Abstract
Background
An alternative reduced-toxicity conditioning regimen for allogeneic transplantation, based on treosulfan and fludarabine, has recently been identified. The rationale for this study was to investigate the efficacy and safety of this regimen prospectively in patients with a primary myelodysplastic syndrome.
Design and Methods
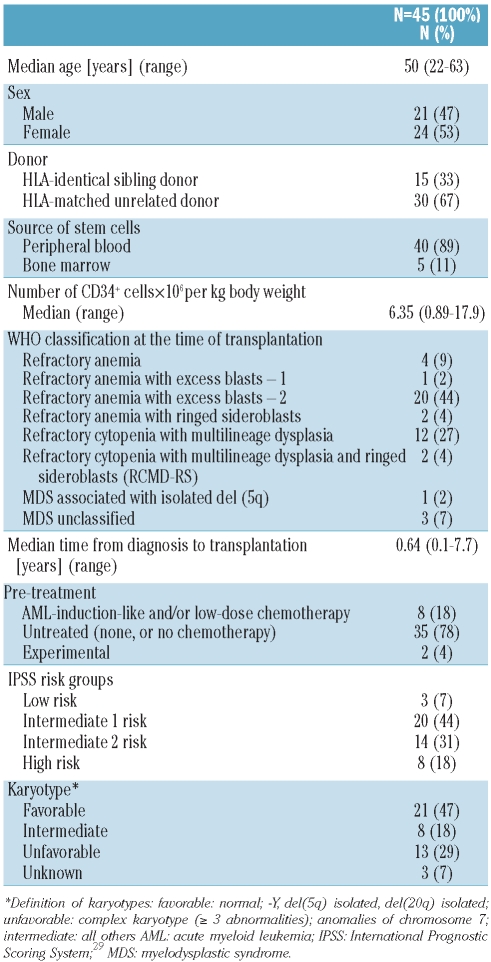
A total of 45 patients with primary myelodysplastic syndromes were conditioned with 3×14 g/m2 treosulfan and 5×30 mg/m2 fludarabine followed by allogeneic hematopoietic stem cell transplantation. Subtypes of myelodysplastic syndromes were refractory anemia with excess blasts-2 (44%), refractory cytopenia with multilineage dysplasia (27%), refractory anemia (9%), refractory anemia with ringed sideroblasts (4%), refractory cytopenia with multilineage dysplasia and ringed sideroblasts (4%), refractory anemia with excess blasts-1 (2%), and myelodysplastic syndrome with isolated del (5q) (2%). The myelodysplastic syndrome was unclassified in 7% of the patients. Forty-seven percent of the patients had a favorable karyotype, 29% an unfavorable one, and 18% an intermediate karyotype. Patients were evaluated for engraftment, adverse events, graft-versus-host disease, non-relapse mortality, relapse incidence, overall survival and disease-free survival.
Results
All but one patient showed primary engraftment of neutrophils after a median of 17 days. Non-hematologic adverse events of grade III–IV in severity included mainly infections and gastrointestinal symptoms (80% and 22% of the patients, respectively). Acute graft-versus-host disease grade II–IV developed in 24%, and extensive chronic graft-versus-host disease in 28% of the patients. After a median follow-up of 780 days, the 2-year overall and disease-free survival estimates were 71% and 67%, respectively. The 2-year cumulative incidences of non-relapse mortality and relapse were 17% and 16%, respectively.
Conclusions
Our safety and efficacy data suggest that treosulfan-based conditioning therapy is a promising treatment option for patients with myelodysplastic syndromes. clinicaltrials.gov identifier: NCT01062490
Keywords: reduced-toxicity conditioning, treosulfan, fludarabine, hematopoietic stem cell transplantation, myelodysplastic syndrome
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative approach for patients with a myelodysplastic syndrome (MDS). The incidence of MDS is age-dependent and the vast majority of MDS patients are older than 50 years. For many years, only a small minority of MDS patients qualified for allogeneic HSCT due to procedure-related toxicity after standard total body irradiation or busulfan-based conditioning, associated particularly with increasing age of the patients. During the past years the number of MDS patients who undergo allogeneic HSCT has increased considerably,1 which might reflect substantial advances in supportive care, but is in particular connected to the development of reduced intensity conditioning (RIC) regimens. Evaluating the benefit of a particular conditioning regimen in MDS is, however, complicated by the diversity of regimens in use as well as inclusion of populations of patients with heterogeneous disease characteristics, such as primary and therapy-related MDS or secondary acute myeloid leukemia (AML) arising from MDS, and different prognostic profiles of the patients. Moreover, most of the published studies on conditioning regimens for allogeneic HSCT in MDS patients are retrospective in nature.2 RIC regimens mainly aim at inducing sufficient immunosuppression to enable engraftment, and rely largely on the graft-versus-malignancy effect for the cure of the patient. Most RIC protocols include fludarabine in combination with reduced doses of either an alkylating agent (e.g., busulfan) or total body irradiation.2 Compared to standard-dose conditioning, the potential, as yet unproven, benefit of RIC relies on the reduction of acute regimen-related non-hematologic toxicity and its inherent mortality. With a few exceptions,3 a major obstacle to successful transplantation after RIC is the higher risk of relapse compared with that after standard conditioning regimens.2,4–8
Treosulfan is a bifunctional alkylating prodrug with proven myelotoxic and immunosuppressive properties and demonstrated strong activity against hematopoietic stem cells.9–11 Treosulfan-based conditioning regimens have recently been shown to have a favorable safety profile and to enable fast and sustained engraftment.12–16 Low transplantation-related morbidity and sustained engraftment have also been reported in children, even in those with non-malignant diseases and at high risk of both regimen-related toxicity and graft failure.17–21 A dose-escalation study in adult patients with a variety of hematologic malignancies qualifying for allogeneic HSCT but with a substantial risk of regimen-related toxicity revealed that a treosulfan dose of 3×14 g/m2 in combination with fludarabine 5×30 mg/m2 was a safe and effective conditioning regimen.22 Based on the available data on low toxicity in combination with myeloablative properties, we consider reduced-toxicity conditioning an appropriate term to characterize this regimen. The current prospective phase II study was designed to investigate the safety and efficacy of this regimen in primary MDS patients with an indication for allogeneic HSCT. The final results of the study are reported after all surviving patients have been observed for at least 1 year after transplantation of the last patient included in the study.
Design and Methods
Patients’ eligibility
Between November 2004 and July 2007, 45 MDS patients were enrolled in this prospective non-randomized phase II study at 11 study centers in four European countries. Written informed consent to all aspects of the study was obtained from all patients before enrollment. The study was approved by the appropriate independent ethics committees and competent authorities and was performed in accordance with the Declaration of Helsinki.
Patients between 18–65 years of age with MDS according to the World Health Organization (WHO) 2001 classification and an indication for allogeneic HSCT according to institutional policy were included. Patients with therapy-related MDS or AML were not to be included. Detailed eligibility criteria are given in the Online Supplementary Appendix.
Outcome parameters were followed until 1 year after transplantation of the last patient included in the study.
Donors and grafts
Either HLA-identical siblings or matched unrelated donors were allowed (matching for eight out of eight antigens, Table 1). Serological typing was required for class I (HLA-A/-B) and molecular typing for class II (HLA-DRB1/-DQB1) antigens.
Table 1.
Patients’ demographic and disease characteristics.
Conditioning regimen and transplantation
All patients received intravenous (IV) fludarabine 30 mg/m2 from day −6 to day −2 (total dose: 150 mg/m2) and treosulfan (medac GmbH, Hamburg, Germany) 14 g/m2 IV on days −6, −5, and −4 (total dose: 42 g/m2) before transplantation. Allogeneic hematopoietic stem cells either from peripheral blood or from bone marrow were given on day 0 (Table 1).
Supportive care
Graft-versus-host disease (GvHD) prophylaxis consisted of cyclosporin (3 mg/kg/day IV) starting 1 day before transplantation in combination with a short-course methotrexate (15 mg/m2 IV on day +1 and 10 mg/m2 IV on days +3 and +6). If an unrelated donor was used, anti-T-cell globulin (ATG-Fresenius (S)®, Fresenius Biotech, Graefelfing, Germany) was administered at a dose of 10 mg/kg IV from day −4 to day −2. Supportive care was given according to center-specific guidelines. The use of human recombinant granulocyte growth factors was not recommended unless clinically indicated.
Engraftment, graft failure and chimerism analysis
Engraftment and graft failure were defined as previously described.22 Chimerism analysis was performed in the total bone marrow according to established methods of the participating institutions.22
Complete chimerism was defined as 95% or more donor cells as quantified by dual color XY chromosome fluorescence in situ hybridization in opposite sex donor-recipient pairs or by variable number of tandem repeats analysis in sex concordant donor-recipient combinations.
Adverse events
Adverse events including serious adverse events were evaluated from the start of conditioning (day −6) to day +28. In addition, serious adverse events occurring after day +28 had to be reported, if at least a possible relation to the conditioning regimen was suspected. Changes in laboratory values were not included in the adverse event analysis, but were documented separately.
Apart from hepatic veno-occlusive disease, all adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 3.0). Hepatic veno-occlusive disease was evaluated according to standard criteria.23,24
Assessment of response/relapse
Treatment response and relapse were evaluated according to standard criteria.25
Graft-versus-host disease
GvHD was diagnosed and graded according to standard criteria.26–28 Depending on whether the GvHD developed before or after day +100, it was classified as acute or chronic.
Sample size and statistical considerations
The primary aim of this study was to evaluate the efficacy and safety profile of a treosulfan-based conditioning regimen. With a sample size of 45 patients, an expected neutrophil engraftment rate of 95% could be estimated with a precision of ± 14 percentage points with a power of 80% using a two-sided exact Clopper-Pearson 95% confidence interval. In addition, any toxicity occurring with a probability of at least 5% had a 90% chance of being seen at least once, whereas any toxicity occurring with a probability of 3.5% had an 80% chance of being seen at least once. Time to engraftment was calculated from day 0 by means of conditional cumulative incidence curves. Day +28 rates were extracted from these curves. Estimates of chimerism, non-relapse mortality, relapse incidence, and acute and chronic GvHD were derived using cumulative incidence rates to accommodate competing risks. Disease-free survival and overall survival were analyzed using product-limit (Kaplan-Meier) estimates. Incidences of selected grade III–IV adverse events reported in the setting of allogeneic HSCT were the primary combined end-point of the study. The incidence of adverse events was calculated as the percentage of patients who experienced at least one adverse event of a certain CTCAE category out of the total number of patients. For exploratory purposes, efficacy data were stratified by type of donor (HLA-identical sibling versus matched unrelated donor), status prior to the conditioning regimen (treated versus untreated), and International Prognostic Scoring System (IPSS) risk score.29 For the comparison of cumulative incidence curves, Gray’s test was applied, whereas log-rank tests were used for the comparison of product-limit estimates. All P-values were derived from two-sided tests. The statistical analyses were performed using SAS software package version 9.1.3 (SAS Institute, Cary, NC, USA) and R version 2.2.1 (The R Foundation for Statistical Computing).
Results
Demographics
A total of 45 MDS patients were included in the study and treated in line with the study protocol. A single patient with AML was erroneously enrolled, but was excluded from the study analysis and was replaced by an additional patient fulfilling the inclusion criteria. Table 1 summarizes the characteristics of the patients and their diseases. Only two patients had significant co-morbidities as assessed by the treating physician.
Engraftment and chimerism
CTCAE grade IV neutropenia, leukocytopenia and thrombocytopenia occurred in virtually all patients. The 28-day conditional cumulative incidences of neutrophil, total white blood cell and platelet recovery reached 96% (95% CI: 85%–100%), 96% (95% CI: 87%–100%), and 87% (95% CI: 76%–98%), respectively. The median time to recovery was 16 days (range, 10–33 days) for white blood cells, 16 days (range, 6–71 days) for platelets and 17 days (range, 10–35 days) for neutrophils. The cumulative incidences of complete donor type chimerism increased from 78% on day +28 to 93% on days +56 and +100. One patient experienced primary graft failure as documented on day +28. Secondary failure of engraftment (pancytopenia) was reported in another patient. Results of engraftment and chimerism analyses are detailed in Online Supplementary Table S1.
Adverse events
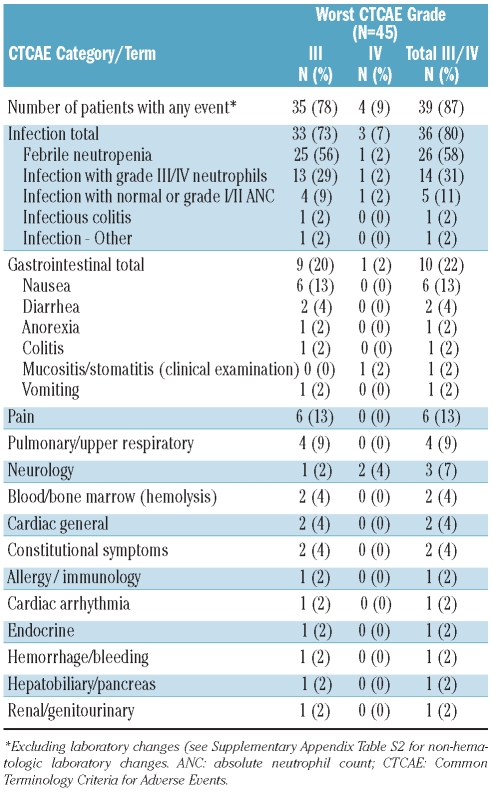
Within the observation period (day −6 to day +28), 39 of 45 patients (87%) experienced at least one episode of grade III–IV adverse events (Table 2). The most frequently reported CTCAE categories were infection (80%) and gastrointestinal events (22%). Infections included grade III–IV febrile neutropenia (58%) and infection either with (31%) or without (11%) neutropenia. Gastrointestinal adverse events were mostly of grade III in severity and included nausea (13%) and diarrhea (4%). Only one patient (2%) experienced grade IV mucositis. All other reported grade III–IV adverse events occurred sporadically.
Table 2.
Frequency of all CTCAE grade III and IV adverse events between start of conditioning and day +28.
The incidences of selected grade III–IV adverse events reported in the setting of allogeneic HSCT were the primary combined end-point of the study. The incidences of these events were 13% (95% CI: 5%–27%) for hyper-bilirubinemia, 2% (95% CI: 0%–12%) for mucositis/stomatitis, and 0% (95% CI: 0%–8%) for seizures. Two patients (4%; 95% CI: 1%–15%) experienced moderate (grade II) veno-occlusive disease, which had resolved by day +20. Changes in laboratory values were within expected ranges (Online Supplementary Table S2).
Beyond day +28 after transplantation, three serious adverse events assessed as at least possibly related to the conditioning regimen were reported. These were secondary graft failure (starting on day +29), reversible grade III cutaneous ulcerations on both legs secondary to skin necrosis (starting on day +29), and breast cancer (starting on day +685).
Non-relapse mortality
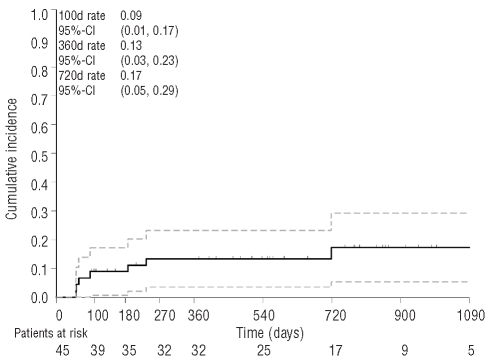
All patients survived the initial 28-day assessment period after transplantation. Thereafter, seven patients (16%) died from transplant-related causes. The main causes of death were infections in two patients and GvHD (acute in one patient, chronic in another). One patient experienced primary graft failure. Eight days later, she developed Epstein-Barr virus lymphoproliferative disease leading to fatal multiorgan failure. Another patient experienced secondary graft failure and developed pneumonia complicated by fatal multiorgan failure. One additional patient died on day +52 of multiorgan failure not further specified. The 100-, 360- and 720-day cumulative incidences of non-relapse mortality were 9%, 13%, and 17%, respectively (Figure 1). Non-relapse mortality was similar among patients transplanted from sibling or. unrelated donors, among patients with IPSS intermediate-1, -2 or high risk disease, and among those with untreated or chemotherapy-pretreated MDS (Online Supplementary Table S3).
Figure 1.
Cumulative incidence of non-relapse mortality and 95% confidence limits.
Relapse incidence
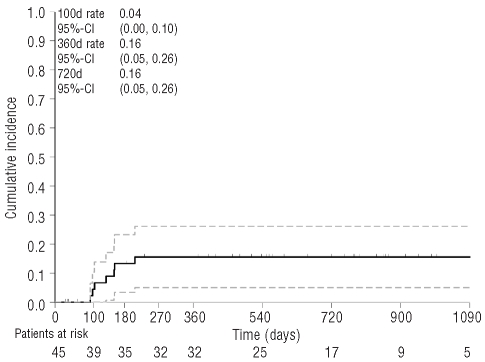
Seven patients relapsed during follow-up, resulting in a cumulative incidence of relapse of 4% at 100 days, and 16% at 360 and 720 days (Figure 2). Five of these patients died of relapse. The 2-year cumulative incidence of relapse was 20% in patients with sibling donors compared to 13% in patients with unrelated donors (P=0.5665, Gray’s test), 10% in patients with intermediate-1 and 13% in patients with high IPSS compared to 29% in patients with intermediate-2 IPSS (P=0.3512, Gray’s test). Patients with previous chemotherapy and untreated patients had relapse incidences of 25% and 14%, respectively (P=0.4956, Gray’s test, Online Supplementary Table S4). An additional analysis stratified by cytogenetic risk revealed comparable 2-year cumulative incidences of relapse for patients with favorable (14%), intermediate (13%), and unfavorable (15%) risk (P=0.9748, Gray’s test).
Figure 2.
Cumulative incidence of relapse and 95% confidence limits.
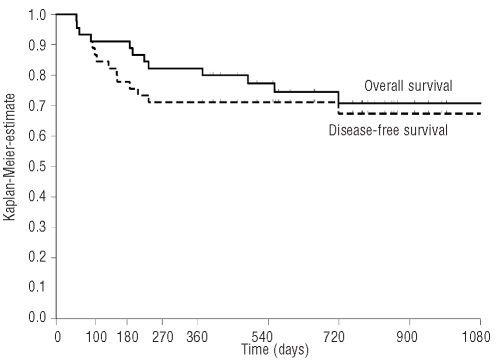
Disease-free survival
The probabilities of disease-free survival were 87% at 100 days, 71% at 360 days, and 67% at 720 days (Figure 3). Patients with sibling donors had a 2-year probability of disease-free survival of 57% compared to 73% of the patients with unrelated donor. However, this difference was not statistically significant (P=0.4684, log-rank test). Comparable disease-free survival probabilities at 2 years were observed for patients within the IPSS groups inter-mediate-1 (70%), intermediate-2 (63%) and high risk (63%), and for previously treated and previously untreated patients (75% versus 67%, respectively; Online Supplementary Table S5). An additional analysis stratified by cytogenetic risk revealed 2-year disease-free probabilities of 88% (95% CI: 66%–100%) for patients with intermediate risk compared to 67% (95% CI: 47%–87%) for patients with favorable risk, and 55% (95% CI: 25%–85%) for patients with unfavorable risk (P=0.4561, log-rank test).
Figure 3.
Kaplan-Meier estimates for disease-free and overall survival.
Survival
After a median follow-up period of 780 days (range of those surviving, 372–1260 days), 33 patients (73%) were alive, and 12 (27%) had died. The overall survival estimates were 91% (95% CI: 83%–99%) at 100 days, 82% (95% CI: 71%–93%) at 360 days, and 71% (95% CI: 56%–85%) at 720 days (Figure 3). A 2-year overall survival rate of 63% was observed for patients with sibling donors compared to 75% for patients with unrelated donors (P=0.5163, log-rank test). In addition, no significant differences were observed among patients within the IPSS groups intermediate-1 (70%), intermediate-2 (68%) and high risk (75%), and for patients previously treated with chemotherapy (80%) compared to untreated patients (70%; Online Supplementary Table S6).
Graft-versus-host disease
The day 100 cumulative incidences of grade I–IV, II–IV, and III–IV acute GvHD were 56%, 24%, and 16%, respectively (Online Supplementary Figure S1). The cumulative incidence of chronic GvHD at 720 days was 59%, while the cumulative incidence of extensive chronic GvHD reached 28% (Online Supplementary Figure S2).
Discussion
The aim of developing a conditioning regimen consisting of treosulfan and fludarabine was to reduce the incidence and severity of non-hematologic acute toxicities frequently observed in allogeneic HSCT recipients after standard conditioning therapy, without compromising the antineoplastic effect. Analyzing the present study results in the context of published data, it is of note that most previous studies included not only patients with primary MDS, but also patients with secondary AML or treatment-related MDS,30 which may substantially affect study results. As anticipated from previous studies with treosulfan-based conditioning, the acute non-hematologic toxicity of the regimen was low.12,15,16,22,31 Grade III–IV hyper-bilirubinemia was noted in 13% of the patients, and only two cases (4%) of moderate veno-occlusive disease were observed. Similarly, the incidence of severe mucositis appears very low when compared to that usually described for standard conditioning regimens. As can be expected for a standard conditioning regimen, severe infections were common as these are primarily connected to the severity and duration of marrow aplasia. Twenty-two percent of the patients had grade III–IV gastrointestinal symptoms. All other CTCAE grade III–IV toxicities occurred with low frequencies. The incidences and severities of acute and chronic GvHD were in the range of those commonly reported for allogeneic HSCT using standard conditioning regimens.5,8,30,32 Our GvHD prophylaxis regimen contained three doses of methotrexate. Using the other common version with four doses might have somewhat influenced the incidence of GvHD, but, on the other hand, also that of engraftment and possibly the occurrence of adverse effects.
The resulting non-relapse mortality rates of 9% at 100 days and 17% at 720 days appear similar to those seen after RIC transplantation5,7,8,30,33 and lower than those reported after standard conditioning in MDS patients, in whom 100-day rates between 19% and 27% have been observed.5,30,34,35
The prognosis of MDS depends strongly on the subtype of the disease as well as on other prognostic factors.2,36 In general, outcome is considered to be more favorable in patients with refractory anemia without excess blasts than in those with an excess of blasts.2,36–39 Certain cytogenetic abnormalities and cytopenia in more than one cell line also indicate an adverse prognosis. The IPSS for the estimation of the likelihood of leukemic transformation and survival includes these variables.29 Our exploratory subgroup analysis revealed no statistically significant influence of the type of donor (sibling or unrelated), IPSS, or previous MDS treatment on relapse incidence, non-relapse mortality, disease-free survival or overall survival. These results should, however, be interpreted cautiously due to the small number of patients in each subgroup. Recent results of a larger prospective randomized transplantation study showed no prognostic influence of IPSS or blast count on survival while advanced age, number of cytopenias and intermediate to high-risk cytogenetic characteristics were adverse prognostic factors.40
A low and encouraging 2-year relapse incidence of only 16% was observed in this study, comparing favorably with incidences in previously published series.5,33,40 Thus far, relapses have occurred only within the first 220 days after transplantation and no relapses have been reported beyond this time. Rare late disease recurrences were noted by Nemecek et al. who used the treosulfan-based conditioning regimen in a prospective allogeneic HSCT protocol, which included several disease entities.31
The 2-year estimates of overall and disease-free survival were 71% and 67%, respectively. Approximately one half of the deaths in this study were caused by relapse. These figures appear superior to those published for the results of allogeneic HSCT using standard conditioning regimens,5,34,35 and compare favorably with those of some studies using RIC. Ho et al., using fludarabine-busulfan conditioning with busulfan 8 mg/kg, reported 1-year overall and disease-free survival rates of 74% and 62%, respectively. However, a considerable proportion of the patients required donor lymphocyte infusions to treat declining donor chimerism.33 In contrast, Alyea et al., using the same conditioning regimen, reported substantially inferior overall and progression-free survival estimates of 39% and 27% at 2 years after transplantation.7 Similar results were observed by Shimoni et al. after RIC with fludarabine and intravenous busulfan. Two-year overall and disease-free survival estimates of 47(49)% and 43(49)%, respectively, were reported. However, that study also included patients with AML, and all MDS patients had an excess of blasts.8 In older AML/MDS patients (≥ 55 years) similar 2-year overall and event-free survival rates of 46% and 44% were reported after RIC with fludarabine and intravenous busulfan, and the 1-year transplant-related mortality rate was 19–20%.41
In preclinical9,11,42 and clinical12,16,22,31,43,44 studies, treosulfan demonstrated strong cytotoxic activity on hematopoietic cells, while non-hematologic toxicity was generally mild. The results of the present study are in good accordance with those reports. Given the prompt engraftment, rapid achievement of full donor hematopoietic chimerism, as well as a comparatively low relapse incidence, the treosulfan/fludarabine conditioning regimen has clearly myeloablative properties, and the term reduced-toxicity conditioning appears most appropriate for this regimen.
In the light of the favorable outcome results with the regimen evaluated in the present study, further investigation of treosulfan and fludarabine conditioning therapy is warranted. A randomized, international phase III study comparing the treosulfan/fludarabine regimen with a conditioning regimen consisting of reduced-dose busulfan and fludarabine in a large cohort of AML and MDS patients stratified by indications is currently under way.
Acknowledgments
The authors would like to thank the nursing staff and all patients who participated in this trial.
Footnotes
Funding: this work was supported by medac GmbH, Hamburg, Germany.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Gratwohl A, Baldomero H, Frauendorfer K, Urbano-Ispizua A. EBMT activity survey 2004 and changes in disease indication over the past 15 years. Bone Marrow Transplant. 2006;37(12):1069–85. doi: 10.1038/sj.bmt.1705377. [DOI] [PubMed] [Google Scholar]
- 2.Barrett AJ, Savani BN. Allogeneic stem cell transplantation for myelodysplastic syndrome. Semin Hematol. 2008;45(1):49–59. doi: 10.1053/j.seminhematol.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Martino R, Valcarcel D, Brunet S, Sureda A, Sierra J. Comparable non-relapse mortality and survival after HLA-identical sibling blood stem cell transplantation with reduced or conventional-intensity preparative regimens for high-risk myelodysplasia or acute myeloid leukemia in first remission. Bone Marrow Transplant. 2008;41(1):33–8. doi: 10.1038/sj.bmt.1705879. [DOI] [PubMed] [Google Scholar]
- 4.Marcondes M, Deeg HJ. Hematopoietic cell transplantation for patients with myelodysplastic syndromes (MDS): when, how and for whom? Best Pract Res Clin Haematol. 2008;21(1):67–77. doi: 10.1016/j.beha.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108(3):836–46. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 6.de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104(3):865–72. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 7.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105(4):1810–4. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 8.Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20(2):322–8. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 9.Van Pel M, van Breugel DW, Vos W, Ploemacher RE, Boog CJ. Towards a myeloablative regimen with clinical potential: I. Treosulfan conditioning and bone marrow transplantation allow induction of donor-specific tolerance for skin grafts across full MHC barriers. Bone Marrow Transplant. 2003;32(1):15–22. doi: 10.1038/sj.bmt.1704094. [DOI] [PubMed] [Google Scholar]
- 10.van Pel M, van Breugel DWJG, Vos W, Ploemacher RE, Boog CJ. Towards a myeloablative regimen with clinical potential: II. Treosulfan induces specific skin graft tolerance across haploidentical MHC barriers. Bone Marrow Transplant. 2004;33(2):153–9. doi: 10.1038/sj.bmt.1704333. [DOI] [PubMed] [Google Scholar]
- 11.Ploemacher RE, Johnson KW, Rombouts EJC, Etienne K, Westerhof GR, Baumgart J, et al. Addition of treosulfan to a nonmyeloablative conditioning regimen results in enhanced chimerism and immunologic tolerance in an experimental allogeneic bone marrow transplant model. Biol Blood Marrow Transplant. 2004;10:236–54. doi: 10.1016/j.bbmt.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Casper J, Knauf W, Kiefer T, Wolff D, Steiner B, Hammer U, et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood. 2004;103(2):725–31. doi: 10.1182/blood-2002-11-3615. [DOI] [PubMed] [Google Scholar]
- 13.Beelen DW, Trenschel R, Casper J, Freund M, Hilger RA, Scheulen ME, et al. Dose-escalated treosulfan in combination with cyclophosphamide as a new preparative regimen for allogeneic haematopoietic stem cell transplantation in patients with an increased risk for regimen related complications. Bone Marrow Transplant. 2005;35(3):233–41. doi: 10.1038/sj.bmt.1704784. [DOI] [PubMed] [Google Scholar]
- 14.Kröger N, Shimoni A, Zabelina T, Schieder H, Panse J, Ayuk F, et al. Reduced-toxicity conditioning with treosulfan, fludarabine and ATG as a preparative regimen for allogeneic stem cell transplantation (alloSCT) in elderly patients with secondary acute myeloid leukemia (sAML) or myelodysplastic syndrome (MDS) Bone Marrow Transplant. 2006;37(4):339–44. doi: 10.1038/sj.bmt.1705259. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Hieber M, Blau IW, Trenschel R, Andreesen R, Stuhler G, Einsele H, et al. Reduced-toxicity conditioning with fludarabine and treosulfan prior to allogeneic stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2007;39(7):389–96. doi: 10.1038/sj.bmt.1705605. [DOI] [PubMed] [Google Scholar]
- 16.Holowiecki J, Giebel S, Wojnar J, Krawczyk-Kulis M, Markiewicz M, Holowiecka-Goral A, et al. Treosulfan and fludarabine low-toxicity conditioning for allogeneic haematopoietic stem cell transplantation in chronic myeloid leukaemia. Br J Haematol. 2008;142(2):284–92. doi: 10.1111/j.1365-2141.2008.07179.x. [DOI] [PubMed] [Google Scholar]
- 17.Bernardo ME, Zecca M, Piras E, Vacca A, Giorgiani G, Cugno C, et al. Treosulfan-based conditioning regimen for allogeneic haematopoietic stem cell transplantation in patients with thalassaemia major. Br J Haematol. 2008;143(4):548–51. doi: 10.1111/j.1365-2141.2008.07385.x. [DOI] [PubMed] [Google Scholar]
- 18.Cutting R, Mirelman A, Vora A. Treosulphan as an alternative to busulphan for myeloablative conditioning in paediatric allogeneic transplantation. Br J Haematol. 2008;143(5):748–51. doi: 10.1111/j.1365-2141.2008.07399.x. [DOI] [PubMed] [Google Scholar]
- 19.Greystoke B, Bonanomi S, Carr TF, Gharib M, Khalid T, Coussons M, et al. Treosulfan-containing regimens achieve high rates of engraftment associated with low transplant morbidity and mortality in children with non-malignant diseases and significant co-morbidities. Br J Haematol. 2008;142(2):257–62. doi: 10.1111/j.1365-2141.2008.07064.x. [DOI] [PubMed] [Google Scholar]
- 20.Slatter MA, Rao K, Amrolia P, Flood T, Abinun M, et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency (PID): UK experience. Blood. 2011;117:4367–75. doi: 10.1182/blood-2010-10-312082. [DOI] [PubMed] [Google Scholar]
- 21.Wachowiak J, Sykora K-W, Cornish J, Chybicka A, Kowalczyk JR, et al. Treosulfan-based preparative regimens for allo-HSCT in childhood hematological malignancies: a retrospective study on behalf of the EBMT pediatric diseases working party. Bone Marrow Transplant. 2011 Feb 07; doi: 10.1038/bmt.2010.343.. Epub. [DOI] [PubMed] [Google Scholar]
- 22.Casper J, Wolff D, Knauf W, Blau IW, Ruutu T, Volin L, et al. Allogeneic hematopoietic stem cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol. 2010;28(20):3344–51. doi: 10.1200/JCO.2009.23.3429. Erratum in: J Clin Oncol 2010;28(23):3797. [DOI] [PubMed] [Google Scholar]
- 23.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–83. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 24.McDonald GB, Hinds MS, Fisher LBG, Schoch HG, Wolford JL, Banaji M, et al. Venoocclusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–67. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. World Health Organization (WHO) International Working Group. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–4. [PubMed] [Google Scholar]
- 26.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestation of graft versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 28.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GvHD grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 29.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 30.Kindwall-Keller T, Isola LM. The evolution of hematopoietic SCT in myelodysplastic syndrome. Bone Marrow Transplant. 2009;43(8):597–609. doi: 10.1038/bmt.2009.28. [DOI] [PubMed] [Google Scholar]
- 31.Nemecek ER, Guthrie KA, Sorror ML, Wood BL, Doney KC, Hilger RA, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(3):341–50. doi: 10.1016/j.bbmt.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura R, Rodriguez R, Palmer J, Stein A, Naing A, Tsai N, et al. Reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation with fludarabine and melphalan is associated with durable disease control in myelodysplastic syndrome. Bone Marrow Transplant. 2007;40(9):843–50. doi: 10.1038/sj.bmt.1705801. [DOI] [PubMed] [Google Scholar]
- 33.Ho AY, Pagliuca A, Kenyon M, Parker JE, Mijovic A, Devereux S, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood. 2004;104(6):1616–23. doi: 10.1182/blood-2003-12-4207. [DOI] [PubMed] [Google Scholar]
- 34.Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23(15):3439–46. doi: 10.1200/JCO.2005.05.694. [DOI] [PubMed] [Google Scholar]
- 35.Scott BL, Sandmaier BM, Storer B, Maris MB, Sorror ML, Maloney DG, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20(1):128–35. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 36.Scott B, Deeg HJ. Hematopoietic cell transplantation as curative therapy of myelodysplastic syndromes and myeloproliferative disorders. Best Pract Res Clin Haematol. 2006;19(3):519–33. doi: 10.1016/j.beha.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA, et al. Conditioning with targeted busulfan and cyclophosphamide for hematopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100(4):1201–7. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JE, Appelbaum FR, Schoch G, Gooley T, Anasetti C, Bensinger WI, et al. Allogeneic marrow transplantation for refractory anemia: a comparison of two preparatory regimens and analysis of prognostic factors. Blood. 1996;87(1):51–8. [PubMed] [Google Scholar]
- 39.Appelbaum FR, Anderson J. Allogeneic bone marrow transplantation for myelodysplastic syndrome; outcomes analysis according to IPSS score. Leukemia. 1998;12(Suppl 1):S25–9. [PubMed] [Google Scholar]
- 40.de Witte T, Hagemeijer A, Suciu S, Belhabri A, Delforge M, Kobbe G, et al. Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European Intergroup Trial. Haematologica. 2010;95(10):1754–61. doi: 10.3324/haematol.2009.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplantation for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2011.02.007. Epub 2011 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjoo F, Hassan Z, Abedi-Valugerdi M, Griskevicius L, Nilsson C, Remberger M, et al. Myeloablative and immunosuppressive properties of treosulfan in mice. Exp Hematol. 2006;34(1):115–21. doi: 10.1016/j.exphem.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Shimoni A, Hardan I, Shem-Tov N, Rand A, Yerushalmi R, Nagler A. Fludarabine and treosulfan: a novel modified myeloablative regimen for allogeneic hematopoietic stem-cell transplantation with effective antileukemia activity in patients with acute myeloid leukemia and myelodysplastic syndromes. Leuk Lymphoma. 2007;48(12):2352–9. doi: 10.1080/10428190701671051. [DOI] [PubMed] [Google Scholar]
- 44.Holowiecki J, Giebel S, Beelen D, Trenschel R, Wandt H, Schaefer-Eckart K, et al. Clinical phase II trial to evaluate the safety and efficacy of low-toxicity treosulfan/fludarabine conditioning prior to allogeneic haematopoietic stem cell transplantation in patients with acute myeloid leukaemia. Blood. 2007;110(11) Abstr. 621. [Google Scholar]