Abstract
The emergence of the novel prion diseases bovine spongiform encephalopathy (BSE) and, subsequently, variant Creutzfeldt-Jakob disease (vCJD) in epidemic forms has attracted much scientific attention. The oral transmission of these disorders, the causative relationship of vCJD to BSE and the resistance of the transmissible agents in both disorders to conventional forms of decontamination has caused great public health concern. The size of the still emerging vCJD epidemic is thankfully much lower than some early published estimates. This paper reviews current knowledge of the factors that influence the development of vCJD: the properties of the infectious agent; the route of inoculation and individual susceptibility factors. The current epidemiological data are reviewed, along with relevant animal transmission studies. In terms of genetic susceptibility, the best characterised is the common single nucleotide polymorphism at codon 129 of prion protein gene. Current biomarkers and future areas of research will be discussed. These issues are important in informing precautionary measures and the ongoing monitoring of vCJD.
Keywords: Variant CJD, prion disease, BSE, transmission, environmental factors, susceptibility, genetic factors, investigations, biomarkers
Introduction
Variant Creutzfeldt-Jakob disease (vCJD) is one of the transmissible spongiform encephalopathies, which affect humans and other mammals. These neurodegenerative conditions, also known as prion diseases, are potentially transmissible, although most human diseases are apparently not acquired (Table 1) [1]. They share a common molecular abnormality: a post-translational conformational change in a normal host protein (the prion protein, encoded in humans by the prion protein (PRNP) gene on chromosome 20). Transmissibility necessitates an infectious agent that has been termed the ‘prion’; its nature has not yet been fully characterised [2]. According to the prion hypothesis, the prion consists entirely or mainly of the abnormally folded prion protein (designated PrPSc, the normal form being designated PrPc). The abnormal prion protein appears to cause a cascade of misfolding in the normal cellular prion, similarly altering its conformational structure and propagating the disease.
Table 1.
Human and animal Transmissible spongiform encephalopathies (TSEs) and the year they were reported
| Human TSEs | First reported | Animal TSEs | First reported |
|---|---|---|---|
| Sporadic CJD | 1921 | Sheep Scrapie | 1730 |
| Familial CJD | 1924 | Goat Scrapie | 1872 |
| Gerstmann-Straussler-Scheinker disease (familial) | 1936 | Chronic wasting disease (Elk/Mule) | 1967 |
| Kuru | 1955 | Bovine spongiform encephalopathy | 1986 |
| Iatrogenic CJD | 1974 | Feline spongiform encephalopathy | 1990 |
| Fatal Familial insomnia | 1986 | ||
| Variant CJD | 1996 |
Given the large numbers of BSE cases in the UK and the great potential exposure of the UK population to BSE infectivity in food between 1985 and 1996 [3], the relatively small number of UK vCJD cases identified to date (N=175) stands in need of explanation [4]. Epidemiological, human clinical and animal experimental studies have led to a greater, although incomplete, understanding of the size of the epidemic and have informed precautionary measures.
BSE and vCJD
In the UK, the National CJD Research and Surveillance Unit was established in Edinburgh in 1990 in response to concerns for public health over the emergence of bovine spongiform encephalopathy (BSE) in cattle. However, there were experimental data suggesting a barrier to transmission of prion diseases between species which provided a degree of reassurance about the risk to the human population [5].
Epidemiology of vCJD
The first cases of vCJD were reported in 1996 [6]. vCJD cases were initially differentiated from sporadic CJD (sCJD) by their younger age of onset and differing pathological features (Table 2) [7].
Table 2.
Clinical differences between Sporadic and Variant CJD
| Sporadic CJD | Variant CJD | |
|---|---|---|
| Mean age at death (years) | 67 | 29 |
| Mean duration of illness (months) | 4 | 13 |
| Rapidly progressive dementia | Common | Rare |
| Psychiatric symptoms at onset | Rare | Common |
| Sensory symptoms | Rare | Common |
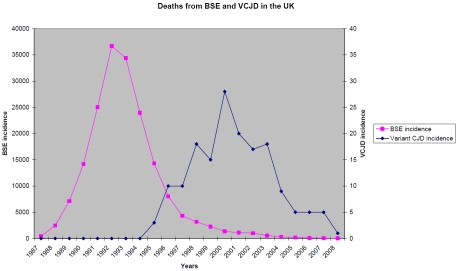
There are documented young onset sCJD cases from as young as 18 years [8], but this is very rare; UK CJD surveillance since 1990 has found only two of 1147 sCJD cases to have been under 30 years of age [4]. Patients with vCJD were initially only found within the UK population, although gradually other countries have reported cases. Currently 218 cases have been reported worldwide, 175 of whom were, or had been, UK residents during the high risk period (Table 3) [4,9]. The temporal relationship to the BSE outbreak in the UK supports the direct link between the two conditions (Figure 1). The epidemiological link between the use of meat and bone meal in bovine feeds and the occurrence of BSE has been established [10]. The annual number of reported deaths from vCJD in the UK has fallen since 2000. One explanation would be the success of the control measures introduced; there have been no cases of vCJD from the UK in those born after the bovine offal ban was introduced in 1989 [11].
Table 3.
Variant CJD cases worldwide (August 2011)
| Number of primary cases (Alive) | Number of secondary cases: blood transfusion (Alive) | |
|---|---|---|
| UK and Northern Ireland | 172 (3) | 3 (0) |
| France | 25 (0) | - |
| Spain | 5 (0) | - |
| Republic of Ireland | 4 (0) | - |
| USA | 3 (0) | - |
| Netherlands | 3 (0) | - |
| Portugal | 2 (0) | - |
| Italy | 2 (0) | - |
| Canada | 2 (1) | - |
| Saudi Arabia | 1 (0) | - |
| Japan | 1 (0) | - |
| Taiwan | 1(0) | - |
Figure 1.
Reported incidence of BSE and vCJD in the UK. Note that the scale on the left is in increments of 5000 cattle. The scale on the right is in increments of 5 vCJD cases.
Infectious agent
Infectious diseases can be characterised in terms of the infective agent, the process of transmission and the characteristics of the individual developing the condition. Our knowledge of the infective agent is limited, however animal studies give us some information about prion disease transmission.
Different types of transmissible prion disease are thought to be due to different strains of the prion, although the basis of agent strain is not fully understood. Successful prion disease transmission is thought to be dependant, at least in part, on the strain of the prion agent and its genetic compatibility with the host. Experimental transmission studies in mice have shown that the strain of agent causing vCJD has identical properties to that of BSE, which differ to that of scrapie or sCJD [12]. Prion strains vary in their ability to be transmitted between different species and in the pattern of disease they cause among species [13].
Sheep scrapie strains can be differentiated by western blot analysis, but BSE and vCJD have the same biochemical properties. vCJD and BSE show a unique isoform on western blot analysis; the glycosylation present after PK digestion is different to that seen in other forms of CJD [14,15]. This suggests that the same agent is responsible for both vCJD and BSE. Unlike some other prion diseases, these agents show remarkable stability following transmission even after multiple passages into different species. Unfortunately, BSE crosses between species more readily than scrapie [15].
A species barrier to transmission of some prion diseases is well established. This may explain why humans apparently have never contracted scrapie [16]. Different species have differing PrP gene sequences and therefore their PrPc structures may vary. These differences in gene sequence may provide some explanation for the species barrier [16]. Of note, transgenic mice expressing human PrP or hamster PrP are susceptible to BSE inoculation, which is not the case in conventional mice [17]. This supports the importance of genetic susceptibility in prion diseases. However, unlike these controlled animal experiments, environmental factors play a role in human transmission.
Environmental factors
Volume of exposure
A case-control study of vCJD revealed a higher reported intake of mechanically recovered beef products in cases than controls. This suggests an increased potential oral exposure to BSE [18] (Chart 2, dietary risk factors). There is a risk of recall bias from the relatives of cases. However, differences in the incidence of cases across the country support the link. A two-fold difference was seen between the rate of development of vCJD in the North compared to South of the UK (rate ratio= 1.94, 95% CI 1.12, 3.36) [19]. Two National Dietary Surveys from the time period demonstrated significant differences in diet between these regions. Consumption of mechanically recovered beef products was much higher in the North, although the overall beef consumption was very similar. This information supports the link between both the consumption of higher risk beef products, and a link to the frequency of their consumption.
Experimental transmission studies have demonstrated that the volume of inoculum affects the likelihood of transmission. In 1995 oral transmission of BSE to sheep and goats was demonstrated with as little as 0.5g of infective bovine brain [10]. Transmission studies in macaques have been successful with 5g of orally introduced BSE-infected brain homogenate [20]. A 2002 study into the lethal challenge required to infect hamsters demonstrated a linear rate of transmission, which fell with increasing dilution of the oral inoculum [21]. This is likely to remain the case in humans, but the volume of necessary tissue can only be speculated.
Route of exposure
The risk of developing vCJD may partly relate to the route of exposure. The commonest route of primary exposure in humans is likely to be oral. We know higher rates of transmission are seen with brain inoculation than with the same infective load given orally. In addition IV transfusion of whole blood from BSE infected sheep into recipient sheep has been shown to transmit infection with success rates of 60% [22].
Since 2003 the Transfusion Medicine Epidemiology Review (TMER) identified four cases of vCJD infection in recipients of blood from donors who later went on to develop clinical vCJD [23,24]. Three cases showed typical MM homozygosity at codon 129 and presented with the same clinical presentation and pathology as other vCJD cases. One however, demonstrated asymptomatic splenic and lymph node infection and was an MV heterozygote at codon 129. These four cases were identified from 66 individuals who had received blood products from vCJD donors, only 28 of whom survived more than five years after their transfusion [25]. Patients receiving blood transfusion are commonly older with more co-morbidity, leading to their requirement for blood and shortened life expectancy. Given the inoculation time in the confirmed vCJD cases varied between 5 and 8.5 years, some of the other patients may have died in the pre-clinical phase. Even the ratio of confirmed symptomatic cases (4/66) suggests a high efficiency of onward infection when compared with the oral route. There is ongoing surveillance of the 18 surviving exposed patients. There is a risk of onwards transmission of the disease during the asymptomatic phase of the illness.
Animal studies comparing vCJD cases contracted via BSE or transfusion suggest no change in the pattern of pathology or clinical presentation following onward transmission into wild-type and transgenic mice [25].
Susceptibility factors
Age
Although the entire UK population is likely to have been exposed to BSE in the food chain, the majority of vCJD cases are aged less than 40. This suggests a higher rate of dietary exposure, increased susceptibility to infection or a reduced incubation period in this age group. One possible explanation is that as part of the development of human immune systems there is a greater volume of gut-associated lymphoid tissues when younger [26]. There is therefore a higher volume of tissue to absorb the infected material. Asymptomatic infection of lymphoid tissue appears to precede neurological disease in animals and humans. This process may explain the broader distribution of PrPSc in vCJD, where infectivity is often found in the tonsils and spleen, unlike familial and sCJD where PrPSc is predominantly only found in neural tissues [27].
Avrahami and Gabizon studied young and old mice inoculated intraperitoneally with prion infected brain tissue [28]. The younger mice developed clinical signs and reached the terminal phase more quickly. They had a shorter incubation period and developed more marked neuro-pathological features. PrPSc levels were maintained at low levels in the spleens of the older mice even at the disease end-point. They suggest the altered peripheral absorption and less marked central nervous system response explain the differences in susceptibility with age. Their paper might also suggest variability in incubation time with increasing age, something that has not been confirmed in humans with vCJD.
Genetic influence
Codon 129: The PRNP gene (OMIM *176640), on chromosome 20, encodes for the production of human PrPC. There are over 20 reported pathological mutations in this gene associated with familial CJD.
In 1991 a single nucleotide polymorphism at codon 129 on the PRNP gene (rs 1799990) was found to be associated with the development of CJD linked to the use of cadaveric pituitary hormones [29]. Subsequently MM homozygosity, at codon 129, was noted to influence the age of presentation of familial CJD [30]. MM homozygosity was also associated with sCJD; where 65% of cases were MM homozygotes compared to 40% of the normal UK population. The difference is most clearly seen in vCJD where all 157 pathologically confirmed cases have had the MM genotype. This is clearly a very strong association, but it is not yet clear whether the polymorphism is the major determinant of patients' susceptibility or only incubation time (Table 4).
Table 4.
PRNP codon 129 distribution in the UK
As discussed earlier there has been one confirmed asymptomatic case with an MV genotype at codon 129 infected with CJD via blood transfusion [31]. A further asymptomatic case in a patient with haemophilia, also of MV genotype, has been reported [32]. They had received multiple blood transfusions and pooled factor VIII from a donor who later died from clinical vCJD . There has been one patient with MV heterozygosity who presented with symptoms suggestive of vCJD. However, their imaging was not typical and the patient did not undergo a post mortem examination [33].
Two of three PrP-positive samples in an anonymous appendix study had the VV genotype (see below) [34]. This suggests the condition can be transmitted at least to the lymphoid tissues of patients of all genotypes. This could result in either a preclinical phase of the illness, before the development of neurological disease or a sub-clinical infection where patients will not progress to develop the condition [35]. Such sub-clinical forms of prion disease have been seen in animal studies with MM transgenic mice, but it is uncertain if this occurs in humans [17]. It has been suggested however, that such cases are probably necessary to explain the observed human data [36].
It is currently unclear whether PRNP genotypes confer only a longer incubation period rather than resistance to the disease. Our knowledge of the other orally acquired prion disease; Kuru, might help answer this question. This disease emerged in the 1950s among a remote linguistic cultural group in Papua New Guinea practicing ritual funerary cannibalism.
We know that the maximum incubation period for Kuru can exceed 50yrs, with a mean incubation period of 12yrs [37]. On examining blood samples from patients from the peak of the epidemic, and more recent samples, an age stratification of codon 129 genotype is seen [38]. The young Kuru patients were mainly of MM or VV genotype and the elderly patients were mostly MV. Eight of eleven of the more recent cases were MV homozygotes; supporting its possible effect on prolonging incubation time given that exposure should have ended over 40 years ago when ritual funerary cannibalism was outlawed [39,40].
Other genetic factors: In addition to MM homozygosity at codon 129 there have been other proposed risk mutations. Bishop et al compared PRNP gene sequencing of 118 confirmed vCJD cases with 970 blood donor controls and 309 sCJD cases [41]. They found four patients with potentially relevant polymorphisms; two with alterations at codon 219 and two at codon 202. The first mutation has been known to influence the clinical phenotype of cases with the P102L mutation in Japan and Korea. The second has been reported in one sporadic and one iatrogenic CJD case in France. A further paper by Bishop et al looked at cathepsin D polymorphisms in patients with vCJD and UK controls [42]. Polymorphisms in this gene have been studied in Alzheimer's disease, where they have been linked to disease susceptibility and amyloid propogation. They found the TT genotype gave a 9.75 odds ratio (CI 1.52-62.47) of developing vCJD compared to the CT genotype and a 10.92 odds ratio (CI 1.80-66.26) compared to the CC genotype. However, there are wide confidence intervals given that only three vCJD cases out of 110 had a TT genotype.
Mead et al looked at the whole genome in their association study with 109 definite vCJD cases and 10 probable cases [38]. They had 3692 controls. Despite looking within a similar cohort of vCJD patients, from the UK population, their genome wide association study implicated two different loci. They found two significant genome wide single nucleotide polymorphisms (p<5×10-7) on allelic testing. One was located in an intron of PRNP with a moderately strong linkage disequilibrium to codon 129. The other occurred in the intergenic region between RARB (retinoic acid receptor, beta; OMIM * 180220) and THRB (thyroid hormone receptors, beta; OMIM * 190160). They suggest retinoic acid may act through the receptor encoded by RARB. PrP expression in cultured neuronal cells is known to be regulated by retinoic acid. The power of their analysis was limited by the small number of vCJD cases and no other significant associations were found.
Predicting the size of the vCJD epidemic
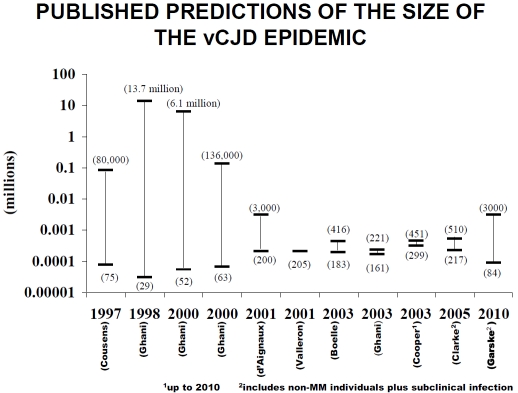
Predicting the number of expected cases of vCJD is difficult, and estimated numbers have fallen in line with the number of reported cases (Figure 2. Predicative modelling of vCJD). The most recent modelling by Ghani (2010) predicts 390 further cases between 2010 and 2179, incorporating the potential for secondary transmission of cases [36]. However, despite active surveillance it is possible that only patients who fit the defined phenotype and pattern of investigations are being diagnosed. The low overall post mortem rate in dementia patients and less intensive investigation of older patients creates the possibility of cases being missed. In addition there may be a number of asymptomatic cases who may or may not develop neurological disease. One way of investigating the general population is to screen lymphoid tissue samples for evidence of prion proteins.
Figure 2.
Published predictions of the size of the vCJD epidemic. The Cooper paper included predictions up to 2010. The Clarke and Garske papers include non-MM cases and subclinical infection as indicated.
In 2004 a study screening 1,427 anonymous tonsil specimens and 11,247 appendix specimens by immuno-histochemistry found three appendixes positive for the presence of disease-associated prion protein [43]. Stored tissue samples from within the high risk 1961-85 birth cohort were screened from two regions; Scotland and South West England. Generalisation of the results suggested a prevalence of 292 per million (95% CI; 60-853). A second larger study of anonymous tonsil specimens conducted in 2008 was more reassuring [44]. They found no abnormal specimens by initially screening with two enzyme immunoassays and following up any positive results with the more proven technique of immuno-histochemistry. This study reviewed tonsil specimens from a later time period, mostly 2006-7 compared to 1995-9 from the appendix study (the peak of the BSE epidemic). Moreover, the average age of patients in the appendicetomy study was four years older; giving more time for abnormal proteins to propagate. The tonsil study reported a prevalence of zero with confidence intervals of 0 to 289 per million in the 1961-85 cohort. This predicted prevalence was therefore lower, but consistent with the earlier study. A P value of 0.09 is given for the comparison of these two prevalence estimates.
The most recent tonsil study showed positive PrP staining in one follicle, from one patient, when looking at 9160 patients from the 1961-1985 birth cohort [45]. The prevalence results are comparable to the initial appendix study if this patient is considered as a positive case or the first tonsil study if they are not. However, all these prevalence results assume the tests are 100% sensitive and specific throughout the incubation period.
Three patients have had removal of lymphoid tissue prior to becoming symptomatic of vCJD. Two patients had positive immunohistochemistry from appendicectomies up to two years prior to developing symptoms [46]. The third patient's appendix was removed ten years earlier, which was negative for PrPSc [43, 46]. Unfortunately, as the average age of appendicetomy is 22 and tonsillectomies is under ten, the majority of prospective specimens will be from out with the highest risk birth cohort limiting further similar studies. Currently we do not have a screening test for patients with sub-clinical infection. We do however have useful CSF bio-markers and imaging features to aid diagnosis in patients once they develop neurological disease.
Biomarkers in vCJD
A large number of CSF biomarkers have been found to be elevated in patients with CJD; including 14-3-3 protein, S-100 β, tau, and neuron-specific enolase (NSE) [47]. The release of these proteins has been shown to follow a bell curve with normal concentrations early and late in the disease process. Most of these biomarkers were initially investigated in sCJD and have lower efficacy in vCJD. Indeed in vCJD, unlike sCJD, biomarkers are not included in the WHO diagnostic criteria [7].
14-3-3 proteins are expressed at the synapse of neurones. These proteins are detected from CSF by western blot, generally relying on visual interpretation of the bands. A high agreement in interpretation of this analysis between laboratories has previously been shown [48]. We know that disorders, including viral and paraneoplastic encephalitis, generalised seizures and strokes lead to release of these proteins into the CSF. The multi-centre study by Sanchez-Juan and colleagues showed an 85% sensitivity (95% CI 83-87%) in 1457 sCJD cases; in 93 vCJD cases its sensitivity fell to 40% (95% CI 30-50%) [49]. The specificity is quoted to be around 92%, but is heavily influenced by the cohort of patients being investigated. Interestingly in an earlier paper by Green et al had shown that the clinical presentation and timing of lumbar puncture did not appear to influence the likelihood of a positive 14-3-3 in vCJD [50].
S-100β, an astrocytic protein, is often used in combination with 14-3-3 protein in sCJD. Green et al found a 78% sensitive and 76% specific in 45 vCJD cases and 34 controls [50]. NSE is even less sensitive occurring in only six of twenty-five vCJD CJD patients, giving a sensitivity of 24% (95%CI: 10-45%) [49].
Tau proteins occur in axons in the central nervous system and are associated with micro-tubules. They are known to become hyperphos-phorylated in neurodegenerative conditions, such as Alzheimer's. The link to vCJD was demonstrated by Tagliavini, who found phosphorylated tau immunoreactive neuritic profiles surrounding PrP deposits in patients with vCJD [51]. In Sanchez-Juan's paper elevated total tau proteins showed a sensitivity of just 24% (95% CI 16-35%) using a cut off of greater than 1,300pg/ml [49]. Elevated total tau was found in 21 of 86 patients tested. However, sensitivities of 80% were shown when using a cut off of greater than 500pg/ml. Green et al used a total CSF tau of >400pg/ml as the cut off in their 2006 paper [52]. In addition phosphorylated tau was also assessed. 51 vCJD and 37 control cases were included in the study, along with 50 sCJD cases and 46 sCJD controls. The control cases had been suspected as having the conditions, but the diagnoses had been excluded by either another diagnosis being reached or their recovery. Interestingly, although levels of CSF tau were highest in the sCJD group, the level of phosphorylated tau was highest among vCJD cases. CSF tau (>400pg/ml) was 90% sensitive and 89% specific in vCJD CJD. Phosphorylated tau was found to be 84% sensitive and 86% specific in this study. The ratio of tau protein to phosphorylated tau could also be useful in differentiating vCJD from sCJD, its most common differential diagnosis. Likewise in small studies, CSF Apolipoprotein E and uric acid levels have also been suggested as useful discriminators between these types of CJD, but not other conditions [53,54]. However, another investigation is better at doing this: MR brain imaging.
Neurological investigations in vCJD
The pattern of investigations in vCJD differs from sCJD. Typical electroencephalogram (EEG) findings of periodic complexes have only rarely been reported in vCJD and then only in the final stages of illness [55]. High signal in the pulvinar area of the thalamus bilaterally on T2 weighted MR imaging is seen in vCJD. Collie et al [56] established that fluid-attenuated inversion recovery and diffusion weighted MRI sequences were better than standard T2 sequences at demonstrating the high signal. By including these new sequences the “pulvinar sign” had a sensitivity of 90%, occurring in 74 out of 82. The pulvinar sign is currently said to be 78-90% sensitivity and is highly specific. There have however been three reported sCJD cases with the MV genotype and predominant pulvinar high sign on MRI [57]. Another potentially useful investigation is tonsil biopsy. In advanced disease it appears to be a sensitive investigation. However, there is the small possibility of misdiagnosing a patient with sub-clinical disease and there are risks from the procedure, particularly in obtunded patients [58].
Conclusion
With the control of the BSE epidemic in the UK we have seen an encouraging fall in the incidence of vCJD. The precautionary measures in beef production and processing have reduced the risk of further vCJD cases developing through dietary exposure to BSE. There is however, the possibility of an increased incubation period in older patients and those with PRNP-129 MV and W genotypes exposed in the dietary at risk period. Early diagnosis of any such patients remains important to reduce the possibility of secondary transmission of the condition via blood donation or surgical procedures. An effective screening test, preferably from blood, is yet to be developed. Progress is being made towards this with a recent publication on an assay for detecting vCJD infection in blood [59]. This involves abnormal PrP being absorbed and concentrated on stainless steel particles, before being detected with a PrP-specific antibody followed by an ELISA coupled with chemiluminescent detection. The assay detected 15 of 21 blood samples from patients with clinically apparent vCJD; all 169 control patients were negative. This will be difficult for others to replicate at present as some of the details of the methodology were not explicitly supplied [60]. Although the techniques may improve, as a diagnostic tests, its sensitivity is currently far lower than that of brain MRI. Larger numbers of samples from healthy individuals would need to be tested to determine the specificity of the assay before it could be considered for blood screening. It is also unclear whether the assay will detect the level of infectivity seen in asymptomatic patients with vCJD in their lymphoid tissues. There is the possibility that some people may have truly sub-clinical infection (ie they may never develop neurological disease). Therefore, even if the assay could detect infectivity in these individuals it would be difficult to know how to advise them following positive results. Such uncertainty could conceivably affect blood donation numbers. Therefore, their findings are interesting and may lead to further developments, but there are a number of issues that still need to be addressed. On the positive side the transmissibility and natural occurrence of prion diseases in animals makes them amenable to further scientific study. Progress is ongoing in our understanding of these conditions and prion biology in general.
Acknowledgments
We are grateful to Dr Mark Head for his support and advice, to Ms Jan McKenzie for compiling the data for Table 3 and Figure 1, and to Dr Hester Ward for allowing the use of Figure 2. This is an independent report commissioned and funded by the Policy Research Programme in the Department of Health, UK. The views expressed in the publication are those of the authors and not necessarily those of the Department of Health.
References
- 1.Zeidler M GC, Meslin F. Geneva: 1998. WHO Manual for stren-ghthening diagnosis and surveillance of CJD. [Google Scholar]
- 2.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 3.Collee JG, Bradley R. BSE: a decade on-Part I. Lancet. 1997;349:636–641. doi: 10.1016/S0140-6736(96)01310-4. [DOI] [PubMed] [Google Scholar]
- 4.The National CJD Surveillance Unit. Variant CJD: current data. March. 2011 http://www.cjd.ed.ac.uk (8.2.11) [Google Scholar]
- 5.Collinge J. Variant Creutzfeldt-Jakob disease. Lancet. 1999;354:317–323. doi: 10.1016/S0140-6736(99)05128-4. [DOI] [PubMed] [Google Scholar]
- 6.Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 7.Heath CA, Cooper SA, Murray K, Lowman A, Henry C, MacLeod MA, Stewart GE, Zeidler M, MacKenzie JM, Ironside JW, Summers DM, Knight RS, Will RG. Validation of diagnostic criteria for variant Creutzfeldt-Jakob disease. Ann Neurol. 67:761–770. doi: 10.1002/ana.21987. [DOI] [PubMed] [Google Scholar]
- 8.Corato M, Cereda C, Cova E, Ferrarese C, Ceroni M. Young-onset CJD: age and disease phenotype in variant and sporadic forms. Funct Neurol. 2006;21:211–215. [PubMed] [Google Scholar]
- 9.Brandel JP, Salomon D, Capek I, Vaillant V, Alperovitch A. [Epidemiological surveillance of Creutzfeldt-Jakob in France] Rev Neurol (Paris) 2009;165:684–693. doi: 10.1016/j.neurol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Ducrot C, Arnold M, de Koeijer A, Heim D, Calavas D. Review on the epidemiology and dynamics of BSE epidemics. Vet Res. 2008;39:15. doi: 10.1051/vetres:2007053. [DOI] [PubMed] [Google Scholar]
- 11.Will B. Variant CJD: where has it gone, or has it? Pract Neurol. 10:250–251. doi: 10.1136/jnnp.2010.223693. [DOI] [PubMed] [Google Scholar]
- 12.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 13.Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos Trans R Soc Lond B Biol Sci. 1994;343:405–411. doi: 10.1098/rstb.1994.0036. [DOI] [PubMed] [Google Scholar]
- 14.Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 15.Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey U, Lantos P. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450. doi: 10.1038/38925. 526. [DOI] [PubMed] [Google Scholar]
- 16.Johnson RT. Prion diseases. Lancet Neurol. 2005;4:635–642. doi: 10.1016/S1474-4422(05)70192-7. [DOI] [PubMed] [Google Scholar]
- 17.Asante EA, Linehan JM, Desbruslais M, Joiner S, Gowland I, Wood AL, Welch J, Hill AF, Lloyd SE, Wadsworth JD, Collinge J. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. Embo J. 2002;21:6358–6366. doi: 10.1093/emboj/cdf653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward HJ, Everington D, Cousens SN, Smith-Bathgate B, Leitch M, Cooper S, Heath C, Knight RS, Smith PG, Will RG. Risk factors for variant Creutzfeldt-Jakob disease: a case-control study. Ann Neurol. 2006;59:111–120. doi: 10.1002/ana.20708. [DOI] [PubMed] [Google Scholar]
- 19.Cousens S, Smith PG, Ward H, Everington D, Knight RS, Zeidler M, Stewart G, Smith-Bathgate EA, Macleod MA, Mackenzie J, Will RG. Geographical distribution of variant Creutzfeldt-Jakob disease in Great Britain, 1994-2000. Lancet. 2001;357:1002–1007. doi: 10.1016/s0140-6736(00)04236-7. [DOI] [PubMed] [Google Scholar]
- 20.Lasmezas C I CE, Hawkins S, Herzog C, Mouthon F, Konold T, Auvre F, Correia E, Lescoutra-Etchegaray N, Sales N, Wells G, Brown P, Deslys J-P. Risk of oral infection with bovine spongiform encephalopathy agent in primates. Lancet. 2005;365:781–783. doi: 10.1016/S0140-6736(05)17985-9. [DOI] [PubMed] [Google Scholar]
- 21.Baier M, Norley S, Schultz J, Burwinkel M, Schwarz A, Riemer C. Prion diseases: infectious and lethal doses following oral challenge. J Gen Virol. 2003;84:1927–1929. doi: 10.1099/vir.0.19037-0. [DOI] [PubMed] [Google Scholar]
- 22.Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, MacKenzie C, Houston F. Transmission of prion diseases by blood transfusion. J Gen Virol. 2002;83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- 23.Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, Linehan JM, Brandner S, Wadsworth JD, Hewitt P, Collinge J. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368:2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 24.Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, Will RG. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 25.Bishop MT, Ritchie DL, Will RG, Ironside JW, Head MW, Thomson V, Bruce M, Manson JC. No major change in vCJD agent strain after secondary transmission via blood transfusion. PLoS One. 2008;3:e2878. doi: 10.1371/journal.pone.0002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Rose SG, Hunter N, Matthews L, Foster JD, Chase-Topping ME, Kruuk LE, Shaw DJ, Rhind SM, Will RG, Woolhouse ME. Comparative evidence for a link between Peyer's patch development and susceptibility to transmissible spongiform encephalopathies. BMC Infect Dis. 2006;6:5. doi: 10.1186/1471-2334-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ironside JW, Head MW. Neuropathology and molecular biology of variant Creutzfeldt-Jakob disease. Curr Top Microbiol Immunol. 2004;284:133–159. doi: 10.1007/978-3-662-08441-0_6. [DOI] [PubMed] [Google Scholar]
- 28.Avrahami D, Gabizon R. Age-related alterations affect the susceptibility of mice to prion infection. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.015. 10.1016/j.neurobiolaging.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Collinge J, Palmer MS, Dryden AJ. Genetic predisposition to iatrogenic Creutzfeldt-Jakob disease. Lancet. 1991;337:1441–1442. doi: 10.1016/0140-6736(91)93128-v. [DOI] [PubMed] [Google Scholar]
- 30.Palmer MS, Dryden AJ, Hughes JT, Collinge J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature. 1991;352:340–342. doi: 10.1038/352340a0. [DOI] [PubMed] [Google Scholar]
- 31.Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364:527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 32.Peden A, McCardle L, Head MW, Love S, Ward HJ, Cousens SN, Keeling DM, Millar CM, Hill FG, Ironside JW. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia. 16:296–304. doi: 10.1111/j.1365-2516.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaski D, Mead S, Hyare H, Cooper S, Jampana R, Overell J, Knight R, Collinge J, Rudge P. Variant CJD in an individual heterozygous for PRNP codon 129. Lancet. 2009;374:2128. doi: 10.1016/S0140-6736(09)61568-3. [DOI] [PubMed] [Google Scholar]
- 34.Ward HJ. Evidence of a new human genotype susceptible to variant CJD. Euro Surveill. 2006;11:E060601–060603. doi: 10.2807/esw.11.22.02965-en. [DOI] [PubMed] [Google Scholar]
- 35.Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Ritchie D, Penney M, Hegazy D, Ironside JW. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol. 2004;203:733–739. doi: 10.1002/path.1580. [DOI] [PubMed] [Google Scholar]
- 36.Garske T, Ghani AC. Uncertainty in the tail of the variant Creutzfeldt-Jakob disease epidemic in the UK. PLoS One. 5:e15626. doi: 10.1371/journal.pone.0015626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadsworth JD, Joiner S, Linehan JM, Asante EA, Brandner S, Collinge J. Review. The origin of the prion agent of kuru: molecular and biological strain typing. Philos Trans R Soc Lond B Biol Sci. 2008;363:3747–3753. doi: 10.1098/rstb.2008.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mead S, Poulter M, Uphill J, Beck J, Whitfield J, Webb TE, Campbell T, Adamson G, Deriziotis P, Tabrizi SJ, Hummerich H, Verzilli C, Alpers MP, Whittaker JC, Collinge J. Genetic risk factors for variant Creutzfeldt-Jakob disease: a genome-wide association study. Lancet Neurol. 2009;8:57–66. doi: 10.1016/S1474-4422(08)70265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collinge J, Whitfield J, McKintosh E, Beck J, Mead S, Thomas DJ, Alpers MP. Kuru in the 21st century–an acquired human prion disease with very long incubation periods. Lancet. 2006;367:2068–2074. doi: 10.1016/S0140-6736(06)68930-7. [DOI] [PubMed] [Google Scholar]
- 40.Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, Thomson V, Tuzi NL, Head MW, Ironside JW, Will RG, Manson JC. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006;5:393–398. doi: 10.1016/S1474-4422(06)70413-6. [DOI] [PubMed] [Google Scholar]
- 41.Bishop MT, Pennington C, Heath CA, Will RG, Knight RS. PRNP variation in UK sporadic and variant Creutzfeldt Jakob disease highlights genetic risk factors and a novel nonsynonymous polymorphism. BMC Med Genet. 2009;10:146. doi: 10.1186/1471-2350-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop MT, Kovacs GG, Sanchez-Juan P, Knight RS. Cathepsin D SNP associated with increased risk of variant Creutzfeldt-Jakob disease. BMC Med Genet. 2008;9:31. doi: 10.1186/1471-2350-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilton DA, Ghani AC, Conyers L, Edwards P, McCardle L, Ritchie D, Penney M, Hegazy D, Ironside JW. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol. 2004;203:733–739. doi: 10.1002/path.1580. [DOI] [PubMed] [Google Scholar]
- 44.Clewley JP, Kelly CM, Andrews N, Vogliqi K, Mallinson G, Kaisar M, Hilton DA, Ironside JW, Edwards P, McCardle LM, Ritchie DL, Dabaghian R, Ambrose HE, Gill ON. Prevalence of disease related prion protein in anonymous tonsil specimens in Britacross sectional opportunistic survey. Brit Med J. 2009;338:b1442. doi: 10.1136/bmj.b1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Marco MF, Linehan J, Gill ON, Clewley JP, Brandner S. Large-scale immunohistochemical examination for lymphoreticular prion protein in tonsil specimens collected in Britain. J Pathol. 222:380–387. doi: 10.1002/path.2767. [DOI] [PubMed] [Google Scholar]
- 46.Hilton DA, Fathers E, Edwards P, Ironside JW, Zajicek J. Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease. Lancet. 1998;352:703–704. doi: 10.1016/S0140-6736(98)24035-9. [DOI] [PubMed] [Google Scholar]
- 47.Van Everbroeck B, Boons J, Cras P. Cerebrospinal fluid biomarkers in Creutzfeldt-Jakob disease. Clin Neurol Neurosurg. 2005;107:355–360. doi: 10.1016/j.clineuro.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Juan P, Sanchez-Valle R, Green A, Ladogana A, Cuadrado-Corrales N, Mitrova E, Stoeck K, Sklaviadis T, Kulczycki J, Hess K, Krasnianski A, Equestre M, Slivarichova D, Saiz A, Calero M, Pocchiari M, Knight R, van Duijn CM, Zerr I. Influence of timing on CSF tests value for Creutzfeldt-Jakob disease diagnosis. J Neurol. 2007;254:901–906. doi: 10.1007/s00415-006-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez-Juan P, Green A, Ladogana A, Cuadrado-Corrales N, Saanchez-Valle R, Mitrovaa E, Stoeck K, Sklaviadis T, Kulczycki J, Hess K, Bodemer M, Slivarichova D, Saiz A, Calero M, Ingrosso L, Knight R, Janssens AC, van Duijn CM, Zerr I. CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2006;67:637–643. doi: 10.1212/01.wnl.0000230159.67128.00. [DOI] [PubMed] [Google Scholar]
- 50.Green AJ, Thompson EJ, Stewart GE, Zeidler M, McKenzie JM, MacLeod MA, Ironside JW, Will RG, Knight RS. Use of 14-3-3 and other brain-specific proteins in CSF in the diagnosis of variant Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2001;70:744–748. doi: 10.1136/jnnp.70.6.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giaccone G, Marcon G, Mangieri M, Morbin M, Rossi G, Fetoni V, Patriarca C, Catania M, Di Fede G, Tagliavini F, Merlin M. Atypical tauopathy with massive involvement of the white matter. Neuropathol Appl Neurobiol. 2008;34:468–472. doi: 10.1111/j.1365-2990.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 52.Goodall CA, Head MW, Everington D, Ironside JW, Knight RS, Green AJ. Raised CSF phospho-tau concentrations in variant Creutzfeldt-Jakob disease: diagnostic and pathological implications. J Neurol Neurosurg Psychiatry. 2006;77:89–91. doi: 10.1136/jnnp.2005.065755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choe LH, Green A, Knight RS, Thompson EJ, Lee KH. Apolipoprotein E and other cerebrospinal fluid proteins differentiate ante mortem variant Creutzfeldt-Jakob disease from ante mortem sporadic Creutzfeldt-Jakob disease. Electrophoresis. 2002;23:2242–2246. doi: 10.1002/1522-2683(200207)23:14<2242::AID-ELPS2242>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 54.Lekishvili T, Sassoon J, Thompsett AR, Green A, Ironside JW, Brown DR. BSE and vCJD cause disturbance to uric acid levels. Exp Neurol. 2004;190:233–244. doi: 10.1016/j.expneurol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Binelli S, Agazzi P, Giaccone G, Will RG, Bugiani O, Franceschetti S, Tagliavini F. Periodic electroencephalogram complexes in a patient with variant Creutzfeldt-Jakob disease. Ann Neurol. 2006;59:423–427. doi: 10.1002/ana.20768. [DOI] [PubMed] [Google Scholar]
- 56.Collie DA, Summers DM, Sellar RJ, Ironside JW, Cooper S, Zeidler M, Knight R, Will RG. Diagnosing variant Creutzfeldt-Jakob disease with the pulvinar sign: MR imaging findings in 86 neuropathologically confirmed cases. AJNR Am J Neuroradiol. 2003;24:1560–1569. [PMC free article] [PubMed] [Google Scholar]
- 57.Meissner B, Kallenberg K, Sanchez-Juan P, Collie D, Summers DM, Almonti S, Collins SJ, Smith P, Cras P, Jansen GH, Brandel JP, Coulthart MB, Roberts H, Van Everbroeck B, Galanaud D, Mellina V, Will RG, Zerr I. MRI lesion profiles in sporadic Creutzfeldt-Jakob disease. Neurology. 2009;72:1994–2001. doi: 10.1212/WNL.0b013e3181a96e5d. [DOI] [PubMed] [Google Scholar]
- 58.Hill AF, Butterworth RJ, Joiner S, Jackson G, Rossor MN, Thomas DJ, Frosh A, Tolley N, Bell JE, Spencer M, King A, Al-Sarraj S, Ironside JW, Lantos PL, Collinge J. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet. 1999;353:183–189. doi: 10.1016/s0140-6736(98)12075-5. [DOI] [PubMed] [Google Scholar]
- 59.Edgeworth JA, Farmer M, Sicilia A, Tavares P, Beck J, Campbell T, Lowe J, Mead S, Rudge P, Collinge J, Jackson GS. Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet. 377:487–493. doi: 10.1016/S0140-6736(10)62308-2. [DOI] [PubMed] [Google Scholar]
- 60.Gregori L. A prototype assay to detect vCJD-infected blood. Lancet. 377:444–446. doi: 10.1016/S0140-6736(11)60057-3. [DOI] [PubMed] [Google Scholar]