Abstract
DCC (deleted in colorectal cancer) is postulated to function as transmembrane receptor for the axon and cell guidance factor netrin-1. We report here that the DCC cytoplasmic domain binds to proteins encoded by mammalian homologs of the Drosophila seven in absentia (sina) gene, as well as Drosophila Sina. Sina has a critical role in R7 photoreceptor development and shows upward of 85% amino acid identity with its mammalian homologs (termed Siahs), but the function of the Sina/Siah proteins has not been defined. We sought, therefore, to characterize further their interaction with DCC. Immunofluorescence studies suggested the Sina/Siah proteins localized predominantly in the cytoplasm and in association with DCC. DCC was found to be ubiquitinated and the Sina/Siah proteins regulated its expression. Proteasome inhibitors blocked the effects of Sina/Siah on DCC, and the Sina/Siah proteins interacted with ubiquitin-conjugating enzymes (Ubcs). A mutant Siah protein lacking the amino-terminal Ubc-binding sequences complexed with DCC, but did not degrade it. The in vivo interaction between Sina/Siah and DCC was confirmed through studies of transgenic Drosophila lines in which DCC and Sina were ectopically expressed in the eye. Taken together, the data imply that the Sina/Siah proteins regulate DCC and perhaps other proteins via the ubiquitin–proteasome pathway.
Keywords: DCC, mammalian homologs, Drosophila sina gene, ubiquitin–proteasome pathway
DCC (deleted in colorectal cancer) was initially identified and cloned from a region of chromosome 18q affected by allelic losses in upward of 70% of colorectal cancers (Fearon et al. 1990). Such losses are thought to indicate the existence of a tumor suppressor gene within the affected chromosomal region (Cavenee et al. 1983; Knudson 1993). DCC expression is reduced markedly or undetectable in the majority of colorectal cancer lines and primary tumors (Fearon et al. 1990; Itoh et al. 1993; Hedrick et al. 1994; Thiagalingam et al. 1996), and the loss of expression has been associated with poor prognosis (Shibata et al. 1996). Somatic mutations in DCC have been identified in some colorectal cancers, but the mechanisms underlying the loss of DCC expression in most cancers remain poorly understood, perhaps in part, because the gene spans >1,350,000 bp (Cho et al. 1994). Frequent DCC allelic losses and loss of its expression, as well as somatic mutations, have also been seen in other malignancies (for review, see Cho and Fearon 1995; Fearon 1996; and Kolodziej 1997).
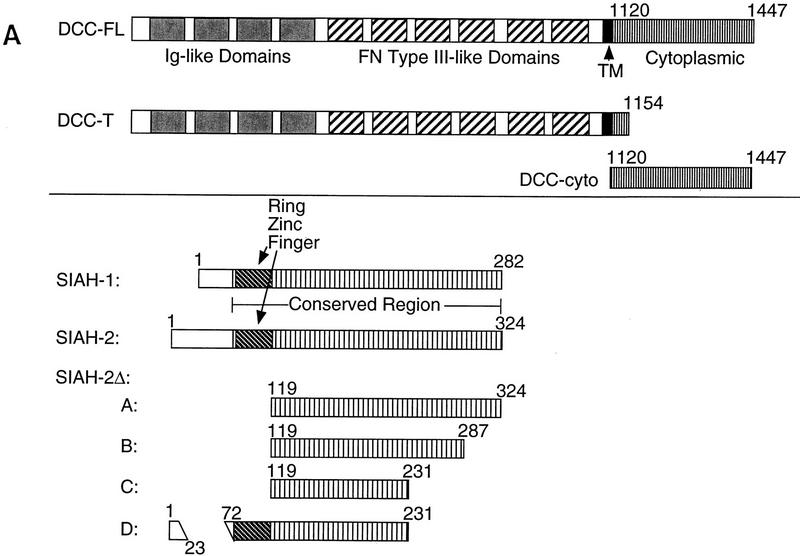
The primary product of the DCC gene is a 1447-amino-acid transmembrane protein with four immunoglobulin-like and six fibronectin type III like extracellular domains and a 325-amino-acid cytoplasmic domain (Hedrick et al. 1994; Reale et al. 1994). Although DCC bears similarity to many immunoglobulin superfamily members, recent studies have established that DCC and closely related proteins comprise a unique subfamily (Kolodziej 1997). The DCC domain structure and sequence are most similar to that of neogenin (NGN), a protein whose expression is dynamically regulated in the developing nervous system and gastrointestinal tract of the chicken (Vielmetter 1994; Meyerhardt et al. 1997). Invertebrate DCC-related proteins have been identified recently, including the Caenorhabditis elegans UNC-40 and Drosophila Frazzled proteins (Chan et al. 1996; Kolodziej et al. 1996).
The specific cellular function of DCC is not known, but there are intriguing clues. Though expressed at low levels in many adult tissues, DCC is most abundant in brain tissue (Hedrick et al. 1994; Reale et al. 1994). DCC is expressed in developing neural tissues of vertebrate embryos (Pierceall et al. 1994a; Cooper et al. 1995) and has been implicated in neuronal differentiation of PC12 cells (Lawlor and Narayanan 1992; Pierceall et al. 1994b). Perhaps most significantly, recent studies have implied that DCC is a receptor for netrin-1, a vertebrate axon and cell guidance factor (Keino-Masu et al. 1996). The critical role of netrins, DCC, and DCC-related proteins in axon guidance and cell migration in the developing nervous systems of vertebrate and invertebrate organisms has been illustrated through a variety of cell biological and genetic studies (Chan et al. 1996; Kolodziej et al. 1996; Serafini et al. 1996; Fazeli et al. 1997; Kolodziej 1997)
To identify proteins that may regulate DCC or transduce signals via its unique cytoplasmic domain, we carried out yeast two-hybrid screens, using DCC cytoplasmic sequences as “baits.” A homolog of the Drosophila seven in absentia (sina) gene was identified independently in both of our screens. Sina function is critical in R7 photoreceptor development (Carthew and Rubin 1990). Genetic studies indicate that Sina either functions downstream of other proteins in the sevenless (sev) pathway, including Ras, Raf, and MAPK, or it functions in an independent pathway that together with the sev pathway specifies R7 formation (Fortini et al. 1992; Lai and Rubin 1992; Brunner et al. 1994; Chang et al. 1994, 1995; Dickson et al. 1995). Recently, defects in tramtrack (ttk) have been found to lead to supernumerary R7 formation even in the absence of Sina (Lai et al. 1996; Yamamoto et al. 1996), suggesting Sina may down-regulate ttk. Despite the substantial interest in the Sev/Ras/MAPK pathway and R7 fate specification, the cellular function of Sina has not yet been defined. Here, we demonstrate that mammalian proteins highly related to Sina, Seven in absentia homologs (Siahs), as well as Drosophila Sina, bind to DCC. DCC was found to be ubiquitinated, and the Sina/Siah proteins appeared to mediate its degradation via proteasome-dependent mechanisms. Our findings indicate that the Sina/Siah proteins function in ubiquitin-mediated proteolysis and that they are candidate regulators of DCC.
Results
Yeast two-hybrid studies identify Siah-2 as a DCC-binding protein
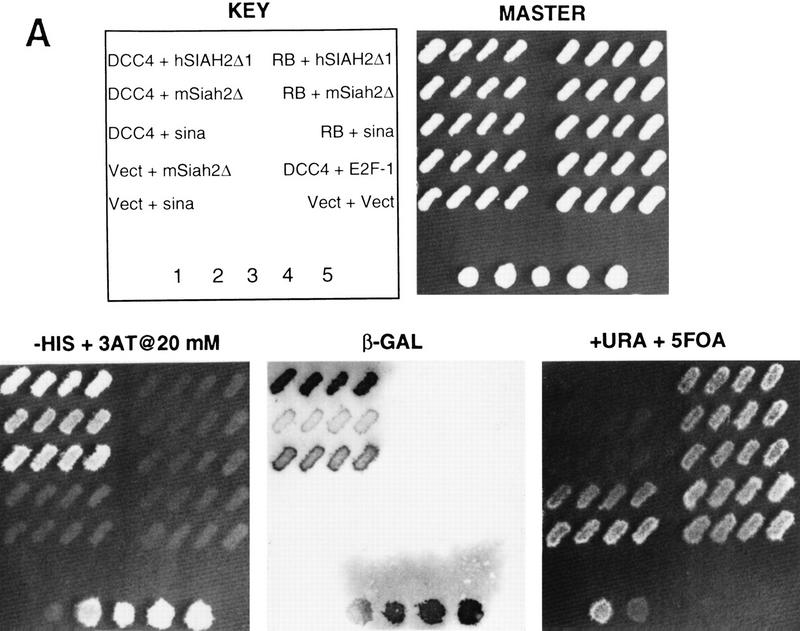
To identify cellular proteins that interact with the DCC cytoplasmic domain, we used a modified yeast two-hybrid system (Fields and Song 1989; Du et al. 1996; Vidal 1997). The full-length DCC cytoplasmic domain strongly activated reporter gene activity when fused to the GAL4 DNA-binding domain (data not shown). Therefore, we generated a series of constructs in which portions of the DCC cytoplasmic domain were fused to the GAL4 DNA-binding domain and characterized their activity. The GAL4–DCC4 construct lacked the proline-rich and acidic carboxyl terminus 55 amino acids of the DCC cytoplasmic domain and had minimal activity (Fig. 1A; data not shown), and it was used in the two-hybrid screens to identify interacting proteins.
Figure 1.
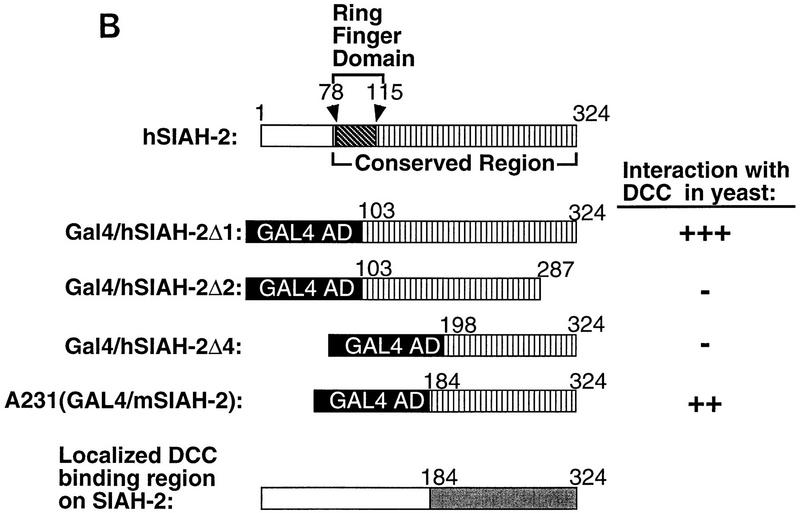
Yeast two-hybrid studies of DCC, Sina, and mammalian Sina homologs (Siahs). (A) Yeast two-hybrid interaction between DCC and Sina/Siah proteins. Yeast strain MaV103 was cotransformed with the indicated GAL4 DNA-binding (DB) and activation domain (AD) fusions. DB fusions were GAL4–DCC4 (DCC4, amino acids 1120–1392) or GAL4DB–p105-RB (RB; amino acids 302–908). AD fusion proteins contained human Siah2Δ1 (amino acids 103–324), mouse Siah2Δ (amino acids 115–325), Drosophila Sina (amino acids 115–314), or human E2F-1 (amino acids 159–437) sequences. Yeast transformants were initially grown on permissive SC–Leu–Trp (master) plates. Reporter assays were performed by replica plating the master plate onto a SC–Leu–Trp–His plate containing 20 mm 3-AT (−His+3AT 20 mm); Hybond N+ membrane on SC–Leu–Trp plate for β-Gal assay (β-Gal); and SC–Leu–Trp plate containing 0.2% 5-fluoro-orotic acid (+URA+5FOA). Positive two-hybrid interactions are indicated by growth on the −HIS + 3AT plate, β-gal activity, and lack of growth on +URA + 5FOA plate. Controls 1–5 are derived from the same yeast strain cotransformed with (1) pPC97–CYH+pPC86; (2) DB–RB+AD–E2F1; (3) DB–Fos+AD–Jun; (4) Gal4+pPC86; and (5) DB-E2F1+AD–DP1. Equivalent expression of the majority of DB and AD fusions was verified by Western blot analysis. (B) Minimal region of Siah-2 needed for interaction with DCC in the yeast two-hybrid assay. Studies to define the Siah-2 sequences were performed as described in A.
Two different cDNA libraries were screened for interaction with the DCC4 construct. One library (Alala I) was generated from cDNAs of murine gestational day 12–13 embryos, and the other library (Glio I) was constructed from cDNAs of glioblastoma xenografts propagated in immunocompromised mice. From each library, 1.5 × 106 transformants were obtained and initially selected for reporter-dependent 3-aminotriazole (3-AT) resistance. After further selection and retesting, nine cDNAs from the Alala I screen and 13 cDNAs from the Glio I screen scored repeatedly positive on all three reporter assays and demonstrated specific interaction with the DNA-binding domain–DCC4 fusion. After sequence analysis, the 22 rescued cDNAs were found to be derived from seven independent open-reading frames (ORFs) (data not shown). The only related cDNAs identified independently in each screen were derived from overlapping fragments of a murine homolog (Siah-2) of the Drosophila sina gene (Della et al. 1993). One cDNA encoded amino acids 115–325 of Siah-2, and the other encoded amino acids 183–325.
As noted above, sina was identified because of its critical role in Drosophila R7 photoreceptor development (Carthew and Rubin 1990). Little is known about the 314-amino-acid Sina protein other than it contains an amino-terminal cysteine-rich domain, similar to the so-called C3HC4 or RING zinc finger domain (Saurin et al. 1996). Three highly conserved murine Siahs, termed Siah-1A, Siah-1B, and Siah-2, have been described (Della et al. 1993). There are two human homologs, termed Siah-1 and Siah-2, displaying 96%–99% amino acid identity with their murine counterparts and ∼77% identity with one another (Nemani et al. 1996; Hu et al. 1997). Drosophila Sina is extremely well conserved with mammalian Siahs, with ∼85% identity between Sina and Siahs over their carboxy terminal 270 amino acids (Della et al. 1993; Hu et al. 1997). Interaction between the DCC cytoplasmic domain and human Siahs was demonstrated in the yeast two-hybrid system (Fig. 1A; data not shown). Given the conservation between Siahs and Sina, we expected that DCC would interact with Sina. This interaction was demonstrated readily (Fig. 1A). No interactions between the Sina/Siah proteins and irrelevant proteins were observed in the two-hybrid assay (Fig. 1A; data not shown). DCC interacted with the carboxy-terminal 140 amino acids of the Sina/Siah proteins (Fig. 1B).
In vitro binding of Sina and DCC
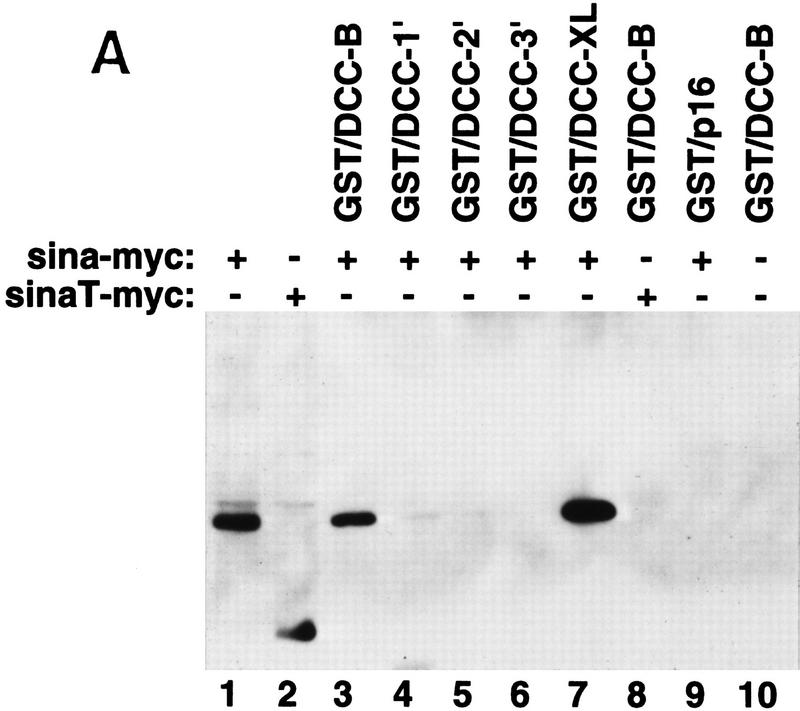
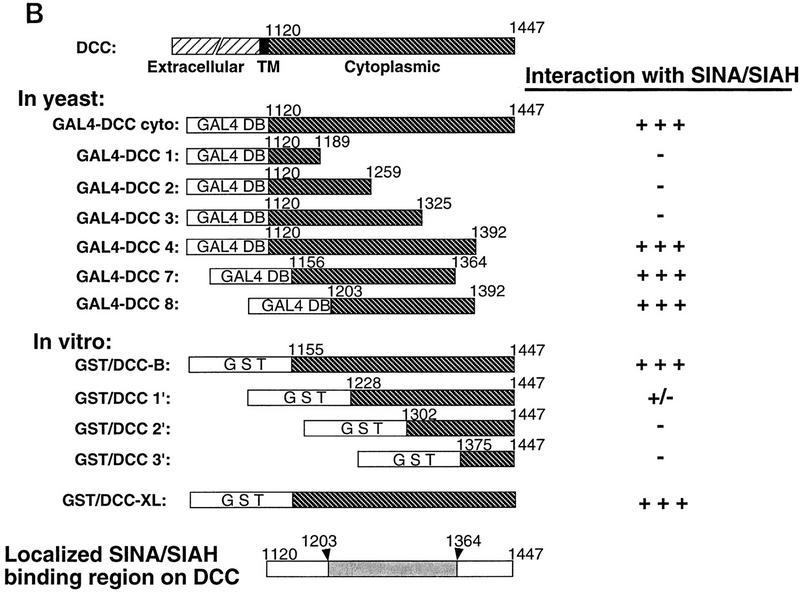
The interaction betwen DCC and Sina was also studied in an in vitro binding assay. Glutathione S-transferase (GST) fusion proteins containing various portions of the DCC cytoplasmic domain were expressed in Escherichia coli and affinity purified. Lysates were prepared from COS-1 cells that had been transfected transiently with an expression vector encoding a full-length Drosophila Sina protein tagged with a c-Myc epitope tag at its amino terminus. The lysates were then incubated with the various GST–DCC fusion proteins or control proteins. Proteins recovered on a glutathione–Sepharose matrix were washed extensively, eluted, and studied by Western blotting with a c-Myc antibody. DCC fusion proteins containing nearly the full-length human DCC cytoplasmic domain (i.e., GST/DCC-B) or the Xenopus DCC cytoplasmic domain (GST/DCC-XL) were readily able to capture Sina protein. Fusion proteins with substantial deletions of the DCC cytoplasmic domain and nonspecific control proteins (e.g., GST–p16) failed to bind Sina (Fig. 2A). Consistent with the two-hybrid results described above, a truncated Sina protein lacking the carboxy-terminal 115 amino acids failed to bind to DCC (Fig. 2A). In vitro binding and yeast two-hybrid studies indicated DCC amino acids 1203–1364 were needed for binding to Sina/Siah (Fig. 2B).
Figure 2.
Association of DCC and Sina in vitro. (A) In vitro binding assay for DCC and Sina. Lysates of COS-1 cells transfected with a c-Myc epitope-tagged sina cDNA (Sina–myc, lanes 1,3–7,9), a carboxy-terminal truncated form (SinaT–Myc, lanes 2,8), or empty expression vector (lane 10) were incubated with 10 μg of each affinity–purified GST fusion protein, including GST/DCC-B (amino acids 1155–1447, lanes 3,8,10); GST/DCC-1′ (amino acids 1228–1447, lane 4); GST/DCC-2′ (amino acids 1302–1447, lane 5); GST/DCC-3′ (amino acids 1375–1447, lane 6); GST/DCC–XL (cytoplasmic domain of Xenopus DCC, lane 7); and GST/p16 (human p16 cyclin kinase inhibitor, lane 9). GST fusion proteins were recovered by incubation with glutathione–Sepharose beads, and bound proteins were released and analyzed by Western blot analysis, using the c-Myc monoclonal antibody 9E10.2 and ECL. Lanes 1 and 2 are COS-1 lysates of Sina-Myc and SinaT–Myc, and represent the abundance of the Sina proteins in one-third of the lysate used for each in vitro binding experiment. (B) Mapping of the minimal region of the DCC cytoplasmic domain required for binding to Sina/Siah in yeast and in vitro.
DCC and Sina proteins colocalize
We sought to establish further the interaction between the DCC and Sina/Siah proteins and undertook immunofluorescence studies to determine if the proteins co-localized in transfected tissue culture cells. Using confocal microscopy, we found that the full-length DCC protein exhibited a membrane localization in Drosophila S2 cells (Fig. 3A), whereas a cDNA encoding only the DCC cytoplasmic domain yielded diffuse staining throughout the cytoplasm (Fig. 3B). Though previous studies had suggested that Sina was predominantly a nuclear protein (Carthew and Rubin 1990), we found that a full-length Sina protein tagged at its amino terminus with a c-Myc epitope localized predominantly in cytoplasmic particles (Fig. 3C). Studies of epitope-tagged Sina and human Siah-1 proteins in Chinese hamster ovary (CHO) cells also indicated a cytoplasmic localization for the Sina/Siah proteins (data not shown). Deletion of the carboxy-terminal 115 amino acids of Sina abrogated its localization to the cytoplasmic particles, resulting in a more uniform distribution of the truncated protein throughout the cell (Fig. 3D).
Figure 3.
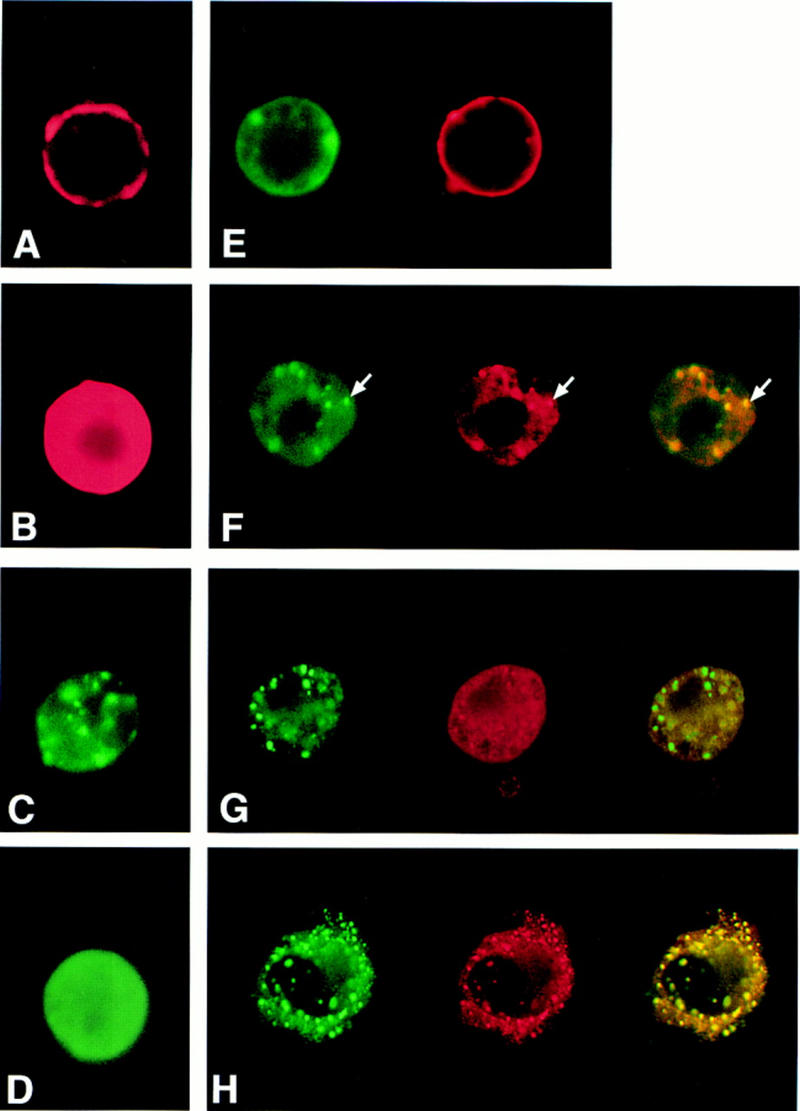
Confocal microscope images of DCC and Sina localization in Drosophila S2 cells and monkey kidney COS-1 cells. Constructs encoding full-length DCC protein (A,E), the DCC cytoplasmic domain (B,F,H) and carboxy-terminal truncated form of the DCC cytoplasmic domain (G) were expressed alone (A,B) or together with Sina (E–H) in Drosophila S2 cells (A–G) or COS-1 cells (H). Full-length Sina (C) or a carboxy-terminal truncated Sina protein (D) were also expressed alone in Drosophila S2 cells. The expression of DCC (red) and Myc-tagged sina (green) was detected by immunofluorescence double staining with a DCC rabbit polyclonal antibody and a c-Myc monoclonal antibody, followed by Texas red-conjugated goat anti-rabbit and FITC-conjugated goat anti-mouse secondary antibodies. The images were captured by confocal microscopy, and pseudo-coloring was performed using the Adobe Photoshop software program. Colocalization of the fluorescence signals was demonstrated by yellow signals when the red and green images were overlaid (e.g., indicated by arrows in F).
In S2 cells expressing both full-length DCC and Sina, we found that Sina protein was many times localized to the membrane (Fig. 3E), consistent with an interaction between DCC and Sina. In cells coexpressing Sina and the cytoplasmic form of DCC, Sina and DCC demonstrated colocalization in cytoplasmic particles (Fig. 3F). The colocalization required specific interaction between DCC and Sina because a further truncated form of the DCC cytoplasmic domain, lacking the Sina-binding sequences, no longer colocalized with Sina (Fig. 3G). Colocalization of DCC and Sina in cytoplasmic particles was also observed following transfection of COS-1 monkey kidney cells (Fig. 3H). However, in Drosophila S2 and COS-1 cells cotransfected with DCC and Sina, only a fraction of the cells expressed DCC, despite the fact that a sizeable proportion of the cells expressed Sina (data not shown). Because DCC was well-expressed on its own (see below), the findings indicated that Sina/Siah proteins might affect DCC expression.
Siah proteins regulate DCC protein expression
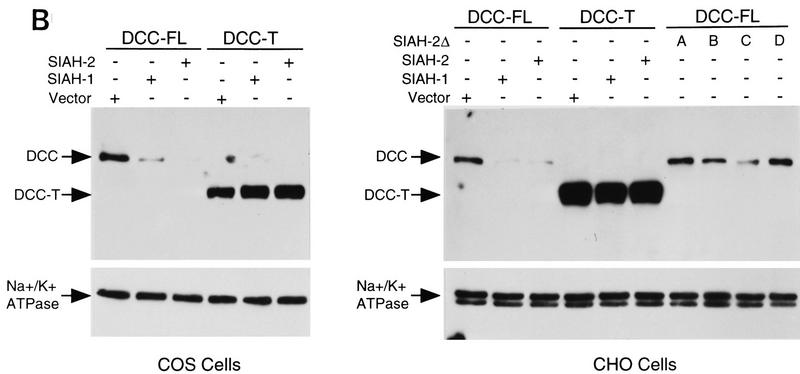
To determine whether the Sina/Siah proteins regulated DCC expression, we assessed their effects on DCC expression using Western blot analysis. COS-1 and CHO cells were cotransfected with mammalian expression vectors containing DCC and SIAH cDNAs (Fig. 4A). After transfection, DCC protein levels were assessed. In both COS-1 and CHO cells, comparison with the control transfection revealed that DCC expression levels were reduced markedly when full-length DCC (DCC-FL) was coexpressed with Siah-1 or Siah-2 (Fig. 4B). Similar findings were obtained when DCC-FL was coexpressed with Sina (data not shown).
Figure 4.
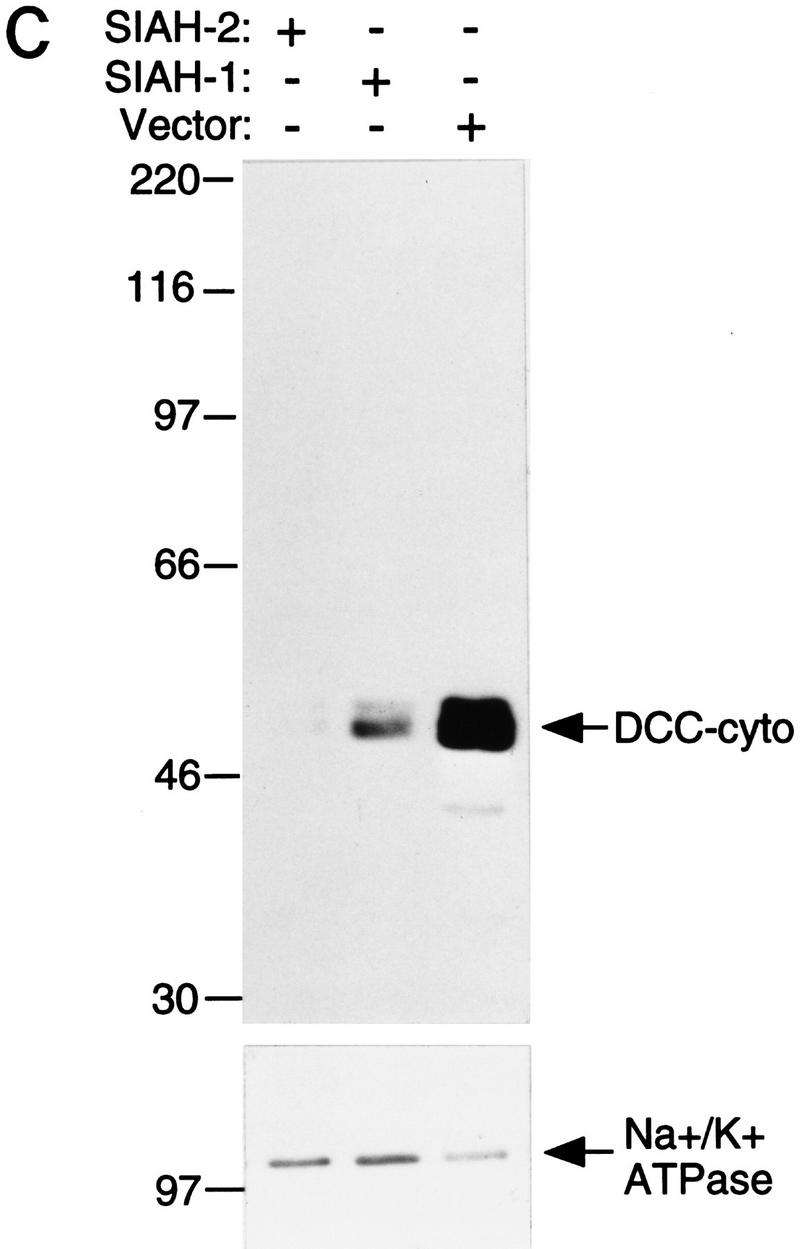
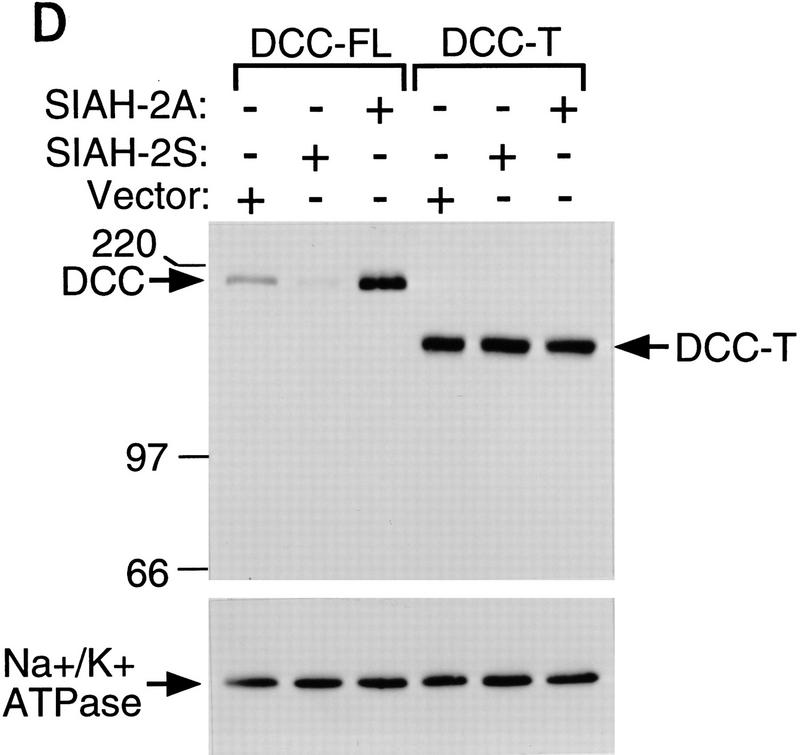
Siahs regulate DCC expression in mammalian cells. (A) Schematic representation of DCC and Siah proteins encoded by the cDNA constructs. The truncated forms of Siah-2 are indicated as Siah-2ΔA, SiahΔB, SiahΔC, SiahΔD. (B) Siah-1 and Siah-2 regulate the expression of full-length DCC (DCC-FL), but not that of a truncated form lacking most of the DCC cytoplasmic domain (DCC-T). As indicated, COS-1 and CHO cells were cotransfected with cDNAs for DCC-FL or DCC-T and Siah-1, Siah-2, Siah-2Δ’s, or the control (empty) expression vector. Forty-eight hours after transfection, Western blot analysis was carried out on the cell lysates, using DCC extracellular domain monoclonal antibody G92-13 and ECL reagents. The membranes were then stripped and ECL–Western blot analysis was performed with a polyclonal antibody against Na+/K+ ATPase to verify the loading. (C) The expression of a cytoplasmic form of DCC is regulated by Siahs. A cDNA encoding the DCC cytoplasmic domain (DCC-cyto) was cotransfected with Siah-1, Siah-2, or the control CMV expression vector (vector). The Western blot analysis of DCC and Na+/K+ ATPase was performed as above, except that ECL–Western blot analysis of DCC was carried out with DCC cytoplasmic domain monoclonal antibody G97–449. The mobility of molecular weight markers is indicated at the left. (D) Antisense SIAH-2 expression in COS-1 cells results in increased expression of DCC-FL, but has no effect on DCC-T expression. The studies were performed with the indicated vectors as described in B.
Ribonuclease protection assays demonstrated that DCC transcript levels were not affected by Siah expression. Moreover, Siah expression failed to affect the expression of a truncated DCC protein (DCC-T) lacking most of the DCC cytoplasmic domain (Fig. 4B). The expression of a control luciferase construct was not affected by Siahs (data not shown). Mutant forms of Siah-2 lacking either the carboxy-terminal DCC-binding sequences or amino-terminal sequences failed to down-regulate DCC expression (Figs. 4A,B). Studies with a vector encoding only the DCC cytoplasmic domain demonstrated that DCC need not be expressed on the cell surface to be down-regulated by Siahs (Fig. 4C). Furthermore, a construct expressing an anti-sense SIAH-2 cDNA led to an increased level of full-length DCC expression in COS-1 cells, but had no effect on the expression of the truncated form of DCC (Fig. 4D), suggesting that the anti-sense SIAH-2 transcripts inhibited the activity of endogenous Siah-2 on DCC expression in COS-1.
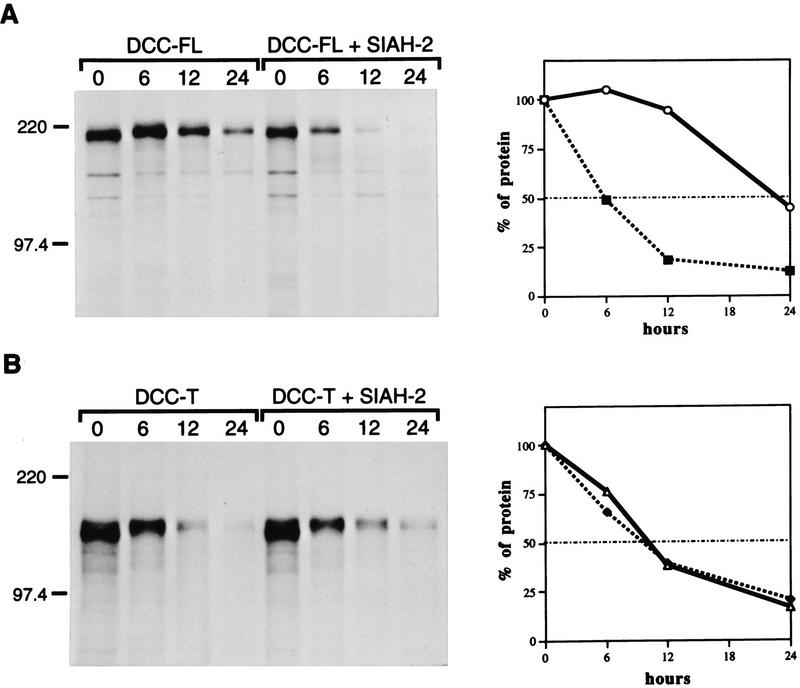
To assess the effects of the Sina/Siah proteins on DCC protein turnover, COS-1 cells were cotransfected with DCC and SIAH-2 cDNAs, and after transfection, the cells were metabolically labeled with a 1-hr pulse of 35S-labeled amino acids. Following varying chase periods, DCC proteins were collected by immunoprecipitation, separated by SDS-PAGE, and fluorography carried out. In cells transfected with full-length DCC and the control vector, the half-life of DCC protein was ∼24 hr (Fig. 5A). In contrast, cotransfection with a SIAH-2 cDNA reduced the half-life of DCC to ∼6 hr. As expected, SIAH-2 had no effect on the half-life of the DCC-T, although we did note that the DCC-T had a decreased half-life compared with full-length DCC (Fig. 5B). Taken together, the findings imply that the Siahs regulate DCC protein stability through interaction with the DCC cytoplasmic domain.
Figure 5.
Siah-2 decreases DCC half-life via its cytoplasmic domain. A pulse–chase analysis was undertaken to determine the half-life of DCC proteins in the presence or absence of Siah-2. COS-1 cells were cotransfected with expression constructs encoding full-length DCC (DCC-FL, A) or a truncated form of DCC lacking the majority of the cytoplasmic domain (DCC-T, B) and human Siah-2 (Siah-2) or the control expression vector. Forty-eight hours after transfection, the cells were pulse-labeled for 1 hr with a [35S]methione/cysteine mix, chased with cold methione/cysteine for the indicated times, and then lysed. DCC proteins were immunoprecipitated with polyclonal antibody 645 and analyzed by SDS-PAGE and fluorography (left). The mobility of molecular weight markers is indicated. The autoradiographic signals of the ∼190 kD DCC protein were quantitated by densitometry and the data were plotted as a function of time (right) (○) DCC-FL; (▪) DCC-FL and Siah-2 in A. (▵) DCC-T; (•) DCC-T and Siah-2 in B.
Ubiquitin-proteasome pathway in Sina/Siah regulation of DCC expression
The ubiquitin–proteasome pathway regulates degradation of many proteins (Varshavsky 1992; Ciechanover 1994; Goldberg 1995), and we sought to determine whether Siahs promoted DCC degradation through this pathway. COS-1 cells were cotransfected with DCC and Siah-2 or a control vector. After transfection, cells were subjected to a 6-hr treatment with various protease inhibitors, and DCC expression was then studied by Western blot analysis. The peptide aldehyde MG132, a potent inhibitor of proteasome function (Rock et al. 1994; Palombella et al. 1994), inhibited the effect of Siah-2 on DCC expression, whereas none of the other protease inhibitors tested displayed such activity (Fig. 6A; data not shown). The other inhibitors studied included calpain inhibitor II, a calcium-dependent cysteine protease inhibitor; E64, another potent cysteine protease inhibitor; chymostatin, a chymotrypsin inhibitor; AEBSF, a serine protease inhibitor; and leupeptin, a serine (and thiol) protease inhibitor. Additional studies demonstrated that 5–20 μm concentrations of MG132 were sufficient for blocking the effects of Siah-2 on DCC stability in COS-1 cells (Fig. 6B). Such concentrations are within the range in which MG132 specifically inhibits proteasome activity in cells (Palombella et al. 1994).
Figure 6.
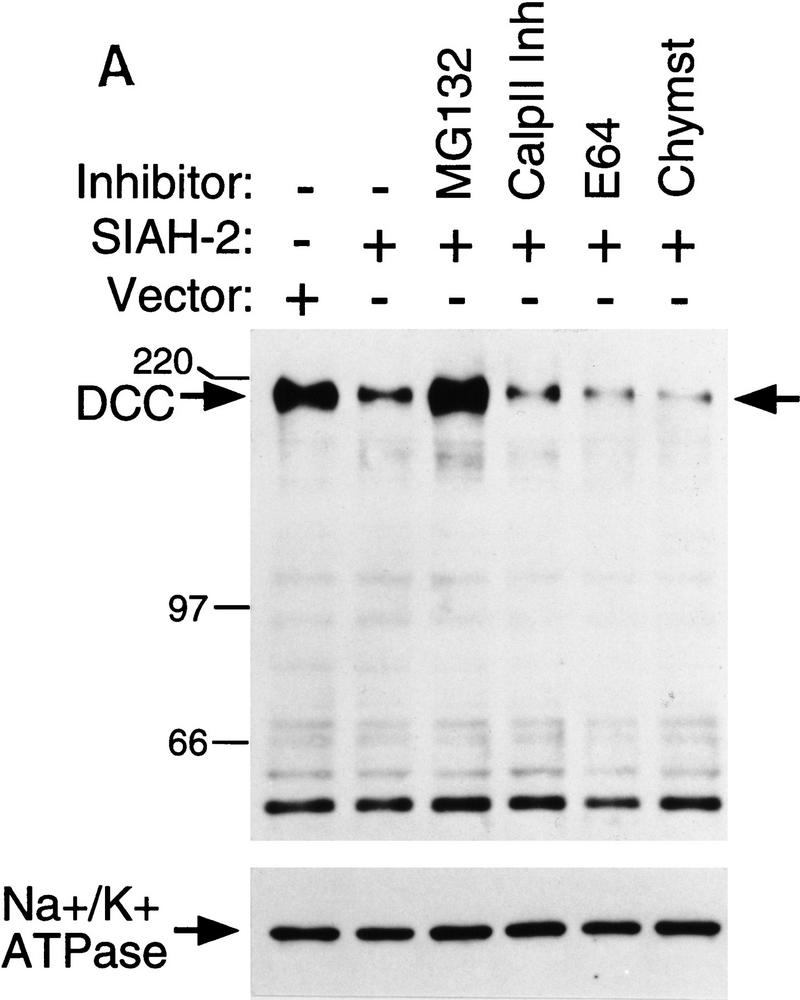
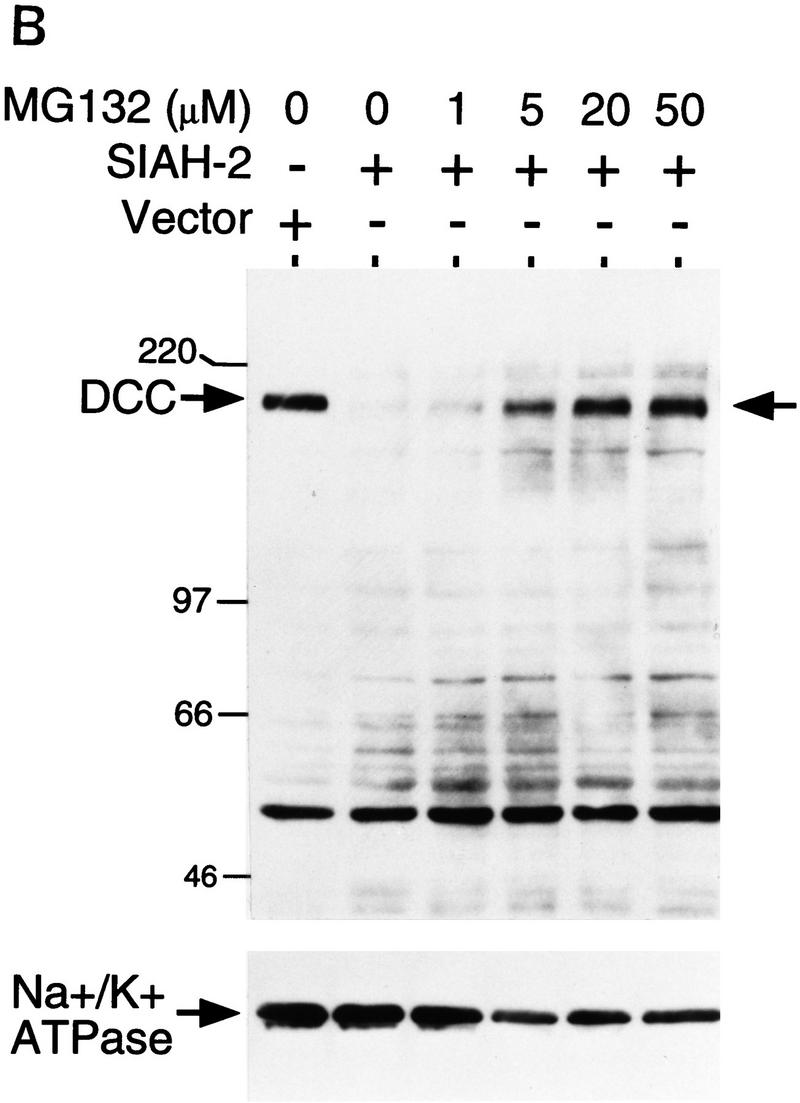
Effects of Siah-2 on DCC expression are blocked by the proteasome inhibitor MG132. (A) Forty-eight hours after co-transfection of COS-1 cells with expression vectors encoding full-length DCC and Siah-2 or a control vector, cells were incubated with dimethyl sulfoxide (DMSO; − inhibitor lane) or various protease inhibitors for 6 hr at 37°C. The final concentrations of the inhibitors in the media were: MG132, 20 μm; Calpain inhibitor II (Calp II Inh), 20 μm; E64, 50 μm; chymostatin (Chymst), 50 μm. After incubation, lysates were prepared and the levels of DCC expression were detected by Western blot analysis using DCC extracellular domain monoclonal antibody G92-13 and ECL reagents. Membranes were then stripped and Western blotted with an antibody against Na+/K+ ATPase to verify loading. Mobility of molecular weight markers is indicated at the left. (B) Dose response of MG132 inhibition of Siah-2-mediated DCC degradation. The studies were carried out as described in A.
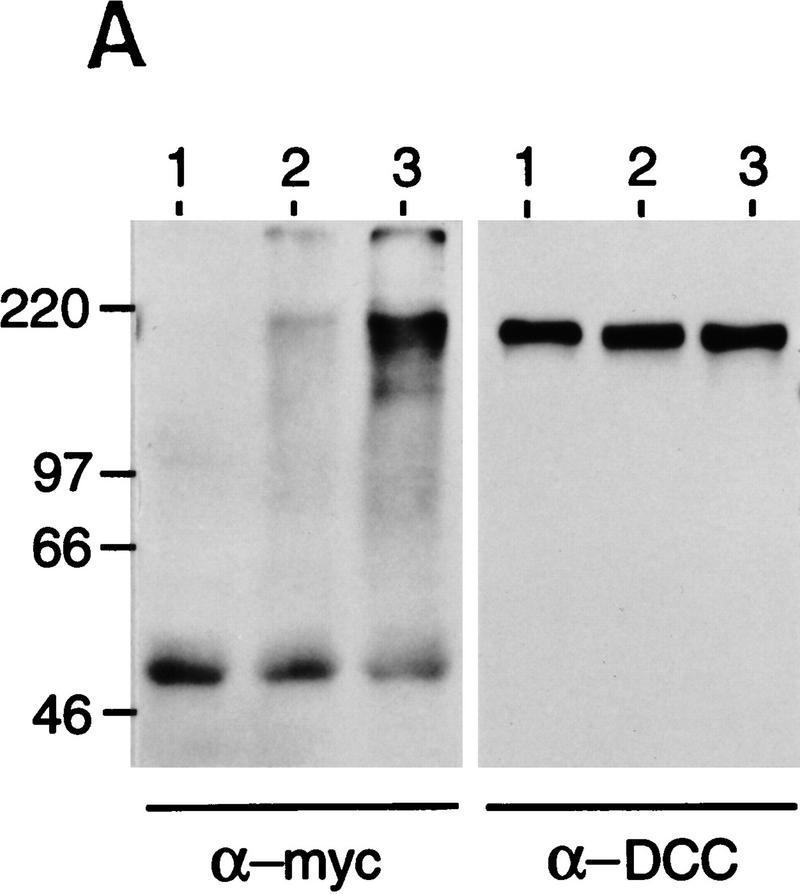
Ubiquitination and polyubiquitination target proteins for degradation by the proteasome (Varshavsky 1992; Ciechanover 1994; Goldberg 1995). To examine whether DCC is ubiquitinated in cells, DCC was coexpressed in COS-1 cells along with a construct encoding a c-Myc epitope-tagged yeast ubiquitin (c-Myc/Ub). Lysates were prepared from the cells and DCC protein was collected by immunoprecipitation. Following electrophoresis, the immunoprecipitates were studied first by Western blot analysis with an antibody against the c-Myc epitope. Following cotransfection of the cells with the DCC and c-Myc/Ub constructs, the c-Myc-epitope-tagged ubiquitin was linked covalently to DCC proteins of ∼220 kDa or greater (Fig. 7A, left panel, lane 2, data not shown). Increased levels of ubiquitin were detected on DCC when the cells were cotranfected with expression constructs for DCC, c-Myc/Ub, and Siah-2 and treated with MG132 (Fig. 7A, left panel, lane 3). Subsequent Western analysis revealed that equivalent amounts of DCC protein were present in all lanes. The findings indicate that DCC can be ubiquitinated in cells.
Figure 7.
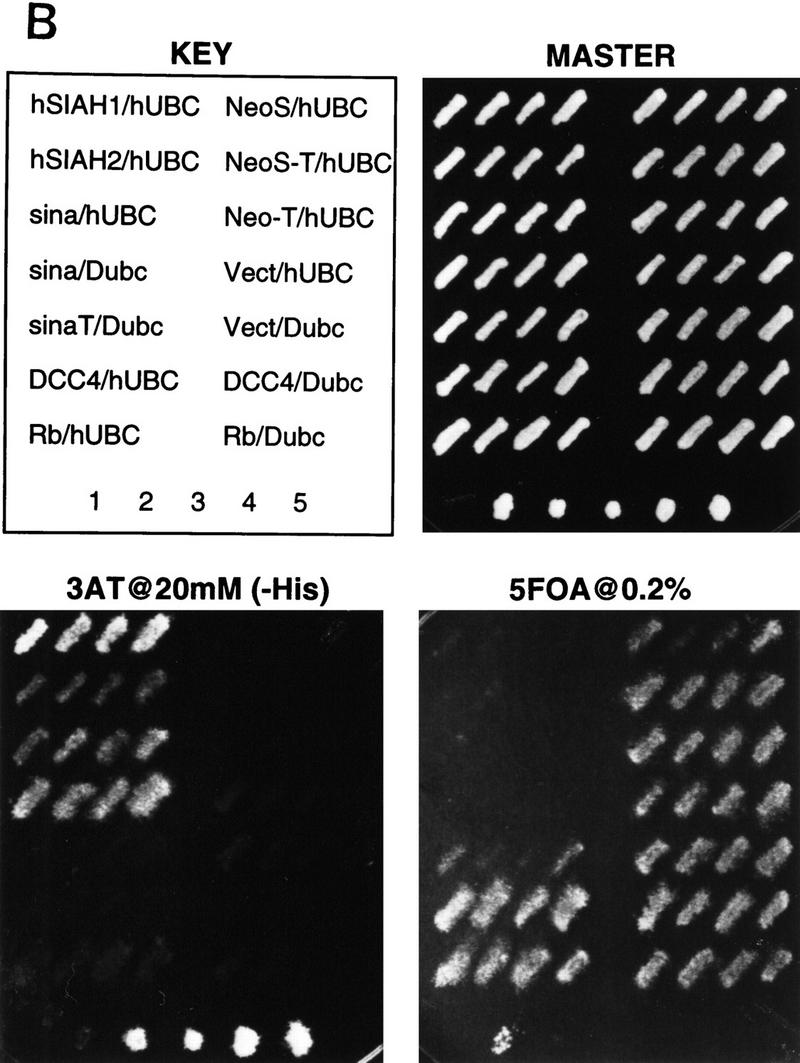
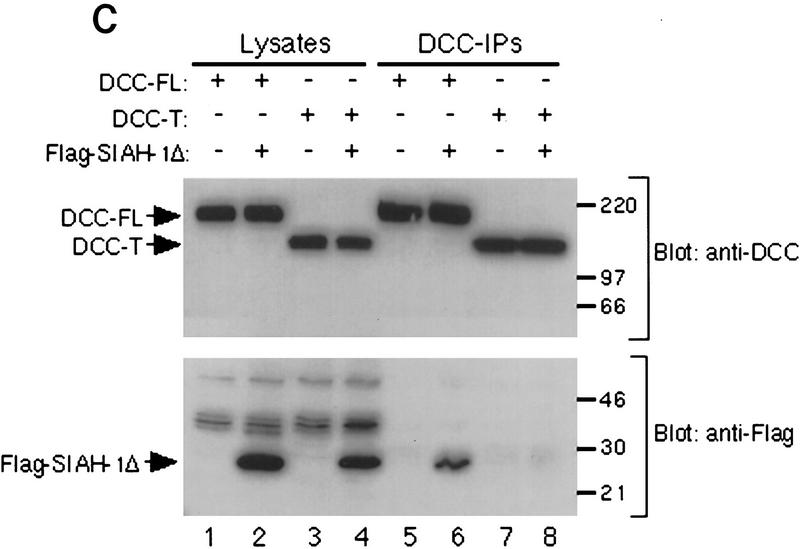
DCC, Sina/Siah, the ubiquitin pathway, and stable interactions between DCC and a mutant form of Siah-1. (A) DCC is ubiquitinated. COS-1 cells were cotransfected with vectors encoding full-length DCC (lanes 1–3), a c-Myc epitope-tagged yeast ubiquitin (lanes 2,3), Siah-2 (lane 3), and control vector (lane 1). Forty-eight hours after transfection, the cells were treated for 6 hr at 37°C with 20 μm MG132 dissolved in DMSO (lane 3) or the same concentration of DMSO (lane 1 and 2). DCC proteins were collected by immunoprecipitation with DCC polyclonal antibodies 645 and 723. The immunoprecipitates were electrophoresed and Western blot analysis was performed to identify DCC proteins to which the c-Myc tagged ubiquitin polypeptide had been covalently attached, using c-Myc monoclonal antibody 9E10.2 and ECL reagents (left panel). After stripping the blot, the abundance of DCC protein in each lane was determined by Western blot analysis with DCC extracellular domain monoclonal antibody G92-13 and ECL reagents (right panel). The mobility of molecular weight markers is indicated at the left. (B) Sina and human Siah homologs interact with ubiquitin-conjugating (Ubc) proteins in the yeast two-hybrid system. DB fusion constructs and AD fusion constructs were cotransformed into yeast strain MaV103. (DB fusions) Full-length human Siah1 and Siah2 (hSiah1 and hSiah2); full-length Drosophila Sina (sina); amino-terminal truncated Sina (sinaT); human DCC cytoplasmic domain (DCC4, amino acids 1120–1392); human retinoblastoma protein (Rb); truncated form of human neogenin cytoplasmic domain (Neo-T); spliced form of human neogenin cytoplasmic domain (NeoS); and the truncated and spliced form of human neogenin cytoplasmic domain (NeoS-T). (AD fusions) Human homolog of yeast UBC9 (hUBC) and Drosophila homolog of UBC9 (Dubc). Yeast transformations, growth, reporter gene studies, and interaction controls 1–5 were as described in Materials and Methods and the legend to Fig. 1. (C) Siah-1 protein lacking amino-terminal Ubc-binding sequences forms a complex with DCC in cells. CHO cells were transfected with vectors encoding the indicated proteins. The pcDNA3 construct for the FLAG epitope-tagged, truncated Siah-1 (i.e., FLAG–Siah1Δ) has the FLAG peptide tag MDYKDDDDK fused to amino acids 77–282 of Siah-1. Lysates were prepared 48 hr after transfection, and a portion of the lysate was used for immunoprecipitation with DCC polyclonal antibody 645, directed against the DCC extracellular domain. The lysates (lanes 1–4) and immunoprecipitates (lanes 5–8) were electrophoresed and ECL–Western blotting was carried out with a monoclonal antibody against the extracellular domain of DCC or against the FLAG epitope. Siah-1 coprecipitated with full-length DCC (DCC-FL) (lane 5) but not with a truncated form of DCC (DCC-T), lacking the DCC cytoplasmic domain (lane 8). The mobilities of molecular weight standards and the DCC and FLAG–Siah1Δ proteins are indicated.
Given that the Sina/Siah proteins appear to regulate DCC via the ubiquitin–proteasome pathway, we sought to obtain more detailed insights into their function. We undertook studies with the yeast two-hybrid system using full-length Drosophila Sina fused to the GAL4 DNA-binding domain to screen a Drosophila SL2 cDNA library. Roughly 0.5 × 106 transformants were obtained and selected for reporter-dependent 3-AT resistance. Further selection and retesting yielded three independent cDNAs that demonstrated specific interaction with Sina. Sequence analysis revealed that one of the three clones encoded a Drosophila homolog of the Saccharomyces cerevisiae Ubc9 (ubiquitin-conjugating) protein (Seufert et al. 1995). Additional studies were undertaken to characterize the interaction of Sina/Siah proteins with Ubc9 homologs. Similar to the interaction between sina and the Drosophila Ubc9 homolog, both human Siah-1 and Siah-2 interacted in the yeast two-hybrid system with a human Ubc9 homolog cloned previously (Kovalenko et al. 1996) (Fig. 7B). Truncation of the amino-terminal 114 amino acids of Sina, including its RING finger domain, inhibited its interaction with Drosophila Ubc9 (Fig. 7B; data not shown), suggesting the amino-terminal region of Sina is critical for the interaction with Ubc9. This finding complements our prior observation that the amino-terminal region of Siah-2 is not needed for binding to DCC in yeast but is critical for down-regulation of DCC expression in mammalian cells (Fig. 4A,B). Furthermore, following their expression in CHO cells, we were able to coprecipitate full-length DCC and an epitope-tagged mutant form of Siah-1 lacking the amino-terminal Ubc-binding sequences (Fig. 7C). As expected, Siah-1 did not coprecipitate with a cytoplasmic domain truncated form of DCC (DCC-T). These findings establish that Sina/Siah proteins can complex with DCC in cells.
Interaction between DCC and Sina in the developing Drosophila eye
We next generated transgenic Drosophila lines in which the human DCC gene was expressed in the developing eye under control of sev promoter/enhancer elements (sev–DCC). Although no obvious phenotype was seen in several independent transgenic lines carrying one copy of sev–DCC, eye phenotypes in flies with multiple copies of sev–DCC were evident. Scanning electron micrograph studies revealed that the two copy (2×) sev–DCC flies had moderately rough eyes and four copy (4×) sev–DCC flies had very rough eyes (Fig. 8; C–F). Eyes of sev–DCC flies were smaller than normal, with apparent fusion of ommatidia and bristle defects. Apical sections through adult retinae demonstrated defects in the orientation of the ommatidia with respect to dorsal/ventral and/or anterior/posterior axes (Fig. 9). sev–DCC flies also had fusion of ommatidia arising apparently, at least in part, from defects in formation of pigment cells at the boundaries between adjacent ommatidia. Only ∼30% of the ommatidia were surrounded fully by pigment cells in the 2× sev–DCC flies (Fig. 9). Interestingly, we noted that ∼10% of the ommatidia in the 2× sev–DCC flies lacked R7 photoreceptor cells (Fig. 9C,D), and in 4× sev–DCC flies, upward of 60%–80% of ommatidia were missing R7 cells (Fig. 9E,F). In a small subset of ommatidia of sev–DCC flies, loss of outer photoreceptor cells was also noted (Fig. 9D).
Figure 8.
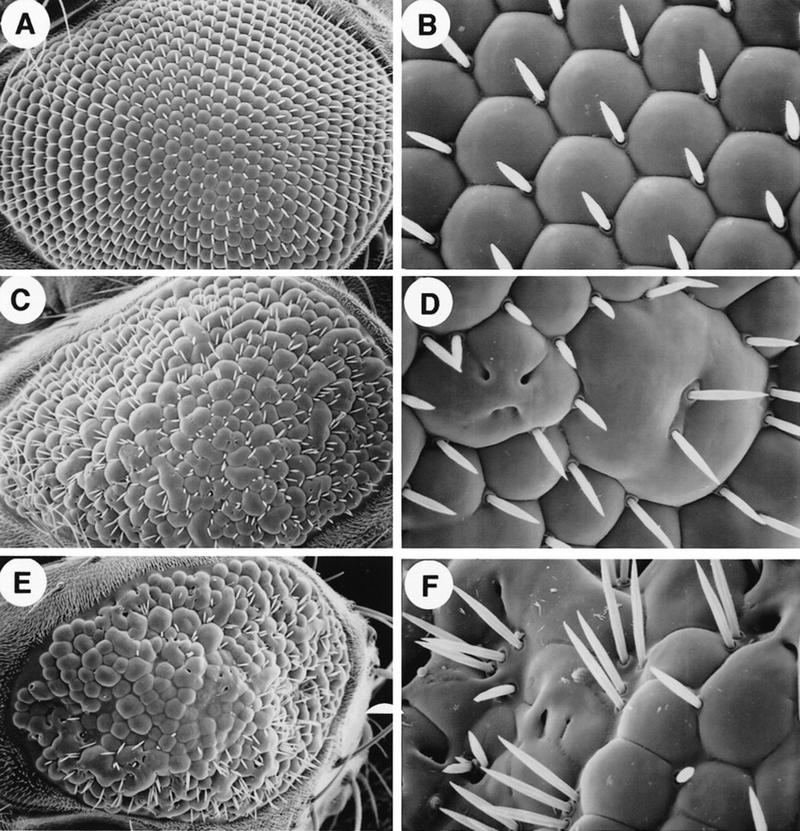
Ectopic expression of human DCC in the Drosophila eye causes rough eye phenotypes. Full-length human DCC cDNA coding sequence were expressed in transgenic flies under the transcriptional control of Drosophila sev promoter and enhancer elements. Shown are low (left panels) and high (right panels) power scanning electron micrographs of adult Drosophila eyes: Wild type (A,B); 2× sev–DCC (C,D); 4× sev–DCC (E,F).
Figure 9.
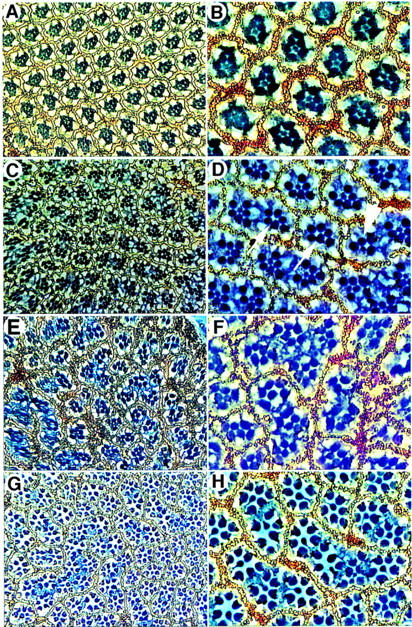
Ommatidial defects are seen in apical retinal sections from sev–DCC flies. Low power (left panels) and high power (right panels) images of apical retinal sections from flies with various genotypes are shown: Wild type (A,B); 2× sev–DCC (C,D); 4× sev–DCC (E,F); and 2× sev–DCC, 1× sev–sina (G,H). Ommatidia with R7 photoreceptor cells missing are indicated by white arrows in D; the arrowhead indicates an ommatidia missing an outer photoreceptor cell.
As noted above, loss of R7 cells is the predominant eye defect seen in flies with sina loss-of-function mutations, although some outer photoreceptors are also lost in flies with strong alleles (Carthew and Rubin 1990). Therefore, we speculated that ectopic expression of human DCC in the developing eye might interfere with Sina function and cause the R7 defects observed in the sev–DCC flies. To test this proposal directly, we generated 2× sev–DCC flies that also had one copy of sev–sina (in addition to their two functional endogenous sina alleles). Eyes of sev-sina flies had no morphological defects (data not shown). In contrast to the absence of R7 cells in ∼10% of the ommatidia of the 2× sev–DCC flies, we found that only ∼2% of ommatidia lacked R7 when the sev–sina transgene was also present (Fig. 9G,H). Three independent eyes were counted for each genotype to determine the effects on R7 formation in the 2× sev–DCC (451 total ommatidia) and the 2× sev–DCC, 1 × sev–sina flies (581 total ommatidia). Overall eye roughness and the ommatidial fusion and orientation defects were not affected significantly by increased expression of sina (Fig. 9; data not shown), suggesting that some eye defects in the sev–DCC flies likely reflect interaction of DCC with other signaling pathways. Nonetheless, the transgenic fly data support further that the in vivo relevance of the interaction between the DCC and Sina/Siah proteins.
Discussion
Recent findings have implicated DCC as a receptor or coreceptor for netrin-1 and have highlighted the important role of netrins, DCC, and DCC-related proteins in the development of the nervous system (Chan et al. 1996; Keino-Masu et al. 1996; Kolodziej et al. 1996; Serafini et al. 1996; Fazeli et al. 1997; Kolodziej 1997). The studies presented here were undertaken with the goal of identifying proteins that regulate DCC or transduce signals through its cytoplasmic domain. As a result of these investigations, we have obtained considerable evidence that Sina/Siah proteins regulate DCC via the ubiquitin–proteasome pathway.
Sina was first identified because its inactivation causes the R7 cell to adopt a cone (non-neuronal) cell fate (Carthew and Rubin 1990). Expression of the sina gene has been detected in various Drosophila cell types, and sina mutations also cause other phenotypes (Carthew and Rubin 1990). The role of Sina in R7 development is much better understood than its role in other cell types, and genetic experiments indicate that Sina functions downstream of defined components of the sev pathway, including Ras, Raf, and MAPK (Fortini et al. 1992; Lai and Rubin 1992; Brunner et al. 1994; Chang et al. 1994; Chang et al. 1995; Dickson et al. 1995). For example, loss-of-function mutations in sina inhibit activated Ras, MAPK, or ectopically expressed phyllopod (PHYL) from inducing R7 development. Recent data suggest that only ttk, which encodes a zinc finger transcription factor, functions downstream of sina (Lai et al. 1996; Yamamoto et al. 1996). No specific cellular function of Sina was known previously.
Cellular proteins targeted for degradation by the proteasome are modified covalently by the attachment of ubiquitin, which is linked to the ε-amino group of one or more lysines of the target protein in a multi-step process involving several key proteins, including an E1 ubiquitin-activating enzyme, an E2 Ubc, and often an E3 ubiquitin–protein ligase (Ciechanover 1994; Goldberg 1995). Ubiquitinated and polyubiquitinated proteins are recognized and proteolyzed by the proteasome, a large ATP-dependent protease complex. The specific means by which the Sina/Siah proteins promote DCC degradation via the ubiquitin–proteasome pathway has not yet been defined. However, we have demonstrated that Sina/Siah proteins interact in yeast via their amino-terminal sequences with Ubc9 homologs. Moreover, mutant Siah proteins lacking these amino-terminal sequences complexed stably with DCC in cells, but failed to promote its degradation.
The processes of ubiquitination and deubiquitination have been implicated previously in neural development and R7 fate specification. The Drosophila bendless (ben) gene has an important role in synaptic connectivity (Muralidhar and Thomas 1993; Oh et al. 1994). Defects in ben mutants include altered morphology of photoreceptor neurons, abnormal projections of the R7 and R8 axons, as well as deformation of the R7 cell. ben encodes a protein with significant similarity to Ubc proteins, including Ubc9 homologs. Inactivation of the fat facets gene results in supernumerary R7 formation (Fischer-Vize et al. 1992). Intriguingly, fat facets encodes a protein that can cleave ubiquitin from ubiquitin–protein conjugates, presumably inhibiting the degradation of specific proteins that repress R7 fate determination (Huang et al. 1995).
As noted above, Sina is expressed in a number of Drosophila tissues and its inactivation leads to other phenotypes besides R7 loss, including defects in sensory bristle formation, a 10-fold reduction in adult lifespan, generally lethargic and uncoordinated adult behavior, and infertility in males and females (Carthew and Rubin 1990). Mammalian Siahs are conserved extremely well with Sina, with upward of 85% amino acid identity, and Siahs are expressed at low levels in many adult tissues (Della et al. 1993; Nemani et al. 1996; Hu et al. 1997). In addition, DCC, DCC-related proteins, and netrins are expressed at reduced levels in many embryonic and adult tissues (Fearon et al. 1990; Choung et al. 1994; Kennedy et al. 1994; Pierceall et al. 1994a; Vielmetter et al. 1994; Chan et al. 1996; Kolodiej et al. 1996; Meyerhardt et al. 1997; Kolodiej 1997). Therefore, the interaction between Sina and DCC may be important in modulating DCC function in a variety of tissues, perhaps regulating the migration and/or differentiation of cells in response to netrin cues. Additional studies of the Sina/Siah proteins and the proteins they regulate are likely to further our understanding of the means by which the ubiquitin–proteasome degradation pathway regulates cell fate decisions in the developing eye and other tissues.
Materials and methods
Molecular biology
Yeast two-hybrid plasmid constructs
The pPC97–CYH2 and the pPC86 plasmids (Chevray and Nathans 1992; Du et al. 1996; Vidal 1997) encode the DNA-binding domain (amino acids 1–147) and the transcriptional activation domain (amino acids 768–881) of yeast GAL4, as well as LEU2 and TRP1 selectable markers, respectively. All DNA-binding domain and transcriptional acitivation domain fusions were generated by cloning cDNA fragments in-frame downstream of the DNA-binding or transcriptional activation domains, and constructs were confirmed by sequencing and/or fusion protein expression and interaction studies. The DNA-binding fusions (pPC97) include pPC97–DCCcyto (DCCcyto), amino acids 1120–1447 of DCC; pPC97–DCCcytoΔ1(DCC1), amino acids 1120–1189 of DCC; pPC97–DCCcytoΔ2 (DCC2), amino acids 1120–1259 of DCC; pPC97–DCCcytoΔ3 (DCC3), amino acids 1120–1325 of DCC; pPC97-DCCcytoΔ4 (DCC4), amino acids 1120–1392 of DCC; pPC97–DCCcytoΔ7 (DCC7), amino acids 1156–1364 of DCC; pPC97–DCCcytoΔ8 (DCC8), amino acids 1203–1392 of DCC; pPC97–Neo, amino acids 1128–1461 of human neogenin; pPC97–NeoT, amino acids 1128–1407 of neogenin; pPC97–NeoS, amino acids 1128–1249/1303–1461 of the spliced form of human neogenin; pPC97–NeoS-T, amino acids 1128–1249/1303–1407 of the spliced form of neogenin; pPC97–Rb, amino acids 302–928 of pRb (19); pPC97–cFos, amino acids 132–211 of cFos (13); pPC97–dE2F, amino acids 342–437. The activation domain fusions (pPC86−) include pPC86–Sina, amino acids 1–314 of Sina (6); pPC86–SinaT, amino acids 115–314 of Sina; pPC86–hSiah1, amino acids 1–282 of human Siah-1; pPC86–hSiah2, amino acids 1–324 of human Siah-2; pPC86–hSiah2Δ1, amino acids 103–324 of human Siah-2; pPC86–hSiah2Δ2, amino acids 103–287 of human Siah-2; pPC86–hSiah2Δ4, amino acids 198–324 of human Siah-2; pPC86–mSiah-2Δ, amino acids 115–325 of mouse Siah-2; pPC86–HUBC9, amino acids 1–158 of human UBC9; pPC86–Dubc, full-coding region of Drosophila UBC9; pPC86–E2F1, amino acids 159–437 of E2F1; pPC86–Jun, amino acids 250–334 of Jun (13); pPC86–dDP, amino acids 1–410 of DP-1 (18).
S2 expression constructs
The Drosophila heat shock-inducible vector pCaSpeR–hs and metallothionein-inducible vector pMT (Busseau et al. 1994) were gifts from Spyros Artavanis-Tsakonas and were used to generate Sina and DCC expression constructs, respectively. Amino-terminal c-Myc epitope-tagged Sina (amino acids 2–314) and SinaT (amino acids 2–199) cDNA fragments were generated by PCR and cloned into the EcoRI and XbaI sites of the pCaSpeR–hs vector (pCaSpeR–hs–SinaNMyc and pCaSpeR–hs–SinaTNMyc). The carboxy-terminal vesicular stomatitis virus glycoprotein (VSVG) epitope-tagged human DCC cytoplasmic domain was inserted into pMT at EcoRI–SalI site to construct pMT–DCCcyto. A 3.4-kb XhoI–BamHI fragment of DCC cDNA was cloned into pMT–DCCcyto to generate a full-length DCC cDNA in pMT (pMT–DCC). pMT–DCCcytoT was made by deleting the EcoRV–ClaI 0.5-kb fragment from pMT–DCCcyto. The sequences of all vectors generated by PCR were verified.
Mammalian expression constructs
The mammalian expression vector pcDNA3 (Invitrogen, San Diego, CA) was used to generate Sina and Siah expression constructs. To generate full-length sense and antisense SIAH-1 and SIAH-2 expression constructs (pcDNA3–Siah1, pcDNA3–Siah2 and pcDNA3–Siah2–AS), a 2.2-kb EcoRI–EcoRI cDNA fragment of human siah-2 and a 2.0-kb EcoRI–EcoRI cDNA fragment of human siah-1 were cloned into pcDNA3. Constructs encoding truncated forms of human Siah-2 [pcDNA3–Siah2–Δ(A, B, C, D)], were obtained by cloning of SIAH-2 cDNA fragments (amino acids 118–324, amino acids 118–287, amino acids 118–231, amino acids 1–23, and 72-231, respectively) into EcoRI–XbaI sites of pcDNA3. The pcDNA3 construct encoding the FLAG epitope-tagged mutant form of Siah-1 (i.e., FLAG–Siah1Δ) was generated by removal of the first 76 amino acids of Siah-1 and introduction of the FLAG peptide tag MDYKDDDDK upstream of amino acid 77. pcDNA3–SinaNMyc and pcDNA3–SinaTNMyc were constructed by subcloning the corresponding EcoRI–XbaI fragments from pCaSpeR–hs–SinaNMyc and pCaSpeR–hs–SinaTNMyc into pcDNA3. Constructs encoding full-length DCC (DCC-FL) and a cytoplamic truncated form (DCC-T) have been described (Pierceall et al. 1994b). The ubiquitin expression construct encoding a His6-, c-Myc-tagged yeast ubiquitin was kindly provided by Dr. Ron R. Kopito (Ward et al. 1995).
Drosophila P-element sev–DCC construct
A full-length human DCC cDNA fragment obtained from pBSII–DCC was inserted into ClaI–XbaI sites downstream of fly sev promoter cassette in pSP/HSS. The sev promoter/DCC fusion was then cloned into the NotI site in the sev enhancer construct pSE8/DM30. Details of the sev promoter/enhancer constructs are described in Dickson et al. (1992).
cDNA libraries
The Glio I cDNA library in the pPC86 vector was prepared using mRNA from human glioblastoma xenograft specimens kindly provided by Dr. Sandra Bigner. Three different tumor specimens with high (656), moderate (643), and absent (542) DCC expression (Ekstrand et al. 1995) were used, and mRNA was prepared from 0.2 grams of each tumor sample with the Micro Fast Track mRNA isolation kit (Invitrogen). cDNA was synthesized using NotI–poly(dT) primers and was blunt end ligated to SalI adaptors, using the SuperScript plasmid system for cDNA synthesis and cloning (GIBCO BRL/Life Technologies, Grand Island, NY). After digestion with NotI, cDNAs were size-selected and ligated into pPC86 at SalI–NotI site. Approximately 1.2 × 106 independent transformants were obtained, amplified, and used for DNA isolation from the Glio I library. The Alala I library was generated from polyA-selected RNA from 12- to 13-day whole murine fetuses. cDNAs were cloned into the EcoRI and SpeI sites of pPC86, using methods similar to those described above for the Glio I library. The library had a complexity of roughly 3.0 × 106 independent clones prior to amplification. The Drosophila SL2 library has been described (Du et al. 1996). To isolate full-length human siah-1 and siah-2 cDNAs, a human fetal brain cDNA library constructed in the λZap phage vector was purchased from Stratagene (La Jolla, CA). Approximately 1.5 × 106 plaques were screened with a mouse siah-2 cDNA fragment, obtained from the two-hybrid screen, as probe. Filters were lifted and hybridized with radiolabeled probe using standard techniques. Plasmids were rescued from the λZap phage by in vivo excision with F1 helper phage (Stratagene).
Yeast two-hybrid studies
All two-hybrid studies were performed with the yeast strain MaV103 (Du et al. 1996; Vidal 1997) and transformation was performed using the standard lithium acetate method. Transformants were first plated onto synthetic complete (SC)–Leu–Trp plates, followed by replica plating onto SC–Leu–Trp–His plates containing 20 mm 3-AT after 2 days. Yeast colonies growing out after additional 3–4 days of incubation were picked as primary positives and further tested in three reporter assays: (1) growth on SC–Leu–Trp–His plates supplemented with 20 mm 3-AT; (2) β-galactosidase activity; and (3) URA3 activation on SC–Leu–Trp plates containing 0.2% 5-fluoro-orotic acid (5-FOA), as a counterselection method (Vidal 1997). Activation domain plasmids were rescued from the selected positive transformants after all three tests. MaV103 cells were retransformed with either rescued activation domain plasmid and the DNA-binding domain fusion or rescued activation domain plasmid and the pPC97–CYH2 vector without a cDNA insert as a control. Activation domain plasmids, repeatedly positive in all three reporter assays, recovered and sequenced either manually or with the automated Applied Biosystems, Inc. system.
Cell culture and DNA transfection
Drosophila SL2 cells were grown as described (Fehon et al. 1990). Transfection of 1 × 106 cells/ml was carried out in 6-well plates with 10 μg of plasmid DNA and Lipofectin (GIBCO BRL). Protein expression was induced with 0.7 mm CuSO4 and two to three rounds of heatshock, as described (Diederich et al. 1994). COS-1 cells obtained from the American Type Culture Collection (ATCC, Rockville, MD) were maintained in Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. CHO cells were obtained from the ATCC and grown in MEMα medium plus 10% FBS and antibiotics. Mammalian cell transfections were carried out by using Opti-MEM reduced serum medium and LipofectAmine reagent, as described by the manufacturer (GIBCO BRL).
In vitro binding assay
All GST–DCC fusions were constructed in the pGX–2TK vector (Pharmacia Biotech, Uppsala, Sweden), and verified by sequencing. The construct for the GST-p16 (human p16 cyclin kinase inhibitor) fusion protein was kindly provided by Dr. Joseph Germino (Yale University, New Haven, CT). GST fusion proteins were expressed and purified as described (Reale et al. 1994). COS-1 cells were transfected with pcDNA3–SinaNMyc or pcDNA3–SinaTNMyc. Forty-eight hours after transfection, the cells were lysed with TBS–Triton lysis buffer (Tris-buffered saline at pH 8.0, 1% Triton, 10 μg/ml of PMSF, 50 μg/ml of antipain, 5 μg/ml of aprotinin, 2 μg/ml of leupeptin). In vitro binding assays were performed by mixing 10 μg of GST fusion proteins with cell lysate and incubated with 15 μl of glutathione–Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) in 500 μl of TBS–Triton buffer supplemented with 1% BSA at 4°C for 4 hr with agitation. After incubation, the mixtures were pelleted, and the pellets were washed with cold TBS–Triton lysis buffer three times. Bound proteins recovered from the beads were separated by SDS-PAGE. After semi-dry transfer (Transblot, Bio-Rad, Hercules, CA) to Immobilon (Millipore, Bedford, MA) membranes, c-Myc epitope-tagged Sina proteins were detected by Western blotting with an anti-Myc monoclonal antibody MYC1-9E10.2 (ATCC) and enhanced chemiluminescence (ECL).
DCC Western blot and immunoprecipitation studies
COS-1 or CHO cells were cotransfected with pCMV–DCC-FL or pCMV–DCC-T constructs and pcDNA3–Sina/Siah constructs or pcDNA3 alone using cationic liposomes (LipofectAMINE reagent, GIBCO/BRL). Approximately 48 hr after transfection, cells were lysed in 0.1% NP-40 buffer containing 50 mm HEPES (pH7.2), 250 mm NaCl, 10% glycerol, 2 mm EDTA and protease inhibitors (50 μg/ml of antipain, 5 μg/ml of aprotinin, 2 μg/ml of leupeptin and 10 μg/ml of phenylmethylsulfonyl fluoride). Cell lysates were separated by SDS-PAGE and transferred to Immobilon membranes (Millipore) followed by ECL–Western blot analysis with DCC polyclonal (Reale et al. 1994) and monoclonal antibodies (G97–449 and G92–13, PharMingen, San Diego, CA). DCC proteins were immunoprecipitated with polyclonal antibody 645 or by combination of DCC polyclonal antibodies 645 and 723 (Reale et al. 1994). A polyclonal antibody against Na+/K+ ATPase (Research Diagnostics, Inc., Flanders, NJ) was used to verify loading of Western blots.
Immunofluorescence studies
Transfected and induced S2 cells were collected by gentle centrifugation and fixed in 250 μl of freshly prepared 2% paraformaldehyde/PBS for 15 min at room temperature. Fixed cells were pelleted gently and washed with PBS twice, followed by staining with primary antibodies (1:25 dilution of anti-Myc mouse monoclonal antibody 9E10.2; 5 μg/ml of rabbit anti-DCC antisera 723 or 645; Reale et al. 1994) in PBS with 0.1% saponin (Sigma, St. Louis, MO) and 1% normal goat serum (Pocono Rabbit Farm, Canadensis, PA) for 1 hr. Subsequently, cells were rinsed twice with PBS and stained with Texas red-conjugated goat anti-rabbit and FITC-conjugated goat anti-mouse antibodies (double-labeling grade, Jackson ImmunoResearch Laborataries, Inc.) for 1 hr in the dark. Cells were rinsed with PBS twice and mounted onto glass slides after resuspension in 90% glycerol, 100 mm Tris at pH 8.0, and 0.5% N-propyl gallate (Sigma). Confocal images were obtained from the Bio-Rad MRC 500 laser scanning confocal system connected to a Zeiss Axiovert compound microscope as described (Fehon et al. 1990; Diederich et al. 1994). Pseudocoloring was performed using the Adobe Photoshop software program.
Drosophila studies
Fly cultures and crosses were performed according to standard procedures. Embryos were injected with the sev–DCC P-element constructs and four sev–DCC transformant lines were established (Spradling and Rubin 1982). Scanning electron microscopy studies were performed as described (Xu and Artavanis-Tsakonas 1990). For retinal section studies, fly eyes were fixed and sectioned according to Tomlinson and Ready (1987) and viewed under phase-contrast optics on a Zeiss Axiphot microscope.
Acknowledgments
We thank Dr. Spyros-Artavanis and his laboratory members for plasmid reagents, advice, and encouragement; Dr. Ron R. Kopito for providing the c-Myc-tagged ubiquitin expression vector; Drs. Gary Nabel and Ross Stein (Proscript, Inc.) for providing MG132; Drs. Ed Harlow and Wei Du for assistance with the two-hybrid studies; Dr. Gerald Rubin for providing sev–sina transgenic flies; and Drs. Kathleen R. Cho, Elizabeth M. Petty, Robert M. Strieter, Bert Vogelstein, David Ginsburg, and Gary Nabel for comments on the manuscript. This work was supported by grant CA70097 to E.R.F.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL efearon@mmg.im.med.umich.edu; FAX (313) 647-7979.
References
- Brunner D, Oellers N, Szabad J, Biggs III WH, Zipursky SL, Hafen E. A gain of function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Busseau I, Diederich RJ, Xu T, Artavanis-Tsakonas S. A member of the Notch group of interacting loci, deltex, encodes a cytoplasmic basic protein. Genetics. 1994;136:585–596. doi: 10.1093/genetics/136.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Rubin GM. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- Cavenee WK, Dryja TP, Phillips RA, Benedict WF, Godbout R, Gallie GL, Murphree AL, Strong LC, White RL. Expression of recessive alleles by chromosomal mechansims in retinoblastoma. Nature. 1983;305:779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chan SS-Y, Zheng H, Su M-W, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- Chang HC, Karim FD, O’Neoill EM, Rebay I, Solomon NM, Therrien M, Wassarman DA, Wolff T, Rubin GM. Ras signal transduction pathway in Drosophila eye development. Cold Spring Harbor Symp Quant Biol. 1994;59:147–153. doi: 10.1101/sqb.1994.059.01.018. [DOI] [PubMed] [Google Scholar]
- Chang HC, Solomon NM, Wassarman DA, Karim FD, Therrien M, Rubin GM, Wolff T. phyllopod functions downstream of Ras1 in the fate determination of a subset of photoreceptors in Drosophila. Cell. 1995;80:1–20. doi: 10.1016/0092-8674(95)90497-2. [DOI] [PubMed] [Google Scholar]
- Chevray PM, Nathans D. Protein interaction cloning in yeast: Identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci. 1992;89:5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KR, Fearon ER. DCC: Linking tumor suppressor genes and altered cell surface interactions in cancer? Curr Opin Genet Dev. 1995;5:72–78. doi: 10.1016/s0959-437x(95)90056-x. [DOI] [PubMed] [Google Scholar]
- Cho KR, Oliner JD, Simons JW, Hedrick L, Fearon ER, Preisinger AC, Hedge P, Silverman GA, Vogelstein B. The DCC gene—structural analysis and mutations in colorectal carcinomas. Genomics. 1994;19:525–531. doi: 10.1006/geno.1994.1102. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Jiang TX, Yin E, Widelitz RB. cDCC (chicken homologue to a gene deleted in colorectal carcinoma) is an epithelial adhesion molecule expressed in the basal cells and involved in epithelial-mesenchymal interaction. Dev Biol. 1994;164:383–397. doi: 10.1006/dbio.1994.1208. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Armes P, Britto J, Gad J, Wilks AF. Cloning of the mouse homologue of the deleted in colorectal cancer gene (mDCC) and its expression in the developing mouse embryo. Oncogene. 1995;11:2243–2254. [PubMed] [Google Scholar]
- Della NG, Senior PV, Bowtell DL. Isolation and characterization of murine homologues of the Drosophila seven in absentia gene (sina) Development. 1993;117:1333–1343. doi: 10.1242/dev.117.4.1333. [DOI] [PubMed] [Google Scholar]
- Dickson B, Sprenger F, Morrison D, Hafen E. Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature. 1992;360:600–603. doi: 10.1038/360600a0. [DOI] [PubMed] [Google Scholar]
- Dickson B, Dominguez M, van der Straten A, Hafen E. Control of Drosophila photoreceptor cell fates by Phyllopod, a novel nuclear protein acting downstream of the Raf kinase. Cell. 1995;80:453–462. doi: 10.1016/0092-8674(95)90496-4. [DOI] [PubMed] [Google Scholar]
- Diederich RJ, Matsuno K, Hing H, Artavanis-Tsakonas S. Cytosolic interaction between deltex and Notch ankyrin repeats implicates deltex in the Notch signaling pathway. Development. 1994;120:473–481. doi: 10.1242/dev.120.3.473. [DOI] [PubMed] [Google Scholar]
- Du W, Vidal M, Xie J-E, Dyson N. A homolog of the retinoblastoma family of proteins regulates E2F activity in Drosophila. Genes & Dev. 1996;10:1206–1218. doi: 10.1101/gad.10.10.1206. [DOI] [PubMed] [Google Scholar]
- Ekstrand BC, Mansfield TA, Bigner SH, Fearon ER. DCC expression is altered by multiple mechanisms in brain tumors. Oncogene. 1995;11:2393–2402. [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Fearon ER. DCC: Is there a connection between tumorigenesis and cell guidance molecules? Biochem Biophys Acta. 1996;1288:M17–M23. doi: 10.1016/0304-419x(96)00023-6. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Cho KR, Nigro, J.M. JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW, Vogelstein B. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch AT, Artavanis-Tsakonas S. Molecular interactions between the protein products of neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Simon MA, Rubin GM. Signaling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature. 1992;355:559–561. doi: 10.1038/355559a0. [DOI] [PubMed] [Google Scholar]
- Fischer-Vize JA, Rubin GM, Lehmann R. The fat facets gene is required for Drosophila eye and embryo development. Development. 1992;116:985–1000. doi: 10.1242/dev.116.4.985. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Functions of the proteasome: The lysis at the end of the tunnel. Science. 1995;268:522–523. doi: 10.1126/science.7725095. [DOI] [PubMed] [Google Scholar]
- Hedrick L, Cho KR, Fearon ER, Wu TC, Kinzler KW, Vogelstein B. The DCC gene product in cellular differentiation and colorectal tumorigenesis. Genes & Dev. 1994;8:1174–1183. doi: 10.1101/gad.8.10.1174. [DOI] [PubMed] [Google Scholar]
- Hu, G., Y.-L. Chung, T. Glover, V. Valentine, A.T. Look, and E.R. Fearon. 1997. Characterization of human homologs of the Drosophila seven in absentia (sina) gene. Genomics (in press). [DOI] [PubMed]
- Huang Y, Baker RT, Fischer-Vize JA. Control of cell fate by a deubiquitinating enzyme encoded by the fat facets gene. Science. 1995;270:1828–1831. doi: 10.1126/science.270.5243.1828. [DOI] [PubMed] [Google Scholar]
- Itoh F, Hinoda Y, Ohe M, Ohe Y, Ban T, Endo T, Imai K, Yachi A. Decreased expression of DCC mRNA in human colorectal cancers. Int J Cancer. 1993;53:260–263. doi: 10.1002/ijc.2910530215. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS-Y, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Knudson AG. Antioncogenes and human cancer. Proc Natl Acad Sci. 1993;90:10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej PA. DCC’s function takes shape in the nervous system. Curr Opin Genet Dev. 1997;7:87–92. doi: 10.1016/s0959-437x(97)80114-1. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Timpe L, Mitchell KJ, Goodman CS, Fried SR, Jan LY, Jan YN. frazzled encodes a member of the immunoglobulin subfamily and is required for CNS axon guidance and motor axon targeting. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- Kovalenko OV, Plug AW, Haaf T, Gonda DK, Ashley T, Ward DC, Radding CM, Golub EI. Mammalian ubiquitin-conjugating enzyme Ubc9 interacts with Rad51 recombination protein and localizes in synaptonemal complexes. Proc Natl Acad Sci. 1996;93:2958–2963. doi: 10.1073/pnas.93.7.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z-C, Rubin GM. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell. 1992;70:609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- Lai Z-C, Harrison SD, Karim F, Li Y, Rubin GM. Loss of tramtrack gene activity results in ectopic R7 cell formation, even in a sina mutant background. Proc Natl Acad Sci. 1996;93:5025–5030. doi: 10.1073/pnas.93.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor K, Narayanan R. Persistent expression of the tumor suppressor gene DCC is essential for neuronal differentiation. Cell Growth Diff. 1992;3:609–616. [PubMed] [Google Scholar]
- Meyerhardt JA, Look AT, Bigner SH, Fearon ER. Identification and characterizaation of neogenin, a DCC-related gene. Oncogene. 1997;14:1129–1136. doi: 10.1038/sj.onc.1200935. [DOI] [PubMed] [Google Scholar]
- Muralidhar MG, Thomas JB. The Drosophila bendless gene encodes a neural protein related to ubiquitin-conjugating enzymes. Neuron. 1993;11:253–266. doi: 10.1016/0896-6273(93)90182-q. [DOI] [PubMed] [Google Scholar]
- Nemani M, Linares-Cruz G, Bruzzoni-Giovanelli H, Roperch J-P, Tuynder M, Bougueleret L, Cherif D, Medhioub M, Pasturaud P, Alavaro V, et al. Activation of the human homologue of the Drosophila sina gene in apoptosis and tumor suppression. Proc Natl Acad Sci. 1996;93:9039–9042. doi: 10.1073/pnas.93.17.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh CE, McMahon R, Benzer S, Tanouye MA. bendless, a Drosophila gene affecting neuronal connectivity encodes a ubiquitin-conjugating enzyme homolog. J Neuroscience. 1994;14:3166–3179. doi: 10.1523/JNEUROSCI.14-05-03166.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Pierceall WE, Reale MA, Candia AF, Wright CVE, Cho KR, Fearon ER. Expression of a homologue of the deleted in colorectal cancer (DCC) gene in the nervous system of developing Xenopus embryos. Dev Biol. 1994a;166:654–665. doi: 10.1006/dbio.1994.1345. [DOI] [PubMed] [Google Scholar]
- Pierceall WE, Cho KR, Getzenberg RH, Reale MA, Hedrick L, Vogelstein B, Fearon ER. NIH3T3 cells expressing the deleted in colorectal cancer tumor suppressor gene product stimulate neurite outgrowth in rat PC12 pheochromocytoma cells. J Cell Biol. 1994b;124:1017–1027. doi: 10.1083/jcb.124.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale MA, Hu G, Zafar AI, Getzenberg RH, Levine SM, Fearon ER. Expression and alternative splicing of the deleted in colorectal cancer (DCC) gene in normal and malignant tissues. Cancer Res. 1994;54:4493–4501. [PubMed] [Google Scholar]
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–777. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Saurin AJ, Borden KLB, Boddy MN, Freemont PS. Does this have a familiar ring? Trends Biochem Sci. 1996;21:208–214. [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Seufert W, Futcher B, Jentsch S. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature. 1995;373:78–81. doi: 10.1038/373078a0. [DOI] [PubMed] [Google Scholar]
- Shibata D, Reale MA, Lavin P, Silverman M, Fearon ER, Steele Jr G, Jessup JM, Loda M, Summerhayes IC. The DCC protein and prognosis in colorectal cancer. N Eng J Med. 1996;335:1727–1732. doi: 10.1056/NEJM199612053352303. [DOI] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JKV, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nature Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Ready DF. Cell fate in Drosophila ommatidium. Dev Biol. 1987;123:264–275. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- Varskavsky A. The N-end rule. Cell. 1992;69:725–735. doi: 10.1016/0092-8674(92)90285-k. [DOI] [PubMed] [Google Scholar]
- Vidal M. The reverse two-hybrid system. In: Bartel P, Fields S, editors. The two-hybrid system. New York: Oxford University Press; 1997. . (In press). [Google Scholar]
- Vielmetter J, Kayyem JF, Roman JM, Dreyer WJ. Neogenin, an avian cell surface protein expressed during terminal neuronal differentiation is closely related to the human tumor suppressor molecule deleted in colorectal cancer. J Cell Biol. 1994;127:2009–2020. doi: 10.1083/jcb.127.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- Xu T, Artavanis-Tsakonas S. deltex, a locus interacting with the neurogenic genes, notch, delta, and mastermind in Drosophila melanogaster. Genetics. 1990;126:665–677. doi: 10.1093/genetics/126.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D, Nihonmatsu I, Matsuo T, Miyamoto H, Kondo S, Hirata K, Ikegami Y. Genetic interactions of pokkuri with seven in absentia, tramtrack and downstream components of the sevenless pathway in R7 photoreceptor induction in Drosophila melanogaster. Roux’s Arch Dev Biol. 1996;205:215–224. doi: 10.1007/BF00365799. [DOI] [PubMed] [Google Scholar]