Abstract
Background
To overcome the increasing resistance of pathogens to existing antibiotics the 10×'20 Initiative declared the urgent need for a global commitment to develop 10 new antimicrobial drugs by the year 2020. Naturally occurring animal antibiotics are an obvious place to start. The recently sequenced genomes of mammals that are divergent from human and mouse, including the tammar wallaby and the platypus, provide an opportunity to discover novel antimicrobials. Marsupials and monotremes are ideal potential sources of new antimicrobials because they give birth to underdeveloped immunologically naïve young that develop outside the sterile confines of a uterus in harsh pathogen-laden environments. While their adaptive immune system develops innate immune factors produced either by the mother or by the young must play a key role in protecting the immune-compromised young. In this study we focus on the cathelicidins, a key family of antimicrobial peptide genes.
Principal Finding
We identified 14 cathelicidin genes in the tammar wallaby genome and 8 in the platypus genome. The tammar genes were expressed in the mammary gland during early lactation before the adaptive immune system of the young develops, as well as in the skin of the pouch young. Both platypus and tammar peptides were effective in killing a broad range of bacterial pathogens. One potent peptide, expressed in the early stages of tammar lactation, effectively killed multidrug-resistant clinical isolates of Pseudomonas aeruginosa, Klebsiella pneumoniae and Acinetobacter baumannii.
Conclusions and Significance
Marsupial and monotreme young are protected by antimicrobial peptides that are potent, broad spectrum and salt resistant. The genomes of our distant relatives may hold the key for the development of novel drugs to combat multidrug-resistant pathogens.
Introduction
Over the past two decades a large number of antimicrobial peptides have been identified in plants, animals and microorganisms. These endogenous antibiotic peptides play a key role in the innate immune system and are a first line of defense in protecting the internal and external body surfaces of the host. The best known antimicrobial peptide gene families include the defensins and the cathelicidins. In this paper we focus on the cathelicidin gene family. Cathelicidin genes are characterized by a conserved signal sequence and pro-peptide region but are highly variable in the C-terminal domain that encodes the mature antimicrobial peptide, which is released by elastase cleavage. The active peptides vary in length both within a species and between species and range from 12–100 amino-acid residues [1], [2]. Cathelicidins are found in neutrophils and macrophages, as well as epithelial cells of the testis, skin, gastrointestinal tract and respiratory tract (reviewed in [2]). Cathelicidins interact with and destroy Gram-positive and Gram-negative bacteria, protozoa and fungi via electrostatic interactions between their positively charged peptides and the negatively charged molecules found in the cell membranes of their targets. Besides their direct antimicrobial function, cathelicidins also play a role in inflammation and in dampening of excessive inflammation, cell proliferation and migration, immune modulation, wound healing, angiogenesis and the release of cytokines and histamine (reviewed in [2], [3]).
The best studied cathelicidin is human LL-37, the only human cathelicidin. It has anti-tumour and anti-HIV activity [4]. Recurrent bacterial infections occur in patients with Chediak-Higaski syndrome, where mature neutrophils lack elastase to cleave cathelicin pro-peptides [5]. Cathelicidin knockout mice are susceptible to Group A Streptococccus, herpes simplex virus, Escherichia coli and vaccinia virus (reviewed in [6]). In the rat, cathelicidins protect against lethal sepsis caused by Gram-negative bacteria [7].
During the course of evolution, nature has developed a vast array of antimicrobial peptides. Each species contains a different set of related genes that reveal signatures of different selective forces. Cathelicidin genes have been identified in the invertebrate hagfish [8] and in a range of vertebrates, including mammals [9], [10], [11], chickens [12], [13], [14], fish [15], [16] and reptiles [17], [18], [19], [20].
Marsupials and monotremes hold an important position in the vertebrate phylogenetic tree because they represent two of the three extant mammalian lineages. Marsupials and eutherian mammals last shared a common ancestor between 130 and 148 million years ago, while monotremes and therian mammals (marsupials and eutherians) diverged about 166 million years ago [21], [22], [23]. Marsupials and monotremes differ from eutherian mammals primarily in their mode of reproduction. Marsupials give birth to underdeveloped (altricial) young after a short gestation, which in the tammar wallaby (Macropus eugenii) is only 26.5 days [24]. At birth, the neonate weighs only 440mg and is 16–17mm long [24]. This developmental stage is roughly equivalent to a 40 day human embryo or a 15 day mouse embryo. The young remains in the pouch for 9–10 months, supported by a long and physiologically sophisticated lactation. Initially the young are permanently attached to a teat, but later they begin to release the teat periodically whilst still in the pouch. The mother's milk undergoes compositional changes over time ensuring that the nutritional supply to the young is specifically matched to each developmental stage [25]. At birth the wallaby does not have a differentiated immune system. Cells involved in adaptive immunity are not seen until about 35 days after birth [26], [27] and immunocompetence develops around 90–100 post partum [28].
Monotremes lay eggs and the young hatch at a very early stage in development similar to that of the marsupial neonate. The platypus (Ornithorhynchus anatinus) lays up to 3 leathery-shelled eggs ∼15–21 days after mating [29]. After a 10 day incubation period, young approximately 15mm in length emerge [30]. Much of the development, including that of the immune system, occurs before weaning. During the three to four months before they leave the burrow the young grow from ∼1.5 cm to ∼40 cm [30]. Platypuses do not have teats but the milk is secreted via mammary patches on the female's abdomen. It is not known exactly when platypus immune tissues reach maturity, but by the time platypuses are adults, they possess a range of lymphoid tissues that are histologically similar to those of therian mammals [31].
Unlike eutherians, whose immune systems develop in the relatively sterile confines of the mother's uterus, the marsupial and monotreme immune systems develop while exposed to a range of pathogens in the pouch and in the burrow. In eutherians, cathelicidins play a crucial role in neonatal defense. They have been detected in skin of neonatal mice and humans, in human milk, and LL-37 is found in the vernix caseosa, a creamy substance that covers the healthy infant at birth (reviewed in [32]). Therefore it is likely that the marsupial and monotreme genomes have also evolved under evolutionary pressure to protect immunologically naïve young with broad spectrum antibiotics and that their genomes will reveal a source of novel antimicrobial peptides that may provide unique answers to antibiotic resistance.
The recent sequencing of the tammar wallaby genome [33] and the platypus genome [23] allow discovery of these divergent peptides using bioinformatics strategies. There are seven cathelicidin genes in the tammar mammary gland EST library [32], expressed in adult leukocytes and in primary and secondary lymphoid organs of the pouch young over the first 120 days of life. An eighth cathelicidin, recently identified, is expressed in spleen and gastrointestinal tract of newborn animals and in most tissues by seven days after birth [34]. We identified eight cathelicidin genes in the platypus genome [23]. All were expressed in adult brain, kidney, liver, lung, spleen and testis [35]. Surprisingly, none of them were expressed in the single platypus milk sample available to us [35]. It is important to note that access to platypus samples for scientific research is difficult. In the wild, adults are trapped in nets without their young, as the young remain in the burrow while the mother forages. Therefore, it is impossible to know the stage of the mother's lactation. It is possible that milk samples from other stages of lactation could have yielded a different result. Due to ethical and conservation concerns, further study of monotreme developmental immunology is not possible at this time and awaits opportunistic sampling.
In this paper we describe functional testing of antimicrobial peptides identified through genome data-mining and support this with expression studies that confirm that marsupials (and probably monotremes) use these powerful broad-spectrum peptides to protect themselves and their young from a diverse range of pathogens found in the pouch (and in the soil).
Results and Discussion
Identification of cathelicidin genes
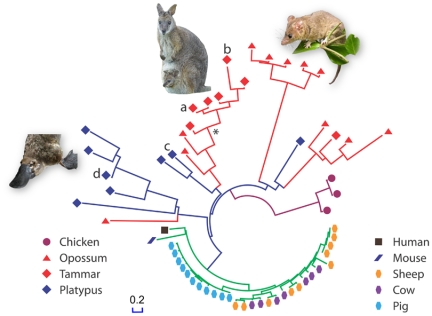
Fourteen divergent cathelicidin genes were identified in the tammar genome, twelve in the opossum and eight in the platypus (Fig. 1). Sequences were identified using a PFAM hidden Markov model and full-length coding sequences were extracted by gene prediction. The phylogenetic relationships of cathelicidins are shown in Fig. 1. Proteins selected for active C-terminal peptide prediction and testing are indicated.
Figure 1. Phylogenetic tree demonstrating superior diversity of antimicrobial peptides in non-eutherian mammals.
Tammar cathelicidin peptides. WAM1 (a) – KRGFGKKLRKRLKKFRNSIKKRLKNFNVVIPIPLPG from MaeuCath1 (Genbank EF624481.1), WAM2 (b) -KRGLWESLKRKATKLGDDIRNTLRNFKIKFPVPRQG from MaeuCath5 (Genbank EF624484.1), Ancestral WAM (*)- RRGFWKRLRRRLRRFGDRIRNRFRNFREKLPDPFPG. Platypus cathelicidin peptides PAM1 (c) – RTKRRIKLIKNGVKKVKDILKNNNIIILPGSNEK from OranCath1 [23] and PAM2 (d) – RPWAGNGSVHRYTVLSPRLKTQ from OranCath2 [23].
Expression of cathelicidin genes
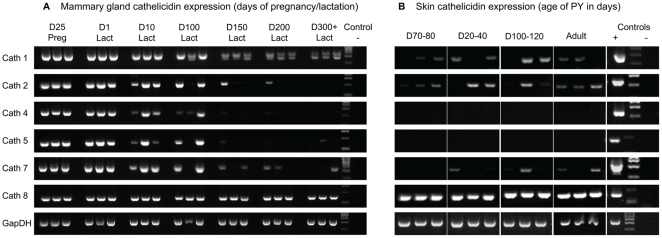
Tammar cathelicidin genes were expressed in the mammary gland throughout lactation, consistent with a role in protection of immuno-naïve young. Five out of six genes tested were down-regulated after 100 days post partum (Fig. 2a) corresponding to the time when the young achieve immune competence. Similar cathelicidin expression also occurred in the skin of pouch young and adult in four of the six genes tested (Fig. 2b).
Figure 2. Tammar cathelicidin gene expression in the mammary gland throughout lactation and in pouch young skin.
D = Day; PY = pouch young. Cath1 and Cath5 correspond to WAM1 and WAM2, respectively. (a) Tammar cathelicidin gene expression in the mammary gland throughout the 350 days of lactation. (b) Tammar cathelicidin gene expression in male D20–D120 pouch young skin and adult lactating female pouch skin. Female pouch young skin and adult non-lactating pouch skin showed similar expression patterns (not shown). Each age group is represented by three different samples and mammary gland and thymus tissues were used as positive controls (+). No template controls (–) were clean.
Evolution of cathelicidin genes
Marsupial and monotreme antimicrobial peptides are ancient, diverging from each other approximately 211 million years ago (101–340 million years, 95% credibility interval (CI)). Mammalian ancestors likely expressed a broad repertoire of cathelicidin peptides to protect their young. Over time, with the evolution of longer gestation periods and the birth of young with greater immune competence, cathelicidin genes were lost from the eutherian lineage, because humans and mice have only one cathelicidin gene. Recent gene duplications in sheep, cows and pigs, ∼53 MYA (27–80, 95% CI) (Fig. 1) were likely driven by increased pathogen pressures in herd animals.
The high level of sequence divergence of the marsupial and monotreme cathelicidin genes (tammar peptides share ∼28% amino-acid identity with each other) suggests that they should have the potential to inhibit a wide range of microbial pathogens.
Activity of tammar and platypus cathelicidin genes
We selected four divergent tammar (wallaby antimicrobial 1 and 2:WAM1 and 2) and platypus antimicrobial 1 and 2 (PAM1 and PAM2) peptides to synthesize and test (Fig 1). All four killed the Gram-positive bacteria Bacillus subtilis, Staphylococcus aureus, Streptococcus uberis and Strep. pyogenes, and the Gram-negative bacteria Escherichia coli, Salmonella choleraesuis and P. aeruginosa (Table 1). PAM2, WAM1 and WAM2 also killed the fungal pathogen Candida albicans (Table 1). Our four divergent peptides were more potent than the human cathelicidin peptide LL-37 which was less effective against the seven bacteria tested and did not kill C. albicans. The platypus peptides had distinct activity profiles, with PAM2 active against C. albicans and PAM1 more potent against S. aureus
Table 1. Antimicrobial activities of cathelicidin peptides.
| Strains | MIC (µM) | |||||
| WAM1 | WAM2 | Ancestral WAM | LL37 | PAM1 | PAM2 | |
| Gram-negative | ||||||
| E. coli DH5α | 0.47 | 1.46 | 0.41 | 5.57 | 0.75 | 0.87 |
| Sal. enterica (ATCC 14028) | 1.14 | 1.58 | 0.96 | 4.30 | 0.47 | 1.11 |
| P. aeruginosa (ATCC 27853) | 0.77 | 1.29 | 2.06 | >56 | 1.89 | 0.73 |
| Gram-positive | ||||||
| B. subtilis | 1.50 | 2.14 | 1.56 | 8.62 | 1.96 | 3.37 |
| S. aureus (ATCC 25923) | 1.01 | 1.39 | 1.42 | 3.01 | 0.56 | 2.42 |
| Strep. pyogenes (ATCC 19615) | 0.66 | 0.39 | 0.68 | >55 | 0.73 | 0.83 |
| Strep. uberis | 1.22 | 0.63 | 0.07 | 2.24 | 0.25 | 0.51 |
| Fungi | ||||||
| C. albicans (ATCC 753) | 1.30 | 1.47 | 6.45 | >56 | >20 | 2.50 |
A phylogenetic approach to designing additional antimicrobial peptides
A phylogenetic approach was used to design additional antimicrobial peptides. An ancestral peptide (WAM = RRGFWKRLRRRLRRFGDRIRNRFRNFREKLPDPFPG) was designed using MaeuCath1, 3, 5, 6 and 7 and three methods: PAML [36], GASP [37] and Ancescon [38]. 6/40 amino acid positions in the putative ancestral peptide were ambiguous and in these positions the Ancescon predicted residues were selected. We predict this peptide originated ∼59 MYA (Fig. 1) and is ancestral to the major clade of marsupial antimicrobial peptides including the modern active peptides WAM1 and WAM2. This ancestral peptide from the Paleocene had broad-spectrum activity and was approximately ten times more effective than LL-37 at killing E. coli, and P. aeruginosa. The archaic peptide had particularly potent activity against the mastitis pathogens Strep. pyogenes and Strep. uberis (Table 1), suggesting that an ancestral function included mammary gland protection. This approach provides a new avenue to investigate ancient peptides and their immune functions.
WAM1- highly potent but not toxic
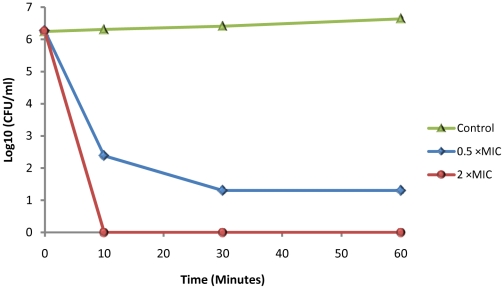
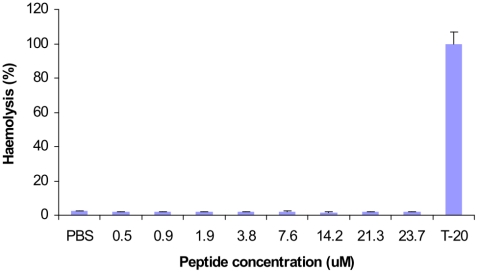
The tammar peptide WAM1 was found to be remarkably potent. It is 3–80 times more effective than LL-37 against each of these microbes and is ten times more effective against E. coli and B. subtilis than antibiotics such as ampicillin, tetracycline and chloramphenicol (Table 2). WAM1 was selected for further studies (results summarized in Table 3) as it was effective against Gram-negative bacteria including P. aeruginosa, a bacterial species known to cause untreatable infections due to its resistance to all current antibiotics. We also tested nineteen clinical isolates of multidrug-resistant microorganisms that are resistant to at least three antibiotics. WAM1 killed multidrug-resistant gram-negative bacteria including P. aeruginosa, A. baumannii and K. pneumoniae (Table 3). This is significant as some of the P. aeruginosa isolates are also resistant to the last-line therapy, colistin [39]. Unlike some other antimicrobial peptides, eg LL37 [40], WAM1 is resistant to inhibition by high (150–200 mM) NaCl concentrations (Table 4), which is relevant as most alpha-helix antimicrobial peptides lose activity at high salt concentrations. The salt resistance of WAM1 suggests that it may be suitable for applications in vivo [41], [42], [43], [44]. WAM1 was rapidly bactericidial, killing 99.9% of K. pneumoniae within 15 minutes (Fig. 3). WAM1 was not hemolytic against human red blood cells indicating potential for parenteral use in humans (Fig. 4). The effectiveness of tammar cathelicidin WAM1 in killing multidrug-resistant P. aeruginosa demonstrates the potential of these compounds for treating life-threatening drug resistant infections.
Table 2. Comparison of WAM1 potency to antibiotics ampicillin, tetracycline and chloramphenicol against E. coli.
| MIC (µM) | Relative potency of WAM1 | |
| Ampicillin | 5.7 | 12 x |
| Tetracycline | 4.5 | 10 x |
| Chloramphenicol | 12.4 | 26 x |
| WAM1 | 0.47 |
Table 3. Activity of WAM1 against antibiotic-resistant gram-negative clinical isolates.
| Isolate description | MIC (µM) |
| P. aeruginosa (ATCC 27853) | 0.95 |
| P. aeruginosa 001* | 1.90 |
| P. aeruginosa 002* | 0.95 |
| P. aeruginosa 003* | 0.47 |
| P. aeruginosa 004* | 1.90 |
| P. aeruginosa 005* (colistin R) | 0.95 |
| P. aeruginosa 006* (colistin R) | 0.95 |
| P. aeruginosa 007* (colistin R) | 0.95 |
| P. aeruginosa 008* (colistin R) | 0.95 |
| P. aeruginosa 009* (colistin R) | 0.95 |
| P. aeruginosa 010* (colistin R) | >30.4 |
| P. aeruginosa 011* | 0.47 |
| P. aeruginosa 012* | 1.90 |
| P. aeruginosa 013* | >30.4 |
| P. aeruginosa 014* | >30.4 |
| A. baumannii (ATCC 19606) | 1.90 |
| A. baumannii 001* | 0.95 |
| A. baumannii 002* | >15.2 |
| A. baumannii 003* | 1.90 |
| K. pneumoniae (ATCC 13883) | 0.47 |
| K. pneumoniae 001* | 1.90 |
| K. pneumoniae 002* | 0.95 |
| K. pneumoniae 003* | 7.59 |
The 19 antibiotic-resistant isolates are marked with an asterisk and those also resistant to colistin are indicated.
Table 4. WAM1 activity in the presence of high concentrations of NaCl.
| MIC (µM)0 mM NaCl | MIC (µM)150 mM NaCl | MIC (µM)200 mM NaCl | |
| P. aeruginosaATCC 27853 | 0.9 | 0.9 | 0.9 |
| A. baumanniiATCC 19606 | 1.9 | 1.9 | 3.8 |
| K. pneumoniaeATCC 13883 | 0.5 | 0.9 | 0.9 |
Figure 3. Time course of K. pneumoniae killing by WAM1 in broth.
Time marked in minutes. [MIC] = 0.47 µM. Antimicrobial assays were performed as described previously [57].
Figure 4. Toxicity of WAM1 on human red blood cells.
Haemolytic activity was determined by treating human red blood cells with different concentrations of WAM1 and haemoglobin release measured by absorbance at 450 nm. Samples were incubated at 37°C for 1 hour. PBS and 1% Tween-20 were used as negative and positive controls, representing 0% and 100% hemolytic activity respectively.
Our data suggests that the function of WAM1 is to protect the altritical neonate at birth, as clinically relevant bacteria affected by WAM1 (such as A. baumanni and E. coli) were detected in the tammar pouch around the time of birth (Table 5). Consistent with previous reports [45], we also identified a number of potentially novel bacterial species in the pouch. This may indicate that WAM1 is able to limit the growth of a broader spectrum of bacterial species, beyond those tested in this study.
Table 5. Bacteria identified by 16S rDNA sequencing from the pouch of the tammar wallaby around the time of birth.
| Time of colony isolation | 16S rDNA identification | Percentage match |
| Day(-6) | Bacillus licheniformis | 100% |
| Day(-5) | Bacillus licheniformis | 100% |
| Day(-5) | Devriesea agamarum a. | 94% |
| Day(-5) | Staphylococcus equorum | 99% |
| Day(-2) | Corynebacterium tuscaniense a. | 95% |
| Day(-2) | Bacillus cereus | 99% |
| Day(0) | Escherichia coli | 100% |
| Day(0) | Acinetobacter baumannii | 100% |
| Day(0) | Corynebacterium urealyticum | 98% |
| Day(0) | Enterobacter aerogenes | 100% |
| Day(0) | Corynebacterium coyleae a. | 95% |
| Day(0) | Devriesea agamarum a. | 95% |
| Day(+1) | Devriesea agamarum a. | 95% |
| Day(+1) | Escherichia coli | 100% |
| Day(+1) | Acinetobacter baumannii | 100% |
The low sequence homology observed in these isolates suggests that they may be a novel bacterial species and/or genus. Day (-6, 5, 2) etc indicates 6, 5 and 2 days before birth; Day(0) is day of birth; Day(+1)is the day after birth.
Conclusions
The dwindling numbers of therapeutic options for bacterial ‘superbugs’ necessitates the development of novel antimicrobials. The development of microbial resistance against naturally occurring antimicrobials peptides is rare [2], and our data suggest that the potent antimicrobial peptide gene expansions in the platypus and tammar genomes are good candidates for discovering natural peptides to fight multidrug resistant microbes. The strong antimicrobial activity, salt insensitivity and low haemolytic activity further suggest that marsupial and monotreme cathelicidin peptides have the potential to provide novel therapeutic antibiotics.
Materials and Methods
Identification of tammar and platypus cathelicidin genes
Genomic search
The PFAM cathelicidin HMMER [46] profile (PF00666) was used to search a six-frame translated assembly of the tammar wallaby genome (1.0). Gene predictions were performed using FGENESH+ [47] based on HMMER results. These were confirmed by BLAST [48] against NCBI's protein nr database. Platypus and tammar cathelicidin sequences can be downloaded from http://hp580.angis.org.au/tagbase/gutentag/. GenBank Accessions for previously published cathlicidin sequences [32], [34] are MaeuCath1 EF624481.1, MaeuCath2 EF624482.1, MaeuCath3 EF624483.1, MaeuCath4 EF624484.1, MaeuCath5 EF624485, MaeuCath6 EF624486.1, MaeuCath7 EF624487.1, MaeuCath8 EU883635.1.
Gene expression of cathelicidin genes
All experiments were approved by the University of Melbourne Animal Experimentation Ethics Committee and the animal handling and husbandry procedures were in accordance with the National Health and Medical Research Council of Australia (2004) guidelines. Pregnancy was initiated in females carrying an embryo in diapause by the removal of their pouch young (RPY) [49], [50]. Adult females carrying fetuses in the final third of gestation (day 19–26/birth) or pouch young (day 0–350) were euthanised either by cervical dislocation or by an anaesthetic overdose (sodium pentobarbitone, 60 mg/ml, to effect) and portions of the suckled mammary gland and pouch skin were collected and snap frozen in liquid nitrogen. Pouch young (PY) skin also was collected from both male and female animals of various ages. Tissues were homogenized and total RNA was extracted from mammary glands using RNeasy Lipid Tissue Minni Kit (QIAGEN, #74804) or from pouch and PY skin using Tri-Reagent (Ambion #AM9738) as described by the manufacturer with a final elution of RNA in 60–80 µl of RNAsecure H2O (Ambion, Geneworks, # AM7005) in a dilution of 1/24 µl. Total RNA was DNase treated (DNA-freeTM, Ambion, # 1906) to remove contaminating genomic DNA, quantified with a nano-spectrometer (NanaDrop ND-1000 Spectrophotometer, NanoDrop Technologies Inc, Wilmington, DE, USA) and cDNA was synthesized with SuperScript III First Strand Synthesis System for RT-PCR (Invitrogen, # 11904-018). Typically 2000 ng or a maximum of 8 µl of total RNA was used in each cDNA synthesis reaction, with 1 µl of Oligo (dT)12–18 (50 µM). cDNA integrity was immediately assessed with GAPDH RT-PCR (Table 1). Approximately 0.5–1 µl (50–100 ng) of template was used with 0.5 µM of each primer (table 1) with GoTaq Green Master Mix (PROMEGA, # M7122) RT-PCR cycles consisted of 94°C for 1 min, followed by 35 cycles of 15 sec at 94°C, 30 sec at 60–63°C, and 40 sec at 72°C, and a final extension at 72°C for 5 min. PCR products were resolved by gel electrophoreses and a band of each cathelicidin was extracted (QIAquick Gel Extraction Kit, Qiagen, # 28704) for direct sequencing. Sequences were assessed with FinchTV (v.1.3.1) DNA sequence chromatogram trace viewer software to confirm correct amplification. Cathelicidin expression RT-PCRs were performed using gene specific primers (Table 6). Three separate samples from each age group were used to assess the expression patterns in the mammary gland throughout lactation and in the skin throughout development. All primers were designed using Primer3 (v. 0.4.0) [51] and synthesised by Sigma-Aldrich.
Table 6. Primer sequences for cathelicidin sequence expression analysis.
| Cathelicidin | Primer | Primer sequence (5′ to 3′) | Length (bp) |
| 3 | Fw | AGTGGGTGAAAAAGTTAAGACCAG | 198 |
| Rv | TATGAGAAGAAGGGTGAGGGTAAG | ||
| 1,2,4,5,6,7 | Fw | CCATACCAGGATGTGCTGAAT | |
| 1 | Rv | ACAGGAGGCTACCCTGGCAGT | 413 |
| 2 | Rv | AGTCAGAATCCCTTCCCAGCC | 245 |
| 4 | Rv | AGTCAGACTCCCTCCCTAGTC | 242 |
| 5 | Fw | CATGCAGGTACTCCTATTGGTGCTG | 190 |
| Rv | AATGCATTGTTTCACCAGCTCCTC | ||
| 7 | Rv | ATCATCCCCGAGTTTCGTCAC | 345 |
| 8 | Fw | ATCTACTCTCCTTCACCCAATCAG | 126 |
| Rv | GGATACTGAGCCTTGACATTCTTT | ||
| GAPDH | Fw | CCTACTCCCAATGTATCTGTTGTGG | 351 |
| Rv | GGTGGAACTCCTTTTTTGACTGG |
Construction of phylogenetic tree shown and divergence dating
The phylogenetic tree was constructed using the prepro region (cathelin domain) of the peptide sequences using the neighbor-joining method with Jones-Taylor-Thornton amino-acid substitution as implemented by MEGA4 [52]. Amino-acid substitution model was identified with ProtTest [53] using Bayesian information criterion. We used partial deletion and gamma distribution for variation among sites (shape parameter = 1.0) in MEGA4 [52]. Divergence dating was performed using BEAST v1.5. Tree prior was defined by Yule process. The BLOSUM 62 amino-acid substitution model was used. We used an uncorrelated log-normal relaxed-clock model to account for lineage-specific heterogeneity. A log-normal calibration node defined at boreoeutheria divergence [54]. Priors for each parameter followed a normal distribution with a standard deviation of 0.5 million years. Monte Carlo stimulations were run for 10,000,000 steps and 10,000 trees were stored and 9,000 trees summarized. Accession numbers: tammar1 - ABV01938.1, tammar2 - ABV01939.1, tammar3 - ABV01940.1, tammar4 - EF624484.1, tammar5 - ABV01941.1, tammar6 - ABV01942.1, tammar7 - ABV01943.1, tammar8 - ACJ76797.1, human - NP_004336.2, mouse - AAB88303.1, chicken1 - NP_001001605.1, chicken2 -Q2IAL7.1, chicken3 - AAZ65841.1, cow1 - P22226, cow2 - P19660, cow3 - P19661, cow4 - P33046, cow5 - P54229, cow6 - P54228, cow7 - P56425, sheep1 - P54230, sheep2 - P82018, sheep3 - P79362, sheep4 - P50415, sheep5 - P49928, sheep6 - P49929, sheep7 - P79361, sheep8 - O19031, sheep9 - O19040, sheep10 - P79360, pig1 - P15175, pig2 - P51524, pig3 - P32194, pig4 - P32195, pig5 - P32196, pig6 - P49933, pig7 - P49934, pig8 - P49930, pig9 - P49931, pig10 - P49932, pig11 - P80054.
Prediction and screening of ancestral tammar cathelicidin mature peptides
A mature peptide consensus sequence was generated using three prediction methods for ancestral sequence: PAML [36], GASP [37] and Ancescon [38]. Previously cloned and sequenced tammar cathelicidin sequences were used as input (ABV01941.1, ABV01938.1, ABV01939.1, ABV01940.1, ABV01942.1, ABV01943.1).
Pouch microbe analysis
Pouch swabs were taken before birth (day 6 – 2), the day of birth (day 0) or one day after birth (day +1) from four wallabies by gently rubbing a sterile cotton swab over the mammary glands and around the bottom of the pouch. Swabs were then used to inoculate Nutrient Agar, Horse Blood Agar and MacConkey Agar. Plates were incubated aerobically for 24–48 hours at 37°C. Selected colonies were then identified by 16S rDNA sequencing. The closest genus and species match for each selected colony (%) are shown in Table 5
Antimicrobial assays
Peptides were synthesized and HPLC purified (AusPep Pty Ltd). The two-stage radial diffusion assay used in this study has been described elsewhere [55]. Briefly, ∼4×106 of mid-logarithmic-phase organisms were mixed into 10 ml of warm 0.8% agarose containing 0.03% (w/v) Trypticase soy broth (TSB) powder buffered with 10 mM sodium phosphate, pH 7.4. The synthetic peptides were serially diluted in 0.01% acetic acid and 5 µl peptide samples were loaded in 2.5 mm diameter wells. The plates were covered, and incubated at 37°C. After 3 hours, a 10 ml overlay gel composed of 6% TSB, 0.8% agarose in 10 mM sodium phosphate buffer (pH 7.4) was poured onto the plates. The plates were incubated overnight to allow the surviving organisms to form microcolonies. The clear zone was measured using a magnified transilluminator and expressed in units (1 mm = 10 U) after subtracting the well diameter. The MIC is defined by the χ intercept of a regression line through zone diameters obtained from a series of serially diluted peptide samples. For multidrug-resistant bacteria, MICs were measured as described by micro-broth dilution in Mueller-Hinton broth (Oxoid, Hampshire, England) according to according to Clinical and Laboratory Standards Institute (CLSI) standards [56]. The MIC measurements were performed in duplicate and the data incorporated into a final MIC using standard methods. All the bacterial strains were tested on at least two different occasions. Mid-logarithmic phase cultures of P. aeruginosa 19056 muc were diluted in Mueller–Hinton broth to yield approximately 106 cfu/ml. Peptides were added at the indicated concentrations and the suspensions were then incubated for different times in a shaking water bath at 35°C. At the end of each incubation time, the samples were serially diluted in buffered saline and plated onto nutrient agar with a spiral plater (Don Whitley Scientific, Australia). Colonies were counted after 17–22 h incubation at 35°C using an automatic colony counter (Don Whitley Scientific, Australia).
Acknowledgments
We thank Kerry and Scott Brownlees for assistance with the animals and Shayne Joseph and Richard Strugnell for assistance in analyzing the tammar pouch microbiota.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Australian Research Council (ARC) Centre of Excellence in Kangaroo Genomics and a University of Sydney grant. MBR was funded by an ARC Federation fellowship, JL by an Australian National Health and Medical Research Council Senior Research Fellow and KB by an Australian Research Council Future Fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tomasinsig L, Zanetti M. The cathelicidins--structure, function and evolution. Curr Protein Pept Sci. 2005;6:23–34. doi: 10.2174/1389203053027520. [DOI] [PubMed] [Google Scholar]
- 2.Bals R, Wilson JM. Cathelicidins--a family of multifunctional antimicrobial peptides. Cell Mol Life Sci. 2003;60:711–720. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KL, Hancock RE. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 5.Cole AM, Shi J, Ceccarelli A, Kim YH, Park A, et al. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood. 2001;97:297–304. doi: 10.1182/blood.v97.1.297. [DOI] [PubMed] [Google Scholar]
- 6.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirioni O, Giacometti A, Ghiselli R, Bergnach C, Orlando F, et al. LL-37 protects rats against lethal sepsis caused by gram-negative bacteria. Antimicrob Agents Chemother. 2006;50:1672–1679. doi: 10.1128/AAC.50.5.1672-1679.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uzzell T, Stolzenberg ED, Shinnar AE, Zasloff M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24:1655–1667. doi: 10.1016/j.peptides.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- 10.Sang Y, Teresa Ortega M, Rune K, Xiau W, Zhang G, et al. Canine cathelicidin (K9CATH): gene cloning, expression, and biochemical activity of a novel pro-myeloid antimicrobial peptide. Dev Comp Immunol. 2007;31:1278–1296. doi: 10.1016/j.dci.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Leonard BC, Chu H, Johns JL, Gallo RL, Moore PF, et al. Expression and activity of a novel cathelicidin from domestic cats. PLoS One. 6:e18756. doi: 10.1371/journal.pone.0018756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goitsuka R, Chen CL, Benyon L, Asano Y, Kitamura D, et al. Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proc Natl Acad Sci U S A. 2007;104:15063–15068. doi: 10.1073/pnas.0707037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao Y, Dai H, Bommineni YR, Soulages JL, Gong YX, et al. Structure-activity relationships of fowlicidin-1, a cathelicidin antimicrobial peptide in chicken. FEBS J. 2006;273:2581–2593. doi: 10.1111/j.1742-4658.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, et al. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem. 2006;281:2858–2867. doi: 10.1074/jbc.M507180200. [DOI] [PubMed] [Google Scholar]
- 15.Chang CI, Pleguezuelos O, Zhang YA, Zou J, Secombes CJ. Identification of a novel cathelicidin gene in the rainbow trout, Oncorhynchus mykiss. Infect Immun. 2005;73:5053–5064. doi: 10.1128/IAI.73.8.5053-5064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CI, Zhang YA, Zou J, Nie P, Secombes CJ. Two cathelicidin genes are present in both rainbow trout (Oncorhynchus mykiss) and atlantic salmon (Salmo salar). Antimicrob Agents Chemother. 2006;50:185–195. doi: 10.1128/AAC.50.1.185-195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhao H, Yu GY, Liu XD, Shen JH, et al. Structure-function relationship of king cobra cathelicidin. Peptides. 31:1488–1493. doi: 10.1016/j.peptides.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 18.de Latour FA, Amer LS, Papanstasiou EA, Bishop BM, van Hoek ML. Antimicrobial activity of the Naja atra cathelicidin and related small peptides. Biochem Biophys Res Commun. 396:825–830. doi: 10.1016/j.bbrc.2010.04.158. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Hong J, Liu X, Yang H, Liu R, et al. Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotics. PLoS One. 2008;3:e3217. doi: 10.1371/journal.pone.0003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Gan TX, Liu XD, Jin Y, Lee WH, et al. Identification and characterization of novel reptile cathelicidins from elapid snakes. Peptides. 2008;29:1685–1691. doi: 10.1016/j.peptides.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Luo ZX. Transformation and diversification in early mammal evolution. Nature. 2007;450:1011–1019. doi: 10.1038/nature06277. [DOI] [PubMed] [Google Scholar]
- 22.Bininda-Emonds OR, Cardillo M, Jones KE, MacPhee RD, Beck RM, et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- 23.Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, et al. Genome analysis of the platypus reveals unique signatures of evolution. Nature. 2008;453:175–183. doi: 10.1038/nature06936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renfree MB, Fletcher TP, Blanden DR, Lewis PR, Shaw G, et al. Physiological and behavioural events around the time of birth in macropodid marsupials. In: Grigg G, Jarman P, Hume ID, editors. Kangaroos, Wallabies and Rat Kangaroos. Sydney: Surrey Beatty & Sons Pty. Ltd; 1989. pp. 323–337. [Google Scholar]
- 25.Green B, Merchant J. The composition of marsupial milk. In: Tyndale-Bisco CH JP, editor. The Developing Marsupial Models for Biomedical Research. Berlin, Germany: Springer-Verlag; 1988. pp. 41–54. [Google Scholar]
- 26.Old JM, Deane EM. The detection of mature T- and B-cells during development of the lymphoid tissues of the tammar wallaby (Macropus eugenii). J Anat. 2003;203:123–131. doi: 10.1046/j.1469-7580.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Old JM, Deane EM. Immunohistochemistry of the lymphoid tissues of the tammar wallaby, Macropus eugenii. J Anat. 2002;201:257–266. doi: 10.1046/j.1469-7580.2002.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Old JM, Deane EM. Development of the immune system and immunological protection in marsupial pouch young. Dev Comp Immunol. 2000;24:445–454. doi: 10.1016/s0145-305x(00)00008-2. [DOI] [PubMed] [Google Scholar]
- 29.Holland N, Jackson SM. Reproductive behaviour and food consumption associated with the captive breeding of platypus (Ornithorhynchus anatinus). Journal of Zoology. 2002;256:279–288. [Google Scholar]
- 30.Grant T. The Platypus A Unique Mammal. In: Dawson T, editor. Sydney: UNSW Press; 1995. [Google Scholar]
- 31.Connolly JH, Canfield PJ, McClure SJ, Whittington RJ. Histological and immunohistological investigation of lymphoid tissue in the platypus (Ornithorhynchus anatinus). J Anat. 1999;195:161–171. doi: 10.1046/j.1469-7580.1999.19520161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly KA, Digby MR, Lefevre C, Nicholas KR, Deane EM, et al. Identification, characterization and expression of cathelicidin in the pouch young of tammar wallaby (Macropus eugenii). Comp Biochem Physiol B Biochem Mol Biol. 2008;149:524–533. doi: 10.1016/j.cbpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Renfree MB, Papenfuss AT. Genome sequence of an Australian kangaroo, Macropus eugenii, provides insight into the evolution of mammalian reproduction and development. Genome Biol in press. 2011 doi: 10.1186/gb-2011-12-8-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carman RL, Old JM, Baker M, Jacques NA, Deane EM. Identification and expression of a novel marsupial cathelicidin from the tammar wallaby (Macropus eugenii). Vet Immunol Immunopathol. 2009;127:269–276. doi: 10.1016/j.vetimm.2008.10.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whittington C, Sharp J, Papenfuss A, Belov K. No evidence of expression of two classes of natural antibiotics (cathelicidins and defensins) in a sample of platypus milk. Australian Journal of Zoology. 2009;57:211–217. [Google Scholar]
- 36.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 37.Edwards RJ, Shields DC. GASP: Gapped Ancestral Sequence Prediction for proteins. BMC Bioinformatics. 2004;5:123. doi: 10.1186/1471-2105-5-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai W, Pei J, Grishin NV. Reconstruction of ancestral protein sequences and its applications. BMC Evol Biol. 2004;4:33. doi: 10.1186/1471-2148-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim LM, Ly N, Anderson D, Yang JC, Macander L, et al. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy. 2010;30:1279–1291. doi: 10.1592/phco.30.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bals R, Wang XR, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisanby MW, Swiecki MK, Dizon BL, Pflughoeft KJ, Koehler TM, et al. Cathelicidin administration protects mice from Bacillus anthracis spore challenge. J Immunol. 2008;181:4989–5000. doi: 10.4049/jimmunol.181.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohgami K, Ilieva IB, Shiratori K, Isogai E, Yoshida K, et al. Effect of human cationic antimicrobial protein 18 Peptide on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2003;44:4412–4418. doi: 10.1167/iovs.03-0246. [DOI] [PubMed] [Google Scholar]
- 43.Zhou H, Dou J, Wang J, Chen L, Wang H, et al. The antibacterial activity of BF-30 in vitro and in infected burned rats is through interference with cytoplasmic membrane integrity. Peptides. 32:1131–1138. doi: 10.1016/j.peptides.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Gronberg A, Zettergren L, Agren MS. Stability of the Cathelicidin Peptide LL-37 in a Non-healing Wound Environment. Acta Derm Venereol. doi: 10.2340/00015555-1102. [DOI] [PubMed] [Google Scholar]
- 45.Chhour KL, Hinds LA, Jacques NA, Deane EM. An observational study of the microbiome of the maternal pouch and saliva of the tammar wallaby, Macropus eugenii, and of the gastrointestinal tract of the pouch young. Microbiology. 2010;156:798–808. doi: 10.1099/mic.0.031997-0. [DOI] [PubMed] [Google Scholar]
- 46.Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14:755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- 47.Salamov AA, Solovyev VV. Ab initio gene finding in Drosophila genomic DNA. Genome Res. 2000;10:516–522. doi: 10.1101/gr.10.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renfree MB, Tyndale-Biscoe C, H . Manipulation of marsupial embryos and pouch young. In: Daniel JC, editor. Methods in Mammalian Reproduction. Academic Press; 1978. [Google Scholar]
- 50.Tyndale-Biscoe CH, Renfree M. Cambridge: Cambridge University Press; 1987. Reproductive physiology of marsupials. [Google Scholar]
- 51.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 52.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 53.Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- 54.Benton MJ, Donoghue PCJ, Asher RJ. Calibrating and constraining the molecular clock. In: Hedges SB, Kumar S, editors. The timetree of life. New York: Oxford University Press; 2009. [Google Scholar]
- 55.Steinberg DA, Lehrer RI. Designer assays for antimicrobial peptides. Disputing the "one-size-fits-all" theory. Methods Mol Biol. 1997;78:169–186. doi: 10.1385/0-89603-408-9:169. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Turnidge J, Milne R, Nation RL, Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45:781–785. doi: 10.1128/AAC.45.3.781-785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, et al. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2008;62:1311–1318. doi: 10.1093/jac/dkn425. [DOI] [PubMed] [Google Scholar]