Myeloid sarcoma (MS), also known as granulocytic sarcoma or chloroma, is a rare extramedullary tumor composed of immature myeloid cells at different stages of differentiation which can involve any site of the body. Most cases of myeloid sarcoma will develop within the first year preceding the occurrence of acute myeloid leukemia (AML), or concomitantly to AML, or at relapse of AML. More rarely, myeloid sarcoma could also be observed in the setting of myelodysplastic syndrome or myeloproliferative neoplasms, or as an isolated myeloid sarcoma (also designated as de novo, non-leukemic or primary MS).1 Common practice suggests that patients with isolated myeloid sarcoma should receive AML-like induction chemotherapy.2 Although a single comparative study has previously shown that isolated myeloid sarcoma may be associated with superior event-free survival and overall survival as compared to AML when patients receive AML-type therapy,3 the outcome of such patients is considered poor; median survival is less than 24 months4–6 The best therapeutic option has not yet been established. Recently, we and others have shown that allogeneic hematopoietic stem cell transplantation (allo-HSCT) may represent a valid treatment option in leukemic myeloid sarcoma.7,8 However, it is still unclear whether allo-HSCT is also a good option for patients with isolated myeloid sarcoma.
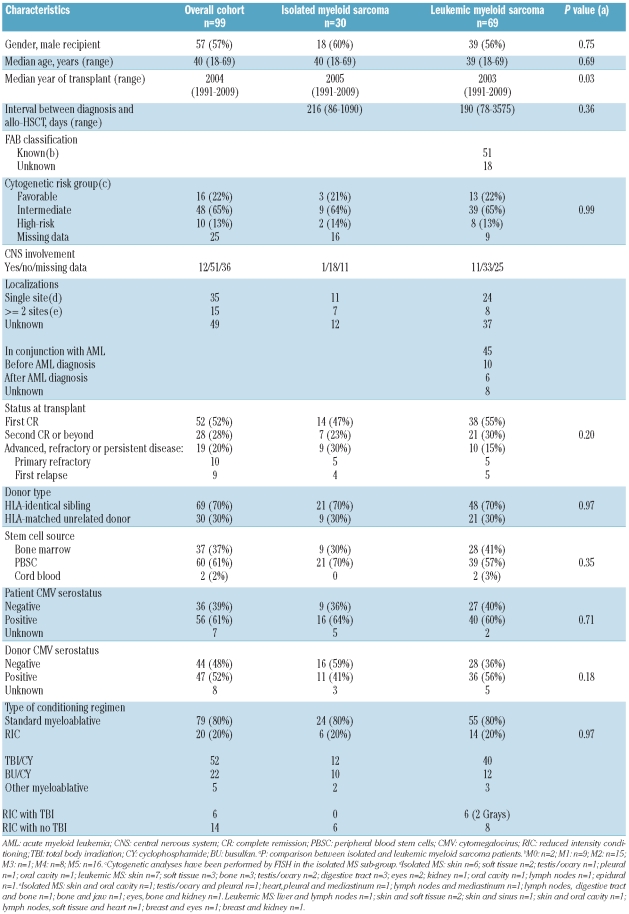
To assess the role of allo-HSCT in patients with isolated myeloid sarcoma and compare the outcome of such patients with leukemic myeloid sarcoma patients, we performed a retrospective multicenter study assessing the results of allo-HSCT in 99 patients reported to the EBMT registry between January 1991 and June 2009 with isolated (n=30) or leukemic (n=69) myeloid sarcoma. Patients’ characteristics and information regarding disease and transplant are summarized in Table 1. The previously published outcome of adult patients8 was updated for this study. Median follow up was 48 months (range 6–213 months). There were no significant statistical differences between the two groups except year of allo-HSCT. Study end points were the probabilities of overall survival, leukemia-free survival (LFS), relapse incidence (RI), non-relapse mortality (NRM) and chronic graft-versus-host disease. Statistical analyses were performed using SPSS 18.0 (SPSS Inc, Chicago, IL) and Splus 8.1 (Math-Soft Inc, Seattle, WA) packages.
Table 1.
Patients’, disease and transplant characteristics.
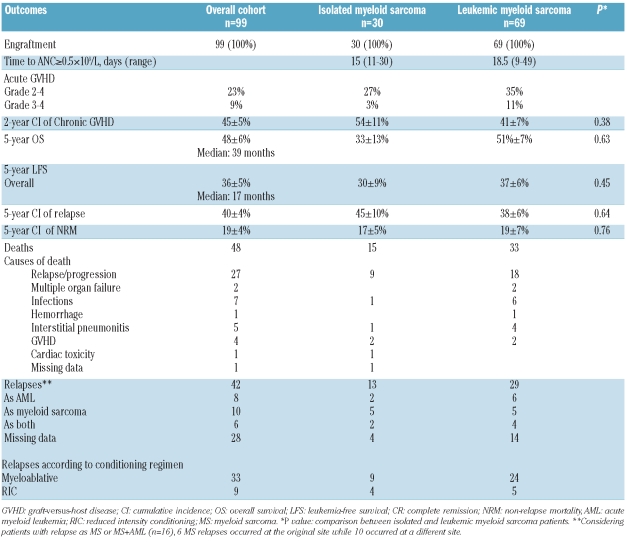
Overall results and comparison of outcomes between isolated and leukemic myeloid sarcoma are given in Table 2. Some patients in the leukemic group had received a second allo-transplant in the leukemic group, and could achieve a second persistent complete remission (CR) as previously reported.8 As outcomes of isolated and leukemic myeloid sarcoma were found to be similar, data for the two groups were pooled to perform univariate and multivariate analyses. Thus, considering the whole cohort (n=99), in univariate analysis, factors associated with higher leukemia-free survival and lower relapse incidence were: CR status at transplant (5-year LFS: 40±6% vs.16±8%; P<0.0001; 5-year RI: 39±6% vs. 68±11%; P=0.0009) and cytogenetics (5-year LFS: good 50±13% vs. intermediate 30±7% vs. poor 20±13%, P=0.06; 5-year RI: good 37±13% vs. intermediate 43±8% vs. poor: 70±16%, P=0.05). The only factor associated with higher non-relapse mortality was patient gender (male: 27±6% vs. female: 10±5%, P=0.04). In multivariate analysis, high-risk cytogenetics remained significantly associated with poorer leukemia-free survival and increased relapse incidence (HR=2.55, 95%CI: 1.14–5.73, P=0.02; HR=2.64, 95%CI: 1.05–6.63, P=0.04, respectively), while a CR status at transplant was associated with improved leukemia-free survival (HR=0.44, 95%CI: 0.22–0.88, P=0.02). There was a trend for a lower incidence of relapse for patients in complete remission (HR=0.49, 95%CI: 0.22–1.07, P=0.07).
Table 2.
Comparison of outcomes between isolated myeloid sarcoma and leukemic myeloid sarcoma.
This study included the largest series of isolated myeloid sarcoma patients having undergone allo-HSCT reported so far. With a 5-year overall survival and leukemia-free survival of 48% and 36%, respectively, this study suggests that allo-HSCT is a potentially efficient treatment for isolated myeloid sarcoma with relatively acceptable toxicity (overall 5-year NRM 17%). When comparing isolated and leukemic myeloid sarcoma, there were no significant differences in term of outcomes. The multivariate analysis showed that CR status at transplant was associated with improved leukemia-free survival while high-risk cytogenetics reduced the chance of long-term leukemia-free survival. If the former result is expected, the latter should be taken with caution, as cytogenetic data were missing in most cases of isolated myeloid sarcoma.
We think our results support the use of allo-HSCT as first-line therapy for myeloid sarcoma, especially in patients achieving complete remission after AML-type therapy, since one should bear in mind that the prognosis of myeloid sarcoma is generally poor with an overall survival of usually less than two years in patients not receiving allo-HSCT.4–6 The latter is in line with recent guidelines from Dohner et al.,2 recommending the use of AML-like induction therapy for myeloid sarcoma, followed by consolidation before proceeding to allo-HSCT. Also, involved field radiation therapy may be considered to improve management of localized tumors.
Besides cytogenetics, the role of new molecular prognostic markers such as NPM1 or FLT3-ITD mutations in myeloid sarcoma should also be investigated, as they will likely influence outcome after chemotherapy and/or allo-HSCT.9 Interestingly, a case report regarding one patient with FLT3-ITD positive myeloid sarcoma suggested a beneficial effect of the kinase inhibitor sorafenib.10
We conclude that allo-HSCT is an effective treatment for patients with myeloid sarcoma. Patients with isolated or leukemic myeloid arcoma have similar outcomes after allo-HSCT. While prospective evaluations are needed, allo-HSCT could be considered the optimal therapy for both isolated and leukemic myeloid sarcoma.
Acknowledgments
We thank all the data managers, especially Benedicte Samey, from the different EBMT participating centers for data management and collection. We also thank co-investigators of the study who recruited patients, provided clinical care, and commented on the manuscript: G Sucak, P Ljungman, B Lioure, J Finke, R Tabrizi, N Contentin, A Gratwohl, L Volin, M Michallet, JP Vernant, JO Bay, I Yakoub-Agha and Arnon Nagler.
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Campidelli C, Agostinelli C, Stitson R, Pileri SA. Extramedullary manifestation of myeloid disorders. Am J Clin Pathol. 2009;132(3):426–37. doi: 10.1309/AJCP1ZA7HYZKAZHS. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 3.Tsimberidou AM, Kantarjian HM, Wen S, Keating MJ, O’Brien S, Brandt M, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukmia. Cancer. 2008;113(6):1370–8. doi: 10.1002/cncr.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol. 1995;13(7):1800–16. doi: 10.1200/JCO.1995.13.7.1800. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi K, Yasuda M. Comparison in treatments of non-leukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer. 2002;94(6):1739–46. doi: 10.1002/cncr.10399. [DOI] [PubMed] [Google Scholar]
- 6.Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17(6):1100–3. doi: 10.1038/sj.leu.2402958. [DOI] [PubMed] [Google Scholar]
- 7.Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinicopathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340–50. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 8.Chevallier P, Mohty M, Lioure B, Michel G, Contentin N, Deconinck E, et al. Allogeneic hematopoietic stem-cell transplantation for myeloid sarcoma: a retrospective study from the SFGM-TC. J Clin Oncol. 2008;26(30):4940–3. doi: 10.1200/JCO.2007.15.6315. [DOI] [PubMed] [Google Scholar]
- 9.Falini B, Lenze D, Hasserjian R, Coupland S, Jaehne D, Soupir C, et al. Cytoplasmic mutated nucleophosmin (NPM) defines the molecular status of a significant fraction of myeloid sarcomas. Leukemia. 2007;21(7):1566–70. doi: 10.1038/sj.leu.2404699. [DOI] [PubMed] [Google Scholar]
- 10.Choschzick M, Bacher U, Ayuk F, Lebeau A. Immuno-histochemistry and molecular analyses in myeloid sarcoma of the breast in a patient with relapse of NPM1-mutated and FLT3-mutated AML after allogeneic stem cell transplantation. J Clin Pathol. 2010;63(6):558–61. doi: 10.1136/jcp.2009.071357. [DOI] [PubMed] [Google Scholar]