Abstract
In this review, we consider the role of endocannabinoids and cannabinoid-1 (CB1) cannabinoid receptors in metabolic regulation and as mediators of the thrifty phenotype that underlies the metabolic syndrome. We survey the actions of endocannabinoids on food intake and body weight, as well as on the metabolic complications of visceral obesity, including fatty liver, insulin resistance and dyslipidemias. Special emphasis is placed on weighing the relative importance of CB1 receptors located in peripheral tissues versus the central nervous system in mediating the metabolic effects of endocannabinoids. Finally, we review recent observations that indicate that peripherally restricted CB1 receptor antagonists retain efficacy in reducing weight and improving metabolic abnormalities in mouse models of obesity without causing behavioural effects predictive of neuropsychiatric side effects in humans.
LINKED ARTICLES
This article is part of a themed issue on Cannabinoids in Biology and Medicine. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: diet-induced obesity, metabolic syndrome, endocannabinoids, anandamide, 2-arachidonoylglycerol, peripheral CB1 receptors
Endocannabinoids are key mediators of the thrifty phenotype
Endocannabinoids are lipid mediators generated on demand in the cell membrane from membrane phospholipid precursors, which then act on cannabinoid receptors in the same or adjacent cells (Pacher et al., 2006). The two most widely studied endocannabinoids are arachidonoyl ethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG), which are ubiquitous as they are present not only in central and peripheral neurons but also in parenchymal cells of various tissues. The well-known effects of smoked marijuana provided early give-aways as to the biological functions of its endogenous counterparts. A case in point is the ‘munchies’, which had prompted a study that provided evidence for endocannabinoids acting via cannabinoid-1 (CB1) receptors (drug/target nomenclature as outlined in Alexander et al., 2008) being part of the leptin-regulated central neural appetitive circuitry as orexigenic mediators (Di Marzo et al., 2001). This accounted for the reported ability of CB1 receptor blockade to inhibit food intake in rodents (Colombo et al., 1998; Simiand et al., 1998; Williams and Kirkham, 1999; Freedland et al., 2000). Together, these findings provided the impetus for testing such compounds as potential treatment for obesity. Paradoxically, subsequent epidemiological studies indicated that although chronic regular marijuana use was associated with increased caloric intake, body/mass index was unchanged (Rodondi et al., 2006) or even reduced in marijuana users (Smit and Crespo, 2001), which may be related to the unexplored role of some of the other cannabinoids present in marijuana that do not interact with CB1 receptors.
Indeed, the first-in-class CB1 antagonist rimonabant proved effective not only in reducing body weight, but also in improving the associated insulin resistance and dyslipidemias in obese/overweight people with the metabolic syndrome (Despres et al., 2005; Van Gaal et al., 2005; Pi-Sunyer et al., 2006; Scheen et al., 2006). In additional human studies, chronic rimonabant treatment reduced intra-abdominal and liver fat in abdominally obese subjects with atherogenic dyslipidemia (Despres et al., 2009), and was also effective in improving glycemic control either as a monotherapy in drug-naïve diabetic patients (Rosenstock et al., 2008) or in type II diabetics receiving insulin (Hollander et al., 2010).
Such findings, along with other ‘energy conserving’ effects of cannabinoids such as hypothermia, hypomotility, reduced sympathetic tone and promotion of sleep (Pacher et al., 2006), could suggest that endocannabinoids are key mediators of the ‘thrifty’ phenotype, which helped evolutionary survival during periods of starvation. However, in our contemporary society of abundant food supplies coupled with a sedentary lifestyle, this phenotype has become the main culprit in the epidemic spread of obesity and its metabolic complications. Indications that blocking endocannabinoid action by rimonabant improves most, if not all, components of the metabolic syndrome, suggest that increased endocannabinoid ‘tone’ may be its unifying pathogenic feature (Di Marzo and Matias, 2005). The alternative explanation that rimonabant's effects reflect inverse agonism rather than enhanced endocannabinoid ‘tone’ is unlikely in view of the finding that treatment of obese mice with a neutral CB1 antagonist produced comparable metabolic benefits (Tam et al., 2010). The correlation of circulating levels of 2-AG with visceral fat mass and indicators of insulin resistance (Bluher et al., 2006; Matias et al., 2006; Di Marzo et al., 2009) also fits into such a concept, although the relationship between CB1 receptor activation and the very low plasma levels of endocannabinoids is unclear.
CB1 expression and anandamide levels positively correlated with visceral adipose mass in obese-hypertensive patients (Bordicchia et al., 2010). Furthermore, adipocyte-derived substances inhibit insulin signalling in human skeletal muscle via CB1 activation (Eckardt et al., 2009). Together, such findings would favour the viewpoint that the metabolic syndrome or ‘syndrome X’ represents a true diagnostic entity, rather than coincident but mechanistically unrelated pathologies (Reaven, 2007).
Development of CB1 antagonists for the pharmacotherapy of the metabolic syndrome
Although appetite reduction was the original rationale for developing CB1 antagonists for the treatment of obesity, it had soon become clear that reduced food intake is not the only – and may not even be the primary – mechanism of weight reduction, at least in preclinical models of obesity. In mice with diet-induced obesity (DIO), tolerance develops rapidly to the reduction in food intake, but not to the decrease in body weight induced by chronic treatment with rimonabant, suggesting food intake-independent effects on energy expenditure (Ravinet Trillou et al., 2003). Although the mechanism(s) involved in the development of tolerance to the anorexigenic effect of CB1 blockade are not known, the observation that endocannabinoids can both increase and decrease appetite via suppressing excitatory glutamatergic or striatal inhibitory GABAergic neurotransmission, respectively (Bellocchio et al., 2010), may be relevant in this regard. Mice lacking CB1 receptors are resistant to DIO and its metabolic consequences, despite similar caloric intake during the diet period (Ravinet Trillou et al., 2004; Osei-Hyiaman et al., 2005), which also points to energy metabolism being directly regulated by endocannabinoids. Indeed, endocannabinoids have been found to promote lipogenesis in adipose tissue (Cota et al., 2003) and liver (Osei-Hyiaman et al., 2005), whereas they inhibit fatty acid oxidation (Jbilo et al., 2005; Herling et al., 2008; Osei-Hyiaman et al., 2008; Flamment et al., 2009) and mitochondrial mitogenesis (Tedesco et al., 2010). Treatment with a CB1 antagonist has opposite effects and also promotes mitochondrial biogenesis (Tedesco et al., 2008) and transdifferentiation of white to brown adipocytes (Perwitz et al., 2010), which could all contribute to an increase in energy expenditure (Herling et al., 2008; Osei-Hyiaman et al., 2008; Tam et al., 2010).
A key question that has arisen as a result of such findings is the relative importance of central versus peripheral CB1 receptors involved in these effects. This question has considerable practical implications in light of observations that a small but significant fraction of individuals treated with rimonabant developed anxiety, depression and/or suicidal ideation (Christensen et al., 2007). This had led not only to the eventual withdrawal of rimonabant from the market, but also discontinuation of the development of all CB1 inverse agonists by Big Pharma and doubts about the therapeutic potential of CB1 blockade (Jones, 2008). The neuropsychiatric, anhedonic side effects of global CB1 blockade should not have been unexpected, given the fact that endocannabinoids and CB1 receptors are obligatory components of the mesolimbic dopaminergic reward pathway that mediates both natural and drug reward (Gardner, 2002). A preclinical counterpart of this is the depression-like phenotype reported in rimonabant-treated rats (Beyer et al., 2010), although in some rodent models of depression, rimonabant displayed antidepressant-like activity (Steiner et al., 2008; Takahashi et al., 2008). Additionally, CB1 null mice were noted to have a reduced life span (Zimmer et al., 1999), which may be related to the early onset of ageing-like changes that appear to be restricted to cognitive abilities and skin structure (Bilkei-Gorzo et al., 2010).
If peripherally located CB1 receptors do contribute to the metabolic benefit of CB1 blockade, then limiting the access of CB1 antagonists to the brain may improve their therapeutic index by reducing or eliminating the potential for CNS-mediated neuropsychiatric side effects, while retaining some or most of their metabolic actions. As for early ageing, there is no evidence that chronic pharmacological blockade, as opposed to life-long absence, of CB1 causes similar effects. Even so, a peripherally restricted CB1 antagonist would be unlikely to influence cognitive functions.
Central versus peripheral sites of the metabolic actions of endocannabinoids
In considering the relative importance of central versus peripheral CB1 receptors in metabolic regulation, there are some general considerations. First, CB1 receptors are highly abundant in the mammalian, including human brain, but are also present at much lower, yet functionally relevant concentrations in many peripheral tissues involved in metabolic regulation, including adipose tissue, liver, skeletal muscle and pancreas (Pacher et al., 2006). The abundance of CB1 receptors in a given tissue is not a good predictor of their functional relevance, as increased efficiency of coupling can offset the effect of low receptor density. This is illustrated by the lack of correlation between CB1 receptor density and CB1-stimulated GTPγS labelling in various brain regions (Breivogel et al., 1997).
Second, in peripheral tissues involved in metabolic regulation, the baseline level of CB1 receptors is very low, but is markedly up-regulated of obesity, as seen in adipose tissue (Bensaid et al., 2003; Jourdan et al., 2010), liver (Osei-Hyiaman et al., 2008; Jourdan et al., 2010; Quarta et al., 2010) and skeletal muscle (see Figure 1, page 76 in Pagotto et al., 2006). This may be an important factor in the apparent increase in endocannabinoid ‘tone’ in obesity, which is also indicated by the ability of CB1 antagonists to affect metabolic parameters under obese, but not under non-obese conditions. The situation is less clear in human obesity, where CB1 expression was reportedly decreased in subcutaneous fat (Engeli et al., 2005), but increased in visceral fat (Bordicchia et al., 2010). In liver, CB1 expression was reduced in the presence of steatosis in immortalized human hepatocytes (De Gottardi et al., 2010), but showed a robust, >30-fold increase along with increased CB1 immunoreactivity in liver tissue from 26 patients with non-alcoholic fatty liver disease, relative to samples of non-fatty livers (R. Bataller, pers. comm.). Although tissue levels of receptors and their ligands are usually inversely related, there are examples of an obesity-related parallel increase in endocannabinoid levels in the same tissues (Osei-Hyiaman et al., 2005; 2008; Bordicchia et al., 2010). This may be related to the ‘autoinduction’ of CB1 expression by its own ligands (Borner et al., 2007; Mukhopadhyay et al., 2010).
Figure 1.
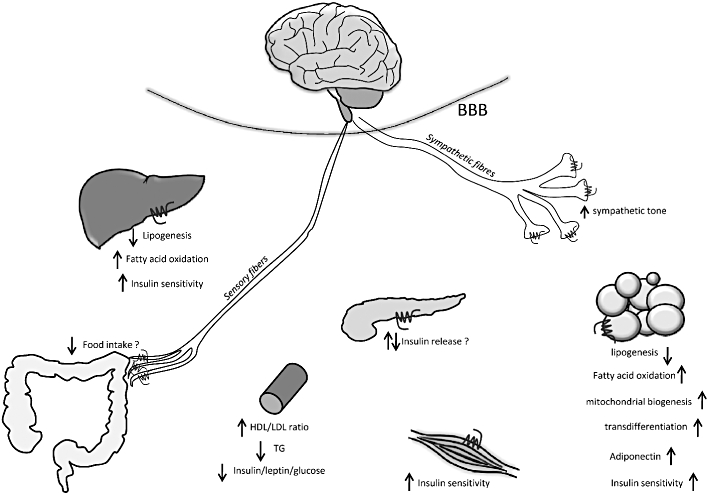
Therapeutically relevant effects of blockade of peripheral cannabinoid 1 (CB1) receptors in visceral obesity/metabolic syndrome. BBB, blood/brain barrier; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TG, triglyceride.
Third, although the CNS obviously exerts regulatory control over both energy intake and peripheral energy metabolism, the neural networks are complex and involve interorgan communication via bidirectional connections among various peripheral tissues and the brain (Uno et al., 2006; Imai et al., 2008; Sabio et al., 2008). This means that the activity in a specific circuit can be modulated, not only in the brain, but also at sensory afferent or autonomic efferent terminals in peripheral tissues. This is particularly relevant for regulation via CB1 receptors, which are prominently expressed in peripheral terminals of sensory neurons (Burdyga et al., 2004) as well as in peripheral sympathetic (Ishac et al., 1996; Niederhoffer et al., 2003) and parasympathetic terminals (Coutts and Pertwee, 1997).
Fourth, in mice with tissue-specific deletion of CB1 receptors, which results in a lean phenotype, the observed changes may be secondary to the resistance of the animal to diet-induced weight gain rather than due to the tissue-specific loss of CB1 receptors. Indeed, this explanation may reconcile seemingly contradictory findings in two recent studies by the same group, where mice lacking CB1 receptors in Ca2+/calmodulin-dependent protein kinase (CAMkinase) IIα-expressing neurons, including peripheral sympathetic nerves, had a similar resistance to diet-induced obesity and metabolic complications (Quarta et al., 2010) as mice with selective deletion of CB1 receptors in adipocytes (Mancini et al., 2010). With the above considerations in mind, let us examine available evidence regarding the potential sites of the metabolic actions of endocannabinoids, schematically illustrated in Figure 1.
Food intake
Endocannabinoids are part of the leptin-regulated neural circuitry in the hypothalamus (Di Marzo et al., 2001), and have been implicated in the control of both the consummatory and appetitive aspects of food intake (Thornton-Jones et al., 2005). Several hypothalamic sites, including the ventromedial nucleus (Jamshidi and Taylor, 2001) and the lateral hypothalamus (Jo et al., 2005), as well as sites in the limbic forebrain (Kirkham et al., 2002) and lower brainstem (Miller et al., 2004) have been implicated in their orexigenic action. CB1 receptors at peripheral sensory nerve terminals may also modulate food intake. In a study in rats, the food intake-reducing effect of rimonabant was abolished by capsaicin-induced sensory deafferentation (Gomez et al., 2002). CB1 receptors have been detected in the nodose ganglion, where cell bodies of sensory neurons projecting from the gut to the hypothalamus are located, and their expression was increased by fasting and reduced by re-feeding (Burdyga et al., 2004), which is compatible with their involvement in the control of food intake. In contrast, selective vagal deafferentation by subdiaphragmatic vagotomy did not affect the anorexic effect of rimonabant (Madsen et al., 2009). It is possible that the loss of the effect of rimonabant in capsaicin-treated animals (Gomez et al., 2002) was due to elimination of non-vagal afferents by capsaicin (Madsen et al., 2009). Interestingly, loss of the food intake-reducing effect of rimonabant in mice with selective knockout of CB1 receptors in CAMkinase IIα-expressing neurons (Quarta et al., 2010) may involve a similar mechanism, given the fact that CAMkinase IIα is prominently expressed not only in forebrain and peripheral sympathetic neurons (Quarta et al., 2010), but also in sensory neurons (Carlton, 2002; Price et al., 2005).
In another study, comparable reductions in food intake in mice were achieved at much lower levels of central CB1 receptor occupancy using the CB1 inverse agonist, SLV-319 (11% occupancy) than using rimonabant (65% occupancy), again suggesting a site of action outside of the brain (Need et al., 2006).
Body weight
The reduction in body weight by chronic CB1 blockade is due to reduced adipose tissue mass, which is unrelated to reduced energy intake, at least in a mouse model of diet-induced obesity (Ravinet Trillou et al., 2004). In high fat-fed dogs that develop abdominal obesity similar to human visceral obesity, chronic rimonabant treatment reduced abdominal fat mass. As in mice, this was unrelated to the transient reduction in food intake and was not associated with any change in basal metabolic rate (Richey et al., 2009), suggesting a peripheral mechanism. Rimonabant has been shown to increase sympathetic tone (Quarta et al., 2010), which could result in decreased adipose mass due to increased β-adrenergic lipolysis. However, lipolysis in the high fat-fed dogs was unaffected by rimonabant (Richey et al., 2009), which argues against a neural mechanism, at least in this model.
Insulin sensitivity
Similar to the effects on energy intake, there is evidence for both central and peripheral sites of action for the modulation of insulin sensitivity by endocannabinoids. The in vivo site of action of insulin itself is a matter of debate. Insulin suppresses hepatic glucose production and increases tissue uptake of glucose, but the question of whether its actions in vivo are primarily via receptors in target tissues (Michael et al., 2000) or via insulin receptors in the brain, which would then influence target organ responses indirectly via neural pathways (Buettner et al., 2005), has not been definitively settled.
Similarly, anandamide may affect hepatic insulin sensitivity via an action in the mediobasal hypothalamus, where it may mediate the insulin resistance induced by short-term overfeeding (O'Hare et al., 2010). On the other hand, mice with liver-specific knockout of CB1 receptors are protected from the insulin resistance induced by chronic exposure to a high-fat diet, even though they become as obese as wild-type mice on the same diet (Osei-Hyiaman et al., 2008). This latter finding indicates that hepatic CB1 receptors play a major role in the obesity-related, weight-independent component of insulin resistance. These two findings are not necessarily mutually exclusive: insulin resistance induced by chronic high-fat diet is associated with up-regulation of hepatic CB1 receptors, and both changes are normalized by CB1 antagonist treatment (Osei-Hyiaman et al., 2008; Jourdan et al., 2010; Quarta et al., 2010). Short-term overfeeding may not be sufficient to induce up-regulation of hepatic CB1 receptors, thus minimizing their involvement in insulin resistance. Insulin resistance in DIO rats was reversed by systemic but not intracerebroventricular administration of rimonabant, which also points to the dominant role of peripheral CB1 receptors in this effect, in established obesity (Nogueiras et al., 2008). The finding that a peripherally restricted CB1 antagonist was equieffective with rimonabant in reversing insulin resistance in DIO mice (14 weeks on high-fat diet) indicates that central endocannabinoid mechanisms play minimal – if any – role in obesity-related insulin resistance (Tam et al., 2010).
Recent preliminary observations indicate that in mice with adipocyte-specific deletion of CB1 receptors, high-fat diet fails to induce glucose intolerance and insulin resistance (Mancini et al., 2010). As pointed out above, these mice also remain lean and do not develop steatosis, so their resistance to diet-induced impairment in insulin sensitivity may be secondary to the absence of the obese phenotype, rather than a direct effect of adipocyte CB1 receptors in the control of insulin sensitivity. One possible mechanism by which adipocyte CB1 receptors may indirectly influence insulin sensitivity is via adiponectin. The adipocyte-derived protein, adiponectin is known to increase insulin sensitivity, and obesity is often associated with reduced plasma adiponectin levels. Rimonabant increases plasma adiponectin levels in obese individuals (Despres et al., 2005), or adiponectin synthesis and secretion in cultured rat adipocytes (Bensaid et al., 2003), although CB1 regulation of adiponectin production has not been documented in human adipocytes. This effect of rimonabant may contribute to the insulin-sensitizing action of CB1 blockade, as suggested by the reduced effectiveness of rimonabant in reversing diet-induced insulin resistance in adiponectin knockout mice (Migrenne et al., 2009; Watanabe et al., 2009). Another possible peripheral mechanism involves the blockade of CB1 receptors in adipose tissue macrophages. Macrophage infiltration into adipose tissue has been implicated in obesity-related insulin resistance (Weisberg et al., 2003). Recent findings indicate that rimonabant inhibits the ability of lipopolysaccharide-activated macrophages to inhibit insulin signalling by reducing their production of the inflammatory cytokine tumour necrosis factor α and increasing the production of the anti-inflammatory cytokine interleukin-10 (Miranville et al., 2010). CB1 antagonists also counteract CB1-mediated increases in reactive oxygen species production by macrophages (Han et al., 2009), or the vascular inflammation that accompanies certain diabetic complications (El-Remessy et al., 2011), although an elevation of pro-inflammatory cytokine levels in the CNS of rimonabant-treated rats has also been reported (Beyer et al., 2010).
Hepatic steatosis
Activation of hepatic CB1 receptors induces lipogenic gene expression (Osei-Hyiaman et al., 2005; Ruby et al., 2008; Son et al., 2009; Jourdan et al., 2010) and promotes de novo hepatic lipogenesis (Osei-Hyiaman et al., 2005), whereas fatty acid oxidation in the liver is inhibited by CB1 receptors (Osei-Hyiaman et al., 2008). These effects, however, only modestly contribute to diet-induced accumulation of triglycerides (TGs) in the liver, because although liver-specific CB1 knockout mice are partially protected from diet-induced steatosis (Osei-Hyiaman et al., 2008), mice with transgenic re-expression of hepatic CB1 receptors on a global CB1 knockout background remain largely resistant to the steatotic effect of a high-fat diet (Tam et al., 2010). The major source of hepatic TGs is likely to be fatty acids transferred from adipose tissue (Jourdan et al., 2010), where they are generated through CB1-mediated activation of adipocyte lipoprotein lipase (Cota et al., 2003) and released via ‘spillover’ into the circulation (Miles et al., 2004). This is also compatible with the recent finding that adipocyte-specific CB1 knockout mice are resistant to diet-induced steatosis (Mancini et al., 2010).
Plasma lipid profile
CB1 antagonist treatment results in improved plasma lipid profile (Despres et al., 2005; Van Gaal et al., 2005; Pi-Sunyer et al., 2006), which is likely mediated via peripheral CB1 receptors. In a mouse model of acutely increased endocannabinoid tone, the increase in tissue 2-AG levels elicited by treatment with a monoacylglyceride lipase inhibitor was associated with elevated plasma TG and cholesterol levels, as well as an accumulation of apolipoprotein E (ApoE)-depleted TG-rich lipoproteins. These effects were absent in CB1−/− and ApoE−/− mice and were reversed by rimonabant in wild-type mice, and could be attributed to reduced TG clearance mediated by peripheral CB1 receptors (Ruby et al., 2008). TG secretion was not affected in this acute model. However, when endocannabinoid tone is chronically increased, such as in DIO mice, a peripherally restricted CB1 antagonist induced an acute increase in secretion of TG-rich very-low-density lipoproteins, which implicated hepatic CB1 receptors in this effect (Tam et al., 2010).
The therapeutic potential of peripherally restricted CB1 antagonists
The likely contribution of peripheral CB1 receptors to the metabolic effects of endocannabinoids coupled with the undisputed role of central CB1 receptors in their hedonic effects has been the motivating factor for the development of second-generation CB1 antagonists with limited brain penetration for the treatment of the metabolic syndrome. Compounds with high CB1 potency (Kd < 10 nM) and low brain penetrance (plasma : brain ratio < 10%) have been reported (McElroy et al., 2008; Receveur et al., 2010; Tam et al., 2010), with 5-(4-[4-Cyanobut-1-ynyl]phenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(1,1-dioxo-thio-morpholino-)-1H-pyrazole-3-carboxamide (AM6545) being the first such compound undergoing detailed pharmacological, metabolic and behavioural assessment in mouse models of obesity.
AM6545 is a structurally modified analogue of rimonabant. Its CB1 binding affinity (Kd: 3.3 nM) and CB1/CB2 selectivity (∼200-fold) is similar to that of rimonabant, but unlike the inverse agonist rimonabant, AM6545 is a neutral antagonist, as revealed by GTPγS binding assays (Tam et al., 2010). AM6545 has very low brain penetrance (3–7% of plasma level following either acute or chronic administration compared with ∼80% for rimonabant), due to reduced lipophilicity as well as P-glycoprotein-mediated extrusion from the brain. Unlike rimonabant, AM6545 does not affect behavioural responses mediated by CB1 receptors in the brain, including catalepsy, hypomotility and the centrally mediated component of cannabinoid-induced hypothermia. It is also devoid of the anxiogenic effect of rimonabant, as tested in the elevated plus maze, and causes only a minor and transient reduction in food intake. At the dose used (10 mg·kg−1 i.p.), AM6545 completely blocked the anandamide-induced inhibition of upper gastrointestinal motility mediated by CB1 on cholinergic terminals innervating the gut, indicating full occupancy of peripheral CB1 receptors.
In DIO mice, chronic treatment with 10 mg·kg−1·day−1 AM6545 for 28 days caused a significant, 12% weight reduction without affecting caloric intake, as compared with a 21% reduction achieved by the same dose of rimonabant, the difference likely due to centrally mediated reduction of food intake by rimonabant. However, AM6545 is equieffective or only slightly less efficacious than rimonabant in improving glucose tolerance and insulin sensitivity, reversing fatty liver and improving the plasma lipid profile of DIO mice. Similar metabolic effects were observed in genetically obese ob/ob mice in which AM6545 did not affect body weight, indicating that the metabolic effects are weight-independent. These effects are due to CB1 blockade in peripheral tissues, including the liver. The role of hepatic CB1 receptors in glycemic control is indicated by the finding that CB1−/− mice with transgenic re-expression of CB1 restricted to hepatocytes develop insulin resistance on a high-fat diet, which is reversed by AM6545 treatment.
The ability of AM6545 to reduce body weight in DIO, but not in leptin-deficient ob/ob mice suggests that this effect is due to the reversal of the peripheral-type leptin resistance that accompanies diet-induced obesity. Leptin is known to suppress lipogenic gene expression in adipose tissue independently of its anorectic effect. Therefore, the observation that AM6545 treatment suppressed lipogenic gene expression in visceral and subcutaneous fat of DIO, but not of ob/ob mice, is compatible with the role of endogenous leptin in these effects. Leptin was found to decrease endocannabinoid levels in adipose tissue (Matias et al., 2006; Buettner et al., 2008), which could be involved in its ability to reduce lipogenic gene expression in adipocytes.
More recently, we tested a highly potent CB1 inverse agonist with very low brain penetrance. Preliminary – as yet unpublished – findings in our laboratory indicate that, similar to the neutral antagonist AM6545, the CB1 inverse agonist is effective in reversing diet-induced hepatic steatosis, glucose intolerance and dyslipidemias in mice without causing behavioural effects that are normally seen following blockade of CB1 receptors in the CNS. Relative to AM6545, the inverse agonist is much more efficacious in reducing body weight and in reversing insulin resistance, suggesting the importance of inverse agonism in these latter effects.
Conclusions
There is growing evidence for an important role of peripherally located CB1 receptors in metabolic regulation, which has gained further support by the pharmacological profile of novel, peripherally restricted CB1 antagonists. Compounds with limited brain penetrance retain efficacy in improving the hormonal/metabolic complications of obesity, but are devoid of behavioural effects that result from blocking CB1 receptors in the brain. Among peripherally restricted compounds, CB1 inverse agonists may offer distinct advantages over neutral antagonists, particularly as far as weight reduction and insulin sensitization are concerned. The improved therapeutic profile of such compound, due to the greatly reduced risk of neuropsychiatric side effects, warrants their clinical testing for the treatment of obesity and its metabolic complications, including fatty liver disease, insulin resistance and dyslipidemias.
Acknowledgments
Work in the authors' laboratory was supported by the National Institutes of Health (intramural funds from National Institute on Alcohol Abuse and Alcoholism to G. Kunos).
Glossary
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AM6545
5-(4-[4-Cyanobut-1-ynyl]phenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(1,1-dioxo-thio-morpholino-)-1H-pyrazole-3-carboxamide
- ApoE
apolipoprotein E
- CAMkinase
Ca2+/calmodulin-dependent protein kinase
- CB1
receptor, cannabinoid-1 receptor
- TG
triglyceride
Conflict of interest
The authors declare that no conflict of interest exists.
Supporting Information
Teaching Materials; Fig 1 as PowerPoint slide.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafenetre P, Cannich A, Cota D, Puente N, Grandes P, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–155. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Drews E, Albayram O, Piyanova A, Gaffal E, Tueting T, et al. Early onset of aging-like changes is restricted to cognitive abilities and skin structure in Cnr1(−/−) mice. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.009. DOI: 10.1016/j.neurobiolaging.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordicchia M, Battistoni I, Mancinelli L, Giannini E, Refi G, Minardi D, et al. Cannabinoid CB1 receptor expression in relation to visceral adipose depots, endocannabinoid levels, microvascular damage, and the presence of the Cnr1 A3813G variant in humans. Metabolism. 2010;59:734–741. doi: 10.1016/j.metabol.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Borner C, Hollt V, Sebald W, Kraus J. Transcriptional regulation of the cannabinoid receptor type 1 gene in T cells by cannabinoids. J Leukoc Biol. 2007;81:336–343. doi: 10.1189/jlb.0306224. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Sim LJ, Childers SR. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- Buettner C, Patel R, Muse ED, Bhanot S, Monia BP, McKay R, et al. Severe impairment in liver insulin signaling fails to alter hepatic insulin action in conscious mice. J Clin Invest. 2005;115:1306–1313. doi: 10.1172/JCI23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM. Localization of CaMKIIalpha in rat primary sensory neurons: increase in inflammation. Brain Res. 2002;947:252–259. doi: 10.1016/s0006-8993(02)02932-3. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts AA, Pertwee RG. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br J Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gottardi A, Spahr L, Ravier-Dall'Antonia F, Hadengue A. Cannabinoid receptor 1 and 2 agonists increase lipid accumulation in hepatocytes. Liver Int. 2010;30:1482–1489. doi: 10.1111/j.1478-3231.2010.02298.x. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Despres JP, Ross R, Boka G, Almeras N, Lemieux I. Effect of rimonabant on the high-triglyceride/ low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arterioscler Thromb Vasc Biol. 2009;29:416–423. doi: 10.1161/ATVBAHA.108.176362. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Verrijken A, Hakkarainen A, Petrosino S, Mertens I, Lundbom N, et al. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur J Endocrinol. 2009;161:715–722. doi: 10.1530/EJE-09-0643. [DOI] [PubMed] [Google Scholar]
- Eckardt K, Sell H, Taube A, Koenen M, Platzbecker B, Cramer A, et al. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia. 2009;52:664–674. doi: 10.1007/s00125-008-1240-4. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Rajesh M, Mukhopadhyay P, Horvath B, Patel V, Al-Gayyar MMH, et al. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia. 2011;54:000–000. doi: 10.1007/s00125-011-2061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamment M, Gueguen N, Wetterwald C, Simard G, Malthiery Y, Ducluzeau PH. Effects of the cannabinoid CB1 antagonist, rimonabant, on hepatic mitochondrial function in rats fed a high fat diet. Am J Physiol Endocrinol Metab. 2009;297:E1162–E1170. doi: 10.1152/ajpendo.00169.2009. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav. 2000;67:265–270. doi: 10.1016/s0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Addictive potential of cannabinoids: the underlying neurobiology. Chem Phys Lipids. 2002;121:267–290. doi: 10.1016/s0009-3084(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84:378–386. doi: 10.1093/cvr/cvp240. [DOI] [PubMed] [Google Scholar]
- Herling AW, Kilp S, Elvert R, Haschke G, Kramer W. Increased energy expenditure contributes more to the body weight-reducing effect of rimonabant than reduced food intake in candy-fed wistar rats. Endocrinology. 2008;149:2557–2566. doi: 10.1210/en.2007-1515. [DOI] [PubMed] [Google Scholar]
- Hollander PA, Amod A, Litwak LE, Chaudhari U. Effect of rimonabant on glycemic control in insulin-treated type 2 diabetes: the ARPEGGIO trial. Diabetes Care. 2010;33:605–607. doi: 10.2337/dc09-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, et al. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322:1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- Jo YH, Chen YJ, Chua SC, Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov. 2008;7:961–962. doi: 10.1038/nrd2775. [DOI] [PubMed] [Google Scholar]
- Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59:926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy J, Sieracki K, Chorvat R. Non-brain-penetrant CB1 receptor antagonists as a novel treatment of obesity and related metabolic disorders. Obesity. 2008;16(Suppl 1):S47. [Google Scholar]
- Madsen AN, Jelsing J, van de Wall EH, Vrang N, Larsen PJ, Schwartz GJ. Rimonabant induced anorexia in rodents is not mediated by vagal or sympathetic gut afferents. Neurosci Lett. 2009;449:20–23. doi: 10.1016/j.neulet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Mancini G, Quarta C, Srivastava RK, Klaus S, Pagotto U, Lutz B. Adipocyte-specific CB1 conditional knock-out mice: new insights in the study of obesity and metabolic syndrome. 2010. 20th Annual Symposium on the Cannabinoids; Research Triangle Park, NC. International Cannabinoid Research Society. p 17.
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Migrenne S, Lacombe A, Lefevre AL, Pruniaux MP, Guillot E, Galzin AM, et al. Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R929–R935. doi: 10.1152/ajpregu.90824.2008. [DOI] [PubMed] [Google Scholar]
- Miles JM, Park YS, Walewicz D, Russell-Lopez C, Windsor S, Isley WL, et al. Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes. 2004;53:521–527. doi: 10.2337/diabetes.53.3.521. [DOI] [PubMed] [Google Scholar]
- Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav. 2004;80:611–616. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Miranville A, Herling AW, Biemer-Daub G, Voss MD. Reversal of Inflammation-Induced Impairment of Glucose Uptake in Adipocytes by Direct Effect of CB1 Antagonism on Adipose Tissue Macrophages. Obesity (Silver Spring) 2010;18:2247–2254. doi: 10.1038/oby.2010.81. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay B, Liu J, Osei-Hyiaman D, Godlewski G, Mukhopadhyay P, Wang L, et al. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-gamma. J Biol Chem. 2010;285:19002–19011. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AB, Davis RJ, Alexander-Chacko JT, Eastwood B, Chernet E, Phebus LA, et al. The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats. Psychopharmacology (Berl) 2006;184:26–35. doi: 10.1007/s00213-005-0234-x. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschop J, Caldwell C, et al. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare J, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes. 2010;60:000–000. doi: 10.2337/db10-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, et al. Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Perwitz N, Wenzel J, Wagner I, Buning J, Drenckhan M, Zarse K, et al. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes Metab. 2010;12:158–166. doi: 10.1111/j.1463-1326.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Price TJ, Jeske NA, Flores CM, Hargreaves KM. Pharmacological interactions between calcium/calmodulin-dependent kinase II alpha and TRPV1 receptors in rat trigeminal sensory neurons. Neurosci Lett. 2005;389:94–98. doi: 10.1016/j.neulet.2005.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, Braulke LJ, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11:273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Reaven GM. The individual components of the metabolic syndrome: is there a raison d'etre? J Am Coll Nutr. 2007;26:191–195. doi: 10.1080/07315724.2007.10719601. [DOI] [PubMed] [Google Scholar]
- Receveur JM, Murray A, Linget JM, Norregaard PK, Cooper M, Bjurling E, et al. Conversion of 4-cyanomethyl-pyrazole-3-carboxamides into CB1 antagonists with lowered propensity to pass the blood-brain-barrier. Bioorg Med Chem Lett. 2010;20:453–457. doi: 10.1016/j.bmcl.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Richey JM, Woolcott OO, Stefanovski D, Harrison LN, Zheng D, Lottati M, et al. Rimonabant prevents additional accumulation of visceral and subcutaneous fat during high-fat feeding in dogs. Am J Physiol Endocrinol Metab. 2009;296:E1311–E1318. doi: 10.1152/ajpendo.90972.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodondi N, Pletcher MJ, Liu K, Hulley SB, Sidney S. Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study) Am J Cardiol. 2006;98:478–484. doi: 10.1016/j.amjcard.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Hollander P, Chevalier S, Iranmanesh A. SERENADE: the Study Evaluating Rimonabant Efficacy in Drug-naive Diabetic Patients: effects of monotherapy with rimonabant, the first selective CB1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes. Diabetes Care. 2008;31:2169–2176. doi: 10.2337/dc08-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby MA, Nomura DK, Hudak CS, Mangravite LM, Chiu S, Casida JE, et al. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proc Natl Acad Sci USA. 2008;105:14561–14566. doi: 10.1073/pnas.0807232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrie P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Smit E, Crespo CJ. Dietary intake and nutritional status of US adult marijuana users: results from the Third National Health and Nutrition Examination Survey. Public Health Nutr. 2001;4:781–786. doi: 10.1079/phn2000114. [DOI] [PubMed] [Google Scholar]
- Son MH, Kim HD, Chae YN, Kim MK, Shin CY, Ahn GJ, et al. Peripherally acting CB1-receptor antagonist: the relative importance of central and peripheral CB1 receptors in adiposity control. Int J Obes (Lond) 2009;34:547–556. doi: 10.1038/ijo.2009.253. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008;33:54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Katayama M, Niimi K, Itakura C. Additive subthreshold dose effects of cannabinoid CB(1) receptor antagonist and selective serotonin reuptake inhibitor in antidepressant behavioral tests. Eur J Pharmacol. 2008;589:149–156. doi: 10.1016/j.ejphar.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco L, Valerio A, Cervino C, Cardile A, Pagano C, Vettor R, et al. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes. 2008;57:2028–2036. doi: 10.2337/db07-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco L, Valerio A, Dossena M, Cardile A, Ragni M, Pagano C, et al. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes. 2010;59:2826–2836. doi: 10.2337/db09-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology (Berl) 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, et al. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem. 2009;284:1803–1812. doi: 10.1074/jbc.M807120200. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


