Abstract
Aims
Down Syndrome (DS), a genetic disease caused by a triplication of chromosome 21, is characterized by increased markers of oxidative stress. In addition to cognitive defects, patients with DS also display hematologic disorders and increased incidence of infections and leukemia. Using the Ts65Dn mouse model of DS, the goal of this study was to examine hematopoietic stem and lymphoid progenitor cell function in DS.
Results
Analysis of hematopoietic progenitor populations showed that Ts65Dn mice possessed fewer functional hematopoietic stem cells and a significantly decreased percentage of bone marrow lymphoid progenitors. Increased reactive oxygen species and markers of oxidative stress were detected in hematopoietic stem cell populations and were associated with a loss of quiescence. Bone marrow progenitor populations expressed diminished levels of the IL-7Rα chain, which was associated with decreased proliferation and increased apoptosis. Modulating oxidative stress in vitro suggested that oxidative stress selectively leads to decreased IL-7Rα expression, and inhibits the survival of IL-7Rα-expressing hematopoietic progenitors, potentially linking increased reactive oxygen species and immunopathology.
Innovation
The study results identify a link between oxidative stress and diminished IL-7Rα expression and function. Further, the data suggest that this decrease in IL-7Rα is associated with defective hematopoietic development in Down Syndrome.
Conclusion
The data suggest that hematopoietic stem and lymphoid progenitor cell defects underlie immune dysfunction in DS and that increased oxidative stress and reduced cytokine signaling may alter hematologic development in Ts65Dn mice. Antioxid. Redox Signal. 15, 2083–2094.
Introduction
Down Syndrome (DS) is a common genetic disease occurring at a frequency of ∼1 in 750 live births. It is caused by a trisomy of chromosome 21, with a triplication of the entire chromosome occurring in greater than 95% of patients with DS. In addition to the recognized impairment of cognitive ability, DS is characterized by numerous hematologic deficiencies such as transient myeloproliferative disease, premature thymic involution, defects in mature lymphocyte composition and function, as well as increased susceptibility to autoimmune disease, infections, and leukemia. Significantly, infections and leukemia are major causes of morbidity and mortality (20). These observations suggest the existence of intrinsic immune defects in DS. Several studies have examined various triplicated genes that may be responsible for leukemogenesis in DS (28, 35). However, the cell physiological and molecular basis underlying the immune deficiency observed in DS has not been thoroughly explored.
Innovation.
Defective hematopoiesis has been reported in Down Syndrome (DS), including inappropriate expansion of myeloid cells and premature aging of lymphoid cells. In addition, patients with DS display impaired adaptive immune responses but the underlying mechanisms for these changes in immune function and development have not been identified. This report directly shows increased oxidative stress in bone marrow and thymic progenitors and diminished expression of IL-7Rα, a requisite cytokine receptor for lymphoid development. On the basis of in vitro data, the two observations are linked in that pro-oxidant conditions led to decreased IL-7Rα surface expression in bone marrow cells, whereas antioxidant treatment enhanced receptor levels. Thus, in IL-7Rα, this study has identified a novel potential therapeutic target in DS and a redox-sensitive modulator of immune response.
Oxidative stress has been linked to the pathology of DS. This is due to the presence of elevated levels of biomarkers of oxidative stress as well as oxidative stress response genes in both fetal and adult tissue (42). Further, DS is associated with the premature onset of degenerative diseases linked to reactive oxygen species (ROS) generation such as cataracts, autoimmune disease, and the development of Alzheimer-like symptoms in the brain. These observations have also led to the hypothesis that the pathology of DS is associated with premature aging (15). Increased levels of oxidants have been shown to impair hematopoietic stem cell (HSC) function and have also been proposed to mediate age-related loss of stem cell function (14, 45). However, the role of oxidative stress in HSC and lymphoid progenitor homeostasis and function has not yet been studied extensively in DS.
The Ts65Dn mouse is the most commonly used model of DS. It is trisomic for the distal end of mouse chromosome 16, which contains 104 genes conserved between mice and humans and is partially syntenic to human chromosome 21. Similar to the human disease, Ts65Dn mice exhibit craniofacial defects and learning and behavioral deficiencies (31, 33). Ts65Dn mice also exhibit hematologic abnormalities such as a progressive myeloproliferative disease, where bone marrow (BM) myeloid progenitor subsets are altered in comparison to euploid controls (18). Further, Ts65Dn mice exhibit premature thymic involution, and elevated levels of ROS have been found in the thymus in response to treatment with pro-apoptotic agents, indicating possible defects in immature T-cell populations (33). Thus, the goal of the current study was to further investigate hematopoietic progenitor dysfunction with a focus upon lymphoid development and the potential role of oxidative stress in Ts65Dn mice.
Materials and Methods
Antibodies
The following antibodies were used in flow cytometry: CD4 biotin (GK1.5), CD5 biotin (Ly-1), CD8α biotin (53-6.7), CD11b biotin (M1/70), TER-119 biotin, and CD135 PE (A2F10.1) were purchased from BD Pharmingen. CD150 PE (TC15-12F12.2) was purchased from BioLegend. All other antibodies were purchased from eBioscience: CD3ɛ biotin (145-2C11), CD48 FITC (HM 48.1), c-kit/CD117 APC-Cy7 (2B8), CD11c biotin (N418), CD19 biotin (1D3), B220 biotin (RA3-6B2), Gr-1 biotin (RB6-8C5), NK1.1 biotin (PK136), CD127 Alexa Fluor 647/PE (A7R34), Streptavidin efluor 450, Sca-1 PE-Cy7/FITC (D7), CD16/32 PE-Cy7 (93), and CD34 PE (RAM34).
Mice
Ts65Dn mice and euploid littermates were purchased from the Jackson Laboratory. Animal care was provided in accordance with Institutional Animal Care and Use Committee procedures approved at the University of Maryland, Baltimore, or at The Jackson Laboratory.
Flow cytometric analysis of hematopoietic progenitor phenotype
BM cells (BMC) were isolated and immediately surface stained after ACK lysis of red blood cells. Cells were gated based upon forward and side scatter profiles, live/dead discrimination with 7-Amino-Actinomycin D (7-AAD), and staining with a pool of antibodies recognizing lineage (Lin) markers. Long-term reconstituting HSCs were defined as Lineage (Lin)− Sca-1+, c-Kit+(LSK), CD48−, CD150+ (16). Multipotent progenitors (MPPs) were defined as LSK, CD48−, CD150− (16). Common lymphoid progenitors (CLPs) were defined as Lin− Sca-1lo, c-Kitlo, IL-7Rα+, Flk-2/Flt-3+ (19). Common myeloid progenitors were defined as Lin− Sca-1−, c-Kit+, IL-7ra−, FcγRlo, CD34+; granulocyte/monocyte progenitors (GMP) were defined as Lin− Sca-1−, c-Kit+, IL-7Rα−, FcγRhi, CD34+. Megakaryocyte/Erythroid Progenitors were defined as Lin− Sca-1−, c-Kit+, IL-7ra−, FcγRlo, CD34−(1): The lineage mix for long-term reconstituting-HSCs, CLPs, and common myeloid progenitors contained antibodies to B220, CD3e, CD4, CD5, CD8, CD11b, CD19, Gr-1, NK1.1, and TER-119. MPPs are Mac-1(lo) and CD4 (lo); therefore, these antibodies were excluded for MPP analysis (26). Compensation settings and lineage gates were based upon single color controls. Analysis was performed with FlowJo (Tree Star, Inc.).
The expression of Ki-67 was detected in BM subsets by intracellular staining as indicated by the supplier, using FITC anti-Ki-67 (BD Biosciences). 3-Nitrotyrosine (3-NT) levels in progenitor subsets were determined through flow cytometry by intracellular staining using a monoclonal FITC anti-NT (Millipore: clone 1A6) antibody. Cells were fixed in 4% paraformaldehyde, permeabilized with the Foxp3 Staining Buffer Set (Ebioscience), and incubated with 2.5 μg/106 cells of antibody for 1 h at room temperature. Protein carbonyls (PC) in progenitor subsets were determined through flow cytometry by intracellular staining. Cells were fixed and permeabilized as for 3-NT staining. PC groups were then derivatized with 10 mM 2,4-dinitrophenylhydrazine in 2.5 M HCl for 45 min at room temperature. After four washes, cells were incubated with 5 μg/106 cells anti-DNP rabbit IgG (Invitrogen A6430) for 1 h, washed with FACS buffer, and then incubated with secondary 0.04 μg/106 cells PE-Cy7 anti-rabbit IgG (Santa Cruz Biotechnology sc-3845) antibody for 20 min. During incubation with the secondary antibody, CD135 PE was added to the cells; PE and PE-Cy7 staining is not compatible with 2,4-dinitrophenylhydrazine treatment. Phosphatidylserine externalization was detected in defined subsets using FITC Annexin V (BD Biosciences). After surface staining, cells were washed in binding buffer (100 mM HEPES pH 7.4, 1.5 M NaCl, 50 mM KCl, 10 mM MgCl2, 18 mM CaCl2), and cells were incubated with Annexin V (1 μl/1×106 cells) for 15 min at room temperature. 7-AAD Viability Staining Solution (eBioscience) was added and cells were immediately analyzed by flow cytometry.
Competitive BM reconstitution
Competitive BM reconstitution of lethally irradiated recipients was performed at The Jackson Laboratory essentially as described (9, 12). Ts65Dn and control donors were males (3–4 months of age) and only those that were H-2b/k were used as donors. Both recipients and competitors were B6C3F1 females, and 2.5- to 3-month-old recipients were lethally irradiated (1100R 137Cs gamma irradiation) before marrow transplants. For competitive repopulation, each recipient received 1 million donor and 1 million competitor marrow cells. Repopulating units (RU) of donor cells were calculated distinguishing donor cells using qPCR for the Y chromosome DNA in total blood lymphocytes separated by density after 3 and 9 months. The data are expressed in RU, where each RU is the repopulating ability of 100,000 fresh competitor marrow cells. For example, in recipients of 1 million donor and 1 million competitor marrow cells, the expected value is 10 for control donors.
The proportion of male-derived donor cells in the blood of recipient mice was determined by measuring the relative concentration of a gene, Sry, which is on chromosome Y (30). The Apob gene, which is on chromosome 12, was used as the control. Briefly, DNA was extracted from nucleated cells after density gradient separation of whole blood. Real-time SYBR Green PCR analysis was performed and the average of three assays were used to calculate the relative concentration of Sry versus Apob, by the formula: 2Ct Apob−Ct Sry. Primer sequences used were as follows: Sry forward: 5′-AGCTGGGATGCAGG TGGAA-3′, Sry reverse: 5′-TGAGGCAACTGCAGGCTGT-3′; for internal control, Apob forward: 5′-AGAAGCTGGACAT GTGGCATT-3′, Apob reverse: 5′-TGACCCGTAAGTGTTTG CCAT-3′. For each analysis, male and female DNA samples were mixed proportionally to make a serial of mixtures that contained 0%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, and 100% male DNA. A standard curve was generated by the real-time PCR assays for the serial mixtures and the proportion of male-derived cells (donor) in female recipients was determined.
Colony forming assays
BMC were isolated from Ts65Dn and euploid mice and were placed at a concentration of 2×105 cells/ml in Methocult MC3534 (StemCell Technologies) methylcellulose media containing SCF, IL-3, and IL-6 and colonies were enumerated after 12 days for myeloid colony-forming assays. For lymphoid colony-forming assays, 10×106 cells/ml were incubated in Methocult MC3630 (StemCell Technologies) methylcellulose media containing IL-7 and colonies were enumerated after 7 days.
In vitro culture of BM progenitors
Lineage-negative [Lin(−)] cells from mouse BM were enriched by magnetic bead negative selection (EasySep; StemCell Technologies) and cultured under conditions that favor lymphoid cell development (6). In separate experiments, enriched Lin(−) BMC were FACS-sorted to produce Lin(−), IL-7Rα-positive and Lin(−) IL-7Rα-negative populations before culture. Pro- or anti-oxidant conditions were established by culturing cells in the presence of buthionine sulfoximine (BSO; 1 and 0.1 mM) or N-acetylcysteine (NAC; 1 and 0.1 mM). Cells were harvested at different time points and survival/expansion of cell subpopulations and cytokine receptor expression were evaluated by flow cytometry as above.
Detection of ROS generation
Intracellular ROS was analyzed by using the oxidation sensitive dye 2′-7′-dichlorofluorescin diacetate (DCFDA). BMC were incubated with 2 μM DCFDA at 37°C for 15 min, washed, and surface stained. As a loading control, parallel samples of BMC were incubated with the oxidized control dye FDA (0.01 μM) at 37°C for 15 min, washed, and surface stained. FACS analysis was performed immediately. DCFDA mean channel fluorescence was normalized to FDA uptake, and the data are shown as the percent increase in DCFDA fluorescence in cells from Ts65Dn mice over euploid controls±SEM.
Measurement of glutathione
Intracellular glutathione (GSH) levels were measured in progenitor subsets by flow cytometry using monochlorobimane (MCB) essentially as previously described (32). Briefly, BMC were surface stained as described above and then incubated for 10 min at room temperature with 20 μM MCB. Cells were washed and analyzed immediately by flow cytometry. The ratio of oxidized (GSSG) and reduced (GSH) glutathione in Ts65Dn mice was determined spectrophotometrically in whole blood using the Bioxytech kit (Oxis Research, 21040) as described by the manufacturer.
Statistical analysis
Statistical significance was analyzed using Student's t-test or Wilcoxon signed rank test. Conditions were deemed significantly different if p<0.05.
Results
Hematopoietic stem and progenitor populations are altered in Ts65Dn BM
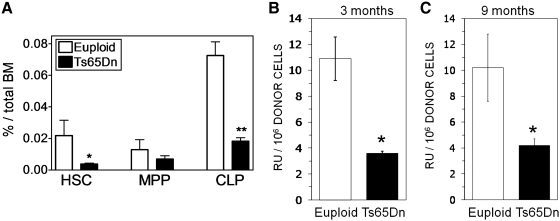
Previous studies have shown that Ts65Dn mice develop a progressive myeloproliferative disease (18). To determine if hematologic abnormalities extend to the lymphoid progenitor and more immature HSC populations, analysis of hematopoietic progenitor phenotype in the BM of Ts65Dn and euploid mice was performed by flow cytometry. No significant difference in total BM cellularity was found (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/ars). Consistent with a previous report (18), there was an expansion of the LSK subset, which contains hematopoietic progenitors and all stem cell activity (43) (Supplementary Fig. S1B). In spite of the expanded LSK population, Ts65Dn mice have an approximately threefold decrease in the percentage of HSCs and CLP cells in their BM in comparison to euploid controls (Fig. 1A). MPP populations were not significantly different. Ts65Dn mice also have an increased percentage and number of GMPs but a decreased percentage and number of megakaryocyte-erythroid progenitors as previously described (Supplementary Fig. S2A, B).4
FIG. 1.
Hematopoietic progenitor populations in the bone marrow of Ts65Dn and euploid mice. (A) Hematopoietic progenitor populations as a percentage of nucleated BMC from euploid (open bars) or Ts65Dn (closed bars) mice. Hematopoietic progenitors [long-term reconstituting hematopoietic stem cell (HSC), multipotent progenitor (MPP), and common lymphoid progenitor (CLP) populations] were assessed ex vivo by flow cytometry as defined and described in the Materials and Methods section (n=6, *p<0.05; **p<0.005). (B, C) Competitive repopulation assay was performed as described in the Materials and Methods section with 1:1 mixture of BMC from Ts65Dn mice (closed bars) or euploid littermates (open bars) mixed with an equal number of wild-type BM. BMC repopulating ability was assessed at (B) 3 months and (C) 9 months and is expressed as repopulating units per million BMC. By definition 105 competitor BMC produce 1 repopulating unit (n=4, *p<0.05). BMC, bone marrow cells.
To assess HSC function in Ts65Dn BM, a competitive reconstitution assay was performed with a 1:1 mixture of 1×106 BMC from male Ts65Dn mice or euploid littermates mixed with an equal number of wild-type female BMC (Fig. 1B, C). Donor contribution to hematopoietic cell generation was determined by measuring Y chromosome content of blood lymphocytes after 3 (Fig. 1B) and 9 (Fig. 1C) months, and RU values were calculated for both the Ts65Dn and euploid mice (12). Ts65Dn mice exhibited an approximately threefold decrease in the number of RUs in comparison to euploid mice in the reconstitution assays. This is consistent with the observed threefold decrease in HSC number determined by flow cytometry.
In vitro colony-forming assays were also performed to determine the number of hematopoietic progenitors in Ts65Dn BM. Ts65Dn BMC were cultured in methylcellulose media containing myeloid-promoting (Methocult M3534: SCF, IL-3, IL-6) cytokines. Ts65Dn BMC formed more granulocyte, granulocyte-monocyte, and total myeloid colonies in comparison to euploid mice (Supplementary Fig. S2C). These results correlate with the increased number and frequency of GMPs as determined by flow cytometry.
Hematopoietic stem and progenitor populations in Ts65Dn BM exhibit increased oxidative stress and proliferative defects
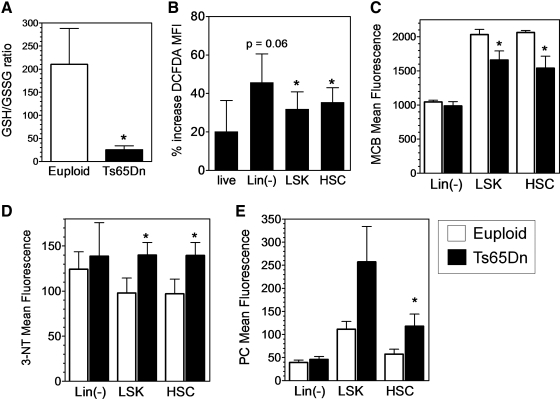
The decreases in hematopoietic stem and progenitor number and function suggested that there are defects in these populations in Ts65Dn mice. Increased ROS levels may contribute to these defects, since oxidative stress has been linked to the pathology of DS (15, 42). Consistent with increased oxidative stress in DS, we found a significantly decreased GSH/GSSG ratio in the blood of Ts65Dn mice (Fig. 2A). To determine if this oxidative stress was also present in BM progenitors, ROS levels were measured in Ts65Dn BMC using the oxidation sensitive dye DCFDA. Intriguingly, the (Lin-) subset that contain hematopoietic progenitors, the LSK subset, and HSC (LSK, Flk2−) populations of Ts65Dn mice all had elevated levels of ROS in comparison to euploid mice, (Fig. 2B) indicating the presence of oxidative stress in these populations. To confirm the presence of oxidative stress, stable markers of ROS exposure were also measured in BM populations. Intracellular GSH, measured with MCB, was significantly decreased in both LSK and HSC populations from Ts65Dn mice (Fig. 2C). Similarly, formation of nitrated proteins (NT–3-NT) and PC as stable markers of protein oxidation was also increased in both the LSK and HSC populations (Fig. 2D, E).
FIG. 2.
Ts65Dn BMC exhibit signs of oxidative stress. (A) Decreased GSH/GSSG ratio in blood cells from Ts65Dn mice. GSH and GSSG were measured spectrophotometrically in fresh blood samples from euploid and Ts65Dn mice as described in the Materials and Methods section (n=5, *p<0.05). (B) 2′-7′-dichlorofluorescin diacetate oxidation was assessed in the indicated bone marrow subpopulations by flow cytometry. These data are expressed as the percent increase in 2′-7′-dichlorofluorescin diacetate fluorescence normalized to uptake of fluorescein diacetate (n=4, *p<0.05). (C) Intracellular GSH levels were measured in the indicated bone marrow subsets from euploid (open bars) and Ts65Dn (closed bars) mice using monochlorobimane as described in the Materials and Methods section (n=5, *p<0.05). (D) Formation of 3-nitrotyrosine (3-NT) was detected in the indicated bone marrow subsets from euploid (open bars) or Ts65Dn (closed bars) mice by intracellular staining and FACS analysis as described in the Materials and Methods section (n=5, *p<0.05). (E) Formation of protein carbonyls (PC) were detected in the indicated bone marrow subsets from euploid (open bars) or Ts65Dn (closed bars) mice by intracellular staining and FACS analysis as described in the Materials and Methods section (n=5, *p<0.05). GSH, glutathione; GSSG, oxidized glutathione.
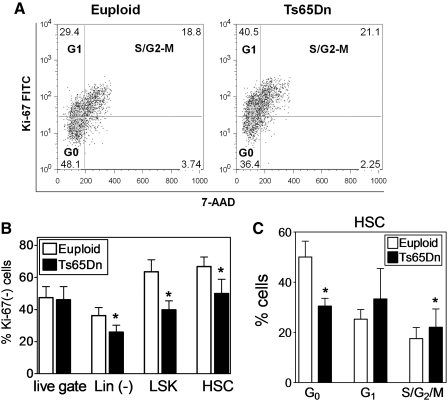
Because increased oxidative stress has been linked to decreased stem cell quiescence (4), the cell cycle status of Ts65Dn BMC was examined by detecting the expression of Ki-67, a protein that is expressed when cells are actively cycling (37). Ts65Dn progenitor cells exhibited a trend toward increased cell cycling, with a lower percentage of quiescent cells lacking Ki-67 expression (Fig. 3B). Double staining with anti-Ki-67 and the DNA binding dye 7-AAD indicated that commensurate with the loss of G0, Ki-67-negative cells in Ts65Dn HSC, there was a concurrent increase in the percentage of cells in G1 and S-G2/M phases of the cell cycle (Fig. 3C). Cell cycle analysis of the hematopoietic progenitor LSK population showed a similar pattern (data not shown). Thus, increased oxidative stress in Ts65Dn BMC is associated with loss of quiescence and stem cell function.
FIG. 3.
Ts65Dn BMC exhibit signs of loss of quiescence. (A) Representative FACS plots of HSC cell cycle analysis using Ki-67 and 7-AAD in Euploid and Ts65Dn mice. (B) Intracellular staining for the Ki-67 antigen was analyzed by flow cytometry as described in the Materials and Methods section. The percentage of cells that are Ki-67(−) were analyzed in total BMC (live gate), lineage-negative (Lin(−)), (Lin−), Sca-1+, c-Kit+ (LSK) population, and the Lin(−), LSK Flk2- (HSC) population. (n=5, *p<0.05). (C) HSC cell cycle analysis by analysis of Ki-67 expression and 7-AAD incorporation showing the percentage of cells in G0, G1, and G2+M phases. (n=4, *p<0.05). 7-AAD, 7-Amino-Actinomycin D.
Expression of the IL-7Rα chain is decreased in Ts65Dn hematopoietic progenitors
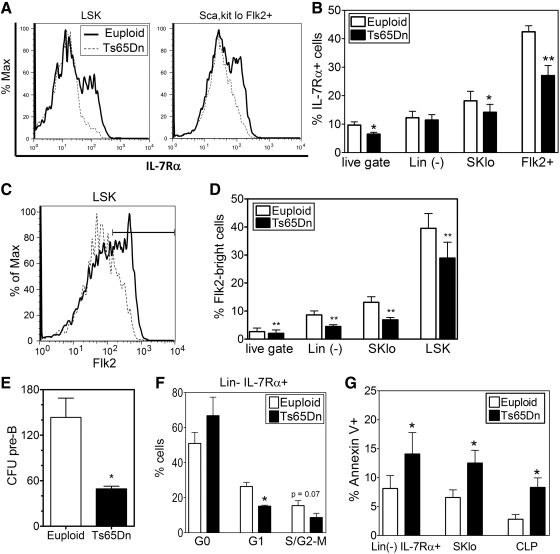
A possible mechanism for changes in lymphoid progenitors is the IL-7/IL-7Rα receptor system, which plays an essential role in lymphoid development and homeostasis by promoting proliferation and inhibiting apoptosis (10, 24). Analysis of Ts65Dn BM showed that the percentage and absolute number of cells expressing the IL-7Rα chain was decreased in total BM, the Lin-, Sca-1/c-kitlo and Lin-, Sca-1/c-kitlo, Flk2+ populations, which encompass the CLP subset, defined as Lin-, Sca-1/c-kitlo, Flk2+ IL-7Rα+ (Fig. 4A, B). The CLP gives rise to all the cells of the lymphoid lineage and may also be able to seed the thymus (39). Previous studies have suggested that a subset in the MPP population described as the lymphoid-primed MPP (LMPP) and defined as LSK, Flk2hi can also seed the thymus (41). Flk2 expression was found to be decreased in BMC from Ts65Dn mice; as a result, there was a significant decrease in the LMPP population (Fig. 4C, D).
FIG. 4.
Downregulation of IL-7Rα and Flk2 expression and function in the bone marrow of Ts65Dn mice. (A, B) IL-7Rα expression in selected bone marrow sub-populations was assessed by flow cytometry. (A) Representative FACS plots of IL-7Rα expression in LSK or the Lin(−), SKlo, Flk2+ populations from euploid (solid line) or Ts65Dn (dashed lines) mice. (B) Analysis of the percent cells that are IL-7Rα+ in total BMC (live gate), Lin(−), Lin(−), Sca-1lo,c-Kitlo (SKlo) population, and the Lin(−), SKlo, Flk2+ (Flk2+) population. (n=6, *p<0.05, **p<0.01). (C, D) Flk2 expression in selected bone marrow sub-populations was assessed by flow cytometry. (C) Representative FACS plots of Flk2 expression in LSK cells from euploid (solid line) or Ts65Dn (dashed lines) mice. Marker indicates Flk2-bright population. (D) Analysis of the percent cells that are Flk2+ in total BMC (live gate), Lin(−), Lin(−), Sca-1lo,c-Kitlo (SKlo) population, and the LSK population (n=6, *p<0.05, **p<0.01). (E) Colony formation of Ts65Dn and euploid pre-B cells. Total BMC were cultured in methylcellulose media containing IL-7 and enumerated after 7 days. CFU pre-B: pre-B cell colony forming unit (n=4, *p<0.05). (F) Cell cycle analysis by Ki-67 expression and 7-AAD incorporation of Lin(−) IL-7Rα+ (Lin- IL-7Rα+) lymphoid progenitor cells from Ts65Dn mice (closed bars) or euploid littermates (open bars) (n=4, *p<0.05). (G) Apoptotic cells were detected by AnnexinV binding in selected bone marrow sub-populations from Ts65Dn mice (closed bars) or euploid littermates (open bars). These data are expressed as the percentage of cells in each population that are AnnexinV+ (n=6, *p<0.05).
To determine the effects of IL-7Rα downregulation in the BM, IL-7Rα responsive cells in the BM were measured using a pre-B cell colony-forming assay. Ts65Dn and euploid BMC were cultured in methylcellulose media containing IL-7 (Methocult MC3630), and colonies were enumerated after 1 week. Interestingly, Ts65Dn BMC formed one-third fewer colonies in comparison to euploid BMC, indicating the presence of fewer IL-7 responsive hematopoietic progenitors or decreased responsiveness to IL-7 (Fig. 4E).
IL-7 signaling induces proliferative and pro-survival signals in cells (24), leading to the activation of the PI3K pathway and expression of anti-apoptotic Bcl-2 family members such as Mcl-1 (23). Cell cycle analysis of the Lin-, IL-7Rα+ lymphoid progenitor population indicated that there was an increase in the percentage of non-proliferating G0, Ki-67-negative cells, with a concurrent decrease in the percentage of proliferating cells in the G1 and S-G2/M phases of the cell cycle (Fig. 4F), consistent with proliferative defects in this population. Concordant with a decrease of pro-survival signals, these same BM subsets, as well as the CLP population display increased apoptosis, as measured by AnnexinV staining (Fig. 4G).
Effects of redox balance on IL-7Rα expression
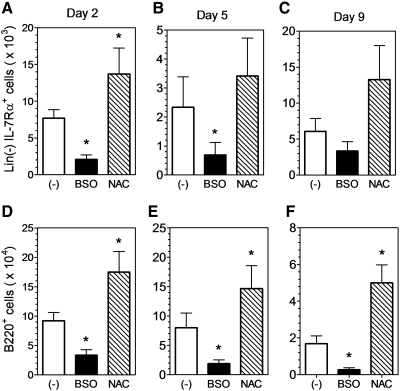
To explore the potential relationship between oxidative stress and IL-7Rα expression in hematopoietic progenitors, Lin(−) BMC from wild-type mice were cultured under lymphoid promoting conditions (6) in the presence of the antioxidant NAC or the pro-oxidant BSO. After 2 days in culture, there were significantly fewer IL-7Rα+ cells in BSO-treated cultures but significantly more IL-7Rα+ cells in NAC-treated cultures in comparison to untreated cells (Fig. 5A). A similar trend was observed after 5 and 9 days in culture (Fig. 5B, C). The same changes were also observed when the data were expressed as the percentage of Lin(−) cells (not shown). The lymphoid promoting culture conditions lead to B cell development in vitro (6), and on days 2, 5, and 9, there were significantly more B220+ cells in the NAC-treated cultures and significantly fewer B220+ cells in BSO-treated cultures in comparison to untreated cells (Fig. 5D–F). Thus, the data suggest that pro-oxidant conditions diminish IL-7Rα expression and/or function and inhibit in vitro lymphoid development in hematopoietic progenitor cells.
FIG. 5.
Redox balance affects IL-7Rα expression and B-cell generation in vitro. IL-7Rα (A–C) and B220 (D–F) expression was assessed by flow cytometry in lymphoid progenitor cultures. Lineage-depleted hematopoietic progenitors were cultured in vitro in lymphoid-promoting media as described in the Materials and Methods section. The pro-oxidant BSO (1 mM) and antioxidant NAC (1 mM) were added to selected cultures. IL-7Rα expression was assessed in Lin(−) cells (Lin(−) IL-7Rα+) on day 2 (A), day 5 (B), and day 9 (C). B220 expression was assessed on day 2 (D), day 5 (E), and day 9 (F). The data are expressed as the number of cells in each sub-population (n=3, *p<0.05). BSO, buthionine sulfoximine; NAC, acetylcysteine.
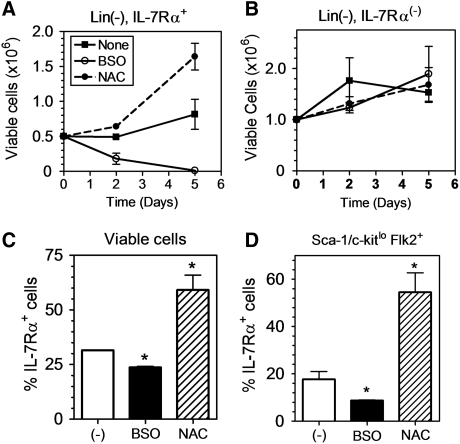
To definitively measure redox-dependent changes in IL-7Rα expression and function in hematopoietic progenitors, BMC were sorted into Lin(−), IL-7Rα+ and Lin(−), IL-7Rα(−) populations before initiation of culture under lymphoid promoting conditions. In the absence of any treatment, in vitro culture induced a slight expansion of the Lin(−), IL-7Rα+ cells after 5 days. This expansion was markedly enhanced in the presence of NAC, whereas the total number of cells was decreased in the presence of BSO (Fig. 6A). This effect was selective for the IL-7Rα+ cells; however, as there was no effect of NAC or BSO on the number of the Lin(−), IL-7Rα(−) sorted cells during culture (Fig. 6B). Furthermore, the in vitro culture conditions led to a rapid loss of IL-7Rα expression in the sorted cells as measured by the percent IL-7Rα+ cells after 2 days in culture. This was accentuated by exposure to BSO, whereas cells treated with NAC retained IL-7Rα expression (Fig. 6C). This effect was especially marked in the Sca-1/c-kitlo, Flk2+ cells, representing the pool of cells defining the CLP population, which are dependent upon the pro-survival effects of IL-7Rα signaling (Fig. 6D). The effects of BSO did not downregulate cell surface expression of all cytokine receptors, as the percent of cells expressing c-kit (receptor for Stem Cell Factor (SCF) or Flk2 (receptor for Flt3-ligand) were not diminished and were even slightly upregulated (Supplementary Fig. S3). Therefore, the data suggest that increased oxidative stress selectively promotes a loss of IL-7Rα expression and decreased survival of IL-7Rα-expressing hematopoietic progenitor and lymphoid cells.
FIG. 6.
Pro/anti-oxidant treatment specifically affects the viability of IL-7Rα-expressing cells. FACS-sorted Lin(−), IL-7Rα+ (A) and Lin(−), IL-7Rα (−) (B) hematopoietic progenitors were cultured in vitro in lymphoid-promoting media. The pro-oxidant BSO (0.1 mM) and antioxidant NAC (1 mM) were added to selected cultures and the number of viable cells in each culture condition was assessed using 7-AAD exclusion by flow cytometry on days 2 and 5 (n=2, *p<0.05). (C, D) The percentage of IL-7Rα+ cells was assessed in FACS-sorted Lin(−), IL-7Rα+ hematopoietic progenitors in the total viable (C) and Sca-1lo c-Kitlo Flk2+ (D) sub-populations by flow cytometry (n=3, *p<0.05).
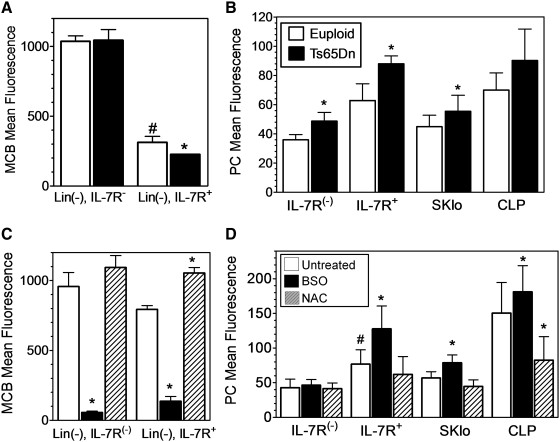
The selective effect of BSO treatment on the viability of IL-7Rα+ cells suggested an enhanced sensitivity to the drug. Consistent with this observation, ex vivo intracellular GSH levels in Lin(−), IL-7Rα+ BMC were more than threefold lower than the Lin(−) IL-7Rα negative cells (Fig. 7A). Similar to the more immature HSC and LSK populations, the IL-7Rα+ cells in the BM also displayed increased markers of oxidative stress ex vivo. Thus, decreased intracellular GSH levels (Fig. 7A) and increased PC formation (Fig. 7B) were also found in the Lin- IL-7Rα+, Sca-1/c-kitlo, and CLP populations of Ts65Dn mice as compared to euploid controls.
FIG. 7.
Oxidative stress in IL-7Rα+ BMC both ex vivo and in vitro. (A, B) BMC from euploid (open bars) and Ts65Dn (closed bars) mice were analyzed ex vivo for (A) intracellular GSH in Lin(−), IL-7Rα(−) or Lin(−), IL-7Rα+ cells or (B) PCs in Lin(−), IL-7Rα(−) (IL-7Rα(−)), Lin(−), IL-7Rα+ (IL-7Rα+), Lin(−), Sca-1lo,c-Kitlo (SKlo), and the Lin(−), SKlo, Flk2+ IL-7Rα+ (CLP) populations as in Figure 2. (C, D) BMC from wild-type mice were cultured in vitro in lymphoid-promoting media in the absence of any addition (open bars), or in the presence of 0.1 mM BSO (solid bars) or 1 mM NAC (hatched bars) for 2 days as in Figure 5. Cells were analyzed for (C) intracellular GSH or (D) PCs in the indicated subsets as defined in A & B (n=5, *significantly different from euploid or untreated controls, p<0.05; #significantly different from IL-7R(−) samples, p<0.05).
GSH levels and PC formation were also assessed during the in vitro culture in lymphoid-promoting conditions. As expected, intracellular GSH levels were depleted in all cells treated with BSO (Fig. 7C), whereas NAC treatment increased intracellular GSH levels significantly only in Lin(−) IL-7Rα+ cells, and not Lin(−) IL-7Rα(−) cells. Remarkably, BSO treatment induced increased PC formation in the Lin- IL-7Rα+, Sca-1/c-kitlo, Lin-, and CLP, but not the Lin(−) IL-7Rα(-) cells (Fig. 7D). Similarly, NAC treatment diminished increased PC formation in the Lin- IL-7Rα+, Sca-1/c-kitlo, Lin-, and CLP, but not the Lin- IL-7Rα- cells (Fig. 7D). Thus, manipulation of GSH/redox balance seemed to have functional consequences selectively in the IL-7Ra+ populations both in vitro and in vivo.
Discussion
The increased risk of developing hematologic disorders such as infections and leukemia has been implicated as a significant cause of morbidity and mortality in DS (7, 11). However, the potential mechanisms underlying the occurrence of these immune defects have not yet been examined in detail. A previous study has reported myelodysplasia and suggested potential HSC abnormalities in Ts65Dn mice (18). However, HSC frequency, HSC function, and possible defects in lymphoid development were not examined. Therefore, in this study, the composition and function of hematopoietic stem and progenitor cells of the Ts65Dn mouse model of DS were explored, with a focus on cells of the lymphoid lineage. Overall, the results suggest defects in hematopoietic progenitor development and function at the level of both HSC and lymphoid progenitors in Ts65Dn mice, with oxidative stress and decreased IL-7Rα expression as possible causes of the changes in these populations.
Phenotypic analysis of HSC by flow cytometry using the well-characterized SLAM markers (16, 46) revealed an approximately threefold decrease in the frequency and absolute number of HSC in Ts65Dn BM in comparison to euploid BM. Functional analysis of HSC by competitive reconstitution assays also showed a threefold decrease in Ts65Dn HSC activity in the BM. Overall, these results demonstrate a decrease in the number of functional HSC in Ts65Dn BM. A previous study also suggested weaker long-term repopulating ability in Ts65Dn BM (18), although the study transferred Ts65Dn BMC into C57Bl/6 recipients, whereas the current study used competitive reconstitution into B6C3F1 recipients to minimize immune rejection.
Potential mechanisms for the defects observed in HSC activity may be the elevated levels of ROS oxidative damage, and loss of quiescence measured in these cells. HSCs are hypothesized to reside in a niche characterized by low levels of O2, and are therefore thought to be especially prone to damage by ROS (29). Significantly, our data show that HSC and LSK cells, which encompass the HSC population, have higher intracellular GSH levels in comparison to more mature hematopoietic progenitor populations. This may be indicative of enhanced antioxidant defenses in HSC to prevent damage from ROS. Previous reports have linked the loss of quiescence in HSC to elevated levels of ROS, which were proposed to induce altered expression of cell cycle regulators such as p53, p16Ink4a, and p19ARF, thus causing aberrant cell cycle progression (3). HSC quiescence has been shown to be required for HSC function, therefore, the oxidative stress in the HSCs of Ts65Dn mice may be inducing a loss of quiescence, resulting in decreased function as measured by repopulating activity (5, 13, 14, 45). Previous reports in Fancc-deficient mice also link oxidative stress with an expansion of the LSK population and loss of self-renewal capacity (38). Therefore, in both Ts65Dn and Fancc-deficient mice, expansion of the LSK population may be indicative of oxidative stress inducing HSC proliferation and differentiation, resulting in decreased self-renewal ability and exhaustion of the functional HSC population.
The threefold decrease in both the frequency and number of CLP cells in the BM of Ts65Dn mice in comparison to euploid mice may simply be a reflection of the decrease in total HSC number and activity. However, the direct precursor of the CLP, the MPP population, is not significantly altered; therefore, it is likely that additional factors or mechanisms may be involved. Significantly, there was a general decrease in IL-7Rα expression in the BM of Ts65Dn mice and this decrease in IL-7Rα expression contributes to the observed decrease in CLP number and frequency. We hypothesize that redox-dependent changes in the IL-7/IL-7Rα receptor system provide a possible link between the global changes in lymphoid progenitor number and composition, because IL-7 signaling plays an essential role in lymphoid development and homeostasis by promoting proliferation and inhibiting apoptosis (10, 24).
The CLP has been hypothesized to be the initial BM-derived thymus-seeding cell, although this is not without controversy (2, 40). The LMPP has also been proposed as a BM-derived population that has the ability to seed the thymus (41). The data show that similar to the CLP population, the LMPP population was also markedly reduced in Ts65Dn mice, suggesting a general decrease in BM-derived T-cell progenitors that have the capability to seed the thymus in Ts65Dn mice. This may correlate with the enhanced thymic involution observed in Ts65Dn mice. Decreased Flk2 expression in this population may also lead to diminished IL-7Rα levels, since a recent report suggested that Flk2 signaling enhances IL-7Rα levels and its signaling capacity (36). The decrease in pre B-cell colony formation of Ts65Dn BM in vitro was consistent with fewer IL-7 responsive cells or decreased responsiveness to IL-7. Fewer IL-7Rα-expressing cells in the BM of Ts65Dn mice as well as decreased cell proliferation and increased apoptosis in BM progenitors that express IL-7Rα suggest that IL-7 responsiveness is clearly diminished in Ts65Dn mice. Thus, the observed reduction in the CLP population of Ts65Dn mice may be linked to the downregulation of IL-7Rα expression and decreased proliferative and pro-survival signals resulting from diminished IL-7 and Flk2 cytokine receptor signaling.
The current results suggest that increased oxidative stress may be a potential mechanism that downregulates IL-7Rα expression and function in hematopoietic progenitors from Ts65Dn mice. In vitro culture of BM progenitors under relatively hyperoxic conditions (atmospheric oxygen) served to diminish IL-7Rα expression and further oxidative stress caused by exposure to BSO exacerbated this effect. Importantly, treatment with the antioxidant NAC prevented IL-7Rα downregulation, and in the presence of IL-7 in the medium, increased survival, proliferation, and differentiation of IL-7Rα+ cells but not IL-7Rα− cells. Additionally, NAC treatment decreased PC formation in IL-7Rα+, and not IL-7Rα− progenitor cells. Because the effects of NAC and BSO seemed to be selective for IL-7Rα+-progenitor cells as opposed to cells lacking the receptor, the data suggest that oxidative stress can modulate hematopoietic development by inhibiting IL-7/IL-7Rα signaling. NAC and BSO both function in modulating the GSH pathway; therefore, it will be interesting to determine whether altering other redox pathways will have a similar effect. The selective effect of NAC and BSO treatment on IL-7Rα+-progenitor cells also suggest that IL-7Rα+ lymphoid cells may be more sensitive to changes in redox balance in comparison to IL-7Rα− myeloid cells.
Modulation of IL-7Rα expression has also been observed during aging, another syndrome associated with oxidative stress. Older individuals were found to have decreased IL-7Rα expression in the EMCD45RA+ CD8+ T-cell population in comparison to younger individuals, leading to defects in survival and signaling in response to IL-7 (17). Significantly, similar to HSC in Ts65Dn mice, HSC from aged mice exhibit cell-intrinsic defects in repopulating activity, and do not effectively generate lymphoid cells (34). This suggests that young adult Ts65Dn HSC may be functionally similar to aged HSC. In aged mice, however, the defect does not appear to be a result of diminished IL-7Rα expression in either T or B cell progenitors. CLP numbers and B cell development decline in aged mice and culture of CLP with IL-7 in vitro resulted in decreased formation of B220+ cells (25). Another study suggested that the B cell progenitors from aged mice displayed a lack of proliferative responses to IL-7 with more cells remaining in G0 phase of the cell cycle (44). These effects would be consistent with the current observations in Ts65Dn BM.
Another possible interpretation of the data is that the increased oxidative stress present in Ts65Dn HSCs may be inducing them to become myeloid-biased. Myeloid-biased (My-bi) HSC have been shown to generate fewer IL-7Rα-expressing cells, leading to the hypothesis that the locus for the lymphoid-specific receptor chain IL-7Rα may already be epigenetically primed in HSC, even though HSC do not express IL-7Rα (27). Therefore, increased oxidative stress may be causing inefficient priming of the IL-7Rα locus in Ts65Dn HSCs, resulting in the generation of myeloid-biased HSC. Alternatively, examination of triplicated genes from MMU-16 in Ts65Dn mice reveals potential candidate genes (GABPA, Runx1, and RCAN1) that may also affect IL-7Rα expression. The transcription factors GABPA (8) and Runx1 (22) both bind to the IL-7Rα promoter, and inhibition of calcineurin (by RCAN1) will downregulate the transcription factor egr-2, which also controls IL-7Rα expression (21). The multi-genic etiology of DS supports potentially complex and independent mechanisms for changes in IL-7Rα expression.
Thus, in conclusion, these data demonstrate significant defects in the hematopoietic stem and lymphoid progenitor cells of Ts65Dn mice, which may be linked to premature thymic involution and abnormal peripheral immunologic development in DS. Further studies need to be conducted to determine if these hematologic abnormalities extend to actual patients with DS. The data also show that modulating redox balance, specifically by changing intracellular GSH levels, alters IL-7Rα expression and selectively influences the survival of IL-7Rα+ cells. Thus, the current study may support intracellular antioxidants and IL-7Rα expression as possible therapeutic targets to treat immune dysfunction in DS and may provide a starting point for approaches that alter oxidative stress as a means to regulate the immune response.
Supplementary Material
Abbreviations Used
- 3-NT
3-nitrotyrosine
- 7-AAD
7-Amino-Actinomycin D
- BM
bone marrow
- BMC
bone marrow cells
- BSO
buthionine sulfoximine
- CFU-G
granulocyte colony forming unit
- CFU-GM
granulocyte-monocyte colony forming unit
- CFU-M
monocyte colony forming unit
- CFU pre-B
pre-B cell colony forming unit
- CLP
common lymphoid progenitor
- CMP
common myeloid progenitor
- DCFDA
2′-7′-dichlorofluorescin diacetate
- DS
Down Syndrome
- GMP
granulocyte-monocyte progenitor
- GSH
glutathione
- GSSG
oxidized glutathione
- HSC
hematopoietic stem cell
- Lin(−)
lineage-negative
- LMPP
lymphoid-primed multipotent progenitor
- LSK
(Lin)− Sca-1+, c-Kit+
- MPP
multipotent progenitor
- NAC
N-acetylcysteine
- PC
protein carbonyl
- ROS
reactive oxygen species
- RU
repopulating units
Acknowledgments
The authors wish to thank Ian M. Kaplan for helpful discussions, Stan Vukmanovic for critical review of article, and Regina Harley for expert assistance in cell sorting. This work was supported by funding from the U.S. Public Health Service (AI070823) (M.S.W.) and the LeJeune Foundation (P.J.Y.). L.P.E.L and S.C. are Ph.D. candidates in the University of Maryland School of Medicine Graduate Program in Life Sciences (GPILS) and this work is submitted in partial fulfillment of the requirement for their Ph.D.
Author Disclosure Statement
No competing financial interests exist.
Author Contributions
L.P.E.L., H.C., K.E.S., R.Y., and S.C. performed experiments; L.P.E.L., R.Y., D.E.H., and M.S.W. analyzed results and made the figures; L.P.E.L., M.S.W., and P.J.Y. designed the research and wrote the article.
References
- 1.Akashi K. Traver D. Miyamoto T. Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 2.Allman D. Sambandam A. Kim S. Miller JP. Pagan A. Well D. Meraz A. Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 3.Bensaad K. Vousden KH. Savior and slayer: the two faces of p53. Nat Med. 2005;11:1278–1279. doi: 10.1038/nm1205-1278. [DOI] [PubMed] [Google Scholar]
- 4.Cheng T. Rodrigues N. Shen H. Yang Y. Dombkowski D. Sykes M. Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 5.Cheshier SH. Morrison SJ. Liao X. Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochrane SW. Zhao Y. Welner RS. Sun XH. Balance between Id and E proteins regulates myeloid-versus-lymphoid lineage decisions. Blood. 2009;113:1016–1026. doi: 10.1182/blood-2008-06-164996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day SM. Strauss DJ. Shavelle RM. Reynolds RJ. Mortality and causes of death in persons with Down syndrome in California. Dev Med Child Neurol. 2005;47:171–176. doi: 10.1017/s0012162205000319. [DOI] [PubMed] [Google Scholar]
- 8.DeKoter RP. Schweitzer BL. Kamath MB. Jones D. Tagoh H. Bonifer C. Hildeman DA. Huang KJ. Regulation of the interleukin-7 receptor alpha promoter by the Ets transcription factors PU.1 and GA-binding protein in developing B cells. J Biol Chem. 2007;282:14194–14204. doi: 10.1074/jbc.M700377200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ertl RP. Chen J. Astle CM. Duffy TM. Harrison DE. Effects of dietary restriction on hematopoietic stem-cell aging are genetically regulated. Blood. 2008;111:1709–1716. doi: 10.1182/blood-2007-01-069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry TJ. Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 11.Goldacre MJ. Wotton CJ. Seagroatt V. Yeates D. Cancers and immune related diseases associated with Down's syndrome: a record linkage study. Arch Dis Child. 2004;89:1014–1017. doi: 10.1136/adc.2003.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison DE. Jordan CT. Zhong RK. Astle CM. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 13.Ito K. Hirao A. Arai F. Matsuoka S. Takubo K. Hamaguchi I. Nomiyama K. Hosokawa K. Sakurada K. Nakagata N. Ikeda Y. Mak TW. Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 14.Ito K. Hirao A. Arai F. Takubo K. Matsuoka S. Miyamoto K. Ohmura M. Naka K. Hosokawa K. Ikeda Y. Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 15.Jovanovic SV. Clements D. MacLeod K. Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic Biol Med. 1998;25:1044–1048. doi: 10.1016/s0891-5849(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 16.Kiel MJ. Yilmaz OH. Iwashita T. Yilmaz OH. Terhorst C. Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Kim HR. Hong MS. Dan JM. Kang I. Altered IL-7Ralpha expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107:2855–2862. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirsammer G. Jilani S. Liu H. Davis E. Gurbuxani S. Le Beau MM. Crispino JD. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome. Blood. 2008;111:767–775. doi: 10.1182/blood-2007-04-085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo M. Weissman IL. Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 20.Kusters MA. Verstegen RH. Gemen EF. de Vries E. Intrinsic defect of the immune system in children with Down syndrome: a review. Clin Exp Immunol. 2009;156:189–193. doi: 10.1111/j.1365-2249.2009.03890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson VJ. Weston K. Maurice D. Egr2 regulates the survival of thymocytes during positive selection. Eur J Immunol. 2010;40:232–241. doi: 10.1002/eji.200939567. [DOI] [PubMed] [Google Scholar]
- 22.Lee HC. Shibata H. Ogawa S. Maki K. Ikuta K. Transcriptional regulation of the mouse IL-7 receptor alpha promoter by glucocorticoid receptor. J Immunol. 2005;174:7800–7806. doi: 10.4049/jimmunol.174.12.7800. [DOI] [PubMed] [Google Scholar]
- 23.Malin S. McManus S. Cobaleda C. Novatchkova M. Delogu A. Bouillet P. Strasser A. Busslinger M. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzucchelli R. Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 25.Miller JP. Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 26.Morrison SJ. Wandycz AM. Hemmati HD. Wright DE. Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Sieburg CE. Cho RH. Karlsson L. Huang JF. Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- 28.Mundschau G. Gurbuxani S. Gamis AS. Greene ME. Arceci RJ. Crispino JD. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood. 2003;101:4298–4300. doi: 10.1182/blood-2002-12-3904. [DOI] [PubMed] [Google Scholar]
- 29.Parmar K. Mauch P. Vergilio JA. Sackstein R. Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pujal JM. Gallardo D. PCR-based methodology for molecular microchimerism detection and quantification. Exp Biol Med (Maywood) 2008;233:1161–1170. doi: 10.3181/0802-RM-35. [DOI] [PubMed] [Google Scholar]
- 31.Reeves RH. Irving NG. Moran TH. Wohn A. Kitt C. Sisodia SS. Schmidt C. Bronson RT. Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 32.Rice GC. Bump EA. Shrieve DC. Lee W. Kovacs M. Quantitative analysis of cellular glutathione by flow cytometry utilizing monochlorobimane: some applications to radiation and drug resistance in vitro and in vivo. Cancer Res. 1986;46:6105–6110. [PubMed] [Google Scholar]
- 33.Richtsmeier JT. Baxter LL. Reeves RH. Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice. Dev Dyn. 2000;217:137–145. doi: 10.1002/(SICI)1097-0177(200002)217:2<137::AID-DVDY1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Rossi DJ. Bryder D. Weissman IL. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007;42:385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell LJ. Capasso M. Vater I. Akasaka T. Bernard OA. Calasanz MJ. Chandrasekaran T. Chapiro E. Gesk S. Griffiths M. Guttery DS. Haferlach C. Harder L. Heidenreich O. Irving J. Kearney L. Nguyen-Khac F. Machado L. Minto L. Majid A. Moorman AV. Morrison H. Rand V. Strefford JC. Schwab C. Tonnies H. Dyer MJ. Siebert R. Harrison CJ. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114:2688–2698. doi: 10.1182/blood-2009-03-208397. [DOI] [PubMed] [Google Scholar]
- 36.Schlenner SM. Madan V. Busch K. Tietz A. Laufle C. Costa C. Blum C. Fehling HJ. Rodewald HR. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Scholzen T. Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Sejas DP. Rani R. Qiu Y. Zhang X. Fagerlie SR. Nakano H. Williams DA. Pang Q. Inflammatory reactive oxygen species-mediated hemopoietic suppression in Fancc-deficient mice. J Immunol. 2007;178:5277–5287. doi: 10.4049/jimmunol.178.8.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serwold T. Ehrlich LI. Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serwold T. Ehrlich LIR. Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sitnicka E. Buza-Vidas N. Ahlenius H. Cilio CM. Gekas C. Nygren JM. Mansson R. Cheng M. Jensen CT. Svensson M. Leandersson K. Agace WW. Sigvardsson M. Jacobsen SE. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- 42.Slonim DK. Koide K. Johnson KL. Tantravahi U. Cowan JM. Jarrah Z. Bianchi DW. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in Down syndrome fetuses. Proc Natl Acad Sci USA. 2009;106:9425–9429. doi: 10.1073/pnas.0903909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spangrude GJ. Heimfeld S. Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 44.Stephan RP. Lill-Elghanian DA. Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- 45.Tothova Z. Kollipara R. Huntly BJ. Lee BH. Castrillon DH. Cullen DE. McDowell EP. Lazo-Kallanian S. Williams IR. Sears C. Armstrong SA. Passegue E. DePinho RA. Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz OH. Kiel MJ. Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.