Abstract
Background
Natural killer cell cytotoxicity is decreased in patients with acute myeloid leukemia in comparison to that in normal controls. Tumor-derived microvesicles present in patients’ sera exert detrimental effects on immune cells and may influence tumor progression.
Design and Methods
We investigated the microvesicle protein level, molecular profile and suppression of natural killer cell activity in patients with newly diagnosed acute myeloid leukemia.
Results
The patients’ sera contained higher levels of microvesicles compared to the levels in controls (P<0.001). Isolated microvesicles had a distinct molecular profile: in addition to conventional microvesicle markers, they contained membrane-associated transforming growth factor-β1, MICA/MICB and myeloid blasts markers, CD34, CD33 and CD117. These microvesicles decreased natural killer cell cytotoxicity (P<0.002) and down-regulated expression of NKG2D in normal natural killer cells (P<0.001). Sera from patients with acute myeloid leukemia contained elevated levels of transforming growth factor-β, and urea-mediated dissociation of microvesicles further increased the levels of this protein. Neutralizing anti-transforming growth factor-β1 antibodies inhibited microvesicle-mediated suppression of natural killer cell activity and NKG2D down-regulation. Interleukin-15 protected natural killer cells from adverse effects of tumor-derived microvesicles.
Conclusions
We provide evidence for the existence in acute myeloid leukemia of a novel mechanism of natural killer cell suppression mediated by tumor-derived microvesicles and for the ability of interleukin-15 to counteract this suppression.
Keywords: exosomes, acute myeloid leukemia, acute myeloid leukemia, TGF-β1, NK cells, interleukin 15
Introduction
Activated natural killer (NK) cells have long been known to mediate killing of a broad range of tumor targets, including human leukemia blasts.1 However, in the peripheral blood of patients with untreated acute myeloid leukemia (AML), NK cells have low cytolytic activity, and their number is decreased relative to that in the circulation of normal donors.2 In addition, NK cells in AML patients have been shown to have low levels of expression of NK activating receptors.3,4
While aberrations in NK cell numbers and function in AML are readily measurable, the mechanisms responsible for them are unknown. It has been suggested in the past that the presence of soluble NK cell receptor ligands derived from leukemic blasts are responsible for decreased NK cell activity.5 We considered the possibility that leukemia blast-derived microvesicles or exosomes could be responsible for NK cell suppression in AML patients.
Microvesicles are endosome-derived organelles (50–100 nm), which are spontaneously secreted from normal and neoplastic cells.6 Tumor cells are generous producers of microvesicles as demonstrated by in vitro studies with tumor cell lines and by large quantities of microvesicles purified from plasma and other body fluids of cancer patients.7–9 Tumor-derived microvesicles exert a broad variety of detrimental effects on immune cells, ranging from apoptosis of activated T cells to the impairment of monocyte differentiation into dendritic cells.10–12 Tumor-derived microvesicles induce accumulation of myeloid-derived suppressor cells (MDSC) in the peripheral circulation of cancer patients13 and up-regulate proliferation as well as suppressor functions of regulatory T cells (Treg).14 It has also been suggested that microvesicles play a key role in tumor progression.8,15 To determine whether microvesicles isolated from sera of patients newly diagnosed with AML contribute to NK cell suppression, we evaluated their effects on phenotypic and functional attributes of normal human NK cells. Here, we show that these microvesicles down-regulated expression of NKG2D activating receptors and interfered with NK cell activity using microvesicle-associated transforming growth factor (TGF)-β to induce suppression.
Design and Methods
Patients with acute myeloid leukemia and healthy volunteers
Samples of venous blood (20–50 mL) were obtained from patients newly diagnosed with AML prior to any treatment (n=19) and age-matched healthy volunteers (n=14). All subjects signed an informed consent form approved by the Institutional Review Board of the University of Pittsburgh. Table 1 lists the characteristics of the patients included in the study. Peripheral blood was collected from all subjects into heparinized vacutainer tubes; the samples were carried by hand to the laboratory and used for experiments immediately after processing. The AML patients were grouped into three cytogenetic risk categories based on published criteria.16 The favorable-risk category included patients with abnormalities (abn) of inv(16)/t(16;16)/del(16q) or t(8;21) without either a del(9q) or being part of a complex karyotype. The intermediate-risk category included patients characterized by +8, Y, +6, del (12p), or a normal karyotype. The unfavorable risk category was defined by the presence of one or more of 5/del(5q), 7/del(7q), inv(3q), abn 11q, 20q, or 21q, del(9q), t(6;9), t(9;22), abn 17p, or complex karyotype defined as three or more abnormalities. Cases of AML were classified as secondary on the basis of a history of previous treatment with chemotherapy or radiotherapy for prior malignancies.
Table 1.
Characteristics of AML patients included in this study.
Isolation of microvesicles
Microvesicles were isolated from sera of normal controls or AML patients using exclusion chromatography and ultracentrifugation, as previously described.9,11 Briefly, aliquots of sera (10 mL) were applied to Bio-Gel A50m columns (Bio-Rad Laboratories, Hercules, CA, USA) packed with Sepharose 2B (Amersham Biosciences, Piscataway, NJ, USA) and were eluted with phosphate-buffered saline (PBS). The protein content was monitored by measuring absorbance at 280 nm. Fractions between 10 and 28 mL (the void volume peak) contained proteins of more than 50 million kDa. Three 9 mL fractions were collected, and after discarding the first fraction, the second and third fractions were combined, placed in a Beckman Optiseal Centrifuge Tube and centrifuged in a Beckman Optima LE-80K Ultracentrifuge (Beckman Coulter) at 100,000g for 3 h at 4°C. The pelleted membrane fragments were resuspended in PBS (500 μL) and analyzed using a Lowry microassay (Bio-Rad Laboratories).
Western blot assays
Isolated microvesicles were characterized for expression of TGF-β1, FasL, MAGE 3/6, MHC class I molecules, MICA/MICB and LAMP-1 using western blots as previously described.8 Each microvesicle fraction equivalent to 25 μg of protein was prepared with lysis buffer containing Halt Protease inhibitor (Pierce, Rockford, IL, USA) and loaded on a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel. The MAGE3/6+ microvesicle fraction of PCI-13 supernatant with a known protein concentration served as a loading control. After electrophoresis, proteins were transferred onto nitrocellulose membranes and blocked with 5% fat-free milk in TBST (0.05% Tween 20 in Tris-buffered saline). Next, protein-loaded membranes were incubated overnight at 4°C with anti-TGF-β1 antibody and anti-LAMP1 (both from Cell Signaling), anti-FasL antibody (Oncogene, Cambridge, MA, USA), anti MICA/MICB (kindly provided by Dr. Soldano Ferrone), anti-MAGE 3/6 antibody (provided by Dr. Spagnoli, Switzerland), or anti-MHC I antibody (also provided by Dr. Ferrone). The membranes were then incubated with the horseradish peroxidase-conjugated secondary antibody at 1:150,000 dilution (Pierce Chemical) for 1 h at room temperature and developed with a SuperSignal chemiluminescent detection system (Pierce Chemical).
Natural killer cell cytotoxicity
Natural killer cell activity was measured in 4 h 51Cr release assays using K562 cells as targets. Briefly, target cells were labeled with 100 μCi of 51Cr for 1 h at 37°C in 5% CO2, washed twice in a complete medium, resuspended in complete medium, and counted. Co-cultures of NK cells and target cells established in triplicate at the effector to target cell ratios of 12, 6, 3, and 1.5:1 were co-incubated for 4 h at 37°C. Controls included targets incubated in medium alone for spontaneous release and targets incubated in 5% (v/v) Triton X-100 in PBS for maximum release. Radioactivity was measured by a Wallac Wizard 1470 Automatic Gamma Counter. The percentage of cytotoxic activity was calculated using the following formula: % specific lysis = (sample cpm − spontaneous cpm)/(maximal cpm − spontaneous cpm) × 100%. Lytic units (LU) were calculated as the number of effector cells needed to lyse 20% of 5×103 target cells, and expressed as LU/107 cells.17,18
Isolation and cultures of natural killer cells
NK cells were isolated from peripheral blood mononuclear cells of normal controls by negative selection using magnetic immunobeads (NK Cell Isolation Kit) and AutoMACS (Miltenyi Biotec, Auburn, CA, USA) or by single-cell sorting. NK cells were cultured with varying concentrations of microvesicles (10–100 μg/mL) isolated from sera of AML patients or normal controls for 24 h at 37°C in 5% CO2 in air with or without interleukin 15 (IL-15) (10–200 ng/mL, Peprotech, Rocky Hill, USA); with or without neutralizing antibodies to TGF-β1 (R&D System) or isotype control IgG (R&D Systems); or with or without recombinant TGF-β1 (0.01–1 ng/mL, R&D Systems).
Flow cytometry
The harvested NK cells were assessed by multiparameter flow cytometry for expression of NK-cell activating and inhibitory receptors. The following anti-human monoclonal antibodies were used for flow cytometry: CD16-FITC and NKG2D-PE (both from BD Pharmingen, San Diego, CA, USA); CD3-ECD, CD56-PC5, CD94-PE, NKp30-PE, NKp44-PE, NKp46-PE, CD158a-PE, CD158b-PE, CD158e1/e2-PE, CD158i-PE, and CD122-PE (all from Beckman Coulter, Miami, FL, USA); and NKG2A-PE and NKG2C-PE (both from R&D System, Minneapolis, MN, USA). The following monoclonal antibodies were used for flow cytometry of the SMAD-TGF-β pathway: rabbit anti-human SMAD1/5/8 and phospho-SMAD1/5/8 (both from Cell Signaling), anti-TGF-β (R&D System) and anti-rabbit FITC-labeled monoclonal antibody (Jackson Immunoresearch). For the SMAD-TGF-β1 studies sorted NK cells were treated with IL-15 with or without microvesicles or TGF-β1 for periods ranging from 5 to 60 min. Cells were then permeabilized with saponin (0.1% in PBS) for 20 min, washed and then monoclonal antibody against SMAD or phospho-SMAD was added.
Flow cytometry was also used to characterize the microvesicles isolated from the AML patients and normal controls. Briefly, microvesicles (10 μg) obtained from sera of AML patients or controls were incubated for 30 min on ice with 5 μL of latex beads, then washed in 2% FSC in PBS and incubated for 30 min with FITC-labeled anti-CD34 antibody, anti-CD33 antibody, anti-CD117 antibody (all from Beckman Coulter) or with appropriate isotype control IgG. After washing, the microvesicle-coated beads were immediately evaluated for expression of these markers by flow cytometry.
Prior to use, all monoclonal antibodies were titrated using normal resting or activated peripheral blood mononuclear cells to establish optimal staining dilutions. Isotype controls were included in all experiments. Flow cytometry was performed using a Beckman Coulter flow cytometer equipped with Expo32 software.
Enzyme-linked immunosorbent assay for transforming growth factor-β1
Human TGF-β1 enzyme-linked immunosorbent assay (ELISA) kits from RayBio (Norcross, GA, USA) and from Invitrogen (Carlsbad, CA, USA) were used, according to the manufacturers’ recommendations, to measure TGF-β1 in sera from patients with AML and from normal controls. These kits use different procedures to activate latent TGF-β1 to the immuno-reactive form. In the former (RayBio), serum samples were first acidified with 2.5 M acetic acid and 10 M urea (1:1) for 10 min at room temperature and then neutralized with an equal volume of 1.2 M NaOH and 0.5M HEPES (pH 7.7). In the latter (Invitrogen), serum samples were first acidified with 1 M HCl (100 μL sample + 50 μL acid) at room temperature for 10 min and then neutralized by adding 50 μL of 1.2 M NaOH. All samples were divided into two aliquots, each processed separately as described above prior to ELISA.
Statistical analysis
Data were summarized by descriptive statistics: mean and standard error (SE) for continued variables, frequency and percentage for categorical variables. Statistical analyses were performed using paired and unpaired two-tailed Student’s t tests. The association between serum microvesicle levels and the percentage of blasts in patients’ bone marrows was tested using Spearman’s correlations. A P value less than 0.05 was considered statistically significant.
Results
Baseline characteristics of the patients with acute myeloid leukemia
The study cohort consisted of 19 AML patients. The patients’ demographics and baseline characteristics are presented in Table 1. The median age was 64 years (range, 33–77 years); 21% of patients had secondary AML. All 19 patients were assessed for cytogenetic risk at diagnosis; 5 (26%) had an unfavorable-risk karyotype, 11 (58%) had an intermediate-risk karyotype and 3 (16%) had a favorable karyotype.
Characterization of microvesicles from patients with acute myeloid leukemia
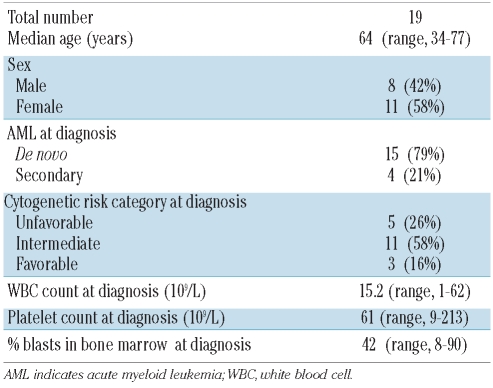
The microvesicle fractions obtained from sera of AML patients had a significantly greater protein content (75±12 μg protein/mL) than those isolated from sera of normal controls (1.2±0.4 μg protein/mL) (P<0.001), as shown in Figure 1A. No correlation was detected between the serum microvesicle levels and percentages of leukemic blasts in the patients’ bone marrows (P<0.92).
Figure 1.
Properties of microvesicles (MV) isolated from sera of AML patients. (A) The protein content in the MV fractions isolated using exclusion chromatography and ultracentrifugation from sera of AML patients and normal controls (NC). (B) Western blot analyses of MV for expression of MHC- class 1, FasL, TGF-β1, LAMP-1 and MICA/MICB. Representative blots are shown. The numbers on the right indicate molecular weights of the detected proteins. (C) MV isolated from AML patients’ sera expressed CD33, CD34, CD117 (solid black lines). The presence of all three markers in MV confirms their origin from myeloid blasts. In contrast, MV isolated from NC expressed only CD33 (dotted lines). Gray-filled histograms indicate isotype control. The representative flow cytometry data were obtained with MV isolated from one NC out of 14 and one AML patient out of 19 examined.
The molecular profile of microvesicles isolated from sera of AML patients was distinct from that of microvesicles isolated from sera of normal controls (Figure 1B). In addition to classical microvesicle markers (LAMP1 or MHC-I), microvesicles isolated from AML patients contained FasL and TGF-β1, which were absent in microvesicles from normal controls. Microvesicles from all patients contained similarly low but detectable levels of MICA/MICB. However, microvesicles isolated from sera of only six out of eight AML patients expressed TGF-β1, and the two negative samples also contained no MAGE3/6, suggesting that blast-derived microvesicles were different in these two AML patients (Figure 1B). In fact, the sera from these two patients contained the highest levels of soluble TGF-β1 measured in this cohort of patients. In contrast to CD33+ microvesicles isolated from sera from normal controls, the microvesicles isolated from sera of 16/19 AML patients expressed the myeloid blast markers CD34 and CD117 (Figure 1C), confirming that these microvesicles were derived from leukemic blasts. In 3/19 AML patients, leukemic blasts did not express these markers.
Effects of microvesicles on natural killer cells
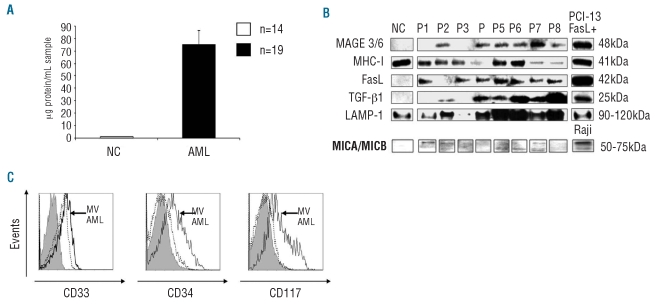
NK cells sorted from peripheral blood of normal controls were co-cultured with microvesicles isolated from sera of the AML patients or controls. The microvesicles isolated from sera of controls did not impair NK cell cytotoxicity. However, a significant decrease in NK cell cytotoxicity was observed after co-incubation with microvesicles isolated from sera of AML patients (2412 LU versus 1640 LU; P<0.002) (Figure 2A). Co-incubation of NK cells with microvesicles from AML sera in the presence of neutralizing anti-TGF-β1 antibodies completely restored NK activity (P<0.004), while isotype control IgG did not (Figure 2A). As expected, the addition of recombinant TGF-β1 to NK cells inhibited their cytotoxicity (Figure 2A).
Figure 2.
Effects of antibodies to TGF-β1 on microvesicles (MV)-mediated effects. (A) MV isolated from sera of AML patients decreased NK cell cytotoxicity as did recombinant TGF-β1. The addition of neutralizing antibodies to TGF-β1 but not of isotype IgG abrogated the effects of MV on NK cell cytotoxicity. MV isolated from normal controls (NC) did not affect NK cell cytotoxicity. The data are mean LU ± SE from experiments with eight AML samples. (B) NK cells were single-cell sorted from the blood of NC (left) and co-incubated with or without MV. NKG2D expression was decreased after co-incubation of NK-cells with MV isolated from AML patients. Antibodies to TGF-β1 abrogated the inhibitory effect of MV on NK cells. Gray-filled histograms indicate an isotype control and the solid line indicates NKG2D expression in NK cells of an AML patient. The representative data were obtained from one AML patient out of 19 examined.
Since NK cell cytotoxicity is controlled through the balance of signals mediated by activating and inhibitory receptors, the effect of microvesicles on the NK-cell receptor repertoire in normal purified NK cells was evaluated (Figure 2B). In the presence of microvesicles isolated from AML patients, down-regulation (P<0.001) in expression of the NK-cell activating receptor, NKG2D, was observed; this expression was restored to normal in the presence of neutralizing anti-TGFβ1 antibodies (Figure 2B) but not iso-type control IgG. Also, the percentage of cells expressing NKG2D was lower after co-incubation with microvesicles from AML patients (57% versus 38%; P<0.001). No significant changes were observed in the expression of the killer immunoglobulin-like receptors, NKG2C, NKG2A or the natural cytotoxicity receptors (NKp30, NKp46) after co-incubation of purified normal NK cells with microvesicles from AML sera (data not shown).
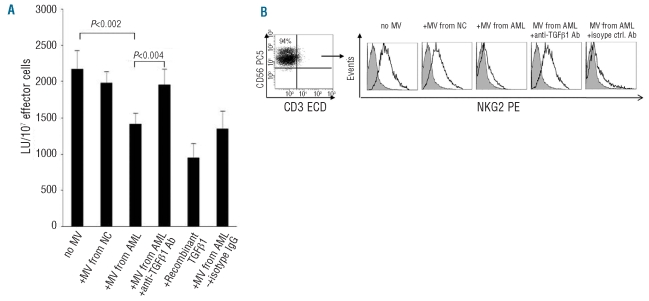
The SMAD pathway is the canonical signaling pathway used by TGF-β family members.20 We, therefore, investigated its involvement in microvesicle-mediated effects on NK cells. Co-incubation of NK cells with microvesicles isolated from sera of AML patients or with recombinant TGF-β1 induced phosphorylation of SMAD1/5/8 in NK cells (Figure 3). The addition of anti-TGF-β1 monoclonal antibodies to the co-culture inhibited microvesicle-induced or TGF-β1-induced SMAD phosphorylation but did not alter expression of SMAD protein itself. Collectively, these data indicate that TGF-β1 expressed on microvesicles obtained from sera of AML patients is responsible for the observed impairment of NK cell activity, down-modulation of NKG2D expression and up-regulation of phospho-SMAD in NK cells.
Figure 3.
Expression of phosphorylated SMAD proteins in NK cells co-incubated with TGF-β1 or microvesicles (MV) derived from AML patients’ sera. Flow cytometry results are from one representative experiment of four performed with different MV preparations co-cultured with NK cells. Upon the addition of recombinant TGF-β1 or MV isolated from serum of an AML patient to NK cells, p-SMAD expression increased. In the presence of neutralizing anti-TGF-β1 antibodies, p-SMAD expression was reduced.
Microvesicle-associated transforming growth factor-β1 in sera of patients with acute myeloid leukemia
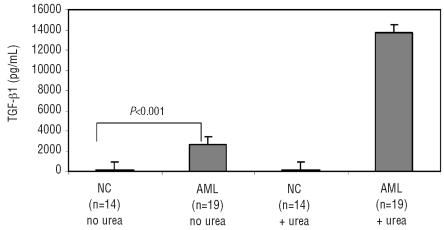
TGF-β1 is a potent immunosuppressive molecule that inhibits NK cell cytotoxicity.19,20 Since TGF-β1 was found to be expressed in microvesicles of AML patients, we further investigated its role in microvesicle-mediated suppression of NK cell activity in these patients. We first measured TGF-β1 concentrations in sera from the newly diagnosed AML patients using two different methods for activating latent TGF-β1 (see Design and Methods section). As shown in Figure 4, the serum concentration of TGF-β1 in the AML patients (mean±SD = 2,600±500 pg/mL) was significantly higher (P<0.0001) than that in the normal controls (94.6±44 pg/mL) when assessed using the Invitrogen kit. The presence of urea in the RayBio kit was presumably responsible for disassociating membrane-bound TGF-β1 on microvesi-cles, resulting in a significant increase (P<0.001) of the measured TGF-β1 levels (mean±SD = 13,700±980 pg/mL) in sera of the AML patients but not in sera from normal controls.
Figure 4.
TGF-β1 levels in the sera of patients with AML and of normal controls (NC). Two different assay methods were used to measure TGF-β1 levels as described in the Design and Methods section. Note that the addition of urea used in one method increased the detectable TGF-β levels in sera of patients with AML but not in sera of NC.
Interleukin-15 protects natural killer cells from microvesicle-mediated effects
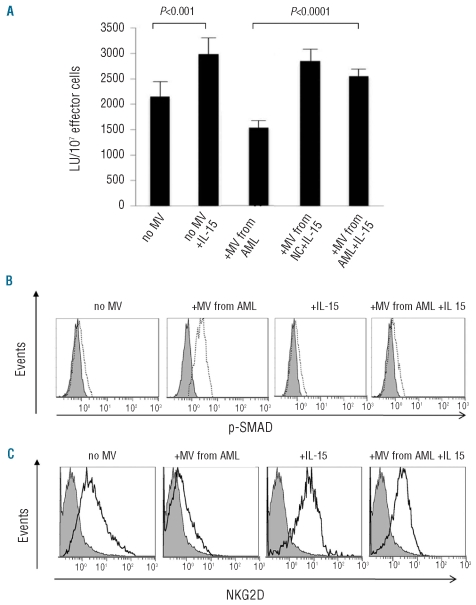
We next evaluated whether IL-15, a NK-cell homeostatic cytokine, could protect NK cells from the microvesicle-induced decrease in NK activity. We first showed that recombinant IL-15 added to NK cells increased their cytotoxicity (P<0.001) (Figure 5A). The addition of IL-15 to co-cultures of microvesicles isolated from sera of AML patients with NK cells from normal controls abrogated the microvesicle-mediated suppression of NK cell activity (P<0.0001). NK cells co-incubated with microvesicles from AML patients up-regulated phospho-SMAD expression. However, the pre-treatment of NK cells with IL-15 followed by co-incubation with microvesicles derived from sera of AML patients prevented microvesicle-induced phosphorylation of the SMAD pathway (Figure 5B). Recombinant IL-15 alone had no effect on phospho-SMAD or SMAD levels in NK cells. Also, when NK cells were co-incubated in the presence of microvesicles isolated from AML patients’ sera NK62D expression was down-regulated (Figures 2B and 5C). However, in the presence of IL-15, the level of NKG2D expression did not decrease in NK cells exposed to microvesicles (Figure 5C). Collectively, these results indicate that IL-15 protects NK cells from microvesicle-associated TGF-β and that it reverses the microvesicle-mediated inhibition of NKG2D expression on NK cells.
Figure 5.
Exogenous IL-15 protects NK cells from microvesicle (MV)-induced loss of cytotoxicity. (A) MV isolated from sera of AML patients decreased NK cell activity while exogenous IL-15 up-regulated it. In the presence of exogenous IL-15 (100 ng/mL for 24 h) NK cell activity was not impaired by the MV obtained from AML patients. MV obtained from normal controls (NC) did not alter NK cell activity. The data are mean LU±SE from experiments with eight AML samples. (A) NK cells obtained from NC were incubated in the presence of IL-15 and then MV isolated from the serum of an AML patient were added and after 60 min p-SMAD expression was measured by flow cytometry. (C) NK cells were incubated with or without MV isolated from an AML patients’ serum for 24 h in the presence or absence of exogenous IL-15 (100 ng/mL). NKG2D expression was measured by flow cytometry. In (A) and (B), gray-filled histograms indicate isotype control IgG.
Discussion
Activated NK cells have long been known to be able to kill a broad range of tumor targets. However, cytotoxicity of NK cells in the peripheral blood of untreated acute leukemia patients was found to be significantly depressed.3,4 This decrease has been variously attributed to: (i) up-regulated expression of MHC class I molecules on leukemia blasts, making them poor NK-cell targets; (ii) decreased expression levels of the ULBP and NCR ligands in AML blasts, impairing their clearance by NK cells; or (iii) decreased expression of the NK-cell activating receptors on NK cells in AML patients.2,21 Although the molecular mechanisms responsible for the reduced receptor expression remain elusive, elevated serum levels of soluble NK cell receptor ligands shed by tumor cells are thought to be responsible and to contribute to the decreased NK cell activity.5 MICA/MICB, tumor-derived soluble NKG2D ligands, have been shown to alter the expression of NKG2D on NK cells.5 Further, exosomes isolated from tumor cell supernatants22,23 or from pleural fluids of patients with malignant mesothelioma23 and carrying the NKG2D ligands were shown to down-modulate NKG2D expression as well as NK cell activity. As both MICA and MICB were detected by western blots in microvesicles derived from sera of AML patients, it is likely that microvesicle-associated MICA/MICB contributed to the down-regulation of NKG2D expression on NK cells of AML patients.
Microvesicles derived from solid tumors are known to have suppressive effects on immune cells.8,15,24 Our studies show for the first time that microvesicles present in sera of patients with newly diagnosed AML play a role in regulating NK activity. Leukemic blasts in newly diagnosed AML patients could be a source of microvesicles responsible for suppression of NK cell activity. Although microvesicle fractions isolated from AML patients’ sera had a consistently high protein content, no correlation was detected between the percentages of blasts in the bone marrow and serum microvesicle levels. Based on the molecular profile of these microvesicles, we believe them to be derived from leukemic blasts. Only the microvesicles isolated from AML patients’ sera were shown to contain biomarkers characteristic of leukemic blasts: CD33, CD34 and CD117. If leukemic blasts are confirmed to be the origin of these microvesicles, this could represent yet another novel mechanism accounting for the reduced NK cell activity in patients with AML.
Microvesicles contain numerous membrane-associated proteins, and their protein content mimics that of surface membranes in the cells from which the microvesicles originate.15,24 By virtue of their virus-like size, tumor-derived microvesicles could transport membrane-associated tumor proteins through the body, delivering them to distant cellular targets. In this fashion, tumors can deliver stimulatory or inhibitory signals to immune cells present in the blood or in tissues.26 While microvesicles are produced by all activated cells by an ATP-dependent process,25,26 those released by tumor cells tend to contain tolerogenic signals, in contrast to, for example, dendritic cell-derived microvesicles which are antigenic and stimulatory.27
TGF-β is an important immunoregulatory molecule that has been shown to impair NK cell activity by reducing NK cell-receptor transcripts and their expression.19 TGF-β is often expressed in human solid tumors, and high serum TGF-β1 levels are usually detected in patients with cancer. Circulating levels of TGF-β1 were found to be inversely correlated with time to tumor recurrence.28 TGF-β1 release by tumors is considered to be one of the mechanisms of tumor escape from the immune system. NK cells are known to be highly sensitive to TGF-β1.29 Our observation that the microvesicles isolated from AML patients’ sera contained TGF-β1, therefore, strongly suggest that microvesicles suppress NK-cell activity using the TGF-β1 pathway. However, TGF-β1 was not detected in microvesicles of two of the AML patients we studied. Possibly this relates to especially high serum TGF-β1 levels measured in these two patients’ sera in the absence of urea, implying a release of soluble TGF-β1. In general, TGF-β1 levels in AML patients’ sera were found to be higher than those in normal controls. Moreover, the use of the membrane-dissociating agent, urea, prior to TGF-β1 measurements allowed for the detection of substantially higher levels of TGF-β1 in patients’ sera but not in sera of normal controls. This finding, reproduced with microvesicles isolated from AML patients’ sera, implies that microvesicle-associated TGF-β is not detected unless urea is used, and that its levels reported to date in cancer patients’ sera may have been underestimated.
IL-15 has been shown to be the major NK cell homeostatic cytokine.30–33 NK cell differentiation is IL-15-dependent, and IL-15 plays a major role in NK-cell expansion and promotes NK-cell survival.30,31 Mice lacking expression of either IL-15 or the IL-15Ra chain fail to develop NK cells, and NK cells transferred into IL-15−/− hosts fail to survive.30–33 AML patients have low NK cell activity, and addition of IL-15 to these cells up-regulates activating receptor expression and NK-cell activity.2 Our current data demonstrate that exogenous IL-15 protects NK cells from the inhibitory effect of tumor-derived microvesicles by interfering with SMAD signaling and that IL-15 can prevent microvesicle-induced down-regulation of NKG2D expression. Recently, it was shown that IL-15 acts as a powerful antagonist of TGF-β signaling by inhibiting the formation of SMAD-DNA complexes34 and thus counteracting down-regulatory effects on T lymphocytes.34 Thus, IL-15/TGF-β crosstalk has important consequences. IL-15 is a pivotal cytokine for the development and survival of NK cells, and our results showing that IL-15 is also able to counteract immunosuppressive effects mediated by TGF-β carried on microvesicles from AML patients are important. They identify another mechanism through which IL-15 can enhance anti-tumor effects of NK cells in cancer patients, including those with AML, and strengthen the potential of IL-15 for AML therapy.
This report presents evidence in support of the role of tumor-derived microvesicles in immune suppression mediated by human leukemic blasts. The demonstration that microvesicle-induced down- regulation of NKG2D expression via TGF-β1 is one of the mechanisms by which microvesicles suppress NK cell activity in AML patients is novel and important. The finding that IL-15 might protect NK cells from microvesicle-mediated TGF-β1 effects, in addition to supporting other NK-cell functions, provides a strong rationale for its incorporation into the future immunotherapy of AML.
Footnotes
Funding: this work was supported in part by the NIH grant PO1 CA109688 to TLW and by the Polish Ministry of Science and Higher Education (NN401047738) and the Foundation for Polish Science (Homing Plus/2010-1/13) grants to MJS.
Presented in part at the 2010 Annual Meeting of ASH in Orlando, FL, USA.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szczepanski MJ, Szajnik M, Welsh A, Foon KA, Whiteside TL, Boyiadzis M. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol Immunother. 2010;59(1):73–9. doi: 10.1007/s00262-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–7. doi: 10.1182/blood.v99.10.3661. [DOI] [PubMed] [Google Scholar]
- 4.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109(1):323–30. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 5.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):679–80. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 6.Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–8. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- 7.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann C, Strauss L, Wieckowski E, Czystowska M, Albers A, Wang Y, et al. Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression. Head Neck. 2009;31(3):371–80. doi: 10.1002/hed.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9(14):5113–9. [PubMed] [Google Scholar]
- 10.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15(1):80–8. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11(3):1010–20. [PubMed] [Google Scholar]
- 12.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183(6):3720–30. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang X, Poliakov A, Liu C, Deng ZB, Wang J, Cheng Z, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–33. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (treg) PLoS One. 2010;5(7):e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67(7):2912–5. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 16.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–83. [PubMed] [Google Scholar]
- 17.Friberg DD, Bryant JL, Whiteside TL. Measurements of natural killer (NK) Activity and NK-cell quantification. Methods. 1996;9(2):316–26. doi: 10.1006/meth.1996.0037. [DOI] [PubMed] [Google Scholar]
- 18.Whiteside TL, Bryant J, Day R, Herberman RB. Natural killer cytotoxicity in the diagnosis of immune dysfunction: criteria for a reproducible assay. J Clin Lab Anal. 1990;4(2):102–14. doi: 10.1002/jcla.1860040207. [DOI] [PubMed] [Google Scholar]
- 19.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100(7):4120–5. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trotta R, Col JD, Yu J, Ciarlariello D, Thomas B, Zhang X, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol. 2008;181(6):3784–92. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowbakht P, Ionescu MC, Rohner A, Kalberer CP, Rossy E, Mori L, et al. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105(9):3615–22. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 22.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, et al. Natural killer cell cytotoxicity is suppresssed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70(2):481–9. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180(11):7249–58. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375–85. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 25.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(pt 19):3365–74. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol Dis. 2006;36(2):315–21. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36(1–3):247–54. doi: 10.1385/IR:36:1:247. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172(12):7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 30.Boyiadzis M, Memon S, Carson J, Allen K, Szczepanski MJ, Vance BA, et al. Up-regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol Blood Marrow Transplant. 2008;14(3):290–300. doi: 10.1016/j.bbmt.2007.12.490. [DOI] [PubMed] [Google Scholar]
- 31.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99(5):937–43. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100(10):3633–8. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 33.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101(12):4887–93. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 34.Benahmed M, Meresse B, Arnulf B, Barbe U, Mention JJ, Verkarre V, et al. Inhibition of TGF-beta signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology. 2007;132(3):994–1008. doi: 10.1053/j.gastro.2006.12.025. [DOI] [PubMed] [Google Scholar]