Abstract
Background
High white blood cell count at presentation is an unfavorable prognostic factor for treatment outcome in intermediate cytogenetic risk acute myeloid leukemia. Since the impact of white blood cell count on outcome of subgroups defined by the molecular markers NPMc+ and FLT3-internal tandem duplication (ITD) is unknown, we addressed this issue.
Design and Methods
We studied the effect of white blood cell count on outcome in a clinically and molecularly well-defined cohort of 525 patients with acute myeloid leukemia using these molecular markers. In addition, since an increased white blood cell count has been associated with an increased FLT3-ITD/FLT3 (wild-type) ratio, we investigated whether the effect of white blood cell count on outcome could be explained by the FLT3-ITD/FLT3 ratio.
Results
This analysis revealed that white blood cell count had no impact on outcome in patients with the genotypic combinations ‘NPMc+ without FLT3-ITD’ and ‘NPM1 wild-type with or without FLT3-ITD’. In contrast, white blood cell count had a significant impact on complete remission rate (P=0.034), event-free survival (P=0.009) and overall survival (P<0.001) in patients with the genotypic combination ‘NPMc+ with FLT3-ITD’. A FLT3-ITD/FLT3 ratio greater than 1 was also associated with a reduced complete remission rate (P=0.066) and significantly reduced event-free survival (P= 0.001) and overall survival (P=0.001) in patients with the genotypic combination ‘NPMc+ with FLT3-ITD’. Multivariable analysis revealed that white blood cell count and FLT3-ITD/FLT3 ratio were independent prognostic indicators for outcome in the subgroup with the genotypic combination ‘NPMc+ with FLT3-ITD’.
Conclusions
Our results demonstrate that both high white blood cell count and FLT3-ITD/FLT3 ratio are prognostic factors in patients with acute myeloid leukemia with the genotypic combination ‘NPMc+ with FLT3-ITD'.
Keywords: acute myeloid leukemia, prognosis, white blood cell count, NPM1, FLT3-ITD
Introduction
Several prognostic factors related to patients’ and disease characteristics have been described for acute myeloid leukemia (AML).1,2 The karyotype at diagnosis is a powerful prognostic factor for treatment outcome in patients with AML.3–7 Various recurrent somatically acquired molecular abnormalities have been identified during the last years.8 Of these molecular abnormalities, mutations in nucleophosmin (NPM1) and internal tandem duplications of the fms-related tyrosine kinase 3 gene (FLT3-ITD) have strong prognostic impact.
Mutations in NPM1 are the most frequently observed molecular abnormalities, present in about 50% of AML cases, and are associated with a favorable outcome.9–13 AML with mutated NPM1 (NPMc+) shows distinctive biological and clinical features, and is, therefore, a provisional entity in the World Health Organization (WHO) 2008 classification of leukemias. FLT3-ITD is another frequent molecular abnormality and can be observed in about 20–25% of patients with AML.14–18 Clinically, AML patients harboring FLT3-ITD frequently have high white blood cell (WBC) counts at presentation.14–18 The presence of FLT3-ITD is generally considered as an unfavorable prognostic factor.14–19 In particular, those cases with a FLT3-ITD mutation/FLT3 wild-type ratio (hereafter referred to as the FLT3-ITD/FLT3 ratio) above the median value have a dismal prognosis.17–19 A high FLT3-ITD/FLT3 ratio points to the absence of the FLT3 wild-type allele.
Mutations in NPM1 and FLT3-ITD are frequently associated. Approximately 40% of patients with NPM1 mutations also carry FLT3-ITD.9 Various studies have shown that the genotypic combination ‘NPMc+ without FLT3-ITD’ represents a subgroup with favorable prognosis.9–13 Nevertheless, the beneficial impact of NPMc+ on prognosis was seen in patients with as well as those without FLT3-ITD and it appeared that both mutations in NPM1 and FLT3-ITD were significant independent predictors of outcome.19
Besides cytogenetic and molecular abnormalities, classically, a high WBC count at presentation is considered to be an independent prognostic factor for poor outcome in both adults and children with AML.1,20–23 The effect of WBC count at diagnosis is most apparent in AML with favorable cytogenetic risk abnormalities, such as t(8;21) and t(15;17).24–25 However, the prognostic effect of WBC count is also present in AML patients with intermediate cytogenetic risk abnormalities.20–23 Multivariable analysis has shown that both WBC count and the genotypic subgroup NPMc+ without FLT3-ITD are independent predictors of outcome.11 Nevertheless, the effect of WBC count at diagnosis on outcome of patients within the four subgroups defined by the molecular markers NPMc+ and FLT3-ITD (within the intermediate cytogenetic risk group) is unknown.
The aim of the present survey was to investigate the prognostic impact of WBC count at diagnosis on outcome within AML subgroups defined by NPMc+ and FLT3-ITD or the FLT3-ITD/FLT3 ratio. Therefore, within a clinically and molecularly well characterized cohort of 525 patients with de novo AML, we compared treatment outcome among patients divided into three groups on the basis of their WBC counts: less than 20×109/L, 20 to 100×109/L, and above 100×109/L.
Design and Methods
Patients
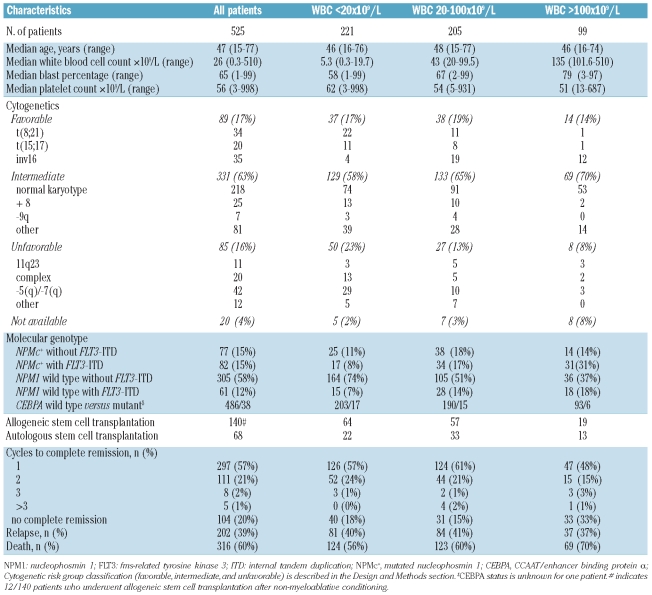
The study cohort consisted of 525 consecutive adult patients with AML who were treated according to the sequential HOVON/SAKK AML-04, -04A, -29, -32, -42, -43 protocols (available at http://www.hovon.nl) and for whom molecular data on NPMc+ and FLT3-ITD status were available.26–29 All patients in this study were newly diagnosed with AML and the diagnosis was established according to WHO criteria. Cell specimens were collected at the time of diagnosis. All patients provided written informed consent in accordance with the Declaration of Helsinki, and the study was approved by all participating institutional review boards. Patients were divided into cytogenetic risk groups (favorable, intermediate, or unfavorable) in accordance with HOVON/SAKK criteria (Table 1). Cytogenetic risk was defined as favorable in patients with t(8;21)(q22;q22), inv(16)(p13.1;q22), or t(16;16)(p13.1;q22) and t(15;17), and unfavorable in patients with complex cytogenetic abnormalities (i.e. three or more distinct clonal abnormalities), −7, −5, del 5q or del 7q, abnormalities of the long arm of chromosome 3 (abn 3q), t(6;9)(q23;q34), t(9;22)(q34;q11), or abnormalities of the long arm of chromosome 11 (abn 11q23). All other cytogenetic abnormalities and AML without cytogenetic abnormalities were considered to indicate an intermediate cytogenetic risk. The median overall survival of the whole cohort was 16.3 months, and the median follow-up of survivors was 61.4 months.
Table 1.
Patients’ characteristics.
Guided by thresholds of WBC counts which are often clinically used for risk stratification, the 525 patients were divided into three groups: those with a WBC count below 20×109/L (n=221), those with a WBC count between 20 and 100×109/L (n=205) and those with a WBC above 100×109/L (n=99). In the current HOVON/SAKK AML study (HOVON102) the following classifications are also used; patients with t(8;21) and WBC greater than 20×109/L are considered at intermediate risk (instead of favorable risk) and patients with a normal karyotype (or only loss of sex chromosomes) and a WBC greater than 100×109/L are considered at unfavorable risk (instead of intermediate risk).
Within our cohort 82 patients had the molecular combination NPMc+ with FLT3-ITD. Six patients of this subgroup had unclassified cytogenetics, and one patient had 5(q)/−7(q) cytogenetics. Therefore, 75 patients with both confirmed intermediate-risk cytogenetics and the genotypic markers NPMc+ with FLT3-ITD were studied.
FLT3-ITD/FLT3-wildtype ratio
Amplification of the FLT3-ITD mutations was performed using primers 11F and 11R, as described by Nakao et al.30 Ratios were determined after agarose gel electrophoresis of the quantitative polymerase chain reaction products.
Statistical analyses
Statistical analyses were performed with SPSS software, release 16.0. Actuarial probabilities of overall survival (with death due to any cause) as well as event-free survival (with failure in case of no complete remission or relapse or death) were estimated according to the Kaplan–Meier method. For quantitative parameters overall differences between the cohorts were evaluated using an F-test (or Student’s-t test in the case of two groups) for normally distributed variables or a Kruskal-Wallis test (or Mann-Whitney-U test in the case of two groups) for variables with a skewed distribution. For qualitative parameters, overall group differences were evaluated using a χ2 test. Cox regression analysis was applied to determine the association of WBC and overall and event-free survivals with adjustment for possible confounding factors such as age at diagnosis, cytogenetic risk profile (i.e. favorable, intermediate or unfavorable), FLT3-ITD, NPMc+ and the transcription factor CCAAT/enhancer binding protein α (CEBPA). All tests were two-tailed, and a P value of less than 0.05 was considered statistically significant.
Results
Patients
The clinicopathological, demographic, and molecular data of the 525 patients with AML in the study cohort are presented in Table 1. Guided by thresholds of WBC counts which are often clinically used for risk stratification, the 525 patients were divided into three groups: those with a WBC count below 20×109/L (n=221), those with a WBC count between 20 and 100×109/L (n=205) and those with a WBC above 100×109/L (n=99). The frequency of patients with a WBC count greater than 100×109/L (often designated as hyperleukocytosis) appeared to be higher in the group of AML patients with intermediate cytogenetic risk abnormalities (21%) than in the group with unfavorable cytogenetic risk abnormalities (9%). Within this study cohort, 49 of 143 (34%) patients with FLT3-ITD and 45 of 159 (28%) patients with NPMc+ presented with hyperleukocytosis (WBC >100×109/L) at diagnosis.
Impact of white blood cell count on complete remission rate, event-free survival and overall survival
When considering all 525 patients, hyperleukocytosis was significantly associated with a lower complete remission rate (67% versus 84%; P<0.001), shorter event-free survival (median 6.8 months versus 11.6 months; P=0.001) and shorter overall survival (median 8.9 months versus 17.2 months; P=0.002) (Online Supplementary Figure S1A,B). Furthermore, within the subgroup of patients with cytogenetically intermediate-risk AML, hyperleukocytosis was significantly associated with a lower complete remission rate (P<0.001), shorter event-free survival (median 7.3 months versus 13.2 months; P=0.009) and shorter overall survival (median 9.2 months versus 19.1 months; P=0.001) (Online Supplementary Figure S1C,D). As expected, in the subgroup of patients with favorable cytogenetic risk, a WBC count below 20×109/L appeared to be significantly associated with a higher complete remission rate (P=0.024), improved event-free survival (median 77.2 months versus 9.7 months) and improved overall survival (median 85.5 months versus 28.9 months; P=0.001) (Online Supplementary Figure S1E,F). Finally, in the subgroup of patients with cytogenetically unfavorable risk AML, WBC count did not affect the complete remission rate (P=0.593), event-free survival (P=0.717) or overall survival (P=0.672) (Online Supplementary Figure S1G,H).
Prognostic value of white blood cell count in the context of additional known risk factors for the whole group of patients with acute myeloid leukemia
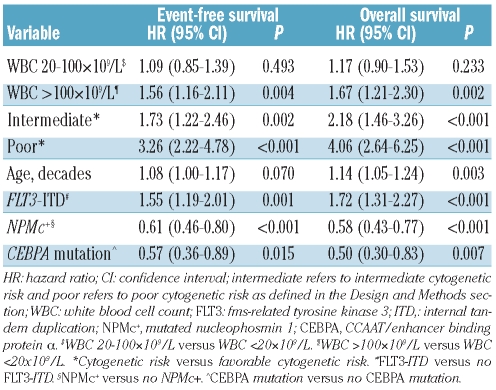
The impact of hyperleukocytosis on event-free and overall survival in all 525 patients with AML was confirmed in univariate analysis. Age at diagnosis, NPMc+, FLT3-ITD, mutated CEBPA and cytogenetic risk group (i.e. unfavorable, intermediate and favorable risk) also correlated with event-free and overall survival (data not shown). When we subsequently considered these variables in a multivariable analysis, hyperleukocytosis maintained its independent prognostic value for both event-free survival (HR: 1.56, 95% CI: 1.16–2.11; P=0.004) and overall survival (HR: 1.67, 95% CI: 1.21–2.30; P=0.002) (Table 2).
Table 2.
Multivariable analysis of WBC count as a prognostic marker for event-free survival and overall survival in all 525 patients.
Prognostic value of white blood cell count in the context of the molecular markers NPMc+ and FLT3-ITD within the intermediate cytogenetic risk group
The impact of WBC count on complete remission rate, event-free survival and overall survival was evident in the intermediate cytogenetic risk group, which contained 63% of the patients of this study cohort. Multivariate analysis established that hyperleukocytosis, NPMc+ and FLT3-ITD were independent predictors for event-free survival as well as overall survival (WBC count >100×109/L: HR: 1.39, 95% CI: 1.01–1.92, P=0.042 for event-free survival: HR: 1.59, 95% CI: 1.14–2.21, P=0.006 for overall survival; FLT3-ITD: HR: 1.58, 95% CI: 1.18–2.13, P=0.002 for event-free survival: HR: 1.80, 95% CI: 1.32–2.45, P<0.001 for overall survival; NPMc+: HR: 0.61, 95% CI: 0.46–0.82, P=0.001 for event-free survival: HR: 0.59, 95% CI: 0.43–0.80, P=0.001 for overall survival). Furthermore, there was a significant positive interaction between NPMc+/FLT3-ITD and WBC in the multivariable model both for event-free and overall survival (P=0.018 and P=0.002, respectively).
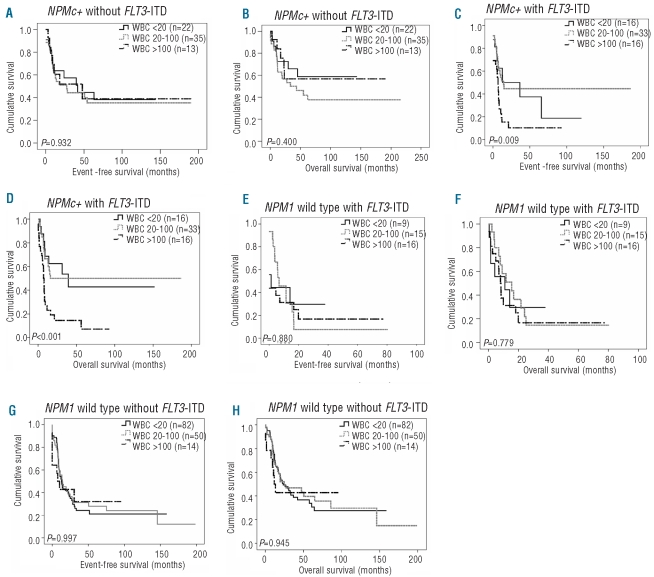
Since we aimed to investigate the impact of WBC count on outcome within the four subgroups defined by the molecular markers NPMc+ and FLT3-ITD (within the intermediate cytogenetic risk group), we subsequently focused on these subgroups. In the most favorable genotypic subgroup, ‘NPMc+ without FLT3-ITD’, WBC count did not significantly affect complete remission rate (P=0.451), event-free survival (P=0.932) or overall survival (P=0.400) (Figure 1A,B). Furthermore, also when WBC count was analyzed as a continuous variable, no significant association was found between WBC count and event-free and overall survival in the subgroup ‘NPMc+ without FLT3-ITD’ (data not shown). Interestingly, however, in the subgroup with the genotypic combination ‘NPMc+ with FLT3-ITD' WBC count did have a significant impact on complete remission rate (P=0.034), event-free survival (P=0.009) and overall survival (P<0.001) (Figure 1C,D). Within this particular subgroup, it appeared that patients with a WBC count less than 20×109/L or between 20–100×109/L had a relatively favorable prognosis with a median overall survival of 21 and 15 months, respectively, and estimated 5-year overall survival rates of 50% and 51%, respectively. In contrast, patients with a WBC greater than 100×109/L evidently had a poor prognosis with a median overall survival of 7 months and an estimated 5-year overall survival rate of 9%. So, based on WBC count (i.e. <100 versus >100×109/L) patients with the genotypic combination ‘NPMc+ with FLT3-ITD’ could be divided into two groups, one with a relatively good prognosis, the other with a very poor prognosis. These results were underscored by univariate Cox regression analyses using WBC count as a continuous variable for event-free survival (HR: 1.006; 95% CI: 1.002–1.010, P=0.001) and for overall survival (HR: 1.009; 95% CI: 1.005–1.013, P<0.001).
Figure 1.
Impact of WBC count on event-free survival (EFS) and overall survival (OS) in cases with cytogenetically intermediate risk AML defined by the molecular markers NPMc+ and FLT3-ITD. (A) EFS according to WBC levels in 70 cytogenetically intermediate risk AML cases with NPMc+ without FLT3-ITD mutation. (B) OS for this group. (C) EFS according to WBC levels in 75 cytogenetically intermediate risk AML cases with NPMc+ and FLT3-ITD. (D) OS for this group. (E) EFS according to WBC levels in 40 cytogenetically intermediate risk AML cases with wild type NPM1 and FLT3-ITD. (F) OS for this group. (G) EFS according to WBC levels in 146 cytogenetically intermediate risk AML cases with wild-type NPM1 without FLT3-ITD. (H) OS for this group. The P value is given for the overall comparison across all three groups.
In the two other subgroups ‘NPM1 wild-type with FLT3-ITD’ and ‘NPM1 wild-type without FLT3-ITD’, WBC count had no evident impact on complete remission rate, event-free survival or overall survival (Figure 1E–H). In addition, when, analyzing WBC count as a continuous variable, no association between WBC count and event-free survival or overall survival was found in the subgroups with the genotypes ‘NPM1 wild-type with FLT3-ITD’ and ‘NPM1 wild-type without FLT3-ITD’ (data not shown). So, when considering the impact of WBC count within the four subgroups defined by the molecular markers NPMc+ with FLT3-ITD, WBC count only had an impact on outcome in patients with the genotypic combination ‘NPMc+ with FLT3-ITD’. Of note, within the subgroup of patients with the genotypic combination ‘NPMc+ with FLT3-ITD’ no difference in age distribution was found between patients with a WBC count below 20×109/L and between 20–100×109/L versus greater than 100×109/L (P=0.412): median age in years (range); 46 (24–68), 51 (18–77) and 50 (19–68), respectively. CEBPA was not taken into account since only two cases had mutated CEBPA within the subgroup of patients with the genotypic combination ‘NPMc+ with FLT3-ITD’.
Prognostic value of FLT3-ITD/FLT3-wildtype ratio among patients with acute myeloid leukemia with the genotypic combination ‘NPMc+ with FLT3-ITD’
It has been shown that the amount of FLT3 signaling is associated with WBC count.17–19 Patients who have lost both FLT3 alleles have higher WBC counts than patients with both wild-type FLT3 and FLT3 -ITD alleles.17–19 We, therefore, wondered whether the gene dosage of wild-type FLT3 was different in patients with the genotypic combination ‘NPMc+ with FLT3-ITD’ depending on whether they had a high or low WBC count. To address this question, we investigated the impact of the FLT3-ITD/FLT3 ratio on clinical outcomes within this particular subgroup of patients. The FLT3-ITD/FLT3 ratio was known for all 75 patients and was categorized as less than 1, 1, or more than 1. Interestingly, the FLT3-ITD/FLT3 ratio was significantly associated with event-free survival (P=0.001) and overall survival (P=0.001) within this subgroup of patients (Figure 2). In detail, patients with an FLT3-ITD/FLT3 ratio greater than 1 had a significantly shorter event-free survival (median 5.6 months versus 15.2 and 13.0 months for patients with a ratio of 1 and <1, respectively) as well as a significantly shorter overall survival (median 7.7 months versus 16.5 and 31.3 months for patients with a ratio of 1 and <1, respectively). Thus, it appears that particularly patients with the genotypic combination ‘NPMc+ with FLT3-ITD’ and an FLT3-ITD/FLT3 ratio greater than 1 had a poor outcome.
Figure 2.
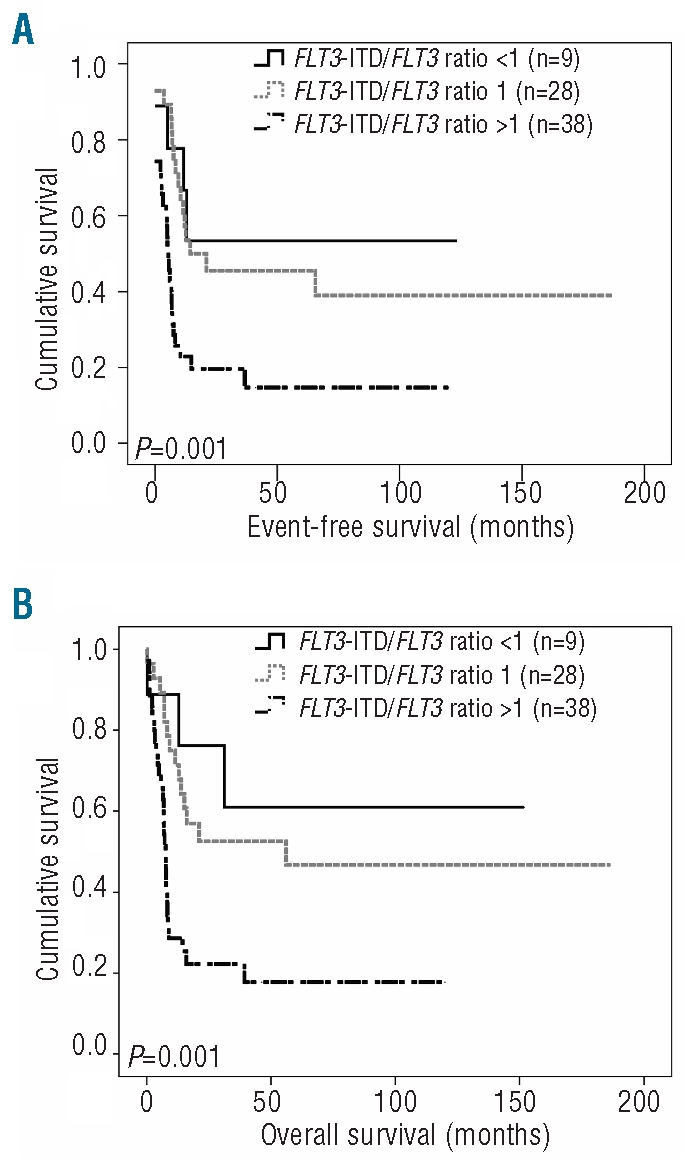
Impact of FLT3-ITD/FLT3 ratio on event-free survival (EFS) and overall survival (OS) in cases with cytogenetically intermediate risk AML with the genotypic combination ‘NPMc+ with FLT3-ITD’. (A) EFS and (B) OS according to FLT3-ITD/FLT3 ratio within 75 cytogenetically intermediate cytogenetic risk AML cases with the genotypic combination NPMc+ with FLT3-ITD. Ratio <1: n=9; ratio 1: n=28; ratio >1: n = 38. The P value is given for the overall comparison across all three groups.
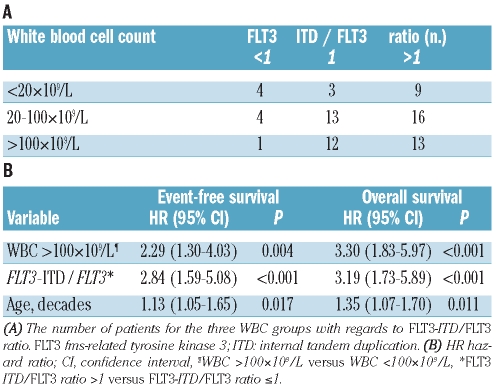
So far, our data indicate that the group of patients with intermediate cytogenetic risk AML with the genotypic combination ‘NPMc+ with FLT3-ITD’ can be further dissected, with the subgroup of patients with hyperleukocytosis or FLT3 ITD/FLT3 ratio greater than 1 having an unfavorable prognosis. However, interestingly, not all patients with hyperleukocytosis had an FLT3-ITD/FLT3 ratio greater than 1 and vice versa (Table 3A). To investigate whether a FLT3-ITD/FLT3 ratio greater than 1 and hyperleukocytosis are indeed independent prognostic factors for patients with AML with the genotypic combination ‘NPMc+ with FLT3-ITD’, a multivariable analysis was performed. This analysis established that FLT3-ITD/FLT3 ratio greater than 1, WBC count greater than 100×109/L and age are independent prognostic factors. In detail, with regards to FLT3-ITD/FLT3 ratio greater than 1, the hazard ratio for event-free survival was 2.84 (P<001) while that for overall survival was 3.19 (P<0.001); for hyperleukocytosis the hazard ratio for event-free survival was 2.29 (P=0.004) and for overall survival 3.30 (P<0.001), and finally, for age (in decades), the hazard ratio for event-free survival was 2.13 (P=0.017) and that for overall survival was 1.35 (P=0.011) (Table 3B).
Table 3.
WBC count and FLT3-ITD/FLT3 ratio in the 75 patients with the genotypic combination ‘NPMC+ with FLT3-ITD’
These data prompted us to propose a model in which patients with intermediate cytogenetic risk AML with the genotypic combination ‘NPMc+ with FLT3-ITD’ were considered to have an unfavorable prognosis if they had a WBC count greater than 100×109/L or an FLT3-ITD/FLT3 ratio greater than 1. Consequently, patients with a WBC count below 100×109/L and an FLT3-ITD/FLT3 ratio of 1 or less were considered to have a favorable prognosis. Within this model, patients with hyperleukocytosis or an FLT3-ITD/FLT3 ratio greater than 1 (n=51) compared unfavorably with patients with a WBC count below 100×109/L and an FLT3-ITD/FLT3 ratio of 1 or less (n=24), with regards to complete remission rate (P=0.010), event-free survival (P<0.001) and overall survival (P<0.001) (Figure 3). In detail, patients with a WBC count below 100×109/L and an FLT3-ITD/FLT3 ratio of 1 or less had median event-free and overall survivals of 24 and 33 months, and estimated 5-year event-free and overall survival rates of 71% and 79%, respectively. In contrast, patients with hyperleukocytosis or an FLT3-ITD/FLT3 ratio greater than 1 had median event-free and overall survivals of 7 and 8 months, and estimated 5-year event-free and overall survival rates of 16% and 18%, respectively. Of note, the median age at diagnosis and the number of patients who had undergone allogeneic stem cell transplantation was not different between these two groups (P=0.55 and P=0.17, respectively).
Figure 3.
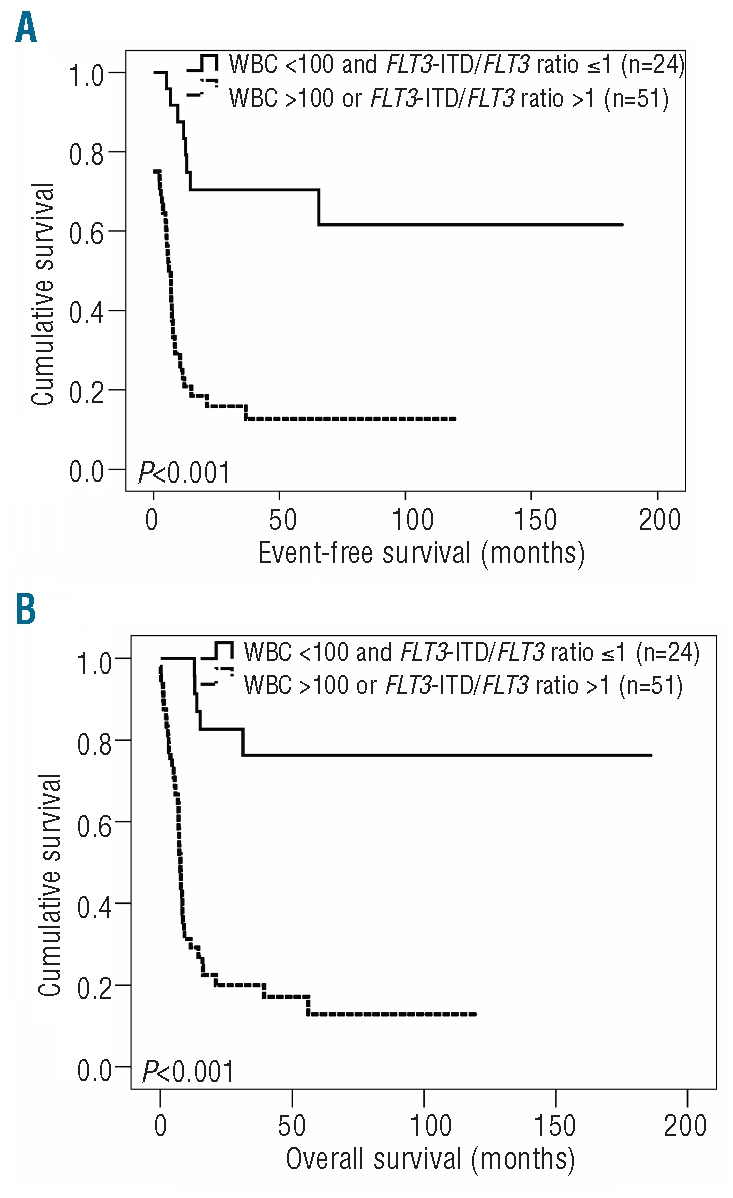
Combined effect of WBC count and FLT3-ITD/FLT3 ratio on event-free survival (EFS) and overall survival (OS) in cases with cytogenetically intermediate risk AML with the genotypic combination ‘NPMc+ with FLT3-ITD’. (A) EFS in patients with WBC count <100x109/L and FLT3-ITD/FLT3 ratio ≤1 (n=24) compared to those with WBC count >100x109/L or FLT3-ITD/FLT3 ratio >1 (n=51) (B) OS in these cases.
Discussion
It is generally accepted, and confirmed in this study, that high WBC count predicts an adverse outcome among AML patients with favorable or intermediate cytogenetic risk.1,2,20–23 In the current study, we have focused on the impact of WBC count at diagnosis on outcome among patients with intermediate cytogenetic risk, divided into subgroups according to the presence of the molecular markers NPMc+ and FLT3-ITD. It was found that the WBC count had an impact on complete remission rate, event-free survival and overall survival only among the patients with the genotypic combination ‘NPMc+ with FLT3-ITD’. Importantly, these results were underscored when using WBC count as a continuous variable. Apparently, the impact of WBC count at diagnosis on treatment outcome is dependent on the molecular genotype of AML blasts since the poor prognostic impact of high WBC count can be bypassed by intrinsic molecular abnormalities of the AML blasts, such as mutated NPM1. This is consistent with observations that NPMc+ AML blasts, independently of FLT3-ITD, have good response to chemotherapy in vivo and in vitro.19,31–33
Analysis of a large cohort of young adult AML patients by the MRC study-group showed that both mutations in NPM1 and FLT3-ITD are significant independent predictors of outcome, implying that the beneficial impact of NPMc+ on prognosis is also seen in patients with FLT3-ITD.19 Although the prognosis of patients with AML is better in the presence of NPMc+, various clinical studies have shown that only patients with the genotypic combination ‘NPMc+ without FLT3-ITD’ have a favorable outcome.9–13 Interestingly, our results show that the subgroup of patients with AML with the genotypic combination ‘NPMc+ with FLT3-ITD’ can be further divided into subgroups with favorable and unfavorable prognosis based on WBC count and FLT3-ITD/FLT3 ratio. It seems that the less favorable clinical course of patients with NPMc+ AML, imposed by the presence of FLT3-ITD, does not apply for those patients with WBC counts below 100×109/L and an FLT3-ITD/FLT3 ratio greater than 1. Indeed, the prognosis of patients with the genotypic combination ‘NPMc+ with FLT3-ITD’ and lower WBC counts and FLT3-ITD/FLT3 ratio greater than 1 appeared comparable to that of patients with the genotypic combination ‘NPMc+ without FLT3-ITD’.
A number of studies suggested that patients with AML harboring FLT3-ITD have a worse outcome than patients without FLT3-ITD.14–18 However, the number of mutated alleles, rather than its presence or the insertion site of the ITD, has been shown to affect outcome.17–19,34,35 Likewise, in our study we also found that patients with the genotypic combination ‘NPMc+ with FLT3-ITD’ and high levels of the mutant allele (i.e. an FLT3-ITD/FLT3 ratio >1) had significantly worse long-term outcome. It is likely that higher levels of FLT3-ITD might trigger pathways involved in chemoresistance more intensively, for example by enhancing DNA repair and salvage of damaged cells.36 Our observations confirm the importance of the FLT3-ITD/FLT3 ratio, representing the FLT3-ITD allelic burden, with regards to prognosis and demonstrate that WBC count is not a surrogate marker for the FLT3-ITD/FLT3 ratio, but indeed is an independent prognostic factor.
The importance of the WBC count and FLT3-ITD/FLT3 ratio as prognostic factors in AML patients with the genotypic combination ‘NPMc+ with FLT3-ITD’ needs to be confirmed in further cohorts of patients. However, for the future it will be of interest to study whether patients with the genotypic combination ‘NPMc+ with FLT3-ITD’ and low WBC count and an FLT3-ITD/FLT3 ratio less than 1 can be excluded from consolidation therapy with allogeneic hematopoietic cell transplantation. This would extend the work of Schlenk et al.,13 who demonstrated that patients with cytogenetically normal karyotype AML bearing the genotypic combination ‘NPMc+ without FLT3-ITD’ had no survival benefit from allogeneic hematopoietic cell transplantation, with an overall survival of 50–60%.
In conclusion, the present study demonstrates that high WBC count and FLT3-ITD/FLT3 ratio are important prognostic factors in patients with AML with the genotypic combination ‘NPMc+ with FLT3-ITD'.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 3.Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26(29):4791–7. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 6.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–83. [PubMed] [Google Scholar]
- 7.Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107(1):69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 8.Dohner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 9.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 10.Schnittger S, Schoch C, Kern W, Mecucci C, Tschulik C, Martelli MF, et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106(12):3733–9. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- 11.Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML) Blood. 2006;107(10):4011–20. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 12.Verhaak RG, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W, et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood. 2005;106(12):3747–54. doi: 10.1182/blood-2005-05-2168. [DOI] [PubMed] [Google Scholar]
- 13.Schlenk RF, Dohner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 14.Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93(9):3074–80. [PubMed] [Google Scholar]
- 15.Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 16.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 17.Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 18.Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a Cancer and Leukemia Group B study. Cancer Res. 2001;61(19):7233–9. [PubMed] [Google Scholar]
- 19.Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–84. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 20.Dutcher JP, Schiffer CA, Wiernik PH. Hyperleukocytosis in adult acute nonlymphocytic leukemia: impact on remission rate and duration, and survival. J Clin Oncol. 1987;5(9):1364–72. doi: 10.1200/JCO.1987.5.9.1364. [DOI] [PubMed] [Google Scholar]
- 21.Hug V, Keating M, McCredie K, Hester J, Bodey GP, Freireich EJ. Clinical course and response to treatment of patients with acute myelogenous leukemia presenting with a high leukocyte count. Cancer. 1983;52(5):773–9. doi: 10.1002/1097-0142(19830901)52:5<773::aid-cncr2820520503>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Rees JK, Gray RG, Swirsky D, Hayhoe FG. Principal results of the Medical Research Council's 8th acute myeloid leukaemia trial. Lancet. 1986;2(8518):1236–41. doi: 10.1016/s0140-6736(86)92674-7. [DOI] [PubMed] [Google Scholar]
- 23.Estey E, Smith TL, Keating MJ, McCredie KB, Gehan EA, Freireich EJ. Prediction of survival during induction therapy in patients with newly diagnosed acute myeloblastic leukemia. Leukemia. 1989;3(4):257–63. [PubMed] [Google Scholar]
- 24.Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the randomized MRC trial. Blood. 1999;93(12):4131–43. [PubMed] [Google Scholar]
- 25.Nguyen S, Leblanc T, Fenaux P, Witz F, Blaise D, Pigneux A, et al. A white blood cell index as the main prognostic factor in t(8;21) acute myeloid leukemia (AML): a survey of 161 cases from the French AML Intergroup. Blood. 2002;99(10):3517–23. doi: 10.1182/blood.v99.10.3517. [DOI] [PubMed] [Google Scholar]
- 26.Lowenberg B, Boogaerts MA, Daenen SM, Verhoef GE, Hagenbeek A, Vellenga E, et al. Value of different modalities of granulocyte-macrophage colony-stimulating factor applied during or after induction therapy of acute myeloid leukemia. J Clin Oncol. 1997;15(12):3496–506. doi: 10.1200/JCO.1997.15.12.3496. [DOI] [PubMed] [Google Scholar]
- 27.Lowenberg B, van Putten W, Theobald M, Gmür J, Verdonck L, Sonneveld P, et al. Effect of priming with granulocyte colonystimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349(8):743–52. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- 28.Lowenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–48. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 29.Ossenkoppele GJ, Graveland WJ, Sonneveld P, Daenen SM, Biesma DH, Verdonck LF, et al. The value of fludarabine in addition to ARA-C and G-CSF in the treatment of patients with high-risk myelodysplastic syndromes and AML in elderly patients. Blood. 2004;103(8):2908–13. doi: 10.1182/blood-2003-07-2195. [DOI] [PubMed] [Google Scholar]
- 30.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–8. [PubMed] [Google Scholar]
- 31.Cilloni D, Messa F, Rosso V, Arruga F, Defilippi I, Carturan S, et al. Increase sensitivity to chemotherapeutical agents and cytoplasmatic interaction between NPM leukemic mutant and NF-kappaB in AML carrying NPM1 mutations. Leukemia. 2008;22(6):1234–40. doi: 10.1038/leu.2008.68. [DOI] [PubMed] [Google Scholar]
- 32.Falini B, Nicoletti I, Martelli MF, Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109(3):874–85. doi: 10.1182/blood-2006-07-012252. [DOI] [PubMed] [Google Scholar]
- 33.Schneider F, Hoster E, Unterhalt M, Schneider S, Dufour A, Benthaus T, et al. NPM1 but not FLT3-ITD mutations predict early blast cell clearance and CR rate in patients with normal karyotype AML (NK-AML) or high-risk myelodysplastic syndrome (MDS) Blood. 2009;113(21):5250–3. doi: 10.1182/blood-2008-09-172668. [DOI] [PubMed] [Google Scholar]
- 34.Breitenbuecher F, Schnittger S, Grundler R, Markova B, Carius B, Brecht A, et al. Identification of a novel type of ITD mutations located in nonjuxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood. 2009;113(17):4074–7. doi: 10.1182/blood-2007-11-125476. [DOI] [PubMed] [Google Scholar]
- 35.Kayser S, Schlenk RF, Londono MC, Breitenbuecher F, Wittke K, Du J, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114(12):2386–92. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]
- 36.Seedhouse CH, Hunter HM, Lloyd-Lewis B, Massip AM, Pallis M, Carter GI, et al. DNA repair contributes to the drug-resistant phenotype of primary acute myeloid leukaemia cells with FLT3 internal tandem duplications and is reversed by the FLT3 inhibitor PKC412. Leukemia. 2006;20(12):2130–6. doi: 10.1038/sj.leu.2404439. [DOI] [PubMed] [Google Scholar]