Abstract
One of the most common microsatellites in eukaryotes consists of tandem arrays of the dinucleotide GT. Although the study of the instability of such repetitive DNA has been extremely fruitful over the last decade, no biological function has been demonstrated for these sequences. We investigated the genetic behavior of a region of the yeast Saccharomyces cerevisiae genome containing a 39-CA/GT dinucleotide repeat sequence. When the microsatellite sequence was present at the ARG4 locus on homologous chromosomes, diploid cells undergoing meiosis generated an excess of tetrads containing a conversion of the region restricted to the region of the microsatellite close to the recombination-initiation double-strand break. Moreover, whereas the repetitive sequence had no effect on the frequency of single crossover, its presence strongly stimulated the formation of multiple crossovers. The combined data strongly suggest that numerous recombination events are restricted to the initiation side of the microsatellite as though progression of the strand exchange initiated at the ARG4 promoter locus was impaired by the repetitive sequence. This observation corroborates in vitro experiments that demonstrated that RecA-promoted strand exchange is inhibited by CA/GT dinucleotide tracts. Surprisingly, meiotic instability of the microsatellite was very high (>0.1 alterations per tetrad) in all the spores with parental and recombinant chromosomes.
Keywords: Microsatellite, homologous recombination, double-strand break repair, genome instability, meiosis, Saccharomyces cerevisiae
The genomes of all eukaryotic species contain tracts of DNA in which a single base or a small number of bases is repeated (microsatellites). Dinucleotide repeats are preferentially located in noncoding regions and display a high instability characterized by small changes in length occurring during cell growth. Tandem-repeat microsatellites have been conserved throughout evolution at an absolute frequency that is 30-fold higher than the expected random frequency; about one repetitive tract per 40 kb in Homo sapiens and Saccharomyces cerevisiae (Levinson and Gutman 1987; Debrauwère 1997). One of the most common microsatellites, the (CA/GT)n microsatellite, consists of tandem arrays of the dinucleotides GT and CA. Such repetitive tracts are unstable (Levinson and Gutman 1987) (Wierdl et al. 1997), frequently undergoing changes in tract length. These changes result in polymorphisms that are useful in genetic mapping studies, and their high frequency is diagnosis of certain types of human tumors (for review, see de la Chapelle and Peltomaki 1995). Although the study of the instability of repetitive DNA has been fruitful over the last decade, no biological function has been demonstrated for these sequences. In this work, we propose that microsatellites could play a fundamental role in genome stability by regulating recombination activity.
In mammalian cells (CA/GT)n microsatellites have been shown to stimulate homologous recombination of transfected DNA (Bullock et al. 1986; Wahls et al. 1990), but do not influence chromosomal intramolecular recombination (Sargent et al. 1996). In yeast, repetitive sequences increase chromosomal recombination (Treco and Arnheim 1986). In bacteria, these sequences stimulate RecA-independent intraplasmid recombination (Murphy and Stringer 1986). In a previous work we showed that Escherichia coli RecA, H. sapiens Rad51, and S. cereviaiae Rad51 recombination proteins bind with high affinity to repetitive single-stranded DNA carrying tracts of GT, CT, or CA dinucleotides (Biet et al. 1999). Moreover, in reactions promoted by the RecA protein, it has been observed that the presence of GT/CA repetitive tracts inhibits strand exchange between fully homologous DNA molecules and leads to accumulation of joint molecules that do not dissociate in vitro (Dutreix 1997). Although the evidence is indirect, it is likely that the inhibition of strand exchange is the consequence of a shifted alignment of repetitive sequences during pairing that is inefficiently corrected because of the tight binding of recombination proteins to these sequences. Because increased binding of recombination proteins is observed in the different species tested, inhibition of strand exchange by microsatellites could be a general process that may modify the recombination activity in the vicinity of the microsatellite. To test this hypothesis we analyzed the effect of a tract of 39 GT/CA dinucleotide repeats on meiotic recombination in S. cerevisiae. Because the amount of meiotic double-strand breaks (DSBs) is correlated with the frequency of gene conversion, it is assumed that meiotic breaks are the site of initiation of recombination events (Alani et al. 1990). Thus, during meiosis, it is possible to identify the site of initiation of one recombination event and to analyze strand-exchange progression and its resolution.
Results
Introduction and analysis of a CA/GT microsatellite at the ARG4 locus
To analyze the effects on recombinational events around ARG4 due to microsatellites, we introduced a sequence of 39 CA/GT dinucleotide repeats (MS) into the ARG4 gene (Fig. 1) at a locus close to a well-characterized meiotic hotspot for recombination (Nicolas et al. 1989; Sun et al. 1989). This hot spot of meiotic recombination is located upstream of the ARG4 coding sequences and is associated with meiosis-specific DSBs that occur in this area. DSBs are detected as transient and heterogeneous DNA fragments in wide-type RAD50 strains, but accumulate as discrete bands in rad50S-K181 mutant strains (Cao et al. 1990). The presence of a microsatellite sequence in the ARG4 coding sequence does not affect the location or the frequency of the breaks induced during meiosis in the area (Fig. 2). Moreover, the processing of DSBs at the ARG4 promoter, estimated by the disappearance of the fragments during incubation, is similar in RAD50 strains carrying or not carrying the microsatellite (data not shown).
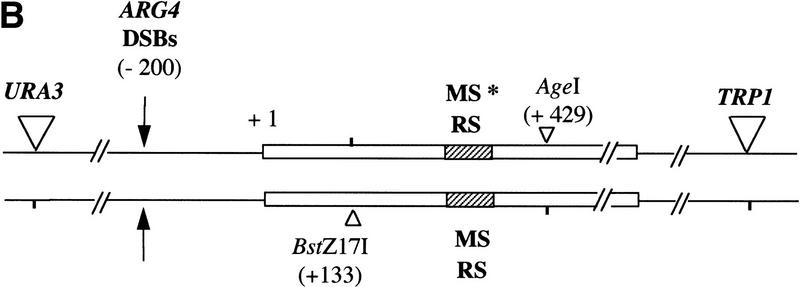
Figure 1.
(A) Physical map of the ARG4 region located on chromosome VIII including the 5′ region of DED82, DED81, and ARG4 and the 3′ end of YSC83. (B) Configuration of ARG4 alleles in strains used for this study. (Horizontal arrows) Orientations of transcripts in this region. (Vertical arrows) Positions of meiotic recombination-initiating double-strand breaks (DED DSBs and ARG4 DSBs) in this region. Numbers in parentheses indicate the site positions respective to the first base of the ARG4 open reading frame. Open triangles indicate modifications that are present as heterozygous markers on chromosomes. Microsatellite (MS) and random (RS) sequences (hatched rectangle) are present. They are inserted into the EcoRV site (+262 bp) of the ARG4 gene coding region as detailed in Materials and Methods. (Asterisk) The chromosome carrying the URA3 and TRP1 genetic markers contains either a sequence of 38 or 39 CA/GT repeats while the homologous chromosome contains a sequence of 39 CA/GT repeats.
Figure 2.
Time-course detection of meiotic double-strand breaks (DSBs) in the ARG4 region in rad50S diploid strains. (Black arrows) Positions of the SnaBI parental fragment (∼12 kb) and the fragments generated by DSB in the DED82–DED81 intergenic region (DED DSBs) and in the ARG4 promoter region (ARG4 DSBs). (Gray arrow)Position of the expected fragment generated by DSBs in the microsatellite sequences, if there were breaks.
To monitor recombination events induced at meiosis we introduced two genetic markers (URA3 and TRP1), and two modifications of restriction sites in the flanking regions of the microsatellite (Fig. 1). During some constructions the microsatellite insertion on the chromosome carrying the URA3 and TRP1 markers lost one repeat. We used this opportunity to monitor the events of instability and recombination at the microsatellite locus. Two diploid strains carrying the microsatellite sequence were used: strains MS(38/39), carrying a one-repeat heterology in the microsatellites and strain MS(39/39), with fully homologous microsatellites. The meiotic products were analyzed for the expression of the URA3 and TRP1 genes on selective media and for the presence of restriction sites by digestion of PCR-amplified DNA fragments. Two kinds of recombination events were monitored: crossovers (CO) that give rise to a reciprocal exchange of homologous chromosome regions (new segregation 2:2) and conversions that result from the correction of the heteroduplex intermediate, which are characterized by three spores carrying the same marker (segregation 3:1). As a control, we inserted a random nonrepetitive 50% GC-rich sequence (RS) in the ARG4 locus with a length identical to that of the microsatellite and with no apparent secondary structure.
High frequency of short conversions do not pass the microsatellite sequence
Tetrad analysis shows that the total number of BstZ171 and AgeI conversion events, measured by digestion of PCR-amplified fragments, is similar in the two strains containing the microsatellite insert and in the strain with the random insert (Table 1). However, strains differ in the number of the short conversions that are restricted to the BstZ171 marker and do not encompass the AgeI marker. As a matter of fact, the number of short conversions is higher in MS diploids (11%–12%) than in RS diploids (3%). After statistical analysis of these values, we found that the difference between short conversion frequencies of the MS and RS diploids was highly significant (χ2 = 8.2; P ≪ 0.002; Table 1). Although the total number of conversions was not significantly higher in the MS strains (χ2 = 0.32; P = 0.13), we do not exclude that the extra short conversions observed in both MS stains result from rare new initiation events. Moreover, there was no apparent bias in the direction of the BstZ171 conversion. Both chromosomes were donors or recipients for the converted sequence as expected if initiation can occur indifferently on each chromosome. The one-repeat heterology in the MS(38/39) diploid did not seem to have any specific effect on the conversions in the MS diploids.
Table 1.
Number of conversion events in strains homozygous for the RS and MS sequences in the ARG4 gene
| Insert
|
Short conversiona
|
Long conversionb
|
Total number of tetrads analysed
|
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Bst+c conversion
|
Bst−d conversion
|
Total |
Bst+ Age−c coconversion
|
Bst− Age+d coconversion
|
Total | ||||
| number
|
frequency
|
number
|
frequency
|
||||||
| RS | 3 (1) | 4 (2) | 7 (3) | 3% | 7 (1) | 33 (14) | 40 (15) | 21% | 205 |
| MS | 12 (3*) | 12 (3**) | 24 (6) | 11% | 14 (8) | 22 (8**) | 36 (14) | 17% | 215 |
| (38/39) | |||||||||
| MS | 10 (2*) | 12 (3*) | 22 (5) | 12% | 7 (4) | 21 (14) | 30 (18) | 16% | 188 |
| (39/39) | |||||||||
Numbers in brackets correspond to conversion events associated with crossover.
Short tract conversion covering only the BstZ17I site (Bst).
Long tract conversion encompassing the BstZ17I (Bst) and AgeI (Age) sites including the inserted sequence. The chromosome carrying the URA3 and TRP1 genetic markers and the AgeI restriction site is either the recipientc or the donord in ARG4 gene conversion events.
One conversion event is associated with a double crossover.
Two conversion events are associated with a double crossover.
The size of the microsatellite sequence for the spores of the converted tetrads in the MS(38/39) strain was analyzed by PCR amplification and sequencing gel electrophoresis. As expected, the microsatellite sequence was always converted with the AgeI and BstZ171 markers in the 18 spores with large conversions that we analyzed. In contrast, among the 11 spores with BstZ171 short conversions that we analyzed, only 2 had converted the microsatellite. This reduction in the number of co-conversion of the BstZ171 marker and the microsatellite suggests that some conversion events would be restricted to the region between the initiation site and the microsatellite and would not be able to extend across the repetitive sequence.
Short conversions without associated crossovers are more frequent in the MS strains
During recombination, strands of homologous chromosomes exchange and form Holliday junctions that are resolved by specific resolvases. According to the junction resolutions, the resulting conversions are associated (+CO) or not (−CO) with an exchange of the adjacent regions. The chromosome reciprocal exchanges in the ARG4 region were first monitored by the segregation of the URA3 and TRP1 markers. Few double crossover events that restore the parental segregation for these markers were also observed (Table 1). We compared the association of conversion events with a crossover in the MS and the RS strains. In all strains, nearly half of the large conversions encompassing the AgeI site were associated with a crossover (Table 1). Similarly, half of the short conversions restricted to the BstZ171 site in the RS strain were associated with a crossover. However, in the MS strains only 23%–25% of the BstZ171 conversions were found to be associated with crossovers (Table 1). This difference could indicate that part of the short conversions that accumulate in the presence of the microsatellite result from mechanisms different from those implicated in the RS strain.
Numerous MS tetrads with parental segregation of the URA and TRP markers have undergone two crossovers
We found that numerous spores that had a parental segregation for the URA3 and TRP1 distal markers display a reciprocal exchange of one or two central markers. These new marker arrangements are probably generated by two crossovers located on both sides of the exchanged region. We located the position of the crossovers in the different spores. They were classified as single and double crossovers according to their association with or without the reciprocal exchange of the distal URA3 and TRP1 markers. We compared the crossover locations in the RS and MS homozygous strains using the four markers (URA3, TRP1, Age1, BstZ171; Table 2). In all strains, single crossovers occurred with roughly the same frequency in the three areas determined by the markers. In contrast, the total number of double crossovers differed significantly between the RS and MS strains. The MS strains produced about threefold more spores having undergone a double crossover than the RS strain (χ2 = 7.44; P < 0.002). Almost all the trends with a double crossover underwent a crossover in the region between URA3 and BstZ171, the second event being located either between BstZ171 and AgeI or between AgeI and TRP1. In the MS(39/39) strain, most of the double crossovers were located on the URA side of the AgeI site, and a significant number of double crossovers (41%) involved the four chromatids. These particular events were less frequent in the MS(38/39) strain. The difference in the location of the second crossover observed between the two MS strains were highly significant (χ2 = 12; P < 0.0002). It is interesting to note that it was the only difference observed between the MS(39/39) and the MS39/38) strains.
Table 2.
Number of single and double crossover at different location in the ARG4 region
| Insert
|
Single crossover
|
Double crossover
|
Total number of tetrads analysed
|
||||||
|---|---|---|---|---|---|---|---|---|---|
|
URA-Bst
|
Bst-Age
|
Age-TRP
|
Total
|
URA-Bst/Bst-Age
|
URA-Bst/Age-TRP
|
Bst-Age/Age-TRP
|
Total
|
||
| RS | 17 | 1 | 24 | 42 (20%) | 6 | 4 | 0 | 10 (5%) | 205 |
| MS | 15 | 5 | 20 | 40 (19%) | 12** | 14 | 2 | 28 (13%) | 215 |
| (38/39) | |||||||||
| MS | 14 | 4 | 22 | 40 (21%) | 22** | 1 | 5* | 28 (15%) | 188 |
| (39/39) | |||||||||
Values in brackets correspond to the percentage of recombinant tetrads. Some double crossovers involve more than two strands.
Three-strand events (one tetrad).
Four-strand events (three MS(38/39) tetrads and nine MS(39/39) tetrads).
Crossovers resolved preferentially at the side of the microsatellite close to the initiation site
Diploid cells with the MS insert generated at meiosis an excess of tetrads containing reciprocally recombined product in the BstZ171 and AgeI area: 9% in the MS(38/39) and l6% in the MS(39/39) tetrads had resolved at least one crossover between the BstZ171 and Age1 restriction sites whereas only 3% of the RS tetrads displayed a crossover at this interval (Table 2). In the MS(38/39) strain, the 5 tetrads with a single crossover and 14 tetrads with a double crossover that had one crossover located in the insert region between the BstZ171 and AgeI restriction sites were analyzed more precisely. We measured the length of the microsatellite associated to the exchange to locate the crossover in relation to the microsatellite sequence. All but two events resolved in the area limited by the BstZ171 site and the microsatellite sequence corresponding to the side of the microsatellite close to the ARG4 initiation DSB. Interestingly, the two crossovers that resolved at the other side of the microsatellite correspond to spores that have undergone double crossovers in which the second crossover is located in the AgeI–TRP1 area. These events could result from repair of DSBs generated at the other side of the microsatellite. It is possible that a small number of meiotic DSBs could occur in the TRP1 region (see Fig. 1).
Instability of the repetitive sequence is increased during meiotic divisions
During the study of the MS(38/39) tetrads we measured the length of the microsatellite to analyze the recombination events. We compared the frequency of microsatellite alterations in the different types of recombinant spores (Table 3). We found that dinucleotide repeat tract alterations were not more frequent in spores having undergone conversion or crossovers than in spores that displayed parental segregation. However, the rate of microsatellite meiotic instability was very high as 3 tetrads out of 32 contained at least one spore with an altered tract length. None of the tetrads displayed the same microsatellite changes on the two copies of one parental chromosome, as would be expected if instability had resulted from a mitotic event. Moreover, we found a frequency of 1/197 tract alterations in the MS diploid cells used for this study. The difference between the pre- and post-sporulation instability frequencies could indicate that, at the ARG4 chromosome locus, the meiotic instability is high relative to the mitotic instability. As already observed in analysis of dinucleotide repeats instability (Henderson and Petes 1992), the changes in length were small (one or two repeats). The number of tract changes was underestimated as we eliminated changes that would generate a 3:1 segregation of the microsatellite, which cannot be distinguished from microsatellite gene conversion (Table 3).
Table 3.
Rates of instability and types of alterations for the microsatellite sequences in parental and recombinant spores
| Genotype of spores
|
Rate of tract instability
|
Number of spores with additions or deletions of base pairs
|
Total
|
|||
|---|---|---|---|---|---|---|
| −2
|
0
|
+2
|
+4
|
|||
| Parental | 1.3 × 10−2 | 0 | 75 | 1 | 0 | 76 |
| Recombinant | 7.7 × 10−2 | 0 | 48* | 3 | 1 | 52 |
Recombinant spores include crossing-over and conversion events encompassing the BstZ17I and/or AgeI restriction sites. The asterix indicates that four of the recombinants classified as microsatellite conversions could be mutants with alterations of the repetitive sequence: Three spores (−2) and one spore (+2).
Discussion
When the microsatellite sequence was introduced into homologous chromosomes at the ARG4 locus, conversion and crossovers were induced in the area between the BstZ171 site and the microsatellite sequence. With a random sequence inserted at the same position there were few events restricted to this small area (129 bp). Interestingly, this region corresponds to the side of the microsatellite close to the DSB initiation site. In MS strains, crossovers located near the microsatellite were mainly observed in tetrads having undergone two crossovers. The increase of multiple events induced by the microsatellite sequence has been already reported by Treco and Arnheim (1986). However, the diploid strain used did not further the precise location of the events. We found that most of the double crossovers in the MS(39/39) strain involved one resolution in the URA3–BstZ171 interval and another in the BstZ171–AgeI. Because the site of the DSB was located in the URA3–BstZ171 interval close to the BstZ171 site (∼330 bp) it is likely that these double crossovers result from the resolution of intermediates formed at each side of the DSB. To locate the second crossover in relation to the microsatellite, we used the MS(39/38) strain, which contains microsatellites at the ARG4 locus with a length differing by one repeat. Most of the crossovers resolved in the BstZ171–AgeI region were located on the DSB initiation side of the microsatellite. Surprisingly, whereas the high frequency of short conversions and the excess of double crossovers were also observed in this strain, half of the second crossovers were resolved in the AgeI–TRP1 intervals. This result suggests that the mismatched repeat could help the strand exchange to progress across the repeated sequence either by recruiting special proteins or by destabilizing the blocked recombination complex. The difference in crossover location was specific to the resolution of a second crossover as single crossovers showed the same distribution along the chromosome in the two MS strains. A large number of double crossovers occurring in the MS strains involved the four strands. Tetrads carrying three and four chromosome recombinations for a single interval have been already observed in chromosomes carrying microsatellite sequences (Treco and Arnheim 1986). In all the MS tetrads with four recombinant strands resulting from two double crossovers located on each chromosomes, one crossover was located in the microsatellite region. In both MS strains, the high number of four-strand exchanges in the URA–Bst/Bst–Age regions can be explained simply by the random occurrence of two independent events that involve all four chromatids within the genetic interval monitored. As a matter of fact, double crossovers occurred at a frequency of 5.6% in MS(38/39) strain and 12% in the MS(39/39) strain so the two events should occur at frequencies of 0.3% and 1.3%, respectively. The observed frequencies of four-strand double crossovers were slightly higher for the two MS strains (1.4% and 4.8%). This difference between observed and calculated frequencies is not significant and could reflect the underestimation of the crossovers in this area. For example, recombination events leading to a double crossover URA–Bst/Bst–Age associated with a BstZ171 conversion would be detected as a short conversion not associated with crossovers. We found an excess of short conversions that were not associated with single crossovers. Given the high frequency of double crossovers in this region, we cannot exclude that part of the short conversions were associated with undetectable double crossovers. Several mechanisms have been proposed to generate conversion events not associated with a reciprocal exchange of the flanking regions (Gilbertson and Stahl 1996). Resolution of DSB repair intermediates can involve the cleavage of two, one, or no Holliday junctions. In the first case, the intermediates should be processed by resolution of the two Holliday junctions, in the same orientation, either both vertically or both horizontally to avoid reciprocal exchange of the distal markers. This model implies that the microsatellite induces a distal constraint and influences the resolution of the Holliday junction formed at the other side of the initiation site. However, this model is attractive as it applies both to the double crossovers and to the conversion not associated to crossovers.
Whatever the resolution model, the high number of events that resolve before the repetitive sequence strongly suggest that progression of strand exchange is inhibited at this level. It may be inhibited by the binding of specific proteins or by a special effect of the sequence on the recombination proteins. This last hypothesis is in agreement with previous in vitro observations indicating that the recombination proteins do not exchange the strands of homologous DNA across such sequences (Dutreix 1997). We found that the highly affinity of recombination proteins for the repeated sequences could impair the reaction (Biet et al.1999). A second possibility is that CA/GT sequences promote crossovers by presenting the meiotic recombination apparatus with a specific structure that could be a signal to initiate genetic exchange nearby. It could be a region where homologous DNA segments are drawn together as the initial step for the meiotic recombination apparatus to recognize homologous chromosomes. Because the analysis of DSBs induced at meiosis did not reveal breaks in the area of the microsatellite, it is unlikely that new breaks could be the initiators of the induced events. Moreover, the absence of a bias in chromatids acting as donors or recipients in genetic exchange between chromosomes that do not contain microsatellites of the same length (this work) or that contain microsatellite sequence on only one chromosome (in prep.) supports a model in which these sequences are not substrates for an endonuclease that nicks or cuts DNA to initiate.
The most important question raised is that of the role of this highly repetitive element in the evolution of eukaryotic genomes. If a CA/GT tract inhibits progression of the strand exchange, then do the 50 to 100 copies of this repetitive sequence that are normally found in the yeast genome serve similar functions? If so, then strand exchange would be limited to regions between such sequences, and the genome would be divided into recombination units. During meiosis the extent of strand exchange seems to be limited and such sequences would have an effect only if they are located close to a DSB. At mitosis, during repair of damage on chromosomes it has been observed that the exchanged regions are longer. Repetitive sequences could prevent their extension and the loss of heterozygosity in large regions of the genome. To support this hypothesis, the effect of microsatellite sequences on mitotic recombination has to be demonstrated. Alternatively, recombination may be an activity that microsatellite sequences influence by virtue of their structure, but this activity may be unrelated to some other function that the repetitive sequence performs within eukaryotic cells.
The high levels of microsatellite instability at meiosis was unexpected. Our results differ from data obtained with a 49-repeat-length tract (Wierdl et al. 1997) or a 15-repeat-length (Strand et al. 1993) microsatellite carried by a plasmid. It is unclear whether the lack of increase of instability at meiosis on plasmid-carried microsatellites represents a chromosome–plasmid difference or an effect of flanking DNA sequences. However, we favor the hypothesis of a plasmid effect because Strand et al. (1993) have already observed that a 29-bp microsatellite has 3-fold higher instability and is 10-fold less sensitive to a defect in mismatch repair when carried by the chromosome rather than by the plasmid. The location and frequency of DSB initiation sites on these plasmids have not been studied and could play an important role in the meiotic instability of microsatellites. Two models have been proposed to explain the instability of simple repeats: unequal recombination (Smith 1973) or DNA polymerase slippage (Streisinger et al. 1996). The first model implies that instability would be higher in recombinant spores. Our data do not allow us to confirm this hypothesis, and a study of a higher number of recombinant tetrads would be necessary to confirm or exclude the role of the recombination in meiotic microsatellite instability. However, several studies in bacteria (Levinson and Gutman 1987) and yeast (Strand et al. 1993) indicate that slippage during replication seems to be the main mechanism of CA/GT repetitive tract instability. Our finding, that this process could be increased during meiosis, raises the questions of the fidelity of the replication complex formed and the efficiency of mismatch repair during meiotic divisions. Because expression of some genes involved in mismatch repair (MSH5, MSH4, and MSH2) and replication (RPA, POL30, POL1, POL3, and POL4) has been shown to be modified during meiosis, it is possible that one (or both) systems could change their fidelity and induce the instability of the microsatellite.
Materials and methods
Plasmids and strains
All plasmids were derived from the L1.1 plasmid. This plasmid is a derivative of the pt92 plasmid and contains the (poly1) substitution of the ARG4 promoter sequence (−316 bp to −139 bp) described previously by de Massy and Nicolas (1993). Two types of large insert were used in this study: The MS (microsatellite) insert,XbaI–PstI fragment from the bacteriophage M13mp19(CA/GT)39 containing a sequence of 39 CA/GT repeats (Dutreix 1997); the RS insert, a random sequence corresponding to the PvuII–PstI fragment of the puc18 plasmid. MS and RS sequences were inserted (out of frame) into the EcoRV (+262 by) site in the ARG4-coding region of L1.1. In the MS constructions, the BstZ171 and AgeI restriction sites were modified by insertion of 2 and 4 bp. The modified ARG4 regions were introduced at the ARG4 locus on chromosome VIII of the strains ORD11-4B and ORD17-47C. Sequences of the constructions were checked by PCR amplification and sequencing using a ABI PRISM system. The poly(CA/GT) tract was oriented such that the poly(CA) repeats were on the transcribed strand.
The S. cerevisiae strains used in this study were derived from the strains ORD11-4B [Matα; ARG4Δ2060; ura3-52; trpl-28; leu2-3; ade2-10; dup KV(DED82-Arg(ΔHpaI)-YSC83] and ORD17-47C[MATa; ARG4Δ2060; his3Δ1; DED82::URA3; ORF83::TRP1; dupKV(DED82-ArgΔHpaI)-YSC83] (Lichten et al. 1990). These strains are derivatives of the strain S288c (N. Schultes, unpubl.). DED82::URA3 and YSC83::TRP1 and correspond to a 1.5-kb EcoRI TRP1 fragment and a 1.2-kb HindIII URA3 fragment inserted into the DED82 BamHI site and the YSC83 BglII site, respectively. To complement deficiencies created upon disruption of the essential genes DED82 and YSC83, a 12-kb fragment was inserted into ApaI–StuI of the URA3 gene at its normal chromosomal position on chromosome V. This insert, designed at dupKV (DED82-Arg(ΔHpaI)-YSC83), contains an ApaI–SnaBI fragment of the ARG4 region deleted of a 2-kb fragment from −316 bp to +1745 bp that carries the ARG4 gene (Δ2060). All strains used in this study bear the poly(I) substitution. The rad50sK181 mutation was introduced into various strains by crosses with the strains ORT329 (MATa; ARG4Δ2060; ura3-52; trp1-289; ade2-101; his3Δ1; rad50s::URA3) and ORT324 (Mat α; ARG4Δ2060; ura3-52; trp1-289; leu2-3; rad50s::URA3) (de Massy and Nicolas 1993).
Media, culture conditions, and genetic analysis
Growth and sporulation of yeast cells were performed by standard methods (deMassy and Nicolas 1993). Cells were grown in YPD media. For sporulation, cells were grown at 30°C in presporulation media (SPS) to a concentration of 2 × 107 to 4 × 107 cells/ml, washed in water, and incubated at the same density in sporulation medium (1% potassium acetate supplemented with the required amino acids) at 30°C. The distribution and frequencies of meiotic recombination implicating the marker genes (URA3 and TRP1) and gene conversion events in the ARG4 gene were studied by tetrad analysis. The segregation of ARG4, URA3, TRP1 and at least four additional markers (LEU2, HIS3, ADE2, and the mating type locus MAT) were examined. All tetrads showing non-Mendelian segregation for URA3, TRP1, or ARG4 were tested for all the markers, using larger patches of cells.
Analysis of the segregation of the restriction sites
The segregation of the BstZ171 and AgeI sites was studied by the polymerase chain reaction with primers complementary to positions +8 bp and +730 bp. DNA was amplified directly from colonies arising from individual spores. The PCR products were digested with the BstZ171 or AgeI restriction enzymes and analyzed by agarose gel electrophoresis on 2% NuSieve GTG (FMS BioProducts, Rockland, Maine) agarose gels.
Analysis of the length of repetitive tracts
The lengths of the tracts in the spores were determined by PCR amplification directly on colonies with primers that flank the repeated tracts (positions +198 bp and +432 bp). The PCR products were run on 6% denaturating polyacrylamide gels with control DNA samples containing poly(GT) tracts of 38 and 39 bp and transferred onto a nylon membrane (Amersham, N+). Membranes were prehybridized for 2 hr and hybidized for 12 hr at 42 °C with a 20-by poly(CA) label probe. Probes were labeled by 3′ end-labeling (terminal transferase; Boehringer Mannheim) with 50 μCi of [α-32P]dCTP, 3000 mCi/mmole for 100 ng of DNA fragment. Unincorporated nucleotides were separated by filtration on a ProbeQuant G-50 Micro column (Amersham). Exposure times were from 20 to 50 min.
Detection of meiotic DSBs and quantification
Cells (200 ml) were grown in sporulation medium and, at the appropriate times (0, 4, 8, 10, and 24 hr), 25-ml aliquots were mixed with 25 ml of ethanol (100% at −20°C) and 1.25 ml of 0.5 m EDTA and kept at −20°C. Chromosomal DNA was extracted and 1.5 μg of DNA was digested by SnaBI restriction enzyme (5 U/μg DNA; twice for 3 hr at 37°C), separated on a 1.2% agarose gel, and transferred under vacuum (LKB 2016 VACUGENE; Pharmacia) onto a nylon membrane (Amersham, Hybond N+). Membranes were prehybridized for 2 hr and hybridized for 24 hr at 65°C with the labeled probe (20 ng/ml). Probes were labeled by random priming (Readyprime Kit; Amersham) with [α-32P]dCTP, 3000 mCi/mmole, 110 TBq/mmole (Amersham). Unincorporated nucleotides were separated by filtration on a ProbeQuant G-50 Micro column. The probe used in the hybridization as the 707-bp PCR product (primers complementary to positions +8 bp and +715 bp) internal to the ARG4-coding region. Quantification of DSB signals were performed as described previously (de Massy and Nicolas 1993) by use of a PhosphorImager (Molecular Dynamics, STORM 860) and the ImageQuant program.
Statistical analysis
All results were tested by statistical analysis. Fisher's exact variant of the chi-square test was used for most comparisons and a P value of <0.05 was considered to be statistically significant.
Acknowledgments
We thank S. Ganglov, A. Nicolas, and F. Fabre for helpful advice and S. Yacine and N. Thiercelin for their efficient technical assistance. We are grateful to A. Nicolas for providing parental yeast strains. This work was supported by the Institut Curie (Program of Genotoxicology).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL marie.dutreix@curie.u-psud.fr; FAX 33 1 6986 9429.
References
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- Biet E, Sun J, Dutreix M. Conserved sequence preference in DNA binding among recombination proteins: An effect of ssDNA secondary structure. Nucleic Acids Res. 1999;27:596–600. doi: 10.1093/nar/27.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock P, Miller J, Botchan M. Effects of poly[d(pGpt)·d(pApC)] and poly[d(pCpG)·d(pCpG)] repeats on homologous recombination in somatic cells. Mol Cell Biol. 1986;6:3948–3953. doi: 10.1128/mcb.6.11.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Alani E, Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990;61:1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A, Peltomaki P. Genetics of hereditary colon cancer. Annu Rev Genet. 1995;29:392–348. doi: 10.1146/annurev.ge.29.120195.001553. [DOI] [PubMed] [Google Scholar]
- de Massy B, Nicolas A. The control in cis of the position and the amount of the ARG4 meiotic double-strand break of Saccharomyces cerevisiae. EMBO J. 1993;12:1459–1466. doi: 10.1002/j.1460-2075.1993.tb05789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrauwère H, Gendrel CG, Lechat S, Dutreix M. Differences and similarities between various tandem repeat sequences: Minisatellites and microsatellites. Biochimie. 1997;79:577–586. doi: 10.1016/s0300-9084(97)82006-8. [DOI] [PubMed] [Google Scholar]
- Dutreix M. (GT)n repetitive tracts affect several stages of RecA-promoted recombination. J Mol Biol. 1997;273:105–113. doi: 10.1006/jmbi.1997.1293. [DOI] [PubMed] [Google Scholar]
- Gilbertson LA, Stahl FW. A test of the double-strand break repair model for meiotic recombination in Saccharomyces cerevisiae. Genetics. 1996;144:27–41. doi: 10.1093/genetics/144.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Petes TD. Instability of simple sequence DNA in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2749–2757. doi: 10.1128/mcb.12.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson G, Gutman GA. High frequencies of short frameshifts in poly-CA/TG tandem repeats borne by bacteriophage M13 in Escherichia coli K-12. Nucleic Acids Res. 1987;15:5323–5338. doi: 10.1093/nar/15.13.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M, Goyon C, Schultes NP, Treco D, Szostak JW, Haber JE, Nicolas A. Detection of heteroduplex DNA molecules among the products of Saccharomyces cerevisiae meiosis. Proc Natl Acad Sci. 1990;87:7653–7657. doi: 10.1073/pnas.87.19.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Stringer JR. RecA independent recombination of poly[d(GT)-d(CA)] in pBR322. Nucleic Acids Res. 1986;14:7325–7340. doi: 10.1093/nar/14.18.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas A, Treco D, Schultes NP, Szostak JW. An initiation site for meiotic gene conversion in the yeast Saccharomyces cerevisiae. Nature. 1989;338:35–39. doi: 10.1038/338035a0. [DOI] [PubMed] [Google Scholar]
- Sargent RG, Merrihew RV, Nairn R, Adair G, Meuth M, Wilson JH. The influence of a (GT)29 microsatellite sequence on homologous recombination in the hamster adenine phosphoribosyltransferase gene. Nucleic Acids Res. 1996;24:746–753. doi: 10.1093/nar/24.4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP. Unequal crossover and the evolution of multigene families. Cold Spring Harbor Laboratory Symp Quant Biol. 1973;31:507–513. doi: 10.1101/sqb.1974.038.01.055. [DOI] [PubMed] [Google Scholar]
- Strand M, Prolla TA, Liskay RM, Petes TD. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993;365:274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- Streisinger G, Okeda Y, Emrich Y, Newton J, Tsugita A, Terzaghi E, Inouye M. Frameshift mutations and the genetic code. Cold Spring Harbor Symp Quant Biol. 1996;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Sun H, Treco D, Schultes NP, Szostak JW. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989;338:87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- Treco D, Arnheim N. The evolutionarily conserved repetitive sequence d(TG·AC)n promotes reciprocal exchange and generates unusual recombinant tetrads during yeast meiosis.Mol. Cell Biol. 1986;6:3934–3947. doi: 10.1128/mcb.6.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls WP, Wallace LJ, Moore PD. The Z-DNA motif d(TG)30 promotes reception of information during gene conversion events while stimulating homologous recombination in human cells in culture.Mol. Cell Biol. 1990;10:785–793. doi: 10.1128/mcb.10.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierdl M, Dominska M, Petes TD. Microsatellite instability in yeast: Dependence on the length of the microsatellite. Genetics. 1997;146:769–779. doi: 10.1093/genetics/146.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]