Abstract
Type-1 cannabinoid receptor (CB1) is the most abundant G-protein-coupled receptor (GPCR) in the brain. CB1 and its endogenous agonists, the so-called ‘endocannabinoids (eCBs)’, belong to an ancient neurosignalling system that plays important functions in neurodegenerative and neuroinflammatory disorders like Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis and multiple sclerosis. For this reason, research on the therapeutic potential of drugs modulating the endogenous tone of eCBs is very intense. Several GPCRs reside within subdomains of the plasma membranes that contain high concentrations of cholesterol: the lipid rafts. Here, the hypothesis that changes in membrane fluidity alter function of the endocannabinoid system, as well as progression of particular neurodegenerative diseases, is described. To this end, the impact of membrane cholesterol on membrane properties and hence on neurodegenerative diseases, as well as on CB1 signalling in vitro and on CB1-dependent neurotransmission within the striatum, is discussed. Overall, present evidence points to the membrane environment as a critical regulator of signal transduction triggered by CB1, and calls for further studies aimed at better clarifying the contribution of membrane lipids to eCBs signalling. The results of these investigations might be exploited also for the development of novel therapeutics able to combat disorders associated with abnormal activity of CB1.
LINKED ARTICLES
This article is part of a themed issue on Cannabinoids in Biology and Medicine. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: cholesterol, CRAC domain, endocannabinoid, membrane lipids, neurodegeneration, neurotransmission, palmitoylation, striatum
Introduction
N-arachidonoylethanolamine [anandamide (AEA)] and 2-arachidonoylglycerol (2-AG) activate type-1 (CB1) (Howlett et al., 2010; Pertwee, 2010) and type-2 (CB2) cannabinoid receptors (Patel et al., 2010), and are the most active ‘endocannabinoids’ (eCBs) as yet characterized (Di Marzo, 2009; Maccarrone et al., 2010a). AEA, but not 2-AG, also binds to and activates type-1 transient receptor potential vanilloid (TRPV1) channels, and hence is considered a true ‘endovanilloid’ (Di Marzo and De Petrocellis, 2010). The nomenclature of the major eCBs-binding receptors (CB1, CB2 and TRPV1) conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2009). Here, the hypothesis that changes in membrane fluidity alter function of the eCBs signalling, as well as progression of particular neurodegenerative diseases, is described. To this end, the impact of membrane cholesterol on membrane properties and hence on neurodegenerative diseases, as well as on CB1 signalling in vitro and on CB1-dependent neurotransmission within the striatum, is discussed.
CB1 receptor is the most abundant G protein-coupled receptor (GPCR) in the brain (Howlett et al., 2010). Together with its endogenous agonists, CB1 forms an ancient neurosignalling system that plays important control functions within the central nervous system (CNS) (Katona and Freund, 2008). Alterations in eCBs signalling have been extensively investigated in a wide range of neurodegenerative and neuroinflammatory disorders, spanning from Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD), to amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS) (Maccarrone et al., 2007; Centonze et al., 2008; Bisogno and Di Marzo, 2010). In addition, prion-related disorders (PrDs) seem to benefit from a nonpsychoactive phytocannabinoid like cannabidiol, that prevents prion accumulation and protects neurons against its toxicity (Dirikoc et al., 2007; Iuvone et al., 2009). Not surprisingly, research on the therapeutic potential of drugs modulating eCBs activity is very intense (Di Marzo, 2009). In recent years it has become increasingly evident the involvement of membrane lipids, especially cholesterol and glycosphingolipids, in regulating the function of GPCRs like β2-adrenergic and serotonin1A receptors, as well as of several other membrane-associated proteins like caveolins (Pontier et al., 2008; Prinetti et al., 2009; Paila et al., 2010; Shrivastava et al., 2010).
In the following sections the effect of cholesterol on membrane properties will be discussed, as well as its involvement in neurodegenerative diseases where eCBs signalling has been shown to play a role. Then, the effect of membrane cholesterol on CB1-dependent signal transduction in in vitro models will be briefly reviewed, and the molecular determinants that underlie CB1, but not CB2, sensitivity to membrane cholesterol perturbation will be presented. Finally, recent evidence that demonstrates a role for membrane cholesterol on CB1-dependent eCBs signalling in striatal neurotransmission will be summarized, in order to support the concept that membrane environment can indeed control eCBs-dependent neurotransmitter networks also in vivo. Incidentally, there evidence that similar changes in plasma membrane fluidity occur in non-neuronal tissues where CB1 receptors are expressed, for instance within the immune system (Bari et al., 2006). Yet, the effect of alterations of membrane cholesterol on peripheral CB1-dependent signalling goes beyond the scopes of this review.
Cholesterol and membrane properties: the ‘lipid raft’ concept
The classical ‘fluid-mosaic’ model of the biological membrane organization (Singer and Nicolson, 1972) has changed in recent years. Membrane proteins are no longer simply floating in a two-dimensional oriented solution, and the solvent is not only a simple viscous phospholipid bilayer. In fact, lateral compartmentalization and membrane asymmetry impose specific constraints to diffusion and partition between the two monolayers of specific hydrophobic molecules, and affect membrane-bound proteins (Forneris and Mattevi, 2008; Lindahl and Sansom, 2008). Some emerging concepts on membranes are that they are patchy structures, with segregated functional regions of various thickness and composition, and that crowding domains and ectodomains limit lipid exposure to the surrounding aqueous regions (Brown and London, 1998; Galbiati et al., 2001). Therefore, it appears increasingly evident that changes in the physicochemical properties of the biological membrane directly affect the structure, and hence the function, of associated proteins. Rather than serving only as a medium through which membrane proteins diffuse, lipid bilayers have now been demonstrated to form compartmentalized subdomains with different biophysical properties.
Among the most interesting specialized microdomains of the plasma membrane are those enriched in cholesterol, sphingolipids, plasmenylethanolamine and arachidonic acid (Brown and London, 1998; Pike, 2005). These domains, characterized by a more tightly packed state responsible for their resistance to solubilization with non-ionic detergents (Brown and London, 1998), have been referred to as ‘lipid rafts’ (LRs). Interestingly, the physicochemical properties of LRs seem to be strictly related to their functional roles (Ostermeyer et al., 1999). Phase separation between lipids in different physical states, most often the solid-like gel phase (β-phase) and the liquid-disordered crystalline phase (α-phase, lc), has been well characterized in model membranes. Sphingolipids differ from most biological phospholipids in that they contain long, largely saturated acyl chains. This allows them to pack tightly together. However, because of the high concentration of cholesterol in the plasma membrane and other membranes in which rafts form, sphingolipids in LRs do not exist in the gel phase. Different kinds of phase separation can occur in binary mixtures of individual phospholipids with cholesterol. In these mixtures, domains in a lc-like phase coexist with domains in a new state, the so-called ‘liquid-ordered’ (lo) phase. Interestingly, acyl chains of lipids in lo phase are extended and tightly packed, as in the gel phase, but they have a high degree of lateral mobility (Brown and London, 1998). LRs obtained from detergent-resistant membranes isolated from cell lysates seem to exist in this lo phase or in a state with similar properties (Ge et al., 1999; Ostermeyer et al., 1999). Thus, in the presence of cholesterol, lipid mixtures can undergo a lo/lc phase separation, instead of the gel/lc distribution observed in the absence of cholesterol. Furthermore, cholesterol seems to promote phase separation, apparently because of favourable packing interactions between saturated lipids and cholesterol itself (Xu and London, 2000). This ‘cholesterol effect’ probably explains LRs formation also in cell membranes that contain low levels of sphingolipids, and clarifies why cholesterol depletion can induce LRs disruption, thus affecting raft function.
In the simplest case, LRs can be viewed as signalling platforms that serve to colocalize the required components, such as receptors, coupling factors, effectors and enzymes, facilitating their interaction and supporting signalling. The functions modulated by LRs include cholesterol transport (Fielding and Fielding, 2001; Uittenbogaard et al., 2002; Smart et al., 2004), organization of signalling protein complexes (Galbiati et al., 2001), endocytosis (Pelkmans, 2005), potocytosis (Anderson et al., 1992) and establishment of cell polarity (Ostrom and Insel, 2004). These lipid microdomains can serve also as specific sites of interaction for certain pathogens and toxins (Fivaz et al., 2002), and they play an important role also in the organization of cell signalling machineries such as receptor tyrosine kinases and GPCRs (Barnett-Norris et al., 2005). More generally, the liquid ordered regions of LRs are thicker than the liquid-disordered bulk of the membrane, and the cholesterol molecules within this region have a slower rate of lateral and interlayer diffusion (flip-flop) (Dainese et al., 2010). These properties of LRs are schematically depicted in Figure 1.
Figure 1.
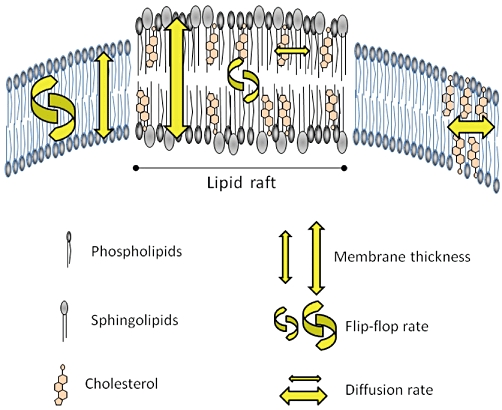
Scheme of a biological membrane containing lipid rafts (LRs). The liquid-ordered regions of LRs are thicker than the liquid-disordered bulk of the membrane, and the cholesterol molecules within this region have a slower rate of lateral and interlayer diffusion (flip-flop). See text for details.
Cholesterol and neurodegenerative diseases
Compared with other tissues, mammalian brain contains the highest levels of cholesterol, that in humans represents ∼25% of the amount in total body (∼20 mg·g−1), although human brain accounts for ∼2% only of total body weight (Dietschy and Turley, 2004; Vaya and Schipper, 2007). Brain cholesterol is mainly synthesized by oligodendrocytes and astrocytes, without a significant contribution by blood circulation because cholesterol in lipoprotein particles [(most often apolipoprotein E (ApoE)] can not cross the blood–brain barrier (Liu et al., 2010). While oligodendrocytes produce cholesterol to form the myelin sheaths, astrocytes supply cholesterol to neurons, by using ATP-binding cassette A1 and G1 transporters (Karten et al., 2006). In addition, astrocytes are the major ApoE-producing cells within the CNS, and cholesterol/ApoE complexes may enter neurons by receptor-mediated endocytosis (Hayashi et al., 2004), followed by transport of cholesterol to different intracellular organelles (e.g. lysosomes, endoplasmic reticulum, Golgi apparatus) and to the plasma membranes.
The relevance of membrane cholesterol in neurodegenerative processes is demonstrated by the number of research papers devoted to this issue: a PubMed search with ‘cholesterol’ and ‘neurodegenerative diseases’ scores more than 1500 entries, 675 of which were released in the last 5 years. Not surprisingly, the topic of cholesterol involvement in the pathogenesis of neurodegenerative diseases has been recently covered by several comprehensive review papers (Adibhatla and Hatcher, 2008; Schweitzer et al., 2009; Liu et al., 2010; Martin et al., 2010; Schengrund, 2010; Zuccato et al., 2010). Besides the effect on membrane properties outlined earlier, it is known that cholesterol in LRs modulates the binding and oligomerization of ‘amyloidogenic proteins’: these are a series of brain proteins with exceptional conformational plasticity and a high propensity for self-aggregation (Fantini and Yahi, 2010). By controlling the balance between unstructured monomers and α or β conformers (the so-called ‘chaperone effect’). LRs can either inhibit or stimulate the oligomerization of amyloidogenic proteins. Cholesterol has a dual role in this process, because it regulates protein-sphingolipid interactions through a fine tuning of sphingolipid conformation (indirect effect), and/or facilitates pore (or channel) formation through direct binding to amyloidogenic proteins (Fantini and Yahi, 2010). Aberrantly folded proteins are hallmarks of amyloidogenic diseases, for instance AD and PrD that, although clinically different, have the same underlying pathogenetic mechanism, that is, an altered protein conformer with high β-sheet structure content: the amyloid β peptide (Aβ) in the case of AD, and the aberrant prion protein, PrPsc, in PrD (Pani et al., 2010). Growing evidence indicates the possible involvement of cholesterol in misfolded protein generation during AD and PrD (reviewed by Pani et al., 2010). Furthermore, Aβ is a product of the amyloid precursor protein (APP) cleavage by secretase, and there is evidence that also the activity of the latter enzyme is modulated by membrane lipid environment (Eckert et al., 2010). Conversely, Aβ disturbs the properties of artificial and isolated biological membranes, as well as those of plasma membranes in living cells. Thus, LRs may be the site where the neurotoxic cascade of Aβ is initiated (Eckert et al., 2010). In addition, also glutamate receptors of the N-methyl-D-asparate (NMDA) subtype show a very complex functional regulation, dependent on neuregulins and receptor tyrosine kinases localized in cholesterol-rich LRs (Schrattenholz and Soskic, 2006). It should be recalled that NMDA receptors are allosteric and ligand-gated calcium channels, with a pivotal role in memory-related signal transduction and other neurotransmission pathways (Chau, 2010). The brain pathologies where membrane cholesterol plays a critical role, and the main targets of cholesterol action, are summarized in Table 1. This table is restricted to neurodegenerative diseases where also eCBs signalling is known to play a major role, whereas other cholesterol-dependent disease conditions, like the Niemann-Pick diseases, were not covered. In fact, Niemann-Pick diseases encompass a heterogenous group of pathologies where lysosomal lipid storage is compromised, leading to cholesterol (and sphingolipids) accumulation in endosomes and lysosomes of neurons due to erroneous cholesterol trafficking (Vance et al., 2006). Yet, for Niemann-Pick disorders a role for eCBs signalling has not yet been recognized, and only a preliminary study showing that sphingomyelin hydrolysis is stimulated by cannabidiol in fibroblasts from a Niemann-Pick patient has been reported (Burstein et al., 1984).
Table 1.
Impact of membrane cholesterol on neurodegenerative diseases
| Disease | Effect | Reference |
|---|---|---|
| Alzheimer's disease | LRs disruption by membrane cholesterol depletion (through MCD) leads to a lower rate of β-amyloid accumulation, that impacts tyrosine kinase Fyn and hence tau phosphorylation. Instead, elevated levels of cholesterol enhance the activity of the amyloid precursor protein (APP)-cleaving enzyme BACE1, and that of γ-secretase, also found in LRs. | Simons et al. (1998) |
| Bhaskar et al. (2005) | ||
| Kalvodova et al. (2005) | ||
| Grimm et al. (2008) | ||
| Parkinson's disease | At least four proteins, which when mutated are associated with PD, reside in LRs: Parkin (an E3 ubiquitin-ligase); α-Synuclein; LRRK2 (leucine-rich repeat kinase 2); PINK1 (PTEN-induced kinase). Mutation of α-synuclein (A30P) leads to a reduced association with LRs. | Fallon et al. (2002) |
| Fortin et al. (2004) | ||
| Kubo et al. (2005) | ||
| Silvestri et al. (2005) | ||
| Hatano et al. (2007) | ||
| Huntington's disease | Mutant huntingtin associates with LRs more strongly than the wild-type protein, and inhibits the expression of several genes involved in cholesterol synthesis, vesicle synthesis and trafficking. In addition, mutant huntingtin increases raft-associated glycogen synthase kinase 3-β, a marker of apoptotic stress. | Sipione et al. (2002) |
| Valenza and Cattaneo (2006) | ||
| Valencia et al. (2009) | ||
| Zuccato et al. (2010) | ||
| Amyotrophic lateral sclerosis | ALRs disruption by membrane cholesterol depletion (through MCD) protects motor neurons against brain-derived neurotrophic factor (BDNF)-induced excitotoxicity due to activation of TrkB receptors. Consistently, increased BDNF was found in muscles from ALS patients at an early stage of the disease. | Kust et al. (2002) |
| Mojsilovic-Petrovic et al. (2006) | ||
| Multiple sclerosis | Lipid peroxidation that occurs in MS may be affected by cholesterol, that regulates membrane fluidity. | Carlson and Rose (2006) |
| Prion-related disorders | LRs are needed for the conversion of normal cellular prion protein (PrPC) into its toxic modified form (PrPSC). Consistently, reduced cholesterol synthesis (by squalestatin) protects cells against prion neurotoxicity. | Johnson and Gibbs (1998) |
| Baron et al. (2002) | ||
| Bate et al. (2004) |
Cholesterol and CB1 signalling in vitro
A role for membrane cholesterol in the functional regulation of CB1 has been well documented in neuronal and non-neuronal cells cultured in vitro (for an updated review see Dainese et al., 2010). LRs disruption by acute cholesterol depletion with methyl-β-cyclodextrin (MCD) has been shown to double CB1-dependent signalling via adenylyl cyclase and mitogen-activated protein kinases in neuronal cells (Bari et al., 2005a,b;). Instead CB2 receptor, which is structurally and functionally related to CB1, and TRPV1 channel, which is also activated by eCBs like AEA (Di Marzo and De Petrocellis, 2010), are completely insensitive to the modulation of membrane cholesterol content (Bari et al., 2006). Consistently, neither CB2 nor TRPV1 reside in cholesterol-rich LRs (Bari et al., 2006; Maccarrone, 2008; Rimmerman et al., 2008). In this context, it seems noteworthy that 2-AG has been shown to be entirely localized in LRs of dorsal root ganglion cells, where also part of AEA (∼30%) can be detected (Rimmerman et al., 2008). However, most of AEA (∼70%) was found in non-LR fractions, and it remains to be clarified whether these eCBs are produced directly within LRs, or are transported to (or accumulated within) these microdomains. The different interaction of AEA and 2-AG with membrane microdomains might have significant implications for eCBs-dependent autocrine and/or retrograde-paracrine signalling pathways, and further studies are needed to clarify which structural determinants are responsible for a different localization of two apparently similar eCBs within lipid bilayers (Maccarrone, 2008). The effects of LRs disruption by cholesterol depletion on eCBs-binding receptors are summarized in Table 2. Recently, some details of the molecular basis for the different response of these two receptor subtypes to membrane cholesterol have been clarified, by showing that both CB1 and CB2 have a cholesterol-binding domain (CRAC, cholesterol recognition amino acid sequence consensus) in helix 7 (Oddi et al., 2011). In this context, it should be recalled that as yet a unique conserved structural determinant for protein interaction with cholesterol has not been identified, however, CRAC [L/V-X(1–5)-Y-X(1–5)-R/K] is a well known motif that enables this interaction (Epand, 2006). For instance, CRAC has been demonstrated in caveolin-1, peripheral-type benzodiazepine receptor (Li and Papadopoulos, 1998; Jamin et al., 2005), and in other proteins targeted to LRs (Xie et al., 2010). Interestingly, by sequence alignment of human CB1 and CB2 we have demonstrated the presence of a CRAC(-like) motif in the last 11 amino acids of the transmembrane helix 7 of both CB1 and CB2, yet with small but very important sequence differences between the two receptor subtypes (Oddi et al., 2011). In particular, we found that in the highly conserved ‘CRAC-like’ region (82% amino acid identity), CB1 differs from CB2 for one residue only: lysine 402 of CB1 corresponds to glycine 304 in CB2 (Oddi et al., 2011). Therefore, CB1 has a true CRAC domain, whereas CB2 has not. In keeping with this observation, we found that the CB1(K402G) mutant where the CRAC sequence of CB1 was converted into that of CB2 had a reduced propensity to reside in cholesterol-rich membrane regions, and lost its sensitivity to membrane cholesterol enrichment (Oddi et al., 2011). Therefore, these data suggest that one residue in complex proteins like GPCRs can be enough to direct their interaction with membrane lipids, thus affecting their localization within LRs and signal transduction thereof. Additionally, we found that the C-terminal component of CB1, that is, the intracellular juxtamembrane helix 8, contains a cysteine residue (C415, in green) that could be constitutively palmitoylated (Dainese et al., 2010). The latter reversible post-translational modification can be used by cells to regulate CB1 targeting to LRs, thus influencing subsequent G protein-dependent signalling. Remarkably, a palmitoylation site corresponding to C415 is absent in CB2 (Dainese et al., 2010), and running experiments in our laboratory are aimed at ascertaining whether also this residue might contribute to LR localization of CB1. The positions of CRAC domain and C415 in the three-dimensional structure of CB1 are depicted in Figure 2.
Table 2.
Effect of LRs disruption by membrane cholesterol depletion on CB1R, CB2R and TRPV1 binding and signalling
| Cholesterol depletion | |||||
|---|---|---|---|---|---|
| Binding | Signalling | ||||
| Receptor | Kd | Bmax | [35S]GTPγSa | ACb | MAPKc |
| CB1R | ↑ | ↑ | ↑ | ↓ | ↑ |
| CB2R | ↔ | ↔ | ↔ | ↔ | ↔ |
| TRPV1 | ↔ | ↔ | nd | na | na |
See text for reference to the original data.
Non-hydrolysable analogue of GTP: it is a measure of the extent of the interaction between receptor and associated G protein.
Adenylyl cyclase.
Mitogen-activated protein kinase.
na, not applicable; nd, not determined.
Figure 2.
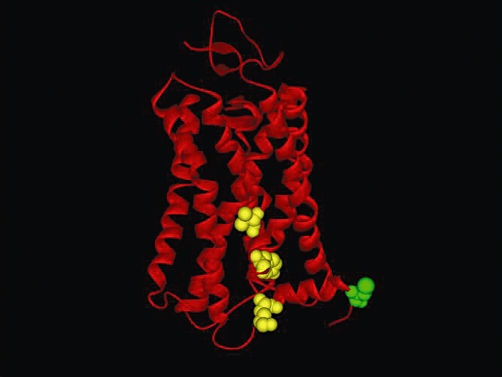
Three-dimensional model of type-1 cannabinoid receptor (CB1), based on sequence alignment with visual rhodopsin in the inactivated state (PDB code: 1F88). The model was obtained using the protein structure homology-modeling server SWISS-MODEL, integrated in the Deep-View program (Dainese et al., 2010). The three residues (V392, Y397, K402) that form the CRAC sequence are represented as yellow spheres, sized to the Van der Waals radii; these residues belong to the transmembrane helix 7 of CB1. Additionally, the C-terminal component of CB1, that is, the intracellular juxtamembrane helix 8, contains a cysteine residue (C415, in green) that could be constitutively palmitoylated. The model was kindly provided by Dr. Enrico Dainese (University of Teramo, Italy). See text for further details.
Overall, we believe that the observation that even a single residue in CB receptors can regulate the activity of eCBs signalling might impact on the therapeutic exploitation of CB1-dependent versus CB2-dependent biological activity of these lipid signals. In the next section, evidence showing that within the striatum cholesterol regulation of eCBs signalling through CB1 can indeed have major consequences on in vivo neurotransmission is presented.
Impact of membrane cholesterol on CB1-dependent neurotransmission within the striatum
The nucleus striatum is a subcortical brain area involved in motor, cognitive and emotional processes (Packard, 2009; Rodriguez-Oroz et al., 2009; Simpson et al., 2010), and is highly enriched in CB1 receptors controlling both glutamate and GABA transmission, mainly through presynaptic mechanisms (Ferréet al., 2010; Rossi et al., 2010a). Direct pharmacological agonists of CB1 receptors, in fact, reduce the release of both transmitters, as demonstrated in several studies (Szabo et al., 1998; Gerdeman and Lovinger, 2001; Huang et al., 2001; Köfalvi et al., 2005; Narushima et al., 2006; Maccarrone et al., 2008). Evidence exists that the two sets of CB1 receptors differ for the preferential eCB activating them, and also have different regulation mechanisms. CB1 receptors controlling striatal glutamate synapses [CB1R(Glu)], but not those regulating GABA transmission [CB1R(GABA)], in fact, seem to be targeted preferentially by AEoA, because we have found that genetic or pharmacological inhibition of the AEA degrading enzyme fatty acid amide hydrolase (FAAH), that selectively increases AEA levels, inhibits glutamate but not GABA transmission in a CB1 receptor-dependent manner (Maccarrone et al., 2008; Rossi et al., 2010b). On the other hand, stimulation of endogenous 2-AG synthesis only affects GABA synapses by activating CB1R(GABA) (Maccarrone et al., 2008).
Several neurotransmitters engage the eCB system in the striatum, and these include dopamine through D2 receptors (Centonze et al., 2004; Yin and Lovinger, 2006), neurotensin (Yin et al., 2008), acetylcholine through M1 receptors (Narushima et al., 2007; Uchigashima et al., 2007; Musella et al., 2010) and glutamate through metabotropic glutamate 5 (mGlu5) receptors (Jung et al., 2005; 2007; Maccarrone et al., 2008). Stimulation of mGlu5 receptor enhances the activity of the 2-AG synthesizing enzyme diacylglycerol lipase (DAGL) (Jung et al., 2005; 2007;), and the resulting 2-AG synthesis and physiological activity on CB1 receptors inhibits GABAergic inhibitory postsynaptic currents (IPSCs) in the striatum (Maccarrone et al., 2008). Of note, elevation of AEA content by pharmacological or genetic inhibition of FAAH regulates the mGlu5 receptor/2-AG interaction, through the stimulation of TRPV1 channels and the resulting inhibition of glutathione metabolism (Maccarrone et al., 2008) (Figure 3). Evidence exists that 2-AG is produced postsynaptically in striatal neurons in response to mGlu5 receptor-dependent DAGL stimulation, and that it acts presynaptically on CB1 receptors to inhibit GABA release (Katona et al., 2006). Accordingly, an ultrastructural study demonstrated that DAGL and mGlu5 receptors are tightly associated on the somatodendritic surface of striatal projection neurons, whereas CB1 receptors are particularly enriched on GABAergic axon terminals of striatal neurons (Uchigashima et al., 2007).
Figure 3.
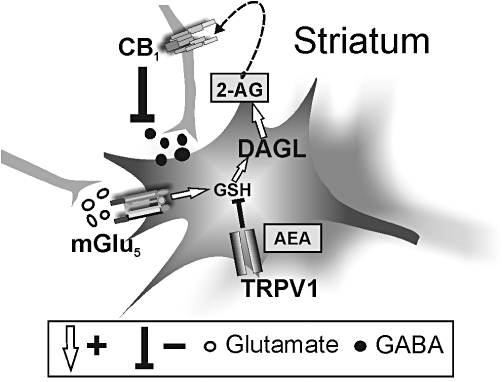
Overall scheme of the interactions between N-arachidonoylethanolamine (AEA), type-1 transient receptor potential vanilloid (TRPV1), 2-arachidonoylglycerol (2-AG) and metabotropic glutamate 5 (mGlu5) receptors in the striatum. Stimulation of mGlu5 receptors increases 2-AG synthesis by enhancing glutathione (GSH) production. 2-AG acts as retrograde signal to limit GABA release through the stimulation of presynaptic type-1 cannabinoid receptor (CB1) receptors. AEA, on the other hand, stimulates TRPV1 channels, presumably located in the somatodendritic region of striatal neurons, and modulates 2-AG metabolism and physiological effects by inhibiting GSH-stimulated diacylglycerol lipase (DAGL) activity (adapted from Maccarrone et al., 2008).
Membrane cholesterol has been recently found to play a substantial role in mGlu5 receptor/eCB coupling. Depletion of membrane cholesterol with MCD, in fact, failed to alter AEA metabolism in the striatum, because both the AEA synthesizing enzyme N-acyl-phosphatidylethanolamine-hydrolysing phospholipase D (NAPE-PLD) and FAAH were unaffected by the treatment. Instead, the activity of DAGL was significantly enhanced in MCD-treated striatal slices, whereas the activity of the 2-AG degrading enzyme monoacylglycerol lipase (MAGL) was not. As a result, 2-AG striatal contents were increased to levels that are able to stimulate CB1 receptors in control slices (Maccarrone et al., 2009). Surprisingly, however, the potentiated 2-AG synthesis observed after cholesterol depletion from striatal neuron membranes was not associated with increased activity of this eCB on CB1 receptors, neither in basal conditions nor after the stimulation of mGlu5 receptors with DHPG. The frequency of spontaneous and miniature GABA-mediated IPSCs was in fact normal in MCD-treated slices, while a frequency reduction of IPSCs normally accompanies the effect of 2-AG on CB1R(GABA) (Maccarrone et al., 2008; 2009;). Furthermore, blockade of CB1 receptors did not increase IPSC frequency, again supporting the conclusion that MCD-mediated elevation of 2-AG did not result in enhanced activity of this eCB on CB1 receptors. We did not observe any CB1 receptor-mediated effect on GABA-mediated IPSCs even after the exacerbation of 2-AG metabolism with DHPG, an effect that was particularly surprising because both CB1 receptor binding and activity were increased, and not down-regulated, in MCD-treated striatal slices. Furthermore, the sensitivity of CB1 receptors was also addressed in physiological experiments, demonstrating that the ability of a synthetic agonist of CB1 receptors to inhibit IPSCs was intact after cholesterol depletion with MCD (Maccarrone et al., 2009).
MCD failed to affect mGlu5 receptor binding, also ruling out that the sensitivity of this receptor was altered following cholesterol depletion (Maccarrone et al., 2009). Instead, by means of double immunofluorescence, we demonstrated a relocalization of both CB1 receptors and mGlu5 receptors on striatal neurons after MCD treatment, while cellular fluorescence densitometry confirmed a significantly higher CB1 receptor expression after MCD treatment (Maccarrone et al., 2009). These results are therefore compatible with the idea that lipid rafts not only influence receptor expression and function in membranes but also their spatial orientation. Accordingly, MCD treatment of striatal slices did not alter the general staining of mGlu1 receptors, which has many pharmacological, signal transduction and physiological homologies with the mGlu5 receptors but is expressed outside lipid rafts in neuronal membranes (Becher et al., 2001).
Given the localization of CB1 receptors (Bari et al., 2005b; 2008;) and possibly of mGlu5 receptors (Moffett et al., 2000; Oh and Schnitzer, 2001; Fourgeaud et al., 2003) in lipid rafts, and the substantial role of these cholesterol-enriched membrane subdomains in the limitation of movements of raft-associated proteins (Lucero and Robbins, 2004; Hanzal-Bayer and Hancock, 2007), it is plausible that MCD disrupts 2-AG-mediated mGlu5 receptor-CB1 receptor interaction by altering CB1 receptor and mGlu5 receptor membrane localization (Maccarrone et al., 2009).
Involvement of striatal eCB signalling in physiological and pathological contexts
The data above assign to membrane cholesterol in lipid rafts a previously unexpected role in the regulation of synaptic transmission, with potentially very relevant consequences for the understanding of the cellular correlates of several physiological states and of pathological conditions. The pharmacological response of raft-associated striatal CB1Rs(GABA) and mGlu5R/2-AG coupling is in fact modulated by a variety of conditions, which include cocaine addiction, stress-induced anxiety, voluntary exercise, caffeine and palatable food assumption, as well as in models of neurological diseases such as HD, MS, ALS and Fragile X syndrome (FXS). It follows therefore that the modulation of cholesterol metabolism in membranes might be considered in the near future as a valuable alternative option for the treatment of frequent and severe neuropsychiatric conditions.
The maximal response of striatal CB1Rs(GABA) is significantly enhanced by chronic cocaine, when rodents develop overt addictive behaviours, but not by a single administration of the psychostimulant (Centonze et al., 2007a; Rossi et al., 2008; De Chiara et al., 2010a,b;). Similar potentiation of CB1R(GABA) signalling is seen in mice drinking chronically another psychoactive compound, caffeine (Rossi et al., 2009), or exposed to running wheel or given access to a drinking solution containing sucrose (De Chiara et al., 2010a). Of note, voluntary running activity has strong rewarding and reinforcing properties in rodents, and shares many neurochemical and behavioural characteristics with drug-induced reward situations, through the activation of dopamine signalling and the modulation of striatal neuron activity (Werme et al., 2000; 2002; Lett et al., 2001; De Visser et al., 2007). Similarly, sweet foods and drinks also have intense rewarding properties (Lenoir et al., 2007), and many commonalities exist between overconsumption of sugars and drug addiction, including the stimulation of the dopamine signalling in the striatum (Levine et al., 2003; Kelley, 2004; Volkow and Wise, 2005). Thus, the evidence that chemical (cocaine) and natural rewards (running wheel and sucrose) share the common property of enhancing CB1R(GABA) responses in the striatum suggest that this receptor subtype is involved in the modulation of complex dopamine- and reward-based behaviours. Accordingly, blockade of dopamine D2 receptors prevents the cocaine-, running- and sucrose-induced sensitization of CB1R(GABA) receptors (De Chiara et al., 2010a,b;).
Because of the ability of rewarding experiences to contrast the behavioural effects of stress, the data presented earlier might indicate that striatal CB1R(GABA) are involved in the mood disorders, such as anxiety and depression. Accordingly, we have demonstrated that stress-induced anxious-depressive behaviour is associated with the complete loss of the sensitivity of striatal CB1Rs(GABA), and that cocaine, caffeine, running wheel or sucrose are able to contrast these effects (Rossi et al., 2008; 2009; De Chiara et al., 2010a,b;).
Membrane cholesterol controls the pharmacological response of striatal CB1Rs(GABA) (Maccarrone et al., 2009), and it can be hypothesized therefore that compounds able to interfere with lipid raft composition could be effective in the management of mood disorders. In agreement with this speculation, recent evidence showed that brain-derived neurotrophic factor (BDNF) levels in the striatum are regulated by dopamine D2 receptors, and that BDNF is able to block CB1R(GABA) activity in the striatum. Importantly, this effect was dependent on increased synthesis and raft concentration of cholesterol mediated by BDNF (De Chiara et al., 2010b) (Figure 4). Importantly, previous evidence showed that striatal infusion of BDNF elicits a depressive behaviour (Eisch et al., 2003), and that the anxious-depressive behaviour induced by social stress is abolished by blockade of BDNF signalling in this brain area (Berton et al., 2006). These observations therefore represent a first indication that raft-associated receptors, such as CB1Rs(GABA), are involved in mood disorders and that inhibition of cholesterol synthesis might be useful to treat anxious depressive symptoms.
Figure 4.
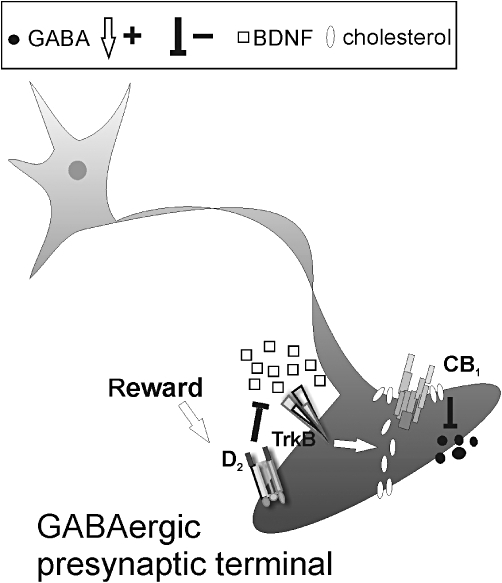
Schematic representation of the interaction between D2 receptors, brain-derived neurotrophic factor (BDNF) and type-1 cannabinoid receptor (CB1) in striatal neurons. Stimulation of dopamine D2 receptors trough chemical (cocaine) and natural rewards (voluntary exercise or sucrose drinking) reduces striatal contents of BDNF, thus reducing cholesterol synthesis and incorporation within CB1 receptor-containing lipid rafts mediated by the activation of the tyrosine receptor TrkB. This effect results in increased sensitivity of CB1Rs(GABA), and is limited to striatal GABAergic neuronal elements. The mechanism by which D2 receptors reduce BDNF concentrations is conversely still unknown (adapted from De Chiara et al. 2010a,b;).
Raft-associated CB1Rs(GABA) in the striatum are also altered in other pathological conditions, and namely neurodegenerative disorders. One of the earliest neurochemical alterations observed in HD patients, in fact, consists in the loss of CB1 receptor binding in the striatum, an alteration that significantly precedes the development of identifiable striatal neuropathology, and that might play a critical role in the development of HD symptoms (Richfield and Herkenham, 1994; Glass et al., 2000). In addition, down-regulation of CB1 receptors also occurs in the striatum of transgenic mouse models of HD prior to the development of either neuropsychiatric symptoms or neuronal degeneration (Denovan-Wright and Robertson, 2000; Lastres-Becker et al., 2002), and environmental enrichment, which delays the onset of HD symptoms in transgenic mice, is associated with a delayed loss of CB1 receptors (Glass et al., 2004). Together, these findings are compatible with the idea that CB1 receptors are heavily implicated in HD pathophysiology and, in line with this idea, we have found in R6/2 HD mice that the sensitivity of CB1Rs(GABA) in the striatum was lost since the early phases of the disease, while the activity of CB1Rs(Glu) was intact (Centonze et al., 2005).
Loss of CB1R(GABA) responses and preserved CB1R(Glu) function is also seen in experimental autoimmune encephalomyelitis (EAE), a model of MS in which the eCB system has been proposed to play a major role (Centonze et al., 2007b; Rossi et al., 2010a). In EAE, in fact, pharmacological stimulation of CB1 receptors with HU210 fails to inhibit GABAergic IPSCs recorded from striatal neurons, while glutamate synapses are inhibited, as in control conditions, by HU210 (Centonze et al., 2007b).
More complex alterations of the eCB system are observed in the striatum of experimental ALS, in which the maximal response of both CB1Rs(GABA) and CB1Rs(Glu) is enhanced (Rossi et al., 2010c), and in the mouse model of FXS (Maccarrone et al., 2009). In FXS mice, altered mGlu5 receptor signalling has been demonstrated in several studies (Huber et al., 2002; Bear et al., 2004). Recently, we have provided evidence that also mGlu5 receptor/2-AG coupling was altered in these mice. In the striatum of mice lacking fragile X mental retardation protein (FMRP), we found in fact enhanced activity of DAGL, associated with altered sensitivity of GABA synapses to the mobilization of 2-AG by mGlu5 receptor stimulation (Maccarrone et al., 2010b). Our data therefore indicate for the first time that mGlu5 receptor-driven eCB signalling in the striatum is under the control of FMRP, and that abnormal mGlu5 receptor/2-AG coupling might play a role in the synaptic defects of FXS.
Conclusions
Modulation of CB1 receptor activity is receiving increasing attention as a novel, promising strategy in the treatment of neuropsychiatric and neurodegenerative disorders. So far, however, Cannabis sativa extracts to activate these receptors (Howlett et al., 2010; Pertwee, 2010), or synthetic antagonists to block them have been of limited clinical utility, because severe psychiatric symptoms have been associated with their use (Hill and Gorzalka, 2009). An alternative option intensely explored is the modulation of eCB activity at these receptors, through the regulation of AEA or 2-AG metabolism (Petrosino and Di Marzo, 2010; Rossi et al., 2010b). It is still fully unexplored whether the modulation of cholesterol/CB1 receptor interaction could be a useful approach to modulate eCB signalling in the brain.
Here, we would like to comment that subtle, yet specific, differences might underpin the differential sensitivity of CB1 and CB2 to membrane cholesterol, possibly explaining the apparent redundancy of having two largely overlapping receptor subtypes that are activated by similar compounds (eCBs) and trigger similar transduction pathways (Di Marzo, 2009; Maccarrone et al., 2010a).
In general, cholesterol may act on the conformation of a membrane receptor by indirectly altering the physicochemical properties of the bilayer, or by directly interacting with the receptor itself, for example, through the CRAC domain and/or palmitoylation sites. More generally, we believe that the comparison between CB1 and CB2 might represent an interesting paradigm that goes well beyond eCB signalling. In fact, the modulation of CB1 by membrane cholesterol might disclose a novel ligand-receptor interaction, where a third player comes into the game: the membrane lipids. As a consequence, the membrane environment might play a role in receptor-dependent signalling, with a potential impact on several neurotransmission pathways, as well as several neurodegenerative/neuroinflammatory diseases where CB1 is known to play a role. It should be recalled that CB1-dependent signalling impacts fundamental processes as different as immune response, energy homeostasis, reproduction and skin differentiation (Di Marzo, 2009; Maccarrone et al., 2010a), thus it can be anticipated that cholesterol-dependent regulation of CB1 can have a physiological relevance well beyond the CNS.
In conclusion, membrane environment seems to be critical for the regulation of signal transduction pathways triggered by G protein-coupled receptors like CB1. Despite the three dimensional complexity of these proteins, we learn from the comparison of CB1 with CB2 that just one amino acid residue can direct receptor functioning, calling for attention on the plasma membrane as a key-player in ligand recognition on the cell surface. Further studies aimed at clarifying the impact of these notions for the treatment of disorders associated with abnormal activity of CB1 are desirable to come in the near future.
Acknowledgments
Financial support from Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2008 grant), and from Fondazione TERCAS (grant 2009-12) is gratefully acknowledged.
Glossary
Abbreviations
- 2-AG
2-arachidonoylglycerol
- Aβ
amyloid β peptide
- AD
Alzheimer's disease
- AEA
N-arachidonoylethanolamine or anandamide
- ALS
amyotrophic lateral sclerosis
- ApoE
apolipoprotein E
- APP
amyloid precursor protein
- BDNF
brain-derived neurotrophic factor
- CB1
type-1 cannabinoid receptor
- CB2
type-2 cannabinoid receptor
- CNS
central nervous system
- DAGL
diacylglycerol lipase
- eCB
endocannabinoid
- FAAH
fatty acid amide hydrolase
- FMRP
Fragile X mental retardation protein
- FXS
Fragile X syndrome
- GPCR
G protein-coupled receptor
- HD
Huntington's disease
- IPSI
inhibitory postsynaptic currents
- lc
liquid-disordered crystalline phase
- lo
liquid-ordered phase
- LR
lipid raft
- MAGL
monoacylglycerol lipase
- MCD
methyl-β-cyclodextrin
- mGlu
metabotropic glutamate
- MS
multiple sclerosis
- NAPE-PLD
N-acyl-phosphatidylethanolamine (NAPE)-hydrolysing phospholipase D
- NMDA
N-methyl-D-aspartate
- PD
Parkinson's disease
- PPAR
peroxisome proliferator-activated receptor
- PrD
prion-related disorder
- PrP
prion protein
- TRPV1
type-1 transient receptor potential vanilloid
Conflict of interest
The authors have no conflict of interest to declare.
Supporting Information
Teaching Materials; Figs 1–4 as PowerPoint slide.
References
- Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. (>4th edn.) 2009;158:S1–S254. [Google Scholar]
- Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- Bari M, Paradisi A, Pasquariello N, Maccarrone M. Cholesterol-dependent modulation of type 1 cannabinoid receptors in nerve cells. J Neurosci Res. 2005a;81:275–283. doi: 10.1002/jnr.20546. [DOI] [PubMed] [Google Scholar]
- Bari M, Battista N, Fezza F, Finazzi-Agrò A, Maccarrone M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem. 2005b;280:12212–12220. doi: 10.1074/jbc.M411642200. [DOI] [PubMed] [Google Scholar]
- Bari M, Spagnuolo P, Fezza F, Oddi S, Pasquariello N, Finazzi-Agrò A, et al. Effect of lipid rafts on Cb2 receptor signaling and 2-arachidonoyl-glycerol metabolism in human immune cells. J Immunol. 2006;177:4971–4980. doi: 10.4049/jimmunol.177.8.4971. [DOI] [PubMed] [Google Scholar]
- Bari M, Oddi S, De Simone C, Spagnuolo P, Gasperi V, Battista N, et al. Type-1 cannabinoid receptors colocalize with caveolin-1 in neuronal cells. Neuropharmacology. 2008;54:45–50. doi: 10.1016/j.neuropharm.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett-Norris J, Lynch D, Reggio PH. Lipids, lipid rafts and caveolae: their importance for GPCR signaling and their centrality to the endocannabinoid system. Life Sci. 2005;77:1625–1639. doi: 10.1016/j.lfs.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Baron GS, Wehrly K, Dorward DW, Chesebro B, Caughey B. Conversion of raft-associated prion protein to the protease-resistant state requires insertion of PrP-res (PrP(Sc)) into contiguous membranes. EMBO J. 2002;21:1031–1040. doi: 10.1093/emboj/21.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C, Salmona M, Diomede L, Williams A. Squalestatin cures prioninfected neurons and protects against prion neurotoxicity. J Biol Chem. 2004;279:14983–14990. doi: 10.1074/jbc.M313061200. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Becher A, White JH, McIlhinney RA. The gamma-aminobutyric acid receptor B, but not the metabotropic glutamate receptor type-1, associates with lipid rafts in the rat cerebellum. J Neurochem. 2001;79:787–795. doi: 10.1046/j.1471-4159.2001.00614.x. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bhaskar K, Yen S-H, Lee G. Disease-related modifications in tau affect the interaction between Fyn and tau. J Biol Chem. 2005;280:35119–35125. doi: 10.1074/jbc.M505895200. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Di Marzo V. Cannabinoid receptors and endocannabinoids: role in neuroinflammatory and neurodegenerative disorders. CNS Neurol Disord Drug Targets. 2010;9:564–573. doi: 10.2174/187152710793361568. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Burstein S, Hunter SA, Renzulli L. Stimulation of sphingomyelin hydrolysis by cannabidiol in fibroblasts from a Niemann-Pick patient. Biochem Biophys Res Commun. 1984;121:168–173. doi: 10.1016/0006-291x(84)90702-2. [DOI] [PubMed] [Google Scholar]
- Carlson NG, Rose JW. Antioxidants in multiple sclerosis: do they have a role in therapy? CNS Drugs. 2006;20:433–441. doi: 10.2165/00023210-200620060-00001. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agrò A, Bernardi G, et al. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Prosperetti C, Tscherter A, Bernardi G, Maccarrone M, et al. Abnormal sensitivity to cannabinoid receptor stimulation might contribute to altered gamma-aminobutyric acid transmission in the striatum of R6/2 Huntington's disease mice. Biol Psychiatry. 2005;57:1583–1589. doi: 10.1016/j.biopsych.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, De Chiara V, Prosperetti C, Battista N, Bernardi G, et al. Chronic cocaine sensitizes striatal GABAergic synapses to the stimulation of cannabinoid CB1 receptors. Eur J Neurosci. 2007a;25:1631–1640. doi: 10.1111/j.1460-9568.2007.05433.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F, et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain. 2007b;130:2543–2553. doi: 10.1093/brain/awm160. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battistini L, Maccarrone M. The endocannabinoid system in peripheral lymphocytes as a mirror of neuroinflammatory diseases. Curr Pharm Design. 2008;14:2370–2382. doi: 10.2174/138161208785740018. [DOI] [PubMed] [Google Scholar]
- Chau PL. New insights into the molecular mechanisms of general anaesthetics. Br J Pharmacol. 2010;161:288–307. doi: 10.1111/j.1476-5381.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainese E, Oddi S, Maccarrone M. Interaction of endocannabinoid receptors with biological membranes. Curr Med Chem. 2010;17:1487–1499. doi: 10.2174/092986710790980087. [DOI] [PubMed] [Google Scholar]
- De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, et al. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010a;35:374–387. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chiara V, Angelucci F, Rossi S, Musella A, Cavasinni F, Cantarella C, et al. Brain-derived neurotrophic factor controls cannabinoid CB1 receptor function in the striatum. J Neurosci. 2010b;30:8127–8137. doi: 10.1523/JNEUROSCI.1683-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Visser L, Van Den Bos R, Stoker AK, Kas MJ, Spruijt BM. Effects of genetic background and environmental novelty on wheel running as a rewarding behaviour in mice. Behav Brain Res. 2007;177:290–297. doi: 10.1016/j.bbr.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Denovan-Wright EM, Robertson HA. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington's disease mice. Neuroscience. 2000;98:705–713. doi: 10.1016/s0306-4522(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17:1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Dirikoc S, Priola SA, Marella M, Zsürger N, Chabry J. Nonpsychoactive cannabidiol prevents prion accumulation and protects neurons against prion toxicity. J Neurosci. 2007;27:9537–9544. doi: 10.1523/JNEUROSCI.1942-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert GP, Wood WG, Müller WE. Lipid membranes and beta-amyloid: a harmful connection. Curr Protein Pept Sci. 2010;11:319–325. doi: 10.2174/138920310791330668. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Epand RM. Cholesterol and the interaction of proteins with membrane domains. Prog Lipid Res. 2006;45:279–294. doi: 10.1016/j.plipres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Fallon L, Moreau F, Croft BG, Labib N, Gu WJ, Fon EA. Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem. 2002;277:486–491. doi: 10.1074/jbc.M109806200. [DOI] [PubMed] [Google Scholar]
- Fantini J, Yahi N. Molecular insights into amyloid regulation by membrane cholesterol and sphingolipids: common mechanisms in neurodegenerative diseases. Expert Rev Mol Med. 2010;12:e27. doi: 10.1017/S1462399410001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Lluís C, Justinova Z, Quiroz C, Orru M, Navarro G, et al. Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br J Pharmacol. 2010;160:443–453. doi: 10.1111/j.1476-5381.2010.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding CJ, Fielding PE. Cellular cholesterol efflux. Biochim Biophys Acta. 2001;1533:175–189. doi: 10.1016/s1388-1981(01)00162-7. [DOI] [PubMed] [Google Scholar]
- Fivaz M, Vilbois F, Thurnheer S, Pasquali C, Abrami L, Bickel PE, et al. Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J. 2002;21:3989–4000. doi: 10.1093/emboj/cdf398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forneris F, Mattevi A. Enzymes without borders: mobilizing substrates, delivering products. Science. 2008;321:213–216. doi: 10.1126/science.1151118. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Bessis AS, Rossignol F, Pin JP, Olivo-Marin JC, Hémar A. The metabotropic glutamate receptor mGluR5 is endocytosed by a clathrin-independent pathway. J Biol Chem. 2003;278:12222–12230. doi: 10.1074/jbc.M205663200. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Ge M, Field KA, Aneja R, Holowka D, Baird B, Freed JH. Electron spin resonance characterization of liquid ordered phase of detergent-resistant membranes from RBL-2H3 cells. Biophys J. 1999;77:925–933. doi: 10.1016/S0006-3495(99)76943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABAA receptor alterations in the human basal ganglia in Huntington's disease. Neuroscience. 2000;97:505–519. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- Glass M, van Dellen A, Blakemore C, Hannan AJ, Faull RLM. Delayed onset of Huntington's disease in mice in an enriched environment correlates with delayed loss of cannabinoid CB1 receptors. Neuroscience. 2004;123:207–212. doi: 10.1016/s0306-4522(03)00595-5. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Tomic I, Beyreuther K, Hartmann T, Bergmann C. Independent inhibition of Alzheimer disease beta- and gammasecretase cleavage by lowered cholesterol levels. J Biol Chem. 2008;283:11302–11311. doi: 10.1074/jbc.M801520200. [DOI] [PubMed] [Google Scholar]
- Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Hatano T, Kubo SI, Imai S, Maeda M, Ishikawa K, Mizuno Y, et al. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum Mol Genet. 2007;16:678–690. doi: 10.1093/hmg/ddm013. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, Vance JE. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J Biol Chem. 2004;279:14009–14015. doi: 10.1074/jbc.M313828200. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Impairments in endocannabinoid signaling and depressive illness. JAMA. 2009;301:1165–1166. doi: 10.1001/jama.2009.369. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Blume LC, Dalton GD. CB1 cannabinoid receptors and their associated proteins. Curr Med Chem. 2010;17:1382–1393. doi: 10.2174/092986710790980023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone T, Esposito G, De Filippis D, Scuderi C, Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther. 2009;15:65–75. doi: 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin N, Neumann JM, Ostuni MA, Vu TK, Yao ZX, Murail S, et al. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol Endocrinol. 2005;19:588–594. doi: 10.1210/me.2004-0308. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Gibbs CJ., Jr Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N Engl J Med. 1998;339:1994–2004. doi: 10.1056/NEJM199812313392707. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, et al. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–621. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, et al. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- Karten B, Campenot RB, Vance DE, Vance JE. Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J Biol Chem. 2006;281:4049–4057. doi: 10.1074/jbc.M508915200. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Katona I, Urbán GM, Wallace M, Ledent C, Jung KM, Piomelli D, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, et al. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S-I, Neman VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, et al. A combinatorial code for the interaction of alphasynuclein with membranes. J Biol Chem. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- Kust BM, Copray JC, Brouwer N, Troost D, Boddeke HW. Elevated levels of neurotrophins inhumanbiceps brachii tissue of amyotrophic lateral sclerosis. Exp Neurol. 2002;177:419–427. doi: 10.1006/exnr.2002.8011. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Berrendero F, Lucas JJ, Martin-Aparicio E, Yamamoto A, Ramos JA, et al. Loss of mRNA levels, binding and activation of GTP-binding proteins for cannabinoid CB1 receptors in the basal ganglia of a transgenic model of Huntington's disease. Brain Res. 2002;929:236–242. doi: 10.1016/s0006-8993(01)03403-5. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72:355–358. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars: hedonic aspects, neuroregulation, and energy balance. Am J Clin Nutr. 2003;78:834S–842S. doi: 10.1093/ajcn/78.4.834S. [DOI] [PubMed] [Google Scholar]
- Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- Lindahl E, Sansom MS. Membrane proteins: molecular dynamics simulations. Curr Opin Struct Biol. 2008;18:425–431. doi: 10.1016/j.sbi.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Liu JP, Tang Y, Zhou S, Toh BH, McLean C, Li H. Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol Cell Neurosci. 2010;43:33–42. doi: 10.1016/j.mcn.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Robbins PW. Lipid rafts-protein association and the regulation of protein activity. Arch Biochem Biophys. 2004;426:208–224. doi: 10.1016/j.abb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Maccarrone M. Good news for CB1 receptors: endogenous agonists are in the right place. Br J Pharmacol. 2008;153:179–181. doi: 10.1038/sj.bjp.0707566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Battista N, Centonze D. The endocannabinoid pathway in Huntington's disease: a comparison with other neurodegenerative diseases. Prog Neurobiol. 2007;81:349–379. doi: 10.1016/j.pneurobio.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, De Chiara V, Gasperi V, Viscomi MT, Rossi S, Oddi S, et al. Lipid rafts regulate 2-arachidonoylglycerol metabolism and physiological activity in the striatum. J Neurochem. 2009;109:371–381. doi: 10.1111/j.1471-4159.2009.05948.x. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Gasperi V, Catani MV, Diep TA, Dainese E, Hansen HS. The endocannabinoid system and its relevance for nutrition. Annu Rev Nutr. 2010a;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A, et al. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology. 2010b;35:1500–1509. doi: 10.1038/npp.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Dotti CG, Ledesma MD. Brain cholesterol in normal and pathological aging. Biochim Biophys Acta. 2010;1801:934–944. doi: 10.1016/j.bbalip.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Jeong GB, Crocker A, Arneja A, David S, Russell D, et al. Protecting motor neurons from toxic insult by antagonism of adenosine A2a and Trk receptors. J Neurosci. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musella A, De Chiara V, Rossi S, Cavasinni F, Castelli M, Cantarella C, et al. Transient receptor potential vanilloid 1 channels control acetylcholine/2-arachidonoylglicerol coupling in the striatum. Neuroscience. 2010;167:864–871. doi: 10.1016/j.neuroscience.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006;24:2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, et al. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi S, Dainese E, Fezza F, Lanuti M, Barcaroli D, De Laurenzi V, et al. Functional characterization of putative cholesterol binding sequence (CRAC) in human type-1 cannabinoid receptor. J Neurochem. 2011;116:858–865. doi: 10.1111/j.1471-4159.2010.07041.x. [DOI] [PubMed] [Google Scholar]
- Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–698. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermeyer AG, Beckrich BT, Ivarson KA, Grove KE, Brown DA. Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells. methyl-betacyclodextrin does not affect cell surface transport of a GPI anchored protein. J Biol Chem. 1999;274:34459–34466. doi: 10.1074/jbc.274.48.34459. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. Anxiety, cognition, and habit: a multiple memory systems perspective. Brain Res. 2009;1293:121–128. doi: 10.1016/j.brainres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Paila YD, Ganguly S, Chattopadhyay A. Metabolic depletion of sphingolipids impairs ligand binding and signaling of human serotonin1A receptors. Biochemistry. 2010;49:2389–2397. doi: 10.1021/bi1001536. [DOI] [PubMed] [Google Scholar]
- Pani A, Mandas A, Dessì S. Cholesterol, Alzheimer's disease, prion disorders: a ménage à trois? Curr Drug Targets. 2010;11:1018–1031. doi: 10.2174/138945010791591386. [DOI] [PubMed] [Google Scholar]
- Patel KD, Davison JS, Quentin J, Pittman OJ, Sharkey KA. Cannabinoid CB2 receptors in health and disease. Curr Med Chem. 2010;17:1394–1410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- Pelkmans L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim Biophys Acta. 2005;1746:295–304. doi: 10.1016/j.bbamcr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem. 2010;17:1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Pontier SM, Percherancier Y, Galandrin S, Breit A, Gales C, Bouvier M. Cholesterol-dependent separation of the beta2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J Biol Chem. 2008;283:24659–24672. doi: 10.1074/jbc.M800778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinetti A, Loberto N, Chigorno V, Sonnino S. Glycosphingolipid behaviour in complex membranes. Biochim Biophys Acta. 2009;1788:184–193. doi: 10.1016/j.bbamem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Herkenham M. Selective vulnerability in Huntington's disease: preferential loss of cannabinoid receptors in lateral globus pallidus. Ann Neurol. 1994;36:577–584. doi: 10.1002/ana.410360406. [DOI] [PubMed] [Google Scholar]
- Rimmerman N, Hughes HV, Bradshaw HB, Pazos MX, Mackie K, Prieto AL, et al. Compartmentalization of endocannabinoids into lipid rafts in a dorsal root ganglion cell line. Br J Pharmacol. 2008;153:380–389. doi: 10.1038/sj.bjp.0707561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, et al. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, et al. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Mataluni G, Sacchetti L, Siracusano A, et al. Caffeine drinking potentiates cannabinoid transmission in the striatum: interaction with stress effects. Neuropharmacology. 2009;56:590–597. doi: 10.1016/j.neuropharm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Rossi S, Bernardi G, Centonze D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp Neurol. 2010a;224:92–102. doi: 10.1016/j.expneurol.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Sacchetti L, Cantarella C, Castelli M, et al. Preservation of striatal cannabinoid CB1 receptor function correlates with the antianxiety effects of fatty acid amide hydrolase inhibition. Mol Pharmacol. 2010b;78:260–268. doi: 10.1124/mol.110.064196. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Cozzolino M, Bernardi G, Maccarrone M, et al. Abnormal sensitivity of cannabinoid CB1 receptors in the striatum of mice with experimental amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010c;11:83–90. doi: 10.3109/17482960902977954. [DOI] [PubMed] [Google Scholar]
- Schengrund CL. Lipid rafts: keys to neurodegeneration. Brain Res Bull. 2010;82:7–17. doi: 10.1016/j.brainresbull.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Schrattenholz A, Soskic V. NMDA receptors are not alone: dynamic regulation of NMDA receptor structure and function by neuregulins and transient cholesterol-rich membrane domains leads to disease-specific nuances of glutamate-signalling. Curr Top Med Chem. 2006;6:663–686. doi: 10.2174/156802606776894519. [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, Krivda JP, D'Souza-Schorey C. Neurodegeneration in Niemann-Pick Type C disease and Huntington's disease: impact of defects in membrane trafficking. Curr Drug Targets. 2009;10:653–665. doi: 10.2174/138945009788680437. [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin1A receptors. Biochemistry. 2010;49:5426–5435. doi: 10.1021/bi100276b. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, et al. Mitochondral import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sipione S, Rigamonti D, Valenza M, Zuccato C, Conti L, Pritchard J, et al. Early transcriptional profiles in huntingtin-inducible striatal cells by microarray analyses. Hum Mol Genet. 2002;11:1953–1965. doi: 10.1093/hmg/11.17.1953. [DOI] [PubMed] [Google Scholar]
- Smart EJ, De Rose RA, Farber SA. Annexin 2-caveolin 1 complex is a target of ezetimibe and regulates intestinal cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:3450–3455. doi: 10.1073/pnas.0400441101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Szabo B, Dörner L, Pfreundtner C, Nörenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uittenbogaard A, Everson WV, Matveev SV, Smart EJ. Cholesteryl ester is transported from caveolae to internal membranes as part of a caveolin-annexin II lipid-protein complex. J Biol Chem. 2002;277:4925–4931. doi: 10.1074/jbc.M109278200. [DOI] [PubMed] [Google Scholar]
- Valencia A, Reeves PB, Sapp E, Li S, Alexander J, Kegel KB, et al. Mutant huntingtin and glycogen synthase kinase 3-β accumulate in neuronal lipid rafts of a presymptomatic knock-in mouse model of Huntington's disease. J Neurosci Res. 2009;29:6106–6116. doi: 10.1002/jnr.22184. [DOI] [PubMed] [Google Scholar]
- Valenza M, Cattaneo E. Cholesterol dysfunction in neurodegenerative diseases: is Huntington's disease in the list? Prog Neurobiol. 2006;80:165–176. doi: 10.1016/j.pneurobio.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Vance JE, Karten B, Hayashi H. Lipid dynamics in neurons. Biochem Soc Trans. 2006;34:399–403. doi: 10.1042/BST0340399. [DOI] [PubMed] [Google Scholar]
- Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J Neurochem. 2007;102:1727–1737. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur J Neurosci. 2000;12:2967–2974. doi: 10.1046/j.1460-9568.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, et al. Delta FosB regulates wheel running. J Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie HQ, Liang D, Leung KW, Chen VP, Zhu KY, Chan WK, et al. Targeting acetylcholinesterase to membrane rafts: a function mediated by the proline-rich membrane anchor (PRiMA) in neurons. J Biol Chem. 2010;285:11537–11546. doi: 10.1074/jbc.M109.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Adermark L, Lovinger DM. Neurotensin reduces glutamatergic transmission in the dorsolateral striatum via retrograde endocannabinoid signaling. Neuropharmacology. 2008;54:79–86. doi: 10.1016/j.neuropharm.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


