Abstract
Calcineurin, a Ca2+/calmodulin dependent protein phosphatase, regulates Ca2+-dependent processes in a wide variety of cells. In the yeast, Saccharomyces cerevisiae, calcineurin effects Ca2+-dependent changes in gene expression through regulation of the Crz1p transcription factor. We show here that calcineurin dephosphorylates Crz1p and that this results in translocation of Crz1p to the nucleus. We identify a region of Crz1p that is required for calcineurin-dependent regulation of its phosphorylation, localization, and activity, and show that this region has significant sequence simlarity to a portion of NF-AT, a family of mammalian transcription factors whose localization is also regulated by calcineurin. Thus, the mechanism of Ca2+/calcineurin-dependent signaling shows remarkable conservation between yeast and mammalian cells.
Calcineurin is a conserved Ca2+/calmodulin-dependent protein phosphatase that plays a critical role in Ca2+ signaling. The activation of T lymphocytes by antigen requires calcineurin, and the immunosuppressive drugs, FK506 and cyclosporin A, act by inhibiting this phosphatase (Friedman and Weissman 1991; Liu et al. 1991a; Clipstone and Crabtree 1992; O’Keefe et al. 1992). NF-AT, a family of mammalian transcription factors, is the critical target of calcineurin-dependent regulation for T-cell activation as well as for other other processes such as cardiac development and hypertrophy (Clipstone and Crabtree 1992; O’Keefe et al. 1992; Rao et al. 1997; de la Pompa et al. 1998; Molkentin et al. 1998; Ranger et al. 1998). Dephosphorylation of NF-AT by calcineurin results in its translocation to the nucleus, where it activates transcription (Flanagan et al. 1991; Jain et al. 1993; Shaw et al. 1995; Beals et al. 1997).
In the yeast Saccharomyces cerevisiae, calcineurin is required for cell viability under specific environmental conditions. Calcineurin is a heterodimer of a catalytic subunit (encoded by the CNA1 and CNA2 genes) and a regulatory subunit (encoded by CNB1) (Cyert et al. 1991; Liu et al. 1991b; Cyert and Thorner 1992). Calcineurin-deficient cells (cna1Δcna2Δ or cnb1Δ) fail to grow in the presence of Na+/Li+, Mn2+, or alkaline pH, and lose viability during prolonged incubation with mating pheromone (Nakamura et al. 1993; Mendoza et al. 1994; Moser et al. 1996; Pozos et al. 1996; Withee et al. 1997). All of these conditions, as well as addition of Ca2+ to the growth medium, induce Ca2+/ calcineurin-dependent gene expression (Mendoza et al. 1994; Mazur et al. 1995; Cunningham and Fink 1996).
Previously, we elucidated the mechanism by which transcription of one target gene, FKS2, is activated in response to Ca2+. A 24 bp region of the FKS2 promoter, the CDRE, is sufficient to direct Ca2+-induced gene expression, and this transcription requires both calcineurin and the Crz1p transcription factor (Stathopoulos and Cyert 1997). crz1Δ mutants fail to activate calcineurin-dependent transcription, and display growth and viability defects similar to those of calcineurin mutants (Matheos et al. 1997; Stathopoulos and Cyert 1997). Thus, many of the physiological functions of calcineurin in yeast are mediated by its regulation of Crz1p.
In this report we describe the mechanism by which calcineurin regulates Crz1p activity. We demonstrate that calcineurin dephosphorylates Crz1p in vitro, and that Crz1p localization changes from cytosolic to nuclear subsequent to calcineurin activation in vivo. Furthermore, we identify a domain of Crz1p that is required for calcineurin-dependent regulation of its phosphorylation, localization, and activity. This domain of Crz1p has significant sequence similarity to a portion of NF-ATc [the serine-rich region (SRR)], which is similarly required for its regulation by calcineurin (Beals et al. 1997). Thus, these studies establish that calcineurin regulates the phosphorylation and localization of Crz1p in yeast, and identify Crz1p as a substrate of calcineurin. Furthermore, our observations reveal that the mechanism by which Ca2+/calcineurin regulates gene expression in yeast and mammalian cells is strikingly similar.
Results and Discussion
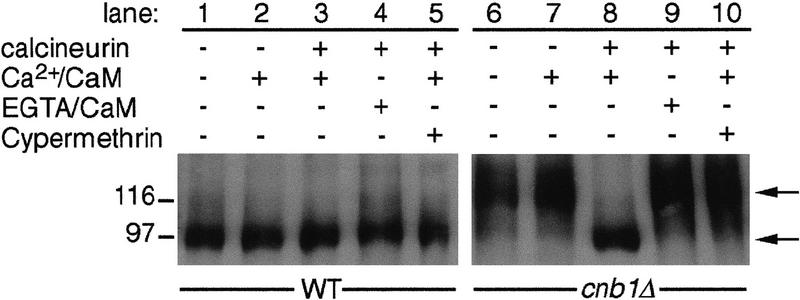
To elucidate the mechanism by which calcineurin controls Crz1p activity, we examined whether Crz1p phosphorylation is modulated by calcineurin. Crz1p isolated from calcineurin-deficient cells migrates on SDS–polyacrylamide gels with a significantly larger apparent molecular mass than Crz1p isolated from wild-type cells (118,000 vs. 97,000; Fig. 1, lane 1 vs. lane 6). Treatment of the larger (118 kD) form of Crz1p with calcineurin, Ca2+ and calmodulin in vitro converted it to the smaller (97 kD) form (Fig. 1, lane 8). This change in apparent molecular mass was due to calcineurin-mediated dephosphorylation, as it was dependent on calcineurin and was blocked either by the addition of the Ca2+ chelator, EGTA, or by the calcineurin inhibitor, cypermethrin (Fig. 1, lanes 7,9,10). Incubation with calcineurin did not alter the apparent molecular mass of Crz1p isolated from wild-type cells (Fig. 1, lane 3). These data indicate that Crz1p is hyperphosphorylated in cells lacking calcineurin, and that it serves as a substrate for calcineurin in vitro.
Figure 1.
Crz1p is dephosphorylated by calcineurin in vitro. HA-Crz1p was purified by immunoprecipitation from crz1Δ strains ASY472 (WT) and ASY475 (cnb1Δ) containing pAMS466 [YEp(HA–CRZ1)], incubated under different conditions as noted, and analyzed by PAGE and Western blotting (see Materials and Methods). (Lanes 1,6) HA–Crz1p incubated with buffer; (lanes 2,7) HA–Crz1p incubated in buffer with 20 mm CaCl2, and calmodulin; (lanes 3,8) HA–Crz1p incubated in buffer with 20 mm CaCl2, 83 units of calmodulin, and 0.8 unit of calcineurin, as described in Materials and Methods; (lanes 4,9) HA–Crz1p incubated in buffer with calcineurin and calmodulin plus 100 mm EGTA; (lanes 5,10) HA–Crz1p incubated in buffer with 20 mm CaCl2 and calmodulin plus calcineurin that was preincubated for 15 min at 30°C with 100 μm cypermethrin.
Next, we sought to identify properites of Crz1p that are modulated by phosphorylation. Calcineurin-mediated dephosphorylation was not required for Crz1p to associate with DNA. A CDRE-specific protein–DNA complex containing the Crz1p protein forms in extracts of wild-type cells (Stathopoulos and Cyert 1997), and an identical complex was observed in extracts of calcineurin deficient cells (data not shown).
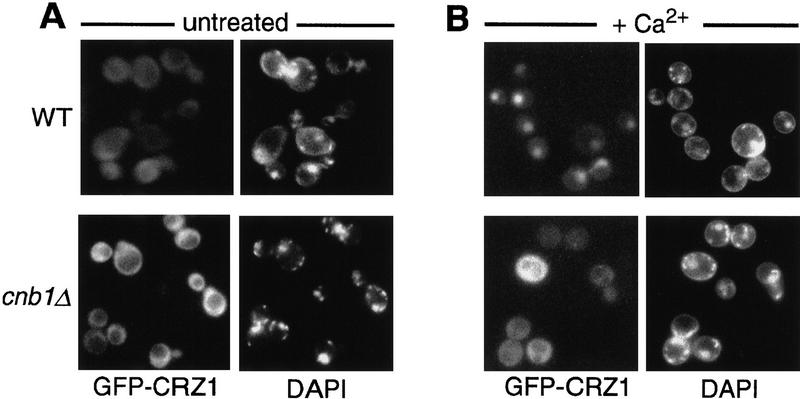
To examine localization of Crz1p, we constructed a fusion protein containing green fluorescent protein (GFP) and Crz1p. GFP–Crz1p fully complements the growth defects of a crz1Δ strain and mediates Ca2+-induced calcineurin-dependent transcriptional activation (data not shown and Fig. 5B, below). As was observed for Crz1p, the apparent molecular mass of GFP–Crz1p is also larger in calcineurin-deficient strains than in wild-type strains (see Fig. 5D, below). Thus, the function and regulation of GFP–Crz1p and Crz1p are indistinguishable. In either wild-type or calcineurin mutant cells cultured under standard conditions, GFP–Crz1p localized to the cytosol (Fig. 2A). However, when wild-type cells were incubated with 200 mm Ca2+, GFP–Crz1p became nuclear within 10 min (Fig. 2B, top). In contrast, in calcineurin-mutant cells treated with 200 mm Ca2+, GFP–Crz1p remained cytosolic (Fig. 2B, bottom). In wild-type cells, nuclear localization of GFP–Crz1p was also induced by addition of 800 mm NaCl or incubation at high temperature (37°C). However, both of these treatments, as well as addition of lower concentrations of Ca2+ (150 mm or less) resulted in <50% cells with complete nuclear localization (data not shown). Thus, several conditions that activate calcineurin/Crz1-dependent transcription (Mendoza et al. 1994; Matheos et al. 1997; Stathopoulos and Cyert 1997; Zhao et al. 1998) also caused nuclear translocation of GFP–Crz1p. Furthermore, other treatments that do not activate calcineurin/Crz1-dependent transcription (i.e., incubation with 200 mm MgCl2 or 800 mm KCl) failed to induce the nuclear translocation of Crz1p (data not shown).
Figure 5.
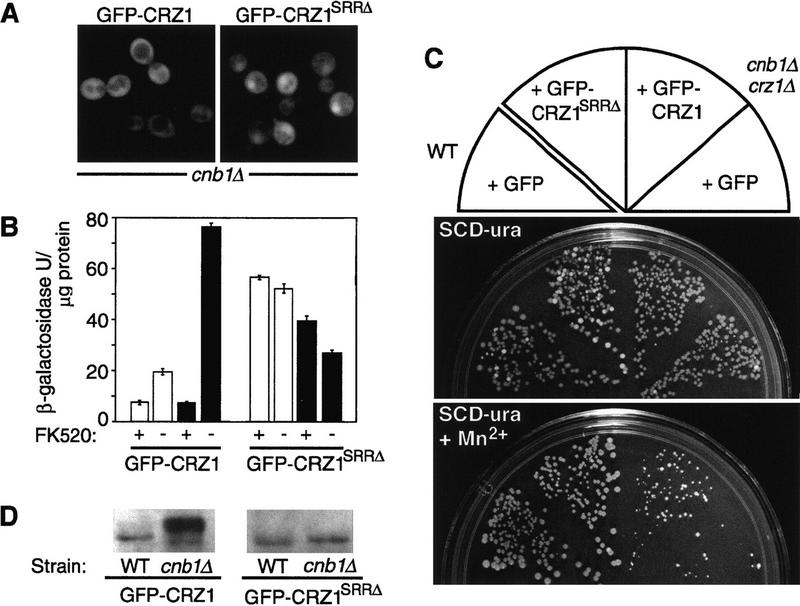
The SRR is required for calcineurin-dependent regulation of Crz1p localization, activity, and phosphorylation. (A) GFP–CRZ1SRRΔ displays partial nuclear localization in cnb1Δ cells. The localization of GFP fusions in crz1Δ cells ASY835 (cnb1Δ) containing either plasmids pAMS463 (GFP–Crz1p) or pAMS490 (GFP–CRZ1SRRΔ is shown. (B) GFP–CRZ1SRRΔ directs calcineurin-independent transcription. Cells of crz1Δ strains ASY834 (WT) or ASY835 (cnb1Δ) containing the CDRE–lacZ reporter (pBJ306) and either plasmids pAMS463 (GFP–Crz1p) or pAMS490 (GFP–CRZ1SRRΔ) were grown in synthetic medium at 21°C for 6 hr with or without FK520 (3 μg/ml) and inducing treatments. β-Galactosidase activity is reported for extract of cells that were either untreated (open bars) or treated with 200 mm CaCl2 (solid bars). β-Galactosidase activities were normalized to the amount of protein in extracts from one representative experiment. Each extract was assayed in triplicate and the s.d. is representative of the error between these samples. (C) GFP–CRZ1SRRΔ confers Mn2+ tolerance to calcineurin-deficient cells. Yeast strains were plated onto synthetic medium containing 4 mm MnCl2 and grown at 30°C for 3 days. (WT + GFP). Strain YPH499 containing pGFP–NFUS; (cnb1Δ crz1Δ + GFP–CRZ1SRRΔ, strain ASY835 containing pAMS490; (cnb1Δ crz1Δ + GFP–CRZ1). Strain ASY835 containing pAMS463. (D) GFP–CRZ1SRRΔ does not show a calcineurin-dependent difference in apparent molecular mass. Equal amounts of extracts from crz1Δ strains ASY834 (WT) or ASY835 (cnb1Δ) containing different GFP–Crz1p constructs [pAMS463 (GFP–CRZ1) or pAMS490 (GFP–CRZ1SRRΔ)] were analyzed by Western blotting (see Methods).
Figure 2.
Translocation of GFP–CRZ1 to the nucleus is induced by Ca2+ addition and requires calcineurin. Living cells of strain ASY472 (WT) or ASY475 (cnb1Δ) containing pAMS463 (GFP–CRZ1) were grown at 21°C, incubated briefly with DAPI to stain DNA, and analyzed by fluorescence microscopy to observe GFP–Crz1p localization (GFP–CRZ1) or nuclear staining (DAPI). (A) Untreated cells; (B) cells incubated with 200 mm CaCl2 for 10 min at 21°C (+ Ca2+).
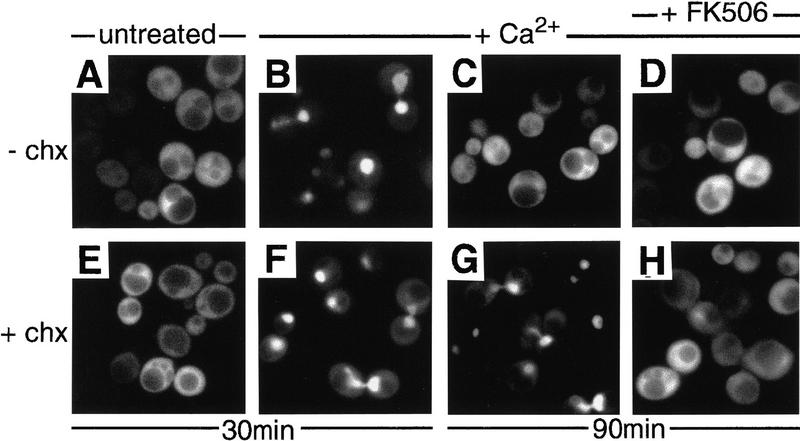
The nuclear localization of Crz1p was transient, and in most cells cytosolic localization of GFP–Crz1p resumed 60–90 min after Ca2+ addition (Fig. 3, cf. B and C). A second addition of Ca2+ to cells at this time induced another cycle of GFP–Crz1p nuclear translocation, suggesting that the loss of GFP–Crz1p nuclear localization reflects the return of cytosolic [Ca2+] to resting levels (data not shown). Yeast cells sequester Ca2+ into intracellular compartments by several mechanisms, and measurements of cytosolic [Ca2+] show that Ca2+ levels decrease quickly after addition of Ca2+ to the medium (Miseta et al. 1999). Consistent with this idea, the return of GFP–Crz1p to the cytosol after Ca2+ addition was delayed in pmc1Δ mutants (data not shown). pmc1Δ cells lack a P-type ATPase that pumps Ca2+ into the yeast vacuole, and are defective in Ca2+ homeostasis (Cunningham and Fink 1994).
Figure 3.
GFP–Crz1 shuttles from the nucleus to the cytoplasm and nuclear localization requires calcineurin activity. Log-phase cultures of strain ASY472 containing GFP–CRZ1 were grown at 21°C and analyzed by fluorescence microscopy to determine GFP–Crz1p localization. (E–H) Cells incubated for 2 hr with cycloheximide prior to other manipulations described (+chx). (A–D) Cells not incubated with cycloheximide (−chx). (B,F) At t = 0 min, CaCl2 was added to one aliquot of each culture (200 mm) and cells were observed at t = 30 min; (A,E) Equivalent cells not exposed to Ca2+. (D,H) At t = 60 min an aliquot of each Ca2+-treated culture was incubated with 3 μg/ml FK506 and observed at t = 90 min. (C,G) Cells incubated with Ca2+ for 90 min, not treated with FK506.
The reversible localization of GFP–Crz1p may be due to its shuttling in and out of the nucleus. Alternatively, Crz1p may be degraded after it translocates to the nucleus. In this case, protein synthesis would be required to reestablish Crz1p cytosolic localization after a [Ca2+] rise. To distinguish between these two possibilites, we examined GFP–Crz1p localization in cells that had been treated with the protein synthesis inhibitor, cycloheximide. In cycloheximide-treated cells, addition of Ca2+ also resulted in rapid translocation of GFP–Crz1p to the nucleus (Fig. 3F). Ninety minutes after addition of Ca2+ to these cells, GFP–Crz1p remained nuclear (Fig. 3G). However, addition of FK506, a calcineurin inhibitor, caused relocalization of GFP–Crz1p to the cytosol (Fig. 3H). These findings establish that GFP–Crz1p enters and exits the nucleus, and that the continuous activity of calcineurin is required to maintain GFP–Crz1p nuclear localization. Surprisingly, inhibiting protein synthesis prolonged the Ca2+-induced nuclear localization of GFP–Crz1p (Fig. 3, cf. C and G). This may be due to impairment of Ca2+ sequestration. Ca2+ induces de novo protein synthesis of Pmc1p (Cunningham and Fink 1996), thus cells treated with Ca2+ and cylcoheximide are deficient for this Ca2+–ATPase. Similarly, cycloheximide may alter the abundance or activity of additional proteins involved in Ca2+ homeostasis.
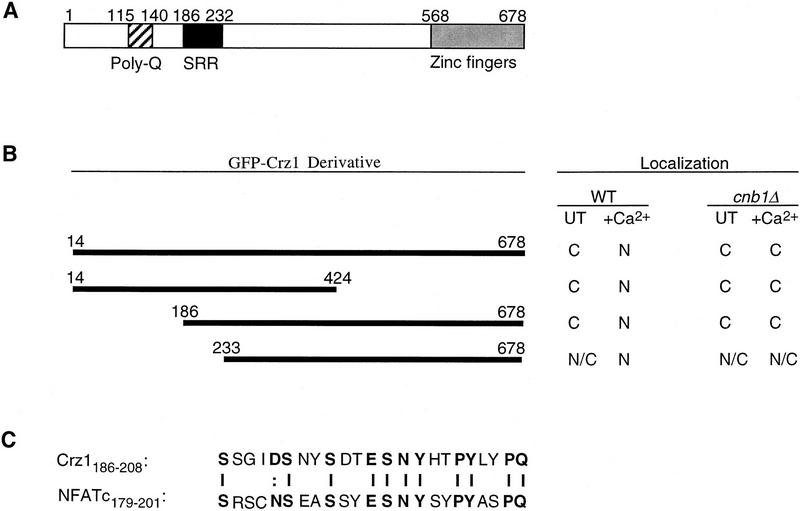
Next, we further investigated Crz1p to determine which regions of this protein (Fig. 4A) are required for calcineurin-dependent regulation of its localization. We constructed a series of GFP fusions containing different amounts of Crz1p and examined the localization of these fusion proteins (Fig. 4B). A GFP–Crz1 fusion lacking either the carboxy-terminal 254 amino acids of Crz1p (GFP–Crz114–424) or the amino-terminal 185 residues of Crz1p (GFP–Crz1186–678) showed Ca2+-induced, calcineurin-dependent nuclear localization that was indistinguishable from that of the full-length protein (Fig. 4B). These proteins also displayed a calcineurin-dependent change in apparent molecular mass (data not shown). Thus, these regions are dispensible for calcineurin-dependent regulation of Crz1p localization and phosphorylation. In contrast, a fusion lacking the amino-terminal 232 amino acids of Crz1 (GFP–Crz1233–678) showed partial localization to the nucleus both in wild-type cells grown under standard conditions and in calcineurinmutant cells grown either under standard conditions or incubated with Ca2+ (Fig. 4B). Thus, for this fusion, nuclear localization was somewhat independent of calcineurin, although addition of Ca2+ to cells containing this protein further increased nuclear localization by a calcineurin-dependent mechanism. Furthermore, the apparent molecular mass of GFP–Crz1233–678 was identical in wild-type and calcineurin-mutant cells, suggesting that the deletion of amino acids 186–232 disrupted the phosphorylation of Crz1p (data not shown). These results suggest that amino acids 186–232 of Crz1p are required for calcineurin-dependent regulation of its localization and phosphorylation. Examination of this region revealed that it shares sequence similarity to a domain of the NF-ATc transcription factor called the SRR, or serine rich region (Beals et al. 1997; Fig. 4C). Several serine residues in the NF-ATc SRR are thought to be dephosphorylated by calcineurin in vivo, resulting in exposure of a nuclear localization sequence (NLS) and import of NF-AT to the nucleus (Beals et al. 1997).
Figure 4.
Localization of GFP–CRZ1 fusions. (A) Diagram of functional domains identified within Crz1p. (Poly-Q) Polyglutamine tract; (SRR), serine-rich region (see text and C); (zinc fingers) sequences that match the consensus for zinc finger DNA-binding motifs (Matheos et al. 1997; Stathopoulos and Cyert 1997). (B) Localization of GFP–Crz1p fusions in crz1Δ strains ASY834 (WT) and ASY835 (cnb1Δ) determined by fluorescence microscopy: (C), predominantly cytoplasmic localization; (N) predominantly nuclear localization; (N/C) localization to both cytosol and nucleus. Localization was observed in resting cells (UT) and cells incubated at 21°C for 10 min with 200 mm CaCl2 (+Ca2+). (C) Crz1p SRR shares sequence similarity with the NF-ATc SRR. Alignment of NF-ATc (Northrop et al. 1994) and Crz1p (Matheos et al. 1997; Stathopoulos and Cyert 1997) amino acid sequences were analyzed by the ALION program (C.G. Nevill-Manning, C. N. Huang, and D. L. Brutlag, pers. comm.; http://motif.stanford.edu/alion/).
We studied the function of the SRR homology region of Crz1p (amino acids 186–232) further by constructing a GFP–Crz1p fusion that lacks only this region (GFP–Crz1SRRΔ). GFP–Crz1SRRΔ partially localized to the nucleus in the absence of Ca2+ and calcineurin (Fig. 5A). In comparison, GFP–Crz1p is cytosolic under these conditions. Next, we investigated the ability of GFP–Crz1SRRΔ to direct expression of a CDRE–lacZ reporter gene. Cells containing GFP–Crz1p produced high levels of β-galactosidase from CDRE–lacZ in the presence of Ca2+ (Fig. 5B). This expression was inhibited by addition of the calcineurin inhibitor, FK520. In contrast, cells containing GFP–Crz1SRRΔ expressed high levels of β-galactosidase from CDRE–lacZ independent of Ca2+ addition, and expression was not decreased by addition of FK520 (Fig. 5B). Note that GFP–Crz1SRRΔ is expressed at a lower level than GFP–Crz1p in vivo, so the absolute amount of transcription directed by these two proteins cannot be compared (data not shown).
We also examined the growth properties of strains expressing either GFP–Crz1p or GFP–Crz1SRRΔ. Strains lacking calcineurin fail to grow on medium containing Mn2+ due, in part, to the absence of calcineurin/Crz1p-dependent transcription (Matheos et al. 1997; Stathopoulos and Cyert 1997). A cnb1Δcrz1Δ mutant strain expressing GFP–Crz1SRRΔ grew significantly better in the presence of Mn2+ than the same strain expressing GFP–Crz1p (Fig. 5C). These observations are consistent with the expression of β-galactosidase from CDRE–lacZ and indicate that GFP–Crz1SRRΔ confers calcineurin-independent gene expression in vivo.
Finally, the apparent molecular mass of GFP–Crz1SRRΔ does not differ significantly in wild-type versus calcineurin-mutant cells, suggesting that its phosphorylation state is not modulated by calcineurin (Fig. 5D). Together, all of the findings concerning GFP–Crz1SRRΔ indicate that the SRR region of Crz1p (amino acids 186–232) plays a critical role in mediating the calcineurin-dependent regulation of Crz1p phosphorylation, localization, and activity.
These studies add significantly to our understanding of Ca2+-dependent signal transduction in yeast by establishing that calcineurin controls gene expression through modulation of Crz1p localization. Furthermore, we demonstrate that the phosphorylation state of Crz1p is regulated by calcineurin in vivo and that calcineurin dephosphorylates Crz1p in vitro. Thus, Crz1p is the first protein identified as a substrate of calcineurin in yeast.
Our observations support the following model for the regulation of Crz1p by calcineurin: In its phosphorylated state, Crz1p resides primarily in the cytosol, and the expression of Crz1-dependent genes is low. Once calcineurin is activated (i.e., in response to addition of Ca2+, high temperature, Na+, etc.) it dephosphorylates Crz1p, which allows its nuclear translocation, and promotes Crz1p-dependent transcription. We cannot yet determine whether, in addition to its localization, other aspects of Crz1p function are also regulated by calcineurin-dependent dephosphorylation. Much like the SRR domain of NF-ATc, the SRR domain of Crz1p serves an inhibitory role, such that when this domain is present, Crz1 remains cytosolic under low Ca2+ conditions or in the absence of calcineurin. The presence of the SRR domain is required not only for calcineurin-dependent regulation of Crz1p localization and activity, but also for calcineurin-dependent regulation of Crz1p phosphorylation. Thus, the SRR domain may contain the major phosphorylation sites of Crz1p that are dephosphorylated by calcineurin or may be required for recognition of Crz1p by one or more protein kinases. Phosphorylation can modify the localization of a protein by regulating its rate of its nuclear import or export or both (Jans and Hubner 1996; Beals et al. 1997; Kaffman et al. 1998a, 1998b). Our future studies of Crz1p will address the mechanism by which the phosphorylation state of Crz1p affects the kinetics of its transport into and/or out of the nucleus.
Ca2+ acts as a signaling molecule in virtually all eukaryotic cells. Although the external conditions that evoke Ca2+ signals vary widely among cell types, many of the intracellular events that occur downstream of a Ca2+ rise are highly conserved. Calcineurin, the Ca2+/calmodulin-regulated phosphatase, effects the nuclear translocation and activation of the Crz1p and NF-AT transcription factors in yeast and mammalian cells, respectively. The mechanisms of these signaling responses are strikingly similar, although the Crz1p and NF-AT transcription factors share only limited sequence similarity. These findings demonstrate that the rapid and reversible control of gene expression via regulation of nuclear localization is a fundamental and highly conserved component of Ca2+-induced calcineurin-mediated signaling pathways.
Materials and methods
Strains and plasmids
All yeast strains have been described previously (Cyert and Thorner 1992; Stathopoulos and Cyert 1997). Yeast medium and culture conditions have also been described previously (Stathopoulos and Cyert 1997). Growth conditions used for GFP localization studies are described below. A stock solution of FK506 or FK520 (Fugisawa, Deerfield, IL, and Merck Co., Rahway, NJ, repectively; 20 mg/ml in 90% ethanol, 10% Tween 20) was also added to liquid medium where noted. The CDRE–lacZ reporter gene within pBJ306 (B. Jiang and M.S. Cyert, in prep.) contains four copies of the CDRE driving lacZ from a CYC1 minimal promoter and was integrated at the URA3 locus after linearization with StuI and selection for tryptophan prototrophy. The construction of plasmids expressing HA–CRZ1 on a high-copy number plasmid has been described previously (Stathopoulos and Cyert 1997). A series of CRZ1 fusions to the carboxyl terminus of GFP (pAMS463, pAMS435, pAMS469, pAMS473, pAMS490) were constructed by cloning and PCR with the GFP fusion vector, pGFP–NFUS (Niedenthal et al. 1996) and portions of the CRZ1 gene from pAM435. Detailed descriptions of plasmids used in this study are available on request.
Cell extract preparation, immunoprecipitation, and Western blotting
For the analysis of Crz1p electrophoretic mobility, whole-cell extracts (∼5 μg/μl) were prepared from cultures of strains ASY635 and ASY635 containing HA-tagged Crz1p (pAMS446) grown at 21°C to log phase essentially as described (Stathopoulos and Cyert 1997) with the following exception: Cells were resuspendend in RIPA buffer [20 mm Tris-HCl (pH 7.6), 2 mm EDTA, 150 mm NaCl, 1% Triton X-100, 1% Na-deoxycholate, 0.1% SDS, 1.0 mm DTT] with protease inhibitors (1 mm benzamidine-HCl, 1 mm PMSF, and 5 μg/ml each pepstatin, aprotinin, and leupeptin), and phosphatase inhibitors (5 mm sodium fluoride, 5 mm sodium phosphate, 10 mm sodium pyrophosphate, 10 mm sodium molybdate, 5 mm EDTA, and 5 mm EGTA) before lysis. For Western blots, 5 μl of extract was separated on a 7.5% polyacrylamide–SDS gel, transferred to nitrocellulose, incubated with anti-GFP antibody (Kahana et al. 1995), HRP-conjugated secondary antibody, and visualized with ECL (reagents from Amersham). For immunoprecipitation, 30 μl of cell extract was mixed with 30 μl of rabbit polyclonal antibody HA.11 (BabCo) and rotated overnight at 4°C. A total of 10 μl of protein-A agarose beads (SIGMA) were added, and after 4 hr at 4°C the beads were washed three times with RIPA buffer, and resuspended in CP buffer [50 mm Tris-HCl, (pH 7.5), 10 mm MgCl2, 5 mm DTT). When noted, some of the following additions were also included with CP buffer: CaCl2 (20 mm), calmodulin (Sigma, 83 units), calcineurin (Sigma, 0.8 units), EGTA (100 mm), and cypermethrin (Calbiochem, 100 μm). Immunoprecipitates were then incubated at 30°C for 30 min, separated on an 8% polyacrylamide gel, and immunoblotted with anti-HA antibodies (Boehringer-Mannheim).
Examination of GFP–CRZ1
Live yeast cells containing GFP–Crz1p fusions were prepared for visualization as follows: Log-phase cultures (OD600 of 1.0–1.5) were grown at 21°C in synthetic medium with 60 μg/ml additional adenine to reduce the background fluorescence of ade2 mutants, ammonium chloride substituted for ammonium sulfate to reduce precipitation on Ca2+ addition, and in the absence of methionine to induce high-level expression from the MET25 promoter of pGFP–NFUS vector. Cells were harvested and concentrated 10- to 20-fold before visualization. When noted, cells were incubated briefly with 1 ng/ml DAPI (Sigma) prior to visualization to stain DNA, or for 2 hr with 10 μg/ml cycloyheximide (Sigma) to inhibit protein synthesis. Cells were viewed at room temperature on a Axioskop microscope (Carl Zeiss, Thornwood, NY) equipped with Nomarski differential interference contrast and fluorescence optics with a 100×/1.3 objective lens and HBO100 mercury lamp. GFP and nuclear staining were visualized with fluorescein and DAPI filter sets, respectively (Chroma Technology, Brattleboro, VT).
Acknowledgments
We thank Jerry Crabtree, Jeremy Thorner, and members of the Cyert laboratory for comments on the manuscript, Fujisawa for supplying FK506, Merck for supplying FK520, and Pam Silver for supplying anti-GFP antibody. We thank Kim Williams for identifying the sequence similarity between Crz1p and NF-AT. We thank Renée Polizotto and John Gerontides for helpful discussion and Tim Stearns for his generous advice concerning fluorescence microscopy. This work was supported from the following sources: National Institutes of Health research grant GM-48728, The National Science Foundation Young Investigator Award MCB-9357017, biomedical scholar award 92-42 from the Lucille P. Markey Charitable Trust, and funds from Proctor and Gamble (all to M.S.C.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL mcyert@leland.stanford.edu; FAX (650) 725-8309.
References
- Beals CR, Clipstone NA, Ho SN, Crabtree GR. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes & Dev. 1997;11:824–834. doi: 10.1101/gad.11.7.824. [DOI] [PubMed] [Google Scholar]
- Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ -ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS, Thorner J. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol Cell Biol. 1992;12:3460–3469. doi: 10.1128/mcb.12.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS, Kunisawa R, Kaim D, Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Nat Acad Sci. 1991;88:7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pompa J, Timmerman L, Takimoto H, Yoshida H, Elia A, Samper E, Potter J, Wakeham A, Marengere L, Langille B, Crabtree G, Mak T. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392:182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- Flanagan W, Corthesy B, Bram R, Crabtree G. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Friedman J, Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: One in the presence and one in the absence of CsA. Cell. 1991;66:799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- Jans DA, Hubner S. Regulation of protein transport to the nucleus: Central role of phosphorylation. Physiol Rev. 1996;76:651–685. doi: 10.1152/physrev.1996.76.3.651. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Rank NM, O’Neill EM, Huang LS, O’Shea EK. The receptor MSN5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature. 1998a;396:482–486. doi: 10.1038/24898. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Rank NM, O’Shea EK. Phosphorylation regulates association of the transcription factor Pho4 with its import receptor Pse1/Kap121. Genes & Dev. 1998b;12:2673–2683. doi: 10.1101/gad.12.17.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana J, Schnapp B, Silver P. Kinetics of spindle pole body separation in budding yeast. Proc Natl Acad Sci. 1995;92:9707–9711. doi: 10.1073/pnas.92.21.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Farmer JJD, Lane WL, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-CsA and FKBP-FK506 complexes. Cell. 1991a;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ishii S, Tokai M, Tsutsumi H, Ohke O, Akada R, Tanaka K, Tsuchiya E, Fukui S, Miyakawa T. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol & Gen Genet. 1991b;227:52–59. doi: 10.1007/BF00260706. [DOI] [PubMed] [Google Scholar]
- Matheos D, Kingsbury T, Ahsan U, Cunningham K. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes & Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P, Morin N, Baginsky W, El-Sherbeini M, Clemas JA, Nielsen JB, Foor F. Differential expression and function of two homologous subunits of yeast 1,3-β-D-glucan synthase. Mol Cell Biol. 1995;15:5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza I, Rubio F, Rodriguez-Navarro A, Pardo JM. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J Biol Chem. 1994;269:8792–8796. [PubMed] [Google Scholar]
- Miseta A, Fu L, Kellermayer R, Buckley J, Bedwell D. The Golgi apparatus plays a significant role in the maintenance of Ca2+ homeostasis in the vps33Δ vacuolar biogenesis mutant of Saccharomyces cerevisiae. J Biol Chem. 1999;274:5939–5947. doi: 10.1074/jbc.274.9.5939. [DOI] [PubMed] [Google Scholar]
- Molkentin J, Lu J, Antos C, Markham B, Richardson J, Robbins J, Grant S, Olson E. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MJ, Geiser JR, Davis TN. Ca2+-calmodulin promotes survival of pheromone-induced growth arrest by activation of calcineurin and Ca2+-calmodulin-dependent protein kinase. Mol Cell Biol. 1996;16:4824–4831. doi: 10.1128/mcb.16.9.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Liu Y, Hirata D, Namba H, Harada S, Hirokawa T, Miyakawa T. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 1993;12:4063–4071. doi: 10.1002/j.1460-2075.1993.tb06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal RK, Riles L, Johnston M, Hegemann JH. Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast. 1996;12:773–786. doi: 10.1002/(SICI)1097-0061(19960630)12:8%3C773::AID-YEA972%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Northrop JP, Ho SN, Chen L, Thomas DJ, Timmerman LA, Nolan GP, Admon A, Crabtree GR. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O’Neill EA. FK506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Pozos TC, Sekler I, Cyert MS. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol Cell Biol. 1996;16:3730–3741. doi: 10.1128/mcb.16.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger A, Grusby M, Hodge M, Gravallese E, de la Brousse F, Hoey T, Mickanin C, Baldwin H, Glimcher L. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392:186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: Regulation and function. Ann Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Shaw K, Ho A, Raghavan A, Kim J, Jian J, Park J, Sharma S, Rao A, Hogan P. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc Natl Acad Sci. 1995;92:11205–11209. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos AM, Cyert MS. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes & Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withee JL, Mulholland J, Jeng R, Cyert MS. An essential role of the pheromone-dependent Ca2+ signal is to activate yeast calcineurin. Mol Biol Cell. 1997;8:263–277. doi: 10.1091/mbc.8.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Jung US, Garrett-Engele P, Roe T, Cyert MS, Levin DE. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol Cell Biol. 1998;18:1013–1022. doi: 10.1128/mcb.18.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]