Abstract
Accumulating evidence documents the initiation of diverse physiologic and biochemical response subsequent to an oral glucose load. However, significant gaps in knowledge exist in the understanding of consequences of glucose load during pregnancy, a state of insulin resistance. Using high dimensional protein arrays, we conducted a pilot proof-of-concept and feasibility study to investigate profiles of 120 plasma proteins in pre- and post- 50-gram oral glucose challenge samples. Participants (N = 10) were selected from among women enrolled in a pregnancy cohort. Differences in plasma protein concentrations between pre- and post-glucose load challenge samples were evaluated using Student's T-test (paired) and mean fold change comparisons. Multiple testing adjusted p-values (i.e., false discovery rate q values) were computed using Benjamini-Hochberg (BH) corrections. Plasma haptoglobulin, epidermal growth factor, hemoglobin, thrombospondin-1, and S100 protein concentrations were two to five fold higher in post-glucose load compared with pre-glucose load samples (all q-values <0.05). Among women aged >31 years (above median), post-load S100 protein was elevated 9.92-fold above pre-load concentrations, while it was elevated 4.10-fold among women aged <31 years (below median). Similarly, among women with post-load glucose concentrations <101mg/dl (below median), S100 was elevated 8.26-fold while it was elevated 3.28 fold among women with post-load glucose concentrations >101mg/dl (above median). Our study findings suggest that post-glucose load changes in plasma biomarkers represent a diverse set of cellular responses including receptor for advanced glycation end products (RAGE), inflammation, oxidative stress and adipogenesis, during mid-pregnancy. Future studies of larger populations and longer periods of follow-up are warranted.
Keywords: Plasma protein, oral glucose load, mid-pregnancy
Introduction
Abnormal glucose tolerance is a heterogeneous disorder consisting of insulin resistance, impaired insulin secretion or both. Gestational diabetes mellitus, is a condition associated with significant morbidity and mortality in the mother and her offspring [1-2]. Understanding normal and abnormal responses to glucose load will enhance characterization of underlying pathological changes that result in gestational diabetes and related complications.
Accumulating evidence from previous, primarily experimental, studies supports initiation of diverse biochemical events subsequent to glucose load [3]. These pathophysiologic events include hemodynamic, metabolic and inflammatory responses. However, significant gaps in knowledge exist in the understanding of consequences of glucose challenge tests during pregnancy, itself, a state of insulin resistance, as well as potential risk factors (e.g. maternal age or body mass index) that potentially influence maternal response to glucose loading during pregnancy [4-5].
As part of antenatal care, pregnant women undergo a glucose challenge screening test (also known as a “glucola test”) between 24-28 weeks of gestation for gestational diabetes screening. This presents a unique opportunity to observe maternal responses to acute glucose loading. Further, recent advances in proteomics have facilitated the development of arrays that allow simultaneous measurement of hundreds of proteins. Using high dimensional protein arrays, we conducted a pilot proof-of-concept and feasibility study to investigate plasma protein profiles in pre- and post- oral glucose challenge plasma samples collected from participants (N=10) of a pregnancy cohort.
Materials and methods
Study participants
Study subjects were selected from participants of the Omega study, a prospective cohort study designed to investigate risk factors of pregnancy complications such as preeclampsia and gestational diabetes. Study population and data collection procedures, described before, were briefly as follows [6-7]. Participants were women who attended prenatal care clinics affiliated with Swedish Medical Center, Seattle, WA. Eligible women were those who began prenatal care before 20 weeks gestation, spoke and read English, were ≥18 years of age, and planned to carry the pregnancy to term and deliver at the study hospital. We randomly selected 10 participants for the Omega study cohort for inclusion in this present pilot and feasibility study. The Institutional Review Board of the Swedish Medical Center approved study protocols. All participants provided written informed consent.
Data collection
During early pregnancy, participants were asked to complete a structured interviewer administered questionnaire regarding socio-demographic characteristics, lifestyle habits, and medical and reproductive histories. Participants also provided non-fasting blood and urine samples. Plasma samples remaining after routine 50-g oral glucose challenge screening tests gestational diabetes (tests administered between 24-28 weeks) were also collected and stored for future analyses. All collected biological samples were immediately processed and stored at -80°C until further processing. Pregnancy outcome information was abstracted from hospital and clinic medical records.
Plasma protein measurement
Plasma pre- and post- glucose load protein profile changes were evaluated using the Whatman Serum Biomarker Chip (WSBC) (Whatman Inc., Piscataway, NJ). The WSBC was specifically designed to conduct a high throughput comparative analysis of abundance of known plasma biomarkers and address a growing need of broad molecular profiling of biological samples. It is a single antibody capture array built on the FAST® Slide dual pad platform. Each slide has two identical arrays of antibodies printed in triplicates. Two-color fluorescent detection permits reproducible profiling of 120 plasma proteins (Table 1).
Table 1.
List of evaluated protein/biomarkers
| Alpha fetoprotein | ErbB2 | IL-6 | Plasminogen |
| alpha1 anthchymotrypsin | E-Selectin | IL-7 | Plasminogen Activator Inhibitor |
| Alpha2 macroglobulin | Estrogen Receptor | IL-8 | Prostatic Acid Phosphatase |
| Angiogenin | Fas | Insulin | PSA (free) |
| Angiopoietin-2 | Fas Ligand | Insulin growth factor binding protein 3 | PSA (total) |
| Angiostatin | Ferritin | Insulin-like Growth Factor 1 | PSA-ACT Complex |
| Apolipoprotein | Fibroblast Growth Factor -7 | Interferon-gamma | RANTES |
| Apolipoprotein J | Fibroblast Growth Factor-basic | IP-10 | S100 |
| beta2 microglobulin | G-CSF | Kallikrein-12 | Serum Albumin |
| Bone Sialoprotein | GM-CSF | Kallikrein-14 | Sialyl Lewis X |
| CA125 | haptoglobulin | Kallikrein-5 | TAG-72 |
| CA15-3 | Hemoglobin | Kallikrein-9 | Tetranectin |
| CA19-9 | Hepatocyte Growth Factor | Laminin | TGF-alpha |
| CA50 | ICAM-1 | low-density lipoprotein | TGF-beta |
| Carcinoembryonic antigen (G 2 specific) | IgA | MCP-1 | Thrombopoietin |
| Carcinoembryonic antigen (G 4 specific) | IgG | MCP-2 | Thrombospondin-1 |
| Cathepsin B | IgM | MCP-3 | thyroglobulin |
| Ceruloplasmin | IL-10 | MCP-4 | TIMP1 |
| Chondroitin Sulfate | IL-12p40 | M-CSF | TIMP2 |
| Chorionic gonadotropin-alpha | IL12-p70 | MIP-1alpha | TNF-alpha |
| Chorionic Gonadotropin-beta | IL-13 | MMP-2 | TNF-beta |
| Chromogranin | IL-17 | MMP-3 | Transferrin |
| Collagen Type 1 | IL-1alpha | MMP-9 | Tumor-Associated Trypsin Inhibitor |
| complement c4 | IL1-beta | Myeloperoxidase | Tyrosinase |
| C-reactive protein | IL-2 | Myoglobin | Urokinase Plasminogen Activator |
| Cyclin-dependent Kinase Inhibitor 2A | IL-2 receptor-alpha | Neuron Specific Enolase | VCAM-1 |
| Cytokeratin Fragment 21-1 (CYFRA21-1) | IL-2 receptor-beta | Osteopontin | VE-Cadherin |
| Eotaxin | IL-3 | PDGF (all isoforms) | VEGF |
| Epidermal Growth Factor | IL-4 | PDGF (BB isoform only) | VEGF-D |
| Epidermal Growth Factor Receptor | IL-5 | Placental Alkaline Phosphatase | Von Willebrand Factor |
Initially, arrayed slides underwent internal QC procedures and were stored desiccated at room temperature until ready for use. Plasma samples were labeled with biotin-Universal Linkage System (ULS™) and fluorescin-ULS™ at 37°C using the Two-Color Labeling and Fluroescent Detection Kit (KeraFAST, Winston-Salem, NC). Labeled lysate was purified using a ULS™-Trap column and incubated with WSBC in an attached dual-pad incubation chamber. The slide was then placed into a FAST Frame for further processing. Images were analyzed using Imaging Research ArrayVision software (Imaging Research Inc., St. Catharines, ON). Initial preprocessing of data involved subtraction of background signal and averaging spot replicates.
Statistical analysis
Study participant characteristics were summarized using mean (standard deviation) for continuous variables and number (%) for categorical variables. Analyses of pre- and post- glucose load protein concentrations were conducted on normalized and log2-transformed data. Differences in plasma protein concentrations between pre- and post-glucose load challenge samples were evaluated using Student's T-test (paired) and mean fold change comparisons. A volcano plot was constructed to demonstrate distribution of Student's T-test p-values (Y-axis: -Log10 [p-value]) and fold change (X-axis: Log2 [fold change]) results. Multiple testing adjusted p-values (also known as false discovery rate q values) were computed using Benjamini-Hochberg (BH) corrections [8].
We conducted stratified analyses that examined plasma concentrations changes of selected proteins (that showed significant differences in the larger group) pre- and post glucose load among subgroups defined by maternal age, pre-pregnancy body mass index (BMI). These analyses were conducted to evaluate differences across sub-groups characterized by potentially different risk status. In these analyses, participants were categorized into two groups based on medians of maternal age (31 years), pre-pregnancy BMI (21.4 kg/m2), and post-load glucose measurements (101 mg/dl). Student's paired T-test and fold change comparisons were repeated for each group. All statistical analyses were conducted using Microsoft Excel (Microsoft, Redmond, WA) and STATA, Version 11 (STATA, College Station, TX).
Results
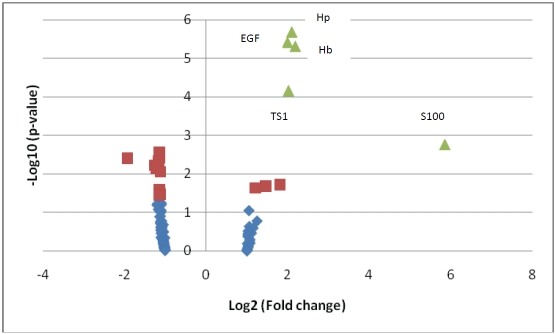
The mean age of participants was 33.2 years (Table 2). The majority of participants were nulliparous, white and married. Volcano plot depicting results of Student's paired T-test and fold change comparisons of pre- and post-glucose load protein (N=120) concentrations indicate significant signal in our data as there were more proteins with extreme p-values than would have been expected by chance (Figure 1). For example, there were 11 proteins with - Log10 [p-value] more extreme than 2.079 (-Log10 [1/120]), when we expected only 1 corresponding to a conservative false discovery rate of 1/120.
Table 2.
Selected participant characteristics
| Characteristics | Number (%) |
|---|---|
| (N=10) | |
| Maternal age, years* | 33.2 (6.1) |
| 20-34 | 7 (70%) |
| 35+ | 3 (30%) |
| Nulliparous | 9 (90%) |
| Non-Hispanic White | 8 (80%) |
| Married | 7 (70%) |
| GA at glucola screening, weeks* | 27.3 (1.6) |
| Family history hypertension | 5 (50%) |
| Family history of diabetes | 3 (30%) |
| Gestational diabetes | 0 (0%) |
| Preeclampsia | 2 (20%) |
| Smoked during pregnancy | 1 (10%) |
| Pre-pregnancy BMI, kg/m2* | 24.5 (8.0) |
| < 25 | 7 (70%) |
| 25-29 | 1 (10%) |
| 30+ | 2 (20%) |
Abbreviations: GA: gestational age, BMI: body mass index;
Mean (standard deviation), otherwise number (%)
Figure 1.
Volcano Plot.Distribution of Students’ T-test p-value (Y-axis: -Log10 [p-value]) and fold change (X-axis: Log2 [absolute fold change]) results comparing pre- and post-glucose load protein concentrations. The volcano plot indicates signal in the data as there were more proteins with extreme p-values than would have been expected by chance. For example, there were 11 proteins with -Log10 [p-value] more extreme than 2.079 (-Log10 [1/120]), when we expected only 1 corresponding to a conservative false discovery rate of 1/120. Green proteins are proteins Students T-test p-values < 0.05 after BH-false discovery rate correction. Purple proteins are proteins with uncorrected t-test p-values <0.05. Abbreviations: hp=haptoglobulin, EGF=epidermal growth factor, hb=hemoglobin, TS1=thrombospondin-1.
Pre- and post- glucose load differences in concentrations of selected proteins are shown in Table 3. Post-glucose load concentrations of these proteins were statistically different from pre-glucose load concentrations before multiple testing adjustments (unadjusted p-values <0.05). Of these, differences in haptoglobulin, epidermal growth factor, hemoglobin, throbmospondin-1, and S100 were significant after BH-based q-value corrections for multiple testing adjustments. Concentrations of these proteins were approximately 2-fold higher among post-challenge samples, except for S100 protein which was elevated more than 5-fold.
Table 3.
Proteins with significant changes in pre and post glucose load comparisons
| Proteins | Fold Change* | Students' T-test p-value | BH Corrected p-value (q value) |
|---|---|---|---|
| Haptoglobulin | 2.09 | 2.07 × 10-6 | 2.49 × 10-4 |
| Epidermal growth factor | 1.99 | 3.83 × 10-6 | 2.30 × 10-4 |
| Hemoglobin | 2.18 | 4.85 × 10-6 | 1.94 × 10-4 |
| Thrombospondin-1 | 2.01 | 6.85 × 10-5 | 2.06 × 10-3 |
| S100 | 5.84 | 1.70 × 10-3 | 4.08 × 10-2 |
| Low-density lipoprotein | -1.15 | 2.76 × 10-3 | 5.51 × 10-2 |
| PDGF (BB isoform only) | -1.15 | 3.78 × 10-3 | 6.49 × 10-2 |
| TAG-72 | -1.93 | 3.98 × 10-3 | 5.98 × 10-2 |
| Angiopietin-1 | -1.17 | 4.64 × 10-3 | 6.19 × 10-2 |
| Estrogen Receptor | -1.26 | 6.00 × 10-3 | 7.18 × 10-2 |
| Tetranectin | -1.23 | 7.37 × 10-3 | 8.04 × 10-2 |
| IL-17 | -1.13 | 8.96 × 10-3 | 8.96 × 10-2 |
Fold change comparing post/pre load samples
In stratified analyses (Table 4), we observed that post/pre glucose load changes in S100 protein concentrations were different among subgroups defined by above-below median values for maternal age (< 31 vs. ≥31 years) or post-load plasma glucose concentrations (< 101 vs. ≥101 mg/dl). Among women aged >31 years, S100 was elevated 9.92-fold while it was elevated 4.10-fold among women aged <31 years. Similarly, among women with post-load glucose concentrations <101mg/dl, S100 was elevated 8.26-fold while it was elevated 3.28 fold among women with post-load glucose concentrations >101mg/dl.
Table 4.
Proteins with significant changes in pre and post glucose load comparisons
| Proteins | Maternal age (years) | Pre-pregnancy | BMI (kg/m2) | Blood glucose (mg/dl) | ||
|---|---|---|---|---|---|---|
| ≤31 | >31 | <21.4 | >21.4 | <101 | >101 | |
| Haptoglobulin | 1.92 (0.000) | 2.37 (0.005) | 2.02 (0.001) | 2.17 (0.003) | 2.05 (0.005) | 1.90 (0.014) |
| Epidermal growth factor | 1.90 (0.000) | 2.12 (0.015) | 1.92 (0.000) | 2.06 (0.006) | 1.94 (0.004) | 1.82 (0.009) |
| Hemoglobin | 2.04 (0.001) | 2.40 (0.007) | 1.96 (0.003) | 2.42 (0.002) | 2.02 (0.020) | 2.08 (0.007) |
| Thrombospondin-1 | 2.00 (0.001) | 2.03** (0.057) | 2.04 (0.002) | 1.98 (0.021) | 1.95 (0.001) | 1.84 (0.038) |
| S100 | 4.10** (0.067) | 9.92 (0.008) | 6.41 (0.021) | 5.32** (0.071) | 8.26 (0.048) | 3.68** (0.179) |
*Fold change comparing post/pre glucose load samples (Students' T-test p-values). Cut offs are based on medians of distributions.
Not statistically significant
Discussion
In this pilot study conducted among pregnant women in mid-gestation, we found that plasma haptoglobulin, epidermal growth factor, hemoglobin, thrombospondin-1, and S100 protein concentrations were two to five fold higher post-glucose load compared with pre-glucose load concentrations. Maternal age and post-load glucose levels appeared to modify changes in S100 protein concentrations.
Investigators have previously described significant metabolic changes that occur after an oral glucose challenge [9-10]. These changes comprise a complex biochemical and physiologic processes involving multiple organs, tissues and systems [9-10]. However, few previous studies have investigated proteomic changes in relation to blood glucose levels in pregnant women [11]. Graca et al demonstrated higher glucose concentrations and lower concentrations of acetate, formate, creatinine and glycerophospho-choline in second trimester amniotic fluid among women who later developed gestational diabetes [12]. Other investigators have noted that placental peptides in maternal serum and amniotic fluid blunt effects of insulin on glucose and possibly play significant roles in development of gestational diabetes and its complications [13]. Much remains unexplained in this area of research given the profound changes expected with glucose load and the unique metabolic and physiologic background of pregnancy.
In our study, we found changes in concentrations of a number of proteins that represent the diverse cellular and likely molecular response to a 50-g oral glucose load. S100, a pro-inflammatory member of the calgranulin family (a calcium binding protein), has been identified to be a ligand of the receptor for advance glycation end products (RAGE) [14-16]. RAGE is a pattern recognition receptor that binds to multiple ligands influencing activation of biochemical cascades that initiate and stimulate chronic stress pathways and repair [15-16]. RAGE activation and regulation have been associated with various chronic disorders including diabetes, vascular diseases, cancer and neurodegenerative diseases, possibly through mechanisms involving inflammatory response [15-16]. The RAGE pathway interacts with cytokine-, lipoplysaccharide-, oxidized LDL-and glucose-triggered cellular reactions to sustain a prolonged cellular inflammatory response driven by activation of the proin-flammatory transcription factor, NFκ-β [16]. In addition, S100 protein, similar to other RAGE ligands, may bind to non-RAGE receptors or act via receptor-independent mechanisms [16-17]. Our finding suggests a role for S100 protein in the relationship between glucose homeostasis and RAGE related inflammatory pathophysiological mechanisms in pregnancy.
Both hemoglobin and haptoglobulin/ haptoglobin were elevated in post-glucose load plasma in our study. Hemoglobin is an iron-containing oxygen-transporting metalloprotein, while haptoglobulin, a plasma protein, binds to hemoglobin to form a stable complex that facilitates heme iron recycling. Free hemoglobin, which catalyzes the generation of reactive oxygen species, and haptoglobulin that binds to it, play key roles in oxidative stress and related complications [18-19]. Reactive oxygen species promote endothelial activation, inflammation, and eventual endothelial dysfunction, events that have been closely linked to pregnancy related disorders including gestational diabetes [18, 20-21]. Member of our team recently reported that early pregnancy maternal urinary 8-hydroxydeoxyguanosine (8-OHdG) concentrations, a biomarker of systemic oxidative DNA damage and repair, were associated with increased GDM risk later in pregnancy [22]. Additionally, we and others have recently reported that maternal diets high in heme iron are positively associated with GDM risk [23-24]. These emerging findings, coupled with our present findings of elevated concentrations of both hemoglobin and haptoglobulin, suggest that oxidative stress (both chronic and acute oxidative stress) is likely to be an important pathophysi-ological process involved in the etiology of gestational diabetes.
Thrombospondin-1, a protein that plays a role in platelet aggregation, angiogenesis and tumorigenesis, is an adipokine that has been correlated with adipose tissue inflammation [25]. Previous reports from lab-based experiments indicate that both glucose and insulin modify thrombospondin-1 expression in primary adipocytes [26]. These reports, along with our findings, support a potential role for thrombospondin-1 in adipocyte-related glucose homeostasis and potential complications during pregnancy.
Epidermal growth factor is an insulin-like growth factor that plays a role in cell growth, proliferation, and differentiation [27]. It is involved in glucose absorption, glucose storage and glucose transport [28]. Investigators have related epidermal growth factor to a number of diabetic complications including diabetic kidney disease [28]. In addition, in pregnancy, epidermal growth factor has been implicated in diabetes related macrosomia [29]. Thus, based on our findings, epidermal growth factor may represent yet another mechanism by which glucose load influences maternal physiology, pregnancy course and/or outcome.
Investigators have put forth thesis supporting presence of individual differences in the type and extent of physiologic or pathophysiologic cascades that are initiated post-glucose load, even when the ultimate objective was achieving glucose homeostasis [9]. These differences could potentially be related to other metabolic characteristics including participants' age and adiposity. For instance, the response that follows interaction of a ligand with RAGE is known to depend on the ligand, the environment or developmental stage of study participants 15-16]. In our study, we found potential evidence for maternal age-related differences in changes S-100 protein concentration in response to the 50-gram oral glucose load. Considerations of these characteristics may strengthen predictive and diagnostic models for gestational diabetes or other glucose related complications of pregnancy [12].
Some limitations of our pilot study deserve mention. Participants of our study included 2 subjects who developed preeclampsia. We conducted sensitivity analyses excluding the 2 affected participants and found comparable results. We had limited statistical power to detect more subtle biochemical changes in response to the glucose challenge. Consequently, we may have missed subtle, though important changes in protein profiles and our findings may be susceptible to a common problem in proteomics studies which is failure of pattern reproducibility in the face of experimental and individual variations [11]. Larger, more highly statistically powered studies, with allowances for replication in independent populations, will address these limitations. Finally, our findings relate only to acute responses to glucose load and we were not able to examine chronic responses.
In summary, we found that plasma biomarkers that represent a diverse set of cellular responses including RAGE, inflammation, oxidative stress and adipogenesis were dysregulated post-glucose load during mid-pregnancy. Such investigations have the potential to aid in classification of metabolic states, revealing new pathways, and potentially improving sensitivity and specificity for detection of abnormalities. Future studies that involve larger study populations and longer periods of follow-up are warranted.
Acknowledgments
The authors are indebted to the participants of the Omega study for their cooperation. They are also grateful for the technical expertise of staff of the Center for Perinatal Studies, Swedish Medical Center. This work was supported, in part, by grants from the National Institutes of Health (R01-HD-055566 and R01-HD-32562). Dr Enquobahrie was supported by a grant from the National Heart, Lung and Blood Institute (K01HL103174).
References
- 1.Catalano PM, Kirwan JP, Haugelde Mouzon S, King J. Gestational diabetes and insulin resis tance: role in short- and long-term implications for mother and fetus. Journal of Nutrition. 2003;133:1674S–1683S. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- 2.Clive J. Petry. Gestational diabetes: risk factors and recent advances in its genetics and treatment. British Journal of Nutrition. 2010;104:775–787. doi: 10.1017/S0007114510001741. [DOI] [PubMed] [Google Scholar]
- 3.Losser M, Bernard C, Beaudeux J, Pison C, Payen D. Glucose modulates hemodynamic, metabolic, and inflammatory responses to lipopolysaccharide in rabbits. J Appl Physiol. 1997;83:1566–1574. doi: 10.1152/jappl.1997.83.5.1566. [DOI] [PubMed] [Google Scholar]
- 4.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30:S112–9. doi: 10.2337/dc07-s202. [DOI] [PubMed] [Google Scholar]
- 5.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 6.Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy maternal plasma lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2005;70:134–142. doi: 10.1016/j.diabres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Qiu C, Frederick IO, Zhang C, Sorensen TK, Enquobahrie DA, Williams MA. Risk of gestational diabetes mellitus in relation to maternal egg and cholesterol intake. Am J Epidemiol. 2011;173:649–58. doi: 10.1093/aje/kwq425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 9.Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, Carr SA, Thadhani R, Gerszten RE, Mootha VK. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Peter A, Fritsche J, Elcnerova M, Fritsche A, Häring HU, Schleicher ED, Xu G, Lehmann R. Changes of the plasma me-tabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296:E384–93. doi: 10.1152/ajpendo.90748.2008. [DOI] [PubMed] [Google Scholar]
- 11.Shankar R, Gude N, Cullinane F, Brennecke S, Purcell AW, Moses EK. An emerging role for comprehensive proteome analysis in human pregnancy research. Reproduction. 2005;129:685–96. doi: 10.1530/rep.1.00524. [DOI] [PubMed] [Google Scholar]
- 12.Graca G, Duarte IF, Barros AS, Goodfellow BJ, Diaz SO, Pinto J, Carreira IM, Galhano E, Pita C, Gil AM. Impact of Prenatal Disorders on the Metabolic Profile of Second Trimester Amniotic Fluid: A Nuclear Magnetic Resonance Metabonomic Study. J Proteome Res. 2010;9:6016–6024. doi: 10.1021/pr100815q. [DOI] [PubMed] [Google Scholar]
- 13.Page NM, Kemp CF, Butlin DJ, Lowry PJ. Placental peptides as markers of gestational disease. Reproduction. 2002;123:487–95. doi: 10.1530/rep.0.1230487. [DOI] [PubMed] [Google Scholar]
- 14.Forbes JM, Soldatos G, Thomas MC. Below the Radar: Advanced Glycation End Products that Detour “around the side” Is HbA1c not an accurate enough predictor of long term progression and glycaemic control in diabetes? Clin Biochem Rev. 2005;25:123–34. [PMC free article] [PubMed] [Google Scholar]
- 15.Han S, Kim Y, Mook-Jung I. RAGE: The Beneficial and Deleterious Effects by Diverse Mechanisms of Actions. Mol Cells. 2011;31:91–97. doi: 10.1007/s10059-011-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009;52:2251–2263. doi: 10.1007/s00125-009-1458-9. [DOI] [PubMed] [Google Scholar]
- 17.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Häring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappa B. Diabetes. 2001;50:2792–808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 18.Mustafa S, Vukovich T, Prikoszovich T, Winzer C, Schneider B, Esterbauer H, Wagner O, Kautzky-Willer A. Hapotglobin phenotype and gestational diabetes. Diabetes Care. 2004;27:2103–2107. doi: 10.2337/diacare.27.9.2103. [DOI] [PubMed] [Google Scholar]
- 19.Thomas L. Haptoglobin/hemopexin. In: Thomas L, editor. In Clinical laboratory Diagnostics. 1st ed. Frankfurt, TH-Books: 1998. pp. 663–667. [Google Scholar]
- 20.Matsuoka H. Endothelial dysfunction associated with oxidative stress in humans. Diabetes Res Clin Pract. 2001;54:S65–S72. doi: 10.1016/s0168-8227(01)00337-0. [DOI] [PubMed] [Google Scholar]
- 21.Anastasiou E, Lekakis JP, Alevizaki M, Papamichael CM, Megas J, Souvatzoglou A, Stamatelopoulos SF. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. DiabetesCare. 1998;21:2111–2115. doi: 10.2337/diacare.21.12.2111. [DOI] [PubMed] [Google Scholar]
- 22.Qiu C, Hevner K, Abetew D, Enquobahrie DA, Williams MA. Oxidative DNA damage in early pregnancy and risk of gestational diabetes mellitus: A pilot study. Clin Biochem. 2011;44:804–808. doi: 10.1016/j.clinbiochem.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu C, Zhang C, Gelaye B, Enquobahrie DA, Frederick IO, Williams MA. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care. 2011;34:1564–1569. doi: 10.2337/dc11-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowers K, Yeung E, Williams MA, Qi L, Tobias DK, Hu FB, Zhang C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care. 2011;34:1557–1563. doi: 10.2337/dc11-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varma V, Yao-Borengasser A, Bodles AM, Rasouli 1N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ, III, Lee M, Fried SK, McGehee RE, Jr, Peterson CA, Kern PA. Throm-bospondin-1 is an adipokine associated with obesity, adipose inflammation and insulin resistance. Diabetes. 2008;57:432–9. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Diaz DF, Arellano AV, Milagro FI, Moreno-Aliaga MJ, Portillo MP, Martinez JA, Campion J. Glucose and insulin modify thrombospondin 1 expression and secretion in primary adipocytes from diet-induced obese rats. J Physiol Biochem. 2011 doi: 10.1007/s13105-011-0081-7. Mar 11. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–12. [PubMed] [Google Scholar]
- 28.Chiarelli F, Gaspari S, Marcovecchio ML. Role of growth factors in diabetic kidney disease. Horm Metab Res. 2009;41:585–93. doi: 10.1055/s-0029-1220752. [DOI] [PubMed] [Google Scholar]
- 29.Forbes K, Westwood M. Maternal growth factor regulation of human placental development and fetal growth. J Endocrinol. 2010;207:1–16. doi: 10.1677/JOE-10-0174. [DOI] [PubMed] [Google Scholar]