Abstract
While tuberculosis (TB) in Canadian cities is increasingly affecting foreign-born persons, homeless persons remain at high risk. To assess trends in TB, we studied all homeless persons in Toronto who had a diagnosis of active TB during 1998–2007. We compared Canada-born and foreign-born homeless persons and assessed changes over time. We identified 91 homeless persons with active TB; they typically had highly contagious, advanced disease, and 19% died within 12 months of diagnosis. The proportion of homeless persons who were foreign-born increased from 24% in 1998–2002 to 39% in 2003–2007. Among foreign-born homeless persons with TB, 56% of infections were caused by strains not known to circulate among homeless persons in Toronto. Only 2% of infections were resistant to first-line TB medications. The rise in foreign-born homeless persons with TB strains likely acquired overseas suggests that the risk for drug-resistant strains entering the homeless shelter system may be escalating.
Keywords: Tuberculosis, homeless persons, epidemiology, molecular epidemiology, clinical medicine, tuberculosis and other mycobacteria, research
In Canada’s major cities, tuberculosis (TB) is increasingly becoming a disease of persons born outside Canada (foreign-born). In 2009 in the city of Toronto in Ontario, 94% of all persons with active TB were foreign-born (1). Although homeless and marginally housed persons represent a smaller proportion of TB case-patients, they remain a persistent high-risk population. Recent TB outbreaks and disease clusters among homeless persons have been reported in many cities in the United States (2–5) and have been associated with transmission at shelters, single-room–occupancy hotels, and rooming houses (which provide inexpensive rooms with shared bathrooms), prisons, and bars (6–10).
Toronto is the largest city in Canada; among its population of 2.5 million persons, ≈50% were born outside Canada (11). Each year in Toronto, ≈29,000 persons use emergency shelters, and on any given night ≈5,000 are without homes (12). During 2001–2002, a large shelter-based TB outbreak occurred among homeless persons in Toronto. A coroner’s inquest into the death of a homeless man in whom pulmonary TB developed during the course of this outbreak revealed the many challenges of diagnosing and managing TB in homeless populations (13). In response to the inquest and resulting jury recommendations, major changes to the management of homeless TB cases occurred in the public health and shelter systems, and local TB clinic capacity expanded. This case resulted in the creation of a public health team dedicated to case management, contact follow-up, advocacy, education, health promotion, and active case finding among the city’s homeless and underhoused population.
A comprehensive review of the population and molecular epidemiology, clinical features, management and health outcomes of homeless persons with TB in Canada is needed but lacking. To better understand and address the extent of disease in this vulnerable population, we studied TB among Toronto’s homeless persons over a 10-year period.
Methods
The study population included all persons in Toronto for whom active TB had been reported to Toronto Public Health from January 1, 1998, through December 31, 2007. Data were extracted from the Reportable Disease Information System and the Integrated Public Health Information System for all case-patients with a risk setting of “shelter/rooming house” or a risk factor of “homeless.” Health case management files were reviewed to ensure accuracy of database entries; additional data were abstracted when necessary. Cases were included in the analysis for persons with active TB who met the following eligibility criteria in the year before diagnosis: 1) any shelter stay, 2) any rooming house stay, 3) no fixed address, or 4) use of services for homeless persons >1× per week. Cases were excluded for persons with active TB who 1) were foreign-born and received a diagnosis of active TB within 1 month of arrival in Canada, 2) received a diagnosis of active TB while in a shelter designed exclusively for resettlement of newly arrived refugees, 3) were not residents of Toronto when they received a diagnosis of active TB, or 4) had incomplete records.
We collected data on patient demographics, clinical features of TB disease, medical management and health outcomes of patients, and molecular fingerprinting of the TB bacterium. Case types were classified according to the Public Health Agency of Canada definition of new and re-treatment TB cases (14). All chest radiographs were interpreted by radiologists. Susceptibility testing for Mycobacterium tuberculosis was performed at the Central Public Health Laboratory of the Ontario Agency for Health Protection and Promotion. All isolates from new TB cases were tested for susceptibility to first-line drugs (isoniazid, rifampin, pyrazinamide, and ethambutol) according to recommended standard protocols, by using the commercial broth system, BACTEC MGIT 960 (Becton, Dickinson and Company, Sparks, MD, USA). Isolates resistant to rifampin or any 2 first-line drugs were also tested for susceptibility to second-line drugs (15). Restriction fragment-length polymorphism was performed for strain genotyping by using established methods (16). Genotypes were analyzed by using Bionumerics 5.0 (Applied Maths, Saint-Martens Latem, Belgium). HIV test results were recorded when available; information about use of antiretroviral therapy for HIV/TB–co-infected patients was not available.
Comparisons were made between Canada-born and foreign-born case-patients and between 5-year periods (1998–2002 and 2003–2007) using the 2-sided Fisher exact test or χ2 test, as appropriate. Kaplan-Meier plots were generated to determine time to death from all causes during the 12 months after TB diagnosis. The log-rank test was used to compare survival curves between the 2 groups. Because of the small cohort size, multivariate regression analyses were not performed. All analyses were performed by using SAS version 9.1.3 (SAS Institute, Cary, NC, USA). Ethics approval was obtained from St. Michael’s Hospital Research Ethics Board.
Results
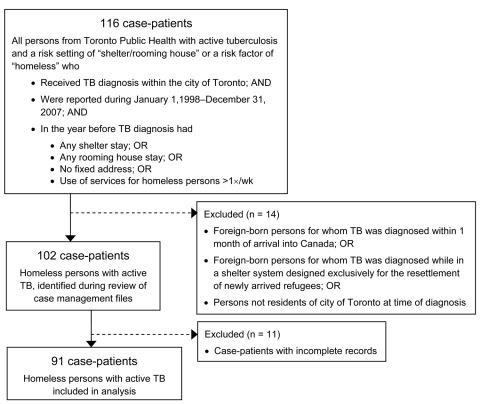
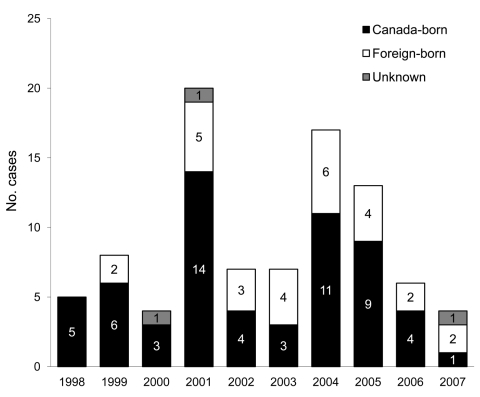
From January 1, 1998, through December 31, 2007, a total of 3,685 active TB cases were reported to Toronto Public Health; among these, 102 (2.8%) met the study inclusion criteria. Incomplete records for 11 patients resulted in a final sample size of 91 (Figure 1). Most patients were absolutely homeless (i.e., living on the street or in a shelter); 86 (95%) patients reported staying in a shelter, having no fixed address, and/or using services for homeless persons >1× per week. Five (5%) patients did not fall into any of these categories but had lived in a rooming house during the past year. Birthplace was available for 88 patients; nearly one third (n = 28; 32%) were born outside Canada (Table 1). The proportion of foreign-born patients increased over time from 24% (n = 10) in 1998–2002 to 39% (n = 18) in 2003–2007 (Table 2). Among the Canada-born homeless persons with TB, 13 (22%) were Aboriginal. The number of reported cases of active TB over the study period by place of birth is shown in Figure 2. Approximately equal numbers of cases were reported during the 2 periods: 44 (48%) during 1998–2002 and 47 (52%) during 2003–2007.
Figure 1.
Inclusion–exclusion criteria for study of active tuberculosis (TB) in homeless persons, Toronto, Ontario, Canada, 1998–2007.
Table 1. Country of origin for 28 foreign-born homeless persons with tuberculosis, Toronto, Ontario, Canada, 1998–2007.
| Country | No. |
|---|---|
| Burundi | 1 |
| Chile | 1 |
| China* | 1 |
| Costa Rica | 1 |
| Ethiopia* | 2 |
| Germany | 1 |
| Guyana | 2 |
| India* | 2 |
| Iraq | 1 |
| Ireland | 2 |
| Nepal | 1 |
| Nigeria* | 1 |
| Philippines* | 1 |
| Poland | 1 |
| Somalia | 2 |
| Tanzania* | 1 |
| Tibet | 1 |
| Turkey | 1 |
| Uganda* | 2 |
| United Kingdom | 1 |
| Yemen | 1 |
| Former Yugoslavia | 1 |
| Zimbabwe* | 2 |
*1 of 22 countries that account for 80% of all new of tuberculosis cases annually (high-burden countries).
Table 2. Demographic and clinical characteristics of 91 homeless persons with tuberculosis, Toronto, Ontario, Canada, 1998–2007*.
| Characteristic | All persons, no. (%) | Canada born, no. (%) | Foreign born, no. (%) | p value† | 1998–2002, no. (%) | 2003–2007, no. (%) | p value‡ |
|---|---|---|---|---|---|---|---|
| Median age, y (IQR) | 47 (38–56) | 49 (42–58) | 38 (30–50) | – | 45 (38–59) | 48 (40–54) | – |
| Male sex |
81 (89) |
53 (88) |
25 (89) |
1.00 |
40 (91) |
41 (87) |
0.74 |
| Origin | 0.15 | ||||||
| Canada born, not Aboriginal | 47 (53) | 47 (78) | NA | 27 (64) | 20 (43) | ||
| Canada born, Aboriginal | 13 (15) | 13 (22) | NA | 5 (12) | 8 (17) | ||
| Foreign born |
28 (32) |
NA |
28 (100) |
|
10 (24) |
18 (39) |
|
| Case type | 0.43 | 1.00 | |||||
| New | 83 (91) | 53 (88) | 27 (96) | 40 (91) | 43 (91) | ||
| Re-treated§ |
8 (9) |
7 (12) |
1 (4) |
|
4 (9) |
4 (9) |
|
| Method of detection | – | – | |||||
| Signs and symptoms | 51 (56) | 30 (50) | 18 (64) | 23 (52) | 28 (60) | ||
| Contact tracing | 19 (21) | 16 (27) | 3 (11) | 11 (25) | 8 (17) | ||
| Diagnosis while under care for other condition | 8 (9) | 8 (13) | 0 | 4 (9) | 4 (9) | ||
| Immigration screening | 6 (7) | NA | 6 (21) | 6 (14) | 0 | ||
| Active case finding (sputum screening) | 3 (3) | 3 (5) | 0 | NA | 3 (6) | ||
| Jail | 1 (1) | 1 (2) | 0 | 0 | 1 (2) | ||
| Other¶ |
3 (3) |
2 (3) |
1 (4) |
|
0 |
3 (6) |
|
| Site(s) of infection | 1.00 | 0.15 | |||||
| Pulmonary only | 67 (74) | 45 (75) | 21 (75) | 33 (75) | 34 (72) | ||
| Extrapulmonary only | 21 (23) | 13 (22) | 6 (21) | 8 (18) | 13 (28) | ||
| Pulmonary and extrapulmonary |
3 (3) |
2 (3) |
1 (4) |
|
3 (7) |
0 |
|
| Chest radiograph at diagnosis# | 0.08 | ||||||
| No abnormalities | 9 (13) | 7 (15) | 2 (9) | 0.86 | 7 (19) | 2 (5) | |
| Abnormal without cavitation | 45 (65) | 30 (64) | 15 (68) | 24 (67) | 21 (62) | ||
| Abnormal with cavitation |
15 (22) |
10 (21) |
5 (23) |
|
5 (14) |
11 (32) |
|
| Self-reported symptoms | 73 (80) | 50 (83) | 20 (71) | 0.26 | 34 (77) | 39 (83) | 0.60 |
| Median time from symptom onset to diagnosis, mo (IQR) |
1.9 (0.6–3.1) |
1.8 (0.6–2.6) |
2.6 (0.8–5.8) |
– |
1.8 (0.6–3.1) |
2.2 (0.6–3.2) |
– |
| Sputum smear results at diagnosis** | 0.39 | 0.58 | |||||
| Negative | 21 (33) | 12 (29) | 9 (43) | 12 (40) | 9 (27) | ||
| Scarce/moderate | 13 (21) | 8 (20) | 5 (24) | 5 (17) | 8 (24) | ||
| Numerous |
29 (46) |
21 (51) |
7 (33) |
|
13 (43) |
16 (49) |
|
| Method of diagnosis | 0.59 | 0.18 | |||||
| Positive culture | 86 (95) | 55 (92) | 28 (100) | 40 (91) | 46 (98) | ||
| Positive AMTD | 3 (3) | 3 (5) | 0 | 3 (7) | 0 | ||
| Clinical | 2 (2) | 2 (3) | 0 | 1 (2) | 1 (2) |
*Birthplace information available for 88 persons. IQR, interquartile range; NA, not applicable; –, no statistical test performed; AMTD, amplified Mycobacterium tuberculosis direct test. †Probability of a significant difference between Canada-born and foreign-born persons for each variable; calculated by using the 2-sided Fisher exact test or χ2 test, as appropriate. ‡Probability of a significant difference between the 2 periods for each variable; calculated by using the 2-sided Fisher exact test or χ2 test, as appropriate. §Public Health Agency of Canada definition: documented evidence or adequate history of previously active tuberculosis (TB) that was declared cured or treatment completed by current standards, AND at least 6 mo have passed since the last day of previous treatment, AND a diagnosis with a subsequent episode of TB that meets the active TB case definition. ¶Includes shelter screening, routine screening at other centers, and other detection methods. #Results for only the 70 persons with pulmonary disease. **Sputum smears available for 63 patients with pulmonary disease.
Figure 2.
Number of reported cases of active tuberculosis in homeless persons, Toronto, Ontario, Canada, 1998–2007.
Demographic information, clinical characteristics, and concurrent medical conditions for patients are presented in Tables 2 and 3. Homeless persons with TB were often highly contagious at the time of diagnosis, as demonstrated by the large proportion of patients who had cavitating pulmonary disease and sputum smears with numerous acid-fast bacilli. The median duration of symptoms for persons with pulmonary disease before diagnosis was 2.5 months (interquartile range 0.6–3.1 months). Pulmonary disease was found in 67 (74%) patients, among whom 29 (46%) showed numerous acid-fast bacilli in sputum smear. The proportion of pulmonary TB patients with cavitary disease increased over time from 14% (n = 5) in 1998–2002 to 32% (n = 11) in 2003–2007.
Table 3. Concurrent conditions of 91 homeless persons with tuberculosis, Toronto, Ontario, Canada, 1998–2007*.
| Condition | All persons, no. (%) | Canada born, no. (%) | Foreign born, no. (%) | p value† | 1998–2002, no. (%) | 2003–2007, no. (%) | p value‡ |
|---|---|---|---|---|---|---|---|
| HIV infection | 0.52 | 0.33 | |||||
| Positive | 11 (12) | 9 (15) | 1 (4) | 5 (11) | 6 (13) | ||
| Negative | 6 (7) | 4 (7) | 2 (7) | 1 (2) | 5 (10) | ||
| Unknown |
74 (81) |
47 (78) |
25 (89) |
|
38 (87) |
36 (77) |
|
| Psychiatric disease§ | 10 (11) | 7 (12) | 2 (7) | 0.71 | 2 (5) | 8 (17) | 0.09 |
| COPD | 8 (9) | 7 (12) | 0 | 0.09 | 6 (14) | 2 (4) | 0.15 |
| Liver disease¶ | 29 (32) | 24 (40) | 4 (14) | 0.03 | 12 (27) | 17 (36) | 0.38 |
| Cancer | 5 (5) | 4 (7) | 1 (4) | 1.00 | 3 (7) | 2 (4) | 0.67 |
| Congestive heart failure | 1 (1) | 0 | 0 | – | 1 (2) | 0 | 0.48 |
| Diabetes | 11 (12) | 5 (8) | 5 (18) | 0.28 | 1 (2) | 10 (21) | 0.01 |
| Chronic alcohol abuse | 29 (32) | 23 (38) | 6 (21) | 0.15 | 12 (27) | 17 (36) | 0.38 |
| Injection drug use | 12 (13) | 10 (17) | 1 (4) | 0.16 | 6 (14) | 6 (13) | 1.00 |
| Noninjection drug use | 6 (7) | 6 (10) | 0 | 0.17 | 0 | 6 (13) | 0.03 |
| Other | 1 (1) | 0 | 1(4) | 0.32 | 0 | 1 (2) | 1.00 |
*Birthplace information available for 88 persons.–, no statistical test performed; COPD, chronic obstructive pulmonary disease. †Probability of a significant difference between Canada-born and foreign-born persons for each variable; calculated by using the 2-sided Fisher exact test or χ2 test, as appropriate. ‡Probability of a significant difference between the 2 periods for each variable; calculated by using the 2-sided Fisher exact test or χ2 test, as appropriate. §Includes schizophrenia, severe mental illness, and dementia. ¶Includes cirrhosis, viral hepatitis B or C.
In terms of treatment information and outcomes, 75% of homeless persons with TB started treatment within 4 days of diagnosis (median 1 day; interquartile range 0–4 days) (Table 4). Most patients received closely monitored treatment within hospitals or as outpatients under directly observed therapy (DOT) (median treatment duration 2.0 and 6.2 months, respectively); few received self-administered therapy for any substantial period of time. Only 1 patient required a court order for treatment in a TB sanitarium.
Table 4. Treatment-associated characteristics of 91 homeless persons with tuberculosis, Toronto, Ontario, Canada, 1998–2007*.
| Characteristic | All persons, no. (%) | Canada-born, no. (%) | Foreign born, no. (%) | p value† | 1998–2002, no. (%) | 2003–2007, no. (%) | p value‡ |
|---|---|---|---|---|---|---|---|
| Days from diagnosis to initiation of treatment, median (IQR) |
1 (0–4) |
0.5 (0–4) |
1 (0–6) |
– |
1 (0–3) |
1 (0–4) |
– |
| Duration of treatment, mo, median (IQR) | |||||||
| Total | 9.9 (6.5–12.7) | 10.0 (6.8–12.5) | 8.6 (6.0–12.9) | – | 9.0 (6.1–12.2) | 10.8 (6.7–13.1) | – |
| Treatment in institution | 2.0 (0.2–4.0) | 2.4 (0.5–3.9) | 0.6 (0–4.2) | 0.9 (0.2–3.9) | 2.2 (0.0–4.1) | ||
| Treatment under directly observed therapy | 6.2 (0.8–8.8) | 6.4 (0.9–9.0) | 6.0 (2.4–7.2) | 5.7 (0.2–7.0) | 6.6 (4.5–9.0) | ||
| Treatment by self-
administered therapy |
0.1
(0.1–0.9) |
0.1
(0.1–0.6) |
0.3
(0.1–2.1) |
|
0.2
(0.0–1.1) |
0.1
(0.1–0.6) |
|
| Admission to hospital | 76 (84) | 55 (92) | 19 (68) | 0.01 | 35 (80) | 41 (87) | 0.40 |
| Treatment under public health order§ | 15 (16) | 9 (15) | 6 (21) | 0.55 | 4 (9) | 11 (23) | 0.09 |
| Court-ordered detention for treatment¶ |
1 (1) |
1 (2) |
0 |
– |
1 (2) |
0 |
– |
| Outcome# | 0.13 | 0.74 | |||||
| Treatment completed | 70 (78) | 45 (75) | 25 (93) | 33 (75) | 37 (80) | ||
| Died while receiving treatment** | 17 (19) | 12 (20) | 2 (7) | 9 (20) | 7 (16) | ||
| Lost to follow-up | 2 (2) | 2 (3) | 0 | 1 (2) | 1 (2) | ||
| Refused further care | 1 (1) | 1 (2) | 0 | 1 (2) | 0 |
*Birthplace information available for 88 persons. IQR, interquartile range; –, no statistical test performed. †Probability of a significant difference between Canada-born and foreign-born persons for each variable; calculated by using the 2-sided Fisher exact test or χ2 test, as appropriate. ‡Probability of a significant difference between the 2 periods for each variable; calculated by using the 2-sided Fisher exact test or χ2 test, as appropriate. §Referred to in Ontario as a Section 22. ¶Referred to in Ontario as a Section 35. #1 patient continues treatment at the time of this report. **One foreign-born case-patient from 2003–2007 period died 14 mo after onset of treatment. Study considers death from all causes within 12 mo of TB diagnosis. After 12 mo, causes of death other than TB become more relevant; therefore, deaths occurring after 12 mo are not included in this estimate.
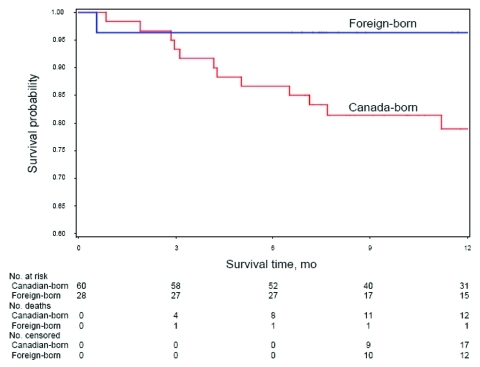
Almost 1 of 5 (n = 17; 19%) patients died (from any cause) within 12 months of diagnosis (Table 4); most (n = 12; 86%) patients who died were born in Canada; 4 were HIV positive, 1 was HIV negative, and the remaining 12 had unknown HIV status. Among patients who survived, most (n = 70; 96%) homeless persons with TB successfully completed treatment and only 3 were lost to follow-up or refused further care. Probability of survival during the 12 months after diagnosis was lower for Canada-born versus foreign-born homeless persons (p = 0.06; Figure 3). No changes in survival probabilities were seen between the 2 periods, 1998–2002 and 2003–2007 (data not shown).
Figure 3.
Probability of death from all causes during the 12-month period after tuberculosis diagnosis among Canada-born and foreign-born homeless persons with active tuberculosis, Toronto, Ontario, Canada, 1998–2007. Birthplace information available for 88 persons. Censored are patients who completed treatment for tuberculosis or were lost to follow up.
TB isolates were genotyped and tested for antimicrobial drug resistance (Table 5). Of the 4 strains known to circulate among Toronto’s homeless population (A, B, C, or D), isolates from ≈90% of Canada-born patients belonged to one of these strains, and isolates from >50% of foreign-born patients belonged to none of them. The proportion of isolates not belonging to these 4 strains increased over time, from 14% (n = 4) during 1998–2002 to 32% (n = 12) during 2003–2007. Almost all (n = 84; 98%) isolates were susceptible to first-line TB medications. Only 2 isolates demonstrated evidence of antimicrobial drug resistance: 1, from a Canada-born patient, was resistant to ethambutol only, and 1, from a foreign-born patient, was resistant to isoniazid only.
Table 5. Characteristics of isolates from 91 homeless persons with tuberculosis, Toronto, Ontario, Canada, 1998–2007*.
| Characteristic | Culture-confirmed case, no. (%) | Canada-born, no. (%) | Foreign born, no. (%) | p value† | 1998–2002, no. (%) | 2003–2007, no. (%) | p value‡ |
|---|---|---|---|---|---|---|---|
| RFLP type | <0.001 | 0.13 | |||||
| Strain A | 32 (48) | 25 (52) | 6 (38) | 17 (59) | 15 (41) | ||
| Strain B | 8 (12) | 7 (15) | 1 (6) | 3 (10) | 5 (14) | ||
| Strain C | 7 (11) | 7 (15) | 0 | 5 (17) | 2 (5) | ||
| Strain D | 3 (5) | 3 (6) | 0 | 0 | 3 (8) | ||
| Other strain | 16 (24) | 6 (13) | 9 (56) | 4 (14) | 12 (32) | ||
| Not tested |
20 |
7 |
12 |
|
11 |
9 |
|
| Antimicrobial drug resistance | 1.00 | 0.21 | |||||
| No | 84 (98) | 54 (98) | 27 (96) | 38 (95) | 46 (100) | ||
| Yes§ | 2 (2) | 1 (2) | 1 (4) | 2 (5) | 0 |
*Birthplace information available for 88 persons. RFLP, restriction fragment-length polymorphism. †Probability of a significant difference between Canada-born and foreign-born persons for each variable; calculated by using the 2-sided Fisher exact test or χ2 test, as appropriate. ‡Probability of a significant difference between the 2 periods for each variable; calculated by using the 2-sided Fisher exact test or χ2 test, as appropriate. §Represents 1 Canada-born person whose isolate was resistant to ethambutol only and 1 foreign-born case whose isolate was resistant to isoniazid only.
Discussion
Homeless persons in our cohort received nearly all health care services for TB in hospital or under careful observation in the outpatient environment. All outpatients received DOT, were accompanied by public health staff to all clinic visits, and received intensive social assistance. Despite these efforts, all-cause mortality rates for our cohort were extremely high. Among Canada-born homeless persons with TB in our study, 20% died within 1 year of their diagnosis; in comparison, among all persons with TB in Toronto during 1999–2002, only 7.4% died (17). All-cause mortality rates for homeless populations in general are disproportionately high; rates among men who use shelters in Toronto are 2–8× higher than rates for the general population (18). Homeless persons often have more concurrent medical conditions (e.g., HIV, liver disease), mental health conditions (e.g., schizophrenia), and/or dependence on substances, any of which may raise their risk for primary or reactivated TB, complicate delivery of health services, and negatively affect treatment outcomes.
Our findings reflect the increased rates of illness and death among homeless persons and suggest that urgent measures are needed to improve TB treatment outcomes for this vulnerable population. Recent research suggests that homeless immigrants in Toronto are in general healthier and possess fewer concurrent illnesses than Canada-born homeless persons, which may explain the lower prevalence of concurrent illnesses among foreign-born TB patients and the differences in mortality rates according to place of birth (19).
Homeless TB patients represent ≈3% of all TB patients in Toronto, of which a growing proportion are foreign-born, likely reflecting the changing demographics in the city overall and in the homeless population itself. Our findings suggest that Canada-born patients with TB were more likely to be infected with strains known to circulate within shelters and other social networks in Toronto. In contrast, active TB in foreign-born patients was more likely to result from reactivation of latent infection with strains acquired overseas (20,21).
To date, drug resistance among homeless TB patients is rare; laboratory evidence of drug resistance was demonstrated for only 2% of homeless TB patients compared with 14% of all culture-positive TB patients in Toronto (1). Because being born outside Canada is a known risk factor for drug-resistant TB, the rise in foreign-born homeless TB patients and the corresponding increase in heterogeneity of strain genotypes are concerning and may pose serious and growing threats to the homeless shelter system (20,22–25). The outbreaks of multidrug resistant TB in New York City during the 1980s and early 1990s highlight the potential dangers of introducing drug-resistant infections into the shelter system and call for increased prevention and control efforts (26).
Despite the increase in foreign-born homeless persons with TB over time, most homeless TB patients in our sample were Canada-born (68%), a substantial proportion of whom were of Aboriginal origin (22%). By comparison, in 2008, only 6% of persons with active TB in Toronto were Canada-born (1). Although TB in Canada is primarily a disease of foreign-born persons (14), our results suggest that TB transmission persists among Canada-born inner city homeless populations. These findings also underscore the need to address TB transmission within the homeless shelter system. Furthermore, the disproportionately high prevalence of Canada-born Aboriginal persons in our sample suggests that further efforts are needed to address the high incidence of TB in this population.
Homeless TB patients tend to seek care when disease is advanced and highly contagious, defined by abnormal chest radiographic findings (cavitation) and numerous acid-fast bacilli in sputum smear. Although many homeless patients had pulmonary disease, the number was proportional to the prevalence of pulmonary disease among all TB patients in Toronto (1). In patients with pulmonary TB, nearly half had numerous acid-fast bacilli in sputum smear. An increasing prevalence of cavitary disease on chest radiographs was observed over time, despite increasing intervention and active case-finding initiatives during the more recent 5-year period of this study. However, the increase in cavitary disease could be related to delays in seeking health care, as indicated by the increase in median time from symptom onset to diagnosis over the 2 periods of the study.
Homeless TB patients often have difficulty accessing the health care system and may prioritize subsistence needs such as food and shelter over health services, especially those perceived as discretionary (27). These factors, as well as cultural and language barriers among foreign-born patients (28,29), may contribute to delays in seeking health care, which lead to advanced disease and hospitalization (27,30,31). For our sample, hospitalization rates were high; >80% of patients were hospitalized. This is noteworthy in Canada, where most TB patients are treated as outpatients, even at the time of diagnosis (32). The inability to isolate infectious homeless patients in outpatient settings such as shelters largely explains the high rate of hospitalization for patients in our sample.
Adherence to treatment is often challenging for patients who are homeless or living in transient, substandard housing and who may have concurrent substance use or mental health problems. Consequently, DOT is usually implemented for homeless persons with TB in Canada (32). In the province of Ontario, all patients with active TB are eligible to receive either inpatient or outpatient TB treatment, regardless of their insurance coverage. Most patients in our sample received their entire treatment closely monitored within hospitals, with outpatient DOT, or both. A few patients self-administered treatment for short periods. Despite the common perception that homeless TB patients are noncompliant with treatment (33–35), ≈80% of patients in our sample completed treatment, which is equivalent to the treatment completion rate for all TB patients in Toronto receiving DOT (1). Intensive case management by public health and clinic staff as well as small incentives and enablers (e.g., food vouchers or cash) helped ensure high completion rates for this population. For most homeless TB patients who did not complete treatment, the reason was that they died; only a few were lost to follow-up or refused further care.
The strength of this study is that it provides a comprehensive description of all cases of TB among homeless persons in a large, ethnically diverse city in Canada over a 10-year period. However, the study also has limitations. Only patients who were residents of the city of Toronto at the time of diagnosis were included in the analysis; consequently, homeless persons with active TB who may have been exposed in Toronto shelters or rooming houses and later moved elsewhere were not detected. Furthermore, homeless persons with TB were not included in the analysis if they had a history of shelter use >1 year before diagnosis with active TB. As a result, some patients who acquired TB infection while homeless but who subsequently acquired housing may have been missed. Furthermore, our retrospective study used public health surveillance data; consequently, our analyses are subject to limitations in how the data were originally collected. We excluded 14 patients with active TB because their records were incomplete; hence, we were unable to determine whether they differed demographically from included patients. This limitation could have influenced the results of our analyses that were stratified by birthplace.
Molecular fingerprinting data were unavailable for 20 isolates, which may have influenced the genotyping trends we observed over time. Because we were unable to definitively determine the number of patients who died directly or indirectly as a result of TB, the mortality rates represent death from all causes in the year after TB diagnosis. Although our study was conducted in a single metropolitan urban center, our findings and recommendations may be relevant to other large cities where levels of immigration and poverty are high.
Prior research among homeless persons in New York City shows decreasing trends in rates of active TB during 1992–2006 and demonstrates that public health prevention and control efforts (e.g., latent TB infection screening) in this population can be effective (36). In our study, several homeless patients were originally identified as tuberculin skin test–positive contacts before active TB developed, but they were unwilling to start treatment for latent TB infection, were deemed poor candidates for treatment because of serious underlying medical or mental health conditions, or could not complete a course of treatment because of adverse drug reactions. Lack of treatment for latent TB occurred despite substantial incentives and enablers for persons to initiate and continue therapy (e.g., cash for attending clinic visits, free passes for taxis or public transit, use of DOT for latent TB infection). Hence, for this cohort the opportunity to mitigate the risk for development of active TB through the treatment of latent TB infection was limited by the above challenges.
Primary prevention efforts should focus on shelter-based control measures, which have proven effective at reducing person-to-person spread of drug-resistant TB in other urban centers (37). Improved ventilation systems at shelters will help reduce the spread of TB during an outbreak (38,39). Smaller shelter sizes and strategies to reduce mobility (e.g., eliminating length of stay restrictions at shelters) may also help limit the extent of transmission. Additionally, expansion of sustainable housing programs for homeless and marginally housed populations will help reduce the number of persons needing to use shelters, subsequently decreasing the likelihood of TB exposure at these congregate settings.
Control of TB in homeless populations within Canada will require further progress in primary prevention (e.g., improved ventilation and other infection control measures in shelters), secondary prevention (e.g., earlier detection and treatment of TB infection or disease through greater access to primary care), and tertiary prevention (e.g., treatment of active TB by health care providers with experience treating TB in homeless persons) (17). Furthermore, Canada’s interconnectedness with the global community, and consequent interdependence with global TB, necessitates continued vigilance to confront the emerging threat of drug-resistant TB in the world (40).
Acknowledgments
This study was supported by the Connor, Clark & Lunn Foundation, the St. Michael’s Hospital Foundation, the Ontario Ministry of Health and Long-Term Care, and the Public Health Agency of Canada.
Biography
Dr Khan is an infectious disease physician and scientist at St. Michael’s Hospital and an associate professor at the University of Toronto. His clinical and research interests pertain to TB and other infectious diseases affecting immigrant and refugee populations.
Footnotes
Suggested citation for this article: Khan K, Rea E, McDermaid C, Stuart R, Chambers C, Wang J, et al. Active tuberculosis among homeless persons, Toronto, Ontario, Canada, 1998–2007. Emerg Infect Dis [serial on the Internet]. 2011 Mar [date cited]. http://dx.doi.org/10.3201/eid1703.100833
References
- 1.Diseases transmitted by direct contact and respiratory routes. Communicable diseases in Toronto, 2009. Toronto: Toronto Public Health; 2008 [cited 2011 Jan 11]. http://www.toronto.ca/health/cdc/communicable_disease_surveillance/statistics_and_reports/annual_reports/pdf/2009/2009_direct_contact_resp_report.pdf
- 2.Centers for Disease Control and Prevention. Public health dispatch: tuberculosis outbreak among homeless persons—King County, Washington, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52:1209–10. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Public health dispatch: tuberculosis outbreak in a homeless population—Portland, Maine, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52:1184. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Tuberculosis transmission in a homeless shelter population—New York, 2000–2003. MMWR Morb Mortal Wkly Rep. 2005;54:149–52. [PubMed] [Google Scholar]
- 5.McElroy PD, Southwick KL, Fortenberry ER, Levine EC, Diem LA, Woodley CL, et al. Outbreak of tuberculosis among homeless persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2003;36:1305–12. 10.1086/374836 [DOI] [PubMed] [Google Scholar]
- 6.Lathan M, Mukasa LN, Hooper N, Golub J, Baruch N, Mulcahy D, et al. Cross-jurisdictional transmission of Mycobacterium tuberculosis in Maryland and Washington, DC, 1996–2000, linked to the homeless. Emerg Infect Dis. 2002;8:1249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukacs J, Tubak V, Mester J, David S, Bartfai Z, Kubica T, et al. Conventional and molecular epidemiology of tuberculosis in homeless patients in Budapest, Hungary. J Clin Microbiol. 2004;42:5931–4. 10.1128/JCM.42.12.5931-5934.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macaraig M, Agerton T, Driver CR, Munsiff SS, Abdelwahab J, Park J, et al. Strain-specific differences in two large Mycobacterium tuberculosis genotype clusters in isolates collected from homeless patients in New York City from 2001 to 2004. J Clin Microbiol. 2006;44:2890–6. 10.1128/JCM.00160-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss AR, Hahn JA, Tulsky JP, Daley CL, Small PM, Hopewell PC. Tuberculosis in the homeless. A prospective study. Am J Respir Crit Care Med. 2000;162:460–4. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PF, Yang Z, Pogoda JM, Preston-Martin S, Jones BE, Otaya M, et al. Foci of tuberculosis transmission in central Los Angeles. Am J Respir Crit Care Med. 1999;159:1081–6. [DOI] [PubMed] [Google Scholar]
- 11.2006 Census: immigration and citizenship. Release no. 4. Ottawa (Canada): Statistics Canada; 2007. Dec 31 [cited 2010 Jan 21]. http://www12.statcan.gc.ca/census-recensement/2006/rt-td/immcit-eng.cfm
- 12.The City of Toronto. The Toronto report card on housing and homelessness 2003. [cited 2010 Dec 31]. http://www.toronto.ca/homelessness/pdf/reportcard2003.pdf
- 13.Government of Ontario. Verdict of coroner’s jury. Teigesser, Joseph. Nov 17, 2003– May 7, 2004. Toronto: Office of the Chief Coroner, Ministry of Community Safety and Correctional Services, Government of Ontario; 2004. [Google Scholar]
- 14.Ellis E, Dawson K, Gallant V, Saunders A, Scholten D. Tuberculosis in Canada. Ottawa (Ontario, Canada): Public Health Agency of Canada; 2007. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. (CLSI, formerly NCCLS). Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. NCCLS document M24-A. Wayne (PA): The Institute; 2003. [PubMed] [Google Scholar]
- 16.Van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan K, Campbell A, Wallington T, Gardam M. The impact of physician training and experience on the survival of patients with active tuberculosis. CMAJ. 2006;175:749–53. 10.1503/cmaj.060124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SW. Mortality among men using homeless shelters in Toronto, Ontario. JAMA. 2000;283:2152–7. 10.1001/jama.283.16.2152 [DOI] [PubMed] [Google Scholar]
- 19.Chiu S, Redelmeier DA, Tolomiczenko G, Kiss A, Hwang SW. The health of homeless immigrants. J Epidemiol Community Health. 2009;63:943–8. 10.1136/jech.2009.088468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long R, Sutherland K, Kunimoto D, Cowie R, Manfreda J. The epidemiology of tuberculosis among foreign-born persons in Alberta, Canada, 1989–1998: identification of high-risk groups. Int J Tuberc Lung Dis. 2002;6:615–21. [PubMed] [Google Scholar]
- 21.McKenna MT, McCray E, Onorato I. The epidemiology of tuberculosis among foreign-born persons in the United States, 1986 to 1993. N Engl J Med. 1995;332:1071–6. 10.1056/NEJM199504203321606 [DOI] [PubMed] [Google Scholar]
- 22.Rivest P, Tannenbaum T, Bedard L. Epidemiology of tuberculosis in Montreal. CMAJ. 1998;158:605–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Hersi A, Elwood K, Cowie R, Kunimoto D, Long R. Multidrug-resistant tuberculosis in Alberta and British Columbia, 1989 to 1998. Can Respir J. 1999;6:155–60. [DOI] [PubMed] [Google Scholar]
- 24.Tuberculosis Prevention and Control, Public Health Agency of Canada. Drug-resistant tuberculosis among the foreign-born in Canada. Can Commun Dis Rep. 2005;31:4446–52. [Google Scholar]
- 25.Long R, Fanning EA, Cowie RL, Hoeppner V, Fitzgerald M. Antituberculosis drug resistance in western Canada (1993 to 1994). Can Respir J. 1997;4:71–5. [Google Scholar]
- 26.Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen GM, Dooley SW. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521. 10.1056/NEJM199302253280801 [DOI] [PubMed] [Google Scholar]
- 27.Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87:217–20. 10.2105/AJPH.87.2.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman LF, Fujiwara PI, Cook SV, Bazerman LB, Frieden TR. Patient and health care system delays in the diagnosis and treatment of tuberculosis. Int J Tuberc Lung Dis. 1999;3:1088–95. [PubMed] [Google Scholar]
- 29.Gardam M, Verma G, Campbell A, Wang J, Khan K. Impact of the patient–provider relationship on the survival of foreign-born outpatients with tuberculosis. J Immigr Minor Health. 2009;11:437–45. 10.1007/s10903-008-9221-8 [DOI] [PubMed] [Google Scholar]
- 30.Marks SM, Taylor Z, Burrows NR, Qayad MG, Miller B. Hospitalization of homeless persons with tuberculosis in the United States. Am J Public Health. 2000;90:435–8. 10.2105/AJPH.90.3.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martell JV, Seitz RS, Harada JK, Kobayashi J, Sasaki VK, Wong C. Hospitalization in an urban homeless population: the Honolulu Urban Homeless Project. Ann Intern Med. 1992;116:299–303. [DOI] [PubMed] [Google Scholar]
- 32.Hoeppner VH, Ward H, Elwood K. Treatment of tuberculosis disease and infection. In: Long R, Ellis E, editors. Canadian tuberculosis standards. 6th ed. Ottawa (Ontario, Canada): Tuberculosis Prevention and Control, Public Health Agency of Canada and Canadian Lung Association/Canadian Thoracic Society; 2007. p. 114–143. [Google Scholar]
- 33.Borgdorff MW, Veen J, Kalisvaart NA, Broekmans JF, Nagelkerke NJ. Defaulting from tuberculosis treatment in the Netherlands: rates, risk factors and trend in the period 1993–1997. Eur Respir J. 2000;16:209–13. 10.1034/j.1399-3003.2000.16b05.x [DOI] [PubMed] [Google Scholar]
- 34.Burman WJ, Cohn DL, Rietmeijer CA, Judson FN, Sbarbaro JA, Reves RR. Noncompliance with directly observed therapy for tuberculosis. Epidemiology and effect on the outcome of treatment. Chest. 1997;111:1168–73. 10.1378/chest.111.5.1168 [DOI] [PubMed] [Google Scholar]
- 35.LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med. 2003;168:443–7. 10.1164/rccm.200303-390OC [DOI] [PubMed] [Google Scholar]
- 36.McAdam JM, Bucher SJ, Brickner PW, Vincent RL, Lascher S. Latent tuberculosis and active tuberculosis disease rates among the homeless, New York, New York, USA, 1992–2006. Emerg Infect Dis. 2009;15:1109–11. 10.3201/eid1507.080410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City: turning the tide. N Engl J Med. 1995;333:229–33. 10.1056/NEJM199507273330406 [DOI] [PubMed] [Google Scholar]
- 38.Canadian Tuberculosis Committee. Housing conditions that serve as risk factors for tuberculosis infection and disease. An Advisory Committee Statement (ACS). Can Commun Dis Rep. 2007;33:1–13. [PubMed] [Google Scholar]
- 39.Menzies D, Fanning A, Yuan L, FitzGerald JM. Hospital ventilation and risk for tuberculous infection in Canadian health care workers. Ann Intern Med. 2000;133:779–89. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance, 2002–2007. Anti-tuberculosis drug resistance in the world. Fourth global report. Geneva: The Organization; 2008. [cited 2011 Jan 11]. http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf