Abstract
The induction of immunity genes in Drosophila has been proposed to be dependent on Dorsal, Dif, and Relish, the NF-κB-related factors. Here we provide genetic evidence that Dif is required for the induction of only a subset of antimicrobial peptide genes. The results show that the presence of Dif without Dorsal is sufficient to mediate the induction of drosomycin and defensin. We also demonstrate that Dif is a downstream component of the Toll signaling pathway in activating the drosomycin expression. These results reveal that individual members of the NF-κB family in Drosophila have distinct roles in immunity and development.
Keywords: Immunity, Drosophila, Dif, NF-κB, Toll, drosomycin
Multicellular organisms share a common burden of defending themselves against the invasion of microorganisms. Recent molecular genetic studies in plants, insects, and mammals reveal conserved pathways that signal host cells of microbial infection and elicit production of protective molecules (Ip et al. 1993; Whitham et al. 1994; Barillas-Mury et al. 1996; Medzhitov et al. 1997; Ryals et al. 1997; Han et al. 1998; Ip and Davis 1998; Yang et al. 1998). In tobacco, the N gene product mediates the resistance to tobacco mosaic virus. The N protein has homology to the intracellular domains of the Drosophila Toll and the mammalian interleukin-1 receptor (IL-1R) (Whitham et al. 1994). Such homology is also observed in the Arabidopsis RPP5 protein, which confers resistance to downy mildew pathogen (Parker et al. 1997). Using the Drosophila Toll sequence, various groups have further identified in human five novel Toll-like receptors (Medzhitov et al. 1997; Rock et al. 1998; Yang et al. 1998). These novel molecules probably represent true homologs of Toll by virtue of having homology in both intracellular and extracellular domains. At least some of these human Toll-like receptors can mediate aspects of immune response (Medzhitov et al. 1997; Rock et al. 1998; Yang et al. 1998). Therefore, the results support the idea that Toll-mediated signaling represents an ancient self-defense pathway. It has also been shown that the stress-activated JNK and p38 MAP kinase pathways, as well as the JAK–STAT pathway, may have similar functions in Drosophila (Han et al. 1998; Ip and Davis 1998; Liu et al. 1998; Mathey-Prevot and Perrimon 1998). Taken together, at least some of the pathways that mediate self-defense response in very diverse species are highly conserved.
The activation of Toll and IL-1R both lead to the mobilization of NF-κB factors, which have been shown to be present in many cell types to regulate genes that are involved in self-protection processes (Verma et al. 1995; Baeuerle and Baltimore 1996). In Drosophila, the first member of the NF-κB family, Dorsal, was identified in a screen for genes required for embryonic development. Dorsal is a key regulator in determining dorsoventral polarity (Drier and Steward 1997). Both Dorsal and NF-κB can bind to similar DNA sequences and have similar gene regulatory functions. Their activities are also modulated by highly conserved signaling pathways (Verma et al. 1995; Baeuerle and Baltimore 1996; Drier and Steward 1997; Wu and Anderson 1997). Despite the striking similarity of the molecular components involved, the biological processes (dorsoventral development vs. immune response) controlled by Dorsal and NF-κB pathways seemed rather disparate. This disparity, however, was reconciled by the implication of NF-κB-like molecules in regulating Drosophila antimicrobial response (Sun and Faye 1992; Engstrom et al. 1993; Ip et al. 1993; Kappler et al. 1993).
Insects battle microbial infection by synthesizing a spectrum of antimicrobial peptides that synergistically lyse invading microorganisms. Whereas >10 different antimicrobial peptides have been identified in different insects, approximately seven genes that encode such peptides have been cloned from Drosophila (Hoffmann et al. 1996; Hultmark 1993). Molecular analyses showed that the induction of these peptides in Drosophila probably involves NF-κB factors, which include Dif and Relish in addition to Dorsal (Ip et al. 1993; Lemaitre et al. 1995a; Petersen et al. 1995; Dushay et al. 1996; Gross et al. 1996). Therefore, previous reports suggest that the Toll–NF-κB signaling pathway represents an evolutionarily conserved cassette utilized in diverse species in the self-defense process. However, the implication of the insect NF-κB factors in immunity has been based on biochemical and molecular experiments. The understanding of how these molecules may function individually and in combination in whole animal requires further analysis. In this report we present genetic evidence demonstrating that Dif is an essential factor for some aspects of insect immunity.
Results
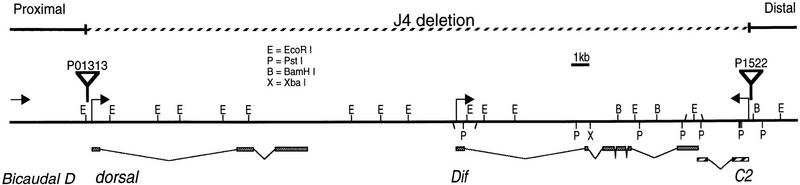
To elucidate the requirements of NF-κB-related factors in Drosophila immunity, we attempted to isolate mutants that were defective in Dif and Dorsal function. Dif was mapped previously near the dorsal locus (Ip et al. 1993). Molecular analysis revealed that the transcription start site of the Dif gene is ∼9 kb distant from the 3′ end of dorsal (Fig. 1). The homology of the Rel domains (Ip et al. 1993) and the similarity of the intron–exon structures (Fig. 1) suggest that the two genes might arise by duplication during evolution. Previous results showed that Dif is expressed at high levels at postembryonic stages, whereas dorsal has high maternal and low zygotic expression (Steward et al. 1984; Ip et al. 1993; Lemaitre et al. 1995a). Nonetheless, it has been demonstrated that Dif can rescue to some extent the dorsal mutant phenotype in the early embryo (Stein et al. 1998). The two transcription factors, therefore, can regulate similar target genes if expressed in the same tissue. Previous reports showed that Dif and Dorsal are present in fat bodies and hemocytes, the insect immune organs (Ip et al. 1993; Lemaitre et al. 1995a, 1996; Petersen et al. 1995; Gross et al. 1996). Moreover, both proteins accumulate in nuclei upon microbial infection (Ip et al. 1993; Lemaitre et al. 1995a; Petersen et al. 1995). Although the induction of immunity genes is normal in dorsal mutants (Lemaitre et al. 1995a, 1996), molecular and biochemical observations raise the possibility that the two factors perform redundant functions during the immune response. Therefore, genetic approach should help to differentiate the roles of these two regulators in vivo.
Figure 1.
Restriction map and intron–exon structure of the Dif–dorsal locus, which includes dorsal, Dif and C2. Bicaudal D is located ∼5 kb upstream of dorsal (Wharton and Struhl 1989). Arrows indicate the start sites and directions of transcription, except the arrow on Bicaudal D shows only the direction of transcription. The exons are represented by rectangular boxes; the introns are represented by the angled lines. All the known EcoRI restriction sites are shown; PstI, BamHI, and XbaI sites are shown only on the Dif and C2 genes. The EcoRI restriction map of the dorsal gene is duplicated from Steward (1987). P-element insertions are indicated by flags. The deletion in the J4 chromosome (broken line), uncovers the three genes but does not affect Bicaudal D.
We employed the strategy of local P element hopping (Tower et al. 1993) to try to isolate insertional mutations of Dif. Because the Dif gene is located close to dorsal, we used a P element strain (P01313) that is allelic to dorsal mutants (Berg and Spradling 1991) and has an insertion mapped to dorsal (Fig. 1). After screening >3000 lines, no placement of the P element into the Dif gene was obtained. Instead, we isolated a new insertion (P1522) into a neighboring transcription unit that we named C2 (Fig. 1). C2 is expressed in the CNS of the developing embryo and encodes a putative protein that has only short stretches of homology with a Saccharomyces cerevisiae gene and a Caenorhabditis elegans gene with no known function (data not shown). We further attempted to mobilize P1522, which is closer to Dif, to isolate an insertion in the Dif locus, but without success. Therefore, we isolated fly lines that might contain deletion in the region by imprecise excision of the P1522 P element. Using this approach, we obtained a deletion strain that we called J4. Molecular mapping by Southern blots using dorsal, Dif, C2, and Bicaudal D cDNA revealed that the J4 deletion uncovered a region extending from the promoter of dorsal to the promoter of C2 (Fig. 1). We surmise that the deletion might have arisen from the recombination of the P element located on the C2 promoter and some remnant P-element sequence from the parent P01313 line. Greater than 99% of the J4 homozygous flies do not survive to adulthood, although we have not tested whether the lethality is associated with the deletion or with other mutations on the chromosome. However, a few homozygous adult escapers can be found consistently. These survivors have no obvious morphological abnormality. We isolated RNA from the homozygous flies and performed Northern analysis. The results in Figure 2 demonstrated that no RNA expression of Dif, dorsal, or C2 was detected, consistent with the molecular mapping data that these three genes are deleted. Therefore, the J4 deletion represents a null mutation of both Dif and dorsal.
Figure 2.
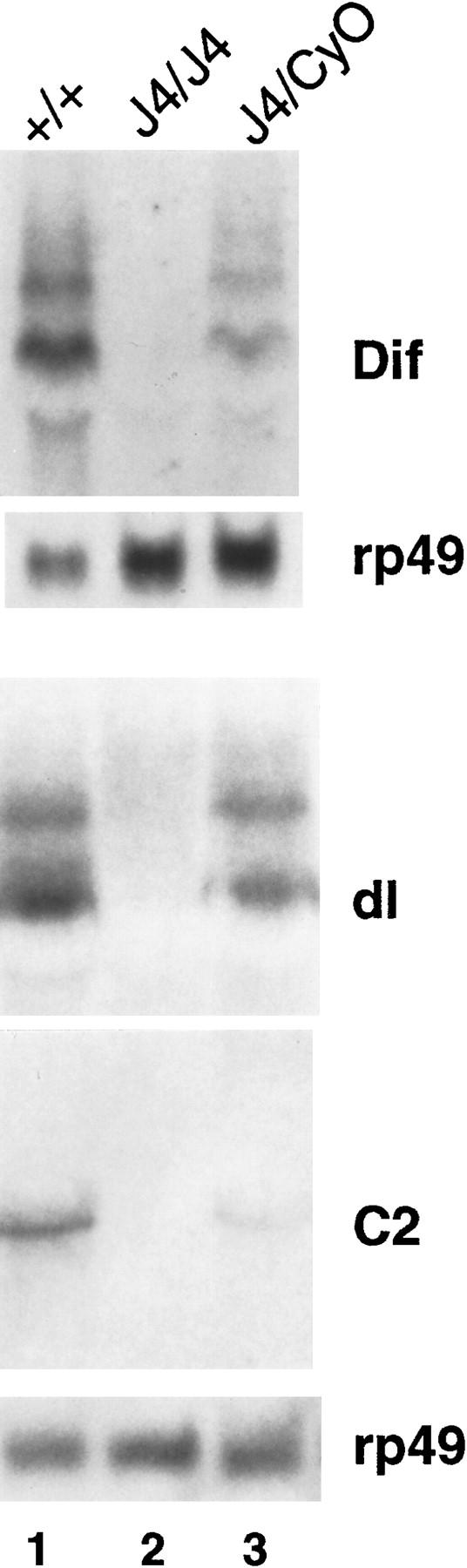
Northern analysis of the expression of the Dif–dorsal locus in the J4 deletion. Total RNAs were isolated from Canton-S wild-type flies (+/+ , lane 1), J4 homozygous flies (J4/J4, lane 2), or heterozygous J4 flies (J4/CyO, lane 3). RNAs were analyzed on denaturing gels, transferred to membranes, and hybridized with radiolabeled probes as indicated (right). (dl) dorsal. The ribosomal protein gene rp49 was used as a loading control.
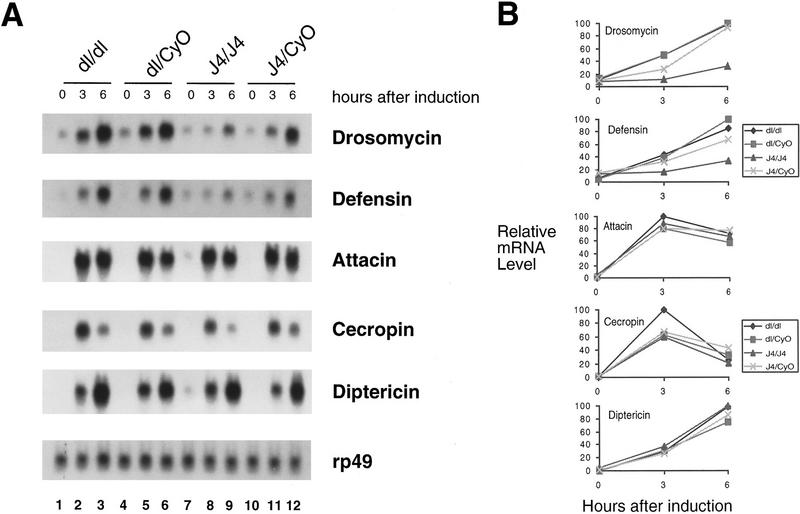
Homozygous J4 flies were collected and used for immunity gene induction experiments. The flies were injected with needles previously dipped into saturated Escherichia coli cultures. The infected flies were allowed to recover for 3 or 6 hr. RNAs were isolated and analyzed by Northern blots. We used five antimicrobial peptide gene cDNAs as probes to assess the induction of the endogenous genes in the infected flies. These genes exhibit different induction kinetics (Fig. 3) (Hoffmann et al. 1996). For instance, cecropin is induced to high levels at 3 hr and the level has declined after 6 hr. drosomycin expression, on the other hand, increases steadily at 3–6 hr after induction. In the parental dorsalP01313 mutant strain, the induction of all five genes were similar to that of wild-type flies, as reported previously (Lemaitre et al. 1995a, 1996; Petersen et al. 1995; also see Fig. 4). The J4 deletion, however, exhibited specific defects in the induction of drosomycin and defensin (Fig. 3A,B). In contrast, induction of cecropin, attacin, and diptericin is not affected in the J4 mutant. These results demonstrate that Dif is required specifically for the induction of only a subset of immunity genes. Although the dorsal mutation alone does not affect drosomycin and defensin induction, as J4 is a Dif dorsal double mutant the results presented so far cannot exclude the possibility that the two regulators have redundant functions.
Figure 3.
Induction of antimicrobial peptide genes in the Dif/dorsal deletion mutant. (A) Total RNAs were isolated from fly strains with the genotype indicated at top. The dorsal (dl) mutant used in this and other experiments was the parental P01313 line. Prior to RNA isolation, the flies were induced by injection of E. coli bacteria and allowed to recover for 3 or 6 hr. The 0-hour represents no injection. The isolated RNAs were analyzed by Northern blot and hybridized with radiolabeled probes indicated at right. Each panel utilized the same set of RNA isolated in parallel, but the times of exposure varied from 12 to 72 hr. This is a representative result of three independent experiments. (B) Quantitative analysis of gene induction. The hybridization signal of the experiments shown in A was quantitated using PhosphorImager (Molecular Dynamics). The relative mRNA levels, normalized with the rp49 signal, were plotted as shown. The value of 100 was assigned to the highest signals of the individual blots; the other signals were calculated as a fraction of this value.
Figure 4.
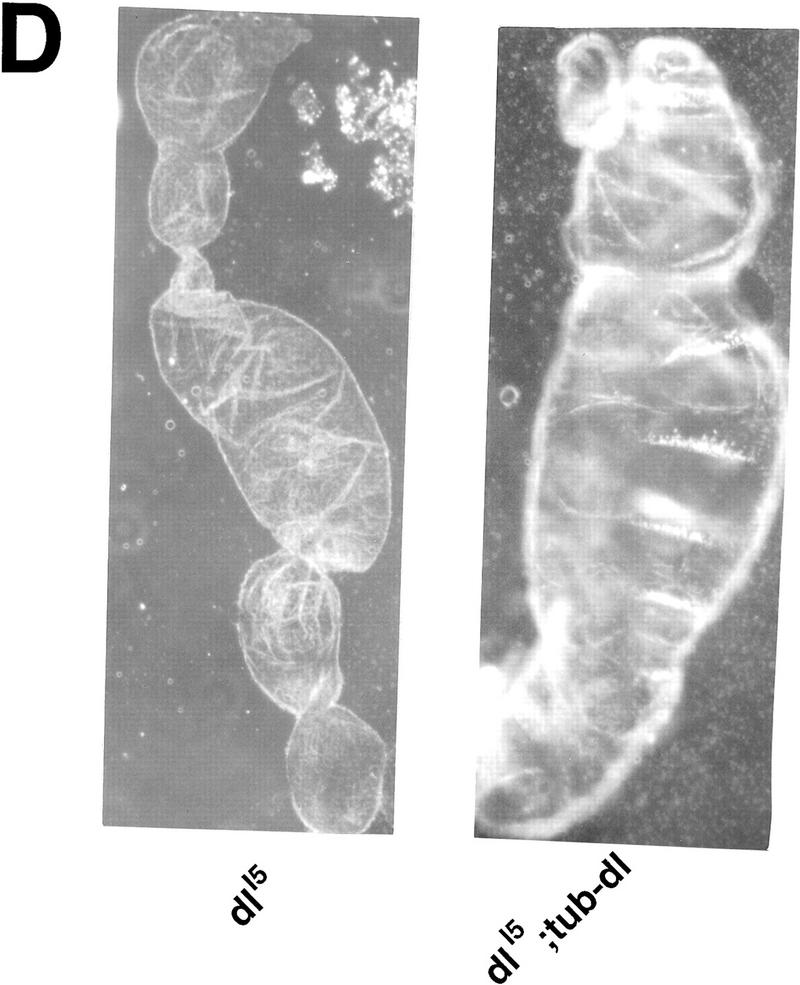
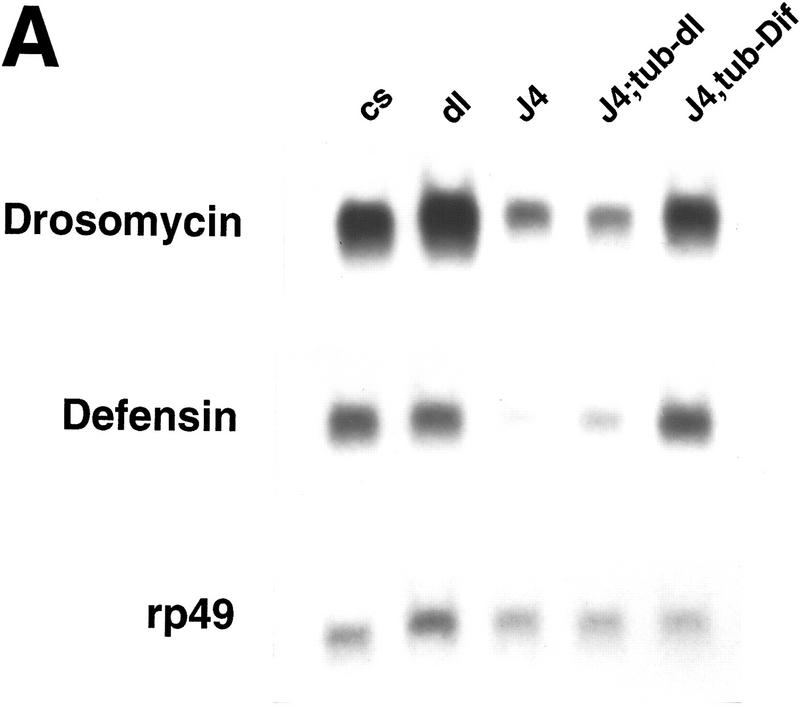
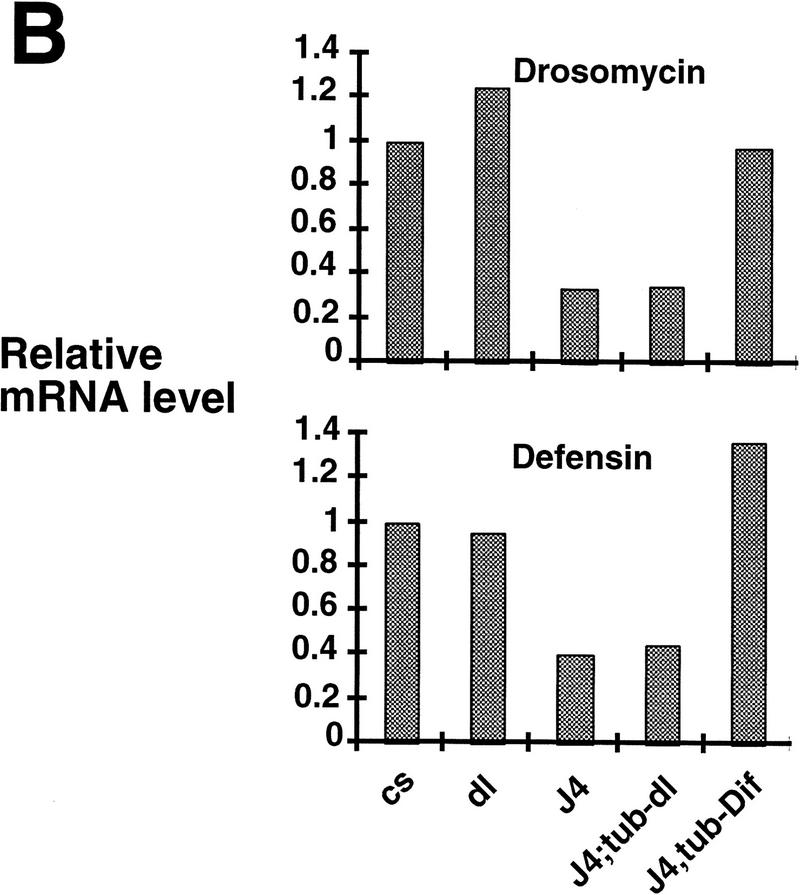
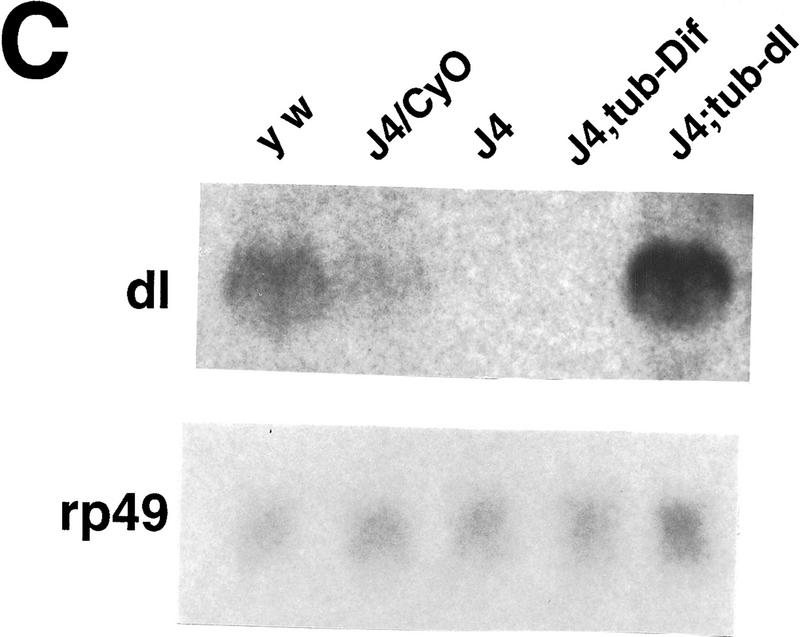
Ubiquitous expression of Dif rescues the defect of immune response. (A) Transgenic flies containing the tubulinα1–dorsal or -Dif constructs were crossed or recombined, respectively, with the J4 chromosome. Flies that had the genotypes indicated were induced by bacterial injection and after 6 hr the RNA were isolated for Northern analysis. The autoradiographs of the blots hybridized with the indicated probes are shown. (B) The blots were quantitated using PhosphorImager, and the drosomycin and defensin signals were normalized with that of rp49. The relative levels of mRNA expression were plotted, with the Canton S (cs) wild-type fly expression levels assigned as 1. The graphs show the average result of two independent experiments. (C) Control experiments were carried out using the transgenic lines to examine the expression of dorsal in adult flies. The tubulin–dorsal transgene drives the expression of dorsal mRNA to a level higher than that in the parental y w flies. (D) The severe cuticle phenotype exhibited in embryos derived from dorsalI5 mutant mothers (left) could be rescued by the tubulin-dorsal transgene (right), demonstrating that the transgene can produce functional Dorsal proteins.
To distinguish the roles of Dif and dorsal in the immune process, we performed a genetic rescue experiment. Rescue plasmids that utilized the tubulinα1 promoter (Basler and Struhl 1994) to direct a ubiquitous expression of Dif or dorsal were constructed. Transgenic flies were obtained for both constructs, and the chromosomal locations of the transgenes were determined by standard genetic crosses. The chromosomes that contained the rescue transgenes were crossed or recombined together with the J4 chromosome. Stable lines were established, and the induction of drosomycin and defensin was examined. The results demonstrate that Dif can rescue the induction of the antimicrobial peptide genes, whereas dorsal cannot (Fig. 4A,B). We have analyzed the RNA expression of the transgenes, and the results show that tubulin–dorsal is expressed to a level higher than the endogenous dorsal (Fig. 4C). To ascertain that the lack of rescue by dorsal was not due to the production of a nonfunctional protein, we crossed the tubulin–dorsal transgene into a well-characterized dorsal mutant background to test for the function. The dorsalI5 allele is a severe mutation, and embryos derived from homozygous mothers exhibit completely dorsalized cuticle phenotype (Fig. 4D, left). The homozygous mothers that also carried the tubulin–dorsal transgene, on the other hand, produced embryos that had well defined dorsoventral polarity and ventral denticle belts (Fig. 4D, right). The tubulin–Dif transgene expressed a relatively lower level of mRNA in adults and could nonetheless rescue the embryonic dorsoventral developmental defect (data not shown). Therefore, we conclude that the lack of rescue of the immune response by tubulin–dorsal is not due to the absence of expression of functional dorsal protein. Instead, it indicates that Dif is sufficient to mediate the induction of drosomycin and defensin, and that dorsal cannot substitute the role of Dif.
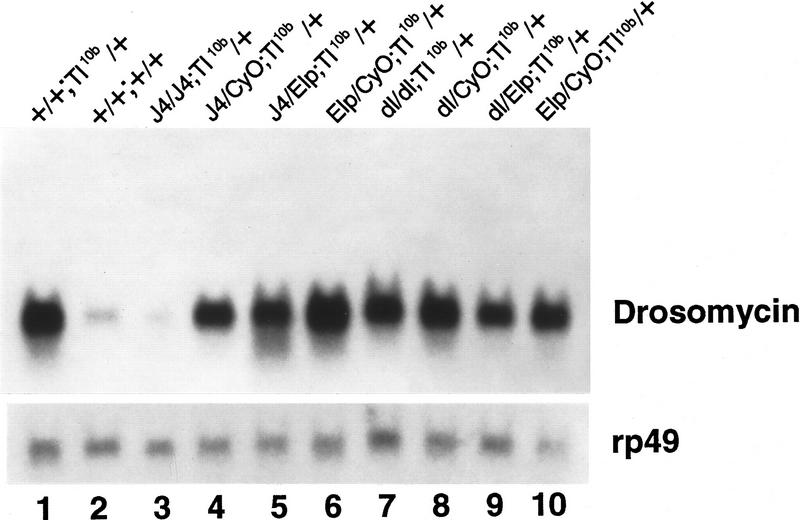
Previous experiments demonstrated that in the gain-of-function Toll10b mutant background, drosomycin is constitutively expressed at high levels, suggesting that the Toll signaling pathway regulates the antifungal gene expression during infection (Lemaitre et al. 1996). This notion was supported further by the analyses of loss-of-function mutants of the Toll signaling pathway components (Lemaitre et al. 1996; Nicolas et al. 1998). Although Dorsal functions downstream of Toll during dorsoventral patterning in the early embryo, it is not clear which of the NF-κB-related proteins are employed in the Toll mediated immune response (Petersen et al. 1995; Gross et al. 1996; Lemaitre et al. 1996;). We carried out a genetic experiment to test whether Dif acts downstream of Toll in regulating drosomycin gene expression. The J4 and dorsal loss-of-function mutants were crossed with the Toll10b gain-of-function mutant. The flies that contained different combinations of marker chromosomes were collected and analyzed for the expression of drosomycin (Fig. 5). In wild-type flies, drosomycin was expressed at a basal level (lane 2), and the expression was much elevated in the Toll10b flies (lane 1). This Toll10b activated expression of drosomycin was clearly suppressed by the homozygous J4 chromosome (lane 3). Other mutants or marker chromosomes in the Toll10b background did not cause any significant decrease of the constitutive, high-level expression of drosomycin. Because the dorsal mutation itself cannot suppress the Toll10b effect (lane 7), the results demonstrate that Dif is an essential component of the Toll signaling pathway in the induction of drosomycin. We have not, however, ruled out the possibility that dorsal can replace the function of Dif in Toll signaling because of the double deletion in the J4 chromosome. Nevertheless, in consideration of the rescue experiments presented above, there is no indication that Dorsal performs essential function downstream of Toll during the immune response.
Figure 5.
Dif is a downstream component of the Toll signaling pathway. Toll10b gain-of-function mutant male flies were mated with yw; BcElp/CyO; Ki/TM6y+ double balancer flies. Similarly, the J4/CyO or dl01313/CyO flies were mated with double balancer flies. F1 flies with the correct markers were mated together to generate the F2 flies (genotype shown above lanes 3–10). The flies were used directly without bacterial injection for RNA isolation and Northern analysis. An autoradiograph after hybridization with the drosomycin probe is shown.
Discussion
The presented results demonstrate that Dif is an essential component of the insect immune response. These results corroborate with recent evidence suggesting that diverse species utilize similar molecules to combat microbial infection (Hoffmann et al. 1996; Wilson et al. 1997; Han et al. 1998; Ip and Davis 1998; Wu and Anderson 1998). Toll and related proteins are present in humans, fruit flies, and tobacco and have been shown to be involved in transmitting signals provoked by infection (Whitham et al. 1994; Lemaitre et al. 1996; Medzhitov et al. 1997; Parker et al. 1997; Williams et al. 1997; Chaudhary et al. 1998; Rock et al. 1998; Yang et al. 1998). Previous reports revealed that in Drosophila the Toll pathway is essential for the induction of the antifungal peptide gene drosomycin and may participate in the induction of some other antibacterial peptide genes (Lemaitre et al. 1996; Wu and Anderson 1998). However, the transcription factor that mediates the Toll response has not been identified unambiguously. Our genetic experiments demonstrate that Dif acts downstream in the Toll signaling pathway for the induction of drosomycin. Furthermore, loss-of-function of Dif causes an impairment in the induction of drosomycin as well as defensin. However, defensin expression is not significantly elevated in the Toll10b mutant (Lemaitre et al. 1996; data not shown). It may be that the activation of Dif is necessary but not sufficient to induce defensin. Such induction may also require other factors that are activated by normal immune challenge but are independent of the Toll pathway.
Earlier results and our experiments do not suggest an essential function of dorsal during immune response. However, it is possible that Dif/Dorsal heterodimer can mediate some in vivo regulation. It is also plausible that some immunity genes that are yet to be identified require dorsal. Because the induction of at least three other antibacterial peptide genes, that is, diptericin, cecropin, and attacin, is not affected in the Dif dorsal double mutant, this indicates that some other NF-κB-related factors, such as Relish, may be involved in regulating the repertoire of immunity genes that contain the κB element (Lemaitre et al. 1995a, 1996; Petersen et al. 1995; Dushay et al. 1996; Gross et al. 1996). Gene knockout experiments in mice revealed that during immune response the mice bearing individual loss-of-function mutations of NF-κB show different defects (Baeuerle and Baltimore 1996; Attar et al. 1997). Our results with Drosophila are reminiscent of these specific requirements of the NF-κB factors in different aspects of self-defense response.
A previous report demonstrated that 18-Wheeler (18W), a member of the Toll and IL-1R family, has a critical function in the Drosophila immune response (Williams et al. 1997). In 18w mutants, the nuclear localization of Dif is blocked, whereas that of dorsal is normal. Furthermore, attacin expression is significantly affected, but that of diptericin is normal. These results suggest that 18W activates Dif, which in turn mediates the induction of attacin. Mutational analysis reported here, however, shows that the attacin induction is not affected in the absence of Dif. It is possible that Dif is only one of the components that functions downstream of 18W. Other members of the family, such as Relish, may be able to substitute Dif in the activation of attacin.
Because Dif localization was not affected in Toll loss-of-function mutations, it was proposed that Toll utilized Dorsal,wherease 18W utilized Dif to mediate some aspects of the immune response (Wu and Anderson 1998). The results presented here suggest that Dif function is required for the activated Toll receptor to induce drosomycin gene expression. Moreover, it has been demonstrated that in Toll10b larvae there are elevated levels of nuclear and cytoplasmic Dif in the fat bodies (Ip et al. 1993). Therefore, at least under some circumstances, Toll utilizes Dif, or Dif/Rel heterodimer where Rel is any other member of the family, as the transcription factor. It is possible that both 18W and Toll can modulate Dif activity, which explains the results that in Toll mutants Dif can translocate to nucleus, as 18W responses to the immune challenge. On the other hand, in 18w mutants, there may still be residual nuclear Dif activity (Williams et al. 1997), probably due to the activation of Toll.
In addition to Toll and 18W, other components may be used to regulate the activity of NF-κB proteins in Drosophila, as genetic screens have identified a number of mutations that are essential for the activation of diptericin and for the regulation of NF-κB proteins (Lemaitre et al. 1995b; Wu and Anderson 1998). These mutations constitute multiple pathways, which differentially regulate the nuclear localization of Dif and Dorsal. Taken together, the data suggest that although some components may have critical roles in regulating specific immunity genes, such as the control of drosomycin by the Toll/Dif pathway, a cross-regulatory network is likely present in inducing the expression of multiple immunity genes.
Materials and methods
Drosophila genetics
Flies were kept in standard yeast/agar/molasses/cornmeal medium. For P element mobilization, the female flies with the P element insertion ry+ were mated with male flies with the genotype w; +/+; TM3,Sb,Δ2-3/Ubx,Δ2-3. Male flies of the F1 generation were mated with BcElp/CyO; ry female balancer flies. F2 males that did not carry Δ2-3 marker chromosomes but expressed ry+ and CyO markers were collected and mated batch-wise with the same balancer flies. After 3–5 days, the male flies were separated and used for DNA isolation and inverse PCR reaction to determine the insertion into the Dif/C2 locus. Individual males of the F3 generation were used to mate with balancer flies to establish stocks and were rescreened for insertion. The imprecise excision of the P element was performed similarly, except that individual ry flies were collected after exposure to Δ2-3. DNAs isolated from the established lines were used for genomic Southern analysis to determine the integrity of the Dif/dl locus. Cuticle preparations were carried out by first collecting embryos that were aged for ∼24 hr. The embryos were dechorionated and incubated in 85% lactic acid at 70°C for 12 hr. Cuticle preparations were photographed using dark-field microscopy.
Molecular analysis
For genomic DNA isolation, ∼25 flies were homogenized with a handheld motorized pestle. The homogenates were extracted with phenol–chloroform organic solvents and precipitated with ethanol. The genomic DNAs were digested with appropriate restriction enzymes for Southern blot analysis or digested with restriction enzymes and then self-ligated for inverse PCR reaction. For RNA isolation, ∼25 flies were homogenized in a buffer containing 50 mm sodium acetate (pH 5.2), 10 mm EDTA, and 1% SDS. Equal volumes of phenol equilibrated with 50 mm sodium acetate (pH 5.2) and 10 mm EDTA were added, and the mixed homogenates were incubated at 65°C for 5 min. The phenol extraction and 65°C incubation were repeated once, and the samples were extracted with phenol–chloroform and precipitated with ethanol. About 40 mg of total RNA was analyzed by formaldehyde–agarose gel electrophoresis. The separated RNAs were blotted onto GeneScreen Plus membrane (NEN) and hybridized with radiolabeled probes as described previously (Ip et al. 1993).
The construction of Dif and dorsal rescue plasmids was performed by PCR amplification of the corresponding cDNA. The PCR products were digested with KpnI and XbaI; these restriction enzyme sites were introduced through the PCR primers. The digested products were then purified and cloned into the pCaSpeR–tubulinα1 vector digested with the same restriction enzymes. The vector contains ∼2.6 kb of the tubulina1 promoter, and an SV40 3′ sequence (Basler and Struhl 1994). The rescue plasmids were injected into embryos of y w flies together with the Δ2-3 helper plasmid to generate germ-line transformants.
Acknowledgments
We thank Roger Davis and Li Zeng for helpful comments on the manuscript. We are indebted to Christine Rushlow for providing part of the Dif genomic sequence, Allan Spradling for the P-element lines, Jin Jiang, Robin Wharton, Dan Hultmark, and Jules Hoffmann for plasmid DNA, and Ruth Steward and Shubha Govind for communicating unpublished results. This work was supported by a grant from the National Institutes of Health (GM53269) and by a Scholar Award of the Leukemia Society of America (to Y.T.I.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Tony.Ip@ummed.edu; FAX (508) 856-4289.
References
- Attar RM, Caamano J, Carrasco D, Iotsova V, Ishikawa H, Ryseck RP, Weih F, Bravo R. Genetic approaches to study Rel/NF-kappaB/I kappaB function in mice. Semin Cancer Biol. 1997;8:93–101. doi: 10.1006/scbi.1997.0060. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-κB: Ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Barillas-Mury C, Charlesworth A, Gross I, Richman A, Hoffmann J A, Kafatos F C. Immune factor Gambif1, a new rel family member from the human malaria vector, Anopheles gambiae. EMBO J. 1996;15:4691–4701. [PMC free article] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Berg CA, Spradling AC. Studies on the rate and site-specificity of P element transposition. Genetics. 1991;127:515–524. doi: 10.1093/genetics/127.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary PM, Ferguson C, Nguyen V, Nguyen O, Massa HF, Eby M, Jasmin A, Trask BJ, Hood L, Nelson PS. Cloning and characterization of two Toll/Interleukin-1 receptor-like genes TIL3 and TIL4: Evidence for a multi-gene receptor family in humans. Blood. 1998;91:4020–4027. [PubMed] [Google Scholar]
- Drier EA, Steward R. The dorsoventral signal transduction pathway and the Rel-like transcription factors in Drosophila. Semin Cancer Biol. 1997;8:83–92. doi: 10.1006/scbi.1997.0059. [DOI] [PubMed] [Google Scholar]
- Dushay MS, Asling B, Hultmark D. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc Natl Acad Sci. 1996;93:10343–10347. doi: 10.1073/pnas.93.19.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom Y, Kadalayil L, Sun S-C, Samakovlis C, Hultmark D, Faye I. κB-like motifs regulate the induction of immune genes in Drosophila. J Mol Biol. 1993;232:327–333. doi: 10.1006/jmbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- Gross I, Georgel P, Kappler C, Reichhart JM, Hoffmann JA. Drosophila immunity: A comparative analysis of the Rel proteins dorsal and Dif in the induction of the genes encoding diptericin and cecropin. Nucleic Acids Res. 1996;24:1238–1245. doi: 10.1093/nar/24.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZS, Enslen H, Hu X, Meng X, Wu I-H, Barrett T, Davis RJ, Ip YT. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol Cell Biol. 1998;18:3527–3539. doi: 10.1128/mcb.18.6.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart J-M, Hetru C. Innate immunity in higher insects. Curr Opin Immunol. 1996;8:8–13. doi: 10.1016/s0952-7915(96)80098-7. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Immune reactions in Drosophila and other insects: A model for innate immunity. Trends Genet. 1993;9:178–193. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun NH2-terminal kinase (JNK)–from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Ip YT, Reach M, Engstrom Y, Kadalayil L, Cai H, Gonzalez-Crespo S, Tatei K, Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75:753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Kappler C, Meister M, Lagueux M, Gateff E, Hoffmann JA, Reichhart J-M. Insect immunity. Two 17 bp repeats nesting a ?B-related sequence confer inducibility to the diptericin gene and bind a polypeptide in bacteria-challenged Drosophila. EMBO J. 1993;12:1561–1568. doi: 10.1002/j.1460-2075.1993.tb05800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Meister M, Govind S, Georgel P, Steward R, Reichhart J-M, Hoffmann JA. Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J. 1995a;14:536–545. doi: 10.1002/j.1460-2075.1995.tb07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci. 1995b;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Liu KD, Gaffen SL, Goldsmith MA. JAK/STAT signaling by cytokine receptors. Curr Opin Immunol. 1998;10:271–278. doi: 10.1016/s0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B, Perrimon N. Mammalian and Drosophila blood: JAK of all trades? Cell. 1998;92:697–700. doi: 10.1016/s0092-8674(00)81396-3. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Nicolas E, Reichhart J-M, Hoffmann JA, Lemaitre B. In vivo regulation of the I kappa B homologue cactus during the immune repsone of Drosophila. J Biol Chem. 1998;273:10463–10469. doi: 10.1074/jbc.273.17.10463. [DOI] [PubMed] [Google Scholar]
- Parker JE, Coleman MJ, Szabo V, Frost LN, Schmid R, van der Biezen EA, Moores T, Dean C, Daniels MJ, Jones JD. The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the Toll and interleukin receptors with N and L6. Plant Cell. 1997;9:879–894. doi: 10.1105/tpc.9.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen U-M, Bjorklund G, Ip YT, Engstrom Y. The dorsal-related immunity factor, Dif, is a sequence-specific trans-activator of Drosophila Cecropin gene expression. EMBO J. 1995;14:3146–3158. doi: 10.1002/j.1460-2075.1995.tb07317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P, Uknes S. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor IκB. Plant Cell. 1997;9:425–439. doi: 10.1105/tpc.9.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D, Goltz JS, Jurcsak J, Stevens L. The dorsal-related immunity factor (Dif) can define the dorsal-ventral axis of polarity in the Drosophila embryo. Development. 1998;125:2159–2169. doi: 10.1242/dev.125.11.2159. [DOI] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogen, c-rel. Science. 1987;238:692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Steward R, McNally FJ, Schedl P. Isolation of the dorsal locus of Drosophila. Nature. 1984;311:262–265. doi: 10.1038/311262a0. [DOI] [PubMed] [Google Scholar]
- Sun S-C, Faye I. Cecropia immunoresponsive factor, an insect immunoresponsive factor with DNA binding properties similar to nuclear factor κB. Eur J Biochem. 1992;204:885–892. doi: 10.1111/j.1432-1033.1992.tb16708.x. [DOI] [PubMed] [Google Scholar]
- Tower J, Karpen GH, Craig N, Spradling AC. Preferential transposition of Drosophila P-elements to nearby chromosome sites. Genetics. 1993;133:347–359. doi: 10.1093/genetics/133.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: Intimate tales of association and dissociation. Genes & Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Wharton RP, Struhl G. Structure of the Drosophila BicaudalD protein and its role in localizing the the posterior determinant nanos. Cell. 1989;59:881–892. doi: 10.1016/0092-8674(89)90611-9. [DOI] [PubMed] [Google Scholar]
- Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B. The product of the tobacco mosaic virus resistance gene N: Similarity to toll and the interleukin-1 receptor. Cell. 1994;78:1101–1115. doi: 10.1016/0092-8674(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Rodriguez A, Kimbrell DA, Eldon ED. The 18-wheeler mutation reveals complex antibacterial gene regulation in Drosophila host defense. EMBO J. 1997;16:6120–6130. doi: 10.1093/emboj/16.20.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I, Vogel J, Somerville S. Signalling pathways: a common theme in plants and animals? Curr Biol. 1997;7:R175–R178. doi: 10.1016/s0960-9822(97)70082-4. [DOI] [PubMed] [Google Scholar]
- Wu LP, Anderson KV. Related signaling networks in Drosophila that control dorsoventral patterning in the embryo and the immune response. Cold Spring Harb Symp Quant Biol. 1997;62:97–103. [PubMed] [Google Scholar]
- ————— Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature. 1998;392:93–97. doi: 10.1038/32195. [DOI] [PubMed] [Google Scholar]
- Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]