Abstract
Background
HLA-C is an important ligand for killer immunoglobulin like receptors (KIR) that regulate natural killer (NK) cell function. Based on KIR specificity HLA-C molecules are allocated into two groups, HLA-C1 or HLA-C2; HLA-C2 is more inhibiting to NK cell function than HLA-C1. We studied the clinical importance of HLA-C genotypes on the long-term graft survival of 760 kidney transplants performed at our centre utilising a population based genetic study and cell culture model to define putative mechanisms.
Methods and Findings
Genotyping was performed using conventional DNA PCR techniques and correlations made to clinical outcomes. We found that transplant recipients with HLA-C2 had significantly better long-term graft survival than transplant recipients with HLA-C1 (66% versus 44% at 10 years, log-rank p = 0.002, HR = 1.51, 95%CI = 1.16–1.97). In in-vitro NK and dendritic cell (DC) co-culture model we made several key observations that correlated with the population based genetic study. We observed that donor derived NK cells, on activation with IL-15, promoted differential HLA-C genotype dependent DC maturation. In NK-DC co-culture, the possession of HLA-C2 by DC was associated with anti-inflammatory cytokine production (IL-1RA/IL-6), diminished DC maturation (CD86, HLA-DR), and absent CCR7 expression. Conversely, possession of HLA-C1 by DC was associated with pro-inflammatory cytokine synthesis (TNF-α, IL-12p40/p70), enhanced DC maturation and up-regulation of CCR7 expression. By immunohistochemistry the presence of donor NK cells was confirmed in pre-transplant kidneys.
Conclusions
We propose that after kidney transplantation IL-15 activated donor derived NK cells interact with recipient DC with less activation of indirect allo-reactivity in HLA-C2 positive recipients than HLA-C1 positive recipients; this has implications for long-term graft survival. Early events following kidney transplantation involving NK-DC interaction via KIR and HLA-C immune synapse may have a central role in long-term kidney transplant outcomes.
Introduction
Kidney transplantation is the standard of care for many people with end stage kidney disease [1]. However, whilst acute rejection rates and early graft loss have improved substantially over the past four decades, progressive chronic allograft injury (CAI) remains a very common cause of late graft loss [2], [3]. A major component of CAI is orchestrated by the adaptive components of the immune system including dendritic cells (DC), T cells and B cells [4]–[8]. Our knowledge of the link between innate and adaptive immunity in CAI including the contribution of NK cells is incomplete. This is an important shortfall, as NK cells have a central role in modulating the development of the adaptive response through interactions with HLA-C molecules on target cells [9], [10]. HLA-C molecules act as ligands for NK cell expressed inhibitory killer immunoglobulin-like receptors (KIR), with subsequent modulation of NK cell function. HLA-C molecules are allocated into two groups based on their KIR specificity: (i) HLA-C group 1 (C1) specific for KIR receptor 2DL2/3; (ii) HLA-C group 2 (C2) specific for KIR receptor 2DL1 [10]. Differential KIR and HLA expression appear to influence clinical outcomes in various diseases including cervical neoplasia [11], pre-eclampsia [12], antiviral immune response [13], hepatitis C [14] and liver transplantation [15]. As the co-expression of KIR 2DL1 and 2DL3 on NK cells occurs in greater than 90% of the population, the major determinant of NK cell inhibition is the differential expression of HLA-C ligands. Functional studies performed by Ahlenstiel and colleagues (2008) investigating antiviral responses in-vitro showed diminished degranulation and cytokine production by NK cells in HLA-C2 compared with HLA-C1 targets. They proposed that NK cell inhibition through interactions between KIR2DL3 and HLA-C1 is weaker than inhibition conferred through KIR2DL1/HLA-C2 interactions [13].
In liver transplantation, we found that HLA-C2 expression by the allograft was protective against the development of chronic rejection, graft cirrhosis and late graft loss [15]. There is limited data on the effect of KIR and KIR ligands on clinical outcomes following kidney transplantation. In a study of 224 kidney transplant recipients, Kunert and colleagues (2007) showed that expression of a HLA-C2 homozygous genotype by the allograft was associated with a reduced incidence of acute rejection [16]. Nowak and colleagues (2010) genotyped 285 recipients of kidney transplants and demonstrated that the presence of HLA-C2 and absence of KIR2DS5 in the recipient correlated with increased episodes of acute allograft rejection. Conversely they found that the presence of both HLA-C1 and KIR2DS5 in the recipient was protective against acute allograft rejection [17]. In a study comprising of 2,757 kidney transplants, Tran and colleagues (2005) investigated the impact of KIR ligand matching on graft survival. Whilst they showed no correlation between KIR ligand matching and graft survival they did not specifically investigate the relationship between differential HLA-C expression and graft outcomes [18].
The predominant subgroup of NK cells (>95% of peripheral blood NK cells) are CD56dimCD16+ and possess KIR receptors (95%) [19], [20]. In addition to their role in the elimination of tumour and virus transformed cells, they also interact with DC. This interaction is contact dependent and bidirectional, involving multiple cytokine synthesis including IFN-γ, TNF-α, IL-12, IL-15, IL-18 and HMGB1 [21]–[25]. During NK-DC co-culture, NKp30 engagement triggers intracellular mechanisms that are further modulated by KIR and HLA-C interactions [26], [27]. Dendritic cells that are matured during NK-DC crosstalk are potent T-cell primers [28] and promote Th1 polarisation [29].
Following kidney transplantation, the allograft undergoes significant ischaemia reperfusion injury (IRI) with release of pro-inflammatory cytokines [30] including IL-15 [31]–[33] and recruitment of recipient monocytes and DCs [34]. Passenger leukocytes transferred in the allograft from donor to recipient augment the allo-immune response. Allogeneic donor derived NK cells transferred by this process may be a predominant activator of recipient DCs. Furthermore DC maturation supersedes cytolysis during IRI as products of cellular damage trigger TLR4 expressed on DC [30]. Even in the presence of conventional immunotherapy, IL-15 will promote NK-DC crosstalk, leading to accelerated maturation of DC with allo-antigen presenting capacity.
In this study, we investigated the influence of HLA-C genotype on graft survival. We found that recipients with HLA-C2 had significantly better long-term graft survival after kidney transplantation than those without HLA-C2 (i.e. HLA-C1 homozygotes). Based on these observations we hypothesised that the interaction between allogeneic donor derived NK cells transferred in the allograft at time of transplantation and recipient DC are differentially modulated by the engagement of donor KIR and recipient HLA-C on DC. This hypothesis was based on the premise that as HLA-C2 is more inhibiting to NK cell function than HLA-C1, DC expressing HLA-C2 should undergo less maturation than those expressing HLA-C1, with a subsequent impact on T-cell priming. This was investigated by an NK-DC co-culture model controlled for confounding factors by utilising: (i) NK cells from donors heterozygous for HLA-C (C1/C2), negative for HLA-Bw4, haplotype AA with one activating KIR2DS4 and with 3 inhibitory KIR2DL1, 2DL3, and 3DL1; (ii) DC from donors negative for HLA-Bw4 and either homozygous for HLA-C1 or HLA-C2. This process of selection excluded the potential confounding effects of HLA-Bw4 through interactions with 3DL1 and allowed direct comparisons to be made between HLA-C1 homozygous and HLA-C2 homozygous DCs. Under these conditions, allogeneic NK-DC co-cultures facilitated an assessment of the mechanisms of the association of HLA-C genotype with graft survival.
Materials and Methods
Ethics Statement
Approvals for all parts of this study were granted by the South Birmingham Research Ethics Committee. Informed consent was not required as data were analyzed anonymously. The ethics committee specifically waived the need for consent.
Population Genetics
Nine hundred and fifty adult kidney transplant recipients were treated at the Queen Elizabeth Hospital, Birmingham between 1996 and 2004. DNA was available for a total of 890 kidney donors and 760 transplant recipients. The final study population therefore comprised 760 renal transplant pairs all with complete long-term follow-up; 640 were deceased donor transplants and 120 were live donor transplants; 652 recipients were first time kidney transplants, 86 were second and 22 were third transplant.
By polymerase chain reaction sequence specific primer (PCR-SSP) technique, donor and recipient DNA were genotyped for the presence of the three major KIR ligand groups, HLA-C1, HLA-C2 and HLA-Bw4. These were assigned directly by using specific oligonucleotide primers to type the codon corresponding to amino acid 80 for HLA-C and codons 80–83 for HLA-Bw4. Similarly, KIR genotyping was performed for inhibitory and activating KIR using PCR-SSP with primers based on those published by Uhrberg [35]; Four inhibitory KIRs (2DL1, 2DL2, 2DL3 and 3DL1) and seven activating KIRs (2DS1, 2DS2, 2DS3, 2DS4, 2DS4v, 2DS5, and 3DS1) were analysed. The clinical outcome data comprised biopsy proven acute rejection, graft survival and patient survival. Graft loss was defined as graft failure requiring dialysis (n = 163). Both death non-censored and death censored graft survival were calculated. For death non-censored graft survival, patient death with a functioning graft was also defined as graft failure and therefore included as graft loss (n = 219). Causes of graft loss and patient death are listed in Table 1 and 2. Patient death (n = 56, Table 2) from all causes was included in the analysis of patient survival. Preliminary power calculations were performed for this study and based on the assumption that 10-year graft survival for kidney transplants at our centre is 65%, then for 760 cases (assuming 40% are HLA-C1 homozygous and the remaining 60% are in the comparator group i.e. the presence of HLA-C2 allele therefore the heterozygous and HLA-C2 homozygous genotype combined) this study size has a 90% power to detect a hazard ratio of 1.49 for graft failure.
Table 1. Causes of graft loss following kidney transplantation.
| Cause of graft loss (n = 163) | Incidence |
| Chronic allograft injury | 124 (76%) |
| Vascular thrombosis | 24 (14.7%) |
| Acute rejection | 7 (4.3%) |
| Recurrent of primary renal disease | 6 (3.7%) |
| Infection of allograft | 2 (1.3%) |
Table 2. Causes of patient death following kidney transplantation.
| Cause of patient death (n = 56) | Incidence |
| Cardiovascular disease | 20 (35.7%) |
| Post-transplant lymphoproliferative disease | 10 (17.9%) |
| Septicaemia | 8 (14.3%) |
| Respiratory failure | 6 (10.7%) |
| Cerebrovascular event | 6 (10.7%) |
| Other malignant disease | 6 (10.7%) |
Immunohistochemistry
Pre-transplant kidney (n = 5) biopsies were available and assessed for the presence of donor derived NK cells. Biopsies were taken prior to transplantation, and fixed in formalin. The method used for the development of slides include dewaxing and antigen retrieval obtained by W-cap system (Bio-Optica) and staining with a mouse monoclonal anti-CD56 antibody (IgG2b Novocastra) used at a dilution of 1∶50 and visualised with the EnVision detection system (DAKO). Negative controls were performed using corresponding isotype antibody staining. Cell counts (degree of infiltration) were performed using light microscopy and counting 10 randomly selected high power fields at a magnification of 400× (area = 0.17 mm2).
In-vitro NK-DC co-culture
In order to analyse the influence of DC HLA-C genotype on NK-cell mediated maturation of DC, we developed a co-culture model to exclude the known confounding factors detailed in the introduction.
Whole blood samples were obtained from healthy laboratory donors for the isolation of purified and enriched NK and DC cell populations from PBMC using magnetic cell separation. The development of an NK-DC co-culture system required two independent steps. Firstly, DC were prepared using a pre-optimised magnetic cell sorting kit (Miltenyi Biotec, Surrey, UK) for the extraction of CD14+ monocytes from PBMC. Following isolation, CD14+ monocytes were treated with 100 ng/ml of GM-CSF (Peprotech, London, UK) and 1000 U/ml of IL-4 (Peprotech, London, UK) in RPMI containing 5% autologous serum and maintained in a 5% CO2 cell culture incubator at 37°C for 5 days. This altered their phenotype to that of immature dendritic cells (iDC) with a population of >90% CD14−CD1a+ iDC cells, comparable with the published literature [21]–[23]. On day 5, NK cells were isolated from PBMC using a pre-optimised magnetic cell sorting kit (Miltenyi Biotec, Surrey, UK) for the extraction of CD56+CD16+ NK cell. Purities of >98% were attained consistent with published literature [21]–[23]. Allogeneic NK and iDC cells were then co-cultured in the presence or absence of 1 ng/ml IL-15 (Peprotech, London, UK) in RPMI containing 10% foetal calf serum (Sigma-Aldrich, Dorset, UK) for 48 hours in a 48 well plate maintained in a 5% CO2 cell culture incubator at 37°C. Co-culture cell ratios of 1∶1 and 1∶5 (NK∶DC) were then studied as these ratios are most favourable for DC maturation [21] and should be consistent with the in-vivo relationship. Typically (at 1∶1) each well contained 0.25×106 of each cell type in a final media volume of 500 µl. The control wells had either DC or NK cells in isolation. Trans-well experiments were performed using the same conditions, but in the presence or absence of a 0.4 µm insert (Costar, Fisher Scientific, UK) to separate NK cells and DC. At the end of co-culture DC were tested for the expression of the maturation markers CD86 (PE, 2331, BD Bioscience) and HLA-DR (FITC, G46-6, BD Bioscience) and the chemokine receptor CCR7 (PE, 150503, R&D Systems). The DC gate on flow cytometry was defined by a combination of scatter plot and CD56 staining to identify NK cells. The Δ Mean Fluorescence Intensity (Δ MFI) per experiment for CD86, HLA-DR and CCR7 was calculated as the difference in MFI for DC in co-culture with NK cells versus DC in isolation (background maturation). Corresponding isotype controls (BD Bioscience) were used. Cells were analysed using a FACSCalibur flow cytometer (Becton Dickinson) with Winmdi 2.9 software (Scripps Research), acquiring information from a total of 5,000 gated cells.
Co-culture supernatants were also collected and tested for the presence of cytokines by multiplex assays [Cytokine 25-plex AB Bead Kit, Human (BioSource™), from Invitrogen; data analysed using Luminex®100™ Analyser].
Comparison of means for ΔMFI and supernatant cytokine synthesis was performed to compare responses for DC with HLA-C1 genotype (n = 4) versus DC with HLA-C2 genotype (n = 4). The NK and DC used in each experiment came from different lab donors thus making these allogeneic interactions.
Statistical analysis
Actuarial data were analysed using the Kaplan-Meier method and the log-rank test. Cox regression models for multivariable analysis were used to identify independent factors contributing to long-term graft loss. Mann-Whitney U test (Exact 2-tailed significance) was used for comparing means of ΔMFI and supernatant cytokines synthesis. All other correlations for variables and comparison of means were performed using Kendall's tau-b and ANOVA tests respectively. Probability (p) values of less than 0.05 were considered significant for our analysis. All statistics were performed using SPSS 14.0.
Results
HLA-C2 positive kidney transplant recipients have significantly better long-term graft survival
Kaplan Meier survival analysis was performed for individual KIR, KIR haplotypes and HLA-C/Bw genotypes for both donor and recipient. Recipient HLA-C genotype was the only factor found to influence survival outcomes and therefore tested further. Frequency of genotypes was consistent with previous published reports. Donor frequency for individual genes: 2DL1 98%, 2DL2 46%, 2DL3 92%, 3DL1 94%, 2DS1 40%, 2DS2 48%, 2DS3 30%, 2DS4 96%, 2DS5 31%, 3DS1 37%, HLA-C1 87%, HLA-C2 62%, HLA-Bw4 35% and Haplotype AA 32%. Recipient frequency for individual genes: 2DL1 95%, 2DL2 36%, 2DL3 97%, 3DL1 89%, 2DS1 28%, 2DS2 44%, 2DS3 28%, 2DS4 83%, 2DS5 33%, 3DS1 34%, HLA-C1 87%, HLA-C2 57%, HLA-Bw4 38% and Haplotype AA 31%.
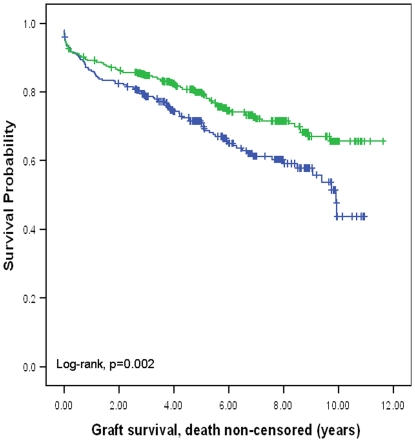
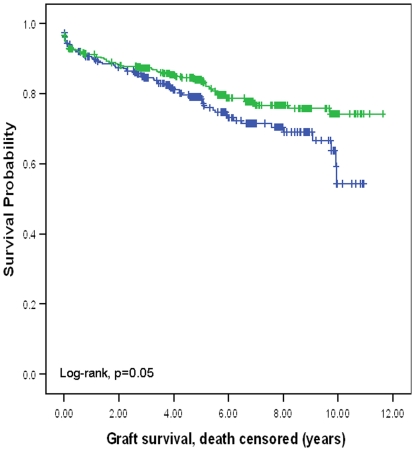
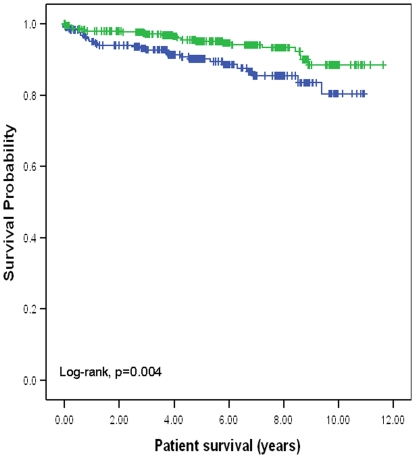
In contrast to liver transplantation, donor HLA-C genotype did not have impact on survival outcomes in kidney transplantation. However, recipient HLA-C genotype did have a major impact on outcomes 10-year death non-censored graft survival was significantly better for recipients with HLA-C2 compared with recipients without HLA-C2 (65.7%. versus 43.8%, p = 0.002; hazard ratio: 1.51, 95% CI: 1.16–1.97) (figure 1). Ten-year death censored graft survival was also significantly better for recipients with HLA-C2 compared with recipients without HLA-C2 (74.2% versus 54.3%, p = 0.05; hazard ratio: 1.36, 95% CI: 1.02–1.85) (figure 2). Ten-year patient survival was significantly better for recipients with HLA-C2 compared with recipients without HLA-C2 (88.5% versus 80.4%, p = 0.004; hazard ratio: 2.12, 95% CI: 1.26–3.58) (figure 3). HLA-C2 homozygous and heterozygous genotypes had comparable survival outcomes, and both groups were superior to HLA-C1 homozygotes (HLA-C2/C2 versus HLA-C1/C2; ten-year death non-censored graft survival 69.1% versus 64.1%, log-rank p = 0.66; ten-year death censored survival 80.5% versus 72.5%, log-rank p = 0.09; ten-year patient survival 88% versus 87%, log-rank p = 0.78). Re-transplants were not excluded from this analysis because analysing re-transplants only demonstrated similar survival benefit seen for recipients with HLA-C2 alleles. For re-transplants 10-year death non-censored graft survival was significantly better for recipients with HLA-C2 compared with recipients without HLA-C2 (60% versus 17.4%, p = 0.02; hazard ration: 2.17, 95% CI: 1.11–4.25). Ten-year death censored graft survival was also significantly better for recipients with HLA-C2 compared with recipients without HLA-C2 (72% versus 33%, p = 0.05; hazard ratio: 1.3, 95% CI: 1.23–1.7). Ten-year patient survival was significantly better for recipients with HLA-C2 compared with recipients without HLA-C2 (77% versus 52%, p = 0.015; hazard ratio: 3.7, 95% CI: 1.19–11.89) (figures not shown). This observation further supports a recipient derived effect. Donor and recipient HLA-Bw4, KIR haplotypes or KIR genotypes did not influence clinical outcomes following kidney transplantation. Furthermore KIR ligand matching did not influence clinical outcomes consistent with previous observations [17].
Figure 1. Kaplan Meier survival curve showing death non-censored graft survival for the presence or absence of HLA-C2 allele in the recipient.
The presence of an HLA-C2 allele in the recipient was associated with a significant improvement in death non-censored graft survival (10-year survival: 65.7% versus 43.8%).
Figure 2. Kaplan Meier survival curve showing death censored graft survival for the presence or absence of HLA-C2 allele in the recipient.
The presence of an HLA-C2 allele in the recipient was associated with a significant improvement in death censored graft survival (10-year survival: 74.2% versus 54.3%).
Figure 3. Kaplan Meier survival curve showing patient survival for the presence or absence of HLA-C2 allele in the recipient.
The presence of an HLA-C2 allele in the recipient was associated with a significant improvement in patient survival (10-year survival: 88.5% versus 80.4%).
Multivariable analysis was performed for variables that may influence clinical outcomes in kidney transplantation (Table 3&4). This confirmed the association of the recipient HLA-C2 allele with improved graft survival for both death non-censored and death censored graft survival. Interestingly, in the multivariable analysis, recipient HLA-C2 allele was not significantly associated with improved patient survival. Other than 4 HLA antigen mismatches we did not observe the relationship between HLA mismatch and graft survival. Whilst HLA mismatching is known to influence graft survival our study was not designed to detect these as the number of cases per HLA mismatch were small.
Table 3. Demographics and distribution of parameters that are known to affect graft and patient survival after kidney transplantation.
| Demographics | Absence of HLA-C2(n = 303) | Presence of HLA-C2(n = 457) | P value |
| Recipient age | 43.0 (CI 41.2–44.8) | 42.3 (CI 40.9–43.6) | 0.49* |
| Donor age | 43.5 (CI 41.6–45.4) | 43.5 (CI 42.1–44.9) | 0.96* |
| Recipient sex M∶F | 191∶112 | 278∶179 | 0.55+ |
| Donor sex M∶F | 180∶123 | 236∶221 | 0.03+ |
| Seropositive CMV in recipient | 169 | 240 | 0.43+ |
| Seropositive CMV in donor | 150 | 212 | 0.41+ |
| Type of transplant; Deceased donor: Live donor | 258∶45 | 382∶75 | 0.60+ |
| Number of transplant | |||
| 1 | 256 | 398 | 0.26+ |
| 2 | 39 | 47 | |
| 3 | 8 | 12 | |
| Acute Rejection | 86 | 129 | 0.96+ |
| Type of CNI; Cyclosporin: Tacrolimus | 290∶15 | 434∶21 | 0.90+ |
| Anti-CD25 monoclonal antibody | 50 | 82 | 0.59+ |
| HLA mismatch (no. of antigens) | |||
| 0 | 44 | 58 | 0.62+ |
| 1 | 31 | 41 | |
| 2 | 128 | 206 | |
| 3 | 71 | 108 | |
| 4 | 15 | 25 | |
| 5 | 10 | 14 | |
| 6 | 4 | 5 | |
| Mean mismatches per patient | 2.09 | 2.15 | |
| DR locus mismatch (no. of antigens) | |||
| 0 | 207 | 297 | 0.42+ |
| 1 | 83 | 142 | |
| 2 | 13 | 18 | |
| Mean mismatches per patient | 0.36 | 0.41 |
As the analysis involved the comparison between absence of HLA-C2 in the recipient versus presence of HLA-C2 in the recipient groups were divided accordingly. Absence of HLA-C2 represents HLA-C1 homozygous whereas presence of HLA-C2 combines both heterozygous and HLA-C2 homozygous groups. CI is 95% confidence interval.
*indicates significance by ANOVA test,
indicates significance by Kendall's tau-b test.
Table 4. Cox regression multivariable analysis for all factors that may influence outcomes after kidney transplantation.
| Significance | Hazard ratio | 95% CI | Significance | Hazard ratio | 95% CI | Significance | Hazard ratio | 95% CI | |
| Graft survival, death non-censored | Graft survival, death censored | Patient survival | |||||||
| Recipient age | 0.29 | 0.99 | 0.98–1.01 | 0.002 | 0.98 | 0.96–0.99 | 0.001 | 1.05 | 1.02–1.08 |
| Donor age | 0.04 | 1.01 | 1.00–1.03 | 0.02 | 1.02 | 1.00–1.03 | 0.69 | 1.01 | 0.98–1.03 |
| Recipient sex | 0.77 | 1.05 | 0.75–1.47 | 0.50 | 1.20 | 0.71–2.05 | 0.88 | 0.95 | 0.49–1.85 |
| Donor sex | 0.61 | 1.09 | 0.79–1.51 | 0.52 | 1.14 | 0.77–1.67 | 0.19 | 0.63 | 0.32–1.25 |
| Seropositive CMV in recipient | 0.18 | 0.78 | 0.55–1.12 | 0.19 | 0.76 | 0.51–1.15 | 0.71 | 0.87 | 0.41–1.84 |
| Seropositive CMV in donor | 0.48 | 0.89 | 0.63–1.24 | 0.41 | 0.85 | 0.57–1.25 | 0.84 | 0.93 | 0.46–1.87 |
| Type of transplant: Deceased versus Live | 0.02 | 2.96 | 1.18–7.44 | 0.11 | 2.17 | 0.85–5.54 | 0.77 | 1.05 | 0.76–1.48 |
| Number of transplant | |||||||||
| Comparisons made to 1st transplant | |||||||||
| 2 | 0.83 | 0.94 | 0.55–1.62 | 0.17 | 0.61 | 0.30–1.24 | 0.11 | 2.10 | 0.86–5.14 |
| 3 | 0.49 | 1.52 | 0.46–5.03 | 0.34 | 1.80 | 0.54–6.08 | 0.99 | 0.00 | 0.00 |
| Acute Rejection | 0.35 | 1.17 | 0.84–1.65 | 0.15 | 1.33 | 0.91–1.95 | 0.38 | 0.70 | 0.32–1.55 |
| Cyclosporin versus Tacrolimus | 0.35 | 0.64 | 0.25–1.65 | 0.57 | 1.53 | 0.35–6.63 | 0.003 | 0.15 | 0.04–0.51 |
| Anti-CD25 monoclonal antibody | 0.62 | 1.15 | 0.66–1.98 | 0.89 | 1.04 | 0.58–1.89 | 0.36 | 2.03 | 0.45–9.13 |
| HLA mismatch (no. of antigens) | |||||||||
| Comparisons made to 0 HLA mismatch | |||||||||
| 1 | 0.07 | 1.90 | 0.96–3.75 | 0.05 | 2.13 | 0.99–4.61 | 0.76 | 1.28 | 0.27–5.96 |
| 2 | 0.29 | 1.37 | 0.77–2.45 | 0.40 | 1.34 | 0.68–2.63 | 0.70 | 1.25 | 0.40–3.87 |
| 3 | 0.86 | 0.94 | 0.46–1.94 | 0.61 | 0.80 | 0.33–1.90 | 0.83 | 1.16 | 0.31–4.36 |
| 4 | 0.04 | 2.46 | 1.05–5.75 | 0.05 | 2.60 | 1.00–6.75 | 0.78 | 1.40 | 0.14–14.28 |
| 5 | 0.63 | 1.56 | 0.25–9.66 | 0.28 | 2.69 | 0.45–16.03 | 0.98 | 0.00 | 0.00 |
| 6 | 0.43 | 3.01 | 0.20–45.16 | 0.23 | 6.67 | 0.30–149.09 | 0.99 | 0.00 | 0.00 |
| DR locus mismatch (no. of antigens) | |||||||||
| Comparisons made to 0 DR mismatch | |||||||||
| 1 | 0.56 | 1.15 | 0.73–1.80 | 0.47 | 1.22 | 0.71–2.08 | 0.99 | 1.01 | 0.42–2.38 |
| 2 | 0.50 | 0.55 | 0.09–3.17 | 0.25 | 0.26 | 0.03–2.55 | 0.18 | 8.53 | 0.39–188.23 |
| HLA-C2 absent in recipient | 0.007 | 1.55 | 1.13–2.13 | 0.02 | 1.53 | 1.06–2.21 | 0.19 | 1.54 | 0.80–2.94 |
| HLA-Bw4 absent in recipient | 0.35 | 1.17 | 0.84–1.65 | 0.50 | 1.20 | 0.71–2.05 | 0.88 | 0.95 | 0.49–1.85 |
Statistically significant associations are indicated in bold.
In table 5 the causes of graft loss were analysed in the context of the absence or presence of HLA-C2. This analysis demonstrated that chronic allograft injury and vascular thrombosis occurred more frequently in the absence of HLA-C2 when compared to the presence of HLA-C2.
Table 5. Causes of graft loss categorized by the absence or presence of HLA-C2.
| Cause of graft loss | Absence of HLA-C2(n = 303) | Presence of HLA-C2(n = 457) | P value |
| Chronic allograft injury | 60 (20%) | 64(14%) | 0.03 |
| Vascular thrombosis | 16 (5.3%) | 8 (1.7%) | 0.01 |
| Acute rejection | 2 (0.7%) | 5 (1.1%) | NS |
| Recurrent of primary renal disease | 2 (0.7%) | 4 (1.1%) | NS |
| Infection of allograft | 1 (0.3%) | 1 (0.3%) | NS |
Figures reported are absolute number of events and figures in brackets represent percentages calculated for events per HLA-C genotype. Statistical analysis was performed by Kendall's tau-b test. NS represents not significant.
HLA-C and KIR genotypes do not influence the incidence of acute rejection
The correlation between the incidence of biopsy proven acute rejection (33% in our kidney transplant population) and various genotypes for donor and recipient was studied. Complete data was available for 657 cases. The remaining 103 cases were excluded because these patients were referred to other treatment centre for their routine transplant follow-up and accurate data regarding acute rejection episodes were not available. The incidence of acute rejection did not correlate with individual KIR genotype, KIR haplotype, KIR ligand genotype (HLA-C or Bw4), or KIR ligand matching.
Donor derived NK cells are present in the allograft at the time of transplant
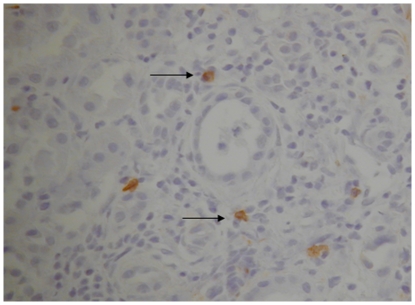
Immunohistochemical staining for CD56 confirms the presence of NK cells in pre-transplant kidney tissue providing evidence that at transplantation donor derived NK cells are present in the allograft (figure 4). Cell counts (degree of infiltration) were performed using light microscopy and counting 10 randomly selected high power fields at a magnification of 400× (area = 0.17 mm2). Mean (±SEM) of 3±2 CD56 positive cells were identified per high power field.
Figure 4. Immunohistochemistry slide demonstrating donor derived CD56 positive cells (anti-CD56 antibody staining brown in colour: arrow as indicator) in pre-transplant kidney biopsy tissue.
In brief, the method for development of this slide included dewaxing and antigen retrieval obtained by W-cap system (Bio-Optica) and staining with mouse monoclonal anti-CD56 antibody (IgG2b Novocastra) used at a dilution of 1∶50 and visualised with the EnVision detection system (DAKO). This image is representative of the observation made for biopsies taken from five different kidney transplants studied. Cell counts (degree of infiltration) were performed using light microscopy and counting 10 randomly selected high power fields at a magnification of 400× (area = 0.17 mm2). Mean (±SEM) of 3±2 CD56 positive cells were identified per high power field.
Optimal DC maturation in allogeneic NK-DC co-culture requires cytokine IL-15 activation and is cell contact dependent
Allogeneic interactions between NK and iDC without cytokine activation led to minimal DC maturation. This was consistent with previous observations [23], [26]. In co-culture, the addition of 1 ng/ml of IL-15 significantly augmented DC maturation with increased expression of CD86, HLA-DR and CCR7. DC maturation occurred optimally at NK-DC ratios of 1∶1 consistent with previous reports [21], [22]. In trans-well experiments where IL-15 activated NK and DC were separated by a porous membrane, DC maturation did not occur, confirming that IL-15 activated NK cell mediated DC maturation is contact dependent.
HLA-C2 expression by DC is associated with inhibited maturation responses in NK-DC co-culture
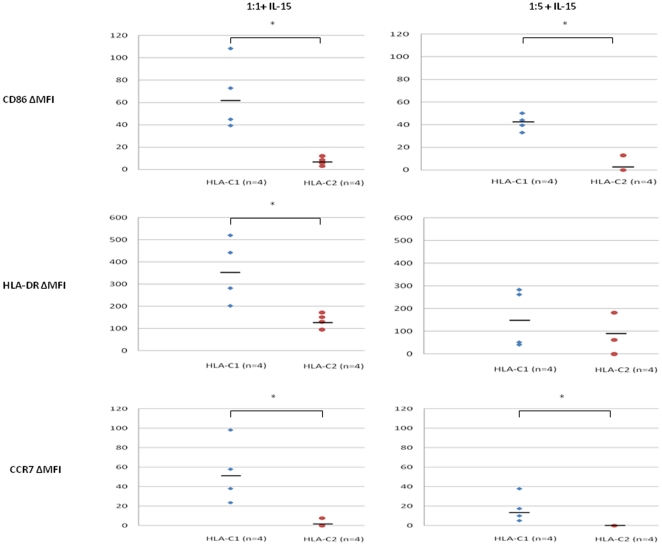
The influence of HLA-C genotype on DC maturation in NK-DC co-culture was assessed at cell ratios of 1∶1 and 1∶5 in the presence of 1 ng/ml IL-15. In NK-DC co-culture at 1∶1 cell ratios, DC maturation (CD86 and HLA-DR) and chemokine expression (CCR7) were significantly greater for HLA-C1 DCs (ΔMFI: CD86 = 61.08±15.40, HLA-DR = 363.67±73.21, CCR7 = 52.35±16.23) compared to HLA-C2 DCs (ΔMFI: CD86 = 6.43±6.12, HLA-DR = 115.81±40.00, CCR7 = 1.91±1.91,) (p<0.05, Mann-Whitney U test) (figure 5 & 6). Similarly at NK-DC cell ratios of 1∶5, CD86 and CCR7 expression were significantly greater for HLA-C1 DCs (ΔMFI: CD86 = 44.78±10.39, CCR7 = 14.35±8.77) than HLA-C2 DCs (ΔMFI: CD86 = 3.22±3.22, CCR7 = 0.00±0.00, p<0.05, Mann-Whitney U test) (figure 5 & 6). However, at 1∶5 cell ratios HLA-DR expression was not significantly different in HLA-C1 DCs compared with HLA-C2 DCs (159.97±64.82 vs 94.42±39.97, p = NS). In general, CD86 and HLA-DR expression by DCs were greater at cell ratios of 1∶1 than 1∶5. In co-culture, CCR7 expression occurred mainly on HLA-C1 DCs expressing alleles (figure 5 & 6). CCR7 expression was more marked at NK-DC co-culture cell ratios of 1∶1 (10.3% versus 1.86%) (figure 6).
Figure 5. Comparisons are made for ΔMFI of CD86, HLA-DR and CCR7 expression between DCs with either HLA-C1 or HLA-C2 homozygous allele.
Data shown are ΔMFI of CD86, HLA-DR and CCR7 expressed by DC in NK-DC co-culture in the presence of 1 ng/ml IL-15 at cell ratios of either 1∶1 or 1∶5. ΔMFI are calculated as the difference of MFI for DC in co-culture versus DC in isolation i.e. spontaneous expression. In NK-DC co-culture, in the presence of IL-15, DC with HLA-C1 homozygous allele express more co-stimulation molecules, and MHC class II molecules than DC with HLA-C2 homozygous alleles. Furthermore expression of trafficking chemokine CCR7 is virtually exclusive to HLA-C1 homozygotes indicating their predominant role in T-cell immune priming in secondary lymphoid tissues. Data shown for 4 independent experiments performed in each group and * indicates statistical significance with p<0.05 by Mann Whitney U test.
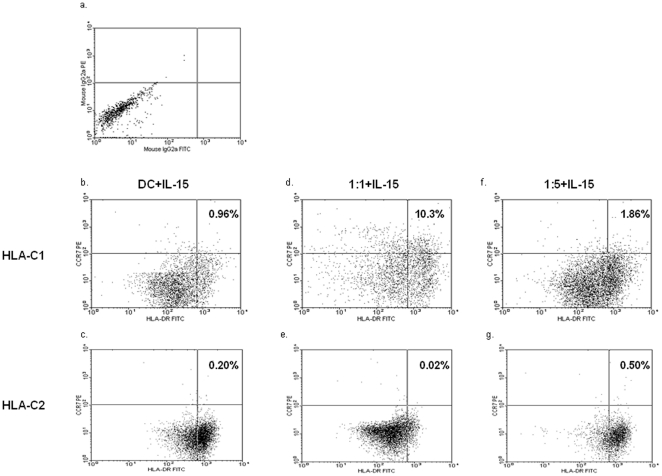
Figure 6. Flow cytometer data illustrating the impact of IL-15 (1 ng/ml) treated NK-DC co-culture on the expression of DC maturation (HLA-DR FITC) and chemokine (CCR7 PE) markers, comparing responses for DCs with either HLA-C1 ( figures 6b, d, f ) or HLA-C2 ( figures 6c, e, g ) homozygous alleles.
(a) shows DC stained with isotype control for HLA-DR & CCR7, (b) & (c) shows background DC staining where DCs are in isolation in the presence of IL-15, (d) & (e) shows HLA-DR & CCR7 expression by DC in NK-DC co-culture at ratios of 1∶1 in the presence of IL-15, (f) & (g) shows DC markers in NK-DC co-culture at ratios 1∶5 in the presence of IL-15. This data clearly demonstrates that in NK-DC co-culture at ratios of 1∶1 in the presence of IL-15, HLA-C1 homozygous DCs undergo significantly greater maturation than HLA-C2 homozygous DCs and attain CCR7 chemokine expression required for trafficking to secondary lymphoid tissues. Results are representative of 4 experiments with 5,000 DC gated events captured.
HLA-C2 expression by DC is associated with an anti-inflammatory cytokine milieu in NK-DC co-culture
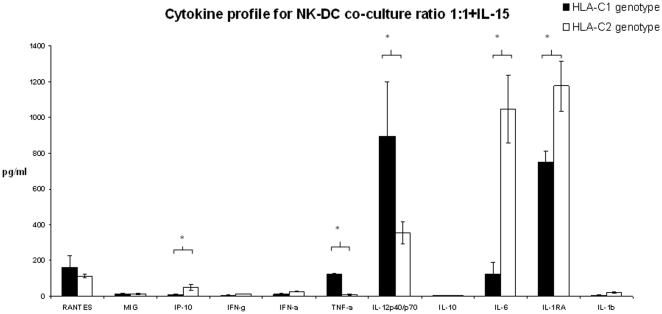
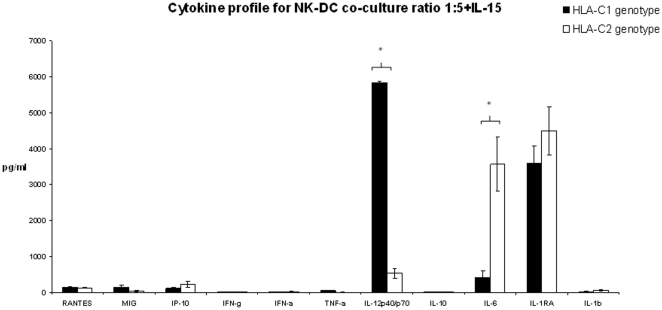
Supernatants were collected from NK-DC co-culture and tested for cytokine synthesis using 25 cytokine multiplex bead immunoassay. From the cytokines analysed, 11 could be detected (as shown on figures 7 and 8). Differential cytokine synthesis was compared between HLA-C1 DC and HLA-C2 DC in co-culture in the presence of 1 ng/ml IL-15. Data presented in figure 7 and 8 are means ± SEM for 4 independent experiments in each arm (HLA-C1 versus HLA-C2) and takes into account background cytokine production when DC/NK cells were in isolation. In NK-DC co-culture at cell ratios of 1∶1 (figure 7), significantly more TNF-α and IL-12 were detected in supernatants from HLA-C1 DC (TNF-α: 123.51±5.1 pg/ml; IL-12: 895.91±302.73 pg/ml) compared to HLA-C2 DC (TNF-α: 10.42±3.02 pg/ml; IL-12: 354.71±64.23 pg/ml, p<0.05). In contrast, significantly more IP-10, IL-6 and IL-1RA were detected in supernatants from HLA-C2 DC (IP-10: 49.43±15.22 pg/ml; IL-6: 1046.16±191.54 pg/ml; IL-1RA: 1175.03±142.25 pg/ml) than HLA-C1 DC (IP-10: 10.92±1.34 pg/ml; IL-6: 126.25±66.48 pg/ml; IL-1RA: 752.11±58.94 pg/ml, p<0.05). Other cytokines including RANTES, MIG, interferon-γ, interferon-α, IL-10 and IL-1β were detected in comparable quantities between groups. In NK-DC co-culture at cell ratios of 1∶5 (figure 8), significantly more IL-12 was detected in supernatants from HLA-C1 DC (IL-12: 5825.28±54.34 pg/ml) compared with HLA-C2 DC (IL-12: 533.99±129.51 pg/ml, p<0.05). In contrast, significantly more IL-6 was detected in supernatants from HLA-C2 DC (IL-6: 3564.48±750.53 pg/ml) than HLA-C1 DC (IL-6: 424.07±179.14 pg/ml, p<0.05). Other cytokines including RANTES, MIG, interferon-γ, interferon-α, TNF-α, IL-10, IL-1RA and IL-1β were detected at comparable quantities between the groups. TNF-α and IL-12 are potent inducers of DC maturation whereas IL-6 and IL-1RA have been shown to inhibit DC maturation. This differential cytokine profile is consistent with the observation that, in co-culture with NK cells, HLA-C1 DC expressed more CD86, HLA-DR and CCR7 than HLA-C2 DC. Diminished TNF-α synthesis during co-culture at cell ratios of 1∶5 (figure 8) may explain the lower expression of CD86, HLA-DR and CCR7 when compared to 1∶1 cell ratios (figure 7).
Figure 7. Supernatants were collected from NK-DC co-culture experiments at cell ratios of 1∶1 treated with IL-15 and tested for cytokine synthesis using 25 cytokine multiplex bead immunoassay.
Data shown are for all the cytokines that were expressed in culture supernatants. Of these, comparisons were made between cytokine synthesis for DCs with either HLA-C1 homozygous allele or DCs with HLA-C2 homozygous alleles. Data shown excludes background synthesis by cells in isolation. These data indicates that DC with HLA-C1 alleles express significantly greater pro-inflammatory cytokines in NK-DC co-culture favouring immune maturation whereas DC with HLA-C2 alleles predominantly express anti-inflammatory cytokines that are inhibiting to DC maturation. Means ± SEM for 4 independent experiments in each group are shown and * indicates statistical significance with p<0.05 by Mann-Whitney U test.
Figure 8. Supernatants were collected from NK-DC co-culture experiments at cell ratios of 1∶5 treated with IL-15 and tested for cytokine synthesis using 25 cytokine multiplex bead immunoassay.
Data shown are for all the cytokines that were expressed in culture supernatants. Of these, comparisons were made between cytokine synthesis for DCs with either HLA-C1 homozygous allele or DCs with HLA-C2 homozygous alleles. Data shown excludes background synthesis by cells in isolation. These data indicates that DC with HLA-C1 alleles express significantly greater pro-inflammatory cytokines in NK-DC co-culture favouring immune maturation whereas DC with HLA-C2 alleles predominantly express anti-inflammatory cytokines that are inhibiting to DC maturation. Means ± SEM for 4 independent experiments in each group are shown and * indicates statistical significance with p<0.05 by Mann-Whitney U test.
Discussion
In contrast to liver transplantation, recipient expression of a HLA-C2 allele had a strong impact on clinical outcomes following kidney transplantation. We found that kidney transplant recipients with a HLA-C2 allele had a 21.9% death non-censored and 19.9% death censored better graft survival at 10 years when compared to recipients without the allele. Recipients with either a HLA-C2 homozygous or heterozygous allele had similar graft survival benefits, suggesting that gene dose does not have a strong effect in this setting. The relative risk of graft loss for kidney transplant recipients without a HLA-C2 allele was 1.5 times that of recipients with a HLA-C2 allele. The benefit of a HLA-C2 allele in the recipient was further confirmed by multivariable analysis. Re-transplants were included because analysing re-transplant cases alone demonstrated similar survival benefits; this observation is fundamentally important as it further emphasizes the reliability of this data, through a recipient derived effect that is maintained even in re-transplantation. 10-year patient survival was also superior in the HLA-C2 positive recipients. However this effect was not significant in the multivariable analysis; this indicates any associations of recipient HLA-C2 allele with patient survival independent of graft loss will be weak. People with kidney transplant failure are treated with dialysis and therefore rarely die from graft failure. Causes of graft loss were analysed in the context of the absence or presence of HLA-C2; this analysis demonstrated that the absence of HLA-C2 was associated with more chronic allograft injury and vascular thrombosis.
Whilst HLA mismatching is known to influence graft survival our study was not designed to detect these as the number of cases per HLA mismatch were small. Other observations made on the multivariable analysis including a lack of a consistent benefit seen with HLA matching, variable effects seen for increasing recipient age with graft and patient survival, and the beneficial effect on patient survival in the tacrolimus group must be interpreted with caution as this study was not designed to investigate these parameters. In particular patients from our clinical centre who were started on tacrolimus were generally younger patients which may have had a direct impact on survival outcomes.
We also tested the impact of the ‘missing self’ model on kidney transplant outcomes as previously performed by Tran and colleagues [18]. Consistent with their observations, we found no correlation between KIR ligand mismatch and graft survival. HLA-C ligand matching also did not influence the rate of acute rejection.
Kunert and colleagues [16] studied the relationship between the incidence of acute rejection after kidney transplantation and various KIR genotypes for a cohort of 224 patients. They found a reduced incidence of acute rejection when the donor was HLA-C2 homozygous. In contrast with their work, we found no significant association between a donor HLA-C2 homozygous genotype and acute rejection. More broadly, we tested both donor and recipient genotypes and found no association between HLA-C ligands, KIR or KIR haplotypes and acute rejection in 657 kidney transplant patients. The explanation for the conflicting findings may relate to their small study size (HLA-C2 homozygous, n = 31, p = 0.052), and reflect a type I statistical error.
The organ specific directional benefit of possession of HLA-C2 allele (recipients with a HLA-C2 allele in kidney transplants and donors with HLA-C2 allele in liver transplants [15]) is of great interest. These observations are consistent with other data that liver and kidney transplants develop immunological responses through different mechanisms [36], [37].
In experimental models of transplantation, liver allografts have been shown to be immune privileged, with evidence for tolerance against T cells [38]–[40]. The mechanisms described include induction of CD8+ T-cell apoptosis [38], inhibition of CD4+ T-cell mediated Th1 differentiation [39] and production of the anti-inflammatory cytokine IL-10 [40]. In clinical transplantation, liver allografts are transplanted without HLA matching and prior knowledge of anti-donor HLA antibodies. HLA matching is not associated with improvement in long-term graft survival after liver transplantation [41]. Therefore current evidence indicates that chronic rejection may be attributable to recipient NK cells targeting donor tissue [15], [37], [42]–[45]. Based on our hypothesis, an absence of HLA-C2 allele on the allograft would lead to NK cell activation with consequent liver transplant damage [15]. Conversely kidney transplants are highly susceptible to all components of the adaptive immune system. CAI occurs through adaptive immune processes involving T cells, B cells and anti-HLA antibodies [46]. Therefore the associations between the recipient HLA-C2 allele and survival benefit may be linked to the maturation of adaptive immunity facilitated by interactions between donor-derived NK cells and recipient antigen presenting cells (stimuli for indirect allorecognition) in the early post-transplant period. Indeed, there is good evidence linking indirect allorecognition to poor long-term graft function in kidney transplantation [47]–[49].
The increased understanding of NK cell biology in the past decade has been based on studies that have assessed interactions between NK cells and DC. DC are professional antigen presenting cells with very potent T-cell priming ability [50]. Recently, activated NK cells were shown to interact with DC to efficiently induce their maturation [51]–[53]. Subsequently, Brilot and colleagues [27] showed polarisation of KIR and KIR ligands to the site of cell-cell contact in NK-DC co-culture indicating that this interaction is involved in NK-DC cross talk. Vitale and colleagues [26] showed that the predominant activation signal in NK mediated DC maturation occurs with engagement of the natural cytotoxic receptor NKp30. This signal was modulated by blocking antibodies against a HLA specific inhibitory receptor (A6/136 IgM), indicating the involvement of KIRs and KIR ligands. Therefore, at the NK-DC immune synapse, HLA-C2 expression by DC may inhibit DC maturation following engagement with KIR 2DL1, with a consequent impact on priming of the adaptive immune system.
Potent NK-DC interactions require a favourable cytokine milieu [51], and one important cytokine in this setting is IL-15 [27]. Kidney tissue constitutively expresses IL-15 [31], [33], with up-regulated expression in the early stages post-transplantation [32], [54]. Unlike IL-2, IL-15 synthesis is not abrogated by anti-CD25 monoclonal antibody treatment [55]. Furthermore, IL-15 induced activation of NK cells is not inhibited by conventional induction immunosuppression with methylprednisolone [56] and encourages potent NK-DC interactions.
We developed an allogeneic NK-DC co-culture model which is relevant to the setting of kidney transplantation. In support of the validity of this model, immunohistochemical studies performed on pre-transplant kidney tissue confirms that donor derived NK cells are present in the allograft at the time of the transplant. Following transplantation, with up-regulation of constitutively expressed IL-15 [31], [33], [54] donor NK cells interact with immature DC. We demonstrated that on in-vitro allogeneic NK-DC co-culture, significant DC maturation occurred following treatment with 1 ng/ml of IL-15 and this process was cell contact dependent. DC maturation occurred at NK-DC cell ratios of either 1∶1 or 1∶5, but not when NK cells were in excess of DC at a ratio of, 5∶1. NK-DC cell ratios of 1∶1 were maximally inductive for DC maturation. This observation is very relevant to the clinical setting, as donor derived cells may be at lower numbers than recipient cells following allograft reperfusion.
We made the novel observation that in NK-DC co-culture, the HLA-C allele expressed by DC influenced the degree of DC maturation. In co-culture, HLA-C1 DC (without HLA-C2 allele) underwent more maturation with greater expression of CD86 (a co-stimulation molecule) and HLA-DR (a MHC class II molecules) when compared to HLA-C2 DC. Furthermore, CCR7, a chemokine receptor required for cell trafficking to secondary lymphoid tissue [57] was exclusively expressed by HLA-C1 DC. CCR7 expression has been shown to enhance DC migratory speed [58], [59] and survival [60]. Therefore HLA-C1 DC (without HLA-C2 allele), matured in-situ in the days following kidney transplantation, acquire the capacity to migrate to secondary lymphoid tissue, where they will present antigen to promote the activation and proliferation of T cells specific for graft antigen [61]. Conversely, DC with HLA-C2 allele express low level CD86 and HLA-DR with no CCR7 expression. This may make them inefficient at T-cell priming but may provide them with tolerogenic potential [62], [63].
Supernatants from NK-DC co-culture were also assessed for the presence of cytokines. At cell ratios of 1∶1 in presence of 1 ng/ml of IL-15, TNF-α and IL-12 were detected at significantly higher concentrations for HLA-C1 DC than HLA-C2 DC. Conversely, IP-10, IL-6 and IL-1 receptor antagonist (IL-1RA) were detected at higher concentrations with HLA-C2 DC than with HLA-C1 DC. These cytokine profiles are consistent with the observation of enhanced cell surface maturation markers for HLA-C1 DC and a failure of efficient maturation for HLA-C2 DC in co-culture. TNF-α and IL-12 are pro-inflammatory cytokines involved in DC maturation [21]–[23]. IL-12 synthesised by DC in NK-DC co-culture has been shown to augment the activation status of NK cells [64]. This in-turn may favour further IFN-γ, TNF-α, and GM-CSF, encouraging DC maturation [65]. The cytokine profiles noted for HLA-C2 DC are anti-inflammatory; the increased IP-10 synthesis may reflect the prolonged contact of ineffective DCs with NK cells. This may be relevant in the process of DC editing. Furthermore, IL-6 inhibits NF-κB binding activity and CCR7 expression [66], therefore inhibiting DC maturation. IL-1 is a powerful pro-inflammatory cytokine profoundly inhibited by IL-1 receptor antagonist (IL-1RA). The role of IL-1RA in human diseases is well described [67]. Up-regulation of IL-1RA may therefore impede DC maturation in HLA-C2 DCs.
Based on these observations, recipients without HLA-C2 alleles are at greater risk of accelerated DC maturation compared with recipients with HLA-C2 alleles. As a consequence recipients without a HLA-C2 allele are subjected to a greater immunological load through T-cells primed by indirect allorecognition.
In conclusion, early events in kidney transplantation involving donor NK cells and recipient DC interactions via KIR and HLA-C immune synapse has a major impact on subsequent immune priming; this is determined by the recipient HLA-C genotype. Graft survival outcomes are related to these processes, which therefore represent a prime target for therapeutic intervention to prolong allograft survival. Furthermore, recipients with the HLA-C2 allele may be at lower immunological risk and may therefore benefit from the minimisation of immunosuppression.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Grants for this study were provided by the University Hospital Birmingham charitable funds. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sayegh MH, Carpenter CB. Transplantation 50 Years Later – Progress, Challenges, and Promises. N Engl J Med. 2004;351:2761–2766. doi: 10.1056/NEJMon043418. [DOI] [PubMed] [Google Scholar]
- 2.2004 Annual report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: transplant data 1994–2003. Rockville, Md.: Health Resources and Services Administration, Division of Transplantation, 2004; [Google Scholar]
- 3.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4(8):1289–95. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 4.Paul LC. Chronic allograft nephropathy: an update. Kidney Int. 1999;56:783–793. doi: 10.1046/j.1523-1755.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 5.Jevnikar AM, Mannon RB. Late kidney allograft loss: what we know about it, and what we can do about it. Clin J Am Soc Nephrol. 2008;Suppl 2:S56–67. doi: 10.2215/CJN.03040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86(3):377–83. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- 7.Bestard O, Nickel P, Cruzado JM, Schoenemann C, Boenisch O, et al. Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol. 2008;19(7):1419–29. doi: 10.1681/ASN.2007050539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najafian B, Kasiske BL. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2008;17(2):149–55. doi: 10.1097/MNH.0b013e3282f4e514. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 10.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 11.Carrington M, Wang S, Martin MP, Gao X, Schiffman M, et al. Hierarchy of resistance to cervical neoplasiamediated by combinations of killer immunoglobulin-like receptor and human leukocyte antigen loci. J Exp Med. 2005;201:1069–1075. doi: 10.1084/jem.20042158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of pre-eclampsia and reproductive success. J Exp Med. 2004;200(8):957–65. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahlenstiel G, Martin MP, Gao X, Carrington M, Rehermann B, et al. Distinct KIR/HLA compound genotypes affect the kinetics of human antiviral natural killer cell responses. J Clin Invest. 2008;118(3):1017–1026. doi: 10.1172/JCI32400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, et al. HLA and NK Cell Inhibitory Receptor Genes in Resolving Hepatitis C Virus Infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 15.Hanvesakul R, Spencer N, Cook M, Gunson B, Hathaway M, et al. Donor HLA-C genotype has a profound impact on the clinical outcome following liver transplantation. Am J Transplant. 2008;8(9):1931–41. doi: 10.1111/j.1600-6143.2008.02341.x. [DOI] [PubMed] [Google Scholar]
- 16.Kunert K, Seiler M, Mashreghi MF, Klippert K, Schönemann C, et al. KIR/HLA ligand incompatibility in kidney transplantation. Transplantation. 2007;84(11):1527–33. doi: 10.1097/01.tp.0000290681.41859.41. [DOI] [PubMed] [Google Scholar]
- 17.Nowak I, Majorczyk E, Wisniewski A, Pawlik A, Magott-Procelewska M, et al. Does the KIR2DS5 gene protect from some human diseases? PLoS One. 2010;5(8):e12381. doi: 10.1371/journal.pone.0012381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran TH, Mytilineos J, Scherer S, Laux G, Middleton D, et al. Analysis of KIR ligand incompatibility in human renal transplantation. Transplantation. 2005;80(8):1121–3. doi: 10.1097/01.tp.0000179110.15304.90. [DOI] [PubMed] [Google Scholar]
- 19.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, et al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31(10):3121–7. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195(3):335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195(3):327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195(3):343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26(4):503–17. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 26.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Aricò M, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106(2):566–71. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 27.Brilot F, Strowig T, Roberts SM, Arrey F, Münz C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J Clin Invest. 2007;117(11):3316–29. doi: 10.1172/JCI31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mocikat R, Braumuller H, Gumy A, Egeter O, Ziegler H, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19(4):561–9. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 29.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171(5):2366–73. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 30.Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6(4):652–8. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 31.Weiler M, Rogashev B, Einbinder T, Hausmann MJ, Kaneti J, et al. Interleukin-15, a leukocyte activator and growth factor, is produced by cortical tubular epithelial cells. J Am Soc Nephrol. 1998;9(7):1194–201. doi: 10.1681/ASN.V971194. [DOI] [PubMed] [Google Scholar]
- 32.Baan CC, Niesters HG, Metselaar HJ, Mol WM, Loonen EH, et al. Increased intragraft IL-15 mRNA expression after liver transplantation. Clin Transplant. 1998;12(3):212–8. [PubMed] [Google Scholar]
- 33.Strehlau J, Pavlakis M, Lipman M, Maslinski W, Shapiro M, et al. The intragraft gene activation of markers reflecting T-cell-activation and -cytotoxicity analyzed by quantitative RT-PCR in renal transplantation. Clin Nephrol. 1996;46(1):30–3. [PubMed] [Google Scholar]
- 34.LaRosa DF, Rahman AH, Turka LA. The innate immune system in allograft rejection and tolerance. J Immunol. 2007;178:7503–7509. doi: 10.4049/jimmunol.178.12.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–63. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 36.Martinez OM, Rosen HR. Basic concepts in transplant immunology. Liver Transpl. 2005;11(4):370–81. doi: 10.1002/lt.20406. [DOI] [PubMed] [Google Scholar]
- 37.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;(3):51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 38.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6(12):1348–54. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 39.Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, et al. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116(6):1428–40. doi: 10.1016/s0016-5085(99)70508-1. [DOI] [PubMed] [Google Scholar]
- 40.O'Connell PJ, Morelli AE, Logar AJ, Thomson AW. Phenotypic and functional characterization of mouse hepatic CD8α+ lymphoid-related dendritic cells. J Immunol. 2000;165:795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 42.Kitchens WH, Uehara S, Chase CM, Colvin RB, Russell PS, et al. The changing role of natural killer cells in solid organ rejection and tolerance. Transplantation. 2006;81(6):811–7. doi: 10.1097/01.tp.0000202844.33794.0e. [DOI] [PubMed] [Google Scholar]
- 43.Obara H, Nagasaki K, Hsieh CL, Ogura Y, Esquivel CO, et al. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am J Transplant. 2005;5(9):2094–103. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maier S, Tertilt C, Chambron N, Gerauer K, Hüser N, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med. 2001;7:557–562. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 45.Uehara S, Chase CM, Kitchens WH, Rose HS, Colvin RB, et al. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol. 2005;175(5):3424–30. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 46.Najafian B, Kasiske BL. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2008;17(2):149–55. doi: 10.1097/MNH.0b013e3282f4e514. [DOI] [PubMed] [Google Scholar]
- 47.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, et al. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001;167(12):7199–206. doi: 10.4049/jimmunol.167.12.7199. [DOI] [PubMed] [Google Scholar]
- 48.SivaSai KS, Smith MA, Poindexter NJ, Sundaresan SR, Trulock EP, et al. Indirect recognition of donor HLA class I peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Transplantation. 1999;67(8):1094–8. doi: 10.1097/00007890-199904270-00002. [DOI] [PubMed] [Google Scholar]
- 49.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13(1):252–9. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]
- 50.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 51.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l'union fait la force”. Blood. 2005;106(7):2252–8. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 52.Moretta A. The dialogue between human natural killer cells and dendritic cells. Curr Opin Immunol. 2005;17(3):306–11. doi: 10.1016/j.coi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Weiler M, Kachko L, Chaimovitz C, Van Kooten C, Douvdevani A. CD40 ligation enhances IL-15 production by tubular epithelial cells. J Am Soc Nephrol. 2001;12:80–87. doi: 10.1681/ASN.V12.1.80. [DOI] [PubMed] [Google Scholar]
- 55.Baan CC, Knoop CJ, van Gelder T, Holweg CT, Niesters HG, et al. Anti-CD25 therapy reveals the redundancy of the intragraft cytokine network after clinical heart transplantation. Transplantation. 1999;67(6):870–6. doi: 10.1097/00007890-199903270-00014. [DOI] [PubMed] [Google Scholar]
- 56.Chiossone L, Vitale C, Cottalasso F, Moretti S, Azzarone B, et al. Molecular analysis of the methylprednisolone-mediated inhibition of NK-cell function: evidence for different susceptibility of IL-2- versus IL-15-activated NK cells. Blood. 2007;109(9):3767–75. doi: 10.1182/blood-2006-07-037846. [DOI] [PubMed] [Google Scholar]
- 57.Sánchez-Sánchez N, Riol-Blanco L, Rodríguez-Fernández JL. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol. 2006;176(9):5153–9. doi: 10.4049/jimmunol.176.9.5153. [DOI] [PubMed] [Google Scholar]
- 58.Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol. 1999;162(7):3859–64. [PubMed] [Google Scholar]
- 59.Riol-Blanco L, Sánchez-Sánchez N, Torres A, Tejedor A, Narumiya S, et al. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol. 2005;174(7):4070–80. doi: 10.4049/jimmunol.174.7.4070. [DOI] [PubMed] [Google Scholar]
- 60.Sánchez-Sánchez N, Riol-Blanco L, de la Rosa G, Puig-Kröger A, García-Bordas J, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104(3):619–25. doi: 10.1182/blood-2003-11-3943. [DOI] [PubMed] [Google Scholar]
- 61.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3(12):984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 62.Coates PT, Duncan FJ, Colvin BL, Wang Z, Zahorchak AF, et al. In vivo-mobilized kidney dendritic cells are functionally immature, subvert alloreactive T-cell responses, and prolong organ allograft survival. Transplantation. 2004;77:1080–1089. doi: 10.1097/01.tp.0000122183.60680.c9. [DOI] [PubMed] [Google Scholar]
- 63.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 65.Marcenaro E, Della Chiesa M, Bellora F, Parolini S, Millo R, et al. IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J Immunol. 2005;174(7):3992–8. doi: 10.4049/jimmunol.174.7.3992. [DOI] [PubMed] [Google Scholar]
- 66.Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-κB binding activity and CCR7 expression. FASEB Journal. 2004;18:1439–1441. doi: 10.1096/fj.03-0969fje. [DOI] [PubMed] [Google Scholar]
- 67.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]