Abstract
Objective
To assess adherence to community-based directly observed treatment (DOT) among Tanzanian tuberculosis patients using the Medication Event Monitoring System (MEMS) and to validate alternative adherence measures for resource-limited settings using MEMS as a gold standard.
Methods
This was a longitudinal pilot study of 50 patients recruited consecutively from one rural hospital, one urban hospital and two urban health centres. Treatment adherence was monitored with MEMS and the validity of the following adherence measures was assessed: isoniazid urine test, urine colour test, Morisky scale, Brief Medication Questionnaire, adapted AIDS Clinical Trials Group (ACTG) adherence questionnaire, pill counts and medication refill visits.
Findings
The mean adherence rate in the study population was 96.3% (standard deviation, SD: 7.7). Adherence was less than 100% in 70% of the patients, less than 95% in 21% of them, and less than 80% in 2%. The ACTG adherence questionnaire and urine colour test had the highest sensitivities but lowest specificities. The Morisky scale and refill visits had the highest specificities but lowest sensitivities. Pill counts and refill visits combined, used in routine practice, yielded moderate sensitivity and specificity, but sensitivity improved when the ACTG adherence questionnaire was added.
Conclusion
Patients on community-based DOT showed good adherence in this study. The combination of pill counts, refill visits and the ACTG adherence questionnaire could be used to monitor adherence in settings where MEMS is not affordable. The findings with regard to adherence and to the validity of simple adherence measures should be confirmed in larger populations with wider variability in adherence rates.
ملخص
الغرض
قياس الامتثال للمعالجة المجتمعية المرتكز تحت الإشراف المباشر بين مرضى السل في تنزانيا باستخدام نظام مراقبة حدث المداواة، وتوثيق مصدوقية التدابير البديلة للامتثال للعلاج في المواقع المحدودة الموارد باستخدام نظام مراقبة حدث المداواة كمعيار مثالي.
الطريقة
أجريت هذه الدراسة الطولانية الارتيادية على 50 مريضاً تم إدراجهم بالتوالي من أحد المستشفيات الريفية، وأحد المستشفيات الحضرية، ومن مركزين صحيين حضريين. وجرى مراقبة امتثالهم للعلاج عن طريق نظام مراقبة حدث المداواة كما قيس مصدوقية التدابير التالية لامتثالهم للعلاج: اختبار الأيزونيازيد البولي، واختبار لون البول، وحرز موريسكي Morisky، واستبيان موجز عن المداواة جرى تكييفه من مجموعة التجارب السريرية عن الإيدز، واستبيان عن الامتثال للعلاج، وتعداد حبوب الدواء، وزيارات إعادة الإمداد بالدواء.
النتائج
كان معدل الامتثال للعلاج بين المُدرَجين في الدراسة 96.3% (والانحراف المعياري: 7.7). وكان الامتثال للعلاج أقل من 100% في 70% من المرضى، وأقل من 95% في 21% منهم، وأقل من 80% في 2%. وكان الاستبيان الخاص بمجموعة التجارب السريرية المُكيَّفة عن الإيدز واختبار لون البول هما الأعلى من حيث الحساسية ولكن الأقل في النوعية. وكان حرز موريسكي وزيارات إعادة الإمداد بالدواء هما الأعلى من حيث النوعية ولكن الأقل في الحسياسية. وقد أدى دمج عدد حبوب الدواء مع زيارات إعادة الإمداد بالدواء، كما هو مستخدم في الممارسة الروتينية، إلى حساسية ونوعية متوسطتين، وقد تحسنت الحساسية عند إضافة استبيان الامتثال الخاص بمجموعة التجارب السريرية عن الإيدز.
الاستنتاج
أظهر المرضى الخاضعون للعلاج القصير الأمد تحت الإشراف المباشر المجتمعي المرتكز في هذه الدراسة امتثالاً جيداً للعلاج. ويمكن استخدام مزيج من عدد حبوب العلاج، وزيارات إعادة الإمداد بالدواء، واستبيان الامتثال الخاص بمجموعة التجارب السريرية عن الإيدز لمراقبة الامتثال للعلاج في المواقع التي لا يتيسر فيها وجود نظام مراقبة حدث المداواة. ويجب التأكد من النتائج الخاصة بالامتثال وتوثيق مصداقية تدابير الامتثال البسيطة على نطاق أوسع من السكان لهم نطاق أوسع من التغيّر في معدلات الامتثال للعلاج.
Resumen
Objetivo
Evaluar el cumplimiento de los tratamientos observados directamente que están dirigidos a la comunidad por parte de los pacientes con tuberculosis en Tanzania, mediante el Sistema de vigilancia de la medicación (Medication Event Monitoring System [MEMS]) y validar medidas alternativas de cumplimiento para los entornos de recursos limitados, empleando los MEMS como método de referencia.
Métodos
Se realizó un estudio piloto longitudinal con 50 pacientes seleccionados consecutivamente de un hospital rural, un hospital urbano y dos centros sanitarios urbanos. El cumplimiento terapéutico se controló con el MEMS y se evaluó la validez de las siguientes medidas de cumplimiento: detección de isoniacida en orina, prueba de color de la orina, test de Morisky, Cuestionario breve de medicación, cuestionario adaptado del cumplimiento terapéutico del Grupo de Ensayos Clínicos sobre el SIDA (ACTG), recuento de la medicación y visitas de aprovisionamiento de medicamentos.
Resultados
La tasa media de cumplimiento en la población del estudio fue de un 96,3% (desviación estándar, DE: 7,7). El cumplimiento fue inferior al 100% en el 70% de los pacientes, inferior al 95% en el 21% de los pacientes e inferior al 80% en el 2% de los pacientes. El cuestionario de cumplimiento ACTG y la prueba de color de la orina registraron los niveles más elevados de sensibilidad y los más bajos de especificidad. El test Morisky y las visitas de aprovisionamiento de medicamentos obtuvieron los niveles más elevados de especificidad y los más bajos de sensibilidad. La combinación del recuento de medicamentos y las visitas de aprovisionamiento, empleada en la práctica habitual, registró una sensibilidad y una especificidad moderadas, si bien la sensibilidad aumentó cuando se añadió el cuestionario de cumplimiento ACTG.
Conclusión
Los pacientes que siguieron un tratamiento observado directamente y dirigido a la comunidad mostraron un cumplimiento correcto en este estudio. La combinación del recuento de medicación, las visitas de aprovisionamiento de medicamentos y el cuestionario de cumplimiento ACTG podría emplearse para controlar el cumplimiento en entornos en los que el uso del sistema MEMS no resulte viable económicamente. Los resultados en cuanto al cumplimiento y a la validez de las medidas sencillas de cumplimiento podrían confirmarse en poblaciones más amplias con una mayor variabilidad de sus tasas de cumplimiento.
Résumé
Objectif
Évaluer l’adhésion au traitement directement observé en milieu communautaire des patients tuberculeux tanzaniens, à l’aide du système de suivi des événements de médication (MEMS, Medication Event Monitoring System) et valider les mesures d’adhésion alternatives dans les configurations de ressources limitées utilisant le MEMS comme critère de référence.
Méthodes
Il s’agissait d’une étude pilote longitudinale sur 50 patients recrutés consécutivement dans un hôpital rural, un hôpital urbain et deux centres de soins urbains. L’adhésion au traitement a été contrôlée par le MEMS et la validité des mesures d’adhésion conséquentes a été évaluée: test urinaire pour détecter la présence d’isoniazide, test de coloration des urines, échelle de Morisky, bref questionnaire sur la médication, questionnaire d’adhésion adapté du Groupe d’essais cliniques du SIDA (ACTG, AIDS Clinical Trials Group), décomptes de pilules et visites de réapprovisionnement en médicaments.
Résultats
Le taux d’adhésion moyen dans la population de l’étude atteignait 96,3% (déviation standard, DS: 7,7). L’adhésion était inférieure à 100% chez 70% des patients, inférieure à 95% chez 21% d’entre eux et inférieure à 80% chez 2% d’entre eux. Le questionnaire d’adhésion de l’ACTG et le test de coloration des urines présentaient les sensibilités les plus élevées, mais les spécificités les plus basses. L’échelle de Morisky et les visites de réapprovisionnement présentaient les spécificités les plus élevées, mais les sensibilités les plus basses. Les décomptes de pilules et les visites de réapprovisionnement en médicaments combinés, utilisés dans la pratique de routine, montraient une sensibilité et une spécificité modérées, mais la sensibilité s’améliorait lors de l’ajout du questionnaire d’adhésion de l’ACTG.
Conclusion
Les patients suivant un traitement directement observé en milieu communautaire ont montré une meilleure adhésion dans cette étude. La combinaison des décomptes de pilules, des visites de réapprovisionnement et du questionnaire d’adhésion de l’ACTG a pu être utilisée pour contrôler l'adhésion dans les cadres où le MEMS n’était pas abordable. Les résultats en termes d’adhésion et de validité des mesures d’adhésion simples devraient être confirmés auprès de populations plus larges, avec une variabilité plus importante des taux d'adhésion.
Резюме
Цель
Оценить приверженность к лечению, проводившемуся на базе общины под непосредственным наблюдением врача, у туберкулезных больных в Танзании с применением Системы электронного мониторирования выдачи препарата (Medication Event Monitoring System, MEMS) и проверить действенность альтернативных мер по контролю приверженности в условиях ограниченности ресурсов, используя MEMS в качестве «золотого стандарта».
Методы
Проведено лонгитюдное пилотное исследование 50 пациентов, отобранных последовательно из сельской больницы, городской больницы и двух городских медицинских центров. Мониторинг приверженности к лечению осуществлялся с помощью MEMS; проводилась также проверка действенности следующих мер по контролю приверженности: анализа мочи при приеме изониазида, контроля цвета мочи, применения шкалы Morisky, Краткого опросника по лекарственным препаратам (Brief Medication Questionnaire, BMQ) и адаптированного варианта «Опросника по приверженности» Группы клинических испытаний по СПИДу (ACTG), а также подсчета таблеток и посещения больных с целью пополнения запаса лекарств.
Результаты
Средний показатель приверженности в исследуемой популяции составлял 96,3% (стандартное отклонение, СО: 7,7). У 70% больных показатель приверженности был ниже 100%, у 21% – ниже 95% и у 2% ниже 80%. При применении опросника ACTG и контроля цвета мочи достигалась максимальная чувствительность при минимальной специфичности. Применение шкалы Morisky и визитов для пополнения запаса лекарств давало максимальную специфичность при минимальной чувствительности. Использование в рутинной практике подсчета таблеток в сочетании с посещениями больных для пополнения запаса лекарств обеспечивали умеренную чувствительность и специфичность, однако при добавлении опросника ACTG чувствительность повышалась.
Вывод
В данном исследовании пациенты, проходившие лечение под непосредственным наблюдением врача (DOT) на уровне общины, продемонстрировали высокий уровень приверженности. Для отслеживания приверженности больных к лечению в условиях, когда применение MEMS недоступно по финансовым причинам, можно применять подсчет таблеток, посещение больных с целью пополнения запаса лекарств и «Опросник по приверженности» ACTG. Полученные результаты, касающиеся приверженности к лечению и действенности простых мер контроля приверженности больных к лечению, необходимо подтвердить на примере более крупных популяций при широком разбросе значений показателя приверженности.
摘要
目的
采用药物治疗活动监控系统(MEMS)评估坦桑尼亚肺结核病人对基于社区直接观察治疗的依从性,并以MEMS为金标准,对资源有限的背景下替代的依从性方法验证。
方法
这是一项在一所农村医院、一所城市医院以及两所城市健康中心连续招募的50位病人开展的纵向试点研究。采用MEMS监控治疗依从性,并评估了以下依从性方法的有效性:异烟肼尿试验、尿颜色试验、Morisky衡量标准、简要药物治疗问卷、经修订的艾滋病临床试验组(ACTG)依从性问卷、药丸数和药物治疗加药就诊数。
结果
研究人群中的平均依从率为96.3%(标准差,SD:7.7)。70%的病人依从率低于100%,21%的病人低于95%,2%的病人低于80%。ACTG依从性问卷和尿颜色试验的敏感性最高,但特异性最低。Morisky衡量标准和加药就诊数的特异性最高,但敏感性最低。常规诊疗药丸数和加药就诊数两者的混合使用敏感性和特异性中等,但添加ACTG依从问卷后,敏感性有所上升。
结论
本研究中基于社区的直接观察治疗(DOT)病人表现出良好的依从性。药丸数、加药就诊数和ACTG依从性问卷可结合用于在无法承担MEMS的情况下进行依从性监控。与依从性和简单依从性方法有效性相关的研究结果应在依从率变化性更大的、更广泛的人群中进行证实。
Introduction
Non-adherence to treatment for tuberculosis is a major barrier to global tuberculosis control. To ensure adherence to treatment by tuberculosis patients, the direct observation of treatment by a trained supervisor is recommended.1 Initially, such directly observed treatment (DOT) was provided in health-care facilities only, but because of workload demands, several countries have started to involve community members in the provision of DOT.2 Studies have shown that community-based DOT is a cost-effective strategy that yields treatment outcomes similar to those obtained with facility-based DOT.3–6 However, community-based DOT has been criticized for being beyond the control of health-care providers and hence conducive to self-administered (unsupervised) treatment and non-adherence.6,7
The actual degree of adherence by patients on community-based DOT has not yet been assessed. Measuring adherence is difficult because most available direct and indirect measures have limitations. Direct adherence measures, such as tests to measure drug levels in plasma or urine, cover brief medication intake periods only. Indirect measures, such as pill counts and self-report questionnaires, cover longer periods but assume rather than prove the patient’s actual medication intake.8
A sophisticated indirect adherence measure is the Medication Event Monitoring System (MEMS). MEMS medication bottles contain a microelectronic chip that registers the date and time of every bottle opening. Assuming that bottle openings represent medication intake, MEMS provides a detailed profile of the patient’s adherence behaviour. MEMS is currently regarded as the gold standard to measure adherence.8 It has been used as such in a wide range of studies on adherence to antihypertensive and lipid-lowering therapy,9,10 therapy for neurologic and psychiatric disorders,11,12 post-transplantation immunosuppressive therapy13,14 and antiretroviral therapy.15–17 Few studies report on the use of MEMS to monitor adherence to tuberculosis treatment.18–21 Because of the high cost involved, MEMS is not feasible for use in routine practice in most settings with a high tuberculosis burden but could be used as a reference standard to validate simple and affordable measures that can be used in patients on community-based DOT.8,21
In this pilot study, we used MEMS to: (i) describe adherence rates among Tanzanian tuberculosis patients on community-based DOT and (ii) determine the validity of several direct and indirect adherence measures of potential use in resource-limited settings.
Methods
Study setting
The study was conducted in the Kilimanjaro region of the United Republic of Tanzania, where the annual tuberculosis case notification rate is 178 per 100 000 population.6 The national tuberculosis programme empowers patients to choose between community- and facility-based DOT. Most patients opt for community-based DOT and those on facility-based DOT are mostly inpatients.6 Patients on community-based DOT have to select a treatment supporter from their community (usually a relative or spouse) who is instructed on how to provide daily DOT at home. Patients on community-based DOT are supposed to collect their medication once a week in the first two months of treatment and once every two weeks in the remaining four months. They should return medication blisters for pill counts and their clinic attendance is registered.22
Study design and procedures
This was a longitudinal pilot study in which treatment adherence among 50 patients on community-based DOT was monitored by MEMS throughout treatment. MEMS was used as a gold standard to validate several other adherence measures (single and in combinations) in this patient group. The adherence measures were selected for their applicability in the Tanzanian setting and included an isoniazid (INH) urine test, a urine colour test for rifampicin, the Brief Medication Questionnaire (BMQ), the Morisky scale, an adapted version of the AIDS Clinical Trials Group (ACTG) adherence questionnaire, pill counts and clinic attendance for medication refills.
The participants were recruited between February and May 2010 from one rural hospital, one urban hospital and two urban health centres. Considering the pilot nature of our study, we chose to enrol 50 patients only. Adult outpatients who consecutively presented at one of the study sites with newly diagnosed tuberculosis and who had chosen community-based DOT were eligible to participate. Eligible patients were informed about the study procedures by trained clinic staff and asked to sign an informed consent form. They were told that their tuberculosis medication would be provided in a medication bottle with a microelectronic chip that registers every bottle opening. They were asked not to use the MEMS bottle for other medication, to open it only to take out medication, and to bring the bottle to every medication refill visit. Patients were informed that they would be under routine treatment and care.
Drug dispensing nurses were responsible for providing patients with medication. Medication refill visits were conducted in accordance with routine practice, except that the medication blisters were cut into pieces to make them fit in the MEMS bottle. The nurses registered the dates of the patients’ clinic visits for medication refills and the number of tablets remaining at each visit. During the visits in weeks 4, 8, 12 and 16, the procedures deviated from routine practice. At these visits, patients submitted a sample of urine and were asked to fill out the BMQ and Morisky scale (in weeks 4, 8 and 12) or the ACTG adherence questionnaire (in week 16). At completion of treatment, the patients filled out a questionnaire about their experience with the use of MEMS bottles.
Treatment adherence measures
Medication Event Monitoring System
MEMS medication bottles (250-ml containers with a 38-mm MEMS 6 TrackCap, AARDEX Ltd, Sion, Switzerland) were used by all participants throughout treatment. MEMS data were used to calculate adherence rates (by dividing the number of days on which at least one bottle opening was registered by the total number of monitored days and multiplied by 100) and to differentiate between adherent and non-adherent patients for validation of the other adherence measures. For the latter purpose, commonly used adherence rate cut-off values of 100%, 95% and 80% were applied.8
Isoniazid urine test
The isoniazid urine test or IsoScreen test (GFC Diagnostics Ltd, Bicester, England) is based on the Arkansas method for the detection of INH in urine and supplied in a ready-to-use plastic testing device. The test was performed in accordance with the directions provided in the accompanying manual. The test result was negative when no colour change was observed after 5 minutes, positive when the colour changed to dark purple and equivocal when the colour turned green. Patients with at least one negative test were regarded as non-adherent.
Urine colour test
Prior to each INH urine test, urine colour was checked for the presence of rifampicin. Orange urine was scored as positive and yellow urine as negative. Patients with at least one negative test were categorized as non-adherent.
Morisky scale
The Morisky scale is a self-report adherence measure with four questions about common barriers to adherence.23 We classified as non-adherent all patients who answered “yes” to at least one of the four questions in at least one of the three repeated tests.
Brief Medication Questionnaire
The BMQ consists of three sections (“screens”) with questions about adherence behaviour, the experienced effects of treatment and other factors that could affect adherence. The screens were scored as described elsewhere.24 Patients with a total score of 1 or more in at least one of the three repeated tests were classified as non-adherent.
AIDS Clinical Trials Group adherence questionnaire
The adapted ACTG adherence questionnaire was developed for patients infected with the human immunodeficiency virus (HIV) who participate in clinical trials25 and is available at www.ghdonline.org/uploads/ACTG_Adherence_Baseline_Questionnaire.pdf (last accessed: 12 May 2011). Our adapted version consisted of three multiple choice items corresponding to sections B (social support), C (possible reasons for non-adherence) and D (adherence behaviour) of the original baseline questionnaire. We scored any answer other than “never” to the questions in sections C and D or less than “somewhat satisfied” to the questions in section B as positive. A positive score was regarded as indicative of non-adherence.
Refill visits and pill counts
The patients’ clinic attendance for medication refills was registered and remaining tablets were counted at every refill visit. Patients who delayed at least once for a medication refill visit and those who had an incorrect number of tablets remaining at least once were classified as non-adherent.
Data analysis
MEMS data were analysed by using Powerview software (AARDEX Ltd, Sion, Switzerland). Periods of “pocket dosing” (i.e. taking out medication for later use) that were identified by the MEMS use questionnaire were excluded from the analysis as non-monitored periods. Statistical analysis was performed in SPSS version 16.0 (SPSS Inc., Chicago, United States of America). Means are presented with standard deviation (SD) and medians with interquartile range (IQR). Means were compared by using the Student t-test. The sensitivity, specificity, positive and negative predictive values and accuracy of single and combined adherence measures were calculated by using MEMS as the gold standard. For combined measures, non-adherent patients were those who were classified as non-adherent by at least one of the single measures in the combination.
Ethical approval
The study was approved by the institutional review board of the Kilimanjaro Christian Medical Centre (Moshi, United Republic of Tanzania) and the National Institute for Medical Research (Dar es Salaam, United Republic of Tanzania).
Results
Patient characteristics and treatment outcomes
We enrolled 31 male and 19 female patients. Their characteristics are summarized in Table 1. Six of the 22 patients who were co-infected with HIV used antiretroviral medication and 14 were on co-trimoxazole prophylaxis. Although all patients were on community-based DOT, seven had no formal treatment supporter.
Table 1. Patient characteristics and treatment outcomes among participants (n = 50) in a study of adherence to tuberculosis treatment, United Republic of Tanzania, 2010.
| Characteristic | No. (%)a |
|---|---|
| Gender | |
| Females | 19 (38) |
| Males | 31 (62) |
| Mean age in years (SD) | 41.6 (14.0) |
| Education | |
| None | 2 (4) |
| Primary | 38 (76) |
| Secondary | 9 (18) |
| Advanced secondary | 1 (2) |
| Marital status | |
| Single | 14 (28) |
| Married | 23 (46) |
| Divorced | 9 (18) |
| Widow | 4 (8) |
| TB diagnosis | |
| Smear positive PTB | 16 (32) |
| Smear negative PTB | 25 (50) |
| EPTB | 9 (18) |
| Treatment clinic | |
| Urban | 44 (88) |
| Rural | 6 (12) |
| Transport to clinic | |
| Foot | 30 (60) |
| Public | 17 (34) |
| Private | 3 (6) |
| Treatment supporter | |
| None | 7 (14) |
| Spouse | 13 (26) |
| Relative | 27 (54) |
| Friend | 3 (6) |
| HIV status | |
| Positive | 22 (44) |
| Negative | 28 (56) |
| Treatment outcome | |
| Favourable | |
| Cured | 12 (24) |
| Treatment completed | 25b (50) |
| Unfavourable | |
| Died | 6 (12) |
| Defaulted | 3 (6) |
| Study drop-out | 4 (8) |
EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis; SD, standard deviation; TB, tuberculosis.
a All except for age, which is expressed as the mean and standard deviation.
b All had smear-negative PTB or EPTB.
Thirty-seven patients successfully completed treatment. Six patients died; all were HIV-positive. Three patients defaulted and four patients dropped out of the study (three were transferred to another region and one developed jaundice and his treatment had to be interrupted).
Treatment adherence according to MEMS
No MEMS data were available for three patients (one defaulter and two who died) because the medication bottle was not returned. For the other 47 patients, a total of 6871 treatment days were monitored by MEMS. On 194 monitored days the MEMS bottle was not opened; the median per patient was 2 days (IQR: 0–5). The mean adherence rate was 96.3% (SD: 7.7) and did not differ significantly between patients with and without a treatment supporter: 96.2% (SD: 8.2) and 97.1% (SD: 3.1), respectively (P = 0.79).
Adherences was less than 100% in 70% of all patients; less than 95% in 21% of them, and less than 80% in 2% (Table 2). Among the patients who completed the six-month treatment course, adherence was less than 100% in 73%, less than 95% in 19% and less than 80% in none, respectively.
Table 2. Median monitored treatment days and adherence rates, and proportions of patients who were less than 100%, 95% and 80% adherent,a as assessed by the Medication Event Monitoring System (MEMS), United Republic of Tanzania, 2010.
| Patientsa | Monitored days |
Adherence rate (%) |
< 100% adherent |
< 95% adherent |
< 80% adherent |
|---|---|---|---|---|---|
| Median (IQR)b | Median (IQR)b | No. (%) | No. (%) | No. (%) | |
| All (47) | 168 (138–172) | 98.4 (95.7–100) | 33 (70) | 10 (21) | 1 (2) |
| Completed treatment (37) | 169 (168–180.5) | 98.4 (95.7–100) | 27 (73) | 7 (19) | 0 (0) |
| Defaulters (2) | 40.0/113c | 50.0/100d | 1 (50) | 1 (50) | 1 (50) |
| Deaths (4) | 63 (29.5–104) | 98.4 (95.6–99.6) | 3 (75) | 1 (25) | 0 (0) |
| Study drop outs (4) | 33 (21–73) | 98.3 (88.4–100) | 2 (50) | 1 (25) | 0 (0) |
IQR, interquartile range.
a Only patients for whom MEMS data were available are included.
b All values given are medians and IQRs except for the values of the two defaulters.
c Number of monitored days for defaulter 1 and defaulter 2, respectively.
d Adherence rates of defaulter 1 and defaulter 2, respectively.
Monthly adherence rates were fairly constant. In the group of patients who completed treatment, the median monthly adherence rate was 100% and the mean monthly adherence rate varied between 95.4% (SD: 7.3) in month 6 and 98.5% (SD: 2.7) in month 3.
Validation of adherence measures
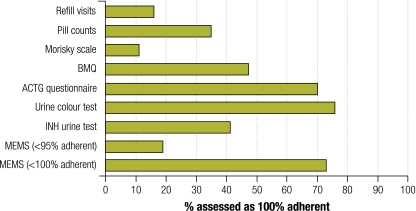
For the validation of the alternative adherence measures only patients who completed the six-month treatment course (n = 37) were included. As shown in Fig. 1, the proportions of non-adherent patients identified by the different measures varied widely. Table 3 shows the sensitivity, specificity, positive and negative predictive value and accuracy of the adherence measures in terms of their ability to differentiate between adherent and non-adherent patients. The ACTG adherence questionnaire and urine colour test had the highest sensitivities but lowest specificities. The Morisky scale and refill visits had the highest specificities but lowest sensitivities. The sensitivities of most measures improved when the cut-off value to differentiate between adherent and non-adherent patients was lowered from 100% to 95% adherence, but the specificities dropped. The positive and negative predictive values were almost reversed by changing the cut-off value from 100% to 95%, reflecting the large difference in the proportions of patients categorized as non-adherent by using the former versus the latter cut-off value. A cut-off value of 80% could not be applied because none of the patients who completed treatment was less than 80% adherent.
Fig. 1.
Non-adherence to tuberculosis treatment among patients who completed treatment (n = 37) as assessed by different adherence measures, United Republic of Tanzania, 2010
ACTG, AIDS Clinical Trials Group; BMQ, Brief Medication Questionnaire; INH, isoniazid; MEMS, Medication Event Monitoring System.
Table 3. Sensitivity, specificity, positive and negative predictive values, and accuracy of individual treatment adherence measures, alone and in combination, in a study of adherence to tuberculosis treatment, United Republic of Tanzania, 2010.
| Adherence measure | Sensitivitya |
Specificityb |
PPVc |
NPVd |
Accuracye |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEMS cut-offf |
MEMS cut-offf |
MEMS cut-offf |
MEMS cut-offf |
MEMS cut-offf |
||||||||||
| < 100% | < 95% | < 100% | < 95% | < 100% | < 95% | < 100% | < 95% | < 100% | < 95% | |||||
| INH urine test | 33 | 43 | 40 | 60 | 60 | 20 | 18 | 82 | 35 | 57 | ||||
| Urine colour test | 74 | 86 | 20 | 27 | 71 | 21 | 22 | 89 | 59 | 38 | ||||
| ACTG questionnaire | 70 | 100 | 30 | 37 | 27 | 27 | 73 | 100 | 60 | 49 | ||||
| BMQ | 54 | 57 | 70 | 55 | 82 | 24 | 37 | 84 | 58 | 56 | ||||
| Morisky scale | 15 | 14 | 100 | 90 | 100 | 25 | 30 | 82 | 38 | 76 | ||||
| Pill counts | 33 | 29 | 60 | 63 | 69 | 15 | 25 | 79 | 41 | 57 | ||||
| Refill visits | 22 | 14 | 100 | 83 | 100 | 17 | 32 | 81 | 43 | 70 | ||||
| Refill visits + pill counts | 41 | 29 | 60 | 57 | 73 | 13 | 27 | 77 | 46 | 51 | ||||
| Refill visits + pill counts + ACTG questionnaire | 79 | 100 | 0 | 20 | 68 | 23 | 0 | 100 | 57 | 35 | ||||
| Refill visits + pill counts + urine colour test | 78 | 86 | 20 | 23 | 72 | 21 | 25 | 88 | 62 | 35 | ||||
| Refill visits + pill counts + BMQ | 69 | 71 | 50 | 38 | 78 | 22 | 39 | 85 | 64 | 44 | ||||
| Refill visits + pill counts + INH urine test | 63 | 71 | 30 | 37 | 71 | 21 | 23 | 85 | 54 | 43 | ||||
| Refill visits + pill counts + Morisky scale | 41 | 29 | 60 | 57 | 73 | 13 | 27 | 77 | 46 | 51 | ||||
ACTG, AIDS Clinical Trials Group; BMQ, Brief Medication Questionnaire; INH, isoniazid; MEMS, Medication Event Monitoring System; NPV, negative predictive value; PPV, positive predictive value.
a Sensitivity: the proportion of non-adherent individuals correctly identified as non-adherent by the measure.
b Specificity: the proportion of adherent individuals correctly identified as adherent by the measure.
c Positive predictive value: the proportion of non-adherent individuals according to the measure who were non-adherent according to MEMS.
d Negative predictive value: the proportion of adherent individuals according to the measure who were adherent according to MEMS.
e Accuracy: the proportion of individuals correctly identified as either adherent or non-adherent by the measure.
f Non-adherence defined as an adherence rate of < 100% (27 patients) or < 95% (7 patients) as assessed by MEMS.
The combination of pill counts and refill visits that is used in routine practice had moderate sensitivity and specificity. Its sensitivity and negative predictive value for the identification of patients who were less than 95% adherent improved by adding any of the other adherence measures except the Morisky scale (Table 3).
Patients’ experience with MEMS use
The questionnaire about the use of MEMS, which was filled out by the 37 patients who completed treatment, revealed that only one patient had correctly understood the purpose of MEMS despite verbal and written information at the onset of the study. Twenty-five patients (68%) stated that the white, bulky appearance of the MEMS bottle reminded them to take the medication. However, the other patients said that the use of MEMS had not influenced their adherence behaviour. Mean adherence rates did not differ between these two groups: 97.5% (SD: 3.0) and 97.3% (SD: 3.2), respectively (P = 0.86).
Eight patients occasionally opened the MEMS bottle to take out medication for later use (resulting in a total of 42 non-monitored treatment days). This usually occurred when patients did not want to take the bottle along on travel occasions.
Discussion
This is the first study in which MEMS was used to assess treatment adherence rates in patients on community-based DOT over the full six-month tuberculosis treatment course. We observed high adherence rates in our pilot study of 50 Tanzanian patients. Almost 80% of the patients were more than 95% adherent and only one patient was less than 80% adherent. These findings do not confirm the concern that patients on community-based DOT are prone to become non-adherent, even though the study did reveal that some patients (i.e. those without a formal treatment supporter) turn community-based DOT into unsupervised treatment.
The participants’ demographic characteristics, such as the ratio of males to females and their treatment outcomes, were comparable to those of the general tuberculosis patient population in the Kilimanjaro region.6 This suggests that we studied a regionally representative patient sample. However, the adherence rates of our patients could have been biased by their participation in the study. Although we tried to deviate as little as possible from routine practice, the repeated adherence questionnaires and urine tests certainly made participants aware of our interest in their adherence behaviour. Two thirds of the patients felt that their adherence behaviour had been influenced by the use of MEMS, but their average adherence rate did not differ from those observed among patients who stated that MEMS had not influenced their behaviour. Findings from other studies suggest that when MEMS is used over long periods, its “interventional effect” disappears.26,27 Since our sample size was small, larger population studies should be conducted to assess the real impact of community-based DOT with and without formal treatment supporter on adherence rates. We did not aim to validate the concept of community-based DOT; studies for this purpose should have a different design.
The main objective of our study was to use MEMS as a reference standard to calculate the validity of several adherence measures whose use is feasible in patients on community-based DOT in resource-limited settings. The high adherence rates in the study population forced us to apply high adherence rate cut-off values to calculate the validity and reliability of the adherence measures. This resulted in wide gaps between the sensitivities and specificities of the measures.8 Combinations of measures were found to be more accurate than single measures in identifying as many true non-adherent patients as possible (reflected in high sensitivities and negative predictive values). The sensitivity and negative predictive value of the routinely used combination of pill counts and clinic attendance for medication refills improved substantially by adding a simple and cheap measure such as the ACTG adherence questionnaire, particularly at an adherence rate cut-off value of 95%.
The rifampicin urine colour test classified more patients as non-adherent than the INH urine test. Since the orange urine colouration caused by rifampicin is of short duration and may be absent altogether,28 it is likely that the urine colour test misclassified some patients with yellow urine as non-adherent. Such misclassifications are difficult to confirm in a study population with high adherence rates.
The adapted ACTG adherence questionnaire yielded more favourable responses than the other self-report measures. Differences in wording in the questionnaires may account for this.8 While patients had to answer either “yes” or “no” to the questions in the Morisky scale, they could answer “often”, “sometimes”, “rarely”, or “never” to comparable questions in the ACTG adherence questionnaire. This wider range of choice options may have evoked more honest replies. The ACTG adherence questionnaire (and to a lesser extent the BMQ) has the added advantage of disclosing factors that cause non-adherence in the individual patient. These factors could be used to design tailored interventions for promoting adherence among non-adherent patients on community-based DOT. We therefore suggest using the triple combination consisting of the ACTG adherence questionnaire, refill visits and pill counts to monitor treatment adherence by Tanzanian patients on community-based DOT. If the results are interpreted carefully, the combination seems valid and its use in routine practice appears feasible.
In conclusion, this study revealed high levels of treatment adherence by Tanzanian tuberculosis patients on community-based DOT. Although caution should be exercised in interpreting the results because this is a pilot study, the findings support the recommendation of using community-based DOT as an alternative to facility-based DOT in settings with overburdened health-care facilities. To monitor adherence among patients on community-based DOT in resource-limited settings where electronic monitoring is not feasible, combinations of simple and affordable adherence measures can be used. Supplementing pill counts and clinic attendance with a self-report measure such as the ACTG adherence questionnaire will help to identify potentially non-adherent patients who could benefit from tailored adherence-promoting interventions. Further studies in larger patient populations are needed to assess the adherence rates of patients on community-based DOT and to confirm the validity of simple and affordable adherence measures.
Funding:
The KNCV Tuberculosis Foundation (the Netherlands) provided financial support for the study but was not involved in the design or conduct of the study, or in the decision to publish the results.
Competing interests:
None declared.
References
- 1.An expanded DOTS framework for effective tuberculosis control (WHO/CDS/TB/2002.297). Geneva: World Health Organization; 2002. [Google Scholar]
- 2.Maher D. The role of the community in the control of tuberculosis. Tuberculosis (Edinb) 2003;83:177–82. doi: 10.1016/S1472-9792(02)00066-5. [DOI] [PubMed] [Google Scholar]
- 3.Lwilla F, Schellenberg D, Masanja H, Acosta C, Galindo C, Aponte J, et al. Evaluation of efficacy of community-based vs. institutional-based direct observed short-course treatment for the control of tuberculosis in Kilombero district, Tanzania. Trop Med Int Health. 2003;8:204–10. doi: 10.1046/j.1365-3156.2003.00999.x. [DOI] [PubMed] [Google Scholar]
- 4.Wandwalo E, Kapalata N, Egwaga S, Morkve O. Effectiveness of community-based directly observed treatment for tuberculosis in an urban setting in Tanzania: a randomised controlled trial. Int J Tuberc Lung Dis. 2004;8:1248–54. [PubMed] [Google Scholar]
- 5.Floyd K, Skeva J, Nyirenda T, Gausi F, Salaniponi F. Cost and cost-effectiveness of increased community and primary care facility involvement in tuberculosis care in Lilongwe District, Malawi. Int J Tuberc Lung Dis. 2003;7(Suppl 1):S29–37. [PubMed] [Google Scholar]
- 6.van den Boogaard J, Lyimo R, Irongo CF, Boeree MJ, Schaalma H, Aarnoutse RE, et al. Community vs. facility-based directly observed treatment for tuberculosis in Tanzania’s Kilimanjaro Region. Int J Tuberc Lung Dis. 2009;13:1524–9. [PubMed] [Google Scholar]
- 7.Frieden TR, Sbarbaro JA. Family observation of antituberculosis treatment. Lancet. 2006;367:2055–6, author reply 2055-6. doi: 10.1016/S0140-6736(06)68913-7. [DOI] [PubMed] [Google Scholar]
- 8.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–90, discussion 1073. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 9.Zeller A, Ramseier E, Teagtmeyer A, Battegay E. Patients’ self-reported adherence to cardiovascular medication using electronic monitors as comparators. Hypertens Res. 2008;31:2037–43. doi: 10.1291/hypres.31.2037. [DOI] [PubMed] [Google Scholar]
- 10.Schwed A, Fallab CL, Burnier M, Waeber B, Kappenberger L, Burnand B, et al. Electronic monitoring of compliance to lipid-lowering therapy in clinical practice. J Clin Pharmacol. 1999;39:402–9. doi: 10.1177/00912709922007976. [DOI] [PubMed] [Google Scholar]
- 11.Grosset KA, Bone I, Grosset DG. Suboptimal medication adherence in Parkinson’s disease. Mov Disord. 2005;20:1502–7. doi: 10.1002/mds.20602. [DOI] [PubMed] [Google Scholar]
- 12.Rivers PH, Ardagh-Walter N, Wright EC. Measurement of anticonvulsant adherence behaviour in the community using a Medication Events Monitoring System (MEMS) Health Care Anal. 1998;6:308–16. doi: 10.1007/BF02678367. [DOI] [PubMed] [Google Scholar]
- 13.Denhaerynck K, Schäfer-Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8:5. doi: 10.1186/1471-2288-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell CL, Owens S, Hamburger KQ, Thompson DA, Leach RR, Cetingok M, et al. Medication adherence and older renal transplant patients’ perceptions of electronic medication monitoring. J Gerontol Nurs. 2009;35:17–21. doi: 10.3928/00989134-20090903-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melbourne KM, Geletko SM, Brown SL, Willey-Lessne C, Chase S, Fisher A. Medication adherence in patients with HIV infection: a comparison of two measurement methods. AIDS Read. 1999;9:329–38. [PubMed] [Google Scholar]
- 16.Hugen PW, Langebeek N, Burger DM, Zomer B, van Leusen R, Schuurman R, et al. Assessment of adherence to HIV protease inhibitors: comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. J Acquir Immune Defic Syndr. 2002;30:324–34. doi: 10.1097/00126334-200207010-00009. [DOI] [PubMed] [Google Scholar]
- 17.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–23. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallab-Stubi CL, Zellweger JP, Sauty A, Uldry C, Iorillo D, Burnier M. Electronic monitoring of adherence to treatment in the preventive chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 1998;2:525–30. [PubMed] [Google Scholar]
- 19.Starr M, Sawyer S, Carlin J, Powell C, Newman R, Johnson P. A novel approach to monitoring adherence to preventive therapy for tuberculosis in adolescence. J Paediatr Child Health. 1999;35:350–4. doi: 10.1046/j.1440-1754.1999.00371.x. [DOI] [PubMed] [Google Scholar]
- 20.Ailinger RL, Black PL, Lima-Garcia N. Use of electronic monitoring in clinical nursing research. Clin Nurs Res. 2008;17:89–97. doi: 10.1177/1054773808316941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruslami R, van Crevel R, van de Berge E, Alisjahbana B, Aarnoutse RE. A step-wise approach to find a valid and feasible method to detect non-adherence to tuberculosis drugs. Southeast Asian J Trop Med Public Health. 2008;39:1083–7. [PubMed] [Google Scholar]
- 22.Manual of the National Tuberculosis and Leprosy Programme in Tanzania Fifth edition. Dar es Salaam: Ministry of Health; 2010. [Google Scholar]
- 23.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. 1999;37:113–24. doi: 10.1016/S0738-3991(98)00107-4. [DOI] [PubMed] [Google Scholar]
- 25.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12:255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 26.Bova CA, Fennie KP, Knafl GJ, Dieckhaus KD, Watrous E, Williams AB. Use of electronic monitoring devices to measure antiretroviral adherence: practical considerations. AIDS Behav. 2005;9:103–10. doi: 10.1007/s10461-005-1685-0. [DOI] [PubMed] [Google Scholar]
- 27.Wagner GJ, Ghosh-Dastidar B. Electronic monitoring: adherence assessment or intervention? HIV Clin Trials. 2002;3:45–51. doi: 10.1310/XGXU-FUDK-A9QT-MPTF. [DOI] [PubMed] [Google Scholar]
- 28.Whitfield R, Cope GF. Point-of-care test to monitor adherence to anti-tuberculous treatment. Ann Clin Biochem. 2004;41:411–3. doi: 10.1258/0004563041731637. [DOI] [PubMed] [Google Scholar]