Abstract
Calcium is a crucial element for striated muscle function. As such, myoplasmic free Ca2+ concentration is delicately regulated through the concerted action of multiple Ca2+ pathways that relay excitation of the plasma membrane to the intracellular contractile machinery. In skeletal muscle, one of these major Ca2+ pathways is Ca2+ release from intracellular Ca2+ stores through type-1 ryanodine receptor/Ca2+ release channels (RyR1), which positions RyR1 in a strategic cross point to regulate Ca2+ homeostasis. This major Ca2+ traffic point appears to be highly sensitive to the intracellular environment, which senses through a plethora of chemical and protein-protein interactions. Among these modulators, perhaps one of the most elusive is Triadin, a muscle-specific protein that is involved in many crucial aspect of muscle function. This family of proteins mediates complex interactions with various Ca2+ modulators and seems poised to be a relevant modulator of Ca2+ signaling in cardiac and skeletal muscles. The purpose of this review is to examine the most recent evidence and current understanding of the role of Triadin in muscle function, in general, with particular emphasis on its contribution to Ca2+ homeostasis.
Keywords: Excitation-contraction coupling, Triadin-null, Calcium release, Ryanodine receptor, FKBP12, Resting calcium
INTRODUCTION
More than two decades after its discovery, and in spite a significant number of studies, our understanding of the role of Triadin in muscle function has remained, for the most part, unclear and elusive. This family of proteins, which are highly abundant and specific to striated muscle, have garnered a significant level of attention fuelled primarily by their ability to interact with the ryanodine receptor (RyR), a Ca2+ release channel that plays a preponderant role in skeletal and cardiac muscle function.
The multiple isoforms of Triadins currently identified in muscle cells seem consistent with the multiplicity of roles credited to these proteins, which include among others, modulation of RyR activity, excitation-contraction (EC) coupling, and Ca2+ homeostasis. The recent development of Triadin-null mouse models have provided us with a critical tool to understand the role of these proteins and have revealed important new insights into the mechanisms that regulate Ca2+ homeostasis in striated muscles.
PROTEIN HETEROGENEITY AND GENE STRUCTURE
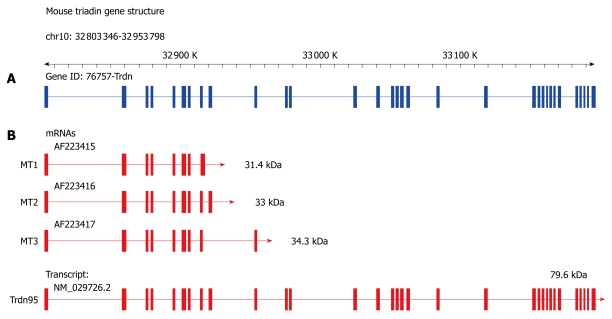
Triadin was originally identified by Caswell et al[1] and Kim et al[2] as a highly enriched 95-kDa protein of the junctional sarcoplasmic reticulum (jSR) in rabbit skeletal muscle. The primary sequence and structure of skeletal Triadin was later deduced from its cDNA sequence, which predicted a 705-amino-acid intrinsic membrane protein containing a short cytoplasmic N terminus, a single membrane-spanning domain, and a long intraluminal C-terminal domain[3,4]. Subsequent studies in rabbit hearts identified three unique cardiac Triadin isoforms with molecular mass of 35, 40 and 92 kDa[5]. Given that all isoforms, skeletal and cardiac, share identical sequences between amino acids 1-264 but have a unique C-terminal region, it appears that all Triadin proteins are products from alternative splicing of a single Triadin (Trdn) gene. Recent sequencing of the whole mouse genome has confirmed this perception (Figure 1). Subsequent studies in other species have revealed that similar tissue-specific patterns of Triadin expression are also present in mouse, canine, rat and human cardiac and skeletal muscle[6-9]. A summary of all Triadin genes currently cloned from cardiac and skeletal muscle is presented in Table 1.
Figure 1.
Genomic structure of mouse Trdn gene. A: Schematic representation of mouse Triadin cDNA structure within the context of the Triadin genomic locus according to the Mouse Genomic Informatics (MGI) gene model[60]; B: Splicing patterns of Triadin. Exons are shown as boxes and introns as lines. The exon splicing pattern that gives rise to the three cardiac Triadin isoforms currently cloned (MT1, MT2 and MT3) as well as the predicted full-length skeletal isoform (Trdn95) are indicated. Size and number of exon boxes in the genomic locus (blue) are not showed in actual scale.
Table 1.
Triadin isoforms cloned from cardiac and skeletal muscle
| Species | Cloned | Isoform |
Mol. mass (kDa) |
Ref. | |
| Predicted | Observed | ||||
| Cardiac muscle | |||||
| Rabbit | 3 | CT1 | 32 | 35 | [5] |
| CT2 | 34.6 | 40 | [5] | ||
| CT3 | 75 | 92 | [5] | ||
| Canine | 2 | CT1 | 30.7 | 35/401 | [6] |
| CT3 | 64.8 | 922 | [6] | ||
| Mouse | 3 | MT1 | 31.4 | 35/381 | [10] |
| MT2 | 33 | 35.5 | [10] | ||
| MT3 | 34.3 | 40 | [10] | ||
| Skeletal muscle | |||||
| Rabbit | 1 | 79.1 | 94 | [4] | |
| Rat | 4 | Trisk 32 | 32.1 | 32 | [9] |
| Trisk 49 | 49.5 | 45 | [9] | ||
| Trisk 51 | 51.3 | 51 | [7] | ||
| Trisk 95 | 77.2 | 95 | [7] | ||
| Canine | 1 | 78.3 | 95 | [6] | |
| Human | 3 | 81.5 | 117 | [8] | |
| Trisk 51 | 51.5 | 51 | [13] | ||
| Trisk 95 | 95 | 952 | [13] | ||
Glycosylated isoform;
Marginally expressed or not detected.
Cardiac muscle appears to express a major Triadin isoform of 32 kDa (CT1 or MT1), which is susceptible to glycosylation and migrates as a secondary 38-kDa band[5,6,10]. A 92-kDa isoform has also been reported in rabbit and canine hearts but its expression is much less prominent than the 32-38-kDa doublet[5,6]. Similarly, it appears that in skeletal muscles Triadin is expressed predominantly as a 95-kDa isoform[1,3,4,6,8,11-13]. However, recent studies in rat skeletal muscle have also identified, and cloned, several shorter Triadin isoforms of 32, 49 and 51 kDa (Trisk-32, Trisk-49 and Trisk-51, respectively)[7,9]. Using the same antibodies generated against the rat skeletal Triadin, the expression of these shorter isoforms has recently been confirmed in mouse skeletal muscle[14], suggesting that multiplicity of isoforms may be a common feature of skeletal Triadin. Whether or not this multiplicity of Triadins is associated with specific functional roles for each isoform is still unknown[15,16]. However, the wide array of Triadin-protein interactions currently reported and the diversity of functional effects directly and indirectly associated with exogenous manipulation of Triadin expression levels seem to support this hypothesis.
PROTEIN-PROTEIN INTERACTIONS
Direct molecular interactions between Triadin and several protein components of the jSR, including the L-Type Ca2+ channel (dihydropyridine receptor, DHPR), RyRs and calsequestrin (Csq), among others, have been consistently reported in skeletal and cardiac muscle. As a result of the importance of many of these components for Ca2+ regulation, it is not surprising that alteration of these interactions has visible functional consequences for Ca2+ homeostasis.
DHPR α1S
In skeletal muscle, early overlay experiments have suggested that Triadin has structural and functional interactions with both the DHPR α1S subunit and RyR1[17,18]. However, with the elucidation of the primary sequence and topological structure of Triadin[4,11], it has become apparent that the DHPR-Triadin interaction involves intraluminal domains of Triadin that are unlikely to be accessible in situ. To date, there has been no new evidence that either supports or rules out direct structural or functional interactions between the cytoplasmic domain of Triadin and the DHPR complex.
RyRs
Immunohistochemistry studies in adult muscles have localized Triadin at the jSR in the vicinity of RyRs, revealing the close association of these two proteins in both skeletal[4,19] and cardiac[20] muscles. However, in skeletal muscle, co-localization with RyR1 only involves the 95-kDa isoform of Triadin, because the lower molecular weight isoforms (Trisk-32 and Trisk-49) appear to be segregated to non-jSR regions of the muscle[9]. Direct RyR1-Triadin interactions have been confirmed using glutathione-S-transferase/Triadin fusion proteins, which suggest that there is a stable multi-protein complex involving Triadin, RyR1 and the Ca2+ binding protein Csq[21]. More recently, mutagenesis analysis has suggested that the Triadin-binding site of RyR1 may reside within the negatively charged residues Asp4878, Asp4907 and Glu4908 of RyR1[22]. Likewise, the corresponding RyR1-binding site of Triadin has been mapped to amino acids 200-232 within the intraluminal domain[22], a region rich in multiple clusters of alternating Lys and Glu residues, known as KEKE motif. This motif is common to all skeletal and cardiac isoforms of Triadin[22-24]. The direct functional effect of Triadin-RyR1 interaction on RyR1 channel activity is discussed below.
Csq and junctin
In addition to binding RyRs, Triadin has been shown to directly interact with Csq and Junctin. Csq, an intra-SR Ca2+ binding protein is thought to be the main Ca2+ buffer protein of the SR[25-27], whereas Junctin is an SR integral membrane protein that shares structural and amino acid sequence similarity with Triadin[10,23,28]. Currently, there seems to be a consensus that Junctin and Triadin interact directly in the jSR membrane and stabilize a quaternary complex that anchors Csq to the RyR, probably through their shared KEKE motifs[21,23,29]. These quaternary complexes have been identified biochemically in both skeletal[21,30] and cardiac[23,24] muscles.
Based on the large Ca2+ binding capacity of Csq and its ability to undergo significant conformational changes over the physiological range of intra-SR Ca2+ concentrations[27,31], Csq has been proposed as an intraluminal Ca2+ sensor that plays a significant role in the ability of RyRs to sense and respond to changes in SR Ca2+ content. Even though in vitro studies in skeletal muscle using purified proteins have suggested that Csq and RyR1 can engage in direct structural/functional interaction[32-35], it is likely that in vivo, this functional crosstalk is primarily mediated through their interaction with Triadin and Junctin[36-38]. Even though the use of purified RyR1 in artificial bilayer membrane (BLM) studies to test the role of Triadin and Junctin in intraluminal Ca2+ regulation of skeletal muscle has recently suggested that Junctin may be the only protein involved in mediating signaling between Csq and RyR1[38], our studies have indicated that this may not be the predominant interaction in vivo. Indeed, Ca2+ imaging studies in Junctin-null mice suggest that, unlike in Triadin-null myotubes that show significant dysregulation of Ca2+ homeostasis, Junctin-null myotubes have a nearly wild-type phenotype, with no significant alteration in SR Ca2+ content or [Ca2+]rest (unpublished data). These results strongly support the idea that Triadin is a key functional component of Ca2+ homeostasis in skeletal muscle.
Histidine-rich Ca2+-binding protein
Histidine-rich Ca2+-binding protein (HRC) is a Ca2+-binding protein found in small amounts in the SR lumen, and shares many biochemical and structural features with Csq[39-42]. Biochemical studies in skeletal muscles have found that HRC can bind to Triadin in a Ca2+-sensitive manner through the same KEKE motif involved in the binding of Triadin to Csq[40,43,44]. Therefore, it is not unlikely that binding of HRC to Triadin could affect RyR activity by disrupting the Triadin/Junctin/RyR/Csq Ca2+ release complex. Although the role of HRC in Ca2+ homeostasis in skeletal muscles is unknown, studies in cardiac cells have suggested that HRC is important for Ca2+ regulation. In the heart, overexpression of HRC is associated with alteration of both SR Ca2+ release and contractility, which coincidently is associated with reduction in Triadin and Junctin expression[41,45,46]. Conversely, HRC-null mice exhibit a significant increase in Triadin expression[47]. However, because cardiac and skeletal muscles express different isoforms of Triadin, the possibility that disruption of the HRC/Triadin interaction in skeletal muscle results in a different functional outcome than that observed in cardiac tissues should not be ruled out. In this regard, our own studies have indicated that, unlike HRC-null mice in which HRC and Triadin expression seem to be interlocked, in Triadin-null mice, the lack of Triadin expression does not seem to affect HRC expression levels (unpublished data).
Overall, these studies suggest that Triadin is positioned to engage in meaningful structural/functional interactions with key modulatory components of Ca2+ release, and thus, seems poised to play a pivotal role in Ca2+ regulation in muscle cells.
EC COUPLING
RyR/DHPR interaction is key for EC coupling. Since the early biochemical studies in rabbit skeletal muscle[1,2,17] showing that Triadin binds to both DHPR α1S and RyR1 and the proposed ternary complex between the proteins, there have been many studies that have linked Triadin to EC coupling in skeletal muscles.
Although the direct interaction between Triadin and DHPR α1S has proven difficult to confirm, the evidence supporting a role for Triadin in modulating depolarization-induced Ca2+ release has been somewhat consistent. Early stopped-flow studies in triad vesicles have demonstrated that the use of an anti-Triadin antibody significantly inhibited depolarization-induced Ca2+ release[48], which supports the idea that Triadin may be involved in the functional coupling between DHPR and RyR1. More recently, in a series of functional studies, with overexpression of different isoforms of Triadin in cultured myotubes, it has been shown that Trisk-95, but not Trisk-55, significantly inhibits depolarization-induced Ca2+ release in rat[49] and C2C12[50] cells, strengthening the idea that Triadin, in particular the 95-kDa isoform, plays a critical regulatory role in skeletal-type EC coupling. Supporting this line of reasoning, Goonasekera et al[51] have shown that expression of mutant RyRs that lack Triadin-binding ability in dyspedic myotubes dramatically impairs electrically evoked Ca2+ transients, nearly ablating skeletal-type EC coupling without noticeable effects on other RyR1 functions. Similarly, Wang et al[52] have shown that the use of siRNAs to knockdown expression of Triadin in cultured myotubes led to a significant reduction in amplitude of K+-induced Ca2+ transients, suggesting that Triadin may play a role in facilitating depolarization-induced Ca2+ release. However, with the recent development of Triadin-null mice, the idea of Triadin playing a critical or direct role in skeletal-type EC coupling has been challenged. Indeed, despite the lack of Triadin expression, homozygous Triadin-null (Trdn-/-) mice do not exhibit embryonic or birth lethality nor demonstrate an obvious gross functional phenotype[12,14] as has been reported for dyspedic[53] and dysgenic mice[54,55], two other mouse models that bear significant disruption of the EC coupling signaling. Triadin-null skeletal muscles, however, have shown a significant decay in strength that confirms the general thought that Triadins are important modulatory components of skeletal muscle function[14].
Interestingly, Ca2+ imaging studies in Triadin-null myotubes have revealed that the absence of Triadin expression results in a noticeable reduction in peak amplitude of depolarization-induced Ca2+ transients[12]. Although, whole-cell patch clamp studies of Triadin-null myotubes have demonstrated that null cells display almost normal bidirectional signaling, with no changes in DHPR Ca2+ current densities and strong voltage-dependent Ca2+ release activity, they do have a moderate reduction in voltage-dependent Ca2+ release amplitude, and therefore, reduced orthograde signaling[56].
Triadin-null myotubes also display a significant alteration of the overall Ca2+ homeostasis driven primarily, but not exclusively, by the disruption of the RyR1/FKBP12 interaction[57]. Overexpression of FKBP12.6 can overcome this faulty interaction and almost completely reverse the effects of lack of Triadin expression on Ca2+ homeostasis. More importantly, overexpression of FKBP12.6 also is sufficient to erase all of the differences in depolarization-induced Ca2+ release observed between wild-type and Triadin-null myotubes[56]. The full restoration of EC coupling signals of Triadin-null myotubes by FKBP12.6 strongly suggests that the effects of Triadin on the orthograde signal are not directly but indirectly mediated by its side effects on the RyR1/FKBP12 interaction. Thus, further supporting the idea that skeletal Triadins are not involved in the bidirectional coupling between DHPR and RyR1.
MYOPLASMIC Ca2+ REGULATION
The well-documented interaction between Triadin and RyR1 has a significant impact on the Ca2+ channel behavior, and consequently, Ca2+ regulation in skeletal muscle cells. The first indication of a direct effect of Triadin on RyR1 function came from studies of Ohkura et al[35], who have reported that purified Triadin has an inhibitory effect on both 3H-ryanodine binding to solubilized RyR1s, and on Ca2+ channel activity of purified RyR1 fused into BLMs. At the same time, Groh et al[58] have shown that a peptide containing a short fragment of the cytoplasmic domain of Triadin not only reduced the open probability of native and purified RyR1 channels in BLMs, but also inhibited the overall Ca2+ release from SR vesicles, identifying one of the first discrete domains of Triadin directly involved in a functional interaction with RyR1.
Our studies on native RyR1 channels reconstituted from Triadin-null skeletal muscles have revealed that the absence of Triadin significantly increases sub-conductance states of RyR channels, which in turn result in elevation of overall open probability. This enhanced channel activity seems to be directly associated with loss of FKBP12 binding capacity of RyR1, because the addition of exogenous FKBP12.6, that has a higher affinity for RyR1 than FKBP12, significantly reduces channel activity[57]. However, in a recent study, Wei et al[38] have found that, unlike previous studies, addition of purified skeletal Triadin had instead an activating effect on the channel activity of purified RyR1 fused into BLMs. It is still unclear whether these differences in functional effect account for differences in experimental protocols or actual functional differences in Triadin-binding sites at the intraluminal and cytoplasmic domain of RyR1. However, what all these reports seem to agree on is the idea that changes in Triadin expression result in modulation of RyR1 Ca2+ channel activity.
Consistent with the enhanced basal activity observed in RyR channels from Triadin-null muscles, Trdn-/- myotubes are characterized by reduced Ca2+ release response to caffeine[12,14,57] and the sarcoplasmic/endoplasmic reticulum calcium pump inhibitor thapsigargin[12,14], both suggestive of alterations in the SR Ca2+ content. Similar observations have been reported in Triadin-knockdown myotubes, where in addition to reduced SR Ca2+ load, there is an increased frequency in Ca2+ spark activity[50]. In agreement with these reports, reduced caffeine-induced Ca2+ release responses are also observed in dyspedic myotubes expressing Triadin-binding-deficient RyRs[51,59]. Overall, these results seem to support the idea that loss of Triadin expression leads to loss of negative regulation on RyR channels, which in turn, results in enhanced SR Ca2+ leakage. Accordingly, overexpression of Triadin in skeletal myotubes, a condition that should increase the negative regulation on RyR1 and suppress SR Ca2+ leakage, appears not to have a detrimental effect on caffeine-induced Ca2+ release and SR Ca2+ load[49,50].
Myotubes and adult muscle fibers from Triadin-null mice also show chronically elevated [Ca2+]rest[12,50,57]. This elevated resting myoplasmic [Ca2+] is partially reversed by inhibitors of RyR1 activity (ryanodine and FKBP12.6) and RyR1 leakage (bastadin-5). Similar effects have been observed with Ca2+ entry blockers (Cd2+ and La3+) and TRPC/Orai-1 blockers (2-APB and BTP-2)[57]. This pharmacological profile is consistent with the idea that elevated resting Ca2+ in Triadin-null muscle cells involves both RyR-mediated SR Ca2+ leakage and enhanced extracellular Ca2+ entry at rest[57]. The effect of 2-APB and BTP-2 on [Ca2+]rest in addition to the elevated intracellular [Na+] observed in Triadin-null cells (unpublished data) strongly suggests that the extracellular Ca2+ entry pathway activated by the lack of Triadin may be, at least partially, mediated by TRP channels or Orai-1.
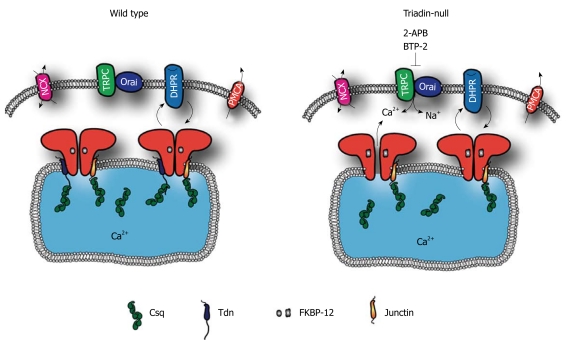
In summary, the current accumulated data from Triadin-null muscle cells support the molecular model depicted in Figure 2 in which the lack of Triadin expression significantly destabilizes the FKBP12/RyR1 interaction, causing increased basal activity of RyR Ca2+ channels. This in turn results in increased SR calcium leakage, which contributes to elevate the myoplasmic resting free Ca2+. On the other hand, this increased Ca2+ leakage leads to partial depletion of SR calcium stores, which drives TRPC- and/or Orai-1-mediated Ca2+ entry, further contributing to elevation of resting Ca2+.
Figure 2.
Proposed model of Ca2+ regulation by Triadin in wild-type and Triadin-null skeletal muscle. Lack of Triadin binding to type-1 ryanodine receptor (RyR1) indirectly affects FKBP12/RyR1 interaction causing, on the one hand, an increase in RyR1 channel gating and, on the other hand, a weakening of the DHPRα1S/RyR1 orthograde signaling. Dysregulation of RyR1 activity of Triadin-null cells leads to enhanced SR Ca2+ leakage and subsequent reduction in SR Ca2+ content. In addition, lack of Triadin expression activates Ca2+ entry pathways that are both store-dependent and store-independent (sensitive to TRPC/Orai-1 inhibitors). Ca2+ entry and SR Ca2+ leakage could contribute independently to elevate myoplasmic [Ca2+]rest.
Although many questions remain, a clear picture of Triadins playing a relevant modulatory role in Ca2+ homeostasis of skeletal muscle has emerged. Triadin control of RyR Ca2+ channel activity has the potential to unravel a cascade of events that can ultimately adjust Ca2+ flux equilibrium in muscle cells, resulting in permanent modification of [Ca2+]rest. Hence, it appears that although not directly involved in Ca2+ transport Triadins may contribute a significant role to fine-tuning Ca2+ homeostasis in skeletal muscles.
Acknowledgments
The author wishes to acknowledge Dr. Paul D Allen for help in editing the manuscript and for financial support in creating the Triadin-null mouse.
Footnotes
Supported by NIH Grant 5K01AR054818 (to Perez CF) and P01AR47605 (to Allen PD)
Peer reviewers: Sheng-Tao Hou, Professor, Institute for Biological Sciences, National Research Council of Canada, 1200 Montreal Road, Bldg M-54, Ottawa, Ontario, K1A 0R6, Canada; Luca Munaron, PhD, Associate Professor, Department of Animal and Human Biology, University of Torino, Via Accademia Albertina 13, 10123 Torino, Italy
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Caswell AH, Brandt NR, Brunschwig JP, Purkerson S. Localization and partial characterization of the oligomeric disulfide-linked molecular weight 95,000 protein (triadin) which binds the ryanodine and dihydropyridine receptors in skeletal muscle triadic vesicles. Biochemistry. 1991;30:7507–7513. doi: 10.1021/bi00244a020. [DOI] [PubMed] [Google Scholar]
- 2.Kim KC, Caswell AH, Talvenheimo JA, Brandt NR. Isolation of a terminal cisterna protein which may link the dihydropyridine receptor to the junctional foot protein in skeletal muscle. Biochemistry. 1990;29:9281–9289. doi: 10.1021/bi00491a025. [DOI] [PubMed] [Google Scholar]
- 3.Knudson CM, Stang KK, Jorgensen AO, Campbell KP. Biochemical characterization of ultrastructural localization of a major junctional sarcoplasmic reticulum glycoprotein (triadin) J Biol Chem. 1993;268:12637–12645. [PubMed] [Google Scholar]
- 4.Knudson CM, Stang KK, Moomaw CR, Slaughter CA, Campbell KP. Primary structure and topological analysis of a skeletal muscle-specific junctional sarcoplasmic reticulum glycoprotein (triadin) J Biol Chem. 1993;268:12646–12654. [PubMed] [Google Scholar]
- 5.Guo W, Jorgensen AO, Jones LR, Campbell KP. Biochemical characterization and molecular cloning of cardiac triadin. J Biol Chem. 1996;271:458–465. doi: 10.1074/jbc.271.1.458. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi YM, Jones LR. Identification of triadin 1 as the predominant triadin isoform expressed in mammalian myocardium. J Biol Chem. 1999;274:28660–28668. doi: 10.1074/jbc.274.40.28660. [DOI] [PubMed] [Google Scholar]
- 7.Marty I, Thevenon D, Scotto C, Groh S, Sainnier S, Robert M, Grunwald D, Villaz M. Cloning and characterization of a new isoform of skeletal muscle triadin. J Biol Chem. 2000;275:8206–8212. doi: 10.1074/jbc.275.11.8206. [DOI] [PubMed] [Google Scholar]
- 8.Taske NL, Eyre HJ, O’Brien RO, Sutherland GR, Denborough MA, Foster PS. Molecular cloning of the cDNA encoding human skeletal muscle triadin and its localisation to chromosome 6q22-6q23. Eur J Biochem. 1995;233:258–265. doi: 10.1111/j.1432-1033.1995.258_1.x. [DOI] [PubMed] [Google Scholar]
- 9.Vassilopoulos S, Thevenon D, Rezgui SS, Brocard J, Chapel A, Lacampagne A, Lunardi J, Dewaard M, Marty I. Triadins are not triad-specific proteins: two new skeletal muscle triadins possibly involved in the architecture of sarcoplasmic reticulum. J Biol Chem. 2005;280:28601–28609. doi: 10.1074/jbc.M501484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong CS, Ji JH, Kim JP, Jung DH, Kim DH. Molecular cloning and characterization of mouse cardiac triadin isoforms. Gene. 2001;278:193–199. doi: 10.1016/s0378-1119(01)00718-1. [DOI] [PubMed] [Google Scholar]
- 11.Marty I, Robert M, Ronjat M, Bally I, Arlaud G, Villaz M. Localization of the N-terminal and C-terminal ends of triadin with respect to the sarcoplasmic reticulum membrane of rabbit skeletal muscle. Biochem J. 1995;307(Pt 3):769–774. doi: 10.1042/bj3070769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen X, Franzini-Armstrong C, Lopez JR, Jones LR, Kobayashi YM, Wang Y, Kerrick WG, Caswell AH, Potter JD, Miller T, et al. Triadins modulate intracellular Ca(2+) homeostasis but are not essential for excitation-contraction coupling in skeletal muscle. J Biol Chem. 2007;282:37864–37874. doi: 10.1074/jbc.M705702200. [DOI] [PubMed] [Google Scholar]
- 13.Thevenon D, Smida-Rezgui S, Chevessier F, Groh S, Henry-Berger J, Beatriz Romero N, Villaz M, DeWaard M, Marty I. Human skeletal muscle triadin: gene organization and cloning of the major isoform, Trisk 51. Biochem Biophys Res Commun. 2003;303:669–675. doi: 10.1016/s0006-291x(03)00406-6. [DOI] [PubMed] [Google Scholar]
- 14.Oddoux S, Brocard J, Schweitzer A, Szentesi P, Giannesini B, Brocard J, Fauré J, Pernet-Gallay K, Bendahan D, Lunardi J, et al. Triadin deletion induces impaired skeletal muscle function. J Biol Chem. 2009;284:34918–34929. doi: 10.1074/jbc.M109.022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen PD. Triadin, not essential, but useful. J Physiol. 2009;587:3123–3124. doi: 10.1113/jphysiol.2009.172015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marty I, Fauré J, Fourest-Lieuvin A, Vassilopoulos S, Oddoux S, Brocard J. Triadin: what possible function 20 years later? J Physiol. 2009;587:3117–3121. doi: 10.1113/jphysiol.2009.171892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt NR, Caswell AH, Wen SR, Talvenheimo JA. Molecular interactions of the junctional foot protein and dihydropyridine receptor in skeletal muscle triads. J Membr Biol. 1990;113:237–251. doi: 10.1007/BF01870075. [DOI] [PubMed] [Google Scholar]
- 18.Fan H, Brandt NR, Peng M, Schwartz A, Caswell AH. Binding sites of monoclonal antibodies and dihydropyridine receptor alpha 1 subunit cytoplasmic II-III loop on skeletal muscle triadin fusion peptides. Biochemistry. 1995;34:14893–14901. doi: 10.1021/bi00045a034. [DOI] [PubMed] [Google Scholar]
- 19.Carl SL, Felix K, Caswell AH, Brandt NR, Brunschwig JP, Meissner G, Ferguson DG. Immunolocalization of triadin, DHP receptors, and ryanodine receptors in adult and developing skeletal muscle of rats. Muscle Nerve. 1995;18:1232–1243. doi: 10.1002/mus.880181104. [DOI] [PubMed] [Google Scholar]
- 20.Carl SL, Felix K, Caswell AH, Brandt NR, Ball WJ, Vaghy PL, Meissner G, Ferguson DG. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995;129:673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo W, Campbell KP. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J Biol Chem. 1995;270:9027–9030. doi: 10.1074/jbc.270.16.9027. [DOI] [PubMed] [Google Scholar]
- 22.Lee JM, Rho SH, Shin DW, Cho C, Park WJ, Eom SH, Ma J, Kim DH. Negatively charged amino acids within the intraluminal loop of ryanodine receptor are involved in the interaction with triadin. J Biol Chem. 2004;279:6994–7000. doi: 10.1074/jbc.M312446200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi YM, Alseikhan BA, Jones LR. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J Biol Chem. 2000;275:17639–17646. doi: 10.1074/jbc.M002091200. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan DH, Wong PT. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci USA. 1971;68:1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meissner G, Conner GE, Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973;298:246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- 27.Ikemoto N, Ronjat M, Mészáros LG, Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989;28:6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- 28.Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995;270:30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- 29.Liu G, Pessah IN. Molecular interaction between ryanodine receptor and glycoprotein triadin involves redox cycling of functionally important hyperreactive sulfhydryls. J Biol Chem. 1994;269:33028–33034. [PubMed] [Google Scholar]
- 30.Glover L, Culligan K, Cala S, Mulvey C, Ohlendieck K. Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle. Biochim Biophys Acta. 2001;1515:120–132. doi: 10.1016/s0005-2736(01)00406-0. [DOI] [PubMed] [Google Scholar]
- 31.Hidalgo C, Donoso P, Rodriguez PH. Protons induce calsequestrin conformational changes. Biophys J. 1996;71:2130–2137. doi: 10.1016/S0006-3495(96)79413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szegedi C, Sárközi S, Herzog A, Jóna I, Varsányi M. Calsequestrin: more than ‘only’ a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem J. 1999;337(Pt 1):19–22. [PMC free article] [PubMed] [Google Scholar]
- 33.Herzog A, Szegedi C, Jona I, Herberg FW, Varsanyi M. Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca(2+) release channel/ryanodine receptor with calsequestrin. FEBS Lett. 2000;472:73–77. doi: 10.1016/s0014-5793(00)01431-9. [DOI] [PubMed] [Google Scholar]
- 34.Beard NA, Sakowska MM, Dulhunty AF, Laver DR. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys J. 2002;82:310–320. doi: 10.1016/S0006-3495(02)75396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkura M, Furukawa K, Fujimori H, Kuruma A, Kawano S, Hiraoka M, Kuniyasu A, Nakayama H, Ohizumi Y. Dual regulation of the skeletal muscle ryanodine receptor by triadin and calsequestrin. Biochemistry. 1998;37:12987–12993. doi: 10.1021/bi972803d. [DOI] [PubMed] [Google Scholar]
- 36.Beard NA, Casarotto MG, Wei L, Varsányi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J. 2005;88:3444–3454. doi: 10.1529/biophysj.104.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beard NA, Wei L, Cheung SN, Kimura T, Varsányi M, Dulhunty AF. Phosphorylation of skeletal muscle calsequestrin enhances its Ca2+ binding capacity and promotes its association with junctin. Cell Calcium. 2008;44:363–373. doi: 10.1016/j.ceca.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Wei L, Gallant EM, Dulhunty AF, Beard NA. Junctin and triadin each activate skeletal ryanodine receptors but junctin alone mediates functional interactions with calsequestrin. Int J Biochem Cell Biol. 2009;41:2214–2224. doi: 10.1016/j.biocel.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofmann SL, Brown MS, Lee E, Pathak RK, Anderson RG, Goldstein JL. Purification of a sarcoplasmic reticulum protein that binds Ca2+ and plasma lipoproteins. J Biol Chem. 1989;264:8260–8270. [PubMed] [Google Scholar]
- 40.Lee HG, Kang H, Kim DH, Park WJ. Interaction of HRC (histidine-rich Ca(2+)-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J Biol Chem. 2001;276:39533–39538. doi: 10.1074/jbc.M010664200. [DOI] [PubMed] [Google Scholar]
- 41.Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, Padmanabhan PA, Mitton BA, Waggoner JR, Del Monte F, et al. Histidine-rich Ca binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol. 2006;40:653–665. doi: 10.1016/j.yjmcc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, Kontrogianni-Konstantopoulos A, Sanoudou D, Kranias EG. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am J Physiol Heart Circ Physiol. 2007;293:H1581–H1589. doi: 10.1152/ajpheart.00278.2007. [DOI] [PubMed] [Google Scholar]
- 43.Sacchetto R, Turcato F, Damiani E, Margreth A. Interaction of triadin with histidine-rich Ca(2+)-binding protein at the triadic junction in skeletal muscle fibers. J Muscle Res Cell Motil. 1999;20:403–415. doi: 10.1023/a:1005580609414. [DOI] [PubMed] [Google Scholar]
- 44.Sacchetto R, Damiani E, Turcato F, Nori A, Margreth A. Ca(2+)-dependent interaction of triadin with histidine-rich Ca(2+)-binding protein carboxyl-terminal region. Biochem Biophys Res Commun. 2001;289:1125–1134. doi: 10.1006/bbrc.2001.6126. [DOI] [PubMed] [Google Scholar]
- 45.Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG. Regulation of myocardial function by histidine-rich, calcium-binding protein. Am J Physiol Heart Circ Physiol. 2004;287:H1705–H1711. doi: 10.1152/ajpheart.01211.2003. [DOI] [PubMed] [Google Scholar]
- 46.Arvanitis DA, Vafiadaki E, Sanoudou D, Kranias EG. Histidine-rich calcium binding protein: the new regulator of sarcoplasmic reticulum calcium cycling. J Mol Cell Cardiol. 2011;50:43–49. doi: 10.1016/j.yjmcc.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaehnig EJ, Heidt AB, Greene SB, Cornelissen I, Black BL. Increased susceptibility to isoproterenol-induced cardiac hypertrophy and impaired weight gain in mice lacking the histidine-rich calcium-binding protein. Mol Cell Biol. 2006;26:9315–9326. doi: 10.1128/MCB.00482-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandt NR, Caswell AH, Brunschwig JP, Kang JJ, Antoniu B, Ikemoto N. Effects of anti-triadin antibody on Ca2+ release from sarcoplasmic reticulum. FEBS Lett. 1992;299:57–59. doi: 10.1016/0014-5793(92)80100-u. [DOI] [PubMed] [Google Scholar]
- 49.Rezgui SS, Vassilopoulos S, Brocard J, Platel JC, Bouron A, Arnoult C, Oddoux S, Garcia L, De Waard M, Marty I. Triadin (Trisk 95) overexpression blocks excitation-contraction coupling in rat skeletal myotubes. J Biol Chem. 2005;280:39302–39308. doi: 10.1074/jbc.M506566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fodor J, Gönczi M, Sztretye M, Dienes B, Oláh T, Szabó L, Csoma E, Szentesi P, Szigeti GP, Marty I, et al. Altered expression of triadin 95 causes parallel changes in localized Ca2+ release events and global Ca2+ signals in skeletal muscle cells in culture. J Physiol. 2008;586:5803–5818. doi: 10.1113/jphysiol.2008.160457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goonasekera SA, Beard NA, Groom L, Kimura T, Lyfenko AD, Rosenfeld A, Marty I, Dulhunty AF, Dirksen RT. Triadin binding to the C-terminal luminal loop of the ryanodine receptor is important for skeletal muscle excitation contraction coupling. J Gen Physiol. 2007;130:365–378. doi: 10.1085/jgp.200709790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Li X, Duan H, Fulton TR, Eu JP, Meissner G. Altered stored calcium release in skeletal myotubes deficient of triadin and junctin. Cell Calcium. 2009;45:29–37. doi: 10.1016/j.ceca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeshima H, Iino M, Takekura H, Nishi M, Kuno J, Minowa O, Takano H, Noda T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994;369:556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- 54.Beam KG, Knudson CM, Powell JA. A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature. 1986;320:168–170. doi: 10.1038/320168a0. [DOI] [PubMed] [Google Scholar]
- 55.Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 56.Eltit JM, Szpyt J, Li H, Allen PD, Perez CF. Reduced gain of excitation-contraction coupling in triadin-null myotubes is mediated by the disruption of FKBP12/RyR1 interaction. Cell Calcium. 2011;49:128–135. doi: 10.1016/j.ceca.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eltit JM, Feng W, Lopez JR, Padilla IT, Pessah IN, Molinski TF, Fruen BR, Allen PD, Perez CF. Ablation of skeletal muscle triadin impairs FKBP12/RyR1 channel interactions essential for maintaining resting cytoplasmic Ca2+ J Biol Chem. 2010;285:38453–38462. doi: 10.1074/jbc.M110.164525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groh S, Marty I, Ottolia M, Prestipino G, Chapel A, Villaz M, Ronjat M. Functional interaction of the cytoplasmic domain of triadin with the skeletal ryanodine receptor. J Biol Chem. 1999;274:12278–12283. doi: 10.1074/jbc.274.18.12278. [DOI] [PubMed] [Google Scholar]
- 59.Lee EH, Song DW, Lee JM, Meissner G, Allen PD, Kim do H. Occurrence of atypical Ca2+ transients in triadin-binding deficient-RYR1 mutants. Biochem Biophys Res Commun. 2006;351:909–914. doi: 10.1016/j.bbrc.2006.10.115. [DOI] [PubMed] [Google Scholar]
- 60.Blake JA, Bult CJ, Kadin JA, Richardson JE, Eppig JT. The Mouse Genome Database (MGD): premier model organism resource for mammalian genomics and genetics. Nucleic Acids Res. 2011;39:D842–D848. doi: 10.1093/nar/gkq1008. [DOI] [PMC free article] [PubMed] [Google Scholar]