Abstract
Reactive oxygen species, including hydrogen peroxide (H2O2), can cause toxicity and act as signaling molecules in various pathways regulating both cell survival and cell death. However, the sequence of events between the oxidative insult and cell damage remains unclear. In the current study, we investigated the effect of oxidative stress on activation of the Receptor for Advanced Glycation End-products (RAGE) and subsequent protection against H2O2-induced pancreatic tumor cell damage. We found that exposure of pancreatic tumor cells to H2O2 provoked a nuclear factor kappa B (NF-κB)-dependent increase in RAGE expression. Further, suppression of RAGE expression by RNA interference increased the sensitivity of pancreatic tumor cells to oxidative injury. Furthermore, targeted knockdown of RAGE led to increased cell death by apoptosis and diminished cell survival by autophagy during H2O2-induced oxidative injury. Moreover, we demonstrate that RAGE is a positive feedback regulator for NF-κB as knockdown of RAGE decreased H2O2-induced activity of NF-κB. Taken together, these results suggest that RAGE is an important regulator of oxidative injury. Antioxid. Redox Signal. 15, 2175–2184.
Introduction
It is well established that cancer interventions with some chemotherapeutic agents and radiation therapies generate ROS in patients (27). Reactive oxygen species (ROS) are reactive molecules containing an oxygen atom and are produced as a normal byproduct of cellular metabolism. In particular, superoxide that leaks from the mitochondria and is converted to hydrogen peroxide (H2O2) is one of the major contributors to oxidative damage (18). Within the cell, catalase and superoxide dismutase mitigate the damaging effects of hydrogen peroxide by converting it into oxygen and water. Excessive ROS production can lead to oxidation of macromolecules and has been implicated in mitochondrial DNA (mtDNA) mutations, aging, and cell death (18). Mitochondrial-generated ROS play an important role in the release of pro-apoptotic proteins, which can trigger caspase activation and apoptosis. Additionally, autophagy, a process by which eukaryotic cells degrade and recycle macromolecules and organelles, also has an important role in the cellular response to oxidative injury (18). Functioning as an anti-aging mechanism, autophagy augments "cellular housekeeping" through enhanced removal of proteins and organelles damaged by ROS, thereby minimizing the formation of potentially harmful stress/age pigments (25). Indeed, there is a complex interplay between autophagy and apoptosis in the cellular response to ROS with the initiation of the respective prosurival or cell death pathway, dependent on the severity of the oxidative damage (18, 35).
The Receptor for Advanced Glycation End-products (RAGE) is a transmembrane receptor from the immunoglobulin super family. RAGE is expressed by cancer cells and cells in the tumor microenvironment including leukocytes, endothelial cells, and fibroblasts (37, 45). RAGE ligands [Advanced glycation end-products (AGEs), High-mobility group box 1 (HMGB1), and S100 protein family] secreted from cancer cells or leukocytes interact with RAGE and other receptors to influence tumor progression (37, 45). Tumor pathogenesis is partially hypothesized to result from ligands binding to RAGE which signals the activation of the nuclear factor kappa B (NF-κB) (2). NF-κB is a dimeric transcription factor that is involved in the regulation of a large number of genes that control various aspects of the immune and inflammatory response. This transcription factor also provides a direct link from inflammation and immunity to cancer development and progression (17). NF-κB is activated by a variety of stimuli including cytokines, radiation, and oxidative stress (such as exposure to H2O2) (28). Interestingly, RAGE is upregulated by NF-κB after lipopolysaccharide treatment (20). Recently, there is increasing evidence supporting the notion that the interaction between an AGE and RAGE generates oxidative stress and subsequently evokes vascular inflammation and thrombosis, in the context of diabetic vascular complications (49).
We have previously demonstrated that expression of RAGE correlates with pancreatic tumor cell survival following cytotoxic insult such as chemotherapy, ultraviolet radiation, and hypoxia (14, 15). However, the expression and function of RAGE in the context of oxidative injury remain largely unknown. Here, we demonstrate that RAGE is upregulated by H2O2 and protects against oxidative injury in pancreatic tumor cells. The mechanism involved in the protection conferred by RAGE upregulation is decreased apoptosis and increased autophagy. Moreover, there is positive feedback between RAGE and NF-κB in cells exposed to oxidative injury. Together, these findings contribute significantly to our understanding of the function of RAGE and uncover a new level of regulation of oxidative injury.
Materials and Methods
Reagents
The antibodies to actin and tubulin were obtained from Sigma (St. Louis, MO). The antibody to RAGE was obtained from R&D Systems Inc (Minneapolis, MN) and Sigma. The antibody to LC3 was obtained from Novus (Littleton, CO). The antibodies to p62 and NF-κB p65 were from Santa Cruz Technology (Santa Cruz, CA). The antibody to COX IV was obtained from Cell Signaling Technology (Danvers, MA). The HMGB1 neutralizing antibody was obtained from Novus (Cat # H00003146-M08). H2O2, curcumin, Bay 11-7082, NAC and 3-MA were obtained from Sigma. The caspase-9 inhibitor (Z-LEHD-FMK) was from EMD Chemicals Inc (Gibbstown, NJ). Gemcitabine was from Eli Lilly (Indianapolis, IN). The HMGB1 enzyme-linked immunosorbent assay (ELISA) kit was from Shino-Test Corporation (Sagamihara-shi, Kanagawa, Japan).
Cell culture
Human [Panc2.03 and Panc1.28] and mouse [Panc02] pancreatic tumor cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, and antibiotic-antimycotic mix in a humidified incubator with 5% CO2 and 95% air.
Cell viability assay
Cells were plated at a density of 4 × 104 cells/well in 96-well plates in 100 μl RPMI. Cell viability was evaluated using the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Tokyo, Japan) according to the manufacturer's instructions. In parallel, analysis of cell viability by trypan blue exclusion assay was performed and yielded similar results.
RNAi
RAGE short hairpin RNA (shRNA), p65 shRNA, and control shRNA (Sigma) were transfected into cells using the Lipofectamine 2000 reagent (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions (15, 16).
Western blot analysis
Proteins in cell lysates were resolved on 4%–12% Criterion XT Bis-Tris gels (Bio-Rad, Hercules, CA) and transferred to a nitrocellulose membrane as previously described (42, 43). After blocking, the membrane was incubated for 2 h at 25°C or overnight at 4°C with various primary antibodies. After incubation with peroxidase-conjugated secondary antibodies for 1 h at 25°C, the signals were visualized by enhanced chemiluminescence (Pierce, Rockford, IL) according to the manufacturer's instruction. The relative band intensities were quantified using the Gel-pro Analyzer® software (Media Cybernetics, Bethesda, MD).
Immunofluorescence analysis
Cells were cultured on glass cover-slips and fixed in 3% formaldehyde for 30 min at room temperature prior to detergent extraction with 0.1% Triton X-100 for 10 min at 25°C, as previously described (48). Cover slips were saturated with 2% bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 1 h at room temperature and processed for immunofluorescence with primary antibodies, followed by Alexa Fluor 488 or Cy3-conjugated IgG (Invitrogen, San Diego, CA), respectively. Nuclear morphology was analyzed with the fluorescent dye Hoechst 33342. Between all incubation steps, cells were washed three times for 3 min with 0.5% BSA in PBS.
NF-κB activation assay
Cells were transiently transfected in a 12-well plate with an NF-κB luciferase reporter plasmid or control empty plasmid using the Lipofectamine 2000 reagent (Life Technologies) according to the manufacturer's instructions. After 24–48 h, the cells were exposed to various agents. The luciferase activity was determined using the luciferase assay system with the reporter lysis buffer obtained from Promega (Madison, WI). The results are expressed as relative NF-κB activity after normalizing to the control empty plasmid.
Quantitative real-time polymerase chain reaction
cDNA from various cell samples were amplified by real-time quantitative PCR with specific primers for RAGE (upper GCCAGGCAATGAACAGGAATGGAA, lower TTCCCATCCAAGTGCCAGCTAAGA) and GAPDH (upper GGTGAAGGTCGGAGTCAACGG, lower GGTCATGAGTCCTTCCACGATACC) using the iQ SYBR Green Supermix (Bio-Rad). Data were normalized to GAPDH expression. The control group was set as 100%.
Apoptosis assays
Apoptosis in cells was assessed using a TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) kit from Roche Applied Science (Stockholm, Sweden), Caspase-3 and −9 Colorimetric Assay Kit (Calbiochem, Gibbstown, NJ), according to manufacturer's instructions. Mitochondrial membrane potential depolarization was measured by flow cytometric analysis utilizing a fluorescent cationic dye, 1,1′3,3′-tetraethylbenzamidazolocarbocyanin iodide (JC-1, Molecular Probes, San Diego, CA). JC-1 is a lipophilic, cationic dye that can selectively enter into mitochondria and reversibly change color from green to red with increases in membrane potential.
Autophagy assays
Autophagic flux assays were performed by Western blotting for LC3-I/II and p62 and by imaging for LC3 punctae, as previously described (15, 44). Images were collected using a laser-scanning confocal microscope (Fluoview FV-1000; Olympus) using a 60x Plan Apo/1.45 oil immersion objective and Fluoview software (FV10-ASW 1.6; Olympus).
Isolation and subcellular fractionation of mitochondria
Subcellular fractionation of tumor cells was carried out with a mitochondria isolation kit obtained from Pierce (Rockford, IL). To confirm that these were the appropriate fractions, the Western blots were probed for COX IV as a mitochondrial marker and tubulin as a cytoplasmic marker.
Statistical analysis
Data are expressed as means ± SD of three independent experiments performed in triplicate. One-way ANOVA was used for comparison among the different groups. When the ANOVA was significant, post hoc testing of differences between groups was performed using an LSD test. A p value <0.05 was considered significant.
Results
Upregulation of RAGE expression in H2O2-induced oxidative injury is NF-κB-dependent
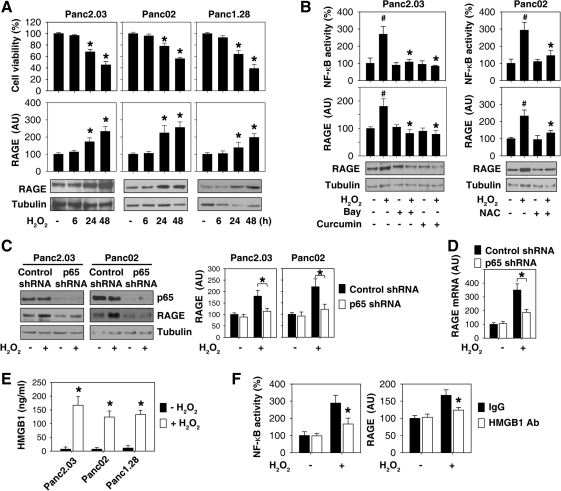
The effects of H2O2 on cell viability were determined by CCK8 and trypan blue exclusion assay. CCK-8 utilizes the highly water-soluble tetrazolium salt, WST-8 [2-(2-methoxy-4-nitrophenyl)-3- (4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium, monosodium salt], which has a detection sensitivity that is higher than other tetrazolium salts such as MTT [3-[4,5- dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide]. Consistent with previous studies (48), at lower (0.0025–0.025 mM) concentrations, H2O2 was not toxic to the pancreatic tumor cell lines (data not shown). However, at higher concentrations (0.25 mM), H2O2 demonstrated time-dependent cytotoxicity in pancreatic tumor cells (Panc2.03, Panc02, and Panc1.28; Fig. 1A).
FIG. 1.
NF-κB-dependent expression of RAGE in H2O2-induced oxidative injury. (A) Time-dependent effects of H2O2 on cell viability and RAGE expression. Pancreatic cancer cells as indicated were treated with H2O2 (0.25 mM) for 6–48 h and cell viability and RAGE expression were subsequently analyzed (n = 3, *p < 0.05 versus untreated group). “AU”: arbitrary unit. (B) NF-κB regulates RAGE expression during oxidative injury. Pancreatic cancer cells as indicated were treated with H2O2 (0.25 mM) for 24 h with or without NF-κB inhibitors: Bay 11-7085 (“Bay”, 10 μM), curcumin (50 μM) and NAC (50 mM). The total protein extracts were used for Western blot analysis (bottom). In parallel, the activity of NF-κB was analyzed by a luciferase reporter assay (n = 3, *p < 0.05 versus H2O2 group, #p < 0.05 versus untreated group). “AU”: arbitrary unit. (C, D) After transfection with p65 shRNA or control shRNA for 48 h, Panc2.03 and Panc02 cells were treated with H2O2 (0.25 mM) for 24 h and then the total protein extracts were used for Western blot analysis (n = 3, *p < 0.05). In parallel, the mRNA level of RAGE in Panc2.03 cells was analyzed by quantitative RT-PCR (n = 3, *p < 0.05). “AU”: arbitrary unit. (E) The indicated pancreatic cancer cells were treated with H2O2 (0.25 mM) for 24 h and HMGB1 release were analyzed by ELISA (n = 3, *p < 0.05 versus untreated group). (F) Panc2.03 cells were treated with H2O2 (0.25 mM) for 24 h with or without HMGB1-neutralizing antibody (10 μg/ml) or control IgG, and then NF-κB activity and RAGE protein expression were analyzed (n = 3, *p < 0.05 versus control IgG group). “AU”: arbitrary unit.
To determine if H2O2 induces RAGE expression, pancreatic tumor cells (Panc2.03, Panc02, and Panc1.28) were treated with H2O2 for various amounts of time and cell lysates were assayed for RAGE expression by Western blot analysis. Indeed, at toxic doses (e.g., 0.25 mM), H2O2 induced a time-dependent increase in RAGE protein levels (Fig. 1A), indicating that RAGE is upregulated after oxidative injury.
It is well established that exogenous H2O2 induces NF-κB activation (28). To explore the potential role for NF-κB in the regulation of H2O2-induced RAGE expression, we evaluated the effects of two potential NF-κB inhibitors, Bay 11-7085 and curcumin (28), on the expression of RAGE in response to oxidative injury. Indeed, both Bay 11-7085 and curcumin attenuated H2O2-induced NF-κB activation by a luciferase reporter assay and RAGE expression by Western blot analysis (Fig. 1B). Similarly, administration of the antioxidant N-acetyl cysteine (NAC) inhibited oxidative stress-induced NF-κB activation and RAGE expression in pancreatic cancer cells (Fig. 1B). Moreover, knockdown of the p65 subunit of NF-κB by shRNA inhibited H2O2-induced RAGE mRNA and protein expression in pancreatic cancer cells (Panc2.03, Panc02) (Figs. 1C and 1D). These data suggest that oxidative injury induces an NF-κB-dependent upregulation of RAGE expression.
The prototypical damage-associated molecular pattern molecule (DAMP) (45), HMGB1 is released with sustained autophagy (22, 23, 40, 41, 47), late apoptosis (1), and necrosis (34). Consistently, HMGB1 was released by pancreatic cancer cells (Panc2.03, Panc02, and Panc1.28) during oxidative injury (Fig. 1E). Moreover, HMGB1-neutralizing antibodies significantly inhibited H2O2-induced NF-κB activation and RAGE expression (Fig. 1F), suggesting that HMGB1 release contributes to NF-κB activation and RAGE expression in response to oxidative injury.
Suppression of RAGE expression by shRNA increases pancreatic tumor cell sensitivity to oxidative injury
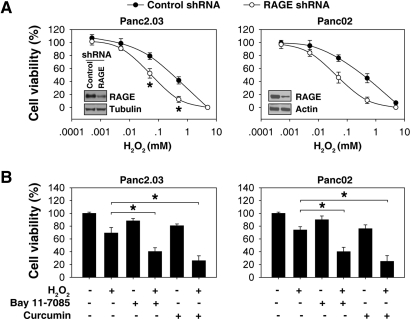
To explore the potential role for RAGE in the regulation of oxidative injury in pancreatic tumor cells, target-specific shRNA against RAGE was transfected into Panc2.03 or Panc02 cells. Transfection of RAGE shRNA at 48 h led to a significant decrease in RAGE expression in cells (Fig. 2A). Depletion of RAGE expression in these cells rendered them significantly more sensitive to H2O2-induced cell injury (Fig. 2A), suggesting that RAGE plays a protective role against oxidative injury.
FIG. 2.
Suppression of RAGE expression by shRNA increases pancreatic tumor cell sensitivity to oxidative injury. (A) Suppression of RAGE expression increases H2O2-induced oxidative cytotoxicity. After transfection with RAGE shRNA or control shRNA for 48 h, Panc2.03 and Panc02 cells were treated with H2O2 at indicated dose for 24 h and then the cell viability was analyzed (n = 3, *p < 0.05 versus control shRNA group). A representative Western blot of RAGE level after shRNA is depicted (inset). (B) NF-κB inhibitors increase H2O2-induced oxidative injury. Panc2.03 and Panc02 cells were treated with H2O2 (0.25 mM) for 24 h with or without NF-κB inhibitors: Bay 11-7085 (“Bay”, 10 μM) and curcumin (50 μM), and the cell viability was analyzed (n = 3, #p < 0.05 versus control shRNA group, *p < 0.05 versus H2O2 group).
To further characterize the role of RAGE in pancreatic tumor cells after oxidative injury, we treated cells with NF-κB inhibitors during H2O2 treatment. Both Bay 11-7085 and curcumin inhibited H2O2-induced RAGE expression (Fig. 1B), and increased oxidative cytotoxicity in Panc2.03 and Panc02 cells (Fig. 2B). These data suggest that cell survival following oxidative injury is dependent on NF-κB transcriptional upregulation of RAGE.
Suppression of RAGE expression increases the mitochondrial apoptotic pathway during oxidative injury
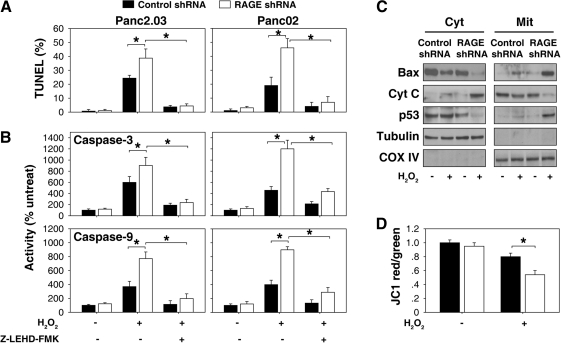
In vitro studies have demonstrated that apoptosis can be triggered by many distinct and different stimuli, including exposure to physical and chemical agents or removal of growth factors (10). Free radicals and oxidative stress have been implicated in apoptosis, although there is uncertainty regarding their degree of importance in this cell death pathway. TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) is a common method for detecting DNA fragmentation that results from apoptotic signaling cascades. Compared with cells treated with control shRNA, suppression of RAGE expression by shRNA increased H2O2 (0.25 mM)-induced apoptosis by TUNEL assay (Fig. 3A) and high levels of caspase-3 and −9 activities (Fig. 3B), suggesting a potential anti-apoptotic role for RAGE in pancreatic tumor cells. Furthermore, the caspase 9 inhibitor, Z-LEHD-FMK, inhibited H2O2-induced apoptosis in RAGE knockdown pancreatic cells. Caspase-9 has been linked to the mitochondrial apoptotic pathway, but not the cell death receptor pathway (10). These data suggest that RAGE inhibits activation of the mitochondrial apoptotic pathway in response to oxidative injury.
FIG. 3.
Suppression of RAGE expression actives mitochondrial apoptotic pathway in response to oxidative injury. (A, B) Panc2.03 and Panc02 cells were treated with H2O2 (0.25 mM, 24 h) with or without caspase-9 inhibitor (Z-LEHD-FMK, 20 μM), then apoptosis levels were assessed by (A) TUNEL and (B) caspase-3 and −9 activity assays (n = 3, *p < 0.05). (C, D) Panc2.03 cells were treated with H2O2 (0.25 mM) for 24 h and then cytosolic (“Cyt” and mitochondrial (“Mit”) Bax were assayed by Western blot analysis (C). Cyt C: cytochrome c. In parallel, the mitochondrial membrane potential was analyzed by flow cytometric analysis (D) utilizing a fluorescent cationic dye JC-1 (n = 3, *p < 0.05). The JC-1 red-to-green fluorescence ratio of the untreated shRNA group was set as 1.
The mitochondrial pathway of apoptosis begins with permeabilization of the mitochondrial outer membrane, which is mainly regulated by Bcl-2 family members. Bax is a pro-apoptotic member of the Bcl-2 family of proteins and plays a critical role in apoptosis. Activation of Bax results in reduction of the mitochondrial membrane and cytochrome c release from mitochondria, which induces a series of biochemical reactions that result in caspase-3 and −9 activation and subsequent cell death. Knockdown of RAGE by shRNA increased mitochondrial Bax translocation and these downstream events resulting in apoptosis (Figs. 3B–3D).
p53 has a direct apoptogenic role at the mitochondria. Mitochondrial p53 directly activates Bax in the absence of other proteins to permeabilize mitochondria and initiate the apoptotic program (5). Our previous study demonstrated that increased apoptosis in RAGE knockdown pancreatic tumor cells is associated with p53 phosphorylation and subsequent translocation to the mitochondria during treatment with chemotherapies (15). Consistently, knockdown of RAGE by shRNA increased H2O2-induced mitochondrial p53 translocation (Fig. 3C). Taken together, these findings suggest that RAGE regulates the p53-Bax mitochondrial pathway of apoptosis.
Suppression of RAGE expression decreases autophagy in response to oxidative injury
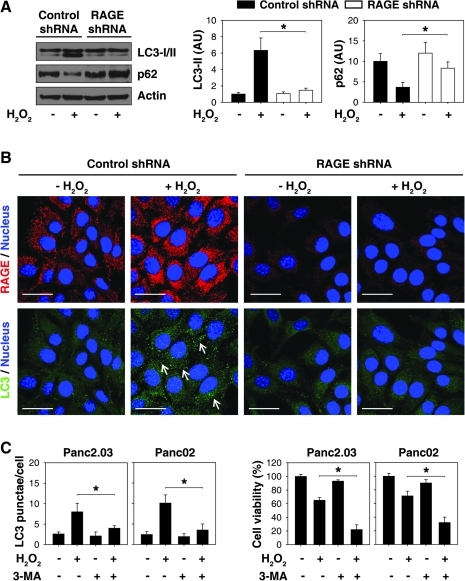
Autophagy has been implicated in many physiologic and pathologic processes, including oxidative injury (4). We therefore investigated whether RAGE regulates autophagy in the context of oxidative injury. During autophagic flux, the cytosolic form of LC3 (LC3-I) is cleaved and then conjugated to phosphatidylethanolamine to form the LC3-phosphatidylethanolamine conjugate (LC3-II), which is recruited to autophagosomal membranes. Detecting microtubule-associated protein light chain 3 (LC3) has become a widely accepted method for monitoring autophagy and autophagy-related processes (24). Indeed, suppression of RAGE expression in Panc2.03 cells decreased H2O2-induced LC3 expression and accumulation of LC3-II by Western blot (Fig. 4A). Furthermore, we observed a loss of endogenous LC3 punctae formation after knockdown of RAGE following oxidative injury by immunofluorescence analysis (Fig. 4B), indicating a specific role for RAGE in the regulation of autophagosome formation during oxidative stress.
FIG. 4.
Suppression of RAGE expression decreases autophagy in the context of oxidative injury. (A, B) Panc2.03 cells were treated with H2O2 (0.25 mM) for 24 h. The total protein extracts were used for Western blot analysis (A). In parallel, LC3 punctae formation was assayed by confocal microscopic analysis (B). Data are representative of three experiments (n = 3, *p < 0.05; Bar = 30 μm). Arrow indicates LC3 punctae. (C) The indicated pancreatic cancer cells were treated with H2O2 (0.25 mM) for 24 h with or without 3-methyladenine (“3-MA”, 10 mM). The LC3 punctae formation was assayed by confocal microscopic analysis. In parallel, the cell viability was assayed by CCK-8 kit (n = 3, *p < 0.05). “AU”: arbitrary unit. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
In addition to LC3, levels of other autophagy substrates such as p62 (SQSTM1/sequestosome-1) can be used to monitor autophagic flux (29). p62 is selectively incorporated into autophagosomes through direct binding to LC3 and is efficiently degraded by autophagy (29). Thus, the total cellular expression of p62 inversely correlates with autophagic activity (24). Consistent with a downregulation of autophagy, knockdown of RAGE inhibited H2O2-induced p62 degradation (Fig. 4A). These autophagic flux assays implicate RAGE in the regulation of autophagic activity.
To explore the role of autophagy in oxidative injury, we use 3-methyladenine (3-MA), a well known inhibitor of autophagy in mammalian cells, in pancreatic cancer cells. 3-MA inhibited H2O2-induced LC3 punctae formation and increased cell death (Fig. 4C). These findings confirm the notion that autophagy promotes tumor cell survival after cell stress.
Suppression of RAGE expression decreases the activity of NF-κB in response to oxidative injury
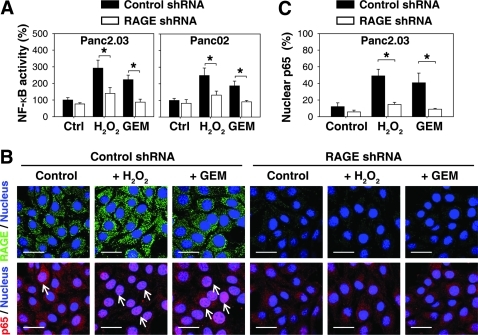
Previous studies have demonstrated that RAGE-mediated NF-κB activation is involved in inflammation and inflammation-associated carcinogenesis (2, 21). Moreover, NF-κB activation leads chemoresistance to gemcitabine, the most widely used first-line chemotherapeutic drug in human pancreatic tumors (11). To determine whether the increase observed in RAGE expression was also responsible for oxidative injury-mediated activity of NF-κB, we determined the activity of NF-κB using a luciferase reporter assay and the location of NF-κB p65 by immunofluorescence analysis in pancreatic cancer cells transfected with RAGE-shRNA and control shRNA. Indeed, knockdown of RAGE inhibited both activity of NF-κB (Fig. 5A) and the nuclear localization of NF-κB p65 (Figs. 5B and 5C) in response to H2O2 and gemcitabine. Since we also found that NF-κB mediates RAGE expression in response to oxidative injury (Fig. 1B), we demonstrate that there is a positive feedback loop between RAGE and NF-κB during oxidative stress (Fig. 6).
FIG. 5.
Suppression of RAGE expression decreases activity of NF-κB during oxidative stress. Panc2.03 and Panc02 cells were treated with H2O2 (0.25 mM) or gemcitabine (“GEM”, 100 nM) for 24 h. (A) The activity of NF-κB was assayed by luciferase reporter analysis (n = 3, *p < 0.05). (B, C) In parallel, the location of NF-κB p65 in Panc2.03 cells was assayed by confocal microscopic analysis. Representative p65 stains are shown in panel (B), and quantification of the percentage of nuclear p65 positive cells is shown in (C). Data are representative of three experiments (n = 3, *p < 0.05; Bar = 30 μm). Arrows indicate nuclear p65 translocation. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
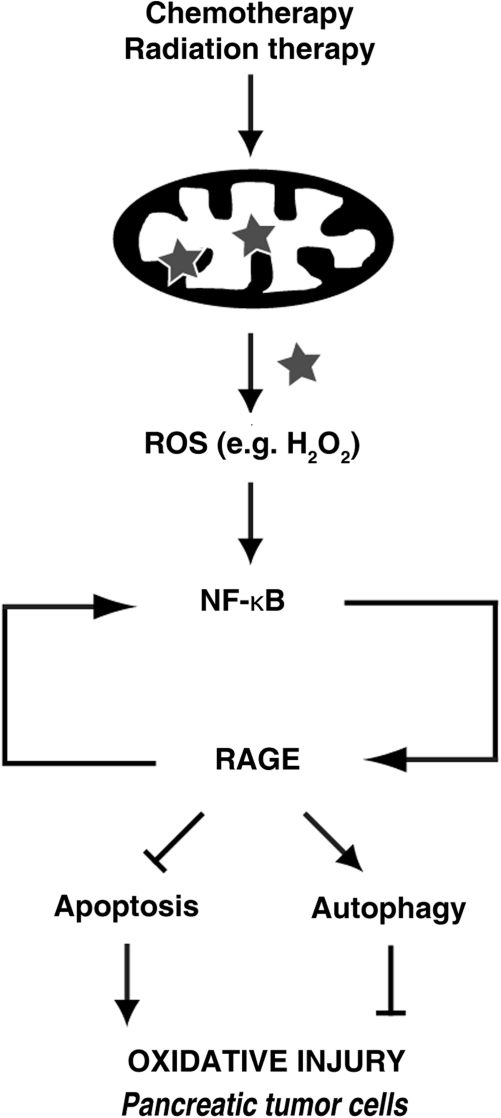
FIG. 6.
Schematic of RAGE/ NF-κB regulation of oxidative injury. The mitochondrial respiratory chain is the major intracellular source of reactive oxygen species (ROS). Radiation therapy and some forms of chemotherapy rely on ROS toxicity to eradicate tumor cells. However, ROS such as H2O2 increase activity of NF-κB and subsequently result in RAGE overexpression. This RAGE upregulation protects pancreatic tumor cells against oxidative injury by increasing programmed cell survival (autophagy) and decreasing programmed cell death (apoptosis). Furthermore, RAGE also sustains NF-κB activation by positive regulation in response to oxidative injury.
Discussion
The Receptor for Advanced Glycation Endproducts (RAGE) is an evolutionarily recent member of the immunoglobulin super-family, encoded in the Class III region of the major histocompatability complex (37). RAGE is highly expressed only in the lung at readily measurable levels but increases quickly at sites of inflammation, largely on inflammatory and epithelial cells (37). RAGE is a multifunctional receptor that binds a broad repertoire of ligands and mediates responses to cell damage and stress conditions (37, 45). We and others have demonstrated that this receptor can be expressed on murine and human pancreatic tumor cell lines (15, 39). Interaction of RAGE ligands (such as S100A8/A9 and HMGB1) with RAGE promotes tumor cell growth (9, 38, 45, 46). Overexpression RAGE inhibits apoptosis under conditions of hypoxia (12) and chemotherapy treatment (15) in tumor cells. Here, we demonstrate that RAGE promotes pancreatic tumor cell survival in H2O2-induced cell death, indicating an important role of RAGE in the regulation of oxidative injury.
During cancer chemotherapy, oxidative stress-induced lipid peroxidation generates numerous electrophilic aldehydes that can attack many cellular targets (27). We have previously demonstrated that RAGE expression promotes pancreatic tumor cell survival following administration of chemotherapeutic agents (15). Thus, we reasoned that RAGE was also involved in the response to oxidative injury since ROS have been proposed as common mediators for chemotherapy-mediated injury (27). To address this possibility, we first observed the expression of RAGE in oxidative injury. Indeed, an increase in RAGE protein expression was tightly associated with H2O2-induced oxidative injury. Consistent with previous findings that RAGE is a NF-κB target gene in lipopolysaccharide-induced bovine aortic endothelial cells and rat vascular smooth cells (20), we demonstrated that H2O2-induced RAGE expression is NF-κB dependent. Although other studies have shown that exogenous H2O2-induced activation of the transcriptional factors HSF (heat-shock transcription factor) (13) and STAT (signal transducer and activator of transcription) (3), which are powerful multifaceted modifiers of carcinogenesis, we do not yet know whether these factors regulate the expression of RAGE under oxidative stress in pancreatic tumors. Moreover, RAGE has several isoforms deriving from alternative splicing, including a soluble form called endogenous secretory RAGE (esRAGE) that acts as a decoy receptor (31). Additionally, there are several reports of cell membrane receptor protein ectodomain shedding (tumor necrosis factor receptor, betacellulin, calcineurin and RAGE) in response to stress (33, 50). RAGE shedding has been confirmed in response to H2O2 exposure in oligodendrocytes (30).
We demonstrate that RAGE promotes pancreatic tumor cell survival following oxidative injury by regulating levels of apoptosis and autophagy. ROS, including H2O2, are important signal molecules that active both apoptosis and autophagy in various cells through different mechanisms. For example, H2O2 induces apoptosis in HeLa cervical cancer cells (36) and T-cells (8) through the mitochondrial pathway involving the release of cytochrome c and activation of caspases-9 and −3. Similarly, we found that knockdown of RAGE in Panc2.03 cells increased H2O2-induced activation of caspases-9 and −3 and subsequently apoptosis levels by TUNEL assay. RAGE limits chemotherapeutic agent-induced apoptosis in pancreatic tumor cells through a p53-dependent mitochondrial pathway (15). Increased apoptosis in RAGE knockdown pancreatic tumor cells is associated with p53 phosphorylation and translocation to the mitochondria (15). Moreover, H2O2-induced apoptosis in glial cells is mediated by p53 induction of Bak (the Bcl-2 homologous antagonist/killer) (19). The Bak protein is a pro-apoptotic member of the Bcl-2 gene family that is involved in initiating apoptosis (19). We found that suppression of RAGE expression by RNAi increased H2O2-induced mitochondrial Bax translocation. Thus, RAGE may interact with p53 to regulate the ROS-mediated mitochondrial apoptosis pathway through the Bcl-2 family.
Autophagy is activated to promote cell survival after endoplasmic reticulum stress, nutrient starvation, chemotherapy, and oxidative stress (4, 7, 26). The primary function of autophagy is to recycle cellular components to sustain metabolism during nutrient deprivation and to prevent the accumulation of damaged toxic proteins and organelles during stress (32). Thus, autophagy may be viewed as a ‘programmed’ cell survival mechanism activated in response to cellular injury. We found that suppression of RAGE expression by shRNA decreased H2O2 and chemotherapy-induced levels of autophagy in pancreatic tumor cells (15), indicating that RAGE may increase the resistance to injury and promote cell survival by promoting cellular clearance of the oxidatively damaged proteins, lipids, carbohydrates, and DNA.
Finally, our experimental data suggest that RAGE may function as a positive feedback regulator for NF-κB, as suppression of RAGE expression also inhibits the activity of NF-κB during oxidative stress. Indeed, the involvement of RAGE in the NF-κB pathway has been demonstrated in many studies (2, 21), although the precise mechanism is unknown. There are two known pathways that lead to NF-κB activation. In the classical pathway, pro-inflammatory stimuli and genotoxic stress leads to IκB kinase (IKK)-β- and IKK-γ-dependent phosphorylation of IκB, which results in proteasomal degradation and subsequent release of the NF-κB dimers (28). The alternative pathway works independently of IKK-β and IKK-γ (28). However, whether RAGE regulates IKK activity is unknown. Interestingly, a recent study suggests that the IKK complex has an NF-κB-independent role in the stimulation of autophagy by physiological and pharmacological stimuli (6), suggesting that IKK has a novel function in regulating autophagy.
In summary, we show that RAGE is upregulated by H2O2 which confers protection from oxidative injury by inhibiting apoptosis and increasing autophagy in pancreatic tumor cells. Moreover, there is a positive feedback loop between RAGE and NF-κB in response to oxidative injury (Fig. 6). These findings suggest that RAGE, in addition to NF-κB promotes pancreatic tumor cell survival in response to H2O2-induced oxidative injury. Today, studies using exogenous H2O2 are occasionally criticized because of the assertion that exogenous addition of H2O2 is always nonphysiological. Thus, future studies should explore the effects of RAGE on oxidative stress/injury in physiological and pathological conditions.
Abbreviations Used
- 3-MA
3-methyladenine;
- AGEs
advanced glycation end-products
- Bak
the Bcl-2 homologous antagonist/killer
- BSA
bovine serum albumin
- CCK-8
cell counting kit-8
- ELISA
enzyme-linked immunosorbent assay
- H2O2
hydrogen peroxide
- HMGB1
high-mobility group box 1
- HSF
heat-shock transcription factor
- IKK
IκB kinase
- LC3
microtubule-associated protein light chain 3
- NAC
N-acetyl cysteine
- NF-κB
nuclear factor kappa B
- PBS
phosphate buffered saline
- RAGE
the receptor for advanced glycation endproducts
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- STAT
signal transducer and activator of transcription
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Acknowledgments
This project was funded by a grant from the NIH 1 P01 CA 101944-04 (Michael T. Lotze) Integrating NK and DC into Cancer Therapy from the National Cancer Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bell CW. Jiang W. Reich CF., 3rd Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 2.Bierhaus A. Schiekofer S. Schwaninger M. Andrassy M. Humpert PM. Chen J. Hong M. Luther T. Henle T. Kloting I. Morcos M. Hofmann M. Tritschler H. Weigle B. Kasper M. Smith M. Perry G. Schmidt AM. Stern DM. Haring HU. Schleicher E. Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 3.Burova EB. Grudinkin PS. Bardin AA. Gamalei NN. [H2O2-induced activation of transcription factors STAT1 and STAT3: The role of EGF receptor and tyrosine kinase JAK2] Tsitologiia. 2001;43:1153–1161. [PubMed] [Google Scholar]
- 4.Chen Y. McMillan–Ward E. Kong J. Israels SJ. Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 5.Chipuk JE. Kuwana T. Bouchier–Hayes L. Droin NM. Newmeyer DD. Schuler M. Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 6.Criollo A. Senovilla L. Authier H. Maiuri MC. Morselli E. Vitale I. Kepp O. Tasdemir E. Galluzzi L. Shen S. Tailler M. Delahaye N. Tesniere A. De Stefano D. Younes AB. Harper F. Pierron G. Lavandero S. Zitvogel L. Israel A. Baud V. Kroemer G. The IKK complex contributes to the induction of autophagy. EMBO J. 2010;29:619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degenhardt K. Mathew R. Beaudoin B. Bray K. Anderson D. Chen G. Mukherjee C. Shi Y. Gelinas C. Fan Y. Nelson DA. Jin S. White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont A. Hehner SP. Hofmann TG. Ueffing M. Droge W. Schmitz ML. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kappaB. Oncogene. 1999;18:747–757. doi: 10.1038/sj.onc.1202325. [DOI] [PubMed] [Google Scholar]
- 9.Ghavami S. Rashedi I. Dattilo BM. Eshraghi M. Chazin WJ. Hashemi M. Wesselborg S. Kerkhoff C. Los M. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol. 2008;83:1484–1492. doi: 10.1189/jlb.0607397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 11.Hering J. Garrean S. Dekoj TR. Razzak A. Saied A. Trevino J. Babcock TA. Espat NJ. Inhibition of proliferation by omega-3 fatty acids in chemoresistant pancreatic cancer cells. Ann Surg Oncol. 2007;14:3620–3628. doi: 10.1245/s10434-007-9556-8. [DOI] [PubMed] [Google Scholar]
- 12.Hiwatashi K. Ueno S. Abeyama K. Kubo F. Sakoda M. Maruyama I. Hamanoue M. Natsugoe S. Aikou T. A novel function of the receptor for advanced glycation end-products (RAGE) in association with tumorigenesis and tumor differentiation of HCC. Ann Surg Oncol. 2008;15:923–933. doi: 10.1245/s10434-007-9698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquier–Sarlin MR. Polla BS. Dual regulation of heat-shock transcription factor (HSF) activation and DNA-binding activity by H2O2: Role of thioredoxin. Biochem J. 1996;318:187–193. doi: 10.1042/bj3180187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang R. Tang D. Lotze MT. Zeh HJ. Apoptosis to autophagy switch triggered by the MHC class III-encoded receptor for advanced glycation endproducts (RAGE) Autophagy. 2011;7:91–93. doi: 10.1038/cdd.2009.149. [DOI] [PubMed] [Google Scholar]
- 15.Kang R. Tang D. Schapiro NE. Livesey KM. Farkas A. Loughran P. Bierhaus A. Lotze MT. Zeh HJ. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–676. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang R. Tang D. Yu Y. Wang Z. Hu T. Wang H. Cao L. WAVE1 regulates Bcl-2 localization and phosphorylation in leukemia cells. Leukemia. 2010;24:177–186. doi: 10.1038/leu.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karin M. Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 18.Kiffin R. Bandyopadhyay U. Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura Y. Ota T. Matsuoka Y. Tooyama I. Kimura H. Shimohama S. Nomura Y. Gebicke–Haerter PJ. Taniguchi T. Hydrogen peroxide-induced apoptosis mediated by p53 protein in glial cells. Glia. 1999;25:154–164. [PubMed] [Google Scholar]
- 20.Li J. Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;272:16498–16506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- 21.Liliensiek B. Weigand MA. Bierhaus A. Nicklas W. Kasper M. Hofer S. Plachky J. Grone HJ. Kurschus FC. Schmidt AM. Yan SD. Martin E. Schleicher E. Stern DM. Hammerling GG. Nawroth PP. Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L. Yang M. Kang R. Wang Z. Zhao Y. Yu Y. Xie M. Yin X. Livesey KM. Lotze MT. Tang D. Cao L. DAMP-mediated autophagy contributes to drug resistance. Autophagy. 2011;7:112–114. doi: 10.4161/auto.7.1.14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L. Yang M. Kang R. Wang Z. Zhao Y. Yu Y. Xie M. Yin X. Livesey KM. Lotze MT. Tang D. Cao L. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25:23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- 24.Mizushima N. Yoshimori T. Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore MN. Autophagy as a second level protective process in conferring resistance to environmentally-induced oxidative stress. Autophagy. 2008;4:254–256. doi: 10.4161/auto.5528. [DOI] [PubMed] [Google Scholar]
- 26.Ogata M. Hino S. Saito A. Morikawa K. Kondo S. Kanemoto S. Murakami T. Taniguchi M. Tanii I. Yoshinaga K. Shiosaka S. Hammarback JA. Urano F. Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozben T. Oxidative stress and apoptosis: Impact on cancer therapy. J Pharm Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 28.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 29.Pankiv S. Clausen TH. Lamark T. Brech A. Bruun JA. Outzen H. Overvatn A. Bjorkoy G. Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 30.Qin J. Goswami R. Dawson S. Dawson G. Expression of the receptor for advanced glycation end products in oligodendrocytes in response to oxidative stress. J Neurosci Res. 2008;86:2414–2422. doi: 10.1002/jnr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raucci A. Cugusi S. Antonelli A. Barabino SM. Monti L. Bierhaus A. Reiss K. Saftig P. Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 32.Rubinsztein DC. Gestwicki JE. Murphy LO. Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson MP. Abbott CA. Tada H. Seno M. Dempsey PJ. Dunbar AJ. Hydrogen peroxide and endothelin-1 are novel activators of betacellulin ectodomain shedding. J Cell Biochem. 2006;99:609–623. doi: 10.1002/jcb.20968. [DOI] [PubMed] [Google Scholar]
- 34.Scaffidi P. Misteli T. Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 35.Simon HU. Haj-Yehia A. Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 36.Singh M. Sharma H. Singh N. Hydrogen peroxide induces apoptosis in HeLa cells through mitochondrial pathway. Mitochondrion. 2007;7:367–373. doi: 10.1016/j.mito.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Sparvero LJ. Asafu–Adjei D. Kang R. Tang D. Amin N. Im J. Rutledge R. Lin B. Amoscato AA. Zeh HJ. Lotze MT. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taguchi A. Blood DC. del Toro G. Canet A. Lee DC. Qu W. Tanji N. Lu Y. Lalla E. Fu C. Hofmann MA. Kislinger T. Ingram M. Lu A. Tanaka H. Hori O. Ogawa S. Stern DM. Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 39.Takada M. Hirata K. Ajiki T. Suzuki Y. Kuroda Y. Expression of receptor for advanced glycation end products (RAGE) and MMP-9 in human pancreatic cancer cells. Hepatogastroenterology. 2004;51:928–930. [PubMed] [Google Scholar]
- 40.Tang D. Kang R. Cheh CW. Livesey KM. Liang X. Schapiro NE. Benschop R. Sparvero LJ. Amoscato AA. Tracey KJ. Zeh HJ. Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang D. Kang R. Livesey KM. Cheh CW. Farkas A. Loughran P. Hoppe G. Bianchi ME. Tracey KJ. Zeh HJ., 3rd Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang D. Kang R. Xiao W. Jiang L. Liu M. Shi Y. Wang K. Wang H. Xiao X. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol. 2007;178:7376–7384. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang D. Kang R. Xiao W. Wang H. Calderwood SK. Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol. 2007;179:1236–1244. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang D. Kang R. Xiao W. Zhang H. Lotze MT. Wang H. Xiao X. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am J Respir Cell Mol Biol. 2009;41:651–660. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang D. Kang R. Zeh HJ., 3rd Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang D. Kang R. Zeh HJ. Lotze MT. HMGB1, Oxidative stress, and disease. Antioxid Redox Signal. 2011;14:1315–1335. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang D. Lotze MT. Zeh HJ., 3rd Kang R. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy. 2010;6:1181–1183. doi: 10.4161/auto.6.8.13367. [DOI] [PubMed] [Google Scholar]
- 48.Tang D. Shi Y. Kang R. Li T. Xiao W. Wang H. Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamagishi S. Advanced glycation end products and receptor-oxidative stress system in diabetic vascular complications. Ther Apher Dial. 2009;13:534–539. doi: 10.1111/j.1744-9987.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z. Oliver P. Lancaster JJ. Schwarzenberger PO. Joshi MS. Cork J. Kolls JK. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]