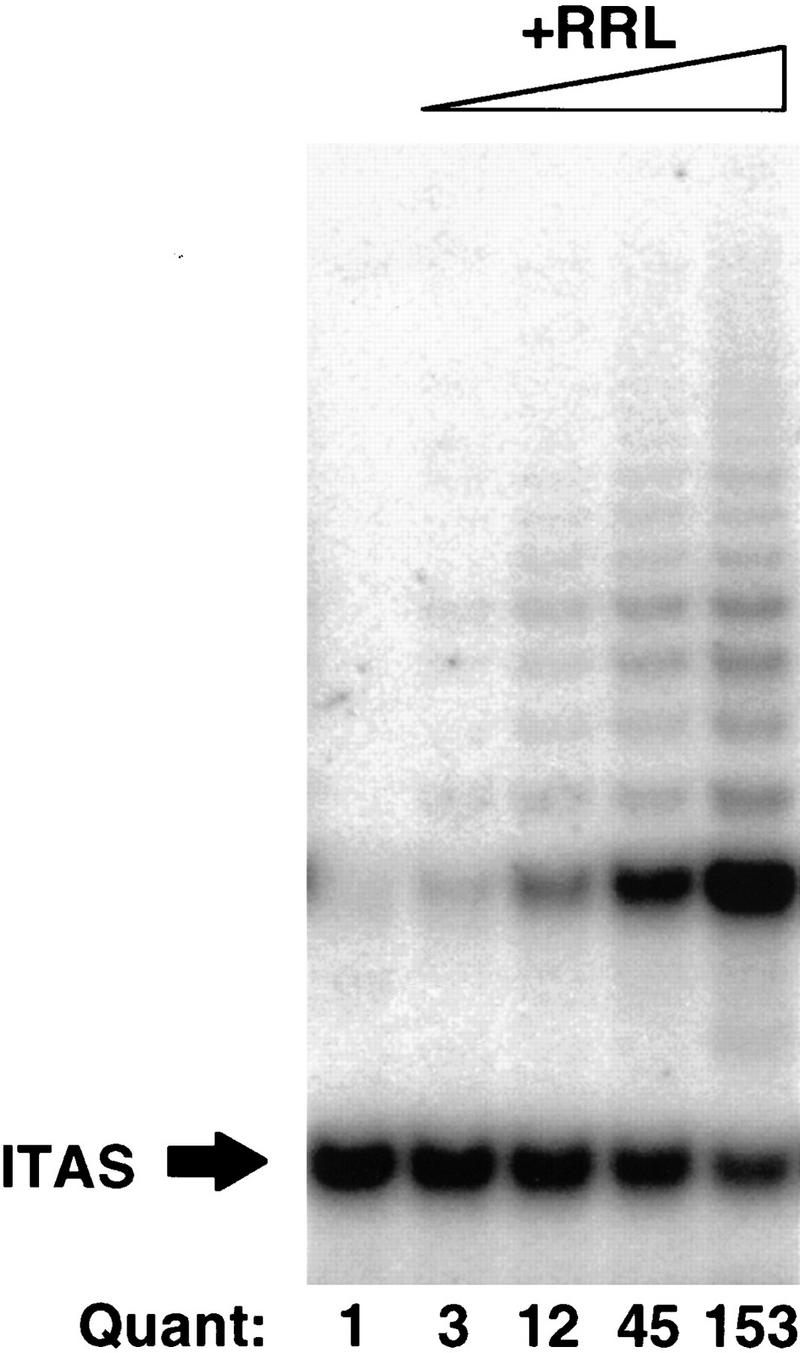
In vitro assembly of active telomerase from hTERT and hTR requires additional components present in reticulocyte lysates. In vitro-transcribed and -translated hTERT was mixed with purified hTR in the presence of increasing concentrations of rabbit reticulocyte lysate (0, 12.5%, 25%, 37.5%, and 50% of reaction volume). Following incubation for 90 min at 30°C, samples were assayed for telomerase activity by TRAP analysis. The ratio of signal from extended products vs. the 36-bp internal TRAP assay standard (ITAS) was determined by densitometetry analysis. Values shown represent these ratios normalized to that observed in the absence of additional RRLs, which was set at 1. This experiment has been repeated numerous times with identical results.
p23 and Hsp90 associate with human telomerase reverse transcriptase
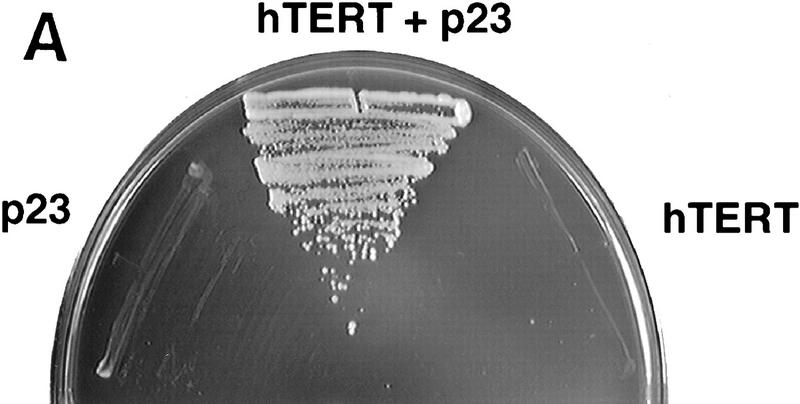
To identify protein cofactors that contribute to the assembly or activity of telomerase, we used the yeast two-hybrid system (Fields and Song 1989) to screen for hTERT-interacting proteins. Expression of hTERT cDNA in yeast did not result in the production of any detectable hTERT protein despite the accumulation of hTERT mRNA (data not shown). This may be due to the presence of a high proportion of codons that require rare yeast tRNAs. We therefore constructed a synthetic coding sequence for hTERT that reflects the codon bias observed in yeast genes. Expression of a synthetic cDNA encoding amino acids 1–195 of hTERT fused to the LexA DNA-binding domain (Vojtek et al. 1993) resulted in readily detectable levels of protein (data not shown). A two-hybrid screen of this bait against a mouse embryo cDNA library (Vojtek et al. 1993) yielded a clone encoding p23 (Fig. 2A). This acidic phosphoprotein associates with Hsp90 in an ATP-dependent manner (Johnson et al. 1994). Together with Hsp90, p23 has been implicated in mediating the formation of receptor/ligand (Hutchison et al. 1995; Pratt and Toft 1997) and protein/RNA (Hu et al. 1997) complexes. The p23 clone did not associate with other ‘irrelevant’ two-hybrid baits including an H-Ras–LexA fusion and a ralGDS–LexA fusion (data not shown).
Figure 2.
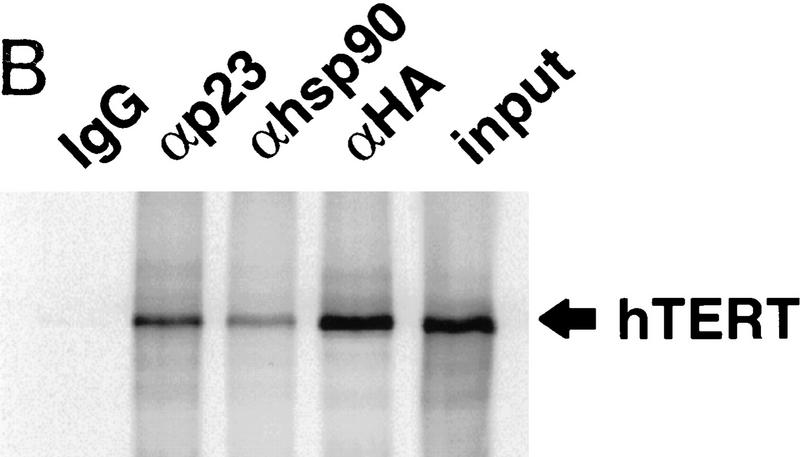
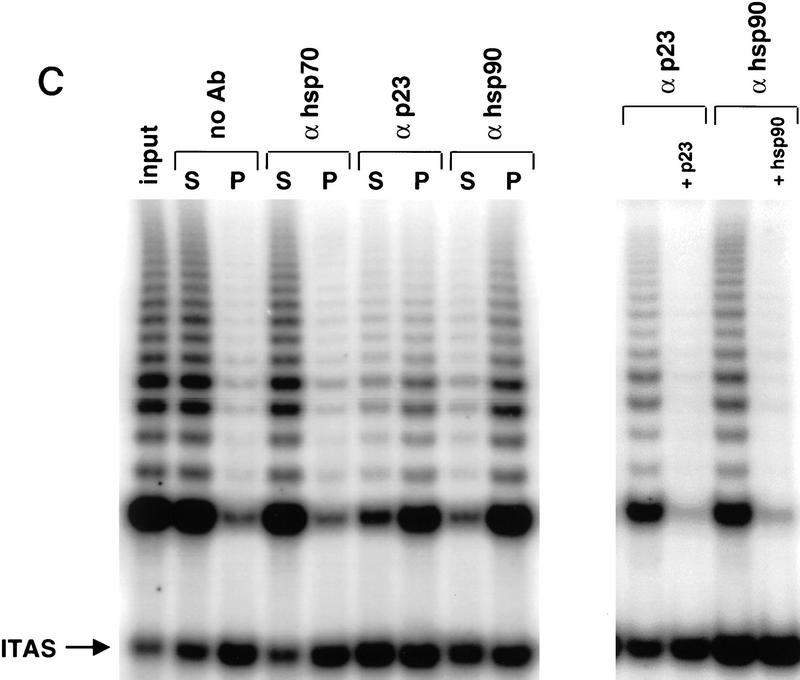
p23 and Hsp90 bind hTERT. (A) Transformants of the yeast two-hybrid reporter strain L40 selected to express LexA–hTERT (1–195) and VP16–p23, alone or together, were tested for the ability to grow on medium lacking histidine. Growth on the selective plate indicates a positive two-hybrid interaction. (B) The indicated antibodies were used to immunoprecipitate proteins from RRLs containing HA-tagged hTERT synthesized with [35S]methionine in the absence of the template RNA. Following extensive washing, the immunoprecipitates were separated by SDS-PAGE, and the presence of coprecipitated hTERT was determined by PhosphorImager analysis. Ten percent of the total translation reaction was loaded to reveal the amount of labeled hTERT present in the reticulocyte lysates. Repeated experiments gave similar results. (C) The indicated antibodies were used to immunoprecipitate proteins from lysates of untransfected HT1080 cells. The immunoprecipitates were washed extensively and assayed for telomerase activity by TRAP. The input lane corresponds to activity present in lysate from 1000 cells. TRAP assays were performed on the pellets (P) and supernatents (S) of immunoprecipitates from lysates of 2000 cells each. Antibodies to p23 and Hsp90 were precoated with saturating levels of purified p23 (+p23) or Hsp90 (+hsp90) to demonstrate specificity of the immunoprecipitation.
Both p23 and Hsp90 are present in RRL, and much of the p23 is bound to Hsp90 (Johnson and Toft 1994). Therefore, we tested for an association of in vitro-transcribed and -translated hTERT with p23 and Hsp90. [35S]Methionine-labeled HA-tagged hTERT was immunoprecipitated with anti-p23, anti-Hsp90, or anti-HA antibodies, demonstrating that full-length hTERT is associated with p23 and Hsp90 in vitro (Fig. 2B). Although <10% was associated, this may simply reflect inefficiency of the immunoprecipitation, as the anti-HA antibody was similarly inefficient. The association of hTERT with p23 and Hsp90 did not require the presence of the telomerase template RNA. The relative in vitro telomerase activity in the immunoprecipitations was proportional to the amount of hTERT protein observed in each sample (data not shown).
Next, we investigated the association of p23 and Hsp90 with telomerase activity in cell extracts. HT1080, a human fibrosarcoma cell line, is immortal and expresses active telomerase (Kim et al. 1994). Lysates of HT1080 cells were prepared by sonication in weak detergent. Antibodies to p23 or Hsp90 bound to agarose beads precipitated telomerase activity from these lysates (Fig. 2C), whereas antibody to the chaperone Hsp70 and a control antibody (MAP1B) (data not shown) failed to precipitate any activity above background levels (Fig. 2C). Preincubation of anti-p23 antibodies with purified p23 prior to incubation with lysates blocked the association of telomerase activity with the precipitates. Similarly, incubation of Hsp90 antibodies with purified Hsp90 blocked immunoprecipitation of telomerase activity (Fig. 2C). Quantitation of telomerase activity present in immunoprecipitates from seven experiments performed in parallel showed that anti-p23 immunoprecipitates contained 20 ± 9% of input telomerase activity, and anti-Hsp90 precipitates contained 37 ± 11% of input. Activity recovered with beads alone, anti-Hsp70, or a control antibody (anti-MAP1) ranged from 2%–5%. Thus p23 and Hsp90 appear to be specifically associated with a significant fraction of the active telomerase in cells. Approximately 90% of the input activity was depleted from the supernatants of anti-p23 and anti-Hsp90 precipitations. Thus, the majority of telomerase in cell lysates may be bound to p23 and Hsp90. The failure to recover corresponding activity in the immunoprecipitates may be due to losses during wash steps, degredation, or partial inhibition of enzyme activity by precipitation.
p23 participates in assembly of functional telomerase in vitro
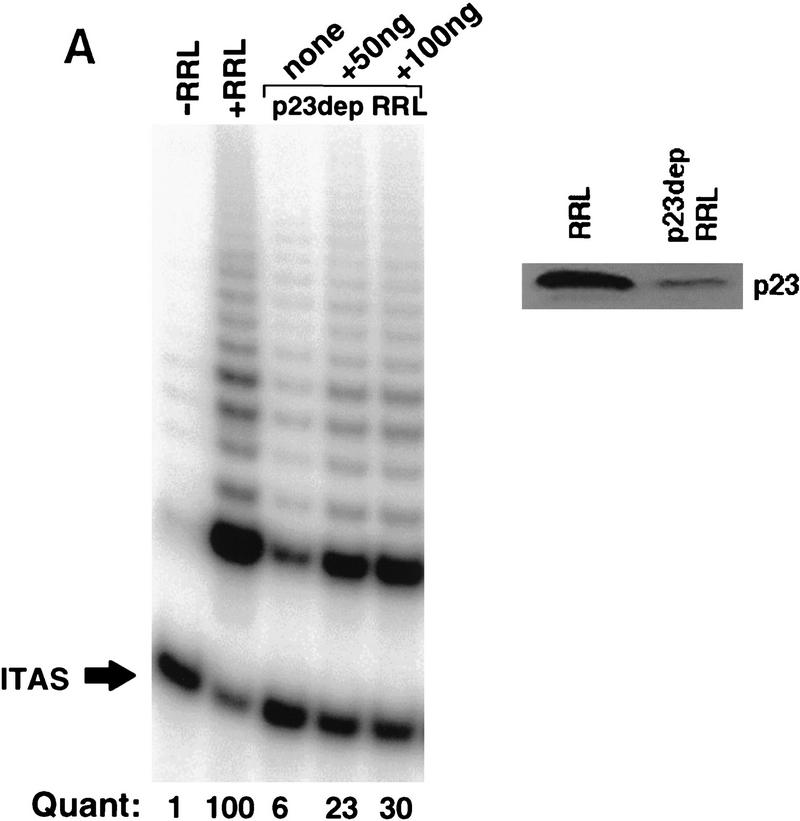
The functional importance of the p23/hTERT association was assessed in telomerase assembly reactions depleted of p23. Following immunodepletion with anti-p23 antibody precoupled to agarose beads, RRL was severely impaired in its ability to stimulate assembly of active telomerase (Fig. 3A). Addition of purified recombinant p23 to the immunodepleted RRL restored activity, demonstrating that p23 promotes efficient assembly of active telomerase in vitro.
Figure 3.
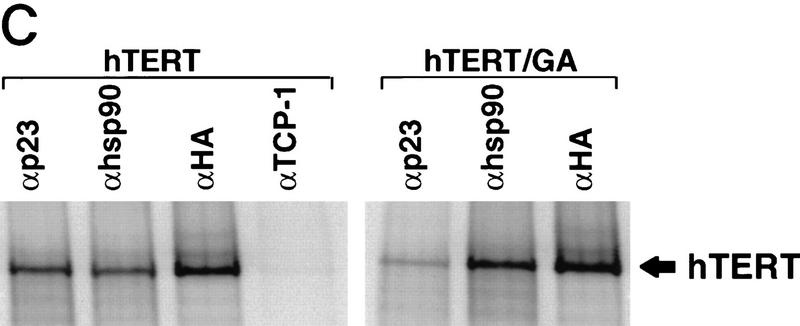
p23 and Hsp90 are required to assemble active telomerase in vitro. (A) In vitro-transcribed and -translated hTERT was diluted 1/20 into buffer (−RRL), fresh RRLs (+RRL), p23-depleted RRLs (p23 dep RRL), or p23-depleted RRLs supplemented with recombinant p23 at the indicated concentrations, and mixed with 0.5 μg of in vitro-transcribed hTR. The mixtures were incubated at 30°C for 90 min to allow reconstitution of hTERT and its hTR template. Aliquots were then removed and assayed for activity by TRAP. Depletion of p23 from RRLs was verified by Western analysis using the anti-p23 antibody (right). The results shown are representative of two independent experiments. Quantitation was performed by densitometry analysis as described in Fig. 1. Among many possibilities, failure to fully reconstitute activity with purified p23 may be due to codepletion of p23-associated factors. (B) Telomerase was reconstituted as described in A except that translated hTERT was diluted in RRLs that had been incubated in the presence of geldanamycin (+GA-pre) (100 μg/ml) or DMSO carrier for 30 min. +GA-Post indicates addition of geldanamycin (100 μg/ml) to the TRAP assay after the reconstitution of hTERT and hTR in normal RRLs. Repeated experiments gave identical results. (C) 35S-labeled HA-tagged hTERT synthesized in the presence of DMSO carrier (hTERT) or 50 μg/ml geldanamycin (hTERT/GA) was assayed for association with p23 and Hsp90 as described in Fig 1. An antibody to TCP-1, an abundant chaperone, was included as an additional control for specificity.
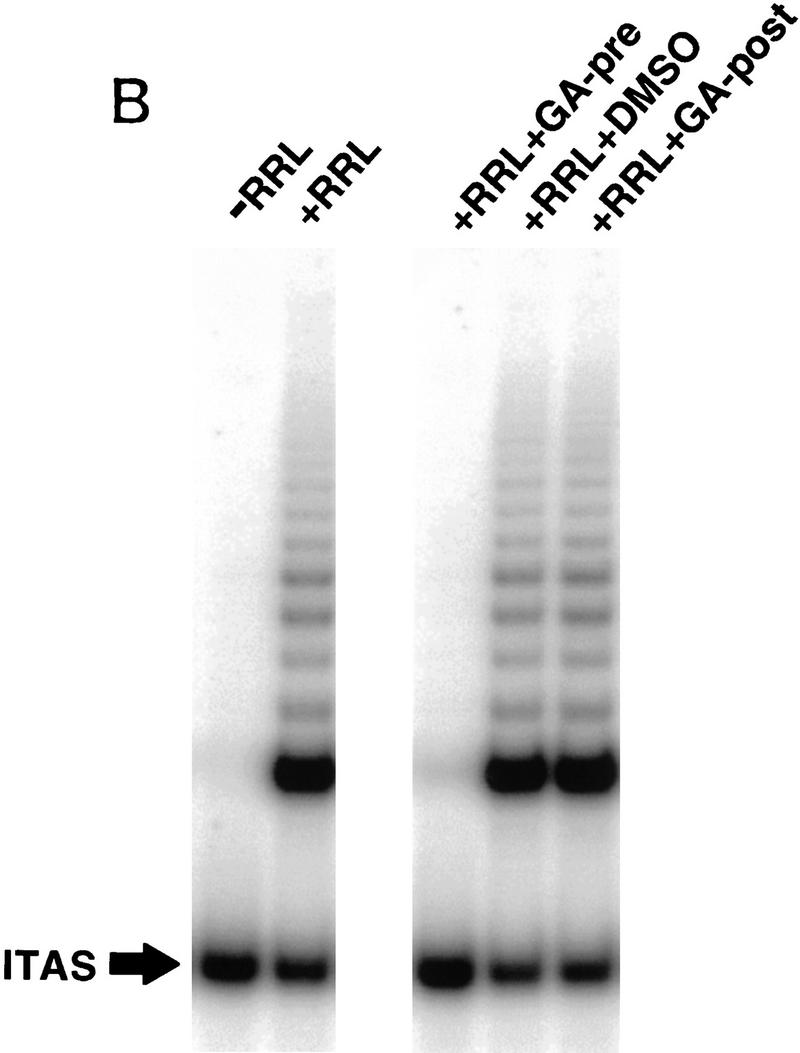
Geldanamycin blocks assembly of active telomerase in vitro
The benzoquinone ansamysin, geldanamycin, inhibits some functions of Hsp90 by binding to its ATP-binding site, and it also blocks ATP-dependent binding of p23 to Hsp90 (Grenert et al. 1997; Prodromou et al. 1997). Pretreatment of RRLs with geldanamycin completely blocked its ability to enhance assembly of active telomerase (Fig. 3B). Addition of geldanamycin after the assembly step but prior to the telomerase activity assay (Fig. 3B) had no effect; thus, geldanamycin did not inhibit association of fully assembled hTERT/hTR with the substrate primer or inhibit in vitro enzymatic activity. Synthesis of hTERT in the presence of 50 μg/ml geldanamycin resulted in a completely inactive enzyme (data not shown). Although Hsp90 association with hTERT is not affected under these conditions, p23 association is reduced significantly (Fig. 3C), suggesting that Hsp90 may recruit p23 to hTERT complexes. Incubation of hTERT in geldanamycin after the assembly step did not affect Hsp90 or p23 association with hTERT (data not shown), suggesting that either Hsp90 complexed with p23 and hTERT is inaccessible to geldanamycin or p23 is associated with hTERT independently of Hsp90 once p23 has been recruited to the complex.
Geldanamycin blocks assembly of active telomerase in vivo
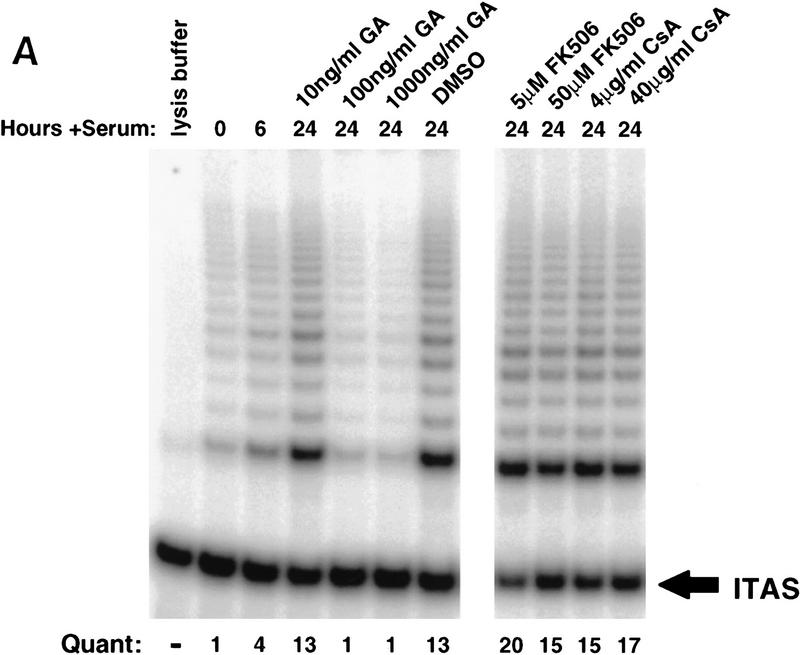
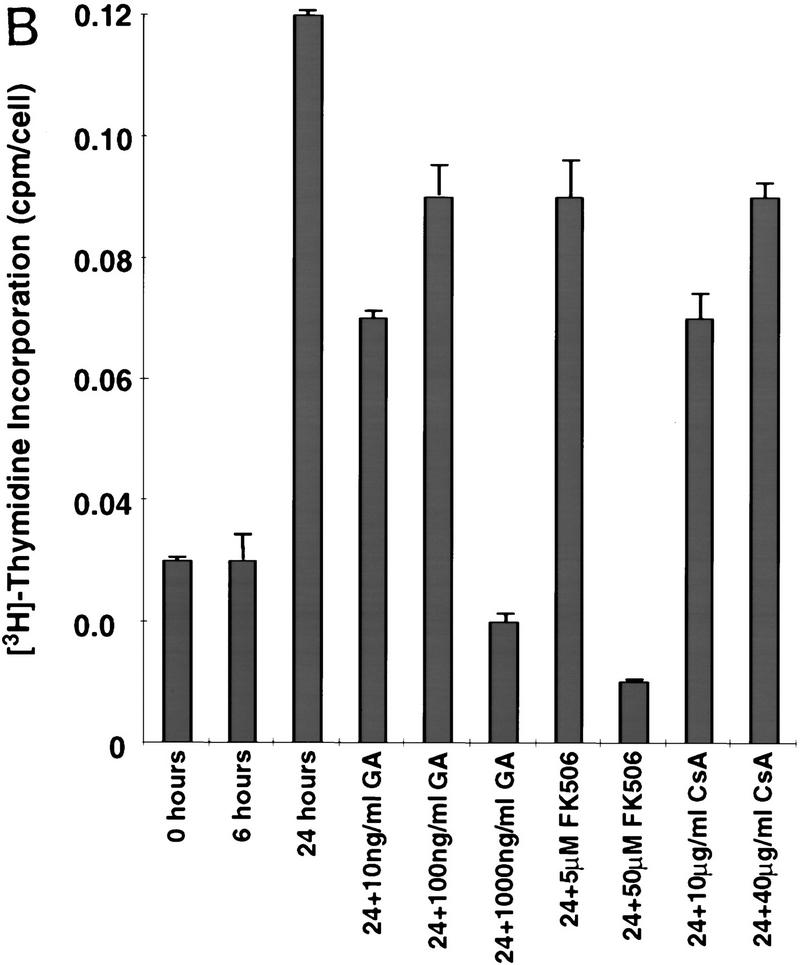
The functional importance of p23 and Hsp90 in telomerase complexes in intact cells was established using geldanamycin to inhibit Hsp90/p23 activity. Telomerase activity in human fibrosarcoma cells (HT1080) is down-regulated when the cells become quiescent and is reinduced upon growth stimulation (Holt et al. 1997). Serum-starved, quiescent HT1080 cells with low levels of telomerase activity were treated with serum plus geldanamycin or the carrier DMSO. Cells treated with carrier alone expressed high levels of telomerase activity 24 hr postserum stimulation (Fig. 4A). In contrast, cells treated with geldanamycin at concentrations of 100 ng/ml or greater failed to express active telomerase in response to serum (Fig. 4A). Consistent with published observations, concentrations of geldanamycin required to inhibit Hsp90 in cells are 1000-fold less than those required to inhibit Hsp90 in vitro (Hu and Seeger 1996). Geldanamycin blocked induction of telomerase activity at concentrations that did not affect S-phase entry or short-term viability (Fig. 4B; data not shown). p23 and Hsp90 have been reported to exist in complexes containing one or more immunophilins including FK506-binding proteins and cyclosporin A binding protein (Freeman et al. 1996; Pratt and Toft 1997). Incubation of HT1080 cells with FK506 or cyclosporin, even at concentrations that blocked induction of DNA synthesis, or that were moderately toxic over the 24-hr incubation (Fig. 4B), did not affect the induction of telomerase activity (Fig. 4A). These results are consistent with a requirement for p23 and Hsp90 but not immunophilin function for induction of active telomerase in vivo.
Figure 4.
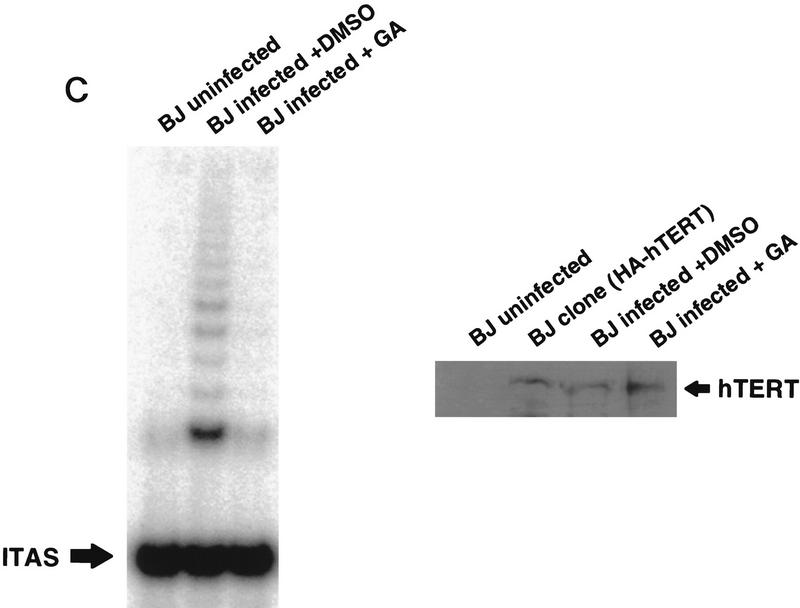
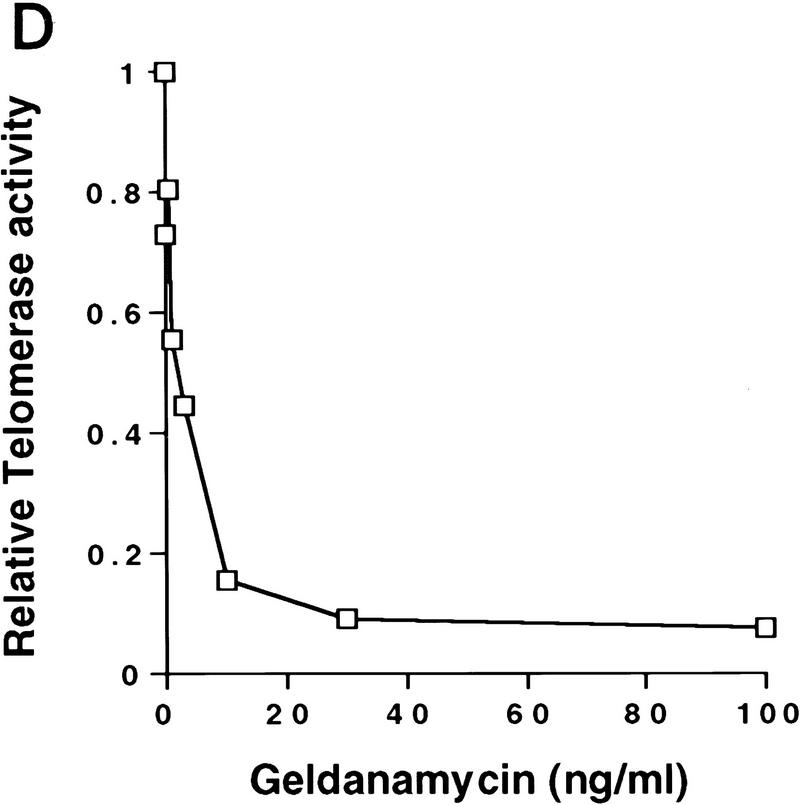
Exposure of cells to an inhibitor of Hsp90 blocks induction of telomerase activity. HT1080 cells were serum starved for 14 days, followed by addition of 10% serum, together with the indicated concentrations of geldanamycin, DMSO carrier, FK506, or cyclosporin A (CsA). (A) Treated cells were lysed and assayed for telomerase activity by TRAP. Data shown are representative of three independent experiments. (B) Parallel plates were assayed for [3H]thymidine incorporation. Effects on plating efficiency were assayed by cell counts before and after stimulation followed by replating. All samples had similar plating efficiencies of 70%–80% except for treatment with 40 μg/ml of CsA, which reduced plating efficiencies to 60% (data not shown). (C) Telomerase negative primary human fibroblasts (BJ cells) were infected with retrovirus expressing HA-tagged hTERT. Four hours postinfection, cells were incubated with 100 ng/ml geldanamycin; 24 hr postinfection, cells were harvested and assayed for telomerase activity by TRAP analysis and expression of the HA-tagged hTERT by Western analysis using the anti-HA (12CA5) antibody. Lysates from a selected population of BJ cells expressing HA-tagged hTERT were used as a positive control for the Western analysis. (D) Cells were treated as described in C with a range of concentrations of geldanamycin. TRAP activity following treatment was quantitated as in Fig 1. Values plotted are the average of two experiments.
We also tested if geldanamycin treatment blocked activity of ectopically expressed HA-tagged hTERT under control of a retroviral promoter. Primary human fibroblasts (BJ) contain no detectable telomerase activity (Bodnar et al. 1998). These cells express hTR but not hTERT (Nakamura et al. 1997). Infection of BJ cells with retrovirus expressing HA-tagged hTERT results in induction of telomerase activity by 24 hr postinfection (Fig. 4C). Treatment of BJ cells with geldanamycin 4 hr after infection with hTERT retrovirus inhibited production of detectable telomerase activity 24 hr later. Incubation in geldanamycin did not block expression of the HA-tagged hTERT (Fig. 4C). The minimal dose of geldanamycin required to inhibit production of active telomerase in the infected BJ cells was <10 ng/ml (Fig. 4D).
Purified recombinant components of a ‘foldosome’ complex mediate telomerase assembly in vitro
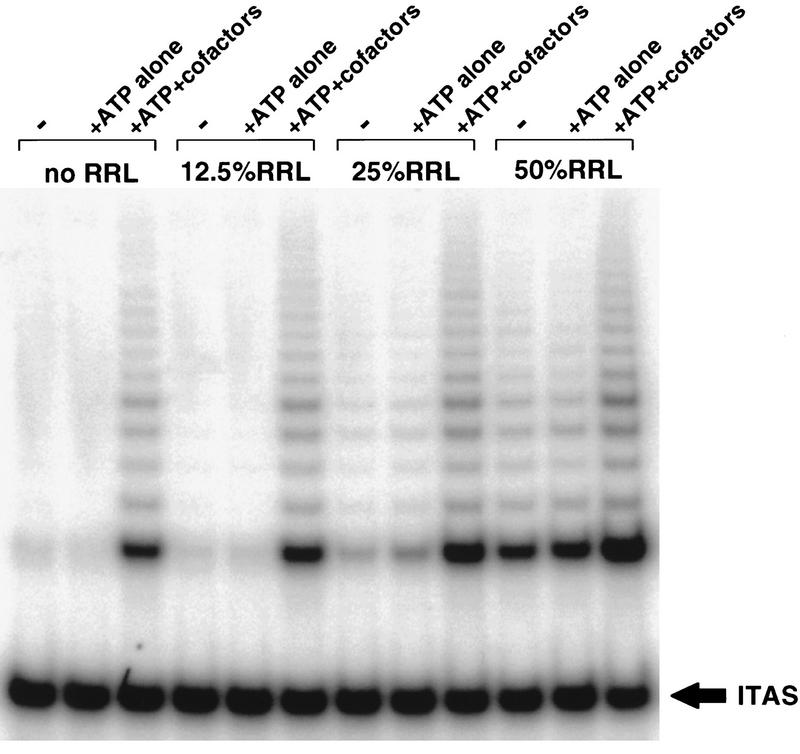
p23 has been studied extensively for its role in stabilizing steroid receptors in a conformation competent to associate with ligand (for review, see Pratt and Toft 1997). Glucocorticoid receptor reconstitution studies have demonstrated the sufficiency of a group of purified chaperone proteins to mediate a multistep complex formation that results in a functionally active receptor. These proteins include Hsp90, p23, Hsp70, Hop (HSP organizing protein), and Hsp40 (Dittmar et al. 1997). Hsp90 and p23, but not Hsp70, Hop, or Hsp40, remain stoichiometricly associated with glucocorticoid receptors prior to ligand binding. However, Hsp70, HOP, and Hsp40 are required to allow association of Hsp90 and p23 with the receptor (Dittmar et al. 1997, 1998). A similar ‘foldosome’ activity has been described for the progesterone receptor (Kosano et al. 1998). Substitution of purified Hsp90 and p23 for RRL in an in vitro telomerase assembly reaction did not enhance the production of active telomerase (data not shown). However, when Hsp70, Hop, and Ydj-1 [the yeast homolog of Hsp40 (Caplan et al. 1992) were added to the assembly reaction in addition to Hsp90 and p23, levels of telomerase activity were obtained that were comparable to that resulting from assembly reactions containing RRLs (Fig. 5). Dropping out any single protein resulted in decreased levels of telomerase activity (data not shown), suggesting that these molecules are core to a minimally sufficient telomerase assembly reaction.
Figure 5.
Purified foldosome components substitute for RRLs to assemble active telomerase in vitro. In vitro-transcribed and -translated hTERT was diluted 1/20 into an assembly reaction containing purified hTR and buffer in the absence (−) or presence (+ATP alone) of 5 mm ATP, or in the presence of 5 mmATP and purified foldosome components: p23, Hsp90, Hop, Hsp70, YDJ1 (+ATP+cofactors). Identical assembly reactions contained increasing concentrations of RRLs. All reactions were incubated for 90 min at 30°C followed by TRAP analysis. The experiment shown is representative of three independent experiments.
Discussion
The reverse transcriptase catalytic core (hTERT) (Meyerson et al. 1997; Nakamura et al. 1997) and the RNA template (hTR) (Feng et al. 1995) components of human telomerase have been cloned. These two components, together with previously unidentified factors present in RRLs are sufficient to assemble active telomerase in vitro (Weinrich et al. 1997; Beattie et al. 1998; this work). To identify the cofactors required to assemble functional telomerase, we screened for hTERT-interacting proteins using the yeast two-hybrid system. From a screen using the amino terminus of hTERT (amino acids 1–195), we identified chaperone p23 as a telomerase-interacting protein. p23 was found to be associated with hTERT proteins synthesized in vitro and functions coordinately with Hsp90 in cells, to which it binds in an ATP-dependent manner (for review, see Toft 1998). Like p23, Hsp90 was found to be associated with hTERT proteins synthesized in vitro. The functional importance of these interactions was revealed by the observation that the presence of p23 and Hsp90 in RRLs was required for assembly of functional telomerase from in vitro-synthesized components.
p23 was first identified as a component of progesterone and glucocorticoid receptor complexes (Johnson et al. 1994; Hutchison et al. 1995). Subsequently, it was found that p23 is associated with Hsp90 in these complexes and that the presence of both molecules is required to maintain these receptors in a high-affinity ligand binding state (Hutchison et al. 1995; Johnson and Toft 1995). A series of studies examining complex formation revealed that it was a multistep process involving additional transiently associated chaperones; Hsp70, Hop, and Hsp40 (Dittmar and Pratt 1997; Dittmar et al. 1997, 1998). These observations led to the concept of a molecular chaperone machine or foldosome that mediates assembly of a biologically active protein complex. Our observations suggest that a similar foldosome functions to assemble active telomerase. Purified recombinant foldosome components substituted for the requirement of RRLs in telomerase assembly reactions. A similar activity is most likely present in vivo, as geldanamycin, a specific inhibitor of Hsp90 (Toft 1998), blocked induction of native telomerase activity in fibrosarcoma cells, and blocked the activity of ectopically expressed hTERT in normal human fibroblasts.
We find that a significant fraction of telomerase activity from cells can be immunoprecipitated with antibodies against p23 or Hsp90. Geldanamycin, an Hsp90 inhibitor, blocks assembly of active telomerase in vivo. These observations, coupled with the in vitro data described above, strongly suggest that p23 and Hsp90 are associated with telomerase in cells and are required to produce functional telomerase at least at the level of assembly of the RNP. The apparent continued association of p23 and Hsp90 with a significant portion of active enzyme from cells hints that these molecules may have a role in telomerase activity in postassembly steps. We do not suspect that p23 and Hsp90 are required for the reverse transcriptase activity of telomerase as measured in vitro by telomerase repeat amplification protocol (TRAP). Preliminary experiments suggest that stripping p23 and Hsp90 from telomerase after assembly of the RNP does not affect the activity of the RNP in vitro (data not shown). However, by analogy to the contribution of Hsp90 and p23 to steroid receptor function, it is tempting to speculate that p23 and Hsp90 mediate conformational changes in the enzyme that may occur during the translocation step, in which telomerase pauses and repositions itself after the addition of every six nucleotides (Morin 1989).
Our observations are in striking parallel to those made with another protein known to associate with p23; hepadnavirus reverse transcriptase (Hu and Seeger 1996; Hu et al. 1997). p23 and Hsp90 are required for the activity of this reverse transcriptase and are stably incorporated into nucleocapsids. The association of hepadnavirus reverse transcriptase with p23 is required for association with its RNA template (Hu and Seeger 1996; Hu et al. 1997). We have found no evidence that p23 and Hsp90 are required to allow physical association of hTERT with hTR, but they may be required to allow functional association (V.M. Tesmer, unpubl.).
In summary, we have identified p23 and Hsp90 as two proteins that are physically and functionally associated with telomerase activity. p23 and Hsp90 bind to hTERT and promote the assembly of active telomerase in vitro and in cells. In addition, these molecules appear to be components of the active holoenzyme and could have important functional roles in telomerase action. Future studies will be directed at determining the role of p23 and Hsp90 in the catalytic activity of telomerase and telomere biogenesis as it impacts cell aging and cancer.
Materials and methods
Plasmids
A gene encoding the amino-terminal 195 residues of hTERT with codon usage optimized for expression in Saccharomyces cerevisiae was assembled from six oligonucleotide pairs (HHMI/Keck Oligonucleotide Facility, Yale University), the ends of which were staggered by 10 bases to create complementary sequences for ligation. The oligonucleotides were gel purified; pairs were annealed by heating to 100°C for 10 min, at 90°C for 10 min, and chilled rapidly. The full-length construct (pJBT1) was built by sequential ligation of adjoining pairs; the ligated fragments were gel-purified prior to each subsequent ligation; the final product was subcloned into pJBT0, a modified form of pBluescript II-SK+ (Stratagene). The BamHI–SalI fragment of pJBT1 was inserted into pBTM116 (Vojtek et al. 1993) to create the bait encoding the first 195 amino acids of hTERT. For in vitro expression, the human hTERT cDNA was inserted into pcDNA3/HisC (Invitrogen) in-frame with three tandem copies of a carboxy-terminal HA epitope. To produce a retroviral construct, this same cDNA was inserted into pBABE Puro (Morgenstern and Land 1990) as an EcoRI–SalI fragment. pTRC3 and pGEM-U2 were described previously (Weinrich et al. 1997).
Antibodies and chemicals
Monoclonal anti-HA antibody (12CA5) was purchased from Boehringer Mannheim. Monoclonal anti-p23 (JJ3), anti-Hsp90 (H9010), and anti-Hsp70 antibodies were described previously (Johnson et al. 1994; Barent et al. 1998). Monoclonal anti-MAP1B antibody was a gift from G. Bloom (University of Texas Southwestern Medical Center). Monoclonal TCP-1 was purchased from Stressgen, and normal mouse IgG was purchased from Santa Cruz Biotechnology. Geldanamycin was purchased from Calbiochem. FK506 was a gift from Fujisawa USA, and cyclosporin A was purchased from Sigma.
Proteins
hTERT was synthesized in the RRL system (TNT, Promega) as described previously (Weinrich et al. 1997) in the presence of [35S] methionine. Human Hsp90β was expressed in SF9 cells and purified to >99% homogeneity, as described previously (Sullivan et al. 1997). Human p23, human Hop, and ydj-1p were expressed in Escherichia coli and purified as described previously (Caplan et al. 1992; Johnson and Tofts 1994; Schumacher et al. 1994). Human Hsp70 was expressed in SF9 cells and purified as described previously for avian Hsp70 (Schumacher et al. 1996) to >97% homogeneity.
Yeast two-hybrid analysis
Yeast two-hybrid screens were carried out using the LexA-dependent reporter strain L40 and a size-selected 16-day mouse embryo library as described (Vojtek et al. 1993).
In vitro telomerase assembly
hTR was produced with the Megascript T7 in vitro transcription system (Ambion). To assemble active telomerase 0.2 μl of in vitro-transcribed and -translated hTERT and 0.5 μg of hTR were mixed together in a 4 μl assembly assay with or without additional fresh RRL and incubated at 30°C for 90 min. Immunodepletion of RRLs with anti-p23 was performed as described previously (Johnson and Tofts 1994). To assemble telomerase with purified cofactors, hTERT and hTR, as above, were mixed with 500 ng of p23, 750 ng of Hsp90, 125 ng of Hsp70, 25 ng of Hop (p60), and 25 ng of YDJ-1 in the presence of 5 mm ATP, 10 mm Tris-HCl, 50 mm KCl, 5 mm MgCl2 and 2 mm DTT (pH 7.5) in a total volume of 5 μl.
Telomerase activity assays
Telomerase activity in all samples was determined by TRAP, as described previously (Weinrich et al. 1997), with minor modifications. The TRAP-eze Telomerase Detection Kit (Intergen), which includes a 36-bp internal standard to allow quantitation of activity, was used as suggested by the manufacturer. After telomerase extension for 30 min at room temperature, extended products were amplified by a two-step PCR (94°C for 30 sec, 60°C for 30 sec) for 27 cycles. Products were separated on 10% polyacrylamide gels and exposed to PhosphorImager screens. Quantitative estimates of telomerase activity were calculated by determining the ratio of the 36-bp internal standard to the 6-bp telomerase-specific ladder (Holt et al. 1997).
Immunoprecipitations
For immunoprecipitation from in vitro assembly reactions, antibodies were added to a final concentration of 0.5 μg/ml and incubated for 1 hr on ice. Protein G–agarose (Boehringer Mannheim) was added, and the mixture was incubated at 4°C for an additional hour with constant rotation. Agarose pellets were subsequently washed three times with 20 mm HEPES (pH 7.6), 20% glycerol, 100 mm NaCl, 0.2 mm EGTA, 1 mm MgCl2, 0.1% NP-40, and 0.1% BSA. To detect proteins, washed pellets were heated to 80°C for 10 min and electrophoresed by SDS-PAGE (7.5%); dried gels were exposed to a PhosphorImager screen (Molecular Dynamics) for 24–48 hr. For immunoprecipitations from cell lysates, HT1080 cells were suspended at a concentration of 1000 cells/μl in lysis buffer [0.01% NP-40, 10 mm Tris at pH 7.5, 50 mm KCl, 5 mm MgCl2, 2 mm DTT, 20% glycerol plus protease inhibitors (CompleteMini EDTA free, Boehringher Mannheim)] and incubated on ice for 20 min followed by a 5-sec pulse of sonication at 50 J/Watt-sec. Lysates were then spun at 13,000 rpm for 15 min, and resulting supernatants used for immunoprecipitation. Approximately 16 μg of each antibody was precoupled to 8 μl of a 50% slurry of protein–G agarose beads by incubating for 1 hr at 4°C with constant rotation. Antibody-coated beads were washed extensively with lysis buffer prior to use in immunoprecipitation reactions. Four microliters of cell lysate and 16 μl of 5% BSA (in lysis buffer) were combined with antibody-beads and rotated for 1 hr at 4°C. Immunoprecipitations were then washed with the lysis buffer 4 × 350 μl for 5 min with rotation at 4°C. For TRAP assays following immunoprecipitations, protein G–agarose pellets were resuspended in a final volume of 8 μl with lysis buffer and 2 μl removed for TRAP assays as described.
Production of retroviral stocks
pBABE Puro containing HA-tagged hTERT was transfected into PE501 cells (Morgenstern and Land 1990) by electroporation. Transfected cells were selected 48 hr post-transfection with 5 μg/ml puromycin. Supernatant from selected cells was harvested, filtered, and placed onto subconfluent PA317 cells. Infected PA317 cells were selected for 48 hr postinfection by addition of 5 μg/ml puromycin. Supernatants from selected cells were harvested, filtered, and frozen in aliquots at −80°C.
Cell lines and culture conditions
HT1080 cells were cultured as described previously (Holt et al. 1997). To test the effects of drugs on telomerase activity, cells were incubated in the absence of serum for 7 days. Serum was then added back to the cultures with one of the following additions: DMSO, geldanamycin, FK506 (gift from Fujisawa USA, Inc.), or cyclosporin A (Sigma), for 24 hr. [3H]Thymidine incorporation was performed as described (Holt et al. 1996). BJ fibroblasts were infected with the hTERT–HA3-containing retrovirus and selected with puromycin to produce a stable population of cells expressing HA-tagged telomerase. For transient expression of hTERT–HA3 in BJ fibroblasts, cells were split into 15-cm dishes at low density 2 days prior to experiment. Retroviral supernatants were thawed at 37°C and placed on plated cells in the presence of 4 μg/ml Polybrene. Four hours after infection, cells were treated with geldanamycin (Calbiochem) or mock treated, and incubated for an additional 20 hr.
Acknowledgments
We thank Nancy McMahon and Bridget Stensgard for protein purification, and Bryan Frank, Barbara Lastelic, and Mike Lombaredi for technical assistance. We also thank the assistance of UT Southwestern Dermatology Research sequencing core facility. This research was supported by grants from the Welch Foundation (M.A.W.), National Institutes of Health (NIH, AG07992) (J.W.S. and W.E.W.), and Geron Corporation. S.E.H. is a National Institute of Aging fellow, and V.M.T. is supported by a NIH oncology training grant.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL white08@utsw.swmed.edu; FAX (214) 648-8694.
References
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF. Analysis of FKBP51/FKBP52 chimeras and mutants for hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–354. doi: 10.1210/mend.12.3.0075. [DOI] [PubMed] [Google Scholar]
- Beattie TL, Zhou W, Robinson M, Harrington L. Reconstitution of human telomerase in vitro. Curr Biol. 1998;8:177–180. doi: 10.1016/s0960-9822(98)70067-3. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1995. [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chu C-P, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AJ, Tsai J, Casey PJ, Douglas MG. Farnesylation of YDJ1p is required for function at elevated growth temperatures in Saccharomyces cerevisiae. J Biol Chem. 1992;267:18890–18895. [PubMed] [Google Scholar]
- Dittmar KD, Pratt WB. Folding of the glucocorticoid receptor by the reconstituted hsp90-based chaperone machinery. J Biol Chem. 1997;272:13047–13054. doi: 10.1074/jbc.272.20.13047. [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Demady DR, Stancato LF, Krishna P, Pratt WB. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. J Biol Chem. 1997;272:21213–21220. doi: 10.1074/jbc.272.34.21213. [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Banach M, Galigniana MD, Pratt WB. The role of DnaJ-like proteins in glucocorticoid receptor-hsp90 heterocomplex assembly by the reconstituted hsp90-p60-hsp70 foldosome complex. J Biol Chem. 1998;273:7358–7368. doi: 10.1074/jbc.273.13.7358. [DOI] [PubMed] [Google Scholar]
- Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, Le S, West MD, Harley CB, Andrews WH, Greider CW, Villeponteau B. The RNA component of human telomerase. Science. 1995;269:1236–1239. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Grenert JP, Sullivan WP, Fadden P, Haystead TAJ, Clark J, Mimnaugh E, Krutzsch H, Ochel H-J, Schulte TW, Sausville E, Neckers LM, Toft DO. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- Harley CB. Telomere loss: Mitotic clock or genetic time bomb? Mutat Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during aging. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K, Tatsumoto N, Kodama T, Shay JW, Yokoyama T. Telomerase activity in human intestine. Int J Oncol. 1996;9:453–458. doi: 10.3892/ijo.9.3.453. [DOI] [PubMed] [Google Scholar]
- Holt SE, Wright WE, Shay JW. Regulation of telomerase activity in immortal cell lines. Mol Cell Biol. 1996;16:2932–2939. doi: 10.1128/mcb.16.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SE, Aisner DL, Shay JW, Wright WE. Lack of cell cycle regulation of telomerase activity in human cells. Proc Natl Acad Sci. 1997;94:10687–10692. doi: 10.1073/pnas.94.20.10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Seeger C. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc Natl Acad Sci. 1996;93:1060–1064. doi: 10.1073/pnas.93.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Toft DO, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997;16:59–68. doi: 10.1093/emboj/16.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KA, Stancato LF, Owens-Grillo JK, Johnson JL, Krishna P, Toft DO, Pratt WB. The 23-kDa acidic protein in reticulocyte lysate is the weakly bound component of the hsp foldosome that is required for assembly of the glucocorticoid receptor into a functional heterocomplex with hsp90. J Biol Chem. 1995;270:18841–18847. doi: 10.1074/jbc.270.32.18841. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Toft DO. A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. J Biol Chem. 1994;269:24989–24993. [PubMed] [Google Scholar]
- ————— Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol Endocrinol. 1995;10:670–678. doi: 10.1210/mend.9.6.8592513. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Beito TG, Krco CJ, Toft DO. Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Mol Cell Biol. 1994;14:1956–1963. doi: 10.1128/mcb.14.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kipling D. The telomere. New York, NY: Oxford University Press; 1995. [Google Scholar]
- Kosano H, Stensgaard B, Charlesworth MC, McMahon N, Toft D. The assembly of progesterone receptor–hsp90 complexes using purified proteins. J Biol Chem. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973. [DOI] [PubMed] [Google Scholar]
- Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- Martin GM, Sprague CA, Epstein CJ. Replicative life-span of cultivated human cells. Effects of donor’s age, tissue, and genotype. Lab Invest. 1970;23:86–92. [PubMed] [Google Scholar]
- Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Lui Q, Bacchetti S, Haber DA, Weinburg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: High titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cells line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin GB. The human telomere transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MDM, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and humans. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- Nakayama J-I, Saito M, Nakamura H, Matsuura A, Ishikawa F. TLP1: A gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell. 1997;88:1–20. doi: 10.1016/s0092-8674(00)81933-9. [DOI] [PubMed] [Google Scholar]
- Norton JC, Gollahon LS, Holt SE, Wright WE, Shay JW. Enhanced detection of telomerase activity from human cells and tumors. DNA Cell Biol. 1997;17:217–219. doi: 10.1089/dna.1998.17.217. [DOI] [PubMed] [Google Scholar]
- Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Schneider EL, Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proc Natl Acad Sci. 1976;73:3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher RJ, Hurst R, Sullivan WP, McMahon NJ, Toft DO, Matts RL. ATP-dependent chaparoning activity of reticulocyte lysate. J Biol Chem. 1994;269:9493–9499. [PubMed] [Google Scholar]
- Schumacher RJ, Hansen WJ, Freeman BC, Alnemri E, Litwack G, Toft DO. Cooperative action of hsp70, hsp90, and DnaJ proteins in protein renaturation. Biochemistry. 1996;35:14889–14898. doi: 10.1021/bi961825h. [DOI] [PubMed] [Google Scholar]
- Sullivan ES, Stensgard B, Caucutt G, Bartha B, McMahon N, Alnemri ES, Litwack G, Toft D. Nucleotides and two functional states of hsp90. J Biol Chem. 1997;272:8007–8012. doi: 10.1074/jbc.272.12.8007. [DOI] [PubMed] [Google Scholar]
- Taylor RS, Ramirez RD, Ogoshi M, Chaffins M, Piatyszek MA, Shay JW. Detection of telomerase activity in malignant and nonmalignant skin conditions. J Invest Dermatol. 1996;106:759–765. doi: 10.1111/1523-1747.ep12345811. [DOI] [PubMed] [Google Scholar]
- Toft DO. Recent advances in the study of hsp90 structure and mechanism of action. Trends Endocrinol Metab. 1998;9:238–243. doi: 10.1016/s1043-2760(98)00060-5. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley CB. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Watson JD. Origin of concatameric T4 DNA. Nature. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Weinrich SL, Pruzan Rl, Ma L, Ouellette M, Tesmer VM, Holt SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB, Taylor RB, Carlos R, Andrews WH, Wright WE, Shay JW, Harley CB, Morin GB. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes & Dev. 1997;11:2801–2809. doi: 10.1101/gad.11.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]