A5224s compared fat changes with abacavir/lamivudine (ABC/3TC) or TenofovirDF/Emtricitabine (TDF/FTC) with efavirenz (EFV) or atazanavir/ritonavir (ATV/r). TDF/FTC- and ABC/3TC-regimens similarly increased limb and visceral fat. ATV/r led to greater gains in limb fat, and a trend towards greater gains in visceral fat than EFV.
Abstract
Background. We compare the effect of 4 different antiretroviral regimens on limb and visceral fat.
Methods. A5224s was a substudy of A5202, a trial of human immunodeficiency virus type 1 (HIV-1)–infected, treatment-naive subjects randomized to blinded abacavir-lamivudine (ABC-3TC) or tenofovir DF-emtricitabine (TDF-FTC) with open-label efavirenz (EFV) or atazanavir-ritonavir (ATV-r). The primary endpoint was the presence of lipoatrophy (≥10% loss of limb fat) at week 96 by intent-to-treat (ITT) analysis. Secondary endpoints included changes in limb and visceral fat. Statistical tests included linear regression, binomial, two-sample t test, and Fisher's exact test.
Results. A5224s enrolled 269 subjects; 85% were male, and 47% were white non-Hispanic. The subjects had a median baseline HIV-1 RNA level of 4.6 log10 copies/mL, a median age of 38 years, a median CD4+ cell count of 233 cells/μL, median limb fat of 7.4 kg, median visceral adipose tissue (VAT) of 84.1 cm2, and VAT: total adipose tissue (TAT) ratio of 0.31. At week 96, estimated prevalence of lipoatrophy (upper 95% confidence interval [CI]) was 18% (25%) for ABC-3TC and 15% (22%) for TDF-FTC (P = .70); this was not significantly less than the hypothesized 15% for both (P ≥ .55 for both). The secondary as-treated (AT) analysis showed similar results. At week 96, the estimated mean percentage change from baseline in VAT was higher for the ATV-r group than for the EFV group (26.6% vs 12.4%; P = .090 in ITT analysis and 30.0% vs 14.5%; P = .10 in AT analysis); however, the percentage change in VAT:TAT was similar by ITT and AT analysis (P ≥ .60 for both). Results were similar for absolute changes in VAT and VAT:TAT.
Conclusions. ABC-3TC– and TDF-FTC–based regimens increased limb and visceral fat at week 96, with a similar prevalence of lipoatrophy. Compared to the EFV group, subjects assigned to ATV-r had a trend towards higher mean percentage increase in VAT.
Clinical Trials Registration. NCT00118898.
Antiretroviral therapy (ART) may be associated with fat wasting (lipoatrophy) and fat accumulation (lipohypertrophy), which are distinct conditions but may coexist. Although lipoatrophy has been linked to nucleoside reverse-transcriptase inhibitor (NRTI)–induced mitochondrial toxicity [1–3], little is known about the cause of lipohypertrophy. Studies of lipohypertrophy have been limited, because few studies have used computed tomography (CT) or magnetic resonance imaging (MRI) scans to discern between subcutaneous and visceral fat. To date, one study compared fat changes with ritonavir-boosted atazanavir (ATV-r) versus efavirenz (EFV) with tenofovir DF-emtricitabine (TDF-FTC) in ART-naive subjects [4]. In addition, no study has compared body fat changes after initiation of abacavir-lamivudine (ABC-3TC) versus TDF-FTC with ATV-r, a protease inhibitor (PI) with minimal metabolic effects [5, 6], or the nonnucleoside reverse-transcriptase inhibitor (NNRTI) EFV.
METHODS
A5224s was a metabolic substudy of A5202, in which ART-naive subjects ≥16 years of age were randomized to blinded ABC-3TC or TDF-FTC with open-labeled ATV-r or EFV. An A5224s co-primary objective assessed prevalence of lipoatrophy (≥10% loss of limb fat from baseline) after ART-initiation with ABC-3TC or TDF-FTC. The second coprimary objective assessing changes in bone density will be reported elsewhere. Secondary objectives compared week 96 changes in limb and visceral fat between ABC-3TC versus TDF-FTC and ATV-r versus EFV. Post hoc endpoints included changes in trunk fat. A5224s exclusion criteria were untreated hypogonadism or thyroid disease, Cushing’s syndrome, diabetes mellitus, and the use of growth hormone, anabolic steroids, or glucocorticoids. Study duration was 96 weeks after the last subject was enrolled. Any subject entering A5202 at a site participating in A5224s and meeting substudy criteria was eligible to enroll. Subjects signed an institutional review board–approved written informed consent.
Substudy evaluations included whole-body dual-energy X-ray absorptiometry (DEXA) scans at baseline and weeks 24, 48, 96, 144, and 192 and a single-slice abdomen CT scan at the L4-L5 level at baseline and week 96. Fat distribution was measured by DEXA in anteroposterior view (with use of Hologic or Lunar scanners). Technicians were instructed to use the same machine on the same subject throughout the study. CT was used to quantify visceral adipose tissue (VAT) and total adipose tissue (TAT). DEXAs and CT scans were standardized and centrally read (at Tufts University) by personnel blinded to subjects characteristics.
On 25 February 2008 [7], the Data Safety and Monitoring Board (DSMB) recommended unblinding the study for subjects with screening human immunodeficiency virus type 1 (HIV-1) RNA levels ≥100,000 copies/mL because of excess virological failures associated with ABC-3TC regimens; subjects receiving ABC-3TC were permitted to modify their NRTI regimen [7].
Statistical Analysis
The primary objective was to assess the prevalence of protocol-defined lipoatrophy at week 96 in those assigned to ABC-3TC or TDF-FTC in addition to ATV-r or EFV. A5224s was powered using a factorial design with 125 subjects per NRTI (combined across the ATV-r and EFV arms), 80% power to conclude that the prevalence of lipoatrophy at week 96 was less than the prespecified 15%, assuming true prevalence was ≤6.5%, 20% loss to follow-up, and 1-sided test with a .05 significance level.
Primary analyses used intent-to-treat (ITT) principles based on randomized treatment assignment in which all available data were used and modifications to randomized treatment and missing values were ignored. Secondary as-treated (AT) analyses excluded values after a change in randomized NRTI component (when comparing NRTI components) and PI-NNRTI component (when comparing NNRTI-PI components). Supplemental analyses included assessing changes by ITT at week 48. Comparisons were performed using a factorial analysis approach in which, after assessing for treatment effect modification by the other factor, the NRTI effect was assessed by combining the EFV and ATV-r arms and vice versa. No significant treatment effect modification was observed in any ITT analyses (P ≥ .48). Missing values were ignored. P values <.05 were interpreted as statistically significant. Analyses were performed with SAS, version 9.1.3 (SAS Institute).
Within each component, tests included 1-sample t or binomial tests. Comparisons between components used 2-sample t, Fisher’s exact, or log-rank tests (with Kaplan-Meier estimation of survival probabilities), as appropriate. Analyses adjusting for baseline factors and exploring associations with baseline factors used linear regression.
RESULTS
Baseline Characteristics
A5224s enrolled 271 subjects from 37 AIDS Clinical Trials Group (ACTG) sites in the United States and Puerto Rico, with 2 subjects excluded from analysis due to eligibility violation. Enrollment spanned from 5 October 2005 to 7 November 2007; 65 subjects were randomized to ATV-r + ABC-3TC, 65 were randomized to ATV-r + TDF-FTC, 70 were randomized to EFV + ABC-3TC, and 69 were randomized to EFV + TDF-FTC. Baseline characteristics are summarized in Table 1; 85% of the subjects were male, and 47% were white non-Hispanics. The median age of the subjects was 38 years, the median body mass index (BMI) was 24.9 kg/m2, median limb fat was 7.4 kg, median trunk fat was 9.4 kg, median VAT was 84.1 cm2, median CD4+ cell count was 233 cells/μL, and median HIV-1 RNA level was 4.6 log10 copies/mL. Baseline characteristics were balanced across arms. A5224s participants and A5202 subjects who did not participate in the substudy were not significantly different at baseline, except that Hispanics were less likely than other subjects to participate in A5224s (16% vs 24%; P = .005).
Table 1.
Baseline Characteristics of Study Subjects by Randomized Arms
| Variable | EFV + TDF-FTC (n = 69) | EFV + ABC-3TC (n = 70) | ATV-rtv + TDF-FTC (n = 65) | ATV-rtv + ABC-3TC (n = 65) | Total (n = 269) |
| Age, y | 40 (33–44) | 39 (31–46) | 38 (30–44) | 37 (29–43) | 38 (31–44) |
| Sex, no. (%) of subjects | |||||
| Male | 58 (84) | 56 (80) | 56 (86) | 59 (91) | 229 (85) |
| Female | 11 (16) | 14 (20) | 9 (14) | 6 (9) | 40 (15) |
| Race/ethnicity, no. (%) of subjects | |||||
| White non-Hispanic | 37 (54) | 34 (49) | 26 (40) | 29 (45) | 126 (47) |
| Black non-Hispanic | 22 (32) | 20 (29) | 21 (32) | 27 (42) | 90 (33) |
| Hispanic (regardless of race) | 8 (12) | 14 (20) | 14 (22) | 8 (12) | 44 (16) |
| Other | 2 (3) | 2 (3) | 4 (6) | 1 (2) | 9 (3) |
| BMI | 24.9 (21.6–27.1) | 24.7 (22.6–28.3) | 24.9 (21.8–28.8) | 25.3 (21.8–28.9) | 24.9 (21.8–28.2) |
| CD4+ cell count, cells/μL | 250 (132–334) | 213 (106–350) | 247 (114–319) | 222 (75–332) | 233 (106–334) |
| HIV-1 RNA (log10 copies/mL) | 4.7 (4.2–4.9) | 4.7 (4.2–4.9) | 4.5 (4.2–4.9) | 4.6 (4.3–5.1) | 4.6 (4.2–4.9) |
| HIV-1 RNA level, no. (%) of subjects | |||||
| < 100,000 copies/mL | 56 (81) | 59 (84) | 52 (80) | 48 (74) | 215 (80) |
| ≥ 100,000 copies/mL | 13 (19) | 11 (16) | 13 (20) | 17 (26) | 54 (20) |
| Limb fat, kg | 7.3 (4.7–9.4) | 7.8 (4.9–10.5) | 7.4 (5.0–11.6) | 6.8 (4.3–10.5) | 7.4 (4.7–10.1) |
| Trunk fat, kg | 8.5 (5.4–12.6) | 10.0 (5.5–13.6) | 9.6 (5.5–14.5) | 9.9 (4.9–12.9) | 9.4 (5.3–13.0) |
| VAT, cm2 | 84.2 (52.0–110.3) | 82.6 (62.8–111.6) | 86.7 (60.2–121.9) | 82.7 (55.2–116.1) | 84.1 (57.2–115.9) |
| VAT:TAT | 0.34 (0.23–0.40) | 0.31 (0.22–0.40) | 0.30 (0.25–0.38) | 0.31 (0.24–0.38) | 0.31 (0.23–0.39) |
NOTE. Data are median value (interquartile range), unless otherwise indicated. ABC, abacavir; ATV-r, atazanavir-ritonavir; BMI, body mass index calculated as the weight in kilograms divided by the square of height in meters; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; TAT, total adipose tissue; TDF, tenofovir DF; VAT, visceral adipose tissue; 3TC, lamivudine.
Subject Disposition
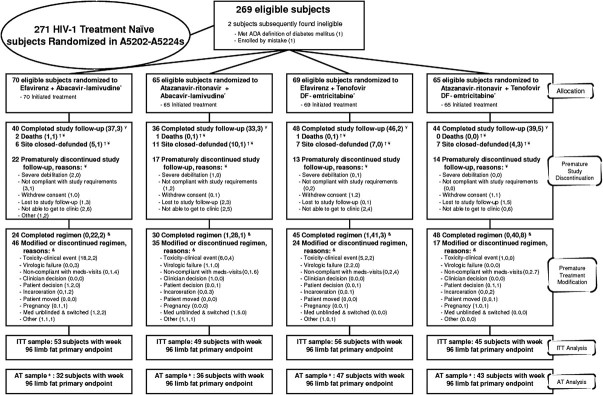
Overall, 66 (25%) of the A5224s subjects prematurely discontinued the substudy, and an additional 4 (1%) died (Figure 1). Reasons for discontinuation were as follows: 27 (10%) were unable to get to the clinic, 16 (6%) were lost to follow-up, 9 (3%) were noncompliant with the study protocol, 7 (3%) withdrew consent, 4 (1%) experienced severe debilitation, and 3 (1%) discontinued the study for other reasons. Seventeen percent of the subjects prematurely discontinued the substudy by week 96. Additionally, 31 (12%) discontinued the study because their ACTG site was defunded; 26 of these 31 subjects had week 96 primary endpoint data available. By study arm, 55% of the ATV-r + ABC-3TC group, 68% of the ATV-r + TDF-FTC group, 57% of the EFV + ABC-3TC group, and 70% of the EFV + TDF-FTC group completed the A5224s protocol. There was no significant difference in time to premature study discontinuation between the NRTI (P = .13) or PI-NNRTI components (P = .86). The median time from randomization to last visit was 165 weeks. Overall, 147 (55%) of the subjects had no modification to their randomized treatment (30 [46%] in the ATV-r + ABC-3TC group, 48 [74%] in the ATV-r + TDF-FTC group, 24 [34%] in the EFV + ABC-3TC group, and 45 [65%] in the EFV + TDF-FTC group). By Kaplan-Meier estimates, 66% of subjects had no modification by week 96. Overall, 23% of subjects (31 in the EFV group and 31 in the ATV-r group) modified the NRTI and PI-NNRTI components within 7 days of each other, and 10% modified only the NRTI component (22% in the ATV-r + ABC-3TC group, 0% in ATV-r + TDF-FTC group, 16% in the EFV + ABC-3TC group, and 1% in the EFV + TDF-FTC group). Of the 102 subjects with NRTI modifications, 26 had modifications that occurred at the time of or after DSMB recommendation.
Figure 1.
Details of disposition and outcome of study subjects. Subjects were to remain in follow-up regardless of having modified antiretroviral therapy. Nucleoside reverse-transcriptase inhibitors (NRTIs) were double-blinded through 25 February 2008 for those with screening human immunodeficiency virus (HIV) RNA levels of ≥100,000 copies/mL and until final visits starting 1 July 2009 for those with screening HIV RNA levels <100,000 copies/mL. Reasons for study discontinuation are split into no. of subjects with and no. of subjects without week 96 limb fat primary endpoint. Site closure was censored for premature study and treatment discontinuation. Death was censored for premature study discontinuation and counted as the reason for treatment discontinuation if there was no prior modification. Reasons for the first treatment modification are split into no. of subjects before, no. of subjects after, and no. of subjects without week 96 limb fat primary endpoint. Subjects in the as-treated sample continued to receive randomized treatment through the week 96 whole-body dual-energy X-ray absorptiometry.
Prevalence of Lipoatrophy at Week 96—Primary Endpoint
Table 2 shows the prevalence of protocol-defined lipoatrophy at week 96 by arm; the overall estimated prevalence was 16.3% (range, 14.3%–18.9%). In factorial analyses combining the ATV-r and EFV groups, within the ABC-3TC arms, prevalence (upper bound of 1-sided 95% confidence interval [CI]) of lipoatrophy was 17.6% (25.0%), which was not significantly less than 15% (P = .81). Within the TDF-FTC arms, the prevalence of lipoatrophy was 14.9% (21.5%; P = .55). There was no significant difference in the week 96 prevalence of lipoatrophy between NRTI components (P = .70) or the PI and NNRTI components (P = 1.0). Similar results were seen on sensitivity analyses (with the last observation carried forward and missing data regarded as treatment failure). In post hoc analysis, the week 96 overall prevalence of subjects who lost ≥20% of limb fat from baseline was 4.9% (95% CI, 2.4%–8.9%), with a range of 0%–8.9% across arms (Table 2) and with no significant differences between NRTI (P = 1.0) or PI-NNRTI components (P = .35). In post hoc analysis, change in limb fat from baseline to week 24 was an independent predictor of protocol-defined lipoatrophy at week 96 (odds ratio, 0.93; 95% CI, 0.90–0.96; P < .001). The odds of week 96 lipoatrophy decrease by 7% for every 1% gain in limb fat at week 24.
Table 2.
Estimated Prevalence of Lipoatrophy by Intent-to-Treat Analysis
| Variable | EFV + TDF-FTC (n = 56) | EFV + ABC-3TC (n = 53) | ATV-r + TDF-FTC (n = 45) | ATV-r + ABC-3TC (n = 49) | Total (n = 203) |
| No. of subjects with ≥ 10% limb fat loss | 8 | 10 | 7 | 8 | 33 |
| Prevalence of ≥ 10% limb fat loss (primary analysis), % (95% CI) | 14.3 (6.4–26.2) | 18.9 (9.4–32.0) | 15.6 (6.5–29.5) | 16.3 (7.3–29.7) | 16.3 (11.5–22.1) |
| No. of subjects with ≥ 20% limb fat loss | 5 | 2 | 0 | 3 | 10 |
| Prevalence of ≥ 20% limb fat loss (post hoc analysis), % (95% CI) | 8.9 (3.0–19.6) | 3.8 (0.5–13.0) | 0.0 (0.0–7.9) | 6.1 (1.3–16.9) | 4.9 (2.4–8.9) |
NOTE. Loss of lipoatrophy defined as ≥ 10% limb fat and ≥ 20% limb fat from baseline to week 96. ABC, abacavir; ATV/r, atazanavir-ritonavir; CI, confidence interval; EFV, efavirenz; FTC, emtricitabine; TDF, tenofovir DF; 3TC, lamivudine.
Changes in Limb Fat (DEXA)—Secondary Endpoint
Table 3 summarizes the estimated changes in limb fat by regimen. Figure 2 plots the changes in limb fat by NRTI and PI-NNRTI components. The estimated mean absolute and percentage limb fat change for all subjects were 1.22 kg and 21%, respectively, at week 48 and were 1.38 kg and 23% at week 96.
Table 3.
Estimated Percentage Changes in Limb Fat, Trunk Fat, VAT, and VAT:TAT Ratio for All 4 Treatment Arms (Intent-to-Treat Analysis)
| Variable | EFV + TDF-FTC(n = 69) | EFV + ABC-3TC(n = 70) | ATV-rtv + TDF-FTC(n = 65) | ATV-rtv + ABC-3TC(n = 65) | Total(n = 269) | |
| Change in limb fat (%) | ||||||
| Wk 0–24 | N | 59 | 62 | 56 | 62 | 239 |
| Mean value (SD) | 10.1 (23.9) | 16.8 (49.5) | 19.7 (29.3) | 19.5 (33.1) | 16.5 (35.5) | |
| Wk 0–48 | N | 58 | 54 | 52 | 53 | 217 |
| Mean value (SD) | 15.6 (29.6) | 19.0 (32.0) | 23.7 (36.7) | 26.6 (37.3) | 21.1 (33.9) | |
| P | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Wk 0–96 | N | 56 | 53 | 45 | 49 | 203 |
| Mean value (SD) | 15.3 (36.7) | 17.7 (30.7) | 27.8 (36.4) | 32.7 (48.0) | 22.9 (38.7) | |
| P | .003 | <.001 | <.001 | <.001 | <.001 | |
| Wk 0–144 | N | 48 | 41 | 42 | 40 | 171 |
| Mean value (SD) | 22.6 (45.8) | 27.5 (39.5) | 23.8 (40.5) | 33.6 (49.7) | 26.7 (43.9) | |
| Wk 0–192 | N | 32 | 30 | 23 | 25 | 110 |
| Mean value (SD) | 28.0 (46.2) | 37.1 (51.9) | 25.0 (46.2) | 39.5 (47.0) | 32.5 (47.7) | |
| Change in trunk fat (%) | ||||||
| Wk 0–24 | N | 59 | 62 | 56 | 62 | 239 |
| Mean value (SD) | 12.1 (28.2) | 19.1 (46.2) | 20.7 (33.0) | 19.9 (36.0) | 18.0 (36.6) | |
| Wk 0–48 | N | 58 | 54 | 52 | 53 | 217 |
| Mean value (SD) | 18.7 (33.2) | 22.0 (38.2) | 24.3 (44.2) | 28.1 (39.9) | 23.2 (38.8) | |
| P | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Wk 0–96 | N | 56 | 53 | 45 | 49 | 203 |
| Mean value (SD) | 20.1 (44.1) | 22.2 (44.6) | 35.9 (50.7) | 37.0 (58.3) | 28.2 (49.7) | |
| P | .001 | .001 | <.001 | <.001 | <.001 | |
| Wk 0–144 | N | 48 | 41 | 42 | 40 | 171 |
| Mean value (SD) | 29.4 (51.6) | 37.6 (61.3) | 32.0 (56.6) | 40.4 (52.9) | 34.6 (55.3) | |
| Wk 0–192 | N | 32 | 30 | 23 | 25 | 110 |
| Mean value (SD) | 32.0 (50.2) | 49.4 (85.0) | 36.0 (68.4) | 46.4 (64.7) | 40.8 (67.5) | |
| Change in VAT (%) | ||||||
| Wk 0–96 | N | 54 | 51 | 45 | 45 | 195 |
| Mean value (SD) | 14.8 (48.7) | 9.9 (45.1) | 29.5 (88.4) | 23.7 (41.4) | 19.0 (58.2) | |
| P | .030 | 0.12 | .031 | <.001 | <.001 | |
| Change in VAT:TAT (%) | ||||||
| Wk 0–96 | N | 54 | 51 | 45 | 45 | 195 |
| Mean value (SD) | −0.2 (19.7) | −1.9 (20.9) | −2.2 (19.1) | −2.3 (21.4) | −1.6 (20.1) | |
| P | .95 | .52 | .44 | .48 | .28 |
NOTE. The duration of the study was 96 weeks since the last subject enrolled in A5202; thus, there was a smaller sample size (N) at later time points. ABC, abacavir; ATV-r, atazanavir-ritonavir; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; SD, standard deviation; TAT, total adipose tissue; TDF, tenofovir DF; VAT, visceral adipose tissue; 3TC, lamivudine.
Figure 2.
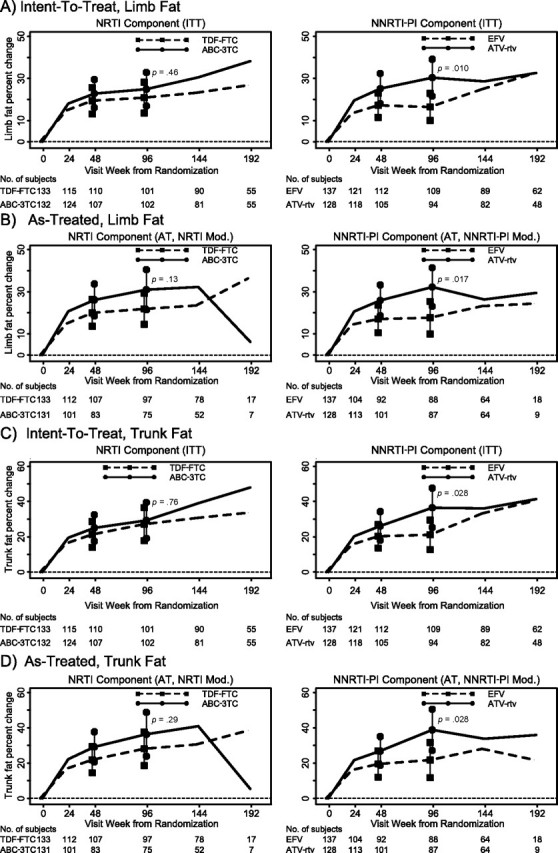
Estimated mean percent changes in limb fat and trunk fat by study week by NRTI components (combining third drugs) and NNRTI/PI components (combining NRTIs) by ITT and AT analyses. Vertical bars are 95% confidence intervals on the mean change.
Changes by NRTI Components
Limb fat significantly increased in all study arms (Table 3). When combining ATV-r and EFV data, the week 96 analysis (ITT) showed mean absolute and percentage changes in limb fat that were not significantly different between the ABC-3TC (1.66 kg and 24.9%) and TDF-FTC (1.11 kg and 20.9%) groups, a difference (Δ) of 0.55 kg (95% CI, −0.14 to 1.24; P = .12) and 4% (95% CI, −6.7% to 14.7%; P = .46). At week 96, AT analysis showed that the ABC-3TC arms had an estimated mean absolute and percentage change in limb fat that was 0.88 kg (95% CI, 0.14–1.61; P = .019) and 9.1% (95% CI, −2.6% to 20.9%; P = .13) larger than the changes in the TDF-FTC arms. At week 48, estimated mean absolute and percentage changes in ABC-3TC arms were not different than those in TDF-FTC arms (P ≥ .30 for both).
Changes by NNRTI-PI Component
When combining ABC-3TC and TDF-FTC, the week 96 ITT analyses showed estimated mean absolute and percentage change in limb fat that was significantly greater in those assigned to ATV-r (1.88 kg and 30.4%) than in those assigned to EFV (0.96 kg and 16.5%); Δ = 0.93 kg (95% CI, 0.24–1.61; P = .008) and 13.9% (95% CI, 3.3%–24.5%; P = .010), respectively. AT analyses showed similar results. At 48 weeks, estimated mean absolute and percentage changes in ATV-r arms (1.46 kg and 25.2%) tended to be larger than those in EFV arms (1.00 kg and 17.3%); Δ = 0.46 kg (95% CI, −0.15 to 1.07; P = .14) and 7.9% (95% CI, −1.1% to 17.0%; P = .086), respectively.
Changes in Trunk Fat (DEXA)—Post Hoc Endpoint
Table 3 summarizes estimated mean percentage change in trunk fat by study arm. Figure 2 plots estimated changes in trunk fat by study week. The overall estimated mean absolute and percentage change in trunk fat was 1.43 kg and 23.1% at week 48 and 1.83 kg and 28.2% at week 96.
Changes by NRTI Components
Trunk fat increased significantly in all arms. At week 96, by ITT, the mean absolute and percentage changes in trunk fat were not significantly different between the ABC-3TC and TDF-FTC groups; Δ = 0.37 kg (95% CI, −0.58 to 1.32; P = .45) and 2.2% (95% CI, −11.6% to 15.9%; P = .76), respectively. At week 96, AT analysis showed mean absolute and percentage changes in trunk fat were not significantly different between the ABC-3TC and TDF-FTC groups; Δ = 0.96 kg (95% CI, −0.07 to 1.99; P = .066) and 8.2% (95% CI, −7.0% to 23.5%; P = .29), respectively. At week 48, estimated mean absolute and percentage changes in the ABC-3TC arms were also not significantly different than in the TDF-FTC arms (P ≥ .34, by ITT).
Changes by PI-NNRTI Component
At week 96, by ITT analysis, the estimated mean absolute and percentage changes in trunk fat were larger in the ATV-r (2.42 kg; 36.5%) than in the EFV (1.33 kg; 21.1 %) study arms; Δ = 1.09 kg (95% CI, 0.15–2.03; P = .023) and 15.4% (95% CI, 1.7%–29.0%; P = .028). Similar results were seen with AT analyses. However, at week 48, mean absolute and percentage changes in trunk fat in the ATV-r arms were not significantly different than in the EFV arms (P ≥.27 for both).
Changes in Visceral Fat by CT Scan—Secondary Endpoint
Table 3 summarizes estimated mean changes in VAT and VAT:TAT ratio by study arm. The overall estimated mean absolute VAT, percentage VAT, and absolute VAT:TAT ratio changes were 10.4 cm2, 19.0%, and −0.01, respectively, at week 96.
Changes by NRTI Components
At week 96, by ITT, estimated absolute and percentage changes in VAT and VAT:TAT ratio were not significantly different between the ABC-3TC and TDF-FTC groups. The ABC-3TC–assigned subjects had an estimated mean difference in VAT absolute change, VAT percentage change, and VAT:TAT ratio change of −2.8 cm2 (95% CI, −12.9 to 7.3; P = .58), −5.1% (95% CI, −21.5% to 11.4%; P = .55), and 0.00 (95% CI, −0.02 to 0.02; P = .94), compared with the TDF-FTC arms.
Changes by PI-NNRTI Component
At week 96, by ITT, the estimated absolute and percentage changes from baseline in VAT tended to be higher in the ATV-r than in the EFV arms; Δ = 7.6 cm2 (95% CI, −2.4 to 17.7; P = .14) and 14.2% (95% CI, −2.2% to 30.6%; P = .090), respectively. The estimated mean change in VAT:TAT was not significantly different between the ATV-r and EFV arms; Δ = 0.00 (95% CI, −0.02 to 0.02; P = .92). Similar results were seen with AT analyses. Interestingly, changes in VAT correlated with changes in limb fat (r = 0.48; P < .001)
Changes in BMI—Post Hoc Endpoint
At week 96, by ITT, there was a trend towards larger gains in mean BMI associated with receipt of ABC-3TC than with receipt of TDF-FTC (Δ = 0.63 kg/m2; 95% CI, −0.12 to 1.38; P = .099) and statistically significant larger gains in mean BMI associated with receipt of ATV-r than with receipt of EFV (Δ =0.88 kg/m2; 95% CI, 0.13–1.62; P = .022).
Changes in Limb and Visceral Fat Adjusted for Baseline Covariates
The ITT analyses of prevalence of lipoatrophy and absolute and percentage changes for limb fat, VAT, and VAT:TAT were adjusted for the following baseline covariates that have been associated with body fat changes, first individually, then jointly using linear regression: NNRTI-PI (NRTI for the NNRTI-PI analyses), limb fat (VAT, VAT:TAT ratio for corresponding analysis), sex, age, race/ethnicity, log10 HIV-1 RNA level, CD4+ cell count, and BMI. All adjusted models led to results similar to those obtained with unadjusted analyses.
Association Between Baseline Factors and Changes in Limb Fat, VAT, and VAT:TAT Ratio at 96 Weeks
Linear regression analyses by ITT were performed to assess baseline factors associated with the changes in limb fat, VAT, and VAT:TAT ratio at week 96 (Table 4). In the multivariable analysis, higher baseline log10 HIV-1 RNA level was associated with significant increases in absolute and percentage limb fat change, and older age was associated with significant decreases. Only higher baseline BMI was associated with significant increases in week 96 percentage VAT change, absolute VAT:TAT ratio change, and a trend towards an increase in week 96 percentage VAT:TAT ratio change.
Table 4.
Univariate and Multivariate Linear Regression Analyses to Assess the Association Between Baseline Factors and 96-Week Absolute and Percent Absolute and Percentage Changes in Limb Fat, VAT, and VAT:TAT Ratio
| 96-Week absolute change |
96-Week percentage change |
||||||||
| Univariate |
Multivariable |
Univariate |
Multivariable |
||||||
| Endpoint | Baseline covariate | Param. est. (95% CI) | P | Param. est. (95% CI) | P | Param. est. (95% CI) | P | Param. est. (95% CI) | P |
| Limb fat (kg) | ABC-3TC (vs TDF-FTC) | 0.55 (−0.14, 1.24) | .012 | 0.51 (−0.15, 1.16) | .013 | 4.0 (−6.7, 14,7) | .046 | 4.7 (−4.6, 14.1) | .032 |
| ATV-r (vs EFV) | 0.93 (0.24, 1.61) | .008 | 0.74 (0.07, 1.42) | .032 | 13.9 (3.3, 24.5) | .010 | 11.1 (1.4, 20.9) | .025 | |
| Male (vs female) | −0.36 (−1.33, 0.60) | .046 | −0.85 (−1.80, 0.10) | .079 | 7.8 (−7.1, 22.7) | .030 | −5.8 (−19.5, 8.0) | .041 | |
| Age, y | −0.04 (−0.08, −0.01) | .003 | −0.04 (−0.07, 0.00) | .003 | −0.7 (−1.2, −0.1) | .002 | −0.59 (−1.1, −0.1) | .002 | |
| Race/ethnicity | N/A | .078a | N/A | .037a | N/A | .024a | N/A | .006a | |
| Black non-Hispanic (vs White non-Hispanic) | 0.00 (−0.81, 0.80) | .098 | −0.21 (−0.99, 0.58) | .060 | −4.4 (−16.7, 7.8) | .048 | −6.4 (−17.7, 4.8) | .026 | |
| Hispanic (White non-Hispanic reference) | −0.07 (−1.09, 0.94) | .089 | −0.69 (−1.66, 0.28) | .016 | −4.1 (−19.6, 11.4) | .060 | −14.5 (−28.5, −0.5) | .004 | |
| Other (White non-Hispanic reference) | 1.18 (−1.10, 3.46) | .031 | 0.99 (−1.15, 3.14) | .036 | 31.3 (−3.5, 66.1) | .008 | 22.2 (−8.6, 53.0) | .016 | |
| HIV-1 RNA, log10 copies/mL | 1.13 (0.61, 1.65) | <.001 | 1.09 (0.50, 1.68) | <.001 | 18.5 (10.6, 26.4) | <.001 | 14.7 (6.2, 23.2) | .001 | |
| CD4+ cell count (50 cells/uL) | −0.16 (−0.27, −0.06) | .003 | −0.06 (−0.18, 0.05) | .28 | −3.2 (−4.8, −1.7) | <.001 | −1.4 (−3.0, 0.2) | .096 | |
| BMI | −0.04 (−0.12, 0.04) | .31 | −0.03 (−0.10, 0.05) | .52 | −2.9 (−4.0, −1.8) | <.001 | −2.5 (−3.6, −1.4) | <.001 | |
| VAT (cm2) | ABC-3TC (vs TDF-FTC) | −2.8 (−12.9, 7.3) | .58 | −2.7 (−12.8, 7.4) | .60 | −5.0 (−21.5, 11.4) | .55 | −5.3 (−21.5, 11.0) | .52 |
| ATV-r (vs EFV) | 7.6 (−2.4, 17.7) | .14 | 5.5 (−5.0, 16.0) | .30 | 14.2 (−2.2, 30.6) | .090 | 11.0 (−5.9, 27.9) | .20 | |
| Male (vs female) | 12.9 (−1.0, 26.7) | .069 | 10.1 (−4.7, 24.9) | .18 | 11.3 (−11.5, 34.1) | .33 | 6.5 (−17.3, 30.4) | .59 | |
| Age, y | −0.3 (−0.8, 0.2) | .23 | −0.3 (−0.8, 0.2) | .28 | −0.8 (−1.7, 0.0) | .055 | −0.7 (−1.6, 0.1) | .096 | |
| Race/Ethnicity | N/A | .78a | N/A | .67a | N/A | .80a | N/A | .77a | |
| Black non-Hispanic (vs White non-Hispanic) | 2.7 (−8.9, 14.3) | .65 | 4.8 (−7.3, 16.9) | .43 | 8.8 (−10.1, 27.7) | .36 | 8.5 (−11.0, 28.0) | .39 | |
| Hispanic (White non-Hispanic reference) | 5.9 (−9.1, 21.0) | .44 | 2.1 (−13.3, 17.4) | .79 | 7.5 (−17.1, 32.1) | .55 | 0.6 (−24.1, 25.3) | .96 | |
| Other (White non-Hispanic reference) | −8.7 (−41.1, 23.7) | .60 | −13.6 (−3.6, 14.9) | .41 | −1.9 (−54.8, 50.9) | .94 | −12.9 (−65.2, 39.4) | .63 | |
| HIV-1 RNA, log10 copies/mL | 7.7 (−0.1, 15.5) | .054 | 5.6 (−3.6, 14.9) | .23 | 9.5 (−3.3, 22.3) | .14 | 4.1 (−10.8, 19.0) | .59 | |
| CD4+ cell count (50 cells/uL) | −1.1 (−2.6, 0.5) | .18 | −0.3 (−2.1, 1.5) | .72 | −2.9 (−5.5, −0.4) | .023 | −2.0c(−4.8, 0.9) | .18 | |
| BMI | −1.1 (−2.2, 0.1) | .066 | −0.8 (−2.0, 0.4) | .20 | −2.5 (−4.3, −0.6) | .009 | −2.0 (−3.9, −0.1) | .044 | |
| VAT:TAT ratio | ABC-3TC (vs TDF-FTC) | 0.00 (−0.02, 0.02) | 0.94 | 0.00 (−0.02, 0.02) | .85 | −1.0 (−6.7, 4.7) | .74 | −1.2 (−6.9, 4.5) | .68 |
| ATV-r (vs EFV) | 0.00 (−0.02, 0.02) | .92 | 0.00 (−0.02, 0.02) | .82 | −1.2 (−6.9, 4.5) | .67 | −0.3 (−6.3, 5.6) | .91 | |
| Male (vs female) | 0.00 (−0.03, 0.03) | >.99 | 0.01 (−0.02, 0.03) | .54 | −0.9 (−8.8, 7.0) | .82 | 1.0 (−7.4, 9.4) | .81 | |
| Age, y | 0.00 (0.00, 0.00) | .25 | 0.00 (0.00, 0.00) | .23 | 0.2 (−0.1, 0.5) | .26 | 0.2 (−0.1, 0.5) | .30 | |
| Race/ethnicity | N/A | .25a | N/A | .19a | N/A | .57a | N/A | .58a | |
| Black non-Hispanic (vs White non-Hispanic) | 0.00 (−0.02, 0.03) | .67a | 0.00 (−0.02, 0.03) | .68 | 0.1 (−6.4, 6.7) | .96 | 0.3 (−6.6, 7.2) | .93 | |
| Hispanic (White non-Hispanic reference) | 0.01 (−0.02, 0.04) | .54 | 0.02 (−0.01, 0.04) | .23 | 0.4 (−8.1, 8.9) | .92 | 2.5 (−6.2, 11.2) | .57 | |
| Other (White non-Hispanic reference) | −0.05 (−0.11, 0.00) | .070 | −0.05 (−0.11, 0.01) | .092 | −12.9 (−31.2, 5.3) | .16 | −11.3 (−29.7, 7.1) | .23 | |
| HIV-1 RNA, log10 copies/mL | −0.01 (−0.03, 0.00) | .12 | −0.01 (−0.03, 0.01) | .31 | −2.7 (−7.2, 1.7) | .22 | −1.6 (−6.8, 3.7) | .55 | |
| CD4+ count (100 cells/uL) | 0.01 (0.00, 0.01) | .031 | 0.00 (0.00, 0.01) | .26 | 1.7 (0.0, 3.5) | .051 | 1.2 (−0.9, 3.2) | .26 | |
| BMI (5) | 0.01 (0.00, 0.02) | .012 | 0.01 (0.00, 0.02) | .046 | 3.7 (0.5, 6.9) | .022 | 3.1 (−0.3, 6.5) | .077 | |
NOTE. Boldface type indicates statistical significance. ABC, abacavir; ATV/r, atazanavir-ritonavir; BMI, body mass index calculated as the weight in kilograms divided by the square of height in meters; CI, confidence interval; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; Param. est., parameter estimate; TAT, total adipose tissue; TDF, tenofovir DF; VAT, visceral adipose tissue; 3TC, lamivudine.
Global test, 3 df.
DISCUSSION
This report details the results of a randomized, controlled substudy comparing the effect that initiation of ABC-3TC or TDF-FTC regimens with either ATV-r or EFV in HIV-infected, ART-naive subjects has on body fat. Our data are consistent with the results of Altair [4] and extend the observation of that 48-week study to 96 weeks and extend the comparison of both TDF-FTC and ABC-3TC as initial combinations.
For our study, we deliberately selected a strict cut-off point to define lipoatrophy, a 10% limb fat loss that is likely to be subclinical [8], because prior studies of TDF- and ABC-containing regimens did not demonstrate the deleterious lipoatrophic effects of thymidine NRTIs (tNRTIs) [9, 10]. With this criteria, lipoatrophy occurred in 16% (95% CI, 12%–22 %) of subjects and was not significantly different between the ABC-3TC– and TDF-FTC–containing regimens, although we could not conclude that lipoatrophy occurred less than the hypothesized level of 15%. In a post hoc analysis, we determined the prevalence of ≥20% loss of limb fat from baseline at 96 weeks, mirroring endpoints of prior studies [4, 11, 12]. Very few subjects in any study arm met this criterion for lipoatrophy, which was a result that was substantially different from the 50%–70% seen in tNRTI-treated subjects [11, 12].
The initial changes (Table 3, Figure 2) in limb fat are consistent with earlier observations of an increase in limb fat after ART initiation, regardless of the type of therapy [11, 13, 14]. However, unlike with tNRTIs [14, 15], no decrease in limb fat was seen after 96 weeks.
In our study, the rate of lipoatrophy was similar in the ATV-r and EFV arms. This is in contrast with A5142, which was a randomized study in which EFV was associated with higher rates of lipoatrophy, compared with lopinavir-ritonavir [11]. However, in our study, limb fat did increase significantly more in the ATV-r than in the EFV arms. Thus, although the rate of lipoatrophy appears to be different than what was seen in A5142, the higher gains in limb fat with ritonavir-boosted PIs may have counteracted the effect that tNRTIs had on lipoatrophy in that study. Also, within the TDF-FTC arms (but not within the ABC-3TC arms), we found a trend towards higher prevalence of ≥20% loss of limb fat in the EFV arms, compared with the ATV-r arms. Additionally, in our study, limb and trunk fat overall increased more in the ATV-r– than EFV-treated subjects. This effect of ritonavir-boosted PIs may explain why, in another study, lipoatrophy was more prevalent with ATV than with ATV-r [12].
The mechanisms involved in changes in body fat after ART initiation have been partially elucidated. The tNRTIs, particularly stavudine, have been strongly implicated in lipoatrophy through mitochondrial toxicity [1–3]. In multivariable analyses exploring baseline factors associated with limb fat changes at week 96, we found that randomization to ATV-r and higher HIV-1 RNA levels were associated with larger increases in limb fat, the latter consistent with the possibility of limb fat increasing after ART initiation being mediated by the return-to-health phenomenon. The only factor independently associated with a decrease in limb fat was older age, as seen by others [16].
The pathogenesis of lipohypertrophy is poorly understood, and the role of PIs is debated. Early studies attributed “lipodystrophy” to PI-based ART [17, 18]; however, recent studies have refuted this association [6, 11, 19]. These discrepancies may be due, in part, to some studies using trunk fat measurement by DEXA, rather than visceral fat measurement by CT or MRI. In addition, in most studies, the backbone NRTIs consisted of tNRTIs, which are known to induce significant metabolic abnormalities, including insulin resistance [20, 21], dyslipidemias [22], and perhaps contribute to visceral fat accumulation [19]. Also, as seen with other complications [5, 23], PIs may differ in their capacity to affect body composition changes, so that our observations pertain only to ATV-r. In our study, ATV-r–containing regimens led to higher gains in limb and trunk fat and a trend towards higher gains in VAT, compared with EFV regimens, with no change in VAT:TAT. Thus, it is likely that the absence of tNRTIs and the lack of resultant limb fat loss allowed us to see a greater net gain in fat in all compartments with ATV-r-based therapy, compared with EFV-based therapy.
In our study, we showed that VAT increased significantly at week 96 in all arms, except for the ABC-3TC + EFV arm (Table 3). In multivariable analysis that explored factors associated with visceral fat changes at week 96, only higher baseline BMI was associated with larger increases in VAT:TAT. This could be linked to the enhanced inflammation associated with obesity [24], which could also play a role in visceral fat accumulation in individuals with HIV-1 infection [25].
Our study has some limitations, including the lack of data on changes in diet, alcohol intake, and physical activity; the open-labeled administration of NNRTIs and PIs; the large number of comparisons performed; and the relatively high amount of missing data. However, sensitivity analyses led to similar results. Lastly, there was a relatively high frequency of changes in NRTIs; however, AT analyses of limb and visceral fat indices yielded results similar to those of the ITT.
In summary, we showed that ART-initiation with ABC-3TC or TDF-FTC as backbone NRTIs over 96 weeks, on average, similarly increased limb fat and visceral fat and caused relatively little lipoatrophy, although we could not conclude that <15% of subjects would have protocol-defined lipoatrophy. In addition, ATV-r–containing regimens induced larger increases in limb and trunk fat, with a trend towards larger percentage increases in visceral fat, compared with EFV-containing regimens. Although lipoatrophy has become rare with decreased use of tNRTIs, lipohypertrophy remains an ongoing complication. Studies aimed at better understanding and preventing lipohypertrophy after ART initiation are needed.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
GlaxoSmithKline and Gilead funded the DEXA and CT scans. Study medications were provided by Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline.
Financial support. The project described was supported by Award Numbers U01 AI068636, U01 AI68634, AI065348, AI38855, AI69501, AI069472, AI0450008, and AI069424 from the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. G. A. M. has served as a scientific advisor for Bristol Myers Squibb, GlaxoSmithKline, Abbott, and Gilead Sciences; has served as a lecturer and on the Speaker bureau for Bristol Myers Squibb, GlaxoSmithKline, and Gilead; has received research grants from Bristol Myers Squibb, GlaxoSmithKline, Abbott, and Merck; and is currently serving as the DSMB Chair for a Pfizer-sponsored study. P. E. S. serves as a consultant for Abbott, Bristol Myers Squibb, Gilead, Merck, Tibotec, and ViiV and receives grant Support from Gilead, Merck, and Tibotec. P. T. has served as a consultant for Merck, Tibotec, Pfizer, and Bristol Myers Squibb; has received grant support from GlaxoSmithKline, Tibotec, Gilead, Bristol Myers Squibb, Tobira, Merck, VGX, VIRxSYS, and Sangamo; and serves on a DSMB for Cyteris and GlaxoSmithKline. C. T. is a member of a DSMB for Tibotec. K. M. is an employee of Gilead Sciences and owns stock in Gilead Sciences. B. H. is an employee of GlaxoSmithKline. E. S. D. serves as a consultant for Bristol Myers Squibb, Gilead, Tibotec, Schering Plough, GlaxoSmithKline, Merck, and ViiV and receives research grant support from Abbott Laboratories, Gilead, ViiV Merck, Pfizer, and GlaxoSmithKline. All other authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
APPENDIX
Acknowledgment for A5224s
Sadia Shaik, MD, and Ruben Lopez, MD,- Harbor-UCLA Medical Center (Site 603) CTU Grant #AI069424, GCRC Grant M01-RR00425
Susan L. Koletar, MD, and Diane Gochnour, RN- The Ohio State University Medical Center (Site 2301) CTU Grant # AI069474
Geyoul Kim, RN, and Mark Rodriguez, RN- Washington University (Site 2101) CTU Grant #U01AI069495; GCRC Grant UL1 RR024992
Elizabeth Lindsey, RN, and Tamara James, BS - Alabama Therapeutics CRS (Site 5801) CTU Grant # U01 AI069452
Ann C. Collier, MD, and Jeffrey Schouten, MD, JD- University of Washington (Site 1401) CTU Grant # AI069434; UL1 RR025014
Jorge L. Santana Bagur, MD, and Santiago Marrero, MD- Puerto Rico-AIDS Clinical Trials Unit (Site 5401) CTU Grant # 5 U0I AI069415-03
Jenifer Baer, RN, BSN, and Carl Fichtenbaum, MD- University of Cincinnati (Site 2401) CTU Grant # AI069513
Patricia Walton, BSN, RN, and Barbara Philpotts, BSN, RN- Case Western Reserve (Site 2501) CTU Grant # AI69501
Princy Kumar, MD, and Joseph Timpone, MD,- Georgetown University (Site 1008) CTU Grant# ACTG Grant # 5U01AI069494
Donna Pittard, RN, BSN, and David Currin, RN- University of North Carolina (Site 3201) CTU Grant # 5-U01 AI069423-03; UNC CFAR # P30 AI050410(-11); UNC CTRC # UL 1RR 025747
Julie Hoffman, RN and Edward Seefried, RN- San Diego Medical Center UC (Site 701) CTU Grant # AI69432
Susan Swindells, MBBS, and Frances Van Meter, APRN- University of Nebraska (Site 1505) CTU Grant # AI 27661
Deborah McMahon, MD, and Barbara Rutecki, MSN, MPH, CRN,P- University of Pittsburgh (Site 1001) CTU Grant # 1 U01 AI069494-01
Michael P. Dube, MD, and Martha Greenwald, RN, MSN,- Indiana University (Site 2601) CTU Grant # 5U01AI025859; GCRC # M01 RR00750
Ilene Wiggins, RN, and Eric Zimmerman, RN- Johns Hopkins University (Site 201) CTU Grant # AI27668; CTSA Grant # UL1 RR025005
Judith Aberg, MD, and Margarita Vasquez, RN,- New York University/NYC HHC at Bellevue Hospital Center (Site 401) CTU Grant # AI27665, AI069532 (new grant number)
Martin McCarter and M. Graham Ray, RN, MSN, - Colorado AIDS Clinical Trials Unit, (Site 6101) CTU Grant # AI69450; RR025780
Mamta Jain, MD, and Tianna Petersen, MS-University of Texas Southwestern Medical Center (Site 3751) CTU Grant # 3U01AI046376-05S4
Emily Stumm, BS, and Pablo Tebas, MD- University of Pennsylvania, Philadelphia (Site 6201) CTU Grant # P30-AI0450008-11; CFAR Grant # UO1-AI069467-04
Mary Albrecht, MD, and Neah Kim, NP- Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) CTU Grant # U01 AI069472-04
Paul Edward Sax, MD, and Joanne Delaney, RN- Brigham and Women's Hospital (Site 107) CTU Grant # UOI AI 069472
Christine Hurley, RN, and Roberto Corales, DO- AIDS Care (Site 1108) CTU Grant # U01AI069511-02; GCRC: UL1 RR 024160
Keith Henry, MD, and Bette Bordenave, RN- Hennepin County Medical Center (Site 1502) CTU Grant # N01 AI72626
Wendy Armstrong, MD, and Ericka R. Patrick, RN, MSN, CCRC- Emory University HIV/AIDS Clinical Trails Unit (Site 5802) CTU Grant # UO1Al69418-01/CFAR P30Al050409
Jane Reid, RNC, MS, and Mary Adams, RN, MPH,- University of Rochester (Site 1101) CTU Grant # U01AI069511-02; GCRC UL1 RR 024160
Gene D. Morse, Pharm.D., FCCP, BCPS- SUNY - Buffalo, Erie County Medical Ctr. (Site 1102) CTU Grant # AI27658
Michael P. Dube, MD, and Martha Greenwald, RN, MSN,- Wishard Memorial Hospital Indiana University (Site 2603) CTU Grant # 5U01AI025859; GCRC # M01 RR00750
Kimberly Y. Smith, MD, MPH and Joan A. Swiatek, APN- Rush University Medical Center (Site 2702) CTU Grant # U01 AI069471
Nancy Hanks, RN, and Debra Ogata-Arakaki, RN -University of Hawaii at Manoa, Leahi Hospital (Site 5201) CTU Grant # AI34853
Ardis Moe, MD, and Maria Palmer, PA-C- UCLA Medical Center (Site 601) CTU Grant # 1U01AI069424-01
Jeffery Meier, MD, and Jack T. Stapleton, MD, - University of Iowa Hospitals and Clinics (Site 1504) CTU Grant # UL1RR024979
Gary Matthew Cox, MD, and Martha Silberman, RN- Duke University Medical Center Adult CRS (Site 1601) CTU Grant # 5U01AI069484-02
Gerianne Casey, RN, and William O’Brien, MD-University of Texas, Galveston (Site 6301) CTU Grant # AI32782
Valery Hughes, FNP, and Todd Stroberg, RN- Cornell CRS (Site 7803, 7804) – CTU Grant# U01 AI069419; CTSC # UL1 RR024996
Nyef El-Daher, MD, -McCree McCuller Wellness Center at the Connection (Site 1107) CTU Grant # U01AI069511-02; GCRC: UL1 RR 024160
Rebecca J. Basham, BS, and Husamettin Erdem, MD,-Vanderbilt Therapeutics CRS (Site 3652) CTU Grant # AI46339-01; MO1 RR 00095
References
- 1.Walker UA, Bickel M, Lutke Volksbeck SI, et al. Evidence of nucleoside analogue reverse transcriptase inhibitor—associated genetic and structural defects of mitochondria in adipose tissue of HIV-infected patients. J Acquir Immune Defic Syndr. 2002;29:117–121. doi: 10.1097/00042560-200202010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 3.McComsey GA, Walker UA. Role of mitochondria in HIV lipoatrophy: insight into pathogenesis and potential therapies. Mitochondrion. 2004;4:111–118. doi: 10.1016/j.mito.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DA. Anthropometric and metabolic outcomes in a 48 week randomized, open-label study of three different combination antiretroviral regimens as initial therapy for HIV infection international workshop on Adverse Drug Reactions and Co-morbidities in HIV. Philadelphia, PA: 2009. [Google Scholar]
- 5.Noor MA, Flint OP, Maa JF, Parker RA. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS. 2006;20:1813–1821. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 6.Jemsek JG, Arathoon E, Arlotti M, et al. Body fat and other metabolic effects of atazanavir and efavirenz, each administered in combination with zidovudine plus lamivudine, in antiretroviral-naive HIV-infected patients. Clin Infect Dis. 2006;42:273–280. doi: 10.1086/498505. [DOI] [PubMed] [Google Scholar]
- 7.Sax PE, Tierney C, Collier AC, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361:2230–2240. doi: 10.1056/NEJMoa0906768. PMCID: 2800041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podzamczer D, Ferrer E, Martinez E, et al. In: Program and abstracts of the 15th Conference on Retroviruses and Opportunistic Infections. Boston, MA:: 2008. How much fat loss is needed for lipoatrophy to become clinically evident? [DOI] [PubMed] [Google Scholar]
- 9.Cassetti I, Madruga JV, Suleiman JM, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials. 2007;8:164–172. doi: 10.1310/hct0803-164. [DOI] [PubMed] [Google Scholar]
- 10.Shlay JC, Visnegarwala F, Bartsch G, et al. Body composition and metabolic changes in antiretroviral-naive patients randomized to didanosine and stavudine vs. abacavir and lamivudine. J Acquir Immune Defic Syndr. 2005;38:147–155. doi: 10.1097/01.qai.0000143599.64234.15. [DOI] [PubMed] [Google Scholar]
- 11.Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–1118. doi: 10.1097/QAD.0b013e32832b4377. PMCID: 2739977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McComsey G, Rightmire A, Wirtz V, Yang R, Mathew M, McGrath D. Changes in body composition with ritonavir-boosted and unboosted atazanavir treatment in combination with lamivudine and stavudine: a 96-week randomized, controlled study. Clin Infect Dis. 2009;48:1323–1326. doi: 10.1086/597776. [DOI] [PubMed] [Google Scholar]
- 13.Dube MP, Komarow L, Mulligan K, et al. Long-term body fat outcomes in antiretroviral-naive participants randomized to nelfinavir or efavirenz or both plus dual nucleosides. Dual X-ray absorptiometry results from A5005s, a substudy of Adult Clinical Trials Group 384. J Acquir Immune Defic Syndr. 2007;45:508–514. doi: 10.1097/QAI.0b013e3181142d26. [DOI] [PubMed] [Google Scholar]
- 14.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–979. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 15.Dube MP, Parker RA, Tebas P, et al. Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS. 2005;19:1807–1818. doi: 10.1097/01.aids.0000183629.20041.bb. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein KA. Redefining lipodystrophy syndrome: risks and impact on clinical decision making. J Acquir Immune Defic Syndr. 2005;39:395–400. doi: 10.1097/01.qai.0000167478.28051.3a. [DOI] [PubMed] [Google Scholar]
- 17.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 18.Carr A, Samaras K, Chisholm DJ, Cooper DA. Abnormal fat distribution and use of protease inhibitors. Lancet. 1998;351:1736. doi: 10.1016/S0140-6736(05)77775-8. [DOI] [PubMed] [Google Scholar]
- 19.Carr A, Ritzhaupt A, Zhang W, et al. Effects of boosted tipranavir and lopinavir on body composition, insulin sensitivity and adipocytokines in antiretroviral-naive adults. AIDS. 2008;22:2313–2321. doi: 10.1097/QAD.0b013e328315a7a5. [DOI] [PubMed] [Google Scholar]
- 20.Blumer RM, van Vonderen MG, Sutinen J, et al. Zidovudine/lamivudine contributes to insulin resistance within 3 months of starting combination antiretroviral therapy. AIDS. 2008;22:227–236. doi: 10.1097/QAD.0b013e3282f33557. [DOI] [PubMed] [Google Scholar]
- 21.De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31:1224–1229. doi: 10.2337/dc07-2013. PMCID: 2746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 23.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Youm YH, Vandanmagsar B, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sevastianova K, Sutinen J, Kannisto K, Hamsten A, Ristola M, Yki-Jarvinen H. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2008;295:E85–E91. doi: 10.1152/ajpendo.90224.2008. [DOI] [PubMed] [Google Scholar]