Abstract
Background
A combination of horse anti-thymocyte globulin and cyclosporine produces responses in 60–70% of patients with severe aplastic anemia. We performed a phase II study of rabbit anti-thymocyte globulin and cyclosporine as first-line therapy for severe aplastic anemia.
Design and Methods
Twenty patients with severe aplastic anemia treated with rabbit anti-thymocyte globulin were compared to 67 historical control cases with matched clinical characteristics treated with horse anti-thymocyte globulin.
Results
Response rates at 3, 6 and 12 months were similar for patients treated with rabbit anti-thymocyte globulin or horse anti-thymocyte globulin: 40% versus 55% (P=0.43), 45% versus 58% (P=0.44) and 50% versus 58% (P=0.61), respectively. No differences in early mortality rates or overall survival were observed. We then performed multivariable analyses of response at 6 months and overall survival and identified the presence of a paroxysmal nocturnal hemoglobinuria clone (P=0.01) and a pretreatment absolute reticulocyte count greater than 30×109/L (P=0.007) as independent predictors of response and younger age (P=0.003), higher pretreatment absolute neutrophil (P=0.02) and absolute lymphocyte counts (P=0.03) as independent predictors of overall survival. None of the immunogenetic polymorphisms studied was predictive of response to immunosupressive therapy.
Conclusions
Despite reports suggesting differences in biological activity of different anti-thymocyte globulin preparations, rabbit and horse anti-thymocyte globulin appear to have a similar efficacy for up-front treatment of severe aplastic anemia. Clinicaltrial.gov: NCT01231841)
Keywords: aplastic anemia, cytokine gene polymorphism, anti-thymocyte globulin
Introduction
Aplastic anemia (AA) is a hematopoietic stem cell disorder characterized by pancytopenia and hypocellular bone marrow. The pathogenesis of most cases of idiopathic AA involves T-cell-mediated destruction of hematopoietic progenitors and stem cells;1 consequently, therapeutic immunosuppression has been effective in a large proportion of patients. The most effective immunosuppressive regimen includes horse anti-thymocyte globulin (hATG) in combination with cyclosporine, a treatment which has been shown to be superior to either ATG or cyclosporine alone.2,3 When used as a first-line treatment for severe AA, 60–70% of patients have a hematologic response.4–6 In one representative study, the actuarial 10-year survival for patients treated with immunosuppressive therapy was 68%.5
ATG can exert various effects on the immune system, including T-cell depletion in the blood and peripheral tissues, down-modulation of adhesion molecules and chemokine receptors,7 and possibly a direct effect on hematopoietic stem cells.8–10 While most studies have been performed with hATG, more recently, rabbit-derived ATG (rATG) has been introduced into clinical practice, particularly in organ transplantation. Although both types of ATG share similar mechanisms of action, rATG may be more potent than hATG at an equivalent concentration based on its capacity to produce more profound and prolonged lym-phocytopenia. Thus, it is not surprising that loads of Epstein-Barr virus have been found to be higher in patients receiving rATG.6 In addition, rATG may selectively promote the expansion of regulatory T cells,11 possibly inducing in vivo immune tolerance through this mechanism.7
While hATG has been the primary treatment used for severe AA, patients with relapsing or refractory AA often receive rATG after a first course of hATG. Although initially reported response rates in this setting approached 77%,12 responses in subsequent studies were more modest, at 30% in hATG-refractory patients and 65% in relapsed primary responders.13 Only limited efficacy data are available for rATG used as initial therapy in severe AA. In a single arm, phase II study, 62% (13/21) of AA patients responded when treated with rATG/cyclosporine up-front.14 When patients were treated with either hALG (lymphoglobulin) or rATG, similar response rates were obtained for both (48% versus 46%).15 Similarly, a prospective study in China enrolled AA patients into four different ATG-based treatment regimens, including hATG and rATG in combination with cyclosporine and growth factors.16 The response rate was 73% (22/30) versus 53% (17/32) (P=0.039), respectively. In a recent, retrospective study, the response rate at 6 months was significantly higher in patients who received hATG (59%) than in those given rATG (34%).17 Of note, the median rATG dose used in this study was only 2.5 mg/kg/day x 5 days. In another cohort of AA patients, 13/14 (93%) AA patients treated with hATG responded, while only 8/17 (47%) responded to rATG.18
We conducted a phase 2 study of the rATG/cyclosporine combination as an initial treatment for patients with severe AA, and compared their responses to those of a historical cohort of patients with severe AA treated with hATG. We also investigated the activity of hATG and rATG as salvage therapy, and analyzed factors associated with response to therapy.
Design and Methods
Patients
We analyzed 89 patients with severe AA who underwent initial therapy with rATG between 2005 and 2009 (n=22) as part of a prospective phase 2 study and compared these patients’ outcomes with those of a retrospective series of well-matched patients treated with hATG between 1996 and 2010 (n=67). Since the characteristics of the patients treated with each therapy were similar, both groups were then pooled to assess prognostic factors. Informed consent was obtained in accordance with protocols approved by the Institutional Review Board of Cleveland Clinic (Cleveland, OH, USA). For this study, severe AA was defined as a bone marrow cellularity of less than 30% associated with at least two of the following peripheral blood count criteria: (i) absolute neutrophil count (ANC) less than 0.5×109/L; (ii) absolute reticulocyte count (ARC) less than 60×109/L (<20×109/L if counted manually); and (iii) platelet count less than 20×109/L.4,19 Baseline laboratory values were defined as the lowest value during the 4 weeks prior to immunosuppressive therapy and were determined prior to transfusions: the prognostic algorithm is, therefore, free of transfusion artifacts.
We also studied 23 AA patients with relapsed (n=12) or refractory disease (n=11) who fulfilled the criteria for severe AA. Out of those who were initially treated with hATG, 13 patients were then treated with rATG while four were treated with repeat hATG. Among the patients who initially received rATG, six were then treated with hATG. Patients were administered hATG (Pfizer, Kalamazoo, MI, USA) at a dose of 40 mg/kg per day intravenously on days 1–4, or rATG (Genzyme, Cambridge, MA, USA) at a dose of 3.5 mg/kg per day intravenously on days 1–5. Cyclosporine was given at a dose between 200 to 400 mg per day following the last day of hATG or rATG infusion and adjusted to maintain a serum level of 200–400 ng/mL or based on renal toxicity or tolerability. At our center, patients typically received corticosteroids, usually methylprednisolone, at a dose of 1 mg/kg per day, from day 1 until day 14; the dose was then tapered down to prevent serum sickness. All patients received supportive care whenever indicated as previously described.4 A chromosome breakage test was done in patients younger than 30 years old to rule out Fanconi’s anemia.
Response criteria
Patients were considered responders if they no longer met the criteria for severe AA in the absence of recent transfusions and administration of granulocyte colony-stimulating factor.4,19 Response was evaluated 3, 6 and 12 months after immunosuppressive treatment. We defined relapse as a need for retreatment with ATG following an initial response.4 Patients who underwent stem cell transplantation prior to 6 months and patients who died within 90 days of therapy were considered as non-responders. The median follow-up period was 792 days (range, 37–1800) for patients treated with rATG and 1174 (range, 8–4492) days for those treated with hATG.
Auxiliary studies
Screening for a paroxysmal nocturnal hemoglobinuria (PNH) clone was done using the flow cytometric analysis of CD55 and CD59 expression on neutrophils or red blood cells as previously reported.20 Patients were considered to have a positive clone in the absence of greater than 1% glycophosphatidylinositol-anchored surface proteins.19 V beta (Vβ) flow cytometric analysis was also done to quantify the percentage of each Vβ family in the CD4 and CD8 lymphocyte populations, as previously described.21 In this study, a significant clonal expansion was defined as one that was greater than the mean plus three standard deviations of that in healthy controls.22 HLA typing was performed for all patients.
Genotyping of cytokine polymorphisms
Cytokine single nucleotide polymorphisms were determined by sequencing the following: interferon-γ (IFN-γ) intron (+874 A/T), transforming growth factor-β (TGF-β) codons (10 C/T and 25 C/G), interleukin (IL)-4 (−1098 G/T, −590 C/T), IL-4Ra (+1902 A/G), tumor necrosis factor-α promoter position (−308 A/G), IL-1α (−889 C/T), IL-1β (−511 C/T and +3962 C/T), IL-1 receptor (+1970 C/T), IL-1Ra (+11100 C/T), IL-2 (−330 G/T, +1662 G/T), and IL-6 (−174 C/G, nt565 A/G).
Statistical analysis
Response at 6 months and overall survival, measured from the date treatment was started to the date of death or last follow-up, were the primary outcomes analyzed; however, other outcomes including response at 3 and 12 months, relapse and early death were also examined. Categorical data were summarized as frequency counts and proportions, measured data were summarized as medians and ranges and duration of response and overall survival were summarized using the method of Kaplan and Meier.
Univariable analyses were performed using Fisher’s exact test and the Cochran-Armitage trend test (categorical data), the Wilcoxon rank sum test (measured data), and the Cox proportional hazards model (overall survival and duration of response). Logistic regression and Cox proportional hazards models were used to assess multiple factors simultaneously. In multivariable analyses a step-up selection algorithm with P=0.1 as the criterion for entry into the model was used to identify independent predictors of response at 6 months and overall survival. For convenience and simplicity a recursive partitioning algorithm was used to identify optimal cut points for measured data such as age at diagnosis and pretreatment blood counts. A bootstrap re-sampling algorithm that employed 1000 simulations was used to provide internal validation of the final response and survival models. All data analyses were performed using SAS version 8.0 (SAS Inc., Cary, NC, USA)
Results
Patients’ characteristics
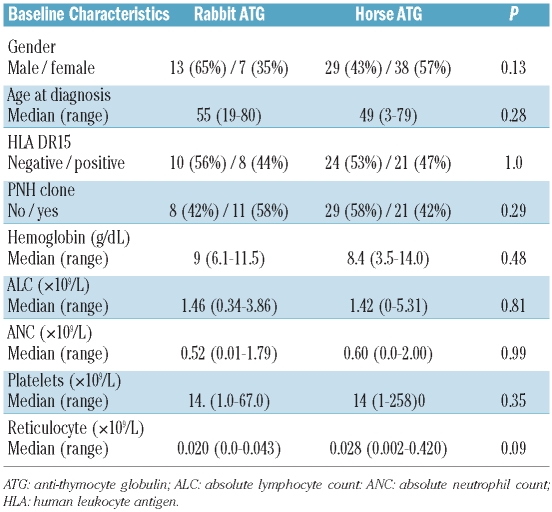
Eighty-nine patients were initially considered for inclusion in the analysis of de novo-treated patients; however, two patients treated off-protocol with rATG were excluded from the analysis. The characteristics of the analyzed patients are summarized in Table 1; 67 patients (77%) were treated with hATG (historical control group) and 20 (23%) were treated with rATG. With the possible exception of a slightly higher pretreatment ARC in the hATG-treated group (median 28×109/L for hATG versus 20×109/L for rATG, P=0.09) there were no statistically significant differences in baseline characteristics between the two groups (Table 1). Fourteen patients with refractory AA underwent stem cell transplantation; ten in the hATG group and four in the rATG group. No excess toxicity was observed in patients who received rATG, which should have resulted in premature termination of the protocol. The difference in the efficacy of rATG and hATG was the primary focus of this study; however, because the characteristics of the patients treated with each therapy were similar, both groups were pooled to assess prognostic factors.
Table 1.
Patients’ characteristics.
Hematologic response following immunosuppressive treatment
Response to theraphy was analyzed 3, 6, and 12 months after the course of immunosuppressive therapy (Table 2). At 3 months, the response rate for patients in the hATG group was 55% (complete response=5%, partial response=50%), while that for patients receiving rATG was 40% (partial response=40%) (P=0.43). At 6 months, the response rate was 58% (complete response=6%, partial response=52%) versus 45% (partial response=45%) (P=0.44) and at 12 months, 58% (complete response=8%, partial response=50%) versus 50% (partial response=50%) (P=0.61), respectively. Six (9%) patients died early (<90 days from the start of immunosuppressive therapy) in the hATG group: four due to infectious complications, one due to renal failure and subsequent pulmonary edema, and one of undetermined cause. In the rATG group, two (10%) patients died early, both due to neutropenic sepsis.
Table 2.
Response after first ATG treatment.
Refractory disease/relapse following immunosuppressive treatment
The relapse rate for patients treated with hATG was 16% while that for patients treated with rATG was 5% (P=0.28). In our center, we maintain patients on cyclosporine as long as they are responding or not developing renal complications. In the hATG cohort, six primary non-responders patients were subsequently treated with rATG and none responded at 6 months. Eleven patients relapsed; seven were then treated with rATG and four with repeated hATG. Of the seven patients subsequently treated with rATG, four responded by 6 months, one patient did not respond and two were not evaluable for response. Of the four patients re-treated with hATG, two responded by 6 months and two did not.
In the rATG group, five primary non-responders were then treated with hATG; one patient responded by 6 months while another patient responded by 12 months. Two patients did not respond and one patient underwent bone marrow transplantation 2 months after the second treatment with ATG. One patient relapsed and was not a responder at 6 months after hATG treatment. Because of very limited number of patients, caution should be exercised in interpreting these data.
Evolution
In our cohort, 15/87 patients (17%) experienced clonal evolution. The median follow-up time was 792 (range, 37–1800) days and 1174 (range, 8–4492) days for patients treated with rATG and hATG, respectively. In the hATG cohort, 11 patients had progressive disease (9 developed a myelodysplastic syndrome, 3 developed acute myeloid leukemia, 1 had clinically manifest PNH) while two patients in the rATG group progressed (1 myelodysplastic syndrome, 1 acute myeloid leukemia). Of these 15 patients, ten had del(7) or del(7q). No factors were found to predict clonal evolution, nor was there a difference in this complication between the rATG and hATG groups.
Correlation of clinical parameters and response
Considering the patients’ baseline factors described in Table 1, higher pretreatment platelet count (≥12×109/L versus <12×109/L; P=0.003), ARC (>30×109/L versus ≤30×109/L; P=0.01), and ANC (>0.5×109/L versus 0.1–0.5×109/L versus <0.1×109/L; P=0.03) were all associated with an increased likelihood of response at 6 months. In addition, there was some suggestion that higher baseline ALC (≥0.75×109/L versus <0.75×109/L; P=0.07) may also be associated with response. In multivariable analyses, which initially considered all the factors in Table 1 (gender, age, HLA DR15, PNH clone, hemoglobin, platelet, ALC, ANC, and ARC), ARC was again seen to be prognostic of response (P=0.007). The presence of a PNH clone, which was not statistically significant in univariable analysis, was seen to be an independent predictor of response (P=0.01, Table 3). Adjusting for these two factors, the difference in response rates between the rATG and hATG groups remained non-significant (P=0.51).
Table 3.
Multivariable analysis for response at 6 months (n=45).
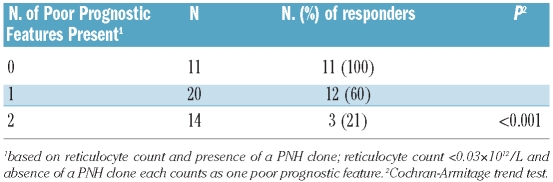
As the point estimates in multivariable analyses of response for the presence of a PNH clone and ARC were similar in magnitude (1.97 and 2.30, respectively), a simple prognostic score could be created by counting the number of poor-risk features present, with the absence of a PNH clone and a pretreatment ARC of less than 30×109/L each counting as one poor feature. Based on this score, three distinct prognostic groups could be defined: 23% (10/44) of patients had the most favorable profile (i.e. no poor risk features - PNH clone and pretreatment ARC ≥30×109/L) and all ten patients responded. In contrast, in patients with one poor-risk feature (i.e. no PNH clone or pretreatment ARC <30×109/L), 11/20 patients (55%) responded and among those patients with an unfavorable profile (i.e. no PNH clone and pretreatment ARC ≤30×109/L), only 3/14 patients (21%) responded (P<0.001) (Table 4). When, in addition to the clinical parameters, the impact of T-cell receptor repertoire at presentation on the quality of subsequent response was analyzed, flow cytometric Vβ typing showed that patients with significantly expanded CD4 T-cell clones at baseline were more likely to have responded by 3 months. Overall, 15/57 had positive CD4 Vβ expansion according to our criteria; these 15 patients with CD4 Vβ expansion comprised 38% (12/32) of the responders versus 12% (3/25) of the non-responders (P=0.03). CD8 expansions had no relationship with response: overall, 35/57 (61%) had a CD8 expansion with the incidence being 15/25 (60%) in non-responders and 20/32 in responders (63%) (P=1.00).
Table 4.
Prognostic grouping for response at 6 months (n=45).
Impact of immunogenetic polymorphisms
Among a large number of immunogenetic polymorphisms studied (see Design and Methods section), only TGF-β codons 10/25, IFN-γ, IL-4 codons 590/1098 and IL4-Ra 1902 were shown to have a higher frequency in our internal AA cohort (data not shown). When these parameters were analyzed within the initial cohort of patients with severe AA (both those treated with rATG and those treated hATG) with regard to the contribution to refractoriness/responsiveness to immunosuppressive therapy, none of these factors was predictive.
Survival analyses
Overall, 36% of the patients have died (hATG=36% and rATG=35%; P=0.54). The median follow-up for all the patients alive at the time of analysis was 43.5 months (range, 3.6–147.3). The median survival has not been reached; however 2- and 5-year estimated survival rates are 76±5% and 64±6%, respectively. Considering the factors in Table 1, younger age (≤18 versus 18–70 versus >70; P<0.001), female gender (P=0.05), pretreatment ALC (≥0.75×109/L versus <0.75×109/L; P<0.001), ANC (>0.5×109/L versus 0.1–0.5×109/L versus <0.1×109/L, P<0.001), and platelet count (>12×109/L versus ≤12×109/L; P=0.03) were all individually associated with improved survival. In multivariable analyses, age (P=0.003), pretreatment ANC (P=0.02), and pretreatment ALC (P=0.03) were the only factors found to be independent predictors of survival (Table 5). After adjusting for these three factors, the difference in survival between patients in the rATG and hATG groups remained non-significant (P=0.73).
Table 5.
Multivariable analysis for survival (n=61).
Like the multivariable analysis of response at 6 months, the point estimates associated with age, and pretreatment ANC and ALC were similar enough in magnitude (1.25, 0.83, and 1.04, respectively) to allow prognostic groups to be determined based solely on the number of poor-risk features present; with age 19–70 years, ANC 0.1–0.5×109/L, and ALC less than 0.75×109/L each counting as one poor-risk feature, and age over 70 years and ANC less than 0.1×109/L being counted as two. Using this scoring system, two distinct prognostic groups could be defined (Table 6): patients with a score of 2 or less and patients with a score of more than 2. Most (44/62) patients (71%) who fell into the favorable prognostic group and had estimated 2- and 5-year survival rates of 85±6% and 71±9%, respectively; 29% of patients (18/61) had an unfavorable profile, with estimated 2- and 5-year survival rates of 37±2% and 16±10%, respectively (P<0.001) (Figure 1).
Table 6.
Prognostic grouping for survival (n=61)
Figure 1.
Survival by number of poor prognostic features present. At 5 years, patients with more than two poor prognostic features (i.e. higher age, lower ANC and ALC) had a 16%+10% survival rate compared to 71%+9%% for patients with two or fewer poor prognostic features (P<0.001).
Discussion
The introduction of a preparation of ATG derived through immunization of rabbits prompted the clinically practical question as to whether this new and likely more potent immunosuppressive therapy would result in improved response rates when used as first-line treatment in patients with severe AA. This issue is the main focus of the current study. Certainly, when used in the salvage setting, rATG shows comparable efficacy to that seen with hATG. We conducted a pilot phase II study of rATG in a primary setting comparing the outcome of this treatment with that of historical controls who received hATG. The dose of 3.5 mg/kg x 5 days was adopted based on results from previous studies on the use of rATG in the refractory setting,12,13 although smaller doses could also be effective, and in one study 2.5 mg/kg was used in older patients.23
Our results suggest that rATG given at a dose of 3.5 mg/kg over 5 days may be similar to 40 mg/kg of hATG administered over 4 days with respect to response, overall survival or the risk of early death. While both prospectively studied patients and the historical group had similar baseline clinical characteristics, reliance on historical control cases treated with hATG is a clear limitation of our study. Nevertheless, our results are comparable to those of a previously published study of a Spanish AA cohort.15 In contrast, preliminary data from a National Institutes of Health, randomized trial showed that rATG was inferior to hATG at 3 months after immunosuppressive therapy.24 The final result of that study will definitively settle the issue of clinical differences between rATG and hATG. Other groups have also reported that the hATG-based regimen is superior to rATG in AA.16,17 The results in patients with myelodysplastic syndrome may be even more confusing since single center studies, mostly using hATG, yielded response rates that varied from 16%–50%.25–27 A prospective study comparing rATG versus hATG in myelodysplastic syndrome yielded similar response rates.28
Previous clinical observations have suggested that rATG may be more potent than hATG, based on the more profound and prolonged lymphocytopenia produced by the former.6 However, this stronger lympholytic activity does not necessarily mean that rATG is more immunosuppressive. In our study, there were no excess infectious complications leading to early death in patients treated with rATG and overall, the toxicity profile was similar to that reported for rATG.
In the present study multivariable analyses showed that the presence of a PNH clone and higher pretreatment ARC were independent predictors of response at 6 months. The National Institutes of Health group19 showed that higher baseline ARC, but not a PNH clone, was strongly predictive of response at 6 months. However, it was previously reported that PNH-positive cells are reliable markers of response to immunosuppressive therapy.29 In general, bio-markers of response are likely to predict the survival as patients with refractory AA were shown to have a poor prognosis.19 Importantly, in the present study combining the effects of PNH clonality and baseline ARC defined three distinct prognostic groups that had 6-month response rates that ranged from 21% to 100%. Other clinical parameters have been shown to predict responsiveness to immunosuppressive therapy. For example, in another recent study, higher ANC was also shown to be predictive of response.30 Baseline ANC was also correlated with response in our study in univariable analysis, but was not seen to be an independent predictor in multivariable analysis once the impact of PNH clone and baseline ARC were taken into account (P=0.64).
Our multivariate analysis of overall survival showed that younger age and higher pretreatment ALC and ANC were independent positive predictors of survival. Higher ALC and ARC were previously correlated with a higher survival rate19 while in another study, only higher ARC was predictive.30 As with response, distinct prognostic groups could be defined by using a simple scoring system to combine the effects of age and pretreatment ANC and ALC. The survival rate at 5 years for patients with two or fewer poor survival features was 71±9% compared to 16±10% for those with more than two poor survival features (P<0.001). Identification of distinct prognostic groups such as those defined here for response and overall survival could have a major clinical impact in terms of therapeutic decision-making by helping to identify patients who may benefit from early stem cell transplantation rather than repeated cycles of ATG or other forms of immunosuppressive therapy. Additional prospective studies in larger groups of patients will, however, be needed to validate these prognostic groups.
HLA-DR15 can serve as an example of an immuno-genetic polymorphism that has been linked to AA and responsiveness to immunosuppressive therapy.31,32 Similarly, allelic polymorphisms within the regulatory regions of cytokine gene promoters can affect their transcription and thereby influence pathogenesis of immune-mediated diseases. For example, IFN-γ and TNF-α suppress the growth of hematopoietic progenitor and stem cells33 and thus “high secretor or high affinity” genotypes could contribute to the pathogenesis of AA. Indeed, a TNF-α promoter/enhancer polymorphism was associated with increased responsiveness after immunosuppressive therapy34 and various other cytokine gene polymorphisms were found to be over-represented in patients with AA or PNH.35,36 However, in our study, none of the wide variety of immunogenetic polymorphisms studied (TGF-β codons 10 and 25, IFN- γ, IL-4 codons 590 and 1098, IL-4Ra) was significantly associated with response to immunosuppressive therapy or survival.
In summary, we conclude that despite reports suggesting differences in biological activity of different ATG preparations, rATG appears to have a similar efficacy to hATG for upfront treatment of severe AA. Irrespectively of the ATG preparation used, the presence of a PNH clone and a higher pretreatment ARC appear to predict response to immunosuppressive therapy while higher pretreatment ANC and ALC correlated with better survival. However, the historical comparison of rATG to established hATG therapy is an obvious limitation of this study and until results of a randomized study are reported, these conclusions should be considered with proper caution.
Footnotes
Funding: this work was supported in part by RO1AI-85578, U54 RR019397, and K24 HL077522, research support from Genzyme Corporation and grant from AA&MDS International Foundation (JPM).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Reference
- 1.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–19. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frickhofen N, Kaltwasser JP, Schrezenmeier H, Raghavachar A, Vogt HG, Herrmann F, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. The German Aplastic Anemia Study Group. N Engl J Med. 1991;324(19):1297–304. doi: 10.1056/NEJM199105093241901. [DOI] [PubMed] [Google Scholar]
- 3.Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101(4):1236–42. doi: 10.1182/blood-2002-04-1134. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–5. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 5.Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2007;92(1):11–8. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- 6.Scheinberg P, Fischer SH, Li L, Nunez O, Wu CO, Sloand EM, et al. Distinct EBV and CMV reactivation patterns following antibody-based immunosuppressive regimens in patients with severe aplastic anemia. Blood. 2007;109(8):3219–24. doi: 10.1182/blood-2006-09-045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114(9):1209–17. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Kook H, Zeng W, Young NS, Maciejewski JP. Is there a direct effect of antithymocyte globulin on hematopoiesis? J Hematology. 2004;5:655–61. doi: 10.1038/sj.thj.6200398. [DOI] [PubMed] [Google Scholar]
- 9.Flynn J, Cox CV, Rizzo S, Foukaneli T, Rice K, Murphy M, et al. Direct binding of antithymoctye globulin to haemopoietic progenitor cells in aplastic anaemia. Br J Haematol. 2003;122(2):289–97. doi: 10.1046/j.1365-2141.2003.04400.x. [DOI] [PubMed] [Google Scholar]
- 10.Killick SB, Cox CV, Marsh JC, Gordon-Smith EC, Gibson FM. Mechanisms of bone marrow progenitor cell apoptosis in aplastic anaemia and the effect of anti-thymocyte globulin: examination of the role of the Fas-Fas-L interaction. Br J Haematol. 2000;111(4):1164–9. doi: 10.1046/j.1365-2141.2000.02485.x. [DOI] [PubMed] [Google Scholar]
- 11.Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111(7):3675–83. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Bona E, Rodeghiero F, Bruno B, Gabbas A, Foa P, Locasciulli A, et al. Rabbit antithymocyte globulin (r-ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Br J Haematol. 1999;107(2):330–4. doi: 10.1046/j.1365-2141.1999.01693.x. [DOI] [PubMed] [Google Scholar]
- 13.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006;133(6):622–7. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- 14.Kadia T, Ravandi F, Garcia-Manero G, Jabbour E, Borthakur G, Estrov Z, et al. Updated results of combination cytokine immunotherapy in the treatment of aplastic anemia and myelodysplastic syndrome (MDS) Blood. 2010;116(22):4422–9. [Google Scholar]
- 15.Vallejo C, Montesinos P, Rosell A, Brunet S, Perez E, Petit J, et al. Comparison between lymphoglobuline- and thymoglobuline-based immunosuppressive therapy as a first-line treatment for patients with aplastic anemia. Haematologica. 2009;94(S. 2):451. [Google Scholar]
- 16.Zheng Y, Liu Y, Chu Y. Immunosuppressive therapy for acquired severe aplastic anemia (SAA): a prospective comparison of four different regimens. Exp Hematol. 2006;34(7):826–31. doi: 10.1016/j.exphem.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Atta EH, Dias DS, Marra VL, de Azevedo AM. Comparison between horse and rabbit antithymocyte globulin as first-line treatment for patients with severe aplastic anemia: a single-center retrospective study. Ann Hematol. 2010;89(9):851–9. doi: 10.1007/s00277-010-0944-y. [DOI] [PubMed] [Google Scholar]
- 18.Maschan MA, Novichkova G, Baidildina D, Suntzova E, Kravchenko E, Goronkova O, et al. Horse ATG (ATGAM) versus rabbit ATG (Fresenius) for treatment of aplastic anemia in children: results of prospective double-blind randomised single centre trial. Bone Marrow Transplant. 2004;33:S3–S79. [Google Scholar]
- 19.Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144(2):206–16. doi: 10.1111/j.1365-2141.2008.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maciejewski JP, Rivera C, Kook H, Dunn D, Young NS. Relationship between bone marrow failure syndromes and the presence of glycophosphatidyl inositol-anchored protein-deficient clones. Br J Haematol. 2001;115(4):1015–22. doi: 10.1046/j.1365-2141.2001.03191.x. [DOI] [PubMed] [Google Scholar]
- 21.Mohan S, Clemente MJ, Afable M, Wlodarski MW, Cazzolli HN, Lichtin AE, Maciejewski JP. Therapeutic implications of variable expression of CD52 on clonal cyto-toxic T cells in large granular lymphocyte leukemia. Haematologica. 2009;94(10):1407–14. doi: 10.3324/haematol.2009.009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wlodarski MW, Gondek LP, Nearman ZP, Plasilova M, Kalaycio M, Hsi ED, Maciejewski JP. Molecular strategies for detection and quantitation of clonal cyto-toxic T-cell responses in aplastic anemia and myelodysplastic syndrome. Blood. 2006;108(8):2632–41. doi: 10.1182/blood-2005-09-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg R, Faderl S, Garcia-Manero G, Cortes J, Koller C, Huang X, et al. Phase II study of rabbit anti-thymocyte globulin, cyclosporine and granulocyte colony-stimulating factor in patients with aplastic anemia and myelodysplastic syndrome. Leukemia. 2009;23(7):1297–302. doi: 10.1038/leu.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheinberg P, Wu C, Scheinberg P, Weinstein B, Nunez O, Sload E, Young N. A randomized trial of horse versus rabbit antithymocyte globulin in severe acquired aplastic anemia. Blood (ASH Annual Meeting Abstracts) 2010 Abstract LBA-4. [Google Scholar]
- 25.Yazji S, Giles FJ, Tsimberidou AM, Estey EH, Kantarjian HM, O’Brien SA, Kurzrock R. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17(11):2101–6. doi: 10.1038/sj.leu.2403124. [DOI] [PubMed] [Google Scholar]
- 26.Molldrem JJ, Caples M, Mavroudis D, Plante M, Young NS, Barrett AJ. Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol. 1997;99(3):699–705. doi: 10.1046/j.1365-2141.1997.4423249.x. [DOI] [PubMed] [Google Scholar]
- 27.Killick SB, Mufti G, Cavenagh JD, Mijovic A, Peacock JL, Gordon-Smith EC, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk’ myelodysplasia. Br J Haematol. 2003;120(4):679–84. doi: 10.1046/j.1365-2141.2003.04136.x. [DOI] [PubMed] [Google Scholar]
- 28.Stadler M, Germing U, Kliche KO, Josten KM, Kuse R, Hofmann WK, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18(3):460–5. doi: 10.1038/sj.leu.2403239. [DOI] [PubMed] [Google Scholar]
- 29.Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, et al. Minor population of CD55-CD59- blood cells predicts response to immunsuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107(4):1308–14. doi: 10.1182/blood-2005-06-2485. [DOI] [PubMed] [Google Scholar]
- 30.Chang MH, Kim KH, Kim HS, Jun HJ, Kim DH, Jang JH, et al. Predictors of response to immunosuppressive therapy with antithymocyte globulin and cyclosporine and prognostic factors for survival in patients with severe aplastic anemia. Eur J Haematol. 2010;84(2):154–9. doi: 10.1111/j.1600-0609.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 31.Maciejewski JP, Follmann D, Nakamura R, Saunthararajah Y, Rivera CE, Simonis T, et al. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001;98(13):3513–9. doi: 10.1182/blood.v98.13.3513. [DOI] [PubMed] [Google Scholar]
- 32.Saunthararajah Y, Nakamura R, Nam J-M, Robyn J, Loberiza F, Maciejewski JP, et al. HLA DR15 (DR2) is over-represented in myelodysplastic syndrome and aplastic anemia, and predicts a response to immunosuppression in myelodysplastic syndrome. Blood. 2000;100(5):1570–4. [PubMed] [Google Scholar]
- 33.Young NS, Maciejewski J. The pathophysiology of acquired aplastic anemia. N Engl J Med. 1997;336(19):1365–72. doi: 10.1056/NEJM199705083361906. [DOI] [PubMed] [Google Scholar]
- 34.Demeter J, Messer G, Schrezenmeier H. Clinical relevance of the TNF-alpha promoter/enhancer polymorphism in patients with aplastic anemia. Ann Hematol. 2002;81(10):566–9. doi: 10.1007/s00277-002-0544-6. [DOI] [PubMed] [Google Scholar]
- 35.Fermo E, Bianchi P, Barcellini W, Pedotti P, Boschetti C, Alfinito F, et al. Immunoregulatory cytokine polymorphisms in Italian patients affected by paroxysmal nocturnal haemoglobinuria and aplastic anaemia. Eur J Immunogenet. 2004;31(6):267–9. doi: 10.1111/j.1365-2370.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 36.Hertenstein B, Wagner B, Bunjes D, Duncker C, Raghavachar A, Arnold R, et al. Emergence of CD52-, phosphatidylinositol-glycan-anchor-deficient T lymphocytes after in vivo application of Campath-1H for refractory B-cell non-Hodgkin lymphoma. Blood. 1995;86(4):1487–92. [PubMed] [Google Scholar]