Abstract
Aims
The mutual interactions between reactive oxygen species, airway inflammation, and alveolar cell death play crucial role in the pathogenesis of chronic obstructive pulmonary disease (COPD). In the present study, we investigated the possibility that hydrogen sulfide (H2S) donor sodium hydrosulfide (NaHS) might be a novel option for intervention in COPD.
Results
We used a mouse model of tobacco smoke (TS)-induced emphysema. Mice were injected with H2S donor NaHS (50 μmol/kg in 0.25 ml phosphate buffer saline, intraperitoneally) or vehicle daily before exposed to TS for 1 h/day, 5 days/week for 12 and 24 weeks. We found that NaHS ameliorated TS-induced increase in mean linear intercepts, the thickness of bronchial walls, and the numbers of total cell counts as well as neutrophils, monocytes, and tumor necrosis factor α in bronchial alveolar lavage. Moreover, NaHS reduced increases in right ventricular systolic pressure, the thickness of pulmonary vascular walls, and the ratio of RV/LV+S in TS-exposed mice. Further, TS exposure for 12 and 24 weeks reduced the protein contents of cystathionine γ-lyase (CGL), cystathionine β-synthetase (CBS), nuclear erythroid-related factor 2 (Nrf2), Pser473-Akt, as well as glutathione/oxidized glutathione ratio in the lungs. TS-exposed lungs exhibited large amounts of 8-hydroxyguanine-positive and terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells. Treatment with NaHS increased Pser473-Akt and attenuated TS-induced reduction of CGL, CBS, and Nrf2 as well as glutathione/oxidized glutathione ratio in the lungs. NaHS also reduced amounts of 8-hydroxyguanine-positive, terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells and active caspase-3 in TS-exposed lungs. Additionally, knocking-down Akt protein abolished the protective effects of NaHS against TS-induced apoptosis and downregulation of Nrf2, CGL, and CBS in pulmonary artery endothelial cells.
Conclusion
These results indicate that NaHS protects against TS-induced oxidative stress, airway inflammation, and remodeling and ameliorates the development of emphysema and pulmonary hypertension. H2S donors have therapeutic potential for the prevention and treatment of COPD caused by TS. Antioxid. Redox Signal. 15, 2121–2134.
Introduction
Chronic obstructive pulmonary disease (COPD) is termed for chronic bronchitis, emphysema, and cor pulmonale. Tobacco smoking is the leading cause of COPD. The pathogenesis of COPD is not fully understood but is attributed to the mutual interaction between reactive oxygen species (ROS), airway inflammation, and alveolar cell death (22). Tobacco smoking induces the production of enormous amounts of ROS, which are released from activated inflammatory cells, including neutrophils, eosinophils, macrophages, or structural cells like epithelial cells in COPD (26, 36). Tobacco smoke (TS) per se also contains large numbers of ROS (14). Oxidative stress occurs when ROS are produced in excess of the antioxidant capacity. Oxidative stress directly damage cellular components such as lipids, proteins, and DNA, which result in lung cell death, activation of metalloproteases, degradation of extracellular matrix, and loss of alveolar unit (4, 11). Oxidative stress also induce inflammatory response in the terminal airway causing squamous and mucous metaplasia of the epithelium, smooth muscle hypertrophy and proliferation, bronchial wall remodeling, and fibrosis, as well as mucosal thickening and mucus hypersecretion (4).
Hydrogen sulfide (H2S), a gaseous molecule, has recently been considered the third member of the gaseotransmitter family, along with nitric oxide and carbon monoxide (37). Immerging evidence has revealed that H2S plays a pivotal role in a variety of physiological and pathological processes. H2S has been shown to cause vasodilation and reduce blood pressure (23, 42). Endogenous and exogenous H2S stimulates endothelial angiogenesis (30). H2S donors protect cells from oxidative injuries caused by ischemia/reperfusion injury (13) and glutamate toxicity (21) and exert anti-inflammatory effects (15). H2S is generated endogenously in mammalian cells from L-cysteine mainly by two enzymes, cystathionine γ-lyase (CGL) and cystathionine β-synthetase (CBS). CGL is the major enzyme producing H2S in the cardiovascular and respiratory system and CBS is mainly responsible for H2S production in the nervous tissue (10, 37).
Several reports indicate that H2S is involved in respiratory processes and diseases, although the mechanisms for these effects have not been completely defined. For example, Aslami et al. (3) and Faller et al. (16) have shown that H2S donor sodium hydrosulfide (NaHS) and inhaled H2S protect against ventilator-induced acute lung injuries. Chen et al. (8) found that endogenous H2S reduces airway inflammation and remodeling in a rat model of asthma. Further, Li et al. (24) and Chunyu et al. (10) reported that NaHS and endogenous H2S prevent the elevation of pulmonary arterial pressure and pulmonary vascular remodeling induced by chronic hypoxia and high pulmonary blood flow. More interestingly, Chen et al. (9) reported that serum H2S levels are significantly lower in patients with acute exacerbation of COPD than those with stable COPD. Moreover, serum H2S level is much lower in smokers than non-smokers (9). These observations led us to speculate that H2S may play a protective role in the pathogenesis of COPD. In the present study, we explored the possibility that H2S donor NaHS might be a novel option for intervention in COPD using a mouse model of TS-induced emphysema. We found that NaHS reduces TS-induced oxidative stress and airway inflammation and remodeling, and ameliorates the development of emphysema and pulmonary hypertension. These protective effects are associated with increased Akt phosphorylation and prevention of down-regulation of anti-oxidant molecules such as nuclear erythroid-related factor 2 (Nrf2).
Innovation.
Hydrogen sulfide (H2S) plays a pivotal role in vasodilation and endothelial angiogenesis and protects against oxidative injuries and inflammation. However, its role in the pathogenesis of chronic obstructive pulmonary disease is not known. This study indicates that H2S donor NaHS reduces tobacco smoke-induced oxidative stress and airway inflammation and remodeling and ameliorates the development of emphysema and pulmonary hypertension. These protective effects are associated with increased Akt phosphorylation and prevention of downregulation of antioxidant molecules such as Nrf2.
Materials and Methods
Mouse model of TS exposure
Eight-week-old male C57BL/6 mice were purchased from the Jackson Laboratory. All experiments were performed in accordance with the guiding principles of the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Georgia Health Sciences University. The mice were divided into four groups: vehicle+room air, vehicle+TS, NaHS+room air, and NaHS+TS. The mice were injected daily with vehicle (deoxygenated phosphate buffer saline, 0.25 ml) or NaHS (50 μmol/kg in 0.25 ml, intraperitoneally [i.p.]) 30 min before exposed to TS. NaHS solution was prepared by dissolving NaHS (Sigma, #161527) in deoxygenated phosphate buffer saline. Choosing this dose was based on previous reports that i.p. injection of NaHS in dose of 50 μmol/kg has anti-inflammatory effect (41) and protects against lung injuries induced by burn and inhalation of cotton-smoke (15). A whole-body smoke inhalation system (Scireq Inc.) was used for TS exposure. Research cigarettes 3R4F (University of Kentucky) were smoked at 1 puff (35 ml, 2 s)/min and 10 puffs/cigarette. The mice were exposed to a mixture of main stream and side stream TS at a schedule of 1 h per day, 5 days per week for 12 or 24 weeks. The body weight was measured every 4 weeks. Concentration of total particle matter in exposure chamber was continuously monitored. The levels of total particle matter were between 450 and 550 mg/m3, which are similar to previously reported level (32).
Measurement of right ventricular systolic pressure and cardiac chamber size
Mice were anesthetized (pentobarbital, 90 mg/kg, i.p.) and the trachea was intubated. The right external jugular vein was surgically exposed and cannulated with a 1.4-F microtip pressure transducer catheter (Millar Instruments). The transducer was advanced into the right ventricle (RV), and the right ventricular pressure was continuously monitored for 10 min. Heart rates (300–450 beats/min were deemed acceptable) and pressure waveforms were monitored to ensure the validity of the pressure measurements. Data were recorded using a PowerLab data-acquisition System (AD Instruments). The mice were then euthanized by using thoracotomy. The blood in pulmonary circulation was rinsed by infusing PBS through pulmonary artery and the heart and lungs were removed. The free wall of the RV, left ventricle (LV), and septum (S) were then carefully dissected and individually weighed to calculate the ratio of RV/LV +S as an index of right ventricular hypertrophy as described previously (29). The measurements of right ventricular pressure were made about 1 h after tobacco smoking.
Measurement of mean arterial pressure and arterial blood gases
Mice were injected daily with vehicle (phosphate buffer saline, 0.25 ml) or NaHS (50 μmol/kg in 0.25 ml, i.p.) 30 min before exposed to TS for 1 h/day for 5 days. Following anesthesia, right femoral artery was cannulated with a catheter filled with saline and heparin. The catheter was connected to a pressure transducer (AD Instruments). Mean arterial pressure was continuously monitored for 10 min. Then, 150 μl of blood was drawn for the measurement of arterial blood gases using GEM Premier 4000 Analyzer (Instrumentation Laboratory). The measurements of arterial blood pressure and arterial blood gases were made about 1 h after tobacco smoking.
Lung morphometric analysis
One side of the lungs was removed and snap-frozen in liquid nitrogen for preparing homogenates, and other sides of the lungs were filled with 4% paraformaldehyde solution with 0.5% agarose at 25 cm H2O and fixed in 4% PFA for 24 h. The fixed lungs were then sliced midsagittally and embedded in paraffin. The slides of 7 μm thickness were stained with hematoxylin and eosin for morphometric analysis. 10 slides of each lung were examined by Olympus BX41 microscope. Olympus DP72 digital camera and ImageJ software were used to analyze slides. Alveolar enlargement and destruction were quantified by the mean linear intercept (Lm) in 10 randomly selected fields per slide. The Lm was obtained by dividing the total length of lines drawn across the lung section by the total number of intercepts encountered in 80 lines per each mouse lung as described previously (25). To quantitate bronchial wall thickness, the lumen area at the level of the basement membrane and total bronchial area at the adventitial border in 10 bronchioles per lung section were outlined and area sizes were measured using ImageJ. The bronchial wall thickness was calculated as following: wall thickness=(total bronchial area – lumen area)/total bronchial area. Similarly, to quantitate pulmonary arterial wall thickness, the lumen area at the level of the basement membrane, and total vascular area at the adventitial border in 10 muscular arteries with diameter of 50–100 μm per lung section were outlined, and area sizes were measured using ImageJ. The vascular wall thickness was calculated as following: wall thickness=(total vascular area – lumen area)/total vascular area.
Immunofluorescence microscopy
Oxidative stress marker 8-hydroxyguanine (8-OHdG) and apoptosis was assessed by immunofluorescence microscopy. The slides of 7 μm thickness were incubated first with a mouse monoclonal antibody against 8-OHdG (sc-100583, 1:100 dilution; Santa Cruz Biotechnology) for overnight and then with goat anti-mouse IgG Alexa Fuor 594 (Invitrogen). The slides were sealed with mounting solution containing antifade reagent and DAPI and were examined using a Zeiss LSM 510 laser scanning confocal microscope. The results were expressed as the ratio of 8-OHdG-positive cell number to total number of cells counted. At least 10 randomly selected fields were counted for each slide.
Determination of cell death
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-based cell death assay was performed using ApopTag® Plus Fluorescein In Situ Apoptosis Detection Kit from Millipore (S7111). The fluorescent staining was examined using a Zeiss LSM 510 laser scanning confocal microscope. The results were expressed as the ratio of TUNEL-positive cell number to total number of cells counted. At least 10 randomly selected fields were counted for each slide.
Assay of active caspase-3
Active caspase-3 in lung homogenates was quantitated using an ELISA kit from R&D Systems according to manufacturer's instruction.
Analysis of bronchial alveolar lavage
In some lungs, bronchial alveolar lavage (BAL) was collected in 1.0 ml of PBS. The total cell numbers were counted and leukocyte differential counts were performed for macrophages, polymorphonuclear neutrophils, and monocytes. In addition, tumor necrosis factor α (TNFα) levels in BALF were determined using TNFα Quantikine ELISA System (R&D Systems) following manufacturer's instruction.
Measurement of H2S content and H2S synthesis in lung tissue
H2S contents in the lungs were measured by using zinc acetate trapping and N,N-dimethyl-p-phenylenediamine reaction as described previously by Xia et al. (39) with slight modification. This method has been widely used for the measurement of H2S in tissues (30, 33, 39), although there are limitations as stated by Kabil and Banerjee (19). Using this method, we were able to detect 1 μM NaHS solution. All solutions were deoxygenated before experiment by passing through nitrogen for 30 min. Lung tissues (20 mg) were homogenized in 0.2 ml of zinc acetate (1%) and mixed with 0.25 ml borate buffer (pH 10.1). Then, 0.25 ml of N,N-dimethyl-p-phenylenediamine (20 mM) and 0.25 ml of FeCl3 (300 mM) were added. Reaction tubes were immediately sealed and incubated at 37°C for 30 min with shaking. After incubation, the samples were centrifuged, and absorbance at 670 nm was measured with a using a SpectraMax M2e spectrophotometer (Molecular Devices Corporation). The H2S concentration was calculated against the calibration curve of the standard NaHS solutions (1–1000 μM) prepared by dissolving NaHS (Sigma; #161527) in deoxygenated PBS.
To determine the capacity for H2S synthesis, lung tissues were homogenized in 50 mM ice-cold phosphate buffer saline (pH 7.4) containing protease and phosphatase inhibitors. The tissue homogenates were mixed with L-cysteine (0 and 2 mM) and pyridoxal 5′-phosphate (2 mM) at final volume of 0.25 ml and incubated at 37°C for 90 min after the reaction tubes were flushed with N2 and sealed. The reactions were stopped by adding 0.125 ml trichloroacetic acid (50%) and then 0.125 ml of zinc acetate (15 mM) and 0.5 ml of borate buffer (pH 10.1). The tubes were then incubated at 37°C for another 60 min. The reaction mixtures were mixed with 0.5 ml of N,N-dimethyl-p-phenylenediamine sulfate (20 mM) and 0.02 ml of FeCl3 (3.0 M) at 37°C for an additional 30 min and then centrifuged at 5,000 g for 5 min. Then, the supernatants were measured for absorbance at 670 nm. The capacity for H2S synthesis was calculated by subtracting H2S concentration containing L-cysteine by those without L-cysteine.
Analysis of reduced and oxidized glutathione content
The ratio of reduced to oxidized glutathione (GSSG) was measured in lung tissue using kit GSH/GSSG-412 (OXIS Research, Inc.) according to manufacturer's instruction.
Western blot analysis
The lysate proteins from lung homogerates (20 to 40 μg) were separated on a 4%–20% Tris-glycline SDS-PAGE and electrophoretically transferred onto nitrocellulose membranes. The membranes were incubated in blocking solution at room temperature for 1–2 h and then hybridized with primary antibodies against CGL, CBS, Nrf2, and Pser473-Akt and total Akt (Santa Cruz Biotechnology) overnight at 4°C. The bands were detected by an immunochemiluminescence method. The density was quantitated by Bio-Rad Quantity One Software.
Cell culture and TS exposure
Bovine pulmonary artery endothelial cells were purchased from ATCC. Third- to eighth-passage cells were maintained in Dulbecco's modified Eagle's medium containing 4% fetal bovine serum and antibiotics (10 μ/ml penicillin, 100 μg/ml streptomycin, 20 μg/ml gentamicin, and 2 μg/ml Fungizone) and were used 2 or 3 days after confluence. Pulmonary artery endothelial cells were transfected with control siRNA or siRNA against Akt for 48 h. After being incubated with or without NaHS (50 μM) for 30 min, cells were exposed to room air or TS for 20 min using smoke inhalation system (Scireq Inc) as described above. Research cigarettes 3R4F (University of Kentucky) were smoked at 2 puff (35 ml, 2 s)/min and 10 puffs/cigarette to produce TS with total particle matter of 1,000 mg/m3. Cells were then left in a CO2 incubator for 24 h after which active caspapse-3 and protein contents of Nrf2, CGL, and CBS were determined as described above.
Knocking-down of Akt protein
Akt protein was knocked down using siRNA against Akt mRNA. The siRNA was obtained from Santa Cruz Biotechnology (#sc-29195). A negative control siRNA (#AM4611; Applied Biosystems) was used as control. The sequences of these siRNAs are not disclosed by the companies. Preconfluent pulmonary artery endothelial cells were transfected with 1 μg of siRNA against Akt mRNA or a control siRNA using RNAiFest transfection reagent (Qiagen) in Dulbecco's modified Eagle's medium containing 4% fetal bovine serum according to the manufacturer's protocol. The ratio of siRNA to transfection reagent was 1:3. 48 h after transfection, and cells were exposed to TS or room air.
Statistical analysis
Results are shown as the mean±SE for n experiments. One-way analysis of variance and t-test analyses were used to determine the significance of differences between the means of different groups. p<0.05 was considered statistically significant.
Results
H2S donor NaHS ameliorates TS-induced emphysema
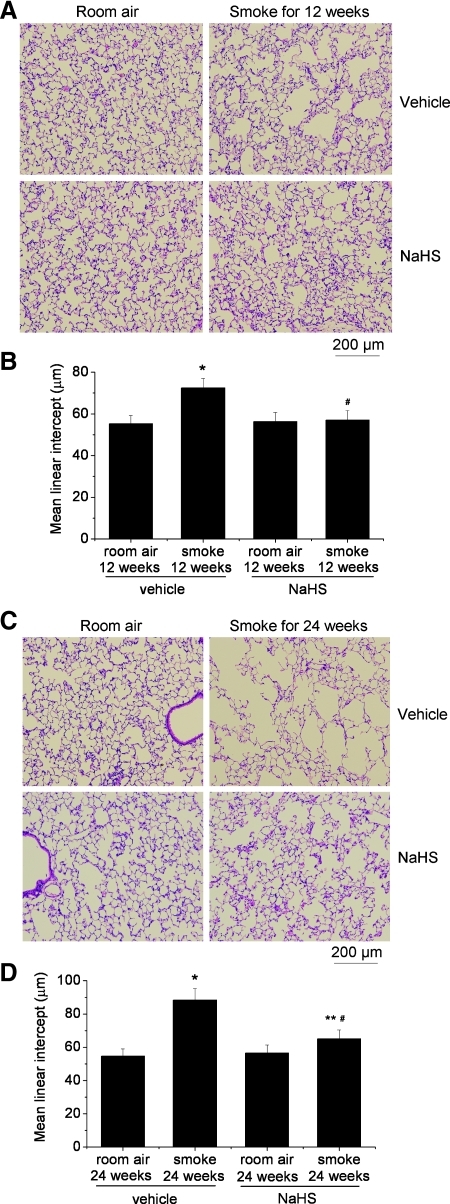
To investigate the effects of H2S on the development of emphysema, mice were injected daily with vehicle or NaHS 30 min before exposed to TS. As shown in Figure 1A and B, the Lms in the lungs of mice exposed to smoke for 12 weeks are much larger than those exposed to room air, suggesting that TS induced emphysema in 12 weeks. The increase in Lms was more evident in mouse lungs exposed to TS for 24 weeks (Fig. 1C, D). More importantly, the Lms in the lungs of mice receiving NaHS treatment were comparable between exposures to TS and room air for 12 weeks (Fig. 1A, B). Moreover, administrations of NaHS significantly reduced the increase in the Lms of the lungs exposed to TS for 24 weeks (Fig. 1C, D). Body weight gains were suppressed starting 12 weeks after exposure to TS, and NaHS did not prevent TS-induced decrease in weight gain (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/ars). Together, these results indicate that H2S donor NaHS ameliorated TS-induced emphysema.
FIG. 1.
H2S donor NaHS ameliorated tobacco smoke-induced emphysema. Mice were injected daily with vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks (A and B) or 24 weeks (C and D). (A) Representative images of lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (B) Changes in mean linear intercepts in the lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (C) Representative images of lung sections of mice exposed to room air or tobacco smoke for 24 weeks. (D) Changes in mean linear intercepts in the lung sections of mice exposed to room air or tobacco smoke for 24 weeks. Results are expressed as mean±SE; n=8 experiments. *p<0.05 versus room air group with vehicle; **p<0.05 versus room air group with NaHS. #p<0.05 versus smoke group with vehicle. H2S, hydrogen sulfide; NaHS, sodium hydrosulfide. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
NaHS attenuates inflammation and remodeling of the airway
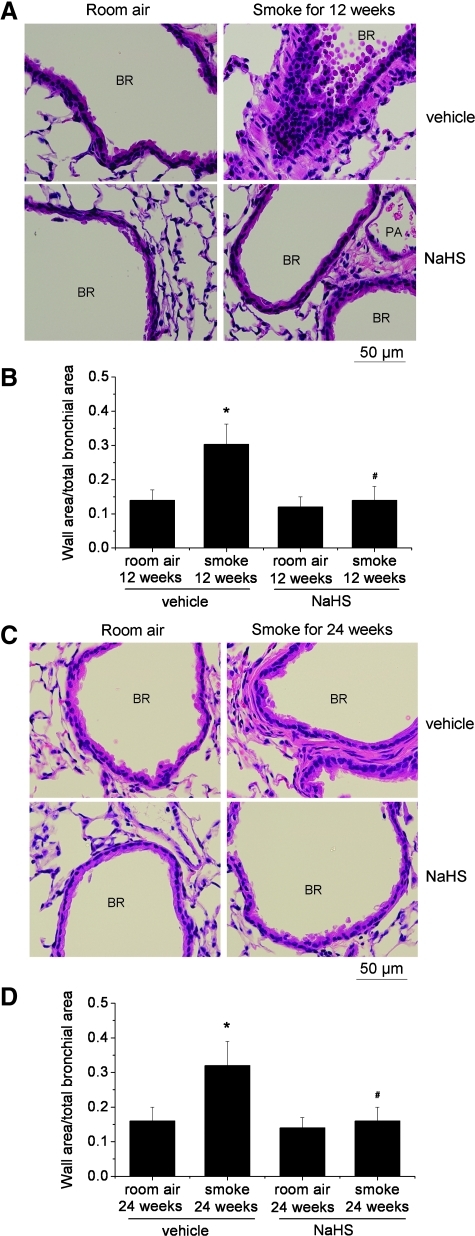
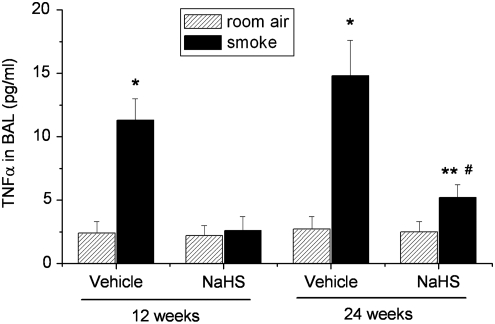
As shown in Table 1, exposure of mice to TS for 12 and 24 weeks caused increases in the total cell numbers in the BAL fluid. The differential counts revealed that polymorphonuclear neutrophils and monocytes were increased in BAL of mice exposed to TS exposure (Table 1). Moreover, exposure of mice to TS for 12 and 24 weeks caused significant increases in the thickness of bronchial walls (Fig. 2). NaHS treatment attenuated the increases in TNFα levels (Fig. 3) and the numbers of total cell counts as well as polymorphonuclear neutrophils and monocytes in BAL (Table 1) and reduced the increases in the thickness of bronchial walls (Fig. 2). These results suggest that NaHS suppresses the inflammation in the airway and reduces bronchial remodeling caused by TS.
Table 1.
Cell Counts in Bronchial Alveolar Lavage Fluid in Control and Smoked Mice Treated With and Without Sodium Hydrosulfide
| |
12 weeks |
24 weeks |
||||||
|---|---|---|---|---|---|---|---|---|
| Room air | Smoke | NaHS | NaHS+smoke | Room air | Smoke | NaHS | NaHS+smoke | |
| Total cells (×104/ml) | 8.2±0.7 | 10.8±0.8a | 7.8±0.6 | 8.0±0.7b | 7.5±0.6 | 13.1±0.9c | 8.5±0.8 | 8.8±0.7d |
| Macrophages (%) | 98.0±2.3 | 83±3.1a | 98.5±3.6 | 98.3±3.8b | 97.5±4.1 | 80.2±3.8c | 98.0±4.5 | 97.0±4.2d |
| PMN (%) | 1.0±0.3 | 6.2±0.8a | 1.0±0.2 | 1.0±0.3b | 1.5±0.4 | 8.4±0.6c | 1.0±0.4 | 1.5±0.4d |
| Monocytes (%) | 1.0±0.2 | 10.8±0.6a | 0.5±0.07 | 0.7±0.08b | 1.0±0.07 | 11.4±0.08c | 1.0±0.05 | 1.5±0.06d |
Values are averages±SE (n=5).
p<0.05 vs. room air group in 12 weeks.
p<0.05 vs. smoke group in 12 weeks.
p<0.05 vs. room air group in 24 weeks.
p<0.05 vs. smoke group in 24 weeks.
PMN, polymorphonuclear neutrophils; NaHS, sodium hydrosulfide.
FIG. 2.
NaHS reduces bronchial remodeling caused by tobacco smoke. Mice were injected daily with vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks (A and B) or 24 weeks (C and D). (A) Representative images of lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (B) Changes in ratio of wall area to total bronchial area in the lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (C) Representative images of lung sections of mice exposed to room air or tobacco smoke for 24 weeks. (D) Changes in ratio of wall area to total bronchial area in the lung sections of mice exposed to room air or tobacco smoke for 24 weeks. Results are expressed as mean±SE; n=8 experiments. *p<0.05 versus room air; #p<0.05 versus smoke group with vehicle. BR, bronchiole; PA, pulmonary arteriole. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 3.
NaHS inhibits tobacco smoke-induced increases in TNFα in BAL of mouse lungs. Mice were injected daily with vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks or 24 weeks. BAL was collected in 1.0 ml of PBS. After centrifugation, TNFα levels in supernatants were determined as described in the Materials and Methods section. *p<0.05 versus room air with vehicle group; **p<0.05 versus room air group with NaHS. #p<0.05 versus smoke group with vehicle. BAL, bronchial alveolar lavage; TNFα, tumor necrosis factor α.
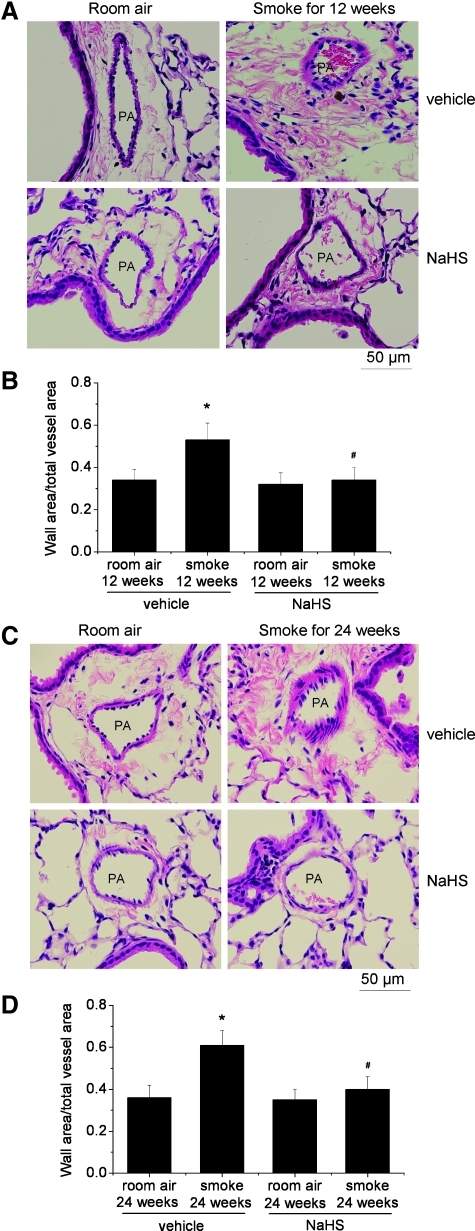
NaHS reduces TS-induced pulmonary hypertension and vascular remodeling
As shown in Table 2, exposure of mice to TS for 12 and 24 weeks caused moderate increases in right ventricular systolic pressure (RVSP). TS also increased the thickness of pulmonary vascular walls (Fig. 4) and the ratio of RV/LV+S, an index of right ventricular hypertrophy (Table 2). Further, treatment with NaHS reduced the increases in RVSP, the thickness of pulmonary vascular walls, and the ratio of RV/LV+S in TS-exposed mice (Table 2 and Fig. 4).
Table 2.
Right Ventricular Systolic Pressure and Right Ventricular Weight in Control and Smoked Mice Treated With and Without Sodium Hydrosulfide
| |
12 weeks |
24 weeks |
||||||
|---|---|---|---|---|---|---|---|---|
| Room air | Smoke | NaHS | NaHS+smoke | Room air | Smoke | NaHS | NaHS+smoke | |
| RVSP (mmHg) | 19.5±2.8 | 24.2±2.5a | 18.1±2.2 | 19.2±2.7b | 19.4±2.1 | 27.2±2.7c | 18.4±1.5 | 22.3±2.3d,e |
| RV (mg) | 21.2±1.9 | 26.8±2.8a | 19.7±3.2 | 20.3±2.4b | 22.5±2.8 | 29.4±2.6c | 20.6±2.2 | 24.7±2.4d,e |
| RV/LV S | 0.21±0.02 | 0.26±0.03a | 0.19±0.04 | 0.21±0.05b | 0.22±0.02 | 0.29±0.04c | 0.18±0.05 | 0.23±0.04d,e |
Values are averages±SE (n=8).
p<0.05 vs. room air group in 12 weeks.
p<0.05 vs. smoke group in 12 weeks.
p<0.05 vs. room air group in 24 weeks.
p<0.05 vs. NaHS group in 24 weeks.
p<0.05 vs. smoke group in 24 weeks.
RVSP, right ventricular systolic pressure; RV, right ventricular weight; LV+S, left ventricular weight plus septal weight.
FIG. 4.
Treatment with NaHS reduces pulmonary vascular remodeling caused by tobacco smoke. Mice were injected daily for vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks (A and B) or 24 weeks (C and D). (A) Representative images of lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (B) Changes in ratio of wall area to total vessel area in the lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (C) Representative images of lung sections of mice exposed to room air or tobacco smoke for 24 weeks. (D) Changes in ratio of wall area to total vessel area in the lung sections of mice exposed to room air or tobacco smoke for 24 weeks. Results are expressed as mean±SE; n=8 experiments. *p<0.05 versus room air; #p<0.05 versus smoke group with vehicle. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Administration of NaHS for 5 days did not affect systemic mean arterial pressure and arterial blood gases
We determined the effect of NaHS on systemic mean arterial pressure and arterial blood gases in mice exposed to TS. As shown in Supplementary Figures S2 and S3, exposure to TS and i.p. injection of NaHS for 5 days did not affect systemic mean arterial pressure and arterial blood gases.
H2S contents and the capacity for H2S synthesis in lung tissues
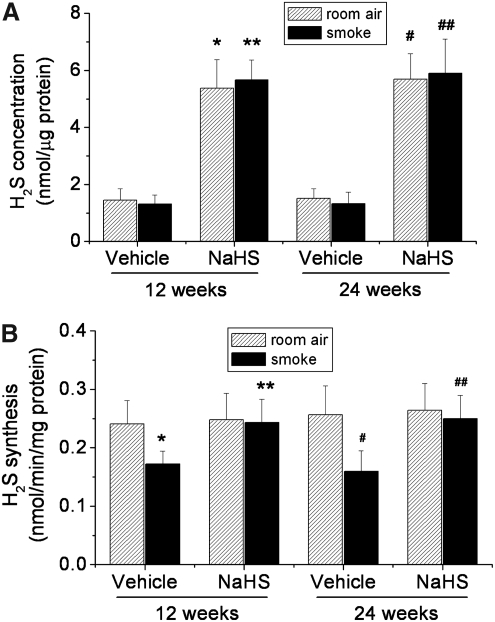
As illustrated in Figure 5A, treatment with NaHS caused significant elevations of H2S contents in lung tissues. However, there is no significant difference in H2S contents in lung tissues between mice exposed to room air and TS (Fig. 5A). To determine whether NaHS and TS affect the capacity for H2S synthesis, we measured the generation of H2S in lung homogenates in the presence of L-cysteine, the substrate of CGL and CBS. We found that TS exposure for 12 and 24 weeks decreased the synthesis of H2S (Fig. 5B). Further, treatment with NaHS prevented the decrease in H2S synthesis in TS-exposed mouse lungs (Fig. 5B).
FIG. 5.
H2S contents and the capacity for H2S synthesis in lung tissues. Mice were injected daily with vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks, and 24 weeks after which H2S contents (A) and the capacity for H2S synthesis (B) were determined as described in the Materials and Methods section. Results are expressed as mean±SE; n=8 experiments. *p<0.05 versus room air for 12 weeks in vehicle group; **p<0.05 versus smoke for 12 weeks in vehicle group; #p<0.05 versus room air for 24 weeks in vehicle group; ##p<0.05 versus smoke for 24 weeks in vehicle group.
NaHS suppresses lung oxidative stress and cell death in TS-exposed mouse lungs
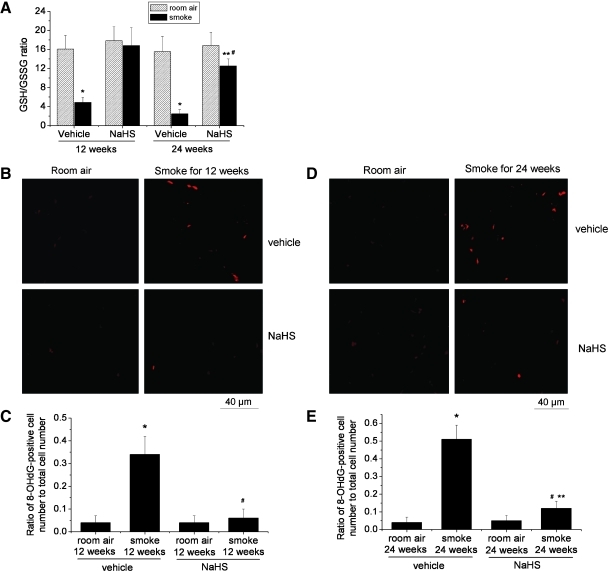
To determine the effects of TS exposure and NaHS on oxidative state, we measure GSH/GSSG ratio and 8-OHdG in mouse lungs. We found that exposure to TS for 12 and 24 weeks significantly reduced GSH/GSSG ratio in mouse lungs (Fig. 6A). Treatment with NaHS prevented the reduction of GSH/GSSG ratio caused by TS (Fig. 6A). Consistent with these data, mouse lungs exposed to TS for 12 and 24 weeks contained large amounts of 8-OHdG, a marker for oxidative stress, and treatment with NaHS attenuated the increases in 8-OHdG in cells of lungs exposed to TS (Fig. 6B–E). These results indicate that NaHS suppresses TS-induced lung oxidative stress.
FIG. 6.
NaHS suppresses oxidative stress in tobacco smoke-exposed mouse lungs. Mice were injected daily with vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks, and 24 weeks after which GSH/GSSG ratio (A) and 8-OHdG (B–E) were determined as described in the Materials and Methods section. (B) Representative images of 8-OHdG assay of lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (C) Changes in ratio of 8-OHdG-positive cell number to total cell number in the lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (D) Representative images of 8-OHdG assay of lung sections of mice exposed to room air or tobacco smoke for 24 weeks. (E) Changes in ratio of 8-OHdG-positive cell number to total cell number in the lung sections of mice exposed to room air or tobacco smoke for 24 weeks. Results are expressed as mean±SE; n=8 experiments. *p<0.05 versus room air group with vehicle; **p<0.05 versus room air group with NaHS; #p<0.05 versus smoke group with vehicle. 8-OHdG, 8-hydroxyguanine; GSH, glutathione; GSSG, oxidized glutathione. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
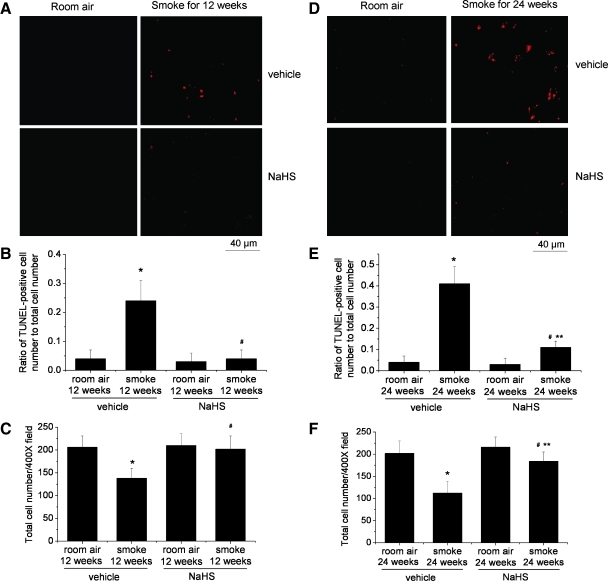
To evaluate whether NaHS-induced suppression of oxidative stress influences TS-induced cell death, we performed a fluorescence TUNEL assay. We found that exposure to TS for 12 and 24 weeks caused significant increases in TUNEL-positive cells (Fig. 7A, B, D, and E) and decreases in total cell numbers in the lungs (Fig. 7C, F). Treatment with NaHS prevented the increases in TUNEL-positive cells and the decreases in total cell numbers in TS-exposed lungs (Fig. 7). These results indicate that NaHS suppresses TS-induced lung cell death.
FIG. 7.
NaHS suppresses tobacco smoke-induced cell death in mouse lungs. Mice were injected daily with vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks (A–C) or 24 weeks (D–F) after which TUNEL assay (A, B, D, and E) and total cell counting was performed as described in the Materials and Methods section. (A) Representative images of TUNEL assay of lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (B) Changes in ratio of TUNEL-positive cell number to total cell number in the lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (C) Changes in total cell number in the lung sections of mice exposed to room air or tobacco smoke for 12 weeks. (D) Representative images of TUNEL assay of lung sections of mice exposed to room air or tobacco smoke for 24 weeks. (E) Changes in ratio of TUNEL-positive cell number to total cell number in the lung sections of mice exposed to room air or tobacco smoke for 24 weeks. (F) Changes in total cell number in the lung sections of mice exposed to room air or tobacco smoke for 24 weeks. Results are expressed as mean±SE; n=8 experiments. *p<0.05 versus room air with vehicle group; **p<0.05 versus room air group with NaHS. #p<0.05 versus smoke group with vehicle. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
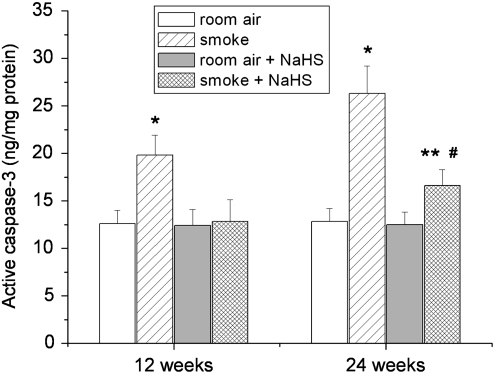
To determine whether the protective effect of NaHS against TS-induced lung cell death occurs via caspase-3, we measured active caspase-3 contents in lungs. As shown in Figure 8, exposure to TS for 12 and 24 weeks caused significant increases in active caspase-3 contents. Treatment with NaHS prevented the increases in active caspase-3 (Fig. 8). These data indicate that the protective effect of H2S against TS-induced lung cell death or apoptosis occurs at the level of caspase-3.
FIG. 8.
NaHS inhibits tobacco smoke-induced increases in active caspase-3 in mouse lungs. Mice were injected daily with vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks or 24 weeks after which active caspase-3 in lung homogenates was quantitated as described in the Materials and Methods section. *p<0.05 versus room air with vehicle group; **p<0.05 versus room air group with NaHS. #p<0.05 versus smoke group with vehicle.
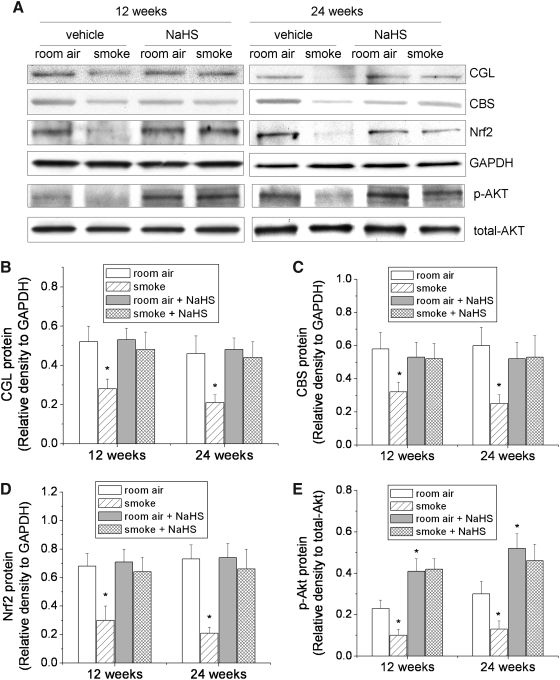
Effects of NaHS and TS on the protein contents of CGL, CBS, Nrf2, and Pser473-Akt
To determine whether TS-induced inhibition of H2S synthesis in lung tissues is due to decreases in the protein contents of CGL and CBS, we measured the protein contents of CGL and CBS. As shown in Figure 9, exposure to TS for 12 and 24 weeks reduced the protein contents of CGL and CBS. NaHS administration prevented TS-induced decrease in CGL and CBS (Fig. 9).
FIG. 9.
Effects of NaHS and tobacco smoke on the protein contents of CGL, CBS, Nrf2, and p-Akt. Mice were injected daily with vehicle or NaHS 30 min before exposed to tobacco smoke for 1 h/day, 5 days/week for 12 weeks and 24 weeks after which the protein contents of CGL, CBS, Nrf2, Pser473-Akt, and total Akt in lung tissues were determined using Western blot analysis. (A) is representative blots of 8 separate experiments. (B–E) are bar graphs depicting the changes in CGL, CBS, Nrf2, and p-Akt proteins. Results are expressed as mean±SE; n=8 experiments. *p<0.05 versus room air group. CBS, cystathionine β-synthetase; CGL, cystathionine γ-lyase; Nrf2, nuclear erythroid-related factor 2.
To determine whether the protective effect of H2S against TS-induced oxidative stress is associated to Nrf2 and Akt phosphorylation, we examined the protein contents of Nrf2 and Pser473-Akt in lung tissues. We found that exposure to TS for 12 and 24 weeks reduced the protein contents of Nrf2 and Pser473-Akt (Fig. 9). NaHS administration increased the protein contents of Pser473-Akt and prevented TS-induced decrease in Nrf2 (Fig. 9). Together, our data suggest that the protective effect of H2S against TS-induced oxidative stress is attributable to inhibition of down-regulation of antioxidant genes in lungs such as Nrf2 due to increased phosphorylation of Akt.
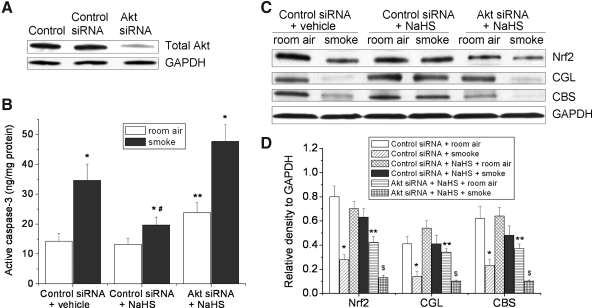
Knocking-down of Akt protein abolishes the protective effects of NaHS against TS-induced apoptosis and down-regulation of CGL, CBS, and Nrf2 in pulmonary artery endothelial cells
As shown in Supplementary Figure S4, incubation of pulmonary artery endothelial cells with NaHS (10–50 μM) for 30 min resulted in increases in Akt phosphorylation. To further confirm the role of Akt in the inhibitory effect of NaHS on TS-induced apoptosis and down-regulation of CGL, CBS, and Nrf2, Akt protein in pulmonary artery endothelial cells was knocked down using siRNA against Akt mRNA. As shown in Figure 10B, NaHS inhibited TS-induced increase in active caspase-3, and knocking-down of Akt prevented the inhibitory effect of NaHS on active caspase-3 in TS-exposed lung endothelial cells. Moreover, NaHS prevented TS-induced decreases in protein contents of Nrf2, CGL, and CBS and knocking-down of Akt abolished the preventative effect of NaHS on TS-induced decreases in Nrf2, CGL, and CBS proteins in lung endothelial cells (Fig. 10C, D). These results support that the preventative effects of H2S against TS-induced apoptosis and inhibition of down-regulation of Nrf2, CGL, and CBS proteins are due to increased phosphorylation of Akt.
FIG. 10.
Knocking-down of Akt protein abolishes the protective effects of NaHS against apoptosis and down-regulation of CGL, CBS, and Nrf2 in pulmonary artery endothelial cells exposed to tobacco smoke. Bovine pulmonary artery endothelial cells were transfected with control siRNA or siRNA against Akt for 48 h. After being incubated with or without NaHS (50 μM) for 30 min, cells were exposed to room air or tobacco smoke for 20 min. Cells were then left in a CO2 incubator for 24 h after which active caspapse-3 and protein contents of Nrf2, CGL, and CBS were determined as described in the Materials and Methods section. (A) Representative blots of 3 separate experiments. (B) Bar graph showing the changes in active caspase-3 contents (n=4). (C) Representative blots of four separate experiments. (D) Bar graphs depicting the changes in Nrf2, CGL, and CBS. Results are expressed as mean±SE; *p<0.05 versus room air group, **p<0.05 versus control siRNA+NaHS+room air, #p<0.05 versus control siRNA+vehicle+smoke, $p<0.05 versus Akt siRNA+NaHS+room air.
Discussion
The major new finding in this study is that H2S donor NaHS protects lungs against oxidative stress and ameliorates TS-induced emphysema. Exposure of mice to TS for 12 and 24 weeks caused oxidative stress and inflammation in the airway. Moreover, TS induces lung cell death, alveolar destruction, and bronchial remodeling. Further, TS exposure for 12 and 24 weeks causes moderate pulmonary hypertension and pulmonary vascular remodeling. More importantly, administration of NaHS increases H2S levels in the lungs, protects lungs against oxidative stress and lung cell death, and suppresses airway inflammation and bronchial wall thickening. Thus, this study provides the first evidence that H2S ameliorates emphysema, pulmonary hypertension, and vascular remodeling induced by TS.
H2S is generated endogenously in mammalian cells from L-cysteine by CGL and CBS (10, 37). We found that both CGL and CBS exist in mouse lung tissue. TS exposure reduces the protein contents of CGL and CBS as well as the capacity for H2S synthesis in the lungs. However, H2S levels in TS-exposed lung are not significantly reduced. This is because TS per se contains H2S (12). In the studies of Chen et al. (9), however, they found that serum H2S level is much lower in smokers than non-smokers. In these studies, the patients have stopped smoking 1 month before the blood was drawn. Serum H2S levels reflect only H2S synthesis in the peripheral and lung tissues. In our animal model, the dose of smoke would be larger than smoker patients, because emphysema was achieved within 12 weeks of smoking. Moreover, the lung tissues were collected about 1 h after smoking. Thus, H2S levels in lungs reflect H2S synthesized in lung tissues and those from TS per se. Although H2S levels in lungs exposed to room air and TS are comparable, GSH/GSSG ratio is lower and oxidative marker 8-OHdG is higher in TS-exposed lungs, indicating that oxidative stress occurs due to overwhelmingly formation of ROS in emphysematous lungs. Our data indicate that elevation of H2S levels in the lungs by injection of NaHS prevents TS-induced decreases in the protein contents of CGL and CBS and the capacity for H2S synthesis. More importantly, administration of NaHS prevents TS-induced decrease in GSH/GSSG ratio and increase in oxidative marker 8-OHdG. These data indicate that H2S ameliorates oxidative stress caused by TS exposure.
Oxidative stress occurs only when ROS are produced in excess of the antioxidant capacity. There are various anti-oxidant defensive mechanisms in the lungs. Nrf2 is the most important protein for the anti-oxidant mechanism in the lungs. As a master antioxidant transcription factor, Nrf2 has been shown to control the expression of more than 100 gene products, including several of the most important antioxidant enzymes (31). Indeed, lungs of COPD patients have lower level and transcriptional activity for Nrf2 (27). Nrf2-null mice have increased susceptibility to TS-induced emphysema (31). Enhancement of Nrf2 expression using synthetic triterpenoid CDDO-Im protects mice against TS-induced emphysema (34). In the present study, we have shown that exposure to TS for 12 and 24 weeks reduces Nrf2 protein contents and that NaHS treatment attenuates the decreases in Nrf2protein contents in TS-exposed lungs. Therefore, the protective effect of H2S against TS-induced oxidative stress is attributable to inhibition of down-regulation of antioxidant genes in lungs such as Nrf2.
Previous reports have shown that H2S increase endothelial angiogenesis and survival via Akt phosphorylation (6, 30). Indeed, appropriate pulmonary angiogenic homeostasis is crucial in the maintenance of normal alveolar structure and protect against emphysema (20). To determine whether H2S-induced inhibition of down-regulation of the antioxidant gene Nrf2 is associated to Akt phosphorylation, Pser473-Akt in mouse lungs was measured. We found that TS exposure for 12 and 24 weeks decreases Akt phosphorylation. NaHS administration increases Akt phosphorylation and prevents TS-induced decrease in Akt phosphorylation and Nrf2 in mouse lungs. Notably, consistent with previous observations (6, 7, 30), our data indicate that NaHS increases Akt phosphorylation in pulmonary artery endothelial cells. Moreover, knocking-down of Akt protein abolishes the preventative effect of NaHS against TS-induced down-regulation of Nrf2 in lung endothelial cells. These data indicate that H2S-induced Akt phosphorylation is responsible for inhibition of down-regulation of the antioxidant gene Nrf2 (Fig. 11). The mechanism for the preventative effect of H2S on TS-induced reductions in CGL and CBS is not clear yet. Our data show that alterations in CGL and CBS in TS- and NaHS-treated lungs and endothelial cells correlate to those in Nrf2. Considering that CGL, CBS, and Nrf2-dependent genes share similar antioxidant function, it is plausible to speculate that CGL and CBS might be direct transcriptional target of Nrf2. Further studies are necessary to confirm this speculation.
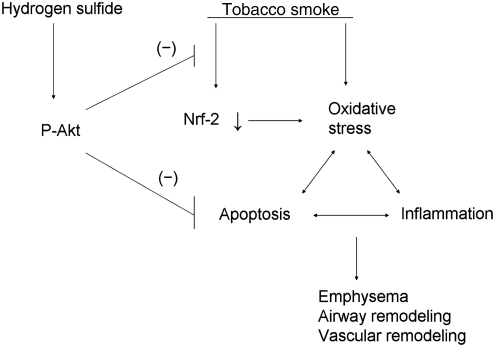
FIG. 11.
A schematic pathway illustrating the protective effects of H2S against tobacco smoke-induced oxidative stress and emphysema. H2S increases phosphorylation of Akt, which is responsible for inhibition of Nrf2 down-regulation and apoptosis, leading to protection against airway inflammation, emphysema, and vascular remodeling in tobacco smoke-exposed lungs.
Oxidative stress plays an important role in the pathological processes in COPD. Oxidative stress directly damage cellular components such as lipids, proteins, and DNA, which result in lung cell death or apoptosis, degradation of extracellular matrix, and loss of alveolar unit as well as inflammatory response in the terminal airway (1, 4, 11). Notably, a series of signaling molecules cascades the apoptotic pathway. Caspase-3 activation plays a key role in initiation of cellular events during the apoptotic process. It is well known that ROS can induce activation of caspase-3 in a number of cell types (28, 35). Our results indicate that the protective effect of H2S against TS-induced lung cell death or apoptosis occurs at the level of caspase-3. Knocking-down of Akt protein abolishes the preventative effect of NaHS against TS-induced increase in active caspase-3 in lung endothelial cells. Thus, H2S-induced amelioration of oxidative stress and Nrf2 down-regulation due to Akt phosphorylation render protection against lung cell death or apoptosis and loss of alveolar unit in TS-exposed lungs (Fig. 11).
H2S has been shown to directly cause vasorelaxation in vitro (42) and inhibit the proliferation of vascular smooth muscle cells in culture (5). In the present study, administration of NaHS for 12 and 24 weeks does not affect RVSP in control mice. Moreover, i.p. injection of NaHS for 5 days does not significantly alter systemic mean arterial pressure in control mice 1 h after injection. However, NaHS does prevent pulmonary hypertension and vascular remodeling induced by TS. Interestingly, Ganster et al. reported that a single intravenous bolus of NaHS (0.2 mg/kg) protects against hemorrhage shock-induced inflammatory response and oxidative stress without affecting systemic arterial pressure in control rats 5 h after injection (17). Further, Zhao et al. reported that single intravenous bolus of H2S provokes a transient (29.5±3.6 s) decrease in systemic arterial pressure (42). These data suggest that the preventative effect of H2S on TS-induced pulmonary hypertension is unlikely to be contributable to its direct vasorelaxant activity. The reduction of RVSP and pulmonary vascular remodeling in TS-exposed mice could be consequences of amelioration of emphysema caused by H2S. It is also feasible that reduction of oxidative stress by H2S contributes to the preventative effects of H2S on pulmonary hypertension and vascular remodeling. ROS and oxidative stress have been shown to mediate pulmonary vascular remodeling and pulmonary hypertension (38).
We acknowledge that the current studies have limitations, but we do not think these issues in any way compromise our conclusions. First, the TUNEL assay used to detect apoptosis may not reliably discriminate between apoptosis, necrosis, and autolysis (18). TS can cause necrosis besides apoptosis (2). However, the changes in caspase-3 activity in TS- and NaHS-treated lungs correlate to those in TUNNEL assay, indicating that H2S does protect lungs from apoptosis and emphysema although we do not exclude the possibility of necrosis. Second, arterial blood gases were measured just in mice treated with TS and NaHS for 5 days. We found that exposure to TS and treatment with NaHS for 5 days did not significantly alter arterial blood gases. Considering the remarkable alterations in the airway and lung parenchyma in the present study, chronic exposure to TS could supposedly cause hypoxemia and hypercapnia in mice as reported by Xu et al. (40).
In summary, we have shown that the H2S donor NaHS protects lungs against oxidative stress and ameliorates emphysema caused by TS exposure. H2S donors have therapeutic potential for the prevention and treatment of COPD caused by TS.
Supplementary Material
Abbreviations Used
- 8-OHdG
8-hydroxyguanine
- BAL
bronchial alveolar lavage
- CBS
cystathionine β-synthetase
- CGL
cystathionine γ-lyase
- COPD
chronic obstructive pulmonary disease
- GSH
glutathione
- GSSG
oxidized glutathione
- H2S
hydrogen sulfide
- i.p.
intraperitoneally
- Lm
mean linear intercept
- LV
left ventricle
- NaHS
sodium hydrosulfide
- Nrf2
nuclear erythroid-related factor 2
- ROS
reactive oxygen species
- RV
right ventricle
- RVSP
right ventricular systolic pressure
- TNFα
tumor necrosis factor α
- TS
tobacco smoke
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Acknowledgments
We thank Dr. William R. Caldwell for critical reading of the article. This work was supported by NIH grant R01HL088261 (to Y.S.), Flight Attendants Medical Research Institute grant 072104 (to Y.S.), and American Heart Association Greater Southeast Affiliate grant 0855338E (to Y.S.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adcock IM. Cosio B. Tsaprouni L. Barnes PJ. Ito K. Redox regulation of histone deacetylases and glucocorticoid-mediated inhibition of the inflammatory response. Antioxid Redox Signal. 2005;7:144–152. doi: 10.1089/ars.2005.7.144. [DOI] [PubMed] [Google Scholar]
- 2.Aoshiba K. Nagai A. Oxidative stress, cell death, and other damage to alveolar epithelial cells induced by cigarette smoke. Tob Induc Dis. 2003;1:219–226. doi: 10.1186/1617-9625-1-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslami H. Heinen A. Roelofs JJ. Zuurbier CJ. Schultz MJ. Juffermans NP. Suspended animation inducer hydrogen sulfide is protective in an in vivo model of ventilator-induced lung injury. Intensive Care Med. 2010;36:1946–1952. doi: 10.1007/s00134-010-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ. Shapiro SD. Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 5.Baskar R. Sparatore A. Del SP. Moore PK. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative inhibit rat vascular smooth muscle cell proliferation. Eur J Pharmacol. 2008;594:1–8. doi: 10.1016/j.ejphar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Cai WJ. Wang MJ. Moore PK. Jin HM. Yao T. Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Calvert JW. Elston M. Nicholson CK. Gundewar S. Jha S. Elrod JW. Ramachandran A. Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YH. Wu R. Geng B. Qi YF. Wang PP. Yao WZ. Tang CS. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine. 2009;45:117–123. doi: 10.1016/j.cyto.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Chen YH. Yao WZ. Geng B. Ding YL. Lu M. Zhao MW. Tang CS. Endogenous hydrogen sulfide in patients with COPD. Chest. 2005;128:3205–3211. doi: 10.1378/chest.128.5.3205. [DOI] [PubMed] [Google Scholar]
- 10.Chunyu Z. Junbao D. Dingfang B. Hui Y. Xiuying T. Chaoshu T. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem Biophys Res Commun. 2003;302:810–816. doi: 10.1016/s0006-291x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 11.Dhami R. Gilks B. Xie C. Zay K. Wright JL. Churg A. Acute cigarette smoke-induced connective tissue breakdown is mediated by neutrophils and prevented by alpha1-antitrypsin. Am J Respir Cell Mol Biol. 2000;22:244–252. doi: 10.1165/ajrcmb.22.2.3809. [DOI] [PubMed] [Google Scholar]
- 12.Dong J-Z. Debusk SM. GC–MS analysis of hydrogen sulfide, carbonyl sulfide, methanethiol, carbon disulfide, methyl thiocyanate and methyl disulfide in mainstream vapor phase cigarette smoke. Chromatographia. 2010;71:259–265. [Google Scholar]
- 13.Elrod JW. Calvert JW. Morrison J. Doeller JE. Kraus DW. Tao L. Jiao X. Scalia R. Kiss L. Szabo C. Kimura H. Chow CW. Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epperlein MM. Nourooz-Zadeh J. Noronha-Dutra AA. Woolf N. Nitric oxide in cigarette smoke as a mediator of oxidative damage. Int J Exp Pathol. 1996;77:197–200. doi: 10.1046/j.1365-2613.1996.9930331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esechie A. Kiss L. Olah G. Horvath EM. Hawkins H. Szabo C. Traber DL. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clin Sci (Lond) 2008;115:91–97. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]
- 16.Faller S. Ryter SW. Choi AM. Loop T. Schmidt R. Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. 2010;113:104–115. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- 17.Ganster F. Burban M. de la BM. Fizanne L. Douay O. Loufrani L. Mercat A. Cales P. Radermacher P. Henrion D. Asfar P. Meziani F. Effects of hydrogen sulfide on hemodynamics, inflammatory response and oxidative stress during resuscitated hemorrhagic shock in rats. Crit Care. 2010;14:R165. doi: 10.1186/cc9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasl-Kraupp B. Ruttkay-Nedecky B. Koudelka H. Bukowska K. Bursch W. Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- 19.Kabil O. Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasahara Y. Tuder RM. Taraseviciene-Stewart L. Le Cras TD. Abman S. Hirth PK. Waltenberger J. Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura Y. Goto Y. Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 22.Kirkham P. Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Koenitzer JR. Isbell TS. Patel HD. Benavides GA. Dickinson DA. Patel RP. rley-Usmar VM. Lancaster JR., Jr. Doeller JE. Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol. 2007;292:H1953–H1960. doi: 10.1152/ajpheart.01193.2006. [DOI] [PubMed] [Google Scholar]
- 24.Li X. Du J. Jin H. Tang X. Bu D. Tang C. The regulatory effect of endogenous hydrogen sulfide on pulmonary vascular structure and gasotransmitters in rats with high pulmonary blood flow. Life Sci. 2007;81:841–849. doi: 10.1016/j.lfs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Luthje L. Raupach T. Michels H. Unsold B. Hasenfuss G. Kogler H. Andreas S. Exercise intolerance and systemic manifestations of pulmonary emphysema in a mouse model. Respir Res. 2009;10:7. doi: 10.1186/1465-9921-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacNee W. Oxidants and COPD. Curr Drug Targets Inflamm Allergy. 2005;4:627–641. doi: 10.2174/156801005774912815. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra D. Thimmulappa R. Navas-Acien A. Sandford A. Elliott M. Singh A. Chen L. Zhuang X. Hogg J. Pare P. Tuder RM. Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Matsura T. Kai M. Fujii Y. Ito H. Yamada K. Hydrogen peroxide-induced apoptosis in HL-60 cells requires caspase-3 activation. Free Radic Res. 1999;30:73–83. doi: 10.1080/10715769900300081. [DOI] [PubMed] [Google Scholar]
- 29.Nisbet RE. Bland JM. Kleinhenz DJ. Mitchell PO. Walp ER. Sutliff RL. Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol. 2010;42:482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papapetropoulos A. Pyriochou A. Altaany Z. Yang G. Marazioti A. Zhou Z. Jeschke MG. Branski LK. Herndon DN. Wang R. Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangasamy T. Cho CY. Thimmulappa RK. Zhen L. Srisuma SS. Kensler TW. Yamamoto M. Petrache I. Tuder RM. Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato A. Hoshino Y. Hara T. Muro S. Nakamura H. Mishima M. Yodoi J. Thioredoxin-1 ameliorates cigarette smoke-induced lung inflammation and emphysema in mice. J Pharmacol Exp Ther. 2008;325:380–388. doi: 10.1124/jpet.107.134007. [DOI] [PubMed] [Google Scholar]
- 33.Stipanuk MH. Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sussan TE. Rangasamy T. Blake DJ. Malhotra D. El-Haddad H. Bedja D. Yates MS. Kombairaju P. Yamamoto M. Liby KT. Sporn MB. Gabrielson KL. Champion HC. Tuder RM. Kensler TW. Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueda S. Masutani H. Nakamura H. Tanaka T. Ueno M. Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 36.van der TM. Rezayat D. Kauffman HF. Bakker SJ. Gans RO. Koeter GH. Choi AM. van Oosterhout AJ. Slebos DJ. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L109–L114. doi: 10.1152/ajplung.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 38.Wedgwood S. Black SM. Role of reactive oxygen species in vascular remodeling associated with pulmonary hypertension. Antioxid Redox Signal. 2003;5:759–769. doi: 10.1089/152308603770380061. [DOI] [PubMed] [Google Scholar]
- 39.Xia M. Chen L. Muh RW. Li PL. Li N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Ther. 2009;329:1056–1062. doi: 10.1124/jpet.108.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu F. Zhuang J. Wang R. Seagrave JC. March TH. Blunted ventilatory response to hypoxia/hypercapnia in mice with cigarette smoke-induced emphysema. Respir Physiol Neurobiol. 2007;158:5–13. doi: 10.1016/j.resp.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zanardo RC. Brancaleone V. Distrutti E. Fiorucci S. Cirino G. Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W. Zhang J. Lu Y. Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.