Abstract
The present natural history study examined the course of CFS from 1995–1997 (Wave 1) to approximately ten years later (Wave 2) from a random, community-based, multi-ethnic population.. The rate of CFS remained approximately the same over the period of time from Wave 1 to Wave 2, although a high level of mortality was found (18% of those with medical or psychiatric exclusions group, 12.5% for the CFS group). Physical measures of disability and fatigue, along with measures of specific somatic symptoms, better differentiate individuals who later are diagnosed with CFS than more psychosocial measures such as stress and coping.
Keywords: Chronic fatigue syndrome, natural history, epidemiology, risk factors
Despite growing knowledge about long-term predictors of chronic fatigue syndrome (CFS) outcomes, many follow-up studies are not prospective in that they either rely on retrospective self-report at a single point in time or they consist of longitudinal data that are analyzed in a cross-sectional manner without taking into account the influence of baseline findings. Moreover, many CFS follow-up studies employ medical care samples instead of random community samples of socioeconomically and ethnically diverse populations (Hill, Tiersky, Scavalla, Lavietes, & Natelson, 1999; Richman, Flaherty, & Rospenda, 1994). Clearly, there is a need for more research on the incidence and course of CFS in ethnically and socioeconomically diverse, community populations.
The Agency for Healthcare Research and Quality of the US Department of Health and Human Services (2001) issued an Evidence Report on CFS, and they concluded that estimating rates of recovery/improvement or relapse from severe fatigue is not possible because there are so few natural history studies available. Clearly, there is a need for longitudinal cohort studies with representative samples that identify risk factors for both recovery/improvement and relapse. Below we review some of the more prominent risk factors that have been associated with CFS.
Several long-term natural history studies are available, but they often did not include a physician examination and psychiatric interview (Harvey, Wadsworth, Wessely, & Hotopf, 2008) or were not based on a representative sample (Friedberg, Dechene, McKenzie, & Fontanetta, 2000). If a physical and psychiatric examination is not included, it is not possible to diagnose CFS using the Fukuda et al. (1994) criteria. The present study examined the course of CFS over approximately a ten year period of time, with a random, community-based, multi-ethnic population. We estimated whether rates of CFS had changed in our sample over time. In our prior studies with the Chicago community-based sample, we have found associations between CFS and life stressors, female gender, older age, minority status, lower SES, and higher fatigue severity. We therefore assessed whether these socio-environmental factors might be associated with CFS status at ten year follow-up.
Method
The present project was carried out in two stages. In Stage 1, we attempted to re-contact the 213 adults who were medically and psychiatrically evaluated from 1995–1997. These adults were previously evaluated in our original Wave 1 CFS epidemiology project (Jason, Jordan et al., 1999). Stage 2 of the study encompasses a structured psychiatric assessment and a complete physical examination and a structured medical history. The original Wave 1 sample is a stratified random sample of several neighborhoods in Chicago specifically selected to contain individuals from different ethnic and socioeconomic profiles. As a whole, Chicago, Illinois is an ethnically and socioeconomically diverse city (See Jason et al., 1999 for more details).
Stage 1
In the first stage of data collection in the original study, procedures developed by Kish (1965) were used to select one adult from each household for subsequent screening for CFS-like illness. The CFS Screening Questionnaire consists of two parts and was administered to all participants. It assesses participants’ sociodemographic characteristics and fatigue characteristics to determine whether any changes have occurred since the first wave of data collection in the original study. The Fatigue Scale (Chalder et al., 1993), was administered in the original Wave 1 study (Jason, Jordan, et al., 1999), and was contained within Part 1 of the CFS Screening Questionnaire. Despite its brevity, the scale has been found to be reliable and valid, possessing good face validity and reasonable discriminant validity (Chalder et al., 1993) (alpha = .85, current sample).
Stage 2
In Stage 2, the Structured Clinical Interview for the DSM-IV (SCID) (Spitzer, Williams, Gibbon, & First, 1995) was administered to assess current psychiatric diagnoses as defined on Axis I of the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV) (American Psychiatric Association, 1994). The SCID is a valid and reliable semi-structured interview guide that approximates a traditional psychiatric interview (First, Spitzer, Gibbon, & Williams, 1995).
Following the structured psychiatric interview, participants were provided a medical history interview and complete medical examination. The Medical Questionnaire is a modified version of The Chronic Fatigue Questionnaire, a structured instrument developed by Komaroff and Buchwald (1991). We also used the Perceived Stress Scale (PSS), which is a reliable four-item revised version of a measure of global perceived stress (alpha = .80, current sample). We also administered the Coping Orientation to Problems Experienced Scale (COPE), which consists of five conceptually distinct scales of problem-focused coping and five conceptually distinct scales of emotion-focused coping (Carver, Scheier, & Weintraub, 1989) (alpha ranged from .61 to .94). We also administered the revised version of the Life Orientation Test (LOT), which provided an overall score of optimism (Scheier & Carver, 1985) (alpha = .76). We also administered the Social Support Questionnaire (SSQ6) (Sarason, Sarason, Shearin, & Pierce, 1987), which assesses the number of available others whom the individual feels he or she can turn to in times of need in a variety of situations (Number or Perceived Availability) and the individual’s degree of satisfaction (Satisfaction score) with the perceived support available in that particular situation (alpha = .99). In addition, the Medical Outcomes Study 36-item Short-Form Survey (Ware & Sherbourne, 1992), a reliable and valid measure, was administered in Stage 2 to discriminate between gradations of disability. A physical and mental composite score was used to assess overall levels of physical and mental functioning (alpha 93, .90, respectively).
Following the medical history interview, the physician conducted a detailed medical examination (see Jason et al., 1999 for more details). At the time of evaluation, the examining physician was blinded to participants’ status with respect to initial classification based upon the Stage 1 screen. Participants were reimbursed $100.00 for the time and effort involved in participation. Participants also signed the Human Subjects Consent Form at DePaul University.
Diagnosing CFS
At the end of Stage 2, a team of physicians was responsible for making final diagnoses. Two physicians independently rated each file according to the current U.S. definition of CFS, Idiopathic Chronic Fatigue (ICF), Exclusionary for CFS due to medically/psychiatrically explained chronic fatigue (Fukuda et al., 1994), or Control (participants with no exclusionary illness and less than 6 months of fatigue). Those with ICF had at least six months duration of fatigue, but with insufficient symptoms or fatigue to meet the case definition of CFS. The Exclusionary group had medically explained chronic fatigue for at least six months duration of fatigue, but with medical explanations of the fatigue, and those with psychiatric explanations of the fatigue (e.g., delusional disorders, schizophrenia, etc). Controls had less than 6 months or fatigue. Reviewing physicians had access to all information gathered on each participant during each of the phases of the study. The review panel was also provided with all results from the physical exam. If a disagreement occurred regarding whether a participant should receive a diagnosis of CFS, Idiopathic Chronic Fatigue, Exclusionary due to medically/psychiatrically explained chronic fatigue, or Controls during the physician review process, the participant’s file was rated by a third physician reviewer, and the diagnosis was determined by majority rule. We used refinements of the Fukuda et al. criteria as recommended by an International Research group and the CDC (Reeves, Lloyd et al., 2003). For example, morbid obesity is exclusionary as it could cause severe fatigue, but the Body Mass Index cut off has been changed to 40 or higher. In addition, a lifetime history of major depressive disorder with melancholic, anorexia nervosa, or bulimia is now not exclusionary if these conditions resolved more than 5 years before the onset of the current chronically fatiguing illness.
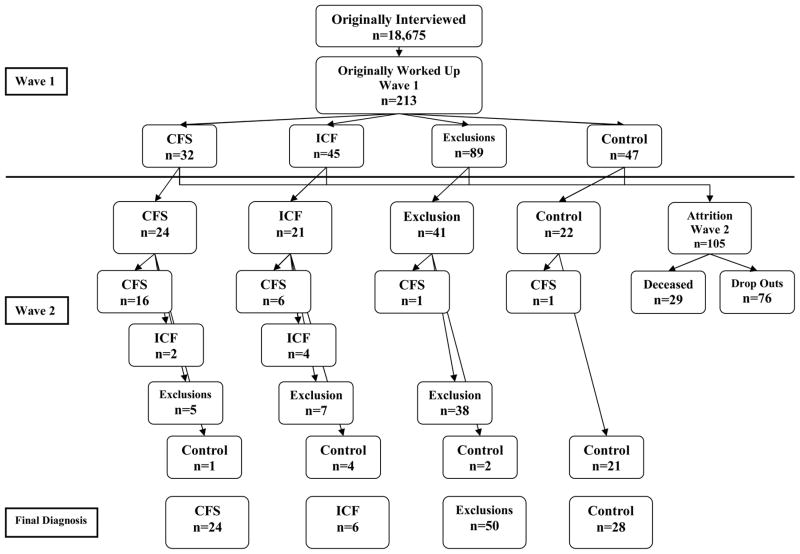
For Wave 1, we had physician consensus diagnoses for the following conditions: 32 with CFS, 45 with ICF, 89 with Exclusionary conditions, and 47 Controls. Figure 1 provides the flow of participants from Wave 1 to Wave 2. By Wave 2, of the original group of 32 individuals with CFS, 4 had died, and 24 were found and agreed to be re-evaluated (completion rate of 24/28=86%). By Wave 2, among the 45 ICF individuals at Wave 1, 4 had died, and we were able to re-evaluate 21 (completion rate of 51%). By Wave 2 among those 89 with an Exclusionary illness at Wave 1, 16 had died and we were able to re-evaluate 41 (completion rate of 56%). Finally, by Wave 2, among the 47 Controls at Wave 1, 5 had died, and we were able to reevaluate 22 (completion rate of 53%). Of course, all participants did not stay within their Wave 1 category over time, so the final four categories consisted of 24 CFS, 6 ICF, 50 Exclusions, and 28 Controls.
Figure 1.
Participant Group Movement from Wave 1 to Wave 2
Statistical Analyses
We used ANOVAs or Chi-squares to test for significant overall effects occurred across the CFS, ICF, Exclusionary or Control groups. If an overall test was significant, for continuous measures, we used post-hoc Tukey tests to determine differences among conditions. We used a Bonferroni correction for dichotomous variables (with 4 groups means and 6 pair wise chi-square comparisons, we used .05/6=.0083 as our criterion for significance).
Results
Sociodemographic Characteristics
We examined Wave 1 differences between those we were able (N = 108) versus those were not able to re-evaluate at Wave 2 (N = 105), and we did not find any significant sociodemographic differences. Given the high attrition rate (49%), this finding suggests that our final Wave 2 sample did not have any systematic bias.
Prevalence of CFS
Of those 24 individuals with CFS at Wave 1 who were re-assessed at Wave 2, 16 continued to be diagnosed with CFS (See Figure 1). In other words, 16 of the 24 (67%) participants with CFS at Wave 1 continued to have CFS in our sample at Wave 2. Of the 21 ICF individuals at Wave 1 who were re-assessed at Wave 2, 6 had developed CFS by Wave 2. Of the 41 with an exclusionary illness at Wave 1 who were re-assessed at Wave 2, 1 was diagnosed with CFS by Wave 2. Finally, among the 22 controls at Wave 1 who were re-assessed at Wave 2, 1 had developed CFS by Wave 2. We compared the number diagnosed with CFS among the sample of 108 Wave 2 participants with the same group of 108 participants at Wave 1. Using McNemar’s test, there was no significant change between Wave 1 and Wave 2 in the proportion of subjects who had confirmed CFS. At Waves 1 and 2, a similar number of individuals (N = 24) within the same sample of 108 had a confirmed diagnosis of CFS. This suggests that the prevalence of CFS had not changed by Wave 2. The reason that the number was similar is that 8 individuals with a Wave 1 diagnosis no longer were counted as a CFS case in Wave 2, and 8 additional cases emerged from the three other groups.
There appeared to be a high level of mortality among the different groups over time, with 18% (16/89) for the Exclusionary group, 12.5% (4/32) for the CFS group, 10.6% (5/47) for the Controls, and 8.9% (4/45) for the ICF group. Among those in the Wave 1 CFS group that died, two had septicemia and one had died of it. Septicemia is the presence of bacteria in the blood and is often associated with severe disease. The other three with CFS had died of lung cancer. The ages of death among those with CFS were 58, 69, 71 and 76. Although the CFS group had higher rates of mortality than those in the Control and ICF groups, the highest mortality was found for the Exclusionary group.
Risk Factors associated with CFS at Wave 2
Sociodemographic characteristics of the four Wave 2 diagnosed groups (i.e., 24 with CFS, 6 with ICF, 50 Exclusionary, and 28 Controls are in Table 1). In this table, the sociodemographic characteristics were from Wave 1. The Exclusionary group in comparison to Controls had significantly lower SES scores, a significantly higher percent women and children, and significantly lower educational levels. A significantly higher percent of the CFS group were women than in the Control group. Those in the Exclusionary group were significantly more likely to have psychiatric co-morbidities than those in the CFS and Control groups. Although the racial variable was not significant, it is interesting to note that 64.3% of Controls and only 37.5% of the CFS group were White, confirming previous findings that CFS is more common among minorities (Jason et al., 1999).
Table 1.
Wave 1 Sociodemographics for Wave 2 Categorization (N=108)
| CFS (n=24) | ICF (n=6) | Exclusion (n=50) | Controls (n=28) | p | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| Age | 40.00 (10.49) | 39.67 (16.50) | 41.46 (10.49) | 39.89 (12.20) | |
| SES (Hollingshead) | 41.13 (17.19) | 36.67 (18.04) | 35.52 (14.28)a | 49.03 (11.53)a | ** |
|
| |||||
| N (%) | N (%) | N (%) | N (%) | ||
| Gender | |||||
| Male | 5 (20.8) | 2 (33.3) | 10 (20.0) | 18 (64.3) | *** |
| Female | 19 (79.2) | 4 (66.7) | 40 (80.0) | 10 (35.7) | |
| Race | |||||
| Black | 5 (20.8) | 1 (16.7) | 11 (22.0) | 5 (17.9) | |
| White | 9 (37.5) | 3 (50.0) | 20 (40.0) | 18 (64.3) | |
| Hispanic/Latino | 8 (33.3) | 2 (33.3) | 16 (32.0) | 2 (7.1) | |
| Other | 2 (8.3) | 0 (0.0) | 3 (6.0) | 3 (10.7) | |
| Marital Status | |||||
| Married | 11 (45.8) | 2 (33.3) | 11 (22.0) | 8 (28.6) | |
| Separated/Widowed/Divorced | 6 (25.0) | 2 (33.3) | 15 (30.0) | 5 (17.9) | |
| Never Married | 7 (29.2) | 2 (33.3) | 24 (48.0) | 15 (53.6) | |
| Children | |||||
| Yes | 14 (58.3) | 4 (66.7) | 30 (60.0) | 8 (28.6) | * |
| No | 10 (41.7) | 2 (33.3) | 20 (40.0) | 20 (71.4) | |
| Education | |||||
| H/S Degree/GED or Less | 8 (33.3) | 2 (33.3) | 26 (52.0) | 6 (21.4) | * |
| Some College/Spec. Training | 4 (16.7) | 1 (16.7) | 12 (24.0) | 4 (14.3) | |
| College/Grad Degree | 12 (50.0) | 3 (50.0) | 12 (24.0) | 18 (64.3) | |
| Work Status | |||||
| Disability/Unemployed/Retired | 5 (20.8) | 1 (16.7) | 16 (32.0) | 4 (14.3) | |
| Student/Homemaker | 7 (29.2) | 2 (33.3) | 7 (14.0) | 2 (7.1) | |
| Part-time | 2 (8.3) | 0 (0.0) | 6 (12.0) | 3 (10.7) | |
| Full-time | 10 (41.7) | 3 (50.0) | 21 (42.0) | 19 (67.9) | |
| Psychiatric Comorbidity | |||||
| Yes | 9 (37.5) | 4 (66.7) | 43 (86.0) | 9 (32.1) | *** |
| No | 15 (62.5) | 2 (33.3) | 7 (14.0) | 19 (67.9) | |
Statistically significant difference at the p < .05 level.
Statistically significant difference at the p<.01 level.
Statistically significant difference at the p<.001 level.
Similar superscript letters across row variables indicate significant differences.
Table 2 presents other measures from Wave 1 for the four Wave 2 participant groups. When examining the measures of disability, fatigue, and psychosocial factors, the CFS group only had significantly more impairment than the Controls for the SF-36 Physical Composite index and the Chalder Fatigue Scale, but for none of the measures of stress, support or coping. In contrast, the Exclusionary group was scored significantly lower than the Controls on the Physical and Mental Composite index, the Chalder Fatigue Scale, Satisfaction with Support, Optimism and Behavioral Disengagement. In regard to other diagnoses, the CFS group had a significantly higher percentage of Irritable Bladder than the Control group. A significantly higher percentage of the Exclusionary group had hypersomnia than the Controls. For the Fukuda et al. symptoms, the CFS and Exclusionary group had significantly more unrefreshing sleep, impaired memory and concentration, and headaches than the Controls. For joint pain, the Exclusionary group had significantly more symptoms than Controls.
Table 2.
Wave 1 Risk Factors for Wave 2 Categorization (N=108)
| CFS (n=24) | ICF (n=6) | Exclusion (n=50) | Controls (n=28) | p | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| MOS | |||||
| Physical Composite Score | 38.82 (10.46)b | 40.03 (5.51) | 37.37 (9.85)a | 46.88 (12.54)ab | ** |
| Mental Composite Score | 40.28 (11.40) | 42.11 (13.43) | 33.86 (9.13)a | 45.76 (11.72)a | *** |
| Chalder Fatigue Scale | 19.79 (5.07)b | 19.83 (7.41) | 20.74 (5.22)a | 14.89 (4.61)ab | *** |
| Perceived Stress Scale | 7.27 (3.91) | 7.83 (2.99) | 9.04 (2.96)a | 5.50 (3.40)a | *** |
| Social Support | |||||
| Number of Social Supports | 2.47 (1.83) | 3.75 (1.65) | 2.00 (1.39) | 2.80 (1.89) | * |
| Satisfaction with Social Supports | 5.19 (1.15) | 5.36 (.73) | 4.73 (1.20)a | 5.58 (.87)a | ** |
| Optimism | |||||
| Life Orientation Test Score | 16.64 (3.24)c | 15.00 (6.32) | 13.09 (4.44)ac | 18.00 (4.03)a | *** |
| Cope Scale | |||||
| Seeking Social Support | 2.40 (.88) | 2.17 (.86) | 2.38 (.90) | 2.89 (.81) | |
| Positive Reinterpretation And Growth | 3.12 (.57) | 2.67 (.56) | 2.81 (.77) | 3.20 (.81) | |
| Acceptance | 3.05 (.52) | 2.79 (.58) | 2.99 (.72) | 2.81 (.65) | |
| Turning to Religion | 2.88 (.99) | 2.75 (1.08) | 2.98 (1.11) | 2.86 (1.29) | |
| Venting | 2.57 (.57) | 2.54 (.68) | 2.82 (.80) | 2.42 (.65) | |
| Denial | 1.88 (.78) | 1.71 (.33) | 2.07 (.92) | 1.60 (.53) | |
| Behavioral Disengagement | 1.80 (.72) | 1.83 (.47) | 2.21 (.84)a | 1.48 (.43)a | ** |
|
| |||||
| % | % | % | % | ||
| Other Diagnoses | |||||
| Muscle Weakness | 77.3 | 80.0 | 67.4 | 40.7 | |
| Multiple Chemical Sensitivities | 56.5 | 50.0 | 54.0 | 28.6 | |
| Insomnia | 52.2 | 66.7 | 53.1 | 25.0 | |
| Irritable Bladder | 47.8 | 0.0 | 24.5 | 3.6 | *** |
| Hypersomnia | 30.4 | 50.0 | 49.0 | 17.9 | * |
| Irritable Bowel Syndrome | 21.7 | 16.7 | 16.3 | 10.7 | |
| Fibromyalgia | 17.4 | 16.7 | 14.0 | 0.0 | |
| Mononucleosis | 13.0 | 33.3 | 10.2 | 0.0 | |
| Hepatitis | 8.7 | 16.7 | 10.2 | 3.6 | |
| Fukuda Symptoms | |||||
| Unrefreshing Sleep | 87.0 | 83.3 | 87.8 | 30.8 | *** |
| Impaired Memory or Concentration | 82.6 | 66.7 | 72.9 | 32.1 | ** |
| Muscle Pain | 81.8 | 83.3 | 73.5 | 42.3 | |
| Headaches | 73.9 | 83.3 | 84.4 | 32.1 | *** |
| Joint Pain | 65.2 | 83.3 | 67.3 | 29.6 | * |
| Post-Exertional Malaise | 50.0 | 33.3 | 50.0 | 30.8 | |
| Sore Throat | 43.5 | 50.0 | 56.3 | 39.3 | |
| Lymph Node Pain | 30.4 | 16.7 | 30.4 | 7.4 | |
Statistically significant difference at the p < .05 level.
Statistically significant difference at the p<.01 level.
Statistically significant difference at the p<.001 level.
Similar superscript letters across row variables indicate significant differences.
Supplementary Analyses
Although sample sizes are relatively small, we were particularly interested in several distinct groups. By Wave 2, 16 cases of CFS at Wave 1 continued to have a CFS diagnosis at Wave 2, and we refer to this group as the Persist group. We also had a group of 27 individuals who did not have a diagnosis of CFS at Waves 1 and 2, and we refer to this group as Non-CFS (we did not include the one control case at Wave 2 that had a CFS diagnosis at Wave 1). The 8 new cases of CFS were referred to as the Incidence group (one came from the control group, one from exclusionary group, and 6 from the ICF group). Finally, there were 4 individuals who had a CFS diagnosis at Wave 1 but no longer had CFS at Wave 2 (we refer to them as the Remit group). For these 4 remitters, all of whom had CFS at Wave 1, but at Wave 2, one was classified as a Control and did not have symptoms, 2 were classified as ICF, and one was classified as an Exclusion. The person in the Exclusion category had a substance abuse disorder but no longer met the Fukuda criteria of CFS. The other 4 individuals in the Exclusion category had both exclusionary illnesses and still met all other CFS criteria as specified by Fukuda et al. (1994).
Sociodemographic characteristics of these four groups are in Table 3. Significant differences in Wave 1 sociodemographics were found for age, with those in the Remit group being significantly younger than the Incidence group. Table 4 presents findings on other risk variables, and the Persist group had significantly lower scores on the physical composite measure than the Incident and Non-CFS; and the Remit and Persist group had significantly higher fatigue scores than Non-CFS group.
Table 3.
Wave 1 Sociodemographics for Wave 2 Conditions (N=55)
| Persist (n=16) | Incidence (n=8) | Remit (n=4) | Non-CFS (n=27) | p | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| Age | 37.1 (9.6) | 45.8 (10.4)e | 25.8 (7.3)e | 40.4 (12.1) | * |
| SES (Hollingshead) | 40.9 (18.2) | 41.5 (16.0) | 37.0 (18.2) | 48.9 (11.7) | |
|
| |||||
| N (%) | N (%) | N (%) | N (%) | ||
| Gender | |||||
| Male | 4 (25.0) | 1 (12.5) | 2 (50.0) | 17 (63.0) | * |
| Female | 12 (75.0) | 7 (87.5) | 2 (50.0) | 10 (37.0) | |
| Race | |||||
| Black | 3 (18.8) | 2 (25.0) | 0 (0.0) | 5 (18.5) | |
| White | 8 (50.0) | 1 (12.5) | 2 (50.0) | 17 (63.0) | |
| Hispanic/Latino | 4 (25.0) | 4 (50.0) | 2 (50.0) | 2 (7.4) | |
| Other | 1 (6.2) | 1 (12.5) | 0 (0.0) | 3 (11.1) | |
| Marital Status | |||||
| Married | 7 (43.8) | 4 (37.5) | 1 (25.0) | 8 (29.6) | |
| Separated/Widowed/Divorced | 3 (18.8) | 3 (37.5) | 0 (0.0) | 5 (18.5) | |
| Never Married | 6 (37.5) | 1 (12.5) | 3 (75.0) | 14 (51.9) | |
| Children | |||||
| Yes | 8 (50.0) | 6 (75.0) | 2 (50.0) | 8 (29.6) | |
| No | 8 (50.0) | 2 (25.0) | 2 (50.0) | 19 (70.4) | |
| Education | |||||
| H/S Degree/GED or Less | 5 (31.2) | 3 (37.5) | 2 (50.0) | 6 (22.2) | |
| Some College/Spec. Training | 3 (18.8) | 1 (12.5) | 0 (0.0) | 4 (14.8) | |
| College/Grad Degree | 8 (50.0) | 4 (50.0) | 2 (50.0) | 17 (63.0) | |
| Work Status | |||||
| Disability/Unemployed/Retired | 4 (25.0) | 1 (12.5) | 0 (0.0) | 4 (14.8) | |
| Student/Homemaker | 6 (37.5) | 1 (12.5) | 1 (25.0) | 2 (7.4) | |
| Part-time | 1 (6.2) | 1 (12.5) | 1 (25.0) | 3 (11.1) | |
| Full-time | 5 (31.2) | 5 (62.5) | 2 (50.0) | 18 (66.7) | |
| Psychiatric Comorbidity | |||||
| Yes | 7 (43.8) | 3 (37.5) | 3 (75.0) | 8 (29.6) | |
| No | 9 (56.3) | 5 (62.5) | 1 (25.0) | 19 (70.4) | |
Statistically significant difference at the p < .05 level.
Similar superscript letters across row variables indicate significant differences.
Table 4.
Wave 1 Risk Factors for Wave 2 Conditions (N=55)
| Persist (n=16) | Incidence (n=8) | Remit (n=4) | Non-CFS (n=27) | p | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| MOS SF-36 | |||||
| Physical Composite Score | 34.19 (8.54)bd | 48.08 (7.53)d | 41.09 (9.34) | 46.78 (12.76)b | ** |
| Mental Composite Score | 38.36 (11.22) | 44.12 (11.60) | 35.82 (8.09) | 45.76 (11.94) | |
| Chalder Fatigue Scale | 19.94 (4.84)b | 19.50 (5.86) | 21.75 (4.79)a | 14.41 (3.91)ab | *** |
| Perceived Stress Scale | 7.07 (3.54) | 7.63 (4.72) | 7.25 (4.35) | 5.67 (3.35) | |
| Social Support | |||||
| Number of Social Supports | 3.45 (1.97) | 1.88 (1.86) | 3.25 (1.79) | 2.69 (1.83) | |
| Satisfaction with Social Supports | 5.74 (.39) | 4.86 (1.36) | 5.13 (1.26) | 5.56 (.88) | |
| Optimism | |||||
| Life Orientation Test Score | 17.00 (3.11) | 16.00 (3.59) | 12.50 (8.96) | 17.85 (4.03) | |
| Cope Scale | |||||
| Seeking Social Support | 2.45 (.87) | 2.31 (.94) | 2.25 (1.14) | 2.86 (.81) | |
| Positive Reinterpretation And Growth | 3.10 (.54) | 3.16 (.65) | 2.38 (.88) | 3.20 (.84) | |
| Acceptance | 3.07 (.58) | 3.00 (.40) | 2.94 (.55) | 2.78 (.64) | |
| Turning to Religion | 2.82 (1.05) | 2.97 (.94) | 2.44 (.97) | 2.94 (1.26) | |
| Venting | 2.60 (.38) | 2.53 (.83) | 2.81 (.52) | 2.41 (.66) | |
| Denial | 1.96 (.83) | 1.72 (.73) | 2.06 (1.20) | 1.63 (.52) | |
| Behavioral Disengagement | 1.80 (.84) | 1.78 (.51) | 2.00 (1.24) | 1.50 (.42) | |
|
| |||||
| % | % | % | % | ||
| Other Diagnoses | |||||
| Muscle Weakness | 86.7 | 57.1 | 50.0 | 42.3 | * |
| Multiple Chemical Sensitivities | 53.3 | 62.5 | 50.0 | 25.9 | |
| Insomnia | 60.0 | 37.5 | 100.0 | 22.2 | ** |
| Irritable Bladder | 53.3 | 37.5 | 0.0 | 3.7 | *** |
| Hypersomnia | 33.3 | 25.0 | 25.0 | 18.5 | |
| Irritable Bowel Syndrome | 20.0 | 25.0 | 25.0 | 11.1 | |
| Fibromyalgia | 20.0 | 12.5 | 0.0 | 0.0 | |
| Mononucleosis | 20.0 | 0.0 | 25.0 | 0.0 | * |
| Hepatitis | 6.7 | 12.5 | 50.0 | 3.7 | * |
| Fukuda Symptoms | |||||
| Unrefreshing Sleep | 93.3 | 75.0 | 75.0 | 28.0 | *** |
| Impaired Memory or Concentration | 100.0 | 50.0 | 50.0 | 29.6 | *** |
| Muscle Pain | 93.3 | 57.1 | 66.7 | 42.3 | * |
| Headaches | 73.3 | 75.0 | 75.0 | 29.6 | ** |
| Joint Pain | 66.7 | 62.5 | 66.7 | 29.6 | |
| Post-Exertional Malaise | 64.3 | 25.0 | 100.0 | 28.0 | ** |
| Sore Throat | 53.3 | 25.0 | 75.0 | 37.0 | |
| Lymph Node Pain | 46.7 | 0.0 | 0.0 | 7.7 | ** |
Statistically significant difference at the p < .05 level.
Statistically significant difference at the p<.01 level.
Statistically significant difference at the p<.001 level.
Similar superscript letters across row variables indicate significant differences.
In regard to other diagnoses, the Persist group had a significantly higher frequency of muscle weakness and Irritable Bladder than Non-CFS. The Remit group compared to Non-CFS had more insomnia, Mononucleosis and Hepatitis than Non-CFS. The Incidence group had a higher frequency of Irritable Bladder than Non-CFS. For the Fukuda et al. symptoms, the Persist group versus Non-CFS had a significantly higher frequency of the following symptoms: unrefreshing sleep, impaired memory and concentration, muscle pain, headaches, and lymph node pain. The Persist group versus the Incidence group had significantly higher frequency of impaired memory and concentration. Finally, the Remit group had significantly less memory and concentration symptoms than the Persist group, and the Remit group had more post-exertional malaise than the Non-CFS.
Discussion
The study’s major finding was that rates of CFS appear to have been relatively stable over the period of time from Wave 1 to 2. As rates of CFS were .42% in Wave 1 (Jason, Richman et al., 1999), estimates from our current natural history study suggest that these rates have stayed relatively constant over the past decade. Sixty-seven percent of participants with CFS at Wave 1 continued to have CFS in our sample at Wave 2, which is comparable to other reports about continued maintenance of CFS over time (Nisenbaum, Jones, Unger, Reyes, & Reeves, 2003). Of the 8 new cases of CFS over time, 6 (75%) came from the ICF group, suggesting that this group is at higher risk of developing CFS. In addition, 50% of the remitters went from a CFS diagnosis to the ICF group, indicating that while remitters no longer met CFS case definition, half were still suffering from chronic severe fatigue. There are few longitudinal studies that have been able to provide such estimates, particularly in culturally diverse, community-based samples.
Among the 108 participants that were followed up in Wave 2, Table 1 indicates that there were a number of sociodemographic differences at Wave 1 among these groups. The Exclusionary group had significantly lower SES scores, less education, more women and children, and higher psychiatric co-morbidity than the Control group. Clearly, the Exclusionary participants represent a high risk group. Those in the CFS group only differed from the Controls in having a higher percentage of women, and this tendency to find a higher percentage of women with CFS was also found in the Wave 1 data (Jason, Richman et al., 1999). In addition, of interest, those in the CFS group did not have higher levels of psychiatric co-morbidity than any of the other groups. Finally, the present study confirms previous findings that CFS is more common among minorities (Jason, Richman et al., 1999).
In Table 2, we examined those with a CFS status versus those who were placed in other diagnostic categories at Wave 2. The CFS group had significantly more impairment than the Controls only for Wave 1 Physical Composite scores and fatigue, but not for any of the other measures of stress, support or coping. It was only for Irritable Bladder that the CFS group had significantly higher percentages than the Control group. In addition, the CFS group did differ from the Controls for unrefreshing sleep, headaches, and memory and concentration. These findings suggest that physical measures of disability and fatigue, along with for Irritable Bladder and measures of specific somatic symptoms (unrefreshing sleep, headaches, and memory and concentration) better differentiated individuals who later are diagnosed with CFS than more psychosocial measures such as stress and coping. When examining all groups, the Exclusionary participants did have more variables in which they differentiated themselves from Controls, including physical and mental measures of disability, fatigue, satisfaction with support, optimism, behavioral disengagement, hypersomnia, unrefreshing sleep, impaired memory and concentration, headaches and joint pain. Therefore, Wave 1 psychosocial measures appear to play a larger role in differentiating the Exclusionary from Controls at Wave 2.
We also examined change over time on disability and fatigue measures for four theoretically interesting groups, those who persisted in having CFS over time, those who remitted, those who developed CFS and the Non-CFS group. Although the sample size of the Remit group was extremely small, we did find those in the Remit group to be significantly younger than the Incidence group. Younger age might be associated with recovery from CFS, and this is consistent with Joyce, Hotopf, and Wessely’s (1997) review article that found older age was a risk factor for poorer CFS prognosis.
Also of interest, the Incidence group that went on to develop CFS had significantly higher Wave 1 scores on the Physical Composite scores than the Persist group. This suggests that physical functioning did not serve as a protective factor for this group that went on to develop CFS. Those who Remitted and Persisted had significantly higher Wave 1 fatigue scores than the Non-CFS group, and this suggests that fatigue scores alone do not appear to be a discriminator as to those who recover versus those who do not. In addition, none of the Wave 1 measures of stress, support, optimism or coping differentiated the four groups.
In comparison to the Non-CFS group, the Persist group had a significantly higher frequency of the following Wave 1 symptoms: Irritable Bladder, muscle weakness, unrefreshing sleep, impaired memory and concentration, muscle pain, headaches, and lymph node pain. This is understandable as the Persist group had CFS at Wave 1 whereas the Non-CFS did not have this illness. Those who went on to develop CFS, the Incidence group, had a significantly higher frequency of Irritable Bladder than the Non-CFS group. It is at least possible that Irritable Bladder might represent a prognostic indicator of being at risk for developing CFS. Those who remitted from a CFS diagnosis had more insomnia, Mononucleosis, Hepatitis, and post-exertional malaise than Non-CFS, and post-exertional malaise than the Non-CFS group. But this Remit group had significantly less memory and concentration symptoms than the Persist group. Even though the Remit group was extremely small, and any conclusions about this group need to be tempered with that limitation, the findings do suggest that those who remit from a CFS diagnosis seem to have a number of symptoms that are more prevalent than the Non-CFS group, indicating that they are highly symptomatic at Wave 1; however, they appear to have at least one symptom, memory and concentration problems at a less prevalent rate than those who Persist with a CFS diagnosis. Finally, the Persist group had a significantly higher frequency of impaired memory and concentration than the Incidence group, and this is explainable by the fact that the Persist group had CFS at Wave 1 whereas the Incidence group had not yet developed this illness.
Although the CFS group had a high rate of follow-up, those in the other groups were much more difficult to track over time. Fortunately, we did not find significant sociodemographic differences at Wave 1 between those we retained in the sample versus those that we were not able to re-contact, and this provides support for the generalizability of the outcomes to the larger sample. Another limitation in our study included the small sample sizes, and particularly with the supplementary analyses. Still, given the low base rates of CFS, this is relatively common in studies on CFS, particularly when dealing with community-based samples. The measures presented in this paper deal with psychological and self-report measures, and future papers will include neuroendocrine and immunologic variables or more qualitative data,
In summary, the present study found that rates of CFS have remained relatively stable over the past decade. In addition, higher death rates than expected were found in all groups over time. There is a dearth of studies that have followed representative samples of patients with CFS over time, and the present study adds to the literature on the natural history of CFS over time.
Acknowledgments
The authors appreciate the financial assistance provided by the National Institute of Allergy and Infectious Diseases (grant number AI055735).
Contributor Information
Leonard A. Jason, DePaul University
Nicole Porter, DePaul University.
Jessica Hunnell, DePaul University.
Alfred Rademaker, Northwestern University.
Judith A. Richman, University of Illinois, Chicago
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 1994. [Google Scholar]
- Bombardier CH, Buchwald D. Outcome and prognosis of patients with chronic fatigue versus chronic fatigue syndrome. Archives of Internal Medicine. 1995;155:2105–2110. [PubMed] [Google Scholar]
- Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology. 1989;56:267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, Wallace EP. Development of a fatigue scale. Journal of Psychosomatic Medicine. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamark T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;37(2):147–153. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient edition. New York: Biometrics Research Department; 1995. [Google Scholar]
- Friedberg F, Dechene L, McKenzie MJ, II, Fontanetta R. Symptom patterns in long-duration chronic fatigue syndrome. Journal of Psychosomatic Research. 2000;48:59–68. doi: 10.1016/s0022-3999(99)00077-x. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Annals of Internal Medicine. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Harvey SB, Wadsworth M, Wessely S, Hotopf M. Etiology of chronic fatigue syndrome: Testing popular hypotheses using a national birth cohort study. Psychosomatic Medicine. 2008;70:488–495. doi: 10.1097/PSY.0b013e31816a8dbc. [DOI] [PubMed] [Google Scholar]
- Hill NF, Tiersky LA, Scavalla VR, Lavietes M, Natelson BH. Natural history of severe chronic fatigue syndrome. Archives of Physical Medicine Rehabilitation. 1999;80:1090–1094. doi: 10.1016/s0003-9993(99)90066-7. [DOI] [PubMed] [Google Scholar]
- Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor R, McCready W, Huang C, Plioplys S. A community-based study of chronic fatigue syndrome. Archives of Internal Medicine. 1999;159:2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- Kish L. Survey Sampling. N.Y.: Wiley; 1965. [Google Scholar]
- Komaroff AL, Buchwald D. Symptoms and signs of Chronic Fatigue Syndrome. Review of Infectious Diseases. 1991;13:S8–S11. doi: 10.1093/clinids/13.supplement_1.s8. [DOI] [PubMed] [Google Scholar]
- Nisenbaum R, Jones JF, Unger ER, Reyes M, Reeves WC. A population-based study of the clinical course of chronic fatigue syndrome. Health and Quality of Life Outcomes. 2003;1:49. doi: 10.1186/1477-7525-1-49. (Available at http://www.hqlo.com/content/1/1/49) [DOI] [PMC free article] [PubMed]
- Richman JA, Flaherty JA, Rospenda KM. Risk factors for chronic fatigue syndrome: Flawed assumptions derived from treatment-based studies? American Journal of Public Health. 1994;84:282–284. doi: 10.2105/ajph.84.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WC, Lloyd A, Vernon SD, Klimas N, Jason L, Bleijenberg G, Evengard B, White PD, Nisenbaum R, Unger ER. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Services Research. 2003;3:25. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support: Practical and theoretical implications. Journal of Social and Personal Relationships. 1987;4:497–510. [Google Scholar]
- Scheier M, Carver C. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-IV – Non-Patient Edition (SCID-NP, Version 2.0) Washington DC: American Psychiatric Press; 1995. [Google Scholar]
- U.S. Department of Health and Human Services. AHRQ Publication No. 02-E001. Rockville, MD: 2001. Defining and managing chronic fatigue syndrome. [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SP-36): Conceptual framework and item selection. Medical Care. 1992 June;:473–483. [PubMed] [Google Scholar]