Abstract
Background
Asthma is a chronic inflammatory disease with a strong genetic predisposition. A major challenge for candidate gene association studies in asthma is the selection of biologically relevant genes.
Methodology/Principal Findings
Using epithelial RNA expression arrays, HapMap allele frequency variation, and the literature, we identified six possible candidate susceptibility genes for childhood asthma including ADCY2, DNAH5, KIF3A, PDE4B, PLAU, SPRR2B. To evaluate these genes, we compared the genotypes of 194 predominantly tagging SNPs in 790 asthmatic, allergic and non-allergic children. We found that SNPs in all six genes were nominally associated with asthma (p<0.05) in our discovery cohort and in three independent cohorts at either the SNP or gene level (p<0.05). Further, we determined that our selection approach was superior to random selection of genes either differentially expressed in asthmatics compared to controls (p = 0.0049) or selected based on the literature alone (p = 0.0049), substantiating the validity of our gene selection approach. Importantly, we observed that 7 of 9 SNPs in the KIF3A gene more than doubled the odds of asthma (OR = 2.3, p<0.0001) and increased the odds of allergic disease (OR = 1.8, p<0.008). Our data indicate that KIF3A rs7737031 (T-allele) has an asthma population attributable risk of 18.5%. The association between KIF3A rs7737031 and asthma was validated in 3 independent populations, further substantiating the validity of our gene selection approach.
Conclusions/Significance
Our study demonstrates that KIF3A, a member of the kinesin superfamily of microtubule associated motors that are important in the transport of protein complexes within cilia, is a novel candidate gene for childhood asthma. Polymorphisms in KIF3A may in part be responsible for poor mucus and/or allergen clearance from the airways. Furthermore, our study provides a promising framework for the identification and evaluation of novel candidate susceptibility genes.
Introduction
The amount of genetic information available with high throughput screens has increased exponentially in the last decade, with the potential for information on three billion base pairs being available through genome wide sequencing. While the genome wide approach has been successfully used to identify numerous genetic variants associated with complex human diseases [1], most variants identified so far confer relatively small increments in risk, and explain only a small proportion of disease heritability. This has led to considerable speculation regarding the sources of the “missing heritability” [2]. Common diseases such as asthma are heterogeneous and may actually be a compilation of disorders with numerous subphenotypes. Current studies are not designed to examine specific subphenotypes of disease due to the large sample sizes required for genome wide approaches. Genome wide approaches require thousands of cases and controls to have sufficient power to properly evaluate such associations. As with many complex diseases such as asthma, analysis of large sample sizes made up of heterogeneous phenotypes may make it more difficult to identify true associations. By focusing on regions with a priori evidence of gene involvement, the candidate gene approach has the advantage of requiring smaller sample sizes as fewer statistical tests are performed. One of the major challenges of the candidate gene approach for genetic studies is selection of appropriate genes for evaluation. Methods that have been utilized thus far include selection of genes based on published biologic function, findings from mouse models of asthma, and chromosomal location in ‘hot-spots’ that have been linked to disease phenotypes in genome wide association studies (GWAS) and linkage studies. While over 600 studies have used these strategies to identify more than 120 different genes to be associated with asthma or its related phenotypes, only a limited number of genes have been replicated leaving much variation yet unexplained [3].
Another promising strategy to narrow the numerous potential disease-associated genes in a less biased way involves examining differences in allele frequencies between populations in conjunction with differences in gene expression within relevant cell types or tissues [4]. Inclusion of an analysis of allele frequency differences between populations may be a beneficial strategy for any disease that shows significant differences in prevalence between groups. A recent study by Frank et. al [5] integrated global gene expression arrays, DNA sequence variation arrays, and public databases to identify new previously untested candidate genes for further testing. This more targeted approach allows researchers to reduce the overall number of genes to be tested and hence increases statistical power to detect an association by lowering the multiple testing burden.
The overall objective of our study was to develop an innovative approach for identifying candidate genes for genetic association studies with complex diseases including asthma. We combined the unbiased characteristics typically obtained using GWAS or expression arrays with the more focused quality of traditional literature-based candidate gene approaches. The basis of our resulting approach takes advantage of our previously published evaluation of nasal epithelial cell-derived RNA from asthmatic and non-allergic children [6], population differences in asthma prevalence, tagging SNPs in the HapMap database, and the published literature. Importantly, nasal epithelial cell samples were used as our source tissue, because of their ability to interface with and function as a physical barrier to the environment, their importance in initiating the immune response to environmental triggers and their role in modulating allergic inflammation [7], [8]. Further, compared to collection of bronchial lavage fluid or bronchial biopsies, nasal epithelial cell collection is less invasive and has been shown to be a good surrogate for the lower airway epithelium [9], [10].
Using our approach, we identified five novel genes that had not been previously implicated in asthma and a sixth gene (PDE4B) that was recently linked to asthma (Table 1) [11]. Upon analysis, we determined that all six genes were nominally associated with pediatric asthma in our discovery population (p<0.05). SNPs in a single gene, KIF3A, were significant even after considering multiple comparisons (p<0.0001). Our results substantiate the validity of our candidate gene selection approach.
Table 1. Selected genes and functions.
| #SNPsa | Gene | Full Gene Name | Chr. | Array Findingsb | Reported Processes and Functionc | Reported Associated Diseases |
| 9 | KIF3A | kinesin family member 3A | 5q31 | Down | protein binding, ATP binding, microtubule motor activity, nucleotide binding | |
| 53 | DNAH5 | dynein, axonemal, heavy polypeptide 5 | 5p15 | Down | microtubule motor activity, ATP binding, ATPase activity, nucleotide binding | primary ciliary dyskinesia, ciliary motility disorders |
| 68 | ADCY2 | adenylate cyclase 2 | 5p15 | Down | adenylate cyclase activity, magnesium ion binding, phosphorus-oxygen lyase activity | |
| 7 | PLAU | plasminogen activator, urokinase | 10q24 | Up | kinase activity, peptidase activity, plasminogen activator activity, serine-type endopeptidase activity | acantholysis, alzheimers rheumatoid arthritis, cancer, endometriosis |
| 53 | PDE4B | phosphodiesterase 4B | 1p31 | Up | 3′,5′-cyclic-AMP phosphodiesterase activity, 3′,5′-cyclic-nucleotide phosphodiesterase activity, catalytic activity, hydrolase activity | chronic kidney failure, schizophrenia |
| 4 | SPRR2B | small proline-rich protein 2B | 1q21 | Up | structural molecule activity, keratinization |
Indicates the total number of genotyped SNPs.
Indicates the direction of gene expression of uncontrolled asthmatics versus non-allergic controls.
Obtained from Gene Ontology website (www.geneontology.org).
Methods
Ethics
The study protocol was approved by the Cincinnati Children's Hospital Medical Center Institutional Review Board. Parents gave written informed consent for the children's participation, and children gave their assent.
Study Populations
The discovery population consisted of a subset of 4 to 17 year old Caucasian participants enrolled in either the Greater Cincinnati Pediatric Clinic Repository (GCPCR) or the Genomic Control Cohort (GCC), a cohort supported by the Cincinnati Children's Hospital Medical Center (CCHMC). The GCPCR includes over 6,200 patients with various diagnoses visiting CCHMC outpatient specialty clinics, the Emergency Department or recruited from the community. Participants completed questionnaires, and provided buccal or saliva samples for genetic analyses. The GCC includes over 1,080 children recruited to be representative of the Greater Cincinnati area. All GCC participants completed a questionnaire that included asthma, allergy and skin questions similar to those included in GCPCR and provided a blood sample for genetic analysis. Non-asthmatic/non-allergic control children were not proactively recruited into the GCPCR for this study due to the availability of appropriate GCC controls. GCC asthmatics were not included in the analyses of the discovery cohort, because their asthma diagnoses were based on parent report alone. Inclusion criteria and case-control definitions are described in Table 2.
Table 2. Inclusion criteria of discovery and replication study populations.
| Population | Race or Ethnicity | Population Source(s) | # asthmatics | # allergic | # controls | Determination of race or ethnicity | Asthma inclusion criteria | Allergic inclusion criteria | Control inclusion criteria |
| Discovery | Caucasian | Greater Cincinnati Pediatric Clinic Repository (GCPCR), CCHMC Genomic Control Cohort (GCC) | 312 | 220 | 246 | Race was self-reported and ascertained by questionnaire; both the participant and his/her parents must have been identified themselves as Caucasian/White; all subjects were non-Hispanic | Children 4–17 years old with confirmed physician diagnoses of asthma based on clinical examination, available pulmonary function test results and respiratory symptom scores at a CCHMC-based specialty clinic | Non-asthmatic children ages 4–17 years old with physician diagnosed allergic rhinitis or atopic dermatitis based on radioallergosorbent testing or skin prick allergy testing; or children with a personal history of either environmental allergies, hay fever or eczema | Non-allergic Caucasian controls were those children ages 4–17 years old that did not meet the criteria to be either an asthmatic or allergic case, had no personal history of food allergies and no family history (parents and siblings) of asthma |
| Replication | African-American | GCPCR, GCC | 182 | 129 | 39 | Race was self-reported and ascertained by vquestionnaire; both the participant and his/her parents must have been identified themselves as African American/Black; all subjects were non-Hispanic | See Discovery Caucasian population above | See Discovery Caucasian population above | See Discovery Caucasian population above |
| Caucasian | GCC, Cincinnati Control Cohort (CCC) | 74 | NAa | 211 | Parents of asthmatics reported their children to be Caucasian by questionnaire; adult controls similarly reported their race to be Caucasian by questionnaire | Caucasian children ages 4 to 18 years with parent-reported asthma from a population-based representative sample from Greater Cincinnati | NA | Caucasian adults ages 24 to 90 years with no personal or family history of asthma (as determined by self-report) from a population-based representative sample from Greater Cincinnati | |
| Puerto Rican | Genetics of Asthma in Latino Americans (GALA) Study | 398 | NA | 712 | Ethnicity was self-reported and ascertained by questions; both biological parents and all biological grandparents of asthmatics had to be identified as being of Puerto Rican ethnicity | Index children at least 8 years of age with physician diagnosed asthma (confirmed by hospital-based medical chart review) and two or more parent-reported asthma symptoms (among wheezing, coughing, and shortness of breath) in the last 2 years were enrolled over a 4-year period in the San Francisco Bay Area, California, New York City, New York , Puerto Rico, and Mexico City, Mexico | NA | One or both biological parents of index asthmatic children were enrolled; allergic status was not a criteria for inclusion or exclusion | |
| Mexican | GALA | 300 | NA | 585 | Ethnicity was self-reported and ascertained by questionnaire; both biological parents of asthmatic children and all biological grandparents had to be identified as being of Mexican ethnicity | See Puerto Rican population above | NA | See Puerto Rican population above |
Not applicable.
In addition to our discovery population, four additional populations were used to replicate our findings (see Table 2). Cincinnati-based African American children selected from the GCPCR and GCC were identified as described in our discovery Caucasian population. Cases for a second Greater Cincinnati Caucasian population consisted of children with parent-reported asthma from the GCC and compared to the Cincinnati Control Cohort (CCC), a population based cohort of Caucasian adults with no personal or family history of asthma (by self-report) representative of Greater Cincinnati.with no personal or family history of asthma (by self-report) from a population-based representative adults sample from Greater Cincinnati. These controls were chosen because we could conclusively indicate their absence of pediatric and adult asthma, unlike similar aged controls that may develop asthma with age. Genotyping data from Affymetrix 6.0 SNP chip was also available for the GCC and CCC. The third and fourth populations consisted of Banked DNA was utilized for genotyping of Puerto Ricans and Mexicans parent- child trios with banked DNA for genotyping participating in the Genetics of Asthma in Latino Americans (GALA) Study [12], a multicenter international collaborative effort designed to identify clinical and genetic risk factors associated with asthma.
DNA Isolation and Genotyping
Genomic DNA was isolated from buccal swabs with either the Zymo Research Genomic DNA II Kit (Zymo Research Corp., Orange, CA) or the Purgene DNA Purification System (Gentra Systems Minneapolis, MN), and from Oragene saliva samples per the kit's instructions. Alternatively, genomic DNA was extracted from blood samples using Manual PerfectPure DNA Blood Kit (Invitrogen, Carlsbad, CA). Genotyping from discovery cases and controls and African American samples was performed using a custom Illumina Golden Gate assay according to manufacturer's protocol (http://www.illumina.com; San Diego, CA). Genotypes were assigned using BeadStudio's genotyping module (BeadStudio v3.2, San Diego, CA). For GALA, genotyping of KIF3A rs7737031 was accomplished using the Roche LightTyper 480 (Roche Diagnostics, Indianapolis, IN) and the KIF3A rs7737031 using TaqMan SNP Genoptyping Assay (assay ID C_25973778_10; Applied Biosystems, Foster City, CA).
Statistical Analysis
Genetic Association
SNPs failing Hardy Weinberg Equilibrium in the non-allergic control group (p<0.0001), having minor allele frequencies below 10%; or missing call rates greater than 10% were excluded. In addition, individuals with more than 20% of their total SNPs missing were excluded. Principal component analyses were performed using the 30 included ancestry-informative markers (AIMs) and the computer program EIGENSTRAT [13], [14] to account for potential population stratification. When examining the 194 SNPs in the six genes, the genomic inflation factor was 1.0, suggesting minimal impact of population stratification for these 30 AIMs. Therefore, no population stratification adjustment was required for analyses of asthmatics versus non-allergic controls in the discovery or African-American populations likely due to the fact that selection of the study population was from a single geographic region. However, the first principal component score was included as a covariate in comparisons of allergic versus non-allergic children in the discovery population as the genomic inflation factor was greater than 1.0 (λ = 1.33 before adjustment and λ = 1.02 after adjustment). Using PLINK [15], associations with asthma were tested adjusting for age and gender using the additive logistic regression model stratified by race. To address multiple testing, we first determined the average pairwise LD (a measured by r2) for all SNP combinations (160 SNPs, correlation = 0.13) and using this correlation, calculated the Bonferroni correction using the freely available Simple Interactive Statistical Analyses Software (http://www.quantitativeskills.com/sisa/). Associations were therefore considered significant at or below the 0.0006 level. For our second Caucasian population, six of the seven SNPs were imputed from the Affymetrix® 6.0 SNP data using MACH and HapMap CEU (release 22) as the reference [16], [17]. Imputed KIF3A SNPs were tested for association with asthma status again using additive logistic regression models in PLINK. The family-based association test (FBAT) [18] was used to assess associations between KIF3A rs7737031 and asthma in the GALA trios. Mexican and Puerto Rican samples were analyzed independently. HWE was tested in parents only. The population attributable risk for KIF3A rs7737031 was estimated using the R software package pARccs (v0.2–2; www.r-project.org) [19].
SNP Imputation
Estimation of SNPs not genotyped in the study was performed to increase statistical power as well as to detect novel associations26. Based on HapMap CEU results (release 22), imputation was performed after filtering out SNPs with genotyping call rates <10%, minor allele frequencies <10%, and HWE p-value<0.0001 using MACH 1.0.16 (http://www.sph.umich.edu/csg/MaCH), which uses a hidden Markov model to estimate an underlying set of unphased genotypes for each subject. We only considered SNPs that could be imputed with relatively high quality (RSQ>0.4). For the replication populations and validation of our approach, the available Affymetrix 6.0 SNP chip genotype data (http://www.ncbi.nlm.nih.gov/gap) from the populations described above were used. Imputed SNPs were tested for association with asthma status using PLINK software as described above.
Validation
To test whether FST is a proxy for minor allele frequency (MAF), we estimated Spearman correlation coefficients, as the MAF and FST were not normally distributed. For the comparison of SNPs with high and low FST values, pair-wise FST was determined using Python (http://www.python.org) scripts and HapMap data [20]. We selected 24 genes containing SNPs with the largest FST values (range 0.25–0.73) and 24 genes containing SNPs with the lowest FST values (range 0.00–0.10). We performed a case control analysis between self-reported child asthmatics from the GCC (n = 74) and controls with no personal or family history of asthma from the GCC (n = 226) using all Affymetrix 6.0 SNPs within 1000 kb of these genes. We then tested whether SNPs with high FST values (FST≥0.1) are more likely to show association with asthma (p≤0.05) than SNPs with low FST values (FST = 0). To test whether our gene selection strategy was superior to random selection, we again evaluated the available Affymetrix data from the 74 GCC asthmatics and 238 CCC controls. From this analysis, four of our six genes exhibited significant evidence of association (p≤0.05). Results from this analysis were then utilized to select six genes from the 161 differentially regulated genes using a random number generator. We noted how many of the six genes had at least one SNP with nominal association (p≤0.05). We repeated this analysis 10,000 times. To determine the empirical level of significance, we determined how many times the random selection obtained evidence in at least four genes added one and divided by the number of replicates (10,000).
Gene Expression Studies
Balb/c mice (Jackson Labs; Bar Harbor, ME) were sensitized twice intraperitoneally with 10 µg house dust mite (HDM, Dermatophagoides pteronyssinus; Greer Laboratories, Lenoir, NC) in 100 µl phosphate buffered saline (PBS) or 100 µl of PBS alone, and then challenged intratracheally with 100 µg HDM in 50 µl PBS or 50 µl PBS alone, euthanized 24 hours later, lung RNA extracted in TRIzol (Invitrogen, Carlsbad, CA) and cDNA prepared (Superscript First Strand cDNA synthesis kit; Invitrogen, Carlsbad, CA). Quantitative PCR on mouse and human cDNA samples collected previously [6] were performed using the Roche Light Cycler 480 SYBR Green 1 Master kit (Mannheim, Germany). The annealing temperature for primers sets was 60°C and the extension time was 5 seconds. After amplification, values from KIF3A were normalized to respective housekeeping genes values (glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or actin). Primers used to amplify both human and mouse KIF3A include: Forward- 5′-GGAGGAGACGAGCTGAG-3′; Reverse- 5′-CTCTGACTTTGCAGCCA-3′. Primers used to amplify human GAPDH: Forward- 5′-AAATCCCATCACCATCTTCC-3′; Reverse- 5′-TCACACCCATGACGAACA-3′. Primers used to amplify mouse actin: Forward - 5′-GGCAATGCGGCTGCAA-3′; Reverse- 5′-GGGTACCCACGCGAATCAC-3′. For the human epithelial gene expression studies, statistical analysis was performed using PRISM software (GraphPad Software Inc., La Jolla, CA) using one-way ANOVA followed by a Tukey-Kramer post-hoc test (for statistical significance between groups). For comparisons showing significant differences, precise p-values were calculated using a two-tailed t-test comparing the two groups under consideration. In the mouse studies, statistical significance was determined using a two-tailed t-test in PRISM.
Results
Gene Selection Approach
We previously published a RNA expression study in nasal epithelial samples from uncontrolled and controlled pediatric asthmatics compared to non-allergic controls [6]. The results of our study revealed that compared to the non-allergic control group (n = 4), the mean gene expression levels of 161 genes (142 known genes) were consistently up or down regulated in the group of uncontrolled asthmatics (n = 4) (one-way ANOVA ; p<0.01) (Fig. 1). To further reduce the pool of 161 candidate genes identified, we compared allele frequencies of SNPs within these genes in two distinct populations shown to have large differences in population prevalence of asthma. We hypothesized that specific alleles in genes with large inter-population frequency differences might be partly responsible for observed variations in asthma susceptibility. In fact, a recently published genome-wide estimation of the fixation index (FST), a measure of population differentiation devised by Wright [21], [22], on approximately 4 million SNPs from the HapMap project, found that genes associated with complex diseases showed a significantly higher mean value of FST suggesting that population genetic differentiation, particularly in genes associated with complex diseases may explain discrepancies in disease prevalence between different populations [23].
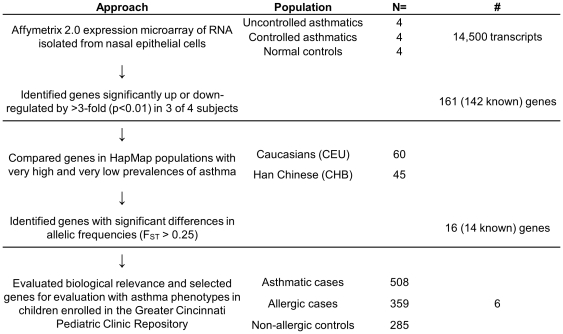
Figure 1. Novel unbiased approach to identify candidate asthma susceptibility genes.
The approach consisted of three stages with an evaluation of RNA expression of 14,500 genes in nasal epithelial samples in stage 1. Next, 142 known genes with >3-fold difference between uncontrolled asthmatics and non-allergic controls (P<0.05) were taken forward to stage 2. In stage 2, using HapMap data, the allelic frequencies of Caucasians (Utah residents of European ancestry; CEU) and the Han Chinese (Beijing, China; CHB) were compared and 14 known genes with a fixation index (FST)>0.25 were identified. Six of these genes, which mapped to chromosomal regions that had been linked to asthma previously, were included in the next phase. In stage 3, tagging SNPs including all CEU and YRI (Yoruban residents of Ibadan, Nigeria) SNPs with minor allele frequency less than 0.05 in the 6 genes were genotyped in children with asthma, allergic rhinitis or atopic dermatitis without asthma, and in non-allergic control children using a custom Illumina Golden Gate SNP Chip. Seven SNPs in a single gene, KIF3A, were significantly associated with asthma after adjusting for multiple comparisons (p-value<0.0006).
For our analysis, we used Wright's FST to quantify genetic differentiation between the Phase I HapMap CEU (Utah residents with Northern and Western European ancestry, n = 60) and the CHB (Han Chinese in Beijing, China, n = 45). We selected these populations based on the largest reported disparity in asthma prevalence in the ISAAC report [24] available at the time among the four Phase I HapMap populations (≥10% asthma prevalence for Caucasians vs. <5% for Chinese, respectively). FST was calculated for individual SNPs (FST = (p1−p2)2/(4p(1−p)), where p1 is the allele frequency in the Caucasian population, p2 is the frequency of the same allele in the Han Chinese population and p is the average allele frequency of each allele across each population [25], [26]. As FST has a theoretical minimum of 0 indicating no genetic differences, and a theoretical maximum of 1 indicating fixation for alternative alleles, we hypothesized that genes with larger FST values between populations that differ in disease prevalence are more likely to be associated with disease. Using this approach, we identified 16/161 genes (or 14/142 known genes) showing differential expression between cases and controls to have relatively large differences in allele frequencies in least one SNP (FST≥0.25; Fig. 1).
Next, these 16 genes were subjected to an extensive literature review using publicly available databases such as PubMed. Our investigation revealed that six of these genes (PDE4B, SPRR2B, ADCY2, KIF3A, DNAH5, and PLAU; see Table 1) were located in chromosomal regions that had been previously linked to asthma or other allergic disease phenotypes and had been shown to be regulated during allergic inflammation [1] (Table 1). Furthermore, we identified five of the same six genes (ADCY2, DNAH5, KIF3A, PDE4B, SPRR2B) when substituting FST values calculated using the HapMap CEU and YRI (Yorubans from Ibadan, Nigeria) populations for the CEU and CHB populations.
Tagging SNP Selection
Consequently and independent of the SNPs evaluated in our analysis of FST, a total of 172 tagging SNPs were selected for inclusion on a custom Illumina Golden Gate platform for the six genes of interest using Haploview and Tagger (http://www.broad.mit.edu/mpg/haploview). All tagging SNPs included were required to have minor allele frequencies greater than 0.05 and patterns of linkage disequilibrium (LD; r2>0.8) in the public HapMap Phase I CEU and YRI populations (http://hapmap.ncbi.nlm.nih.gov) [27], [28], [29].The rationale for using tagging SNPs is that genetic variants that are near each other and in LD tend to be inherited together as a result of shared ancestry. The strong correlations between markers within haplotype blocks help to enable accurate representation of a gene region by a small number of tagging SNPs and further ensures the efficient capture all the common genetic variation in the genes selected. We selected SNPs from the CEU and YRI populations because our discovery population consisted of children of Caucasian ancestry, and the availability of African American children for a replication cohort. We also included an additional 18 non-synonymous SNPs, four promoter SNPs, and 30 unlinked ancestry-informative markers (AIMs) [30] to estimate global population structure. AIMs were selected based on the criterion previously described by Rosenberg et al., 2003 [30]. Twenty of these AIMs were included to specifically distinguish between Northern and Southern Europeans [31] and the remaining ten AIMs were chosen to specifically distinguish between Europeans and people of African descent.
Asthma and Allergic Disease Genetic Associations
Genotyping was performed first on 790 Caucasian children from the Cincinnati Metropolitan area. Our SNP call rate was uniformly >95%. SNPs with call rates <10% (N = 9) or minor allele frequencies <10% (N = 25) were removed from the analysis. None of the SNPs failed Hardy-Weinberg Equilibrium in the non-allergic control population (p<0.001). When the more conservative cut off of 0.01 was used, only three additional SNPs would have been excluded. Individuals with more than 20% of their total SNPs missing were also excluded from the analysis (N = 12; Table 3). The mean age of the remaining children was slightly, but significantly less for asthmatic (p = 3.9×10−9) and allergic (p = 1.5×10−6) children compared to the non-allergic control group (Table 3).
Table 3. Characteristics of the discovery Caucasian population.
| Asthmatic Children | Allergic Children | Non-allergic Controls | |
| Total children, N | 317 | 227 | 246 |
| Children after exclusions, Na | 312 | 220 | 246 |
| Mean age (years)± SD | 10.04±3.44b | 10.21±3.54b | 11.79±3.40 |
| Male gender, N (%) | 170 (54.5) | 126 (57.3%) | 121(49.2) |
Indicates the number children after children with missing call rates above 20% were removed.
Indicates significant differences (p<0.05) with non-allergic control children.
Using a case-control study design, associations with asthma were evaluated. Notably, seven of nine KIF3A SNPs were associated with asthma with more than a 2-fold increase in the odds ratio for asthma (p<10−4) even after applying an LD adjusted Bonferroni correction for 160 pairwise comparisons with 0.13 correlation (α = 6×10−4; Table 4). We retrospectively compared the FST estimates of differentiation between HapMap (Phase III, Build 26) CEU and CHB populations for each of the KIF3A SNPs genotyped (Table 4). Our analysis revealed that all seven of the asthma-associated KIF3A SNPs had FST values≥0.32, while the remaining 2 SNPs had much lower FST estimates, suggesting that FST may be useful in identifying those SNPs most likely to be associated with asthma. Interestingly, at least one genetic variant in each of the six identified epithelial genes was nominally associated with asthma at p-value<0.05 (Fig. 2A).
Table 4. KIF3A SNP associations with asthma.
| Population = | Discovery Caucasiana | African-Americana | Secondary Caucasianb | Puerto Ricanc | Mexicanc | |||||
| # cases/# controls = | 312/246 | 182/39 | 74/211 | 398 | 300 | |||||
| KIF3A SNP | Major/Minor Allele | FST e | OR | P-value | OR | P-value | OR | P-value | P-value | P-value |
| rs12186803 | G/A | 0.33 | 2.08 | 0.00011 | 1.79 | 0.032 | 3.21 | 0.005 | ||
| rs1080001 | A/G | 0.32 | 2.08 | 0.00009 | 1.83 | 0.029 | 2.76 | 0.011 | ||
| rs7737031 | C/T | 0.34 | 2.18 | 0.00003 | 1.83 | 0.028 | 2.76 | 0.011 | 0.0458 | 0.471 |
| rs9784675 | A/Gd | 0.33 | 2.09 | 0.00007 | 1.61 | 0.064 | 3.32 | 0.002 | ||
| rs3798130 | G/Ad | 0.33 | 2.16 | 0.00004 | 1.56 | 0.086 | 3.21 | 0.005 | ||
| rs2299011 | C/Gd | 0.32 | 2.19 | 0.00003 | 1.52 | 0.101 | 3.21 | 0.005 | ||
| rs12514685 | C/Td | 0.32 | 2.16 | 0.00004 | 1.52 | 0.100 | 2.76 | 0.011 | ||
| rs1468216 | G/A | 0.01 | 0.78 | 0.16870 | 0.68 | 0.191 | 1.08 | 0.810 | ||
| rs17691077 | A/C | 0.06 | 0.85 | 0.41000 | - | - | - | - | ||
The discovery Caucasian population and African American population consisted of asthmatic children from the GCPCR and non-asthmatic/non-allergic controls from the GCPCR and GCC. Associations between asthmatics and controls were tested using an additive model. Odds ratios (OR) were determined using logistic regression based on the minor allele after adjusting for age, gender and population stratification. Bolding indicates the SNP associations <0.05. 7 of 9 SNPs were significant even after considering multiple comparisons (p<0.0006).
The Secondary Caucasian population consisted of asthmatic children from the GCC and non-asthmatic adult controls from the CCC. OR and p-values were determined using HapMap CEU results (release 22) and Affymetrix data from each cohort for imputation analysis.
The Puerto Rican and Mexican populations consisted of mother, father, child trios enrolled in the GALA Study. The value provided is the frequency of childhood asthmatics within those trios.
Major and minor alleles are reversed in African Americans;
Value of fixation index (FST) between CHB (China/Beijing) and CEU (Caucasian Europe, as represented by Utah) HapMap populations.
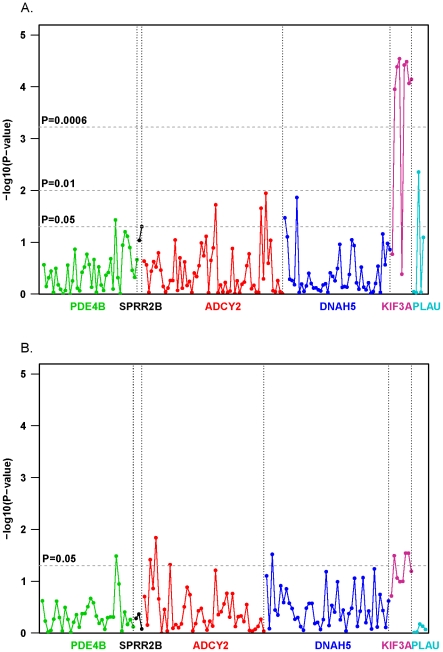
Figure 2. Genetic associations with childhood asthma.
A. We evaluated associations in our discovery Caucasian population between asthma and 160 directly genotyped SNPs within the six epithelial genes using the additive model after adjusting for age and gender. The upper dashed line corresponds to a p-value of 0.0006, the Bonferroni adjustment after considering LD correlation between SNPs. SNPs significant at this level (all in KIF3A) include rs12186803 (p = 0.00011), rs3798130 (p = 0.00004), rs2299011 (p = 0.00003), rs12514685 (p = 0.00004), rs7737031 (p = 0.00003), rs1080001 (p = 0.00009), and rs9784675 (p = 0.00007). The lower dashed line corresponds to a p-value of 0.05. SNPs significant at this level include rs11747117 (p = 0.0188), rs7714830 (p = 0.0219), and rs13174121 (p = 0.0113) in ADCY2, rs2896111 (p = 0.0335) and rs17263496 (p = 0.0136) in DNAH5, rs12060491 (p = 0.0369) in PDE4B, rs6693927 (p = 0.0496) in SPRR2B and rs2227562 (p = 0.0044) in PLAU. B. Associations between asthma and 160 directly genotyped SNPs within the six epithelial genes were evaluated among African American children from Cincinnati using an additive model after adjusting for age and gender. The dashed line corresponds to a p-value of 0.05. SNPs significant at this level include rs11742602 (p = 0.038), rs2017214 (p = 0.014) and rs1032719 (p = 0.048) in ADCY2, rs30168 (p = 0.030) in DNAH5, rs11208834 (p = 0.032) in PDE4B, rs12186803 (p = 0.032), rs1080001 (p = 0.029) and rs7737031 (p = 0.028) in KIF3A.
To further verify our findings and substantiate the approach, we evaluated associations with asthma in 362 African American children also from the Cincinnati Metropolitan area. We found that ADCY2, PDE4B, DNAH5 and KIF3A were again associated with asthma (p<0.05) at the gene level (Fig. 2B). Interestingly, seven KIF3A SNPs were also significantly associated with other allergic diseases such as allergic rhinitis and eczema (p-values≤0.008 and p-value≤0.023, respectively) in both our discovery Caucasian and African American populations (Table 4).
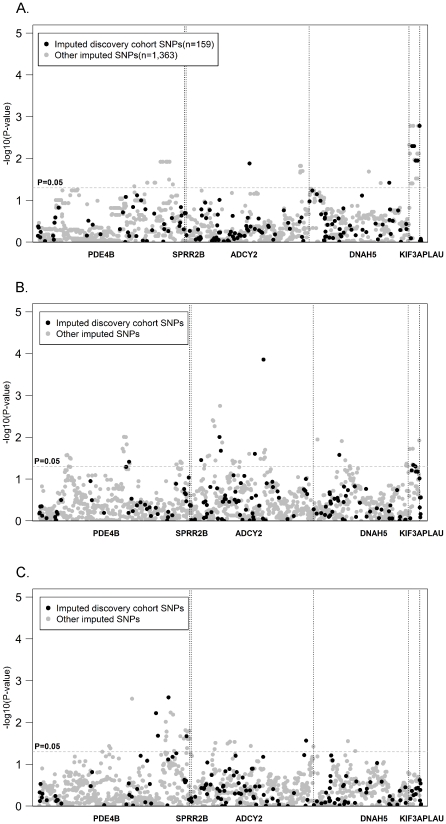
In both our discovery Caucasian and our African American populations, asthma was diagnosed according to ATS criteria [32] by hospital-based specialists at CCHMC. In order to validate the results we had obtained from these subsets, we utilized childhood and adult population-based cohorts for which Affymetrix 6.0 gene chip genome-wide genotyping data was available. We evaluated associations between our candidate genes and parent reported diagnosis of asthma in 74 Caucasian childhood asthmatics and 211 adults with no personal or family history of asthma living in Cincinnati. We performed imputation analysis to directly assess associations of tagging SNPs previously genotyped in our discovery Caucasian population as well as other SNPs not previously genotyped. Our analysis revealed all 7 previously associated KIF3A SNPs (as well as several other imputed SNPs) were significantly associated with asthma in this population (p-value≤0.01; Table 4). Furthermore, at least one previously associated SNP within ADCY2 and DNAH5 was associated with asthma (Fig. 3A). Imputed SNPs within PDE4B not previously genotyped were also significantly associated with asthma.
Figure 3. Associations with asthma in three additional independent populations.
SNPs not genotyped in the study were imputed based on HapMap CEU results (release 22) with MACH software using the available from the Affymetrix 6.0 genotype data. Imputed SNPs were then tested for associations with asthma using PLINK. The dashed line corresponds to a p-value of 0.05. A. Associations in a second Cincinnati-based Caucasian population. B. Asthma associations in the GALA Puerto Rican trios. C. Associations in the GALA Mexican trios.
We next examined associations between imputed SNPs and asthma in 398 Puerto Rican and 300 Mexican family trios enrolled in the Genetics of Asthma in Latino Americans (GALA) Study [12]. Puerto Ricans living in the US have higher asthma prevalence (26%), morbidity, and mortality rate than Caucasians living in the US, and Mexicans living in the US have a lower asthma prevalence (10%), morbidity, and mortality rate [33], [34], [35], [36], [37], [38]. Our analysis provided evidence for gene level replication of ADCY2, DNAH5, KIF3A, and PDE4B (Fig. 3B, C, respectively). As only KIF3A achieved significance after correction for multiple testing, this replication is essential to minimize false positive findings. To further support our findings, we directly genotyped KIF3A rs7737031, the SNP with the most significant association in our discovery population, and again observed a significant association with asthma in the Puerto Rican (p-value = 0.0458), but not the Mexican trios (p-value = 0.471) (Table 4).
Validation of Candidate Gene Selection
To validate our approach, we tested whether identifying candidate genes based on higher values of FST is a proxy for identifying more common SNPs with more power to detect an association, or whether information is gained from measures of population differentiation. We found that FST values for the 161 differentially expressed genes in uncontrolled asthma were not significantly correlated with minor allele frequency (Spearman rho = 0.05, p-value = 0.52), suggesting that including measures of FST to identify candidate genes is not simply identifying SNPs with greater power to detect an association, but rather provides information beyond that of minor allele frequency.
Second, we compared the HapMap CEU and CHB populations and determined whether SNPs with high FST values were more likely to exhibit association with asthma than those SNPs with low FST values. We evaluated Affymetrix 6.0 data of 74 asthmatic and 238 non-allergic control children from Cincinnati. Of the 161 differentially regulated genes identified by our expression array, we determined that the 241 SNPs within the 24 genes with the highest FST values (FST≥0.1; including SNPs within the final six genes selected - KIF3A, ADCY2, DNAH5, PDE4B, PLAU, SPRR2B) were significantly more likely (p = 0.0008, Fisher's Exact Test; 6% of SNPs reached p≤0.05) to be associated with asthma than the 190 SNPs in the 24 genes with the lowest FST values (FST = 0; 0.5% of SNPs reached p≤0.05).
Finally, again using the Affymetrix 6.0 data of the 74 Caucasian asthmatic children and 238 control children described above, we randomly selected six genes among those differentially expressed in asthmatics versus controls and asked how often we would observe at least four of the six genes having at least one SNP significantly associated with asthma (p-value = 0.05). Notably, we found that the random selection of genes using differences in expression alone was superior to our proposed selection approach (e.g. fewer genes were identified using random selection) in only 48 out of 10,000 permutations (p = 0.0049), and conclude that the addition of FST and the published literature search together provides a valuable tool for candidate gene selection.
RNA Expression Analyses
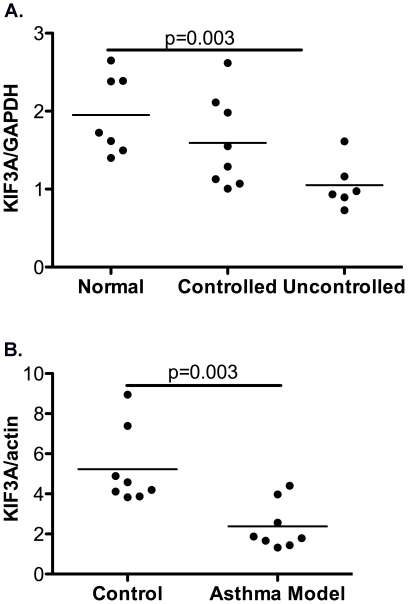
As we found the most significant results with KIF3A and as our earlier expression array findings indicate that KIF3A expression was down-regulated in nasal epithelial children with acute asthma [6], we wanted to corroborate this observation. To this end, quantitative PCR for KIF3A was performed on nasal epithelial RNA samples from the asthmatic children and controls previously included on the array (Fig. 4). Consistent with our array data, we observed that KIF3A expression was significantly down-regulated (p<0.05). As an additional independent validation of our results, we also measured expression of KIF3A in lung RNA isolated from a house dust mite-induced murine asthma model. Our results indicate that KIF3A expression is significantly reduced compared to phosphate buffered saline-treated controls [6] (Fig. 4).
Figure 4. KIF3A gene expression of human epithelial RNA from children with uncontrolled asthma and of mouse models with asthma is decreased compared to controls.
Gene expression of KIF3A in nasal epithelial-derived RNA from non-allergic children, controlled and uncontrolled asthmatics was determined by quantitative PCR. GAPDH was used to normalize for expression. To induce an asthma-like phenotype, wild type Balb/c mice were sensitized and challenged with 100 µg of house dust mite (asthma model) and compared to mice sensitized and challenged with phosphate buffered saline (control). One day post-treatment, RNA was extracted from lungs and gene expression measured by quantitative PCR. Actin was used to normalize for gene expression.
Discussion
A major challenge for candidate gene association studies is the selection of biologically relevant genes for evaluation. Our unique approach utilized multiple lines of evidence to select relevant candidate genes (Fig. 1). First, as nasal epithelial cells have been previously shown to be a good surrogate for bronchial epithelial cells [10], we took advantage of expression microarray data from these cells to identify genes differentially expressed in asthmatics and non-asthmatic controls. Next, using HapMap, we compared the allelic variation across each of these genes in populations with marked differences in asthma prevalence (CEU vs. CHB), reviewed the published literature to identify specific genes having potential application in the lung and genotyped tagging SNPs across these genes. Using this approach, we determined that SNPs within six genes were associated with asthma (Fig. 2A). Many of these associations were also observed in one or more of our 4 independent populations either at the SNP or gene level (Fig. 2B and Fig. 3), and the association with KIF3A was associated in three of four populations examined in addition to our discovery population. The Puerto Rican and Mexican GALA trios not only allowed us to evaluate our results across racial/ethnic groups, but to evaluate genetic associations in populations with potentially different environmental exposures.
For candidate gene selection, researchers have used gene expression results [6], FST [21], [22], and biologic relevance as gleaned from the literature [1], but no study has combined these strategies. We found that the combination of approaches for candidate gene selection was superior to using one type of data. Indeed, in an independent screen of SNPs in genes found to have dysregulated epithelial gene expression in uncontrolled asthmatics, we found that SNPs with high FST values were more likely to be associated with asthma than those with low FST values, including the six genes evaluated in this study, supporting the validity of our gene selection approach. Further, when we randomly selected genes from those that were differentially expressed, we found our selection method was statistically superior. These data point to a strong relationship between epithelial cells and asthma and substantiate the validity of this approach to identify genetic biomarkers of complex disease. Collectively, our approach may be applicable to other complex diseases that show varying prevalences across human populations, and may be a useful tool to select novel gene candidates for assessment of disease associations in other pertinent cell types and tissues.
Using this approach, we have identified KIF3A as a possible susceptibility gene for childhood asthma and allergic disease. We found that seven of nine KIF3A SNPs genotyped were significantly associated with asthma in our discovery Caucasian population and in independent Caucasian and African American populations – all from Cincinnati (Table 4). SNPs in KIF3A were also to a lesser degree, associated with other allergic diseases independent of asthma demonstrating the importance of appropriate phenotyping and selection of controls in genetic studies of asthma (Table 5). If we had merely compared asthmatics to non-asthmatics without determining the non-asthmatics allergic status, we might have missed the association with KIF3A. Importantly, all of the disease associated KIF3A SNPs and neither of the two non-associated KIF3A SNPs displayed significant population differentiation in HapMap CEU and CHB populations (individual FST values>0.25; Table 4). The observed association was strongest for KIF3A rs7737031 which had a PAR for asthma of >18%. Carriers of rs7737031 have more than double the odds of having asthma. Similar to the PAR of this KIF3A variant, the PAR for myocardial infarction was 21% in individuals with the previously reported rs10757278 variant located in adjacent CDKN2A and CDKN2B genes [39]. We also directly genotyped this SNP and evaluated its association with asthma in Puerto Rican and Mexican populations in the GALA Study. Puerto Ricans and Mexicans living in the US have higher and lower asthma prevalences, morbidity, and mortality rates than Caucasians, respectively. Interestingly, our analysis reveals that KIF3A rs7737031 was significantly associated in Puerto Ricans and not Mexicans.
Table 5. KIF3A SNP associations with allergic disease.
| KIF3A | Allergic vs. Non-allergic Controlsa | ||||
| Discovery Caucasian | African American | ||||
| Frequency cases/controls = | 220/246 | 129/39 | |||
| SNP | Major/Minor Allele | OR | P-value | OR | P-value |
| In Caucasians | |||||
| rs12186803 | G/A | 1.84 | 0.003 | 2.11 | 0.013 |
| rs1080001 | A/G | 1.84 | 0.003 | 2.11 | 0.013 |
| rs7737031 | C/T | 1.83 | 0.004 | 2.11 | 0.013 |
| rs9784675 | A/Gb | 1.72 | 0.008 | 2.13 | 0.009 |
| rs3798130 | G/Ab | 1.83 | 0.003 | 1.89 | 0.023 |
| rs2299011 | C/Gb | 1.82 | 0.003 | 1.92 | 0.017 |
| rs12514685 | C/Tb | 1.82 | 0.004 | 1.85 | 0.023 |
| rs1468216 | G/A | 0.70 | 0.076 | 0.78 | 0.416 |
| rs17691077 | A/C | 0.91 | 0.661 | ||
The discovery Caucasian population and African American population consisted of asthmatic children from the GCPCR and non-asthmatic/non-allergic controls from the GCPCR and GCC. Associations between allergic children and controls were tested using an additive model. Odds ratios (OR) were determined using logistic regression based on the minor allele after adjusting for age, gender and population stratification. Bolding indicates the SNP associations <0.05.
Indicates major and minor alleles are reversed in African Americans.
KIF3A is a heterotrimeric member of the kinesin superfamily of microtubule associated motors that are important in the transport of protein complexes within cilia and flagella [40], [41] among other roles. Cilia, together with mucus and the airway surface liquid layer, make up the mucociliary apparatus that clears inhaled allergens or other particles from the lung. Defective mucociliary clearance is a characteristic feature of several genetically linked airway diseases including asthma and cystic fibrosis [42], [43], yet the mechanisms responsible for poor mucus and/or allergen clearance from the airways remain largely unknown.
As KIF3A is located on 5q31, immediately upstream of IL-4, we also noted a strong LD between IL-4 and KIF3A SNPs in our discovery cohort, a finding that is consistent with another published report [44]. Examination of International HapMap data [45] indicates that this LD exists across many other populations as well making it difficult to determine if one or both genes confer risk. Numerous studies have reported associations with IL-4, asthma and other allergic diseases [29], [46]. It is possible that some of the previous asthma associations reported between asthma and IL-4 may reflect the LD with KIF3A. Therefore, we further examined the biologic plausibility of KIF3A as an asthma susceptibility gene by examining gene expression in the lungs of mice. We found expression of KIF3A was significantly reduced in HDM-treated mice compared to controls (Fig. 4). We speculate that in asthmatics specifically, diminished KIF3A expression might be important in allowing the lung to repair itself after exacerbation. Alternatively, diminished KIF3A expression may contribute to the lungs' inability to clear mucus and remove inhaled particles and aeroallergens therefore exacerbating the asthma and/or allergic phenotypes. Individuals with polymorphic cilia genes may have further reduced cilia gene expression, diminished ciliary function and increased allergen exposure resulting in even greater susceptibility to asthma and allergic disease. Regardless of the mechanism of action, KIF3A is clearly down-regulated in asthma, supporting a role for this novel gene in the pathogenesis of this disease. While our analyses of tagging SNPs provide strong evidence of a gene-disease association, it will be important to investigate the combined effects of IL-4 and KIF3A in future studies and to identify specific causal variants in KIF3A.These findings emphasize the importance of evaluating multiple genes and critically exploring the biological relevance of genes previously unknown to influence disease susceptibility.
In summary, our study took advantage of our previously published evaluation of nasal epithelial cell-derived RNA from asthmatic and non-allergic children [6], population differences in asthma prevalence, tagging SNPs in the HapMap database, and the published literature to identify six genes (ADCY2, DNAH5, KIF3A, PDE4B, PLAU, SPRR2B) for detailed and targeted genetic testing. The most strongly associated gene, KIF3A, was first reported by our group as having a role in asthma in 2009 [47]. KIF3A has also been associated with aspirin sensitive asthma [48]. Here, we verify that KIF3A is a novel candidate gene for childhood asthma and show that its expression is down regulated in nasal epithelial cells in asthmatics. Our success supports the validity of our approach for identifying asthma candidate genes with a high likelihood of exhibiting association in specific variants. As the level of genomic data continues to increase, it will be imperative to develop methods which can help focus studies. By demonstrating that we can identify associations using this approach and that these associations can be replicated, we have provided a novel framework for the identification of candidate genes.
Acknowledgments
We thank the physicians, nurses and staff of Cincinnati Children's Hospital Medical Center Allergy and Immunology clinics, Pulmonary clinics, Dermatology clinics, Headache Center clinics, Dental clinics, Orthopedic clinics and Emergency Department as well as the investigators and staff of the Cincinnati Children's Hospital Genomic Control Cohort. We thank all the patients and their families who participated in this study. We thank the investigators and staff of the Cincinnati Control Cohort and the Genetics of Asthma in Latino Americans Study for the use of their specimens and/or data. We thank Dr. Daniel Nebert, MD, in the Department of Environmental Health, University of Cincinnati and Dr. Jessica Woo, PhD in the Division of Biostatistics and Epidemiology at Cincinnati Children's Hospital Medical Center for their critical review of this manuscript.
Footnotes
Competing Interests: Mr. Chris Gignoux has indicated that he holds stock in 23andMe, Inc. The other authors do not have any conflicts of interest or financial disclosures, including declarations of financial interest, to report.
Funding: This work was supported by the National Institutes of Health [U19A170235 to GKKH, R21016830 to MBK, and U19AI77439 to EGB]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weiss ST, Raby BA, Rogers A. Asthma genetics and genomics 2009. Curr Opin Genet Dev. 2009;19:279–282. doi: 10.1016/j.gde.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 4.Akey JM, Zhang G, Zhang K, Jin L, Shriver MD. Interrogating a high-density SNP map for signatures of natural selection. Genome Res. 2002;12:1805–1814. doi: 10.1101/gr.631202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franke L, Jansen RC. eQTL analysis in humans. Methods Mol Biol. 2009;573:311–328. doi: 10.1007/978-1-60761-247-6_17. [DOI] [PubMed] [Google Scholar]
- 6.Guajardo JR, Schleifer KW, Daines MO, Ruddy RM, Aronow BJ, et al. Altered gene expression profiles in nasal respiratory epithelium reflect stable versus acute childhood asthma. J Allergy Clin Immunol. 2005;115:243–251. doi: 10.1016/j.jaci.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Frieri M. Asthma concepts in the new millennium: update in asthma pathophysiology. Allergy Asthma Proc. 2005;26:83–88. [PubMed] [Google Scholar]
- 8.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol. 2004;4:978–988. doi: 10.1038/nri1500. [DOI] [PubMed] [Google Scholar]
- 9.Gaga M, Lambrou P, Papageorgiou N, Koulouris NG, Kosmas E, et al. Eosinophils are a feature of upper and lower airway pathology in non-atopic asthma, irrespective of the presence of rhinitis. Clin Exp Allergy. 2000;30:663–669. doi: 10.1046/j.1365-2222.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- 10.McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, et al. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39:560–568. doi: 10.1165/rcmb.2007-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himes BE, Hunninghake GM, Baurley JW, Rafaels NM, Sleiman P, et al. Genome-wide association analysis identifies PDE4D as an asthma-susceptibility gene. Am J Hum Genet. 2009;84:581–593. doi: 10.1016/j.ajhg.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burchard EG, Avila PC, Nazario S, Casal J, Torres A, et al. Lower bronchodilator responsiveness in Puerto Rican than in Mexican subjects with asthma. Am J Respir Crit Care Med. 2004;169:386–392. doi: 10.1164/rccm.200309-1293OC. [DOI] [PubMed] [Google Scholar]
- 13.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 14.Narayanaswamy CRaR, D. Principal Component Analysis for Large Dispersion Matrices. App Stat. 1991;40:309–316. [Google Scholar]
- 15.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nothnagel M, Ellinghaus D, Schreiber S, Krawczak M, Franke A. A comprehensive evaluation of SNP genotype imputation. Hum Genet. 2009;125:163–171. doi: 10.1007/s00439-008-0606-5. [DOI] [PubMed] [Google Scholar]
- 18.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, et al. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsch C, Pfahlberg AB, Gefeller O. Point and interval estimation of partial attributable risks from case-control data using the R-package ‘pARccs’. Comput Methods Programs Biomed. 2009;94:88–95. doi: 10.1016/j.cmpb.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 20.McKeigue PM. Mapping genes that underlie ethnic differences in disease risk: methods for detecting linkage in admixed populations, by conditioning on parental admixture. Am J Hum Genet. 1998;63:241–251. doi: 10.1086/301908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright S. 1978. Variability within and among Natural Populations: University of Chicago Press.
- 22.Wright S. The genetic structure of populations. Ann Eugen. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- 23.Amato R, Pinelli M, Monticelli A, Marino D, Miele G, et al. Genome-wide scan for signatures of human population differentiation and their relationship with natural selection, functional pathways and diseases. PLoS One. 2009;4:e7927. doi: 10.1371/journal.pone.0007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 25.Nei M. Definition and estimation of fixation indices. Evolution. 1986;40:643–645. doi: 10.1111/j.1558-5646.1986.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 26.Weir B. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 27.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, et al. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ke X, Cardon LR. Efficient selective screening of haplotype tag SNPs. Bioinformatics. 2003;19:287–288. doi: 10.1093/bioinformatics/19.2.287. [DOI] [PubMed] [Google Scholar]
- 29.Sebastiani P, Lazarus R, Weiss ST, Kunkel LM, Kohane IS, et al. Minimal haplotype tagging. Proc Natl Acad Sci U S A. 2003;100:9900–9905. doi: 10.1073/pnas.1633613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg NA, Li LM, Ward R, Pritchard JK. Informativeness of genetic markers for inference of ancestry. Am J Hum Genet. 2003;73:1402–1422. doi: 10.1086/380416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet. 2008;17:R143–150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 33.Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115:1254–1260. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- 34.Arif AA, Delclos GL, Lee ES, Tortolero SR, Whitehead LW. Prevalence and risk factors of asthma and wheezing among US adults: an analysis of the NHANES III data. Eur Respir J. 2003;21:827–833. doi: 10.1183/09031936.03.00054103a. [DOI] [PubMed] [Google Scholar]
- 35.Beckett WS, Belanger K, Gent JF, Holford TR, Leaderer BP. Asthma among Puerto Rican Hispanics: a multi-ethnic comparison study of risk factors. Am J Respir Crit Care Med. 1996;154:894–899. doi: 10.1164/ajrccm.154.4.8887582. [DOI] [PubMed] [Google Scholar]
- 36.Carter-Pokras OD, Gergen PJ. Reported asthma among Puerto Rican, Mexican-American, and Cuban children, 1982 through 1984. Am J Public Health. 1993;83:580–582. doi: 10.2105/ajph.83.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flores G, Fuentes-Afflick E, Barbot O, Carter-Pokras O, Claudio L, et al. The health of Latino children: urgent priorities, unanswered questions, and a research agenda. JAMA. 2002;288:82–90. doi: 10.1001/jama.288.1.82. [DOI] [PubMed] [Google Scholar]
- 38.Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117:43–53. doi: 10.1542/peds.2004-1714. [DOI] [PubMed] [Google Scholar]
- 39.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Hancock WO. The two motor domains of KIF3A/B coordinate for processive motility and move at different speeds. Biophys J. 2004;87:1795–1804. doi: 10.1529/biophysj.104.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 43.Mall MA. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J Aerosol Med Pulm Drug Deliv. 2008;21:13–24. doi: 10.1089/jamp.2007.0659. [DOI] [PubMed] [Google Scholar]
- 44.Kleinrath T, Gassner C, Lackner P, Thurnher M, Ramoner R. Interleukin-4 promoter polymorphisms: a genetic prognostic factor for survival in metastatic renal cell carcinoma. J Clin Oncol. 2007;25:845–851. doi: 10.1200/JCO.2006.07.8154. [DOI] [PubMed] [Google Scholar]
- 45.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabesch M, Tzotcheva I, Carr D, Hofler C, Weiland SK, et al. A complete screening of the IL4 gene: novel polymorphisms and their association with asthma and IgE in childhood. J Allergy Clin Immunol. 2003;112:893–898. doi: 10.1016/j.jaci.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 47.Sivaprasad U, Gibson AM, Wang N, Hershey GKK. Expression of Cilia Structural Genes is Downregulated in Asthma and Single Nucleotide Polymorphisms in These Genes Correlate with Asthma. The Journal of Allergy and Clinical Immunology. 2009;123:S81. [Google Scholar]
- 48.Kim JH, Cha JY, Cheong HS, Park JS, Jang AS, et al. KIF3A, a cilia structural gene on chromosome 5q31, and its polymorphisms show an association with aspirin hypersensitivity in asthma. Journal of Clinical Immunology. 2011;31:112–121. doi: 10.1007/s10875-010-9462-x. [DOI] [PubMed] [Google Scholar]