Abstract
Background
Advances in acute graft-versus-host disease therapy are needed.
Design and Methods
We examined the efficacy of sirolimus as primary therapy for acute graft-versus-host disease in 32 patients.
Results
Acute graft-versus-host disease involved the skin in 53% of cases, gastrointestinal tract in 66%, liver in 16%. The syndrome was overall grade 1 in 12% cases, grade 2 in 75%, and grade 3 in 13%. Sirolimus was targeted to achieve serum trough levels of 5–14 ng/mL. Sixteen (50%) patients achieved sustained, complete resolution of acute graft-versus-host disease with sirolimus alone. In contrast, 19 of 32 (59%) matched historical controls treated with standard 1 mg/kg steroids achieved complete response (P=0.47). With median follow-up time for surviving patients of 16 (range 6–26) months, one year overall survival was 56% (95% CI 38–74%). The cumulative incidence of relapse at one year was 37% (95% CI 23–60%), and mortality in remission was 20% (95% CI 10–42%). The cumulative incidence of chronic graft-versus-host disease was 55% (95% CI 39–79%). Thrombotic microangiopathy occurred in 3 cases (grade 1 n=1; grade 2 n=2), and responded to dose reduction of calcineurin inhibitor.
Conclusions
In this retrospective series, sirolimus demonstrates activity comparable to that of high-dose glucocorticoids in the primary therapy of acute graft-versus-host disease. Confirmation of this activity requires prospective clinical trials.
Keywords: sirolimus, acute GVHD, glucocorticoids, primary therapy
Introduction
Prednisone at 1–2 mg/kg constitutes the present standard primary therapy for acute graft-versus-host disease (GVHD) following allogeneic hematopoietic cell transplantation (HCT). However, complete response to this therapy is achieved in 40–50% of cases.1–4 Patients with glucocorticoid-refractory acute GVHD have limited response to available rescue therapies and have inferior survival compared to those with glucocorticoid-responsive disease.5–7 In the therapy of acute and chronic GVHD, morbidity from glucocorticoid therapy is observed in HCT recipients.8–12 The limited efficacy and associated toxicity of glucocorticoid therapy provide the rationale for novel approaches in the therapy of acute GVHD.
Previous reports of combined therapy with glucocorticoids and additional immunosuppressive agents were disappointing.13,14 The combination of glucocorticoids and mycophenolate mofetil may offer the promise of improved GVHD control,15 but this additional immunosuppression may enhance the risk of opportunistic infection and primary malignancy relapse after HCT. These and historical efforts have been based on the assumption that glucocorticoids constitute a necessary component of primary therapy for acute GVHD.
In a previous report, we suggested that sirolimus is active as sole primary therapy of acute GVHD.16 This approach may allow the dual benefit of acute GVHD control and avoidance of systemic glucocorticoids. Given the antitumor activity of mTOR inhibitors demonstrated in related literature,17–20 as well as evidence that its use as prophylaxis is associated with decreased lymphoma relapse post transplant,21 sirolimus may be a particularly attractive therapy for acute GVHD in those HCT recipients with high risk for relapse after transplant. As further evidence of the activity of sirolimus in the primary therapy of acute GVHD, we report an expanded cohort of HCT recipients treated with sirolimus as primary therapy for acute GVHD with extended follow up.
Design and Methods
Through retrospective review, 32 HCT recipients were identified who were treated with sirolimus as primary therapy of acute GVHD; biopsy confirmation of acute GVHD was available in 31 of 32 cases. In older patients, primary glucocorticoid therapy was not given to avoid toxicity, and in patients with active malignancy, sirolimus was preferred for its dual activity as immunosuppressant and anti-cancer drug. All patients received tacrolimus in combination with methotrexate (MTX) or mycophenolate mofetil (MMF) as acute GVHD prophylaxis. The target serum level of tacrolimus was reduced to 3–7 ng/mL while receiving concomitant sirolimus to reduce the risk for thrombotic microangiopathy (TMA). Sirolimus was administered orally with a target serum level of 4–12 ng/mL by mass spectrometry or 5–14 ng/mL by the Architect assay (Abbott, Abbott Park, IL, USA). In the absence of ongoing acute GVHD, tacrolimus was tapered with empiric dose reductions.
Baseline characteristics were summarized with descriptive statistics. Established consensus criteria were utilized to score acute GVHD weekly from onset to sustained complete resolution or last follow up.19 Complete response (CR) to sirolimus as primary therapy was defined as sustained complete resolution of acute GVHD manifestations for at least four weeks without the addition of systemic glucocorticoids or other systemic immunosuppressive agents. The association between complete response to sirolimus alone and acute GVHD characteristics, including organ involvement and severity, was examined using logistic regression analysis. Chronic GVHD was scored according to the proposed National Institutes of Health (NIH) consensus criteria.20 Indication, initial dose, and duration of any glucocorticoid therapy was recorded and considered a failure of primary therapy with sirolimus. The cumulative incidence of disease relapse, non-relapse mortality, and cGVHD was estimated, accounting for competing risk.22 All outcomes were assessed from the initiation date of sirolimus. Overall and relapse free survival were calculated by the Kaplan-Meier method; primary disease relapse or death were considered events in the estimation of relapse free survival. Survival was estimated from date of sirolimus initiation. The incidence of cytomegalovirus (CMV) reactivation, and TMA are reported; TMA was scored according to proposed CTN consensus definitions.23 This study was approved as a retrospective review by the University of South Florida Institutional Review Board (IRB). While sirolimus was utilized as primary therapy of acute GVHD outside a prospective clinical trial, patients were adequately informed prior to therapy of the rationale for avoiding high-dose steroid therapy, of the potential risks associated with sirolimus, and of the monitoring required to achieve and sustain therapeutic sirolimus levels. Sirolimus was utilized in this setting with patients who had been adequately counseled on the anticipated risks, benefits and alternatives to this approach. All patients had signed an IRB-approved consent form indicating willingness to participate in long-term follow-up studies.
Results
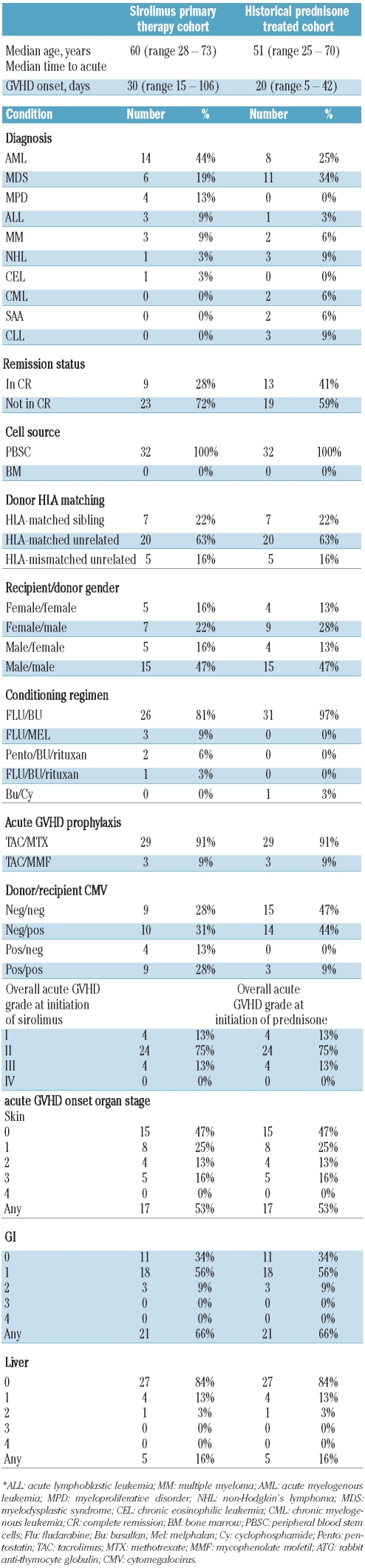
Baseline characteristics of this series are summarized in Table 1. The cohort is notable for advanced age (median 60 years, range 28–73 years), and for high-risk malignancy, as 23 (72%) were not in remission at the time of HCT. Primary acute GVHD prophylaxis consisted of either tacrolimus plus methotrexate (n=29) or tacrolimus plus mycophenolate mofetil (n=3). Four patients with an HLA mismatched donor received additional thymoglobulin 7.5 mg/kg ending on day −1. Patients were treated with sirolimus as the primary therapy for acute GVHD at a median of 30 (range 15–106) days after HCT. Sirolimus was administered orally as a median loading dose of 6 mg (range 2 to 9 mg), followed by maintenance dosing to sustain the desired target therapeutic levels. Therapeutic sirolimus levels were achieved in all cases, including patients with gastrointestinal involvement. When used in prophylaxis of acute GVHD, mycophenolate mofetil was continued with the initiation of sirolimus; however, tacrolimus was reduced to target a range of 3–7 ng/mL.
Table 1.
Baseline characteristics of study sample and historical control.
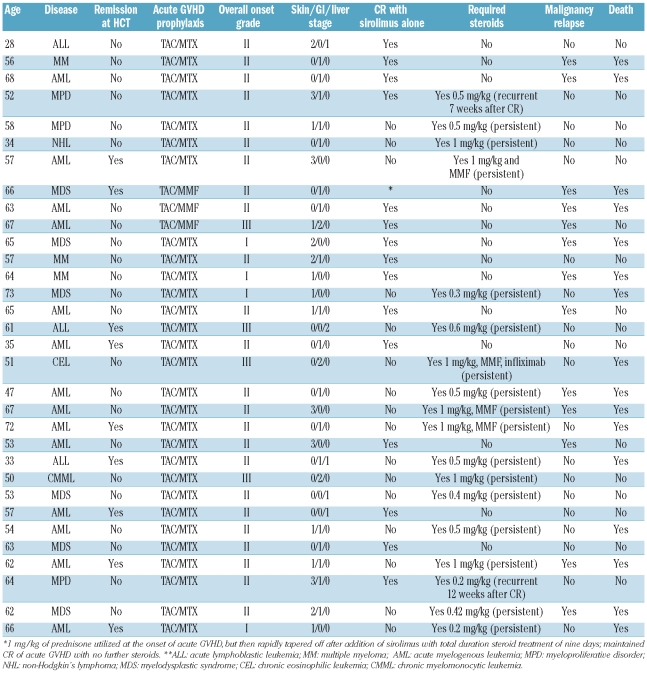
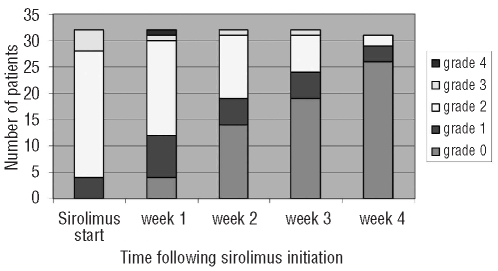
Sixteen (50%) patients achieved complete response of acute GVHD following primary therapy with sirolimus without the addition of systemic glucocorticoids or any other systemic immunosuppressive agents (Table 2). Among these 16 patients who achieved complete resolution of acute GVHD with sirolimus alone, initial overall response (composite of partial and complete response) was achieved at a median of seven days (range 5–21 days) and complete response was achieved by a median of 14 days (range 5–28 days). In 2 of these cases, recurrent acute GVHD developed 7–12 weeks after initial complete response; resolution of recurrent acute GVHD was achieved in both with the addition of 0.2–0.5 mg/kg body weight of prednisone. In the remaining 16 cases, systemic glucocorticoids were initiated at a median of nine days (range 2–28 days) after initiation of sirolimus with a prednisone-equivalent median dose of 0.5 mg/kg (range 0.2–1 mg/kg), and 12 achieved resolution of acute GVHD (Figure 1). Importantly, uniform criteria for initiation of systemic steroids after first-line sirolimus therapy were not employed. Among these cases, prednisone was started after initial sirolimus therapy for persistent acute GVHD manifestations of unchanged severity in 6 cases (median nine days from sirolimus initiation, range 2–19 days), grade progression in 6 cases (median nine days from sirolimus initiation, range 2–16 days), in the setting of partial response in 2 cases (6–7 days after sirolimus initiation), and in 2 cases for recurrent acute GVHD within four weeks after initial complete response to sirolimus. Four patients (12%) had persistent acute GVHD that was treated with mycophenolate mofetil. Of these 4 patients, one died following primary disease relapse, 2 died from non-relapse causes (sepsis, and refractory acute GVHD with sepsis), and one is alive following resolution of acute GVHD and without relapse. On logistic regression analysis, neither acute GVHD severity nor organ involvement were significantly associated with complete response to sirolimus.
Table 2.
Summary of individual patient outcomes.
Figure 1.
Weekly overall acute GVHD grade following initiation of sirolimus as primary therapy.
To further qualify our results, we performed a retrospective comparison of complete response rate following sirolimus primary therapy to complete response following standard primary glucocorticoid therapy in a historical matched cohort treated at our center. These control subjects all had biopsy-confirmed acute GVHD, and were identified by matching to cases at each of the following variables: acute GVHD organ involvement and severity, donor relation, HLA matching, stem cell source, and acute GVHD prophylaxis agents utilized. All matched control subjects were treated with 1 mg/kg of prednisone as primary therapy. Nineteen of these 32 subjects (59%) achieved complete remission, as compared to 16 of 32 (50%) of those treated with sirolimus primary therapy (Fisher’s exact test, P=0.47). These retrospective comparative data suggest comparable complete response rates achieved with sirolimus primary therapy as compared with standard 1 mg/kg of prednisone.
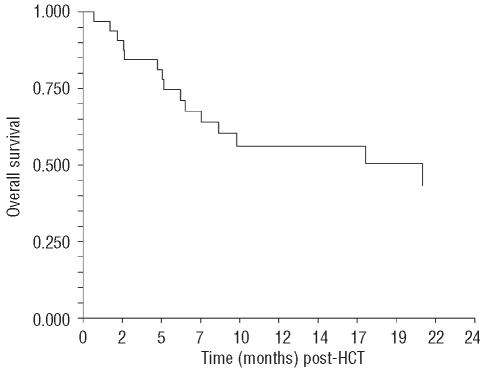
The cumulative incidence of chronic GVHD was 55% (95% CI 39–79%), with maximal NIH criteria global chronic GVHD score of mild in 5, moderate in 9, and severe in 3 patients, respectively. With a median follow up for living patients of 16 months (6–26 months), one year overall survival was 56% (95% CI 38–74%) (Figure 2), and one year relapse free survival was 37% (95% CI 19–55%). Causes of death include: relapse (n=9), sepsis (n=3), veno-occlusive disease and CMV pneumonitis (n=1), cardiomyopathy (n=1), and chronic GVHD (n=1). The one year cumulative incidence of relapse was 37% (95% CI 23–60%), and the one year cumulative incidence of non-relapse mortality was 20% (95% CI 10–42%). None of the 16 patients with complete response to sirolimus died in remission, compared to 4 of 12 (33%) of those with complete response to sirolimus plus systemic glucocorticoids, and 2 of 4 (50%) of those with persistent acute GVHD. By time of last follow up, no patients in this series had successfully discontinued all immune suppression.
Figure 2.
Overall survival from date of sirolimus initiation.
TMA occurred in 3 cases; per CTN consensus criteria, this was grade 1 in one case, and grade 2 in 2 cases. TMA resolved with dose reduction of tacrolimus in all cases. In one case, all immunosuppression was withdrawn due to primary disease relapse. CMV infection or reactivation differed according to donor/recipient serostatus: 0/9 for negative/negative; 4/10 for negative/positive; 1/4 for positive/negative; and 4/9 for positive/positive.
Discussion
High-dose glucocorticoid therapy induces complete resolution in a minority of cases, and imposes a well characterized burden of early and late complications. Given this limited efficacy and marked toxicity, advances in primary therapy of acute GVHD are needed. We have previously reported early experience of sirolimus as a sole primary therapy of acute GVHD in a series of 10 patients deemed high risk for steroid toxicity and primary malignancy relapse.16 In this report, we have examined the activity of sirolimus in a larger cohort including the original 10, and with extended follow up. These more mature data demonstrate the efficacy of sirolimus alone in the induction of sustained complete remission of acute GVHD in 50% of cases. Complete responses were observed across diverse organ involvement and acute GVHD severity. Importantly, these patients achieved the major therapeutic goal of acute GVHD resolution, while being spared the toxicity of systemic glucocorticoids. An additional 38% (total 88%) achieved complete resolution of acute GVHD with the addition of glucocorticoid doses which were mainly less than 1 mg/kg.
The low non-relapse mortality observed is encouraging in this group of older adults who mainly received unrelated donor and mismatched unrelated donor allografts. This was particularly striking in those who achieved complete remission of aGVHD with sirolimus alone. The efficacy in aGVHD with this approach was also balanced by a favorable toxicity profile. Sirolimus was safely administered with tacrolimus with an overall low incidence of TMA, which was successfully managed with dose reduction or discontinuation of tacrolimus. This evidence further complements the durable efficacy of sirolimus in the primary therapy of acute GVHD.
These are encouraging results. However, the study has several limitations. First, patients were treated according to the physician’s discretion, introducing selection bias. This likely had a significant impact on the distribution of acute GVHD severity among the included patients. Also, uniform criteria for utilization of systemic glucocorticoids after first-line sirolimus therapy were not employed. Accordingly, patients received steroids for a variety of indications, including persistent but stable manifestations, progressive severity, recurrent manifestations after initial complete response, and in the setting of partial response. As those with stable manifestations and partial response may have ultimately experienced complete response with sirolimus alone if additional time were allowed, the true failure rate or requirement for systemic glucocorticoids after primary sirolimus therapy is not clear from these data.
Additionally, while we did not detect significant differences in complete response rate according to organ involvement and acute GVHD severity, these analyses are necessarily limited by small numbers. In particular, the cohort does not include grade 4 aGVHD. The absence of this severity of acute GVHD in our series precludes any conclusion on the activity of sirolimus in primary therapy of grade 4 acute GVHD. While complete response rate would be anticipated to be less in grade 4 disease, this limited efficacy remains the unfortunate reality of available immune suppressive therapeutic agents. Another major concern is the potentially limited efficacy of sirolimus in advanced gastrointestinal tract (GI) involvement given the exclusively oral formulation of the drug. While we have observed therapeutic serum levels of sirolimus and clinical response in cases with gastrointestinal tract involvement, this effect may be undermined in the setting of vomiting and diarrhea of greater severity. Given this limitation, oral sirolimus may not be an effective primary intervention in an acute GVHD syndrome manifesting large volume diarrhea. While the data reported here are encouraging, these limitations would be best addressed through a prospective clinical trial of sirolimus as primary therapy for acute acute graft-versus-host disease.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol. 2006;43(1):32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387–94. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 3.Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76(8):1464–72. [PubMed] [Google Scholar]
- 4.Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75(4):1024–30. [PubMed] [Google Scholar]
- 5.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–26. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin PJ, Schoch G, Fisher L, Byers V, Appelbaum FR, McDonald GB, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77(8):1821–8. [PubMed] [Google Scholar]
- 7.Pidala J, Anasetti C. Glucocorticoid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(11):1504–18. doi: 10.1016/j.bbmt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Duell T, van Lint MT, Ljungman P, Tichelli A, Socie G, Apperley JF, et al. Health and functional status of long-term survivors of bone marrow transplantation. EBMT Working Party on Late Effects and EULEP Study Group on Late Effects. European Group for Blood and Marrow Transplantation. Ann Intern Med. 1997;126(3):184–92. doi: 10.7326/0003-4819-126-3-199702010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kiss TL, Abdolell M, Jamal N, Minden MD, Lipton JH, Messner HA. Long-term medical outcomes and quality-of-life assessment of patients with chronic myeloid leukemia followed at least 10 years after allogeneic bone marrow transplantation. J Clin Oncol. 2002;20(9):2334–43. doi: 10.1200/JCO.2002.06.077. [DOI] [PubMed] [Google Scholar]
- 10.Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104(12):3501–6. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 11.Worel N, Biener D, Kalhs P, Mitterbauer M, Keil F, Schulenburg A, et al. Long-term out-come and quality of life of patients who are alive and in complete remission more than two years after allogeneic and syngeneic stem cell transplantation. Bone Marrow Transplant. 2002;30(9):619–26. doi: 10.1038/sj.bmt.1703677. [DOI] [PubMed] [Google Scholar]
- 12.Pidala J, Kim J, Kharfan-Dabaja MA, Nishihori T, Field T, Perkins J, et al. Dysglycemia following glucocorticoid therapy for acute graft vs. host disease adversely affects transplantation outcomes. Biol Blood Marrow Transplant. 2011;17(2):239–48. doi: 10.1016/j.bbmt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Zahrieh D, Agura E, MacMillan ML, Maziarz RT, McCarthy PL, Jr, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104(5):1559–64. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 14.Levine JE, Paczesny S, Mineishi S, Braun T, Choi SW, Hutchinson RJ, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111(4):2470–5. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511–7. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pidala J, Kim J, Anasetti C. Sirolimus as primary treatment of acute graft-versus-host disease following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(7):881–5. doi: 10.1016/j.bbmt.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dancey JE, Curiel R, Purvis J. Evaluating temsirolimus activity in multiple tumors: a review of clinical trials. Semin Oncol. 2009;36(Suppl 3):S46–58. doi: 10.1053/j.seminoncol.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol. 2009;36(Suppl 3):S3–S17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor A, Figlin RA. Targeted inhibition of mammalian target of rapamycin for the treatment of advanced renal cell carcinoma. Cancer. 2009;115(16):3618–30. doi: 10.1002/cncr.24409. [DOI] [PubMed] [Google Scholar]
- 20.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease pro-phylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26(35):5767–74. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals Of Statistics. 1988;16:1141–54. [Google Scholar]
- 23.Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–5. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]