Abstract
The risk factors for and incidence of central nervous system involvement at first relapse in adult patients with acute myeloid leukemia have not been established. This single-center study analyzed the prognostic factors for and cumulative incidence of meningeal relapse in 458 adult patients achieving complete remission. Before 1990, patients received old chemotherapy approaches without stem cell transplantation that often included prophylactic intrathecal chemotherapy. Since 1990, modern protocols included stem cell transplantation without intrathecal prophylaxis. Meningeal relapse occurred in 6 patients (overall 5-year cumulative incidence 1.3%). The 5-year cumulative incidence of meningeal relapse in patients treated with old and modern protocols were 3.9% and 0.3%, respectively. Univariate and multivariate analyses showed that the chemotherapy approach was the main prognostic factor for central nervous system relapse (P=0.02). This study shows an extremely low incidence of meningeal relapse in adult patients with acute myeloid leukemia treated with modern protocols including stem cell transplantation without intrathecal prophylaxis.
Keywords: central nervous system relapse, acute myeloid leukemia, incidence, risk factors
Introduction
Central nervous system involvement (CNS) at first relapse can be a serious complication during the clinical course of patients with acute myeloid leukemia (AML).1 To our knowledge, this complication has been critically assessed in patients with acute promyelocytic leukemia (APL)2 and children with acute myeloid leukemia.3 However, the prevalence of and risk factors for central system involvement in adult patients with acute myeloid leukemia have been analyzed in only a few studies carried out in the 1980s4–8 and 1990s.9,10 No studies to date have analyzed the incidence of and risk factors for central nervous system relapse using the most appropriate statistical methods, i.e. cumulative incidence (CI) and multivariate analysis. Therefore, there is a lack of reliable information about central nervous system relapse in adult patients with acute myeloid leukemia, particularly in those treated with modern chemotherapy protocols.
In this study, we assessed the cumulative incidence and outcome of and risk factors for central nervous system involvement at first relapse in a large cohort of adult patients diagnosed with non-promyelocytic acute myeloid leukemia who were treated with intensive chemotherapy protocols during the past three decades at a single institution.
Design and Methods
This study included all adult patients over 13 years of age who were diagnosed consecutively with acute myeloid leukemia in a single institution (Hospital Universitari i Politècnic La Fe, València, Spain) between 1979 and 2009, and who achieved complete remission (CR) after intensive induction chemotherapy. The diagnosis and classification of AML were made according to the French-American-British (FAB) criteria,11,12 and were based on the World Health Organization 2002 criteria.13 Patients with acute promyelocytic leukemia were excluded from the study. The study was approved by the Research Ethics Board, and all patients provided informed consent according to institutional guidelines.
The chemotherapy schedules used in the first patients from 1979 to 1985 (group 1; n=56) included adriamycin (cumulative doses [CD] of 180 mg/m2), cytarabine (CD 3,150 mg/m2), and 6-thioguanine or vincristine as the induction and consolidation therapy, which was followed by maintenance with polychemotherapy for 1–2 years. From 1986 to 1990 (group 2; n=73), therapy comprised induction and consolidation with the combination of cytarabine (CD 2,800 mg/m2) and anthracycline (daunorubicin or mitoxantrone, CD 180 mg/m2 and 30 mg/m2, respectively) followed by intensification with cytarabine (CD 12,000 mg/m2) plus L-asparaginase (n=22) or daunorubicin (n=51, CD 135 mg/m2). From 1990 to the present, therapy comprised induction and consolidation with the combination of cytarabine (CD ranging from 1,400 to 8,000 mg/m2) and anthracycline (idarubicin: n=149, CD 72 mg/m2; or mitoxantrone: n=59, CD 60 mg/m2; or daunorubicin: n=68, CD from 270 to 360 mg/m2) (group 3; n=276). Patients with secondary AML received induction therapy with fludarabine, cytarabine, and idarubicin followed by consolidation with idarubicin plus cytarabine (n=42).14 All the above protocols included intensification therapy with autologous or allogeneic stem cell transplantation (SCT) when feasible or an additional cycle of intensive chemotherapy. The remaining 11 patients received other therapeutic schedules comprising intensive chemotherapy followed by allogeneic stem cell transplantation (group 4).
Lumbar puncture to examine cerebrospinal fluid (CSF) was not performed at the time of AML diagnosis except in patients with clinical suspicion of central nervous system infiltration. Lumbar puncture was also performed to administer central nervous system relapse prophylaxis with intrathecal (IT) chemotherapy (methotrexate plus hydrocortisone alternating with cytarabine) in patients with FAB-M4 or M5 and a white blood cell (WBC) count over 20×109/L treated with protocols used to treat groups 1 and 3.
The diagnosis of central nervous system relapse was confirmed by lumbar puncture and cytological examination of the cerebrospinal fluid. When central nervous system relapse occurred, treatment comprised weekly intrathecal chemotherapy with methotrexate plus hydrocortisone alternating with cytarabine. Some patients received further craniospinal irradiation or systemic chemotherapy at the physician’s discretion.
The remission induction response was assessed according to the recently revised criteria of Cheson et al.15 We analyzed the incidence of and risk factors for central nervous system involvement at first relapse, either isolated or simultaneous with bone marrow or other organ involvement, in patients who achieved a complete remission.
Fifty-one patient and disease characteristics were examined to establish their relationship with central nervous system relapse. In addition to those listed in Table 1, the following variables were also analyzed: weight, height, fever, tumoral symptoms, skin involvement, hepatomegaly and splenomegaly, adenopathies, gingival hypertrophy, monocyte, myelocyte, and blast cell count in the peripheral blood, glucose serum levels, creatinine, urea nitrogen, uric acid, calcium, phosphate, proteins, albumin, aspartate aminotransferase, alanine aminotransferase, bilirubin, and alkaline phosphatase, the presence of disseminated intravascular coagulopathy, serum fibrinogen level, Quick index, activated partial thromboplastin time, and bone marrow characteristics such as the presence of fibrosis, blast percentage, blast positivity to alpha-naphthyl acetate esterase, peroxidase and Sudan black staining, and Auer rods. The results of the cytogenetic studies were categorized according to the Medical Research Council classification.16 Patients were classified as having secondary acute myeloid leukemia when there was documented pre-leukemic disease or exposure to leukemogenic agents.
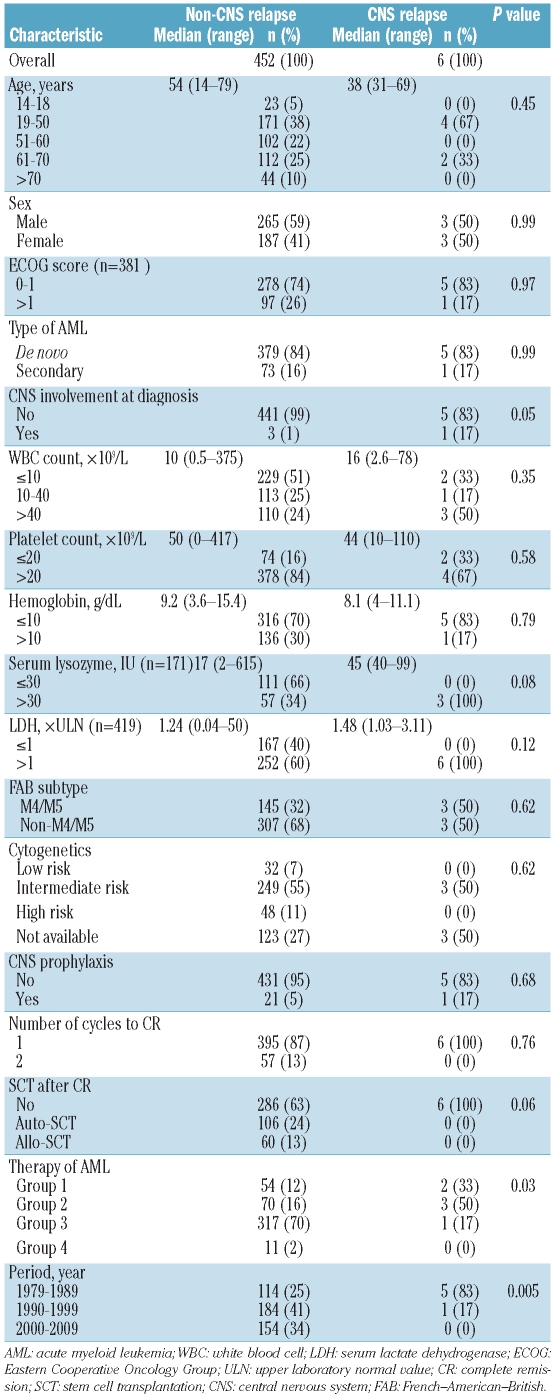
Table 1.
Main demographic and baseline characteristics of 458 AML patients who achieved CR.
The probability of overall survival was estimated by the Kaplan-Meier method,17 and of central nervous system involvement at first relapse by the cumulative incidence method.18 Isolated bone marrow relapse and death in complete remission were each considered to be competing risk events. Characteristics selected for inclusion in the multivariate analysis were those showing a significant association in the univariate analysis (P<0.05). Multivariate analysis for cumulative incidence was performed using the Fine and Gray model.19 Computations were performed using the BMDP statistical library20 and R 2.9.2 software package.
Results and Discussion
Four hundred and fifty-eight of 859 adult patients with a diagnosis of non-promyelocytic acute myeloid leukemia undergoing induction chemotherapy achieved a first complete remission. Their main characteristics are shown in Table 1. Central nervous system infiltration at diagnosis was documented by lumbar puncture in 4 patients (1%). Twenty-two patients received prophylactic intrathecal chemotherapy; 18 of 129 in groups 1 and 2 (14%), and 4 of 329 in groups 3 and 4 (1%). One hundred and sixty-six patients (37%) received autologous (n=106) or allogeneic (n=60) stem cell transplantation in first complete remission. The median patient follow up was 96 months (range 11–317 months).
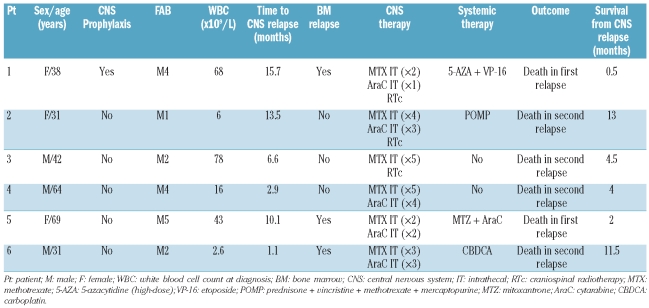
The characteristics of the patients who experienced central nervous system relapse are summarized in Table 2. Central nervous system involvement at first relapse was documented in 6 patients: 2 had isolated cases, one had another extramedullary relapse location, and half had simultaneous bone marrow involvement, as reported in other studies.6,10 The median time to central nervous system relapse was seven months (range 1–16 months) compared with nine months (range 1–167 months) for isolated bone marrow relapse. The median time to central nervous system relapse reported here is sooner than the 12 and 19 months reported by Castagnola et al.10 and Holmes et al.,7 respectively, in adult AML patients, but similar to the four months described in a pediatric series.3
Table 2.
Pre-treatment characteristics, time of occurrence, treatment, and outcomes of patients with CNS relapse.
The present study showed an overall 5-year cumulative incidence of central nervous system relapse of 1.3% (either isolated or combined with bone marrow relapse), which is lower than the previously reported incidence of 3–11%.4–10 The different statistical methods used to calculate the incidence in our study (competing risk analysis using the CI) compared with others (crude incidence) may explain, at least in part, our different rate of central nervous system relapse. Another explanation is that most previous studies were performed exclusively in patients treated with old chemotherapy protocols in the 1980s,4–9 whereas in our series most of the patients were treated with modern protocols. In fact, the cumulative incidence of relapse was 3.9% in groups 1 and 2 (old protocols) which is consistent with the previously published series,4–9 compared with 0.3% in groups 3 and 4 (modern protocols). Unlike other studies,4,6–10 patients with biological entities requiring characteristic treatment, such as those with acute promyelocytic leukemia,2 were excluded from the present analysis. Our study included a series of unselected, consecutively diagnosed, adult acute myeloid leukemia patients and, therefore, shows an unbiased incidence of central nervous system relapse. However, the study was not designed to analyze the impact of therapeutic interventions on the development of central nervous system relapse, in contrast to other studies performed in the context of controlled randomized multicenter trials.8,9 Besides, the 5-year cumulative incidence of isolated bone marrow relapse was 51%, and the relative frequency of central nervous system involvement among relapsed patients overall was 2.5% (6 of 239 relapses).
The treatments for and outcomes of central nervous system relapse are shown in Table 2. All patients treated for central nervous system relapse received several lumbar punctures, as recommended,1 and 4 were treated with concomitant systemic chemotherapy (including the 3 patients with simultaneous bone marrow relapse). Three patients with isolated central nervous system relapse achieved complete clearance of blasts in the cerebrospinal fluid and relief of symptoms. However, bone marrow involvement in subsequent relapses occurred invariably after central nervous system relapse, suggesting that systemic chemotherapy should be administered independently of the bone marrow status at the time of central nervous system relapse.10 As reported previously,4,6,10 the outcome after central nervous system relapse was very poor in our study (median survival from central nervous system relapse of four months, range 0.5–13 months).
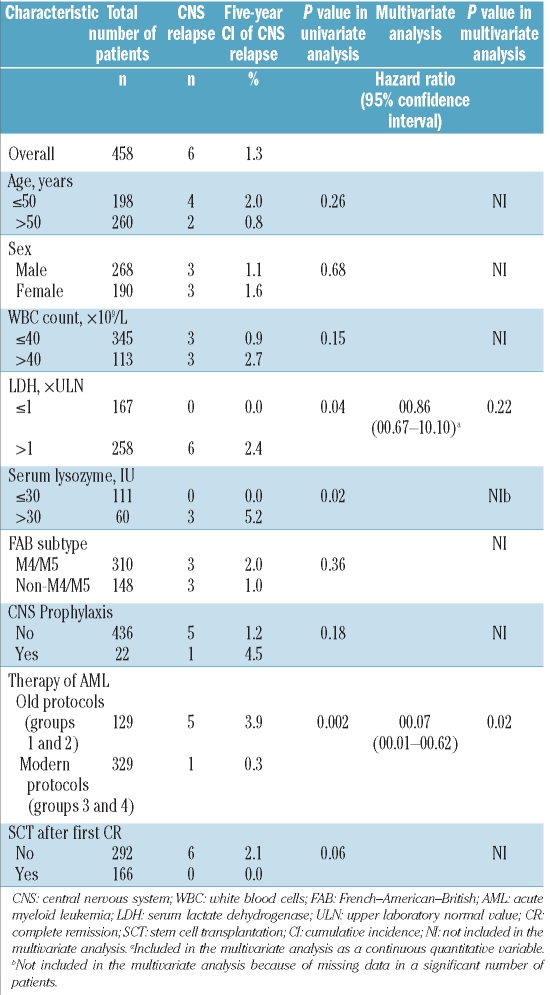
The univariate analysis identified the following characteristics as associated with a low risk of central nervous system relapse: modern protocols (groups 3 and 4)(P=0.002), serum lysozyme 30 IU or below (P=0.02), and lactate dehydrogenase (LDH) concentration less or equal than the upper laboratory normal value (P=0.04). A trend was observed for stem cell transplantation after the first complete remission (P=0.06) (Table 3). Multivariate analysis identified modern protocols as the sole independent prognostic factor for central nervous system relapse (P=0.02; hazard ratio 0.07 [95% confidence interval 0.01–0.62]) (Table 3).
Table 3.
Univariate and multivariate analysis of cumulative incidence of CNS relapse in the study cohort.
Our analysis of the prognostic factors suggests that the up-front chemotherapy protocols for acute myeloid leukemia treatment might influence the development of central nervous system involvement at first relapse. We hypothesize that modern chemotherapies including frequent stem cell transplantation might reduce the risk of central nervous system relapse by eliminating the residual leukemic cells in the central nervous system or in the bone marrow, or both. On the other hand, we were not able to confirm the previously reported associations between central nervous system relapse and FAB M4/M5 sub-types,3,5,10,21 the cytogenetic abnormality inv(16),7 male sex,4 and high white blood cell count at diagnosis.3,4,7 Elevated serum lysozyme concentration, a marker of monocytic acute myeloid leukemia,11 and increased lactate dehydrogenase serum concentration, as described by Stewart et al.,6 were both significantly associated with central nervous system relapse. However, lactate dehydrogenase concentration was not confirmed to be an independent risk factor, and lysozyme concentration was not included in the multivariate analysis because this parameter was available in only a limited number of patients.
Recently published guidelines for the treatment of adult patients with acute myeloid leukemia recommend consideration of lumbar puncture screening22 or the use of specific central nervous system prophylaxis,1 especially in those patients with monocytic or hyperleukocytic forms. However, these recommendations were based presumably on early studies of adults4–10 or on more recent data from pediatric trials.3 Our data showing an extremely low incidence of central nervous system relapse in adult patients treated with modern chemotherapy protocols without intrathecal prophylaxis suggest that the recommendation of central nervous system screening and prophylaxis to prevent central nervous system relapse should be reconsidered. The burden for the patients and the potential risks of additional medical complications (e.g. hemorrhage or neurological toxicity23) following lumbar puncture would be unlikely to outweigh the possible benefits. We note that from 1979 to 1985, patients with monocytic and hyperleukocytic acute myeloid leukemia received prophylactic intrathecal chemotherapy, but this measure apparently did not prevent central nervous system relapse in this cohort of patients. This finding is consistent with two previously published randomized trials that showed similar9 or even increased8 rates of central nervous system relapse in patients receiving prophylactic intrathecal chemotherapy.
In conclusion, the cumulative incidence of central nervous system involvement at first relapse in adult acute myeloid leukemia patients treated with modern chemotherapy protocols including allogeneic stem cell transplantation who did not receive central nervous system prophylaxis was extremely low. In contrast, the cumulative incidence of central nervous system relapse in patients treated with old chemotherapy approaches without allogeneic stem cell transplantation, which often included intrathecal prophylaxis, was relatively high. Our results do not support the use of systematic or selective central nervous system prophylaxis in adult acute myeloid leukemia patients.
Acknowledgments
The authors thank Shirley Weiss, Paula Petruskevicius, Carlos Pastorini and David Pellicer for data collection and management.
Footnotes
Funding: this study was supported in part by the Fundación para la Investigación Hospital Universitario La Fe-Ayudas Bancaja (grant 2006/0137), Red Temática de Investigación Cooperativa en Cáncer (RD06/0020/0031).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Döhner H, Estey EH, Amadori S, Applebaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 2.Montesinos P, Díaz-Mediavilla J, Debén G, Prates V, Tormo M, Rubio V, et al. Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal prophylaxis. Haematologica. 2009;94(9):1242–9. doi: 10.3324/haematol.2009.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston DL, Alonzo TA, Gerbing RB, Lange BJ, Woods WG. Risk factors and therapy for isolated central nervous system relapse of pediatric acute myeloid leukemia. J Clin Oncol. 2005;23(36):9172–8. doi: 10.1200/JCO.2005.02.7482. [DOI] [PubMed] [Google Scholar]
- 4.Cassileth PA, Sylvesteer LS, Bennett JM, Begg CB. High peripheral blast count in adult AML is a primary risk factor for CNS leukemia. J Clin Oncol. 1988;6(3):495–8. doi: 10.1200/JCO.1988.6.3.495. [DOI] [PubMed] [Google Scholar]
- 5.Brinch L, Evensen SA, Stavem P. Leukemia in the central nervous system. Acta Med Scand. 1988;224(2):173–8. doi: 10.1111/j.0954-6820.1988.tb16756.x. [DOI] [PubMed] [Google Scholar]
- 6.Stewart DJ, Keating MJ, McCredie KB, Smith TL, Youness E, Murphy SG, et al. Natural history of central nervous system acute leukemia in adults. Cancer. 1981;47(1):184–96. doi: 10.1002/1097-0142(19810101)47:1<184::aid-cncr2820470130>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Holmes R, Keating MJ, Cork A, Broach Y, Trujillo J, Dalton WT, et al. A unique pattern of central nervous system leukemia in acute myelomonocytic leukemia associated with inv(16)(p13q22) Blood. 1985;65(5):1071–8. [PubMed] [Google Scholar]
- 8.Rees JK, Gray RG, Swirsky D, Hayhoe FG. Principal results of the Medical Research Council’s 8th acute myeloid leukaemia trial. The Lancet. 1986;2(8518):1236–41. doi: 10.1016/s0140-6736(86)92674-7. [DOI] [PubMed] [Google Scholar]
- 9.Morrison FS, Kopecky KJ, Head DR, Athens JW, Balcerzak SP, Gumbart C, et al. Late intensification with POMP chemotherapy prolongs survival in acute myelogenous leukemia - Results of a Southwest Oncology Group study of rubidazone versus adriamycin for remission induction, prophylactic intrathecal therapy, late intensification, and levamisole maintenance. Leukemia. 1992;6(7):708–14. [PubMed] [Google Scholar]
- 10.Castagnola C, Nozza A, Corso A, Bernasconi C. The value of combination therapy in adult acute myeloid leukemia with central nervous system involvement. Haematologica. 1997;82(5):577–80. [PubMed] [Google Scholar]
- 11.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French–American–British (FAB) co-operative group. Br J Haematol. 1976;33(4):451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 12.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French–American–British Cooperative Group. Ann Intern Med. 1985;103(4):620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 13.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 14.de la Rubia J, Regadera A, Martín G, Cervera J, Sanz G, Martínez J, et al. FLAG-IDA regimen (fludarabine, cytarabine, idarubicin and G-CSF) in the treatment of patients with high-risk myeloid malignancies. Leuk Res. 2002;26(8):725–30. doi: 10.1016/s0145-2126(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: Analysis of 1612 patients entered into MRC AML 10 trial. The Medical Research Council and Children’s Leukemia working parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimations from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 18.Gray RJ. A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–54. [Google Scholar]
- 19.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Lee ET. Statistical methods for survival data analysis. Vermont, CA: Wadsworth Inc.; 1980. [Google Scholar]
- 21.Champlin R, Gale RP. Acute myelogenous leukemia: recent advances in therapy. Blood. 1987;69(6):1551–62. [PubMed] [Google Scholar]
- 22.NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesTM) Acute Myeloid Leukemia. Version 2. 2011. [[accessed February 16, 2011]]. Available from URL: http://www.nccn.org/index.asp.
- 23.Atra A, Pinkerton CR, Bouffet E, Norton A, Hobson R, Imeson J, et al. Acute neurotoxicity in children with advanced stage B-non-Hodgkin’s lymphoma and B-acute lymphoblastic leukaemia treated with the United Kingdom children cancer study group 9002/9003 protocols. Eur J Cancer. 2004;40(9):1346–50. doi: 10.1016/j.ejca.2004.02.011. [DOI] [PubMed] [Google Scholar]