Abstract
Inductive interactions between cells of distinct fates underlie the basis for morphogenesis and organogenesis across species. In the Drosophila embryo, somatic myotubes form specific interactions with their epidermal muscle attachment (EMA) cells. The establishment of these interactions is a first step toward further differentiation of the EMA cells into elongated tendon cells containing an organized array of microtubules and microfilaments. Here we show that the molecular signal for terminal differentiation of tendon cells is the secreted Drosophila neuregulin-like growth factor Vein produced by the myotubes. Although vein mRNA is produced by all of the myotubes, Vein protein is secreted and accumulates specifically at the muscle–tendon cell junctional site. In loss-of-function vein mutant embryos, muscle-dependent differentiation of tendon cells, measured by the level of expression of specific markers (Delilah and β1 tubulin) is blocked. When Vein is expressed in ectopic ectodermal cells, it induces the ectopic expression of these genes. Our results favor the possibility that the Drosophila EGF receptor DER/Egfr expressed by the EMA cells functions as a receptor for Vein. We show that Vein/Egfr binding activates the Ras pathway in the EMA cells leading to the transcription of the tendon-specific genes, stripe, delilah, and β1 tubulin. In Egfr1F26 mutant embryos that lack functional Egfr expression, the levels of Delilah and β1 tubulin are very low. In addition, the ability of ectopic Vein to induce the expression of Delilah and β1 tubulin depends on the presence of functional Egfrs. Finally, activation of the Egfr signaling pathway by either ectopically secreted Spitz, or activated Ras, leads to the ectopic expression of Delilah. These results suggest that inductive interactions between myotubes and their epidermal muscle attachment cells are initiated by the binding of Vein, to the Egfr on the surface of EMA cells.
Keywords: Muscle, Drosophila, mesoderm, epidermal muscle attachment cell, receptor tyrosine kinase, Egfr/DER, Vein, neuregulin
Organogenesis is a multistep process, in which, at various stages of development, a defined set of cells influences the differentiation of other nearby cell populations to form tissues and organs. The basis for organ morphogenesis stems from inductive interactions occurring continuously between cells of epithelial and mesenchymal origin. Often, the mesenchyme-derived cells serve as the source for secreted signals that affect the state of differentiation of the neighboring target epithelial cells (Birchmeier et al. 1995).
The differentiation of tendon cells and the formation of muscle–tendon interactions represent an attractive model system to study the nature and hierarchy of molecular and cellular instructive interactions between distinct cell types that are responsible for the correct connections of body musculature to the skeleton. Here too, equivalent instructive interactions between distinct cell types, including muscles, tendon cells, and bones may regulate the process.
Muscle–tendon interactions in Drosophila occur during development of the embryo as well as the adult fly. In both processes myotubes migrate and bind specifically to their attachment cells. The larval somatic muscle tissue develops from mesodermal cells in the anterior compartment of mesodermal segments, determined by autonomous pair–rule gene activity in the mesoderm (Azpiazu et al. 1996). These cells express high levels of the basic helix–loop–helix (bHLH) protein, Twist (Baylies and Bate 1996). The different somatic muscles are formed by an array of 30 different types of myotubes that develop, through the second half of embryonic development, in close proximity to the basal surfaces of the epidermis (Bate 1990). The identity of each of these somatic myotubes is thought to be determined by inductive patterning mechanisms that define a single founder cell with a given specificity (Bate 1993; Baker and Schubiger 1995; Rushton et al. 1995). The founder cell then fuses to somatic mesodermal cells that, upon fusion, acquire the specificity of the primary founder cell. The specificity of the fused myotube, manifested by a distinct pattern of gene expression, determines the spatial and temporal development of a given myotube, the number of myoblasts to be fused, and the polarity of the fused myotube (Bate 1993; Abmayr et al. 1995). During extension, the myotube sends elongated filopodia at its leading edge, which facilitate its pathfinding toward the epidermal attachment cells (Bate 1990).
The final targeting of the muscle toward its specific epidermal muscle attachment (EMA) cells depends on these target cells (Volk and VijayRaghavan 1994). The initial differentiation of the EMA cells is induced by the activity of Stripe, an early growth response (EGR)-like putative transcription factor (Lee et al. 1995) expressed specifically in the EMA cells. Stripe is necessary and sufficient for the induction of EMA cell-specific genes including groovin, alien, and delilah and, in addition, positively autoregulates its own transcription. In stripe mutant embryos all of the characteristic EMA-specific genes are not expressed, and the muscle pattern is significantly deranged. Ectopic expression of Stripe induces ectopic expression of the EMA-specific genes in all of the tissues tested (Frommer et al. 1996; Becker et al. 1997). Groovin, a large membrane-associated extracellular protein may mediate adhesion between the muscles and their EMA cells (D. Strumpf and T. Volk, unpubl.). The function of Delilah, a bHLH protein characteristic of the EMA cells (Armand et al. 1994), as well as the function of Alien [a protein with homology to the human TRIP15 gene, which interacts with vertebrate thyroid receptor (Goubeaud et al. 1996)], are yet to be elucidated. The EMA cells attract and direct the leading edge of the myotube toward its target attachment cells. Specific adhesion between the myotube and the epidermal attachment cells is followed by the formation of extensive adherens-type junctions between the two cell types, mediated by the integrin receptors (Tepass and Hartenstein 1994).
Recent evidence suggests that there are two waves of gene expression in the EMA cells (Becker et al. 1997). The first wave is muscle independent and results from the initial induction of stripe in the future tendon cells. The second wave maintains Stripe and Groovin expression only in EMA cells to which myotubes are connected and, hence, is defined as muscle-dependent regulation of gene expression in these cells. The expression of Stripe and Groovin in the EMA cells that are not bound to muscles gradually diminishes. Recently, Buttgereit et al. (1996) reported that the expression of β1 tubulin, a structural protein characteristic of tendon cells during their terminal differentiation, depends on muscle insertion. These observations suggest that the establishment of EMA–muscle cell interactions is a prerequisite for terminal differentiation of the tendon-like cells, manifested by the expression of specific genes in these cells.
This paper elucidates the molecular mechanism mediating the inductive interactions between muscles and their specific attachment cells, responsible for terminal differentiation of the EMA cells into tendon cells. We show that muscle-dependent terminal differentiation of tendon cells is induced by activation of the Drosophila epidermal growth factor (EGF)-like receptor (Egfr/DER) in the EMA cells, by the neuregulin-like growth factor Vein secreted from the muscle cells.
Results
Elevated levels of Delilah and β1 tubulin are induced in the EMA cells as a result of muscle binding
Targeting of the myotube to encounter its counterpart muscle attachment cell in the epidermis is achieved through molecular cross talk between the two cell types. We showed previously that Stripe is the key factor in the induction of EMA cell fate determination (Frommer et al. 1996; Becker et al. 1997). Stripe is initially expressed in ectodermal cells, from which a subset of cells will develop into tendon cells. Although the initial expression of Stripe precedes muscle binding and appears to be independent of signals coming from the muscles, the maintenance of Stripe expression is actively induced only in cells that are bound to muscles.
To further analyze the dependence of gene expression in the EMA cells on muscle binding, we examined the timing of gene expression of typical EMA cell-specific genes, relative to the muscle binding process. Two classes of genes were identified: Groovin and Alien follow the temporal and spatial expression of Stripe throughout embryonic development, whereas Delilah and β1 tubulin expression in embryonic stages 12–14 is relatively low and becomes significantly elevated in embryos at stage 16 and beyond, when binding of somatic muscles is established (Fig. 1A–D). In heartless mutant embryos [induced by expressing the extracellular domain of Heartless, dominant-negative (DN)-Htl, under the upstream activating sequence (UAS)/Gal4 system] some of the muscles are missing and Delilah and β1 tubulin expression is detectable only in those EMA cells that are bound to muscles (Fig. 1E,F). Thus, although the initial expression of Delilah is muscle independent (Armand et al. 1994), it appears that muscle binding induces a significant elevation in Delilah expression in the EMA cells (Fig. 1). β1 tubulin expression is also significantly elevated following muscle binding (this study; Buttgereit et al. 1996).
Figure 1.
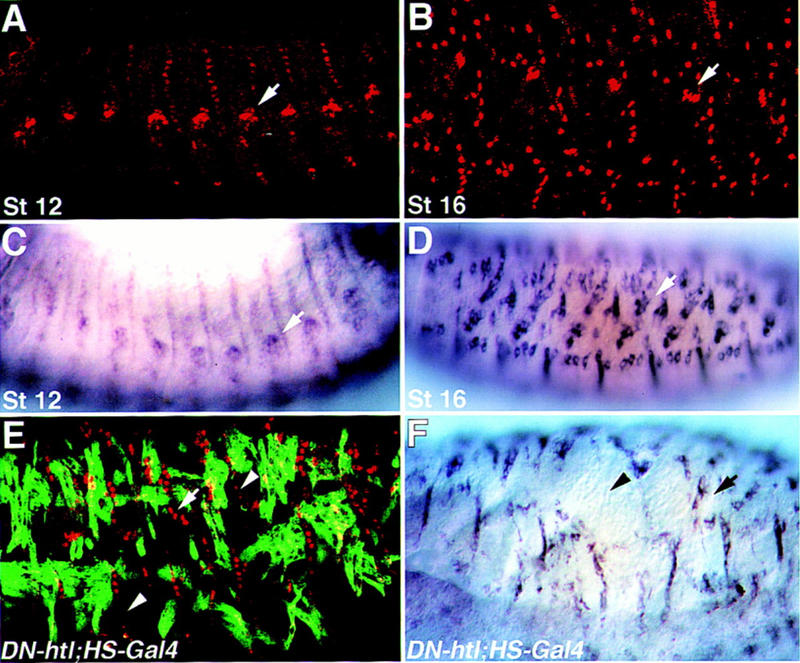
Muscle-independent, and muscle-dependent expression of Delilah and β1 tubulin. Wild-type embryos at stage 12 (A,C) or stage 16 (B,D) were stained with anti-Delilah antibody (A,B) or hybridized with a β1 tubulin antisense probe (C,D). At stage 12 of embryonic development, somatic muscles are not yet connected to their EMA cells, and the expression of Delilah and β1 tubulin is weak, relative to the strong expression of both markers in embryos at stage 16 when binding of somatic myotubes has been established. In mutant embryos carrying UAS–DN–Htl, and HS–Gal4 (Gal4 under a heat shock promoter), which were heat-shocked at 4–5 hr after egg laying, some of the muscles are missing (E,F). Embryos were double-labeled for Delilah and Myosin (E), or hybridized with a β1 tubulin probe (F). Note that the absence of somatic muscles leads to reduced expression of Delilah and β1 tubulin. Arrows mark chordotonals; arrowheads in E and F mark regions in which Delilah or β1 tubulin expression is abnormal.
Thus, in the following experiments high expression of Delilah and β1 tubulin was taken as a marker for muscle-dependent gene expression within the tendon cells.
Vein mutant embryos show abnormal differentiation of muscle attachment cells
To identify genes involved in the process of muscle–EMA cell interactions, we screened a collection of lethal P-element mutations (Karpen and Spradling 1992) and selected a mutant strain (P1749) that exhibits an abnormal muscle phenotype. The myotubes of P1749 mutant embryos are elongated and continuously send filopodia in random directions. This phenotype is most prominent in the ventral oblique muscles and in some dorsal longitudinal muscles (Fig. 2B). The ventral longitudinal muscles are less affected. The overall pattern of the mesoderm in P1749 mutant embryos at earlier stages of development, including segregation into different mesodermal tissues and subdivision of the somatic mesoderm, appears to be close to normal, although some slight aberrations are observed. However, the expression of the typical epidermal muscle attachment-specific genes in P1749 mutant embryos is abnormal. Although the levels of Stripe and Groovin do not show significant alterations, the levels of Delilah and β1 tubulin are significantly reduced (Fig. 2D,F). We used the expression of Delilah and β1 tubulin in the chordotonal organs as an internal control, and the intensity of staining in the EMA cells was always compared to that of the chordotonal organs (Fig. 2). Because of the dependence of the expression of Delilah and β1 tubulin on the process of muscle-dependent differentiation of tendon cells described earlier, we suspected that the mutated locus in P1749 may be directly involved in that process. A defected differentiation of the EMA cells may lead to continuous formation of filopodia at the myotube leading edge, a phenotype characteristic of P1749.
Figure 2.
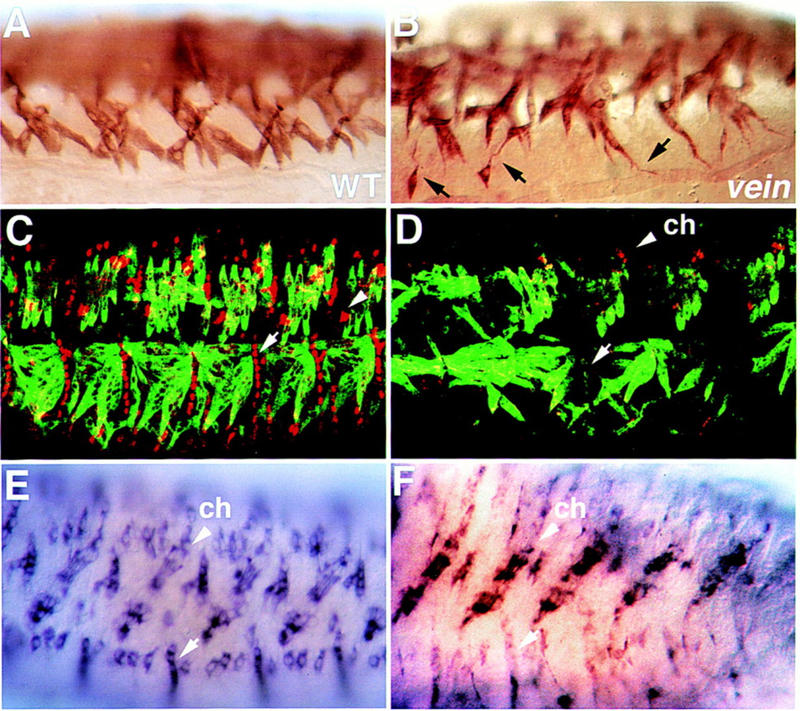
Somatic muscles and tendon cells are defective in vein mutant embryos. Wild-type (A,C,E) and vein mutant (B,D,F) embryos were stained with anti-Myosin antibody (A,B), double-labeled with anti-Myosin (green), and anti-Delilah (red) antibody (C,D), and hybridized with a β1 tubulin antisense probe (E,F). Notice that the somatic myotubes in the vein mutant embryo send elongated random filopodia (black arrows in B) and that the expression of Delilah or β1 tubulin in the tendon cells is significantly reduced (cf. white arrows in D and F to C and E, respectively). Arrowheads in C, D, E, and F show a high level of Delilah and β1 tubulin expression in the chordotonal organs (ch).
To gain molecular insight into the gene that is defective in P1749 mutation, genomic sequences flanking the P element were rescued and used to screen an embryonic cDNA library. Several partially overlapping cDNAs were isolated and sequenced. All of the cDNAs species analyzed were identical in their sequence to vein, a gene described recently by Schnepp et al. (1996), which is involved in wing disc development. The hallmarks of the Vein protein demonstrated by Schnepp et al., and confirmed by our analysis, include a signal peptide at the amino-terminal domain followed by PEST sequences, an immunoglobulin-like domain, and a carboxy-terminal EGF-like domain (see Fig. 3A). Genetic evidence described in Schnepp et al. supports the idea that Vein protein complements the activity of Spitz, the major embryonic ligand of Egfr, the Drosophila homolog of the mammalian EGFR. Much of the regulation of the Egfr signaling cascade is thought to be controlled by the localized release of its various ligands, as Egfr is widely expressed in all germ layers throughout embryonic development (Perrimon and Perkins 1997; Schweitzer and Shilo 1997). Three putative Egfr ligands have been described; these include two membrane-bound proteins, Spitz (Rutledge et al. 1992), Gurken (Neuman-Silberberg and Schüpbach 1993), and Vein (Schnepp et al. 1996), a neuregulin-like secreted protein. The similarity of Vein to vertebrate neuregulins (shown to bind various types of erbB receptors) suggests that Vein may function as a ligand of Egfr.
Figure 3.
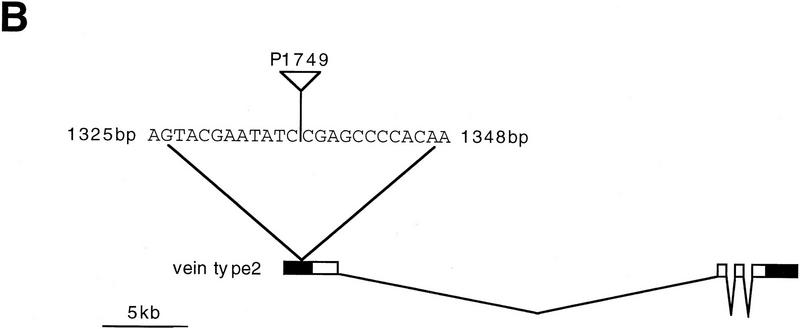
(A) Schematic illustration of the molecular structure of Vein. A signal peptide (SP) is present at its amino-terminal domain, followed by PEST sequences, a single immunoglobulin-like (Ig) domain, and an EGF domain at its carboxyl terminus. (B) Localization of the P1749 construct within the first non-coding exon of the vein gene. Solid boxes represent noncoding sequences; open boxes represent coding exons.
Several lines of evidence indicate that the vein gene is affected by the P1749 insertion and suggest that the defects in vein alone can account for the embryonic phenotype. (1) Sequence analysis of genomic sequences flanking the P1749 mutation from the proximal and distal regions indicates that the P element is inserted within the first noncoding exon of vein cDNA (see Fig. 3B). (2) Antibodies to Vein protein [raised against a glutathione S-transferase (GST)–Vein fusion protein] do not stain the P1749 mutant embryos (Fig. 4F), suggesting that Vein protein is not expressed in this mutant allele. (3) The lacZ expression pattern of the P element closely resembles the expression pattern of vein mRNA (not shown). (4) Chromosomal mapping performed using the rescued genomic DNA as a probe reveals a labeled band at chromosomal location 64F, where vein is mapped. This location was also confirmed with DfXAS96 (uncovers 64E–65C1–3), which does not complement P1749. (5) Precise excision of the P element completely reverses the muscle phenotype and results in full viability of the flies, indicating that P-element insertion caused the phenotype. (6) The differentiation of the EMA cells, visualized by the levels of Delilah expression, is deranged in an ethylmethane sulfonate (EMS)-induced allele of vein (vndddL6/DfXAS96; not shown), as in the P1749 allele, verifying that this phenotype is a direct consequence of the loss of Vein functionality.
Figure 4.
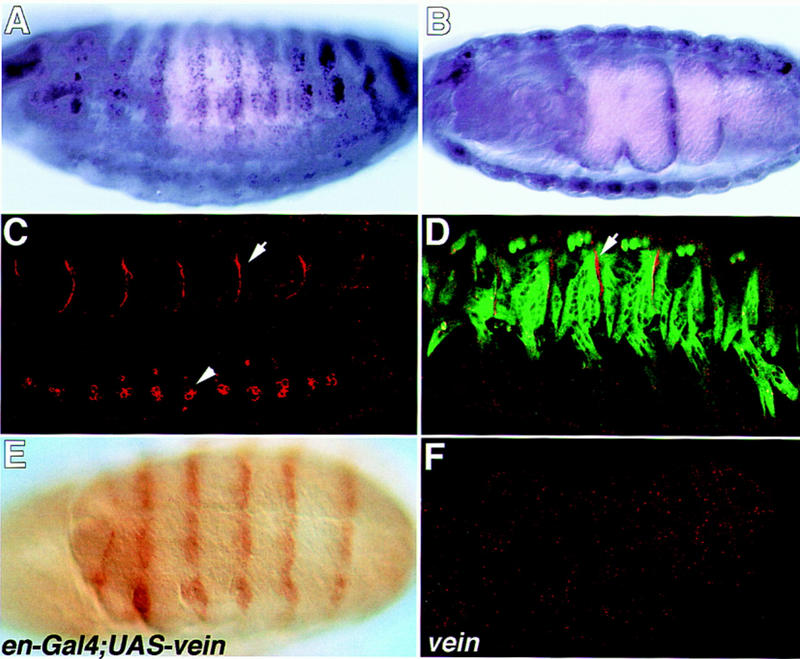
The mRNA and protein expression pattern of Vein. Wild-type embryos (A–D) were hybridized with Vein antisense probe (A,B) or with anti-Vein antibody (red in C and D). The embryo in D is double labeled for Myosin (green) and vein (red). Note that although vein mRNA is expressed throughout the somatic and visceral muscles, Vein protein is concentrated in the muscle–tendon junctional site (arrows in C and D). Vein protein staining is also noted in a cluster of cells along the CNS (arrowhead in C). The embryo in E labeled with anti-Vein antibody, carries gal4 under the engrailed promoter and UAS–vein. The engrailed pattern is prominent. The embryo in F is a veinΔ25/DfXAS96 mutant embryo labeled with anti-Vein antibody and shows no positive staining.
Expression of Vein during embryonic development
The expression of vein mRNA analyzed by in situ hybridization was described by Schnepp et al. (1996). High levels of mRNA expression are observed in somatic and visceral muscles during late stages (14–17) of embryonic development (also shown in Fig. 4A,B). To enable us to track Vein expression, antibodies were raised against a GST–Vein fusion protein. The specificity of the antibodies is verified by the following two observations: (1) Vein protein is detected when expressed in ectopic cells utilizing the Gal4/UAS expression system; and (2) no staining is detected in vein (Δ25/DfXAS96) mutant embryos (Fig. 4F). Figure 4E shows an embryo expressing ectopic Vein induced by the engrailed–gal4 inducer and stained with anti-Vein antibody. The typical engrailed striped expression pattern is detected with the anti-Vein antibody.
Although vein mRNA is prominent in the muscles (Fig. 4A,B), Vein protein expression is restricted to the sites of contact between muscles and their epidermal attachment cells (Fig. 4C,D). The pattern of staining appears to form a fine line composed of small dots. This pattern may represent small protein aggregates of Vein at the junction site. Staining is also noted along segmental repeated clusters of three cells located along the central nervous system (CNS). Here too, the staining appears to be composed of small dots, but unlike the muscle–EMA junctional localization, the CNS staining seems to surround the entire cell periphery. Thus, Vein may be produced and secreted from the muscles and is localized specifically at the muscle–tendon junction sites. The mechanism of Vein localization is not known.
The protein localization of Vein at the junctional site between muscles and EMA cells, together with the abnormal muscle-dependent differentiation of the EMA cells in vein mutant embryo, support the possibility that Vein activity is required for tendon cell maturation.
Tendon cell differentiation in spitz group mutant embryos
The molecular structure of Vein, together with the genetic analysis performed by Schnepp et al. (1996), suggests that Vein may be an Egfr ligand. If Vein activity in tendon cell differentiation is mediated through Egfr, it is expected that this process would be abnormal in Egfr mutant embryos. We used the mutant allele Egfr1F26, which is an embryonic lethal allele of the Egfr (at 29°C). Delilah expression in Egfr1F26 mutant embryos, deficient in Egfr, was examined. It should be noted that in Egfr1F26 mutant embryos the muscle pattern is severely disrupted. However, some of the muscles appear to be normal and to attach to EMA cells. Similarly, although the ectoderm is severely disrupted, competent EMA cells are present (not shown). Egfr1F26 mutant embryos were stained for Delilah. Whereas the expression of Delilah in the EMA cells is almost eliminated, the expression in the chordotonal organs remains high (Fig. 5A). The abnormal differentiation of tendon cells manifested by the reduced expression of Delilah is a phenotype shared by vein and Egfr1F26 mutant embryos, supporting the possibility that Egfr is a functional receptor of Vein.
Figure 5.
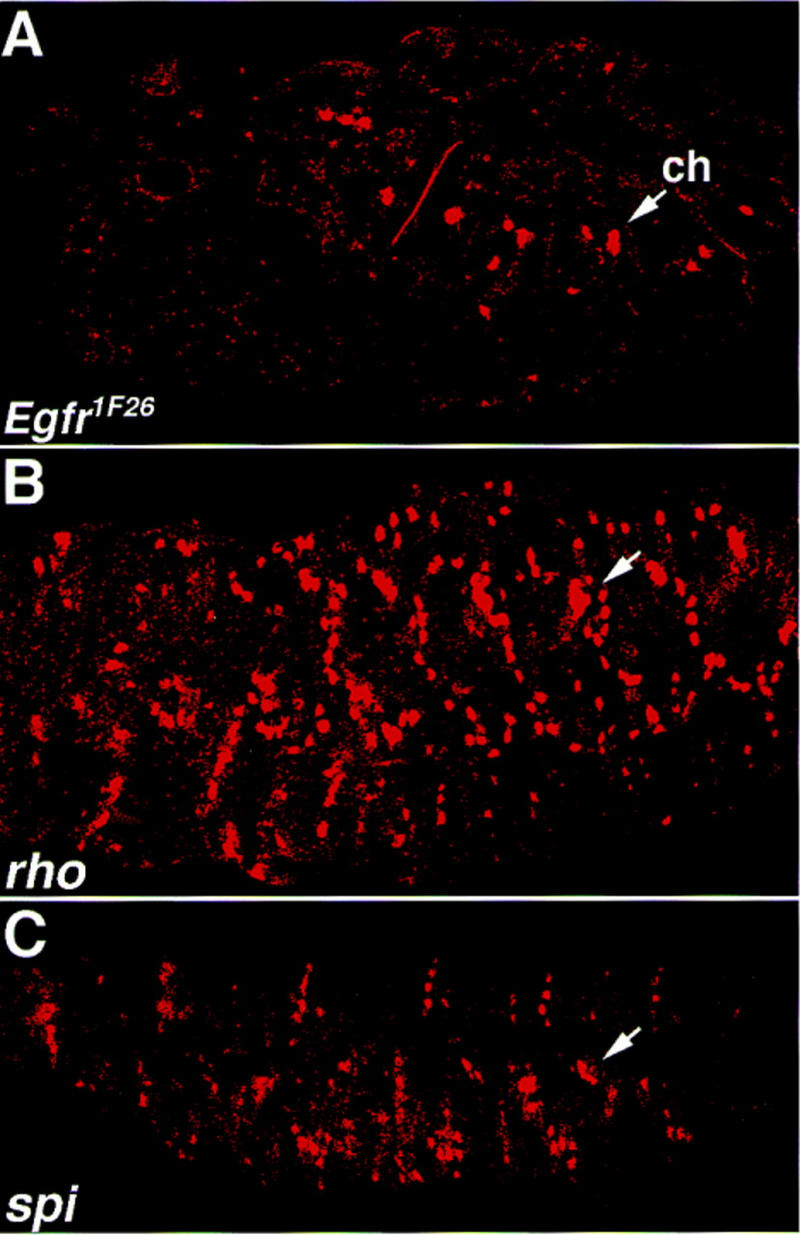
The expression of Delilah in spitz group mutant embryos. Egfr1F26 mutant embryos (A), rho mutant embryos (B), or spitz (spi) mutant embryos were labeled with anti-Delilah antibody. While in rho and spitz mutants, Delilah expression in the tendon cells is maintained, in the Egfr1F26 mutant, Delilah expression is significantly reduced. Compare the expression of Delilah in the EMA cells to that in the chordotonal organs (arrows).
The spitz group includes a number of genes [such as Egfr, single-minded (sim), spitz, star, rhomboid (rho), and pointed (pnt); Mayer and Nüsslein Volhard (1988)] that affect differentiation of the ventral ectoderm. Previous analysis indicated that Egfr encodes for an EGF receptor homolog (Price et al. 1989; Schejter and Shilo 1989), spi codes for a transforming growth factor-α (TGFα) membrane-bound ligand of Egfr (Rutledge et al. 1992), and rho codes for a seven transmembrane domain protein (Bier et al. 1990) that is presumably required for Spitz processing (Schweitzer et al. 1995). To examine the possible involvement of spi and rho in tendon cell differentiation, in addition to that of vein, we analyzed the expression of Delilah in spi and rho mutant embryos.
Unlike the reduction of Delilah expression in Egfr1F26 mutant embryos, its expression in spi and rho mutant embryos remains high, not only in the chordotonals but also in residual EMA cells that presumably are bound to muscles (Fig. 5B,C). These results are consistent with the idea that Vein, but not Spitz, is necessary for the process of tendon cell differentiation.
Vein can induce tendon cell differentiation
The data presented above favor the possibility that Vein/Egfr interactions underlie the basis for muscle-dependent tendon cell differentiation. We wished to prove that Vein is not only necessary, but also sufficient, to induce Delilah expression. Because Egfr is expressed in all ectodermal cells and Vein is a secreted protein, we used the UAS/Gal4 expression system to express Vein in ectopic cells in the ectoderm and followed tendon-specific gene expression in those cells. Transgenic flies carrying vein under UAS control were produced and crossed to either a ubiquitous ectoderm Gal4 inducer (69B) or to an engrailed–gal4 strain. Ectopic expression of Vein in the ectoderm leads to ectopic expression of Stripe, Delilah, and β1 tubulin (Fig. 6A–D). Because Stripe is sufficient to induce Delilah and β1 tubulin expression (Becker et al. 1997), it appears that Vein activates the expression of tendon cell-specific genes, through the induction of high levels of stripe transcription. The possibility that a direct activation of delilah transcription is also induced by ectopic Vein is not ruled out. Embryos expressing ectopic Vein under the engrailed–gal4 inducer show a nonautonomous effect of Vein outside of the engrailed domain (Fig. 6B, in accordance with the possibility that Vein is a secreted protein and can affect neighboring cells). These results, combined with the loss-of-function phenotype of vein mutants, indicate that Vein/Egfr association mediates the muscle–EMA cell inductive interactions required for terminal differentiation of tendon cells.
Figure 6.
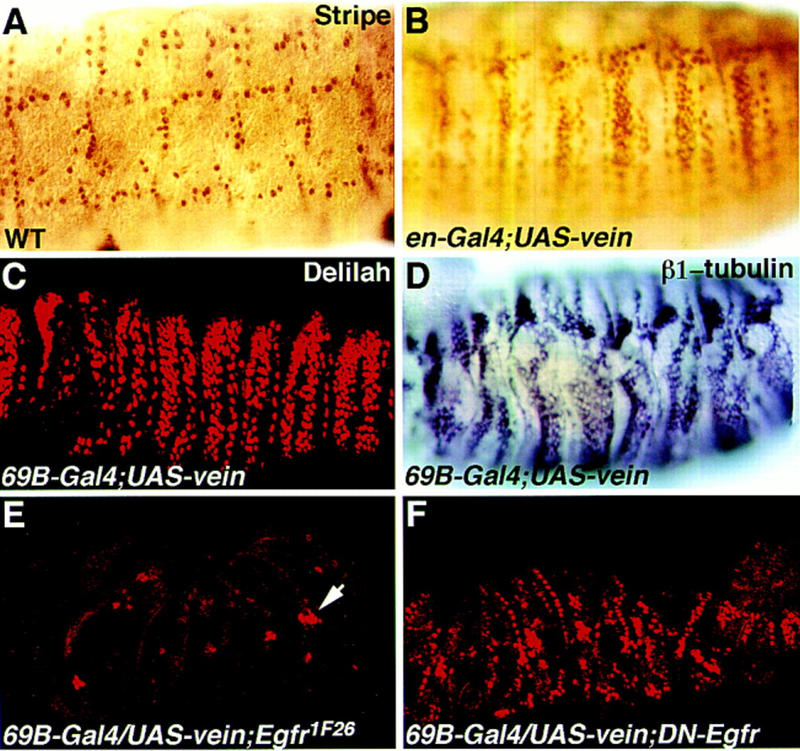
Ectopic expression of Vein induces ectopic expression of tendon-specific genes. Embryos carrying the UAS–vein construct (B–F) in combination with either the 69B–gal4 construct (C–F) or the engrailed–gal4 construct (B) were stained with anti-Stripe antibody (B) or with anti-Delilah antibody, or with a β1 tubulin antisense probe. Note that ectopic Vein induces the ectopic expression of Stripe (cf. embryo in B to a wild-type embryo stained for Stripe in A). Ectopic expression of Vein also induces Delilah (C) and β1 tubulin (D). The embryo in E carries a UAS–vein construct in combination with flb1F26 (null mutation for Egfr) and the 69B–gal4 inducer, and was stained with anti-Delilah antibody. The ectopic expression of Delilah in this embryo is eliminated (the arrow marks the chordotonal organs). The embryo in F carries a UAS–vein construct in combination with the UAS–DN–Egfr construct and the 69B–gal4 inducer, and is stained with anti-Delilah antibody. The ectopic expression of Delilah is significantly reduced.
Ectopic expression of Delilah induced by Vein requires Egfr and is mediated through the Ras pathway
The effect of ectopic Vein on Delilah expression was further used as a tool to address whether Egfr functions as a receptor for Vein in this process. If the effect of ectopic Vein is eliminated in the absence of Egfr, this would strongly favor the possibility that Egfr functions as a receptor for Vein in Delilah induction. To address this question, two types of embryos were constructed: (1) Egfr1F26;UAS–vein;gal4–69B; and (2) UAS–DN–Egfr;UAS–vein;gal4–69B (see Materials and Methods for details). In the first combination, ectopic Vein is induced in an Egfr1F26 homozygous mutant background. In the second combination, Vein is induced in embryos, whereas Egfr activity is reduced by the expression of the dominant-negative construct. Both of these phenotypes are coinduced only in cells where Gal4 is expressed. Mutant embryos were collected and stained for Delilah protein. No Delilah expression was detected in Egfr1F26-ectopic Vein mutant embryos (Fig. 6E). Interestingly, in the second combination, in which DN–Egfr was activated by Gal4–69B, we did find embryos expressing residual ectopic Delilah (Fig. 6F). Because both UAS lines share the same Gal4 inducer, the residual ectopic expression of Delilah could be explained either by residual activity of Egfr and/or by the nonautonomous effect of Vein on neighboring cells expressing wild-type Egfr. The results from both experiments strongly support the possibility that Egfr is a direct receptor for Vein in the induction of EMA to tendon cell differentiation.
If Vein directly activates Egfr in the tendon cell, leading to Delilah expression, it is possible that ectopic activation of Egfr by other ligands can induce ectopic expression of Delilah. We tested whether ectopic expression of the activated form of secreted Spitz can induce Delilah expression in the ectoderm. Flies carrying the active secreted form of Spitz controlled by UAS were crossed to flies carrying the general ectoderm inducer gal4–69B, and their embryos were collected and stained for Delilah. The results indicate that ectopic expression of secreted Spitz induces ectopic expression of Delilah in the ectoderm (Fig. 7B).
Figure 7.
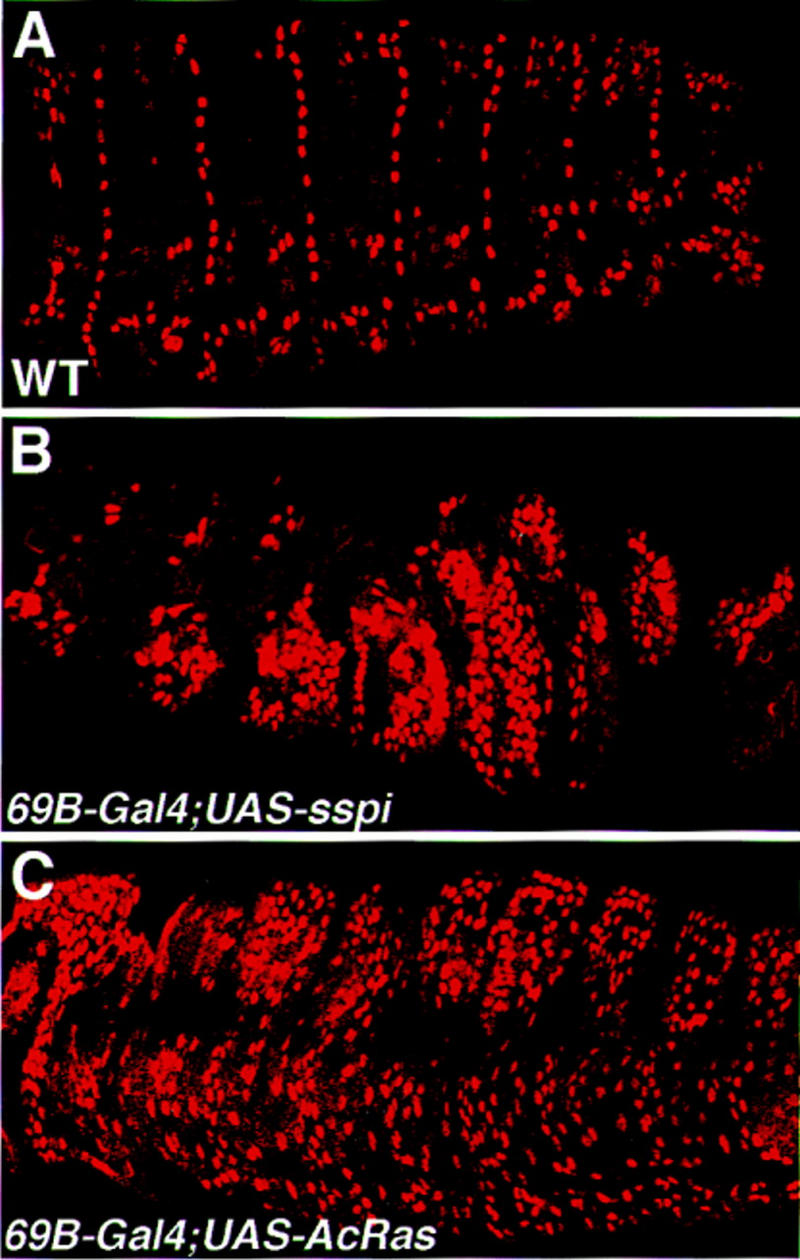
Activation of the Ras pathway is sufficient to induce ectopic expression of Delilah. Embryos carrying a UAS-secreted spitz construct (B), or UAS-activated ras1 construct (C) in combination with the whole ectoderm gal4 inducer 69B were collected and stained with anti-Delilah antibody. Note that in both genetic combinations the induction of ectopic Delilah expression is prominent (cf. to the wild-type expression of Delilah shown in A).
To examine the possibility that activation of the Ras pathway is sufficient to induce Delilah expression, we expressed the activated form of Ras in the whole ectoderm. Flies carrying the activated form of Ras under UAS control were crossed to flies carrying Gal4–69B, and their embryos were collected and stained for Delilah. The resulting embryos show ectopic expression of Delilah (Fig. 7C).
Taken together, these experiments provide strong evidence supporting the idea that Egfr functions as the receptor for Vein in muscle-dependent tendon cell differentiation and that binding between Vein and Egfr leads to Delilah expression through activation of the Ras pathway.
Discussion
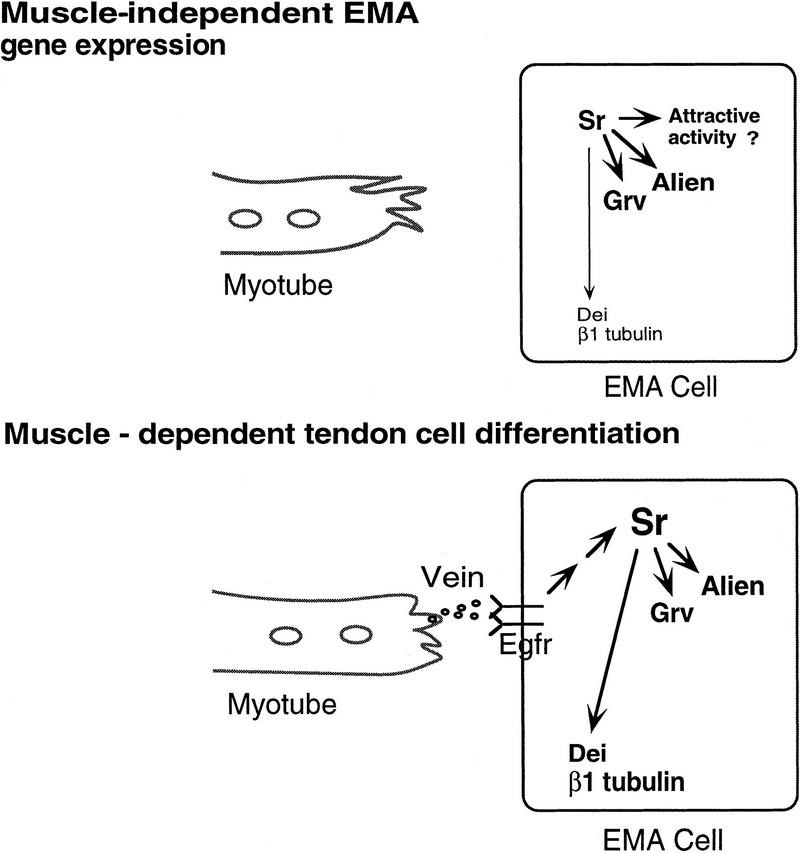
Inductive interactions performed between myotubes and their attachment cells underlie the basis for terminal differentiation of the epidermal muscle attachment cells into tendon cells. Differentiation of the EMA cells may be divided into two phases, namely muscle-independent and muscle-dependent differentiation (shown schematically in Fig. 8). In the first phase, the expression of the putative transcription factor Stripe is induced in a subset of ectodermal (EMA) cells from which tendon cells will develop. Stripe is sufficient to activate the expression of Groovin, Alien, Delilah, and β1 tubulin. The initial low expression of Delilah and β1 tubulin could be explained by the requirement for high levels of Stripe (which are not available initially), to activate Delilah and β1 tubulin expression. Direct activation of β1 tubulin by Delilah is a possibility. The presence of a putative inhibitory activity on Delilah promoter during the initial stages of EMA differentiation cannot be excluded. In addition to the activation of these genes, Stripe presumably triggers an attractive activity that guides the myotube to interact specifically with the EMA cell. The molecular nature of this activity is yet to be elucidated.
Figure 8.
Summary of the genetic circuitry in the EMA cell before and following muscle binding. In the first phase of EMA gene expression, Stripe (Sr) activates the expression of Alien, Groovin (Grv), and low levels of Delilah (Dei) and β1 tubulin. In the second muscle-dependent phase of gene expression, Vein/Egfr interactions activate high levels of Stripe, Groovin, Alien, Delilah, and β1 tubulin.
In the second muscle-dependent phase, activation of the Egfr pathway by Vein leads to a second wave of gene expression in the EMA cells, leading to elevated levels of Stripe, Groovin, Delilah, and β1 tubulin. Myotube binding to the EMA cell is accompanied by the formation of adherence type junctions between the two cell types. Analysis at the electron microscopy level revealed an electron-dense material deposited in the extracellular domain of these adherens type junctions (Tepass and Hartenstein 1994 ). It is possible that this extracellular matrix deposition is crucial for the accumulation and localization of Vein protein that is produced and secreted from the myotube at the muscle–EMA junctional site. This localization may also prevent rapid degradation of Vein protein, thereby facilitating its binding to Egfr.
Although the possibility that Egfr is the receptor for Vein was raised by Schnepp et al. (1996), the experiments in this paper provide additional evidence for this notion; in both vein and Egfr1F26 mutant embryos, Delilah and β1 tubulin expression is significantly reduced, and the effect of ectopic Vein is not exerted in the absence of functional Egfr. It also appears that Vein binding to Egfr activates the Ras pathway, as ectopic expression of activated Ras could induce an effect similar to the ectopic expression of Vein. Finally, although ectopic expression of secreted Spitz leads to ectopic expression of Delilah, it appears that Spitz is not an integral part of the inductive interactions of the muscle–EMA cells, as spitz mutant embryos do not show reduced levels of Delilah expression. Schnepp et al. (1996) suggested that Vein might synergize with Spitz to achieve the maximal effect of Egfr activation. Our experiments indicate that Vein function in tendon cell differentiation is independent of Spitz.
Delilah appears to be a key factor in terminal differentiation of tendon cells
Previous analysis of the gene expression hierarchy in EMA cells indicated that stripe is a key gene in the determination of specific EMA cell fate. Stripe induces the transcription of an array of EMA-specific genes, including groovin, alien, delilah, and β1 tubulin and possibly regulates its own transcription as well. Our results indicate that Delilah may be the key transcription factor to be activated as a result of muscle–EMA inductive interactions. The regulation of Delilah activity could occur at different levels: At the transcriptional level, it appears that only high levels of Stripe are capable of activating delilah transcription. At the functional level, the effect of Delilah on transcription may be modulated by the presence of additional unknown positive or negative regulators. Interestingly, the HLH inhibitory protein Extramacrochaetae (Emc) is expressed in muscle attachment cells and may counteract Delilah activity in these cells (Cubas et al. 1994). emc mutants also show defective muscle patterns, in line with the possibility that this protein may play a role in muscle-dependent, tendon-specific differentiation.
Additional signaling pathways, including the integrin signaling cascade, may contribute to the differentiation process of tendon cells as well. The integrin receptors are expressed on both sides of the adherens type junctions formed between the muscles and EMA cells, and their function has been attributed mainly to the formation and maintenance of these intercellular junctions. Because vertebrate integrins have been implicated in signal transduction events, in addition to their function in mediating intercellular adhesion, it is possible that integrins at the muscle–EMA junctional site in the Drosophila embryo contribute to the muscle-dependent tendon cell differentiation. In that case, they may act in parallel or downstream to the Ras pathway.
Nonautonomous activation of the Ras pathway is common to various types of intercellular inductive interactions
The Ras pathway appears to be a universal signaling pathway that is continuously utilized in numerous differentiation and cell fate determination processes during embryonic development (Dickson and Hafen 1994). It has been suggested that the specific differentiation event mediated by the activation of this signaling cascade depends on the context of the cell to be triggered. An interesting functional correlation between the nonautonomous activation of the Egfr pathway by Vein and the activity of ARIA (achetylcholine receptor-inducing activity) at the site of neuromuscular synapse may be noted. Here too, a neuregulin growth factor (ARIA) is secreted from the nerve terminals to induce activation of the erbB receptor at the neuromuscular junction (Sanes 1997). This activation leads to elevated transcription of acetylcholine receptor in the nucleus closest to the neuromuscular synapse domain. In both examples, activation of the receptor tyrosine kinase (RTK) pathway is achieved as a result of close apposition of the two cell types.
An additional level of regulation is achieved through localization or ligand presentation. In the process of myotube-dependent tendon cell differentiation, this localization appears to be an important factor and may be regulated by an unknown matrix protein deposited at the junctional site. To this end, two mechanisms may contribute to the specific localization of Vein in the muscle–tendon junctional sites: (1) association of the protein with specific extracellular factor, and (2) rapid degradation of the protein at sites other than the junction. The PEST sequences located at the amino-terminal domain of Vein may contribute to its rapid degradation. The immunoglobulin-like domain located at the carboxyl terminus of the protein may be important for its association with other proteins at the junctional sites.
In conclusion, inductive interactions among different cell types underlie the basis for organ morphogenesis in a wide variety of species. Common signaling mechanisms, including activation of the Ras pathway in combination with other pathways, may regulate these interactions.
Materials and methods
Fly stocks
The UAS/Gal4 system used is based on Brand and Perrimon (1993). The following gal4 inducers were used: engrailed–gal4, 69B–gal4 [A. Brand, Wellcome/Cancer Research Campaign (CRC) Institute, Cambridge, UK]. In addition, the following strains were used: y w (wild-type strain); UAS–DN–Htl (B. Shilo, Weizmann Institute, Rehovot, Israel); P1749 (Karpen and Spradling 1992); Df(3L)XAS96 (W.A. Johnson, University of Iowa, Iowa City); Egfr1F26 (B. Shilo); spitz (N. Perrimon, Harvard Medical School, Boston, MA); rho (E. Bier, University of California at San Diego, La Jolla), UAS-secreted–spitz (B. Shilo); UAS–ras (C. Klambt, University of Köln, Germany). vndddL6 (A. Simcox, Ohio State University, Columbus; A. Shearn, Johns Hopkins University, Baltimore, MD). ΔP25 was produced by imprecise excision of P1749 in our laboratory. UAS–vein flies were constructed by ligating a 3.4-EcoRI fragment of vein cDNA with pUAST (Brand and Perrimon 1993), digesting with EcoRI, and introducing this construct into the fly germ line by a standard P-element transformation method. Egfr1F26;UAS–vein;gal4–69B flies were produced as follows: Egfr1F26 flies were crossed to 69B–gal4 flies to produce Egfr1F26;gal4–69B strain, and UAS–vein flies were crossed to Egfr1F26 to produce Egfr1F26;UAS–vein. These two strains were then crossed to produce the Egfr1F26;UAS–vein;gal4–69B flies. Egfr1F26 flies were balanced over a blue balancer, and the Egfr1F26 mutants were identified by negative β-gal staining. UAS–DN–Egfr;UAS–vein;gal4–69B flies were produced by crossing UAS–DN–Egfr flies to UAS–vein flies, to produce UAS–DN–Egfr;UAS–vein flies, and this strain was crossed with 69B–gal4 flies to produce the UAS–DN–Egfr;UAS–vein;gal4–69B strain. The DN–Egfr embryos were identified because of the nonretracted germ band phenotype.
Immunochemical reagents
To visualize embryonic muscles, we used anti-Myosin heavy chain polyclonal antibody, provided by P. Fisher (State University of New York, Stony Brook). The serum was usually preadsorbed on 0- to 2-hr-old embryos and diluted 1:1000 for staining. Vein was visualized either by in situ hybridization with a digoxygen (DIG)-labeled 0.5 kb vein DNA fragment or by antibody against a GST–Vein fusion protein, raised in rats. The serum was diluted 1:200 for staining. Stripe was visualized with anti-GST–StripeB fusion protein raised in guinea pigs (serum dilution of 1:500), and Groovin with monoclonal anti-Groovin hybridoma supernatant (dilution of 1:2). Anti-Alien antibody was obtained from A. Paululat (University of Marburg, Germany); Delilah protein was visualized with anti-GST–Delilah fusion protein raised in rats (dilution of 1:200). β1 tubulin expression was monitored by in situ hybridization using β1 tubulin cDNA as a probe (D. Butgereit, University of Marburg, Germany). Anti-β-galactosidase antibodies were purchased from Cappel (USA).
Secondary antibodies included horseradish peroxidase-, fluorescein- or rhodamine-conjugated goat anti-rabbit or donkey anti-rat IgG, anti-guinea pig IgG, and anti-mouse IgM (Jackson Laboratories, USA), and anti-Dig–AP antibody (Boehringer Mannheim, Germany).
Whole-mount embryonic staining
In addition to HRP (see below) staining to determine expression of the appropriate markers, we routinely stained the embryos collected from the different mutant lines for β-galactosidase to identify homozygous mutant embryos. Staining was performed essentially as described (Ashburner 1989). In brief, embryos were collected and incubated as indicated, dechorionated, and fixed with a mixture of 3% paraformaldehyde and heptane. Following two washes with PBT (PBS containing 0.1% Triton X-100), embryos were incubated in the X-gal staining solution until blue staining was visible (15–30 minutes at 37°C) and then washed and devitellinized with a methanol–heptane mixture. Permeabilization was performed by incubation in PBT containing 10% BSA for 2–3 hr, and incubation with primary antibody was usually performed for 16 hr at room temperature.
In situ hybridization was performed by the method of Tautz and Pfeifle (Tautz and Pfeifle 1989) with minor modifications. β-galactosidase staining, if needed, was performed before the devitellinization step. The DNA fragments used as probes were labeled by the random priming method, using DIG (Boehringer Mannheim, Germany).
Confocal microscopy
Fluorescent-labeled preparations were imaged using a Bio-Rad MRC 1024 confocal microscope coupled to a Zeiss Axiovert 135M microscope. Bright-field and fluorescent digital images were processed using Photoshop (Adobe Systems, Inc).
Cloning of vein cDNA
A 2.5-kb plasmid rescue DNA fragment was used to screen a partial EcoRI-restricted Drosophila genomic library (Levine et al. 1994). A single genomic clone of ∼30 kb in length was isolated. Genomic DNA fragments on both sides of the P element were analyzed by in situ hybridization. A 0.5-kb genomic fragment gave a similar pattern of expression as the lacZ pattern of the original P-element mutation and therefore was used to screen an embryonic (9–12 hr) Drosophila λgt11 cDNA library (Zinn et al. 1988). Several clones were isolated, all of which correspond to partial overlapping sequences. Sequence analysis of the cDNAs isolated revealed that the gene isolated is identical to vein. Sequencing was performed by an automated sequencer (Applied Biosystems). The sequences were analyzed by AutoAssembler DNA sequence assembly software package (v. 1.0.3; Applied Biosystems). The predicted amino acid sequences were analyzed by the program package of the University of Wisconsin Genetics Computer Group (UWGCG; v. 8.1–UNIX). We searched the nonredundant GenBank, PIR, and EMBL databases with Vein amino acid sequence using the Mail Server Utility (MSU, v. 1.4), choosing various programs to identify the various conserved domains. The MOTIFS program of the UWGCG package was used for identification of putative post-translational modifications. Additional potential post-translational modifications were identified manually.
Acknowledgments
We thank A. Brand, E. Hafen, B. Shilo, C. Klambt, A. Simcox, and A. Shearn for various fly strains; P. Fisher, D. Buttgereit, and M. Cole for antibodies and cDNA probes; B. Shilo and S. Schwarzbaum for critical reading of the manuscript and helpful comments; M. Krasnow for making the collection of embryos from the lethal P-element screen available to us; and S. Becker for making the anti-Delilah antibody. This work was supported by a grant from the Israel Cancer Research Fund (T.V.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL lgvolk@wiccmail.weizmann.ac.il; FAX 972-8-9344108.
References
- Abmayr SM, Erickson MS, Bour BA. Embryonic development of the larval body wall musculature of Drosophila melanogaster. Trends Genet. 1995;11:153–159. doi: 10.1016/s0168-9525(00)89030-7. [DOI] [PubMed] [Google Scholar]
- Armand P, Knapp AC, Hirsch AJ, Wieschaus EF, Cole MD. A novel basic helix-loop-helix protein is expressed in muscle attachment sites of the Drosophila epidermis. Mol Cell Biol. 1994;14:4145–4154. doi: 10.1128/mcb.14.6.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 44–49. [Google Scholar]
- Azpiazu N, Lawrence PA, Vincent JP, Frasch M. Segmentation and specification of the Drosophila mesoderm. Genes & Dev. 1996;10:3183–3194. doi: 10.1101/gad.10.24.3183. [DOI] [PubMed] [Google Scholar]
- Baker R, Schubiger G. Ectoderm induces muscle-specific gene expression in Drosophila embryos. Development. 1995;121:1387–1398. doi: 10.1242/dev.121.5.1387. [DOI] [PubMed] [Google Scholar]
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. doi: 10.1242/dev.110.3.791. [DOI] [PubMed] [Google Scholar]
- ————— . The mesoderm and its derivatives. In: Bate M, Martines Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1013–1090. [Google Scholar]
- Baylies MK, Bate M. twist: A myogenic switch in Drosophila. Science. 1996;272:1481–1484. doi: 10.1126/science.272.5267.1481. [DOI] [PubMed] [Google Scholar]
- Becker S, Pasca G, Strumpf D, Min L, Volk T. Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development. 1997;124:2615–2622. doi: 10.1242/dev.124.13.2615. [DOI] [PubMed] [Google Scholar]
- Bier E, Jan LY, Jan YN. rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster [published erratum appears in Genes & Dev. 1990 4: 680–681]. Genes & Dev. 1990;4:190–203. doi: 10.1101/gad.4.2.190. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Meyer D, Riethmacher D. Factors controlling growth, motility, and morphogenesis of normal and malignant epithelial cells. Int Rev Cytol. 1995;160:221–266. doi: 10.1016/s0074-7696(08)61556-9. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Buttgereit D, Paululat A, Renkawitz Pohl R. Muscle development and attachment to the epidermis is accompanied by expression of beta 3 and beta 1 tubulin isotypes, respectively. Int J Dev Biol. 1996;40:189–196. [PubMed] [Google Scholar]
- Cubas P, Modolell J, Ruiz Gomez M. The helix-loop-helix extramacrochaetae protein is required for proper specification of many cell types in the Drosophila embryo. Development. 1994;120:2555–2566. doi: 10.1242/dev.120.9.2555. [DOI] [PubMed] [Google Scholar]
- Dickson B, Hafen E. Genetics of signal transduction in invertebrates. Curr Opin Genet Dev. 1994;4:64–70. doi: 10.1016/0959-437x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Frommer G, Vorbruggen G, Pasca G, Jäckle H, Volk T. Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 1996;15:1642–1649. [PMC free article] [PubMed] [Google Scholar]
- Goubeaud A, Knirr S, Renkawitz-Pohl R, Paululat A. The Drosophila gene alien is expressed in the muscle attachment sites during embryogenesis and encodes a protein highly conserved between plants, Drosophila and vertebrates. Mech Dev. 1996;57:59–68. doi: 10.1016/0925-4773(96)00532-1. [DOI] [PubMed] [Google Scholar]
- Karpen GH, Spradling AC. Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics. 1992;132:737–753. doi: 10.1093/genetics/132.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, VijayRaghavan K, Celniker SE, Tanouye MA. Identification of a Drosophila muscle development gene with structural homology to mammalian early growth response transcription factors. Proc Natl Acad Sci. 1995;92:10344–10348. doi: 10.1073/pnas.92.22.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Bashan Ahrend A, Budai Hadrian O, Gartenberg D, Menasherow S, Wides R. Odd Oz: A novel Drosophila pair rule gene. Cell. 1994;77:587–598. doi: 10.1016/0092-8674(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Mayer U, Nüsslein-Volhard C. A group of genes required for pattern formation in the ventral ectoderm of the Drosophila embryo. Genes & Dev. 1988;2:1496–1511. doi: 10.1101/gad.2.11.1496. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- Perrimon N, Perkins L A. There must be 50 ways to rule the signal: The case of the Drosophila EGF receptor. Cell. 1997;89:13–16. doi: 10.1016/s0092-8674(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Price JV, Clifford RJ, Schupbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 1989;56:1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- Rushton E, Drysdale R, Abmayr SM, Michelson AM, Bate M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. doi: 10.1242/dev.121.7.1979. [DOI] [PubMed] [Google Scholar]
- Rutledge BJ, Zhang K, Bier E, Jan YN, Perrimon N. The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal-ventral axis formation and neurogenesis. Genes & Dev. 1992;6:1503–1517. doi: 10.1101/gad.6.8.1503. [DOI] [PubMed] [Google Scholar]
- Sanes JR. Genetic analysis of postsynaptic differentiation at the vertebrate neuromuscular junction. Curr Opin Neurobiol. 1997;7:93–100. doi: 10.1016/s0959-4388(97)80126-2. [DOI] [PubMed] [Google Scholar]
- Schejter ED, Shilo BZ. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989;56:1093–1104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- Schnepp B, Grumbling G, Donaldson T, Simcox A. Vein is a novel component in the Drosophila epidermal growth factor receptor pathway with similarity to the neuregulins. Genes & Dev. 1996;10:2302–2313. doi: 10.1101/gad.10.18.2302. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Shilo BZ. A thousand and one roles for Drosophila EGF receptor. Trends Genet. 1997;13:191–196. doi: 10.1016/s0168-9525(97)01091-3. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Shaharabany M, Seger R, Shilo BZ. Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes & Dev. 1995;9:1518–1529. doi: 10.1101/gad.9.12.1518. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Volk T, VijayRaghavan K. A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development. 1994;120:59–70. doi: 10.1242/dev.120.1.59. [DOI] [PubMed] [Google Scholar]
- Zinn K, McAllister L, Goodman CS. Sequence analysis and neuronal expression of fasciclin I in grasshopper and Drosophila. Cell. 1988;53:577–587. doi: 10.1016/0092-8674(88)90574-0. [DOI] [PubMed] [Google Scholar]