Abstract
H19 and Igf2 are expressed in a monoallelic fashion from the maternal and paternal chromosomes, respectively. A region upstream of H19 has been shown to regulate such imprinted expression of both genes in cis. We have taken advantage of a loxP/cre recombinase-based strategy to delete this region in mice in a conditional manner to determine the temporal requirement of the upstream region in initiating and maintaining the imprinted expression of H19 and Igf2. Analysis of allele-specific expression of H19 and Igf2 and DNA methylation at the H19 promoter demonstrates that this region controls the monoallelic expression of the two genes in different ways, suggesting that it harbors two functionally distinct regulatory elements. Continued presence of the region is required to silence maternal Igf2 in accordance with its proposed role as an insulator. However, it does not have a direct role in keeping the paternal H19 promoter silenced. Instead, on the paternal chromosome, the upstream element mediates epigenetic modifications of the H19 promoter region during development, leading to transcriptional silencing of H19. Thereafter, its presence is redundant for preventing transcription. Presently, this temporal requirement of the silencing element appears to be a unique cis activity in the mammalian system. However, it is likely that other cis-acting elements, positive and negative, have the ability to effect stable changes in the chromatin structure and are not constantly required to give signals to the transcriptional machinery.
Keywords: Genomic imprinting, conditional deletion, DMR, H19, Igf2
Certain loci in the mammalian genome exhibit functional inequivalence of the two alleles. Depending on the parent of origin, some genes are expressed exclusively from the maternal chromosome and others exclusively from the paternal chromosome (Efstratiadis 1994; Bartolomei and Tilghman 1997; Brannan and Bartolomei 1999). To achieve parent-of-origin-specific expression, three aspects are of prime importance. During gametogenesis, a mark must be set to make paternal and maternal alleles distinct from each other at the molecular level. Subsequently, as the embryo develops via cell division and cell differentiation, the distinction must be maintained. Finally, the transcriptional machinery must be able to recognize this distinguishing mark such that it manifests as allele-specific expression. Failure at any of the three steps will lead to loss of parentally imprinted expression.
H19 and Igf2 are part of a cluster of imprinted genes on mouse chromosome 7 (syntenic to human chromosome 11p15.5). The genes exhibit reciprocity in allele-specific expression. Only the maternal allele of H19 is expressed (Bartolomei et al. 1991) whereas for Igf2, it is the paternal allele that is active (DeChiara et al. 1991). The two genes are almost identical in their spatial and temporal expression patterns. In fact, expression in endodermal tissues has been demonstrated to be dependent on a common set of enhancers located between 7 and 9 kb downstream of the H19 promoter (Leighton et al. 1995). Also, the imprinting of the two genes is mechanistically linked. Deletion of H19 and the ∼10-kb region upstream of it leads to biallelic expression of Igf2 (Leighton et al. 1995). Molecular studies implicate sequences upstream of H19 as important for monoallelic expression of both H19 and Igf2. Maternal chromosome-specific hypersensitivity to nuclease digestion has been demonstrated at two regions that are ∼ 2.4 kb and 3.8 kb upstream of the H19 promoter (Hark and Tilghman 1998; Khosla et al. 1999). Also, this region displays paternal chromosome-specific hypermethylation (Tremblay et al. 1995) that extends from approximately −4.0 kb to −2.0 kb. The differential methylation patterns are evident at the gamete stage and are retained through development (Tremblay et al. 1997). Therfore, methylation of this region has been suggested to be responsible for controlling the imprinted expression of H19 and Igf2. Strong support in favor of this role also comes from mutant studies in which DNA methyltransferase gene (dnmt) has been deleted. In these mutants, H19 was shown to be expressed in a biallelic manner, whereas Igf2 expression was completely lost (Li et al. 1993).
Deletion of a part of the differentially methylated region (DMR) encompassing −3.7 kb to −2.1 kb upstream of H19 resulted in biallelic expression of H19 when paternally inherited, and biallelic expression of Igf2 when maternally inherited (Thorvaldsen et al. 1998). This experiment has provided genetic proof of the crucial role played by this region in regulating monoallellic expression of H19 and Igf2. Although these experiments demonstrate the importance of the DMR at the H19/Igf2 locus, they do not determine the exact role of the DMR. The effect of germ-line inheritance of this mutation was examined at the level of transcription and DNA methylation in differentiated tissue. Therefore, loss of monoallelic expression could be interpreted as a failure to set the imprint during gametogenesis, as a failure to maintain the imprint during cell division, or as the loss of a signal important for transcriptional regulation. To address these distinct functions, it is necessary to delete the DMR at different stages of development. A loxP/cre recombinase-based strategy (Gu et al. 1993, 1994) was used to delete the DMR in the germ line, in the zygote, and in differentiated tissue to discern its exact role.
Transcriptional regulation at the H19 locus has been investigated earlier using transgenic mice. These studies demonstrated that sequences downstream of −0.8 kb are sufficient to direct normal H19 expression (Pfeifer et al. 1996). Sequences between −4 kb and −0.8 kb were necessary to direct monoallelic expression of H19 transgenes and to induce methylation of the upstream region in a parent-specific manner. However, imprinting of these transgenes was observed only when they were present in multiple copies, suggesting that not all of the genetic information required for the imprinting of the H19 locus was present on these constructs (Pfeifer et al. 1996; Elson and Bartolomei 1997). More recent studies have shown that BAC transgenes carrying the sequences −7.0 kb to +133 kb are imprinted even when present in single copies (C. Kaffer and M. Srivastava, in prep.). Thus, upstream sequences only up to −7.0 kb were inferred to be necessary and sufficient for imprinting H19 and plausibly Igf2. DMRflox mutants were generated in which loxP sites flank the region −7 kb to −0.8 kb upstream of H19 (Fig. 1), enabling the deletion of this region dependent on expression of cre recombinase. The region includes the entire DMR (−4 to −2 kb), both clusters of hypersensitive sites (−3.8 and −2.4 kb), and all of the sequences sufficient for imprinting single-copy transgenes. On the basis of the analysis of these conditional mutants of mice, we demonstrate that the region harbors two regulatory elements that act in distinct ways to regulate monoallelic expression of Igf2 and H19 due to parental imprinting.
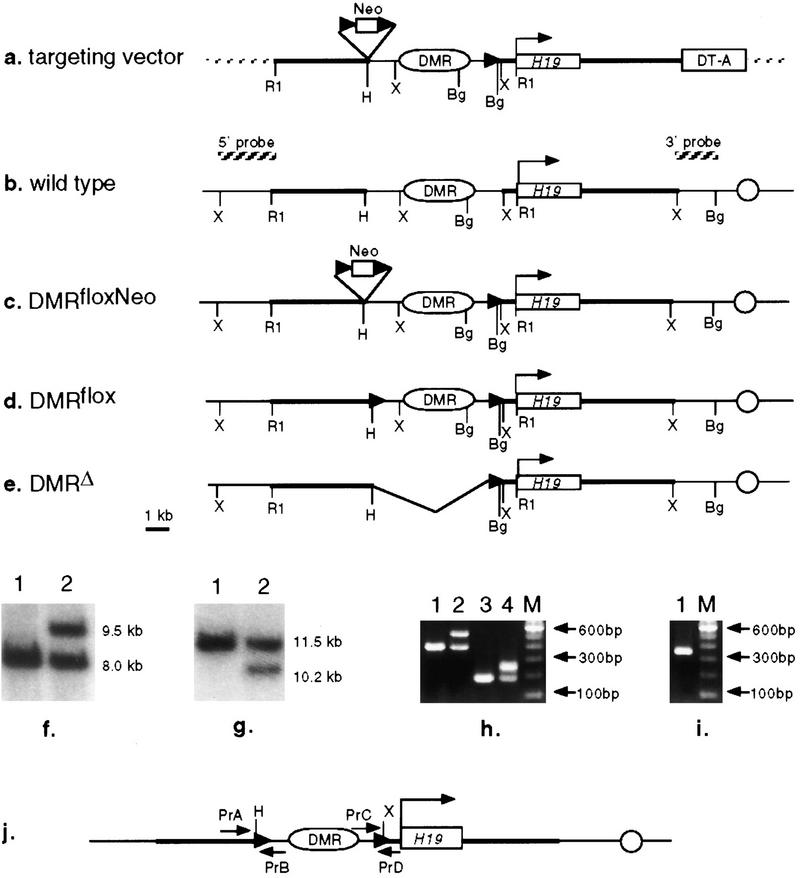
Figure 1.
Strategy for conditional deletion of the −7.0-kb to −0.8-kb region upstream of H19. (a) Targeting vector; (b) wild-type chromosome; (c) targeted chromosome, DMRfloxNeo; (d) targeted chromosome DMRflox after excision of the Neor selection marker; (e) the DMRΔ chromosome after cre recombinase-mediated excision at specific stages. (f,g) Southern hybridization of ES cell DNA to confirm the correctly targeted clones (DMRfloxNeo). Genomic DNA isolated from the ES cells was digested with XbaI (f) and hybridized to an EcoRI/XbaI fragment from the 5′ end of the targeted locus. Clones that have undergone homologous recombination (lane 2) yield a 9.5-kb band in addition to a 8.0-kb band derived from the endogenous non-targeted allele (lane 1). Similarly, BglII digests of the genomic DNA (g) hybridized to an XbaI/BglII fragment from the 3′ end of the targeted locus yield 11.5-kb and 10.2-kb bands from the wild-type (lane 1) and targeted alleles (lane 2), respectively, due to the presence of the additional BglII site at the −0.8-kb position. (h) DNA amplified from ES cell clones (DMRflox) correctly recombined by cre recombinase in vitro. The DMRflox allele was a result of cre recombinase-mediated excision of the Neo resistance gene. This was identified by amplifying the region around the −7.0-kb HindIII site (primers PrA and PrB). The endogenous locus (lane 1) gives a PCR product of 387 bp and the presence of the loxP site as a result of the correct excision event increases the size of this fragment to 520 bp. Presence of the other loxP site at −0.8-kb XbaI was confirmed by amplifying the region around it (Primers PrC and PrD). Wild-type (lane 3) and loxP carrying DNA samples yield PCR products of 177 and 234 bp, respectively. The correct clones carrying the DMRflox allele (lanes 2, 4) carry the loxP sites at both of these positions. (i) DNA amplified from genomic DNA of neonates (DMRΔ), in which DMR has been excised due to in vivo expression of cre recombinase under the control of different promoters (described in text). The excision was verified by PCR amplification (primers PrA and PrD) to give a 340-bp product. The authenticity of all of the PCR products was confirmed by sequencing. (j) Positions of the primers used for the PCR- based amplifications shown in h and i. Enhancers (circles), loxP sites (solid triangles), regions used as flanks (thick lines), transcription start site for H19 (arrow above H19 gene) and fragments used as probes (hatched lines above b) are shown. (DTA) Diptheria toxin A gene; (Bg) BglII; (H) HindIII; (R1) EcoRI; (X) XbaI; (M) DNA size markers.
Results
Generation of conditional mutants
To generate a mutation upstream of the H19 gene, a targeting vector (Fig. 1) was constructed that carried a loxP site at the −0.8-kb XbaI site and a neomycin-resistance gene flanked by loxP sites at the −7.0-kb HindIII site. Distances given are relative to the transcriptional start of H19. Correctly targeted clones of embryonic stem cells were identified by Southern hybridization. Cell lines heterozygous for this mutation, DMRfloxNeo, were subsequently electroporated with a supercoiled plasmid that directs the expression of cre recombinase. Resultant clones were analyzed using a PCR-based strategy to identify the recombination event that resulted in the excision of the neomycin-resistance gene without deleting the sequences between the −7.0-kb HindIII and −0.8-kb XbaI sites. Consequently, the region between the HindIII and XbaI sites was flanked by loxP sites (Fig. 1), generating the DMRflox allele. Thus, mice generated from such cell lines carried an allele of chromosome 7 in which the −7.0- to −0.8-kb region upstream of H19 could be deleted dependent on the in vivo expression of cre recombinase to generate DMRΔ. Successful deletion was monitored by a PCR assay (see Materials and Methods) and quantified, as needed, by Southern analysis.
Strategy for the assay of allele-specific expression
For all experiments, matings were set up such that two types of progeny were obtained. Experimental progeny carried a mutant allele of the DMR on a domesticus chromosome and a wild-type allele on a castaneus chromosome. Control littermates also carried a domesticus chromosome and a castaneus chromosome, but not the mutation under investigation.
To analyze the relative expression from the two chromosomes, we designed a single nucleotide primer extension (SNuPE) assay taking advantage of polymorphisms between domesticus and castaneus mice at the H19 locus. RNA from the tissue of interest was reverse transcribed and amplified for H19. A primer immediately upstream of the polymorphic base (+ 2395 of H19 relative to the transcription start) was extended using radioactively labeled dATP or dGTP in the absence of any other nucleotides. When the H19 primer is extended, relative dATP and dGTP incorporations give an estimate of cDNA derived from domesticus and castaneus alleles, respectively, and hence reflect the relative expression of H19 from the two parental alleles.
To authenticate the ability of the SNuPE assay to estimate the relative contribution of the two alleles, we assayed DNA samples from pure domesticus and pure castaneus mice and also their F1 progeny. As expected, pure domesticus DNA incorporated dATP and pure castaneus DNA incorporated dGTP, whereas F1 DNA incorporated both of these nucleotides (Fig. 2a). Theoretically, incorporation of dGTP and dATP in F1 DNA should be equivalent. Instead, we noticed a bias in favor of dATP. This bias could be attributed to a higher affinity of Taq polymerase for dATP compared with dGTP and/or to a difference in the specific activity of the two nucleotides. Considering this bias, we noted the requirement to include multiple F1 controls with each assay that we performed. On the basis of the relative incorporation of the two nucleotides by the F1 DNA, a correction factor was deduced specifically for each experiment (see Materials and Methods).
Figure 2.
SNuPE assays for H19 with control DNA templates. (a) DNA from castaneus (cas), domesticus (dom) and from castaneus × domesticus F1 (F1) mice. Castaneous DNA incorporates dGTP and domesticus DNA incorporates dATP. F1 DNA, representing a 1:1 mix of the castaneus and domesticus templates, incorporates both of the nucleotides. dATP incorporation in F1 is higher than dGTP incorporation, although, theoretically, it should be equivalent (see text). The experimentally observed relative incorporation of the two nucleotides by F1 DNA was used to derive a correction factor (see Materials and Methods) for dGTP incorporation. (b) Castaneus and domesticus DNA mixed in known proportions as indicated, amplified, and assayed by SNuPE. The expected values of A/(A+G) on the basis of relative concentrations of domesticus and castaneus DNA used in the mix are compared with those observed.
Next, DNA amplified from pure domesticus and pure castaneus animals was mixed in known proportions (castaneus:domesticus ratio varying from 8:1 to 1:8) and subjected to SNuPE (Fig. 2b). We observed a linear increase in the dATP incorporation and a linear decrease in the dGTP incorporation as the relative proportion of domesticus DNA increased. Relative incorporation of the two nucleotides was calculated as A/(A+G) taking the F1 correction into account and compared with the theoretically expected A/(A+G) values. The observed values were found to be comparable with the expected values, indicating that the SNuPE assay does reflect the relative concentration of DNA from the two alleles. Thus, we conclude that the SNuPE assay is capable of quantifying the relative abundance of RNA from domesticus and castaneus alleles in a given RNA sample and, therefore, enables an estimate of the paternal and maternal contributions to total H19 RNA.
A similar assay was used for estimation of the relative expression of Igf2 from the maternal and paternal alleles. In this case, a primer immediately upstream of the polymorphic base at position +440 of exon 6 was extended. This primer incorporates dGTP from the domesticus allele and dATP from the castaneus allele.
Effect of loxP sites insertion on expression of H19 and Igf2
Because the insertion of loxP sites may, even without deletion of the intervening sequences, interfere with allele-specific expression of H19 and Igf2, we investigated H19 and Igf2 expression in DMRflox mutants. Domesticus DMRflox males were mated to wild-type females carrying a castaneus allele at the H19/Igf2 locus. In +/DMRflox neonates, expression of H19 continues to be solely maternal (Fig. 3b; Table 1). DMRflox/+ progeny from a reverse cross, with maternal inheritance of the DMRflox, exhibited normal imprinting of Igf2 (Fig. 3g; Table 1), that is, solely paternal expression was observed. Thus, insertion of loxP sites did not lead to activation of either paternal H19 or maternal Igf2. In addition, the overall levels of expression of H19 and Igf2 in +/DMRflox and DMRflox/+ neonates were unaltered in liver, heart, and muscle as determined by Northern hybridization (data not shown).
Figure 3.
Effect of the DMR deletion mutations on H19 and Igf2 allele-specific expression. Skeletal muscle RNA samples were DNase treated, amplified for H19 and Igf2 by RT–PCR, and analyzed by SNuPE assays. (a,f) Demonstration of the efficiency of the assay for H19 and Igf2 respectively, by testing pure DNA templates, castaneus (cas), domesticus (dom), and DNA from castaneus × domesticus F1 mouse (F1), which represents a 1:1 mix of the templates. On the basis of a polymorphism between castaneus and domesticus alleles, the relative incorporation of the radiolabeled nucleotides dATP and dGTP during primer extension gives an estimate of relative abundance of the DNA from the two parental alleles. H19 expression was analyzed in neonates carrying a mutated paternal allele (b–e). Igf2 expression was analyzed in neonates carrying mutated maternal allele (g–j). Control littermates are either wild-type or DMRflox as indicated. The experimental chromosome (paternal for H19 and maternal for Igf2) is always domesticus, whereas the other chromosome is wild-type castaneus. For each RNA sample analyzed, RT-minus controls were used. These exhibited no amplification products, demonstrating the absence of any DNA contamination in the DNase treated RNA samples.
Table 1.
Allele specific expression of H19 and Igf2 in DMR mutants
|
|
% contribution of paternal allele total H19 mRNA
|
% contribution of maternal allele to total Igf2 mRNA
|
|---|---|---|
| Wild type | 2.5 ± 0.2 | 4.0 ± 0.9 |
| DMRflox | 2.9 ± 0.3 | 4.7 ± 1.3 |
| DMRΔG | 42.9 ± 6.2 | 34.7 ± 5.0 |
| DMRΔZ | 29.8 ± 2.5 | 40.5 ± 1.3 |
| DMRΔS | 1.7 ± 0.4 | 20.0 ± 0.2 |
Samples were analyzed by SNuPE assays. The values are from representative experiments and are an average derived from at least three skeletal muscle RNA samples. Similar results were noted from heart RNA samples in all cases. Pure castaneous DNA gives a background of 2 ± 0.5% for H19 and 5 ± 2% for Igf2. The values have not been corrected for this background but are corrected for differences in incorporation of dATP and dGTP by F1 DNA (see Materials and Methods).
Analysis of allele-specific expression of H19
Deletion of the DMR in the entire mouse, including the germ-line tissues, was afforded by mating DMRflox males with females expressing cre recombinase during early embryogenesis under the control of the EIIa promoter (Lakso et al. 1996). Mutants heterozygous for the deleted allele DMRΔG (for germ-line deletion) were then mated to wild-type mice homozygous for the castaneus allele of H19. When the deletion was inherited paternally, activation of paternal H19 was observed (Fig. 3c; Table 1). As suggested by the biallelic expression, an increase in the total H19 RNA was also observed by Northern analysis (data not shown). These results confirm the role of the DMR in imprinted expression of H19 as reported earlier (Thorvaldsen et al. 1998).
To address the role of the DMR after gametogenesis, we examined H19 expression in mice in which the DMR was intact in germ cells but was deleted after fertilization. Zygotes were obtained by mating males carrying the DMRflox allele with females homozygous for the wild-type castaneus allele. These zygotes were injected with plasmid pCAGGS-cre to induce transient expression of cre recombinase from the β-actin promoter (Araki et al. 1995). Neonates in which the DMR was successfully deleted were identified. DMRΔZ mutants (for zygotic deletion), like DMRΔG mutants, showed an activation of the paternal H19 (Fig. 3d; Table 1). As described earlier, setting up of the imprint during gametogenesis is normal in DMRflox males. Hence, these results demonstrate that the DMR plays a crucial role in maintaining the imprint of the locus or in transcriptional silencing.
To investigate the role of the DMR in transcription per se, we examined H19 expression in mice in which the region was deleted from the paternal chromosome late in development, at the final steps of differentiation. Such mice were obtained by mating males carrying the DMRflox allele with females expressing cre recombinase under the control of the muscle creatin kinase (MCK) promoter (Bruning et al. 1998) and carrying castaneus alleles of H19. The MCK promoter is expressed in terminally differentiated skeletal and cardiac muscle cells (Lyons et al. 1991; Sternberg et al. 1988) and has been demonstrated to cause cre recombinase-mediated excision at a high efficiency in these tissues (Bruning et al. 1998). Southern analysis demonstrated that excision of the DMR occurred in at least 50% of the cells in the muscle preparations and in a greater proportion of cells in hearts from neonates (data not shown). The partial excision observed was expected because the dissected muscle or heart tissue also contains non-muscle cells coming with connective tissue and blood vessels that should not express MCKcre. Strikingly, despite the DMR excision, expression of H19 in these DMRΔS (for somatic deletion) mutants remained completely monoallelic in muscle (Fig. 3e; Table 1) and heart (data not shown). We conclude that the DMR sequences and the imprint they carry are not required to actually prevent transcription of the paternal H19 allele. Rather, it appears that the DMR in its imprinted state must have directed changes during development, possibly in the H19 promoter region, that led to silencing of the paternal H19.
Analysis of DNA methylation around H19 promoter
At the gamete stage, paternal methylation of the H19 locus is restricted to the DMR. During early embryogenesis, however, the methylated region on the paternal chromosome spreads to encompass the H19 promoter and structural gene (Bartolomei et al. 1993; Brandeis et al. 1993; Ferguson-Smith et al. 1993; Tremblay et al. 1995, 1997). Considering the strong correlation between the silencing of H19 and methylation of its promoter region, we investigated the requirement of the DMR for this promoter methylation using restriction enzymes sensitive to methylation (Fig. 4). Genomic DNA derived from DMRΔG mutants inheriting the mutation either paternally or maternally was digested with BamHI and BglII and probed with an XbaI/BamHI probe as shown in Figure 4a. The DMRΔG allele yields a band of 1.5 kb as opposed to 2.5 kb from the wild-type DNA. When this digest was additionally digested with HpaII or HhaI, the wild-type paternal chromosome showed partial resistance to these methylation-sensitive enzymes, whereas the wild-type maternal chromosome was completely digested. In contrast, the DMRΔG allele on the paternal chromosome was digested completely and was indistinguishable from a maternal chromosome in this respect. DMRΔZ mutants gave identical results (data not shown). The methylation status of DMRΔS was investigated by digesting the genomic DNA with BamHI and HindIII using the XbaI/BamHI fragment as a probe. In this case wild-type, undeleted DMRflox and deleted DMRΔS alleles give 2.5-, 2.6-, and 1.6-kb bands, respectively. Additional digestion with methylation-sensitive enzymes HpaII and HhaI was performed. The paternal DMRΔS allele, like the wild-type paternal allele, was partially resistant to HpaII and HhaI and thus distinct from the maternal DMRΔS, indicating that the promoter stays methylated despite the absence of the DMR when deletion is effected in differentiated cells.
Figure 4.
CpG methylation in the H19 promoter region as assayed by digestion with methylation-sensitive enzymes. (a) Restriction map of the region. Wild-type and DMRflox alleles are distinguished by the presence of a BglII site at −0.8-kb (top). Cre recombinase mediated excision of the DMR keeps the BglII site and also brings in juxtaposition a HindIII site (bottom). (Thick lines) The probes used for hybridization; (*) BglII site present only on the DMRflox and DMRΔ alleles; (**) HindIII site present only on DMRΔ allele; (arrow) transcription start site for H19. (b) Maternal and paternal inheritance of DMRΔG. DNA prepared from skeletal muscle of neonates inheriting DMRΔG either maternally or paternally was digested with BamHI and BglII. Subsequent hybridization with a 1.4-kb probe (see a) reveals 2.5- and 1.5-kb bands representing the wild-type and mutant chromosomes, respectively. Additional digestion with HpaII and HhaI was performed to monitor methylation. (c) Deletion of the DMR mediated by MCK-cre recombinase. DNA was prepared from neonates in which the DMRΔS mutation was generated on the maternal or paternal chromosome. Control DNA is from +/DMRflox mutants not carrying the MCK-cre transgene. Digestion with BamHI and HindIII and subsequent hybridization as above reveals three bands of 2.6, 2.5, and 1.6 kb representing DMRflox, wild-type, and DMRΔS alleles, respectively. Portions of the enzyme digest were also digested with HpaII and HhaI and methylation of the DMRΔS allele was monitored by the disappearance of the 1.6-kb band. (B) BamHI; (Bg) BglII; (H) HindIII; (Hh) HhaI; (Hp) HpaII, and (X) XbaI.
The analysis of DNA methylation on the basis of digestion with methylation-sensitive enzymes and Southern hybridization gives an estimate of the population-averaged status of methylation at some specific enzyme sites. Bisulphite-based cytosine methylation analysis was performed to derive information about additional CpG residues in the H19 promoter region and to determine methylation patterns of individual chromosomes. Genomic DNA from the skeletal muscle of wild-type and mutant neonates was digested with BamHI, treated with bisulphite, amplified, cloned, and sequenced. Bisulphite treatment modifies all unmethylated cytosines to thymidines (Frommer et al. 1992). Methylated cytosines escape this conversion and hence can be recognized directly. Most of the 19 CpG residues between −170 and +167 bp were found to be methylated on the wild-type paternal chromosome, although the methylation at each individual residue was variable (Table 2) as has been reported earlier (Tremblay et al. 1997). However, hypermethylation was missing on the paternal chromosomes from which the DMR was deleted either in the germ line (DMRΔG) or at the zygotic stage (DMRΔZ). In DMRΔS mutants, hypermethylation on the mutated paternal chromosome was very similar to that of the wild-type paternal chromosome. Thus, a paternal DMR is required at least in the early embryo to direct CpG methylation of the H19 promoter region. However, once established, this promoter methylation is not dependent on the continued presence of the DMR. Because the DMR deletion in DMRΔS mutants occurs in terminally differentiated cells, our results do not address the stability of the promoter methylation on multiple cell divisions in the absence of the DMR.
Table 2.
Status of CpG methylation around the H19 promoter on paternal chromosome in DMR mutants
| CpG
|
wild type
|
DMRΔG
|
DMRΔZ
|
DMRΔS
|
|---|---|---|---|---|
| −170 | 5/8 | 0/7 | 0/5 | 11/14 |
| −164 | 6/8 | 0/7 | 0/5 | 13/14 |
| −147 | 6/8 | 0/7 | 1/5 | 13/14 |
| −143 | 5/8 | 0/7 | 0/5 | 10/14 |
| −139 | 6/8 | 0/7 | 0/5 | 13/14 |
| −131 | 3/8 | 0/7 | 0/5 | 9/14 |
| −106 | 3/8 | 0/7 | 0/5 | 12/14 |
| −97 | 5/8 | 0/7 | 0/5 | 11/14 |
| −94 | 6/8 | 0/7 | 0/5 | 12/14 |
| −59 | 7/8 | 0/7 | 0/5 | 13/14 |
| −46 | 8/8 | 0/7 | 0/5 | 13/14 |
| −20 | 3/8 | 0/7 | 0/5 | 6/14 |
| −6 | 3/8 | 0/7 | 0/5 | 8/14 |
| +3 | 6/8 | 0/7 | 0/5 | 9/14 |
| +43 | 0/8 | 0/7 | 1/5 | 13/14 |
| +81 | 3/8 | 0/7 | 0/5 | 11/14 |
| +90 | 8/8 | 0/7 | 2/5 | 12/14 |
| +102 | 3/8 | 0/7 | 1/5 | 12/14 |
| +167 | 8/8 | 2/7 | 1/5 | 14/14 |
Position of the CpG residues are in relation to the transcriptional start site of H19. Fraction of clones found to be methylated is shown.
We note that all four mutations DMRflox, DMRΔG, DMRΔZ, and DMRΔS, when present on the maternal chromosome, did not have any effect on monoallelic expression of H19, which continues to be solely maternal (data not shown). This result was expected on the basis of the cis-acting nature of the DMR (Thorvaldsen et al. 1998), (C. Kaffer and M. Srivastava, in prep.).
Analysis of allele-specific Igf2 expression
Allele-specific expression of Igf2 was also investigated subsequent to deletion of the DMR at different stages using protocols similar to those described above for H19. Deletion of the DMR from the maternal chromosome either in the germ line, DMRΔG, or in the zygote, DMRΔZ, led to biallelic expression of Igf2 (Fig. 3h,i; Table 1). When the DMR was deleted in differentiated cells using an MCK-promoter driven cre recombinase (DMRΔS), the excision efficiency in skeletal muscle and heart was >50% as described earlier. In contrast to what we observed for H19, excision of the DMR in differentiated cells results in a loss of imprinting of Igf2. The mutant maternal DMRΔS allele expressed Igf2 (Fig. 3j; Table 1). These results demonstrate that the DMR is continually required on the maternal chromosome to keep Igf2 silenced. Its deletion at any stage leads to biallelic expression of Igf2.
The presence of any of the four mutations, DMRflox, DMRΔG, DMRΔZ, and DMRΔS, on the paternal allele did not interfere with monoallelic paternal expression of Igf2 (data not shown) consistent with the idea that it is a cis-acting element (Thorvaldsen et al. 1998), (C. Kaffer and M. Srivastava, in prep.).
Discussion
Imprinting of H19 and Igf2 is regulated by a cis-acting element present upstream of H19 (Thorvaldsen et al. 1998). In the absence of this element, normally silent paternal H19 and maternal Igf2 alleles are transcribed. Such a loss of monoallelic expression will be manifest if the region is responsible for setting up the imprint during gametogenesis, maintaining the imprint in cells during development, or providing appropriate signals to the transcriptional machinery for monoallelic transcription. To understand the role of DMR in these distinct processes, we deleted the DMR during different stages that could be important for imprinted expression. The DMR was deleted in the germ line, in the zygote, and in terminally differentiated muscle cells. Analysis of the deleted mutants revealed that maternal Igf2 and paternal H19 are silenced by two distinct mechanisms, suggesting the presence of two regulatory elements in the deleted DMR (Fig. 5). Whether the two regulatory elements are structurally distinct or share any sequences remains to be determined.
Figure 5.
A model to explain H19 and Igf2 imprinting. Hypermethylation and other associated changes (close vertical bars) at the DMR on the paternal chromosome accomplish two functions. They inactivate the insulator function residing in the DMR to allow Igf2 promoter-enhancer interaction and also direct epigenetic modification (sparse vertical bars) of the H19 promoter region leading to its transcriptional silencing. The shared endodermal enhancers for H19 and Igf2 expression have been mapped from the +7- to +9-kb region downstream of H19 (Leighton et al. 1995). The enhancers for mesodermal expression of H19 are located downstream to +12 kb of H19 and on the basis of identical expression patterns of the two genes, are presumably shared by Igf2. (D) DMR; (E) enhancer.
Activation of the paternal H19 was observed when the DMR was deleted in the paternal germ line as was expected on the basis of earlier studies (Thorvaldsen et al. 1998). When setting up of the imprint was normal and the DMR was deleted only in the zygote, activation of paternal H19 was still observed, showing a requirement of this region post-fertilization. However, when the DMR was deleted in the terminally differentiated cells, H19 expression remained solely maternal just as in the wild-type mice. Because the DMR is required subsequent to fertilization but not continually required to prevent transcription from the H19 promoter, it must direct certain epigenetic modifications of the region during development that lead to the silencing of the H19 promoter. Once silencing has been achieved, it is stable despite the removal of DMR, indicating that the DMR and associated proteins do not interact directly with the transcriptional machinery to prevent transcription from the H19 promoter. Whether DMR-mediated changes are stable during mitosis was not addressed by these experiments.
Additionally, the H19 promoter was found to be methylated when silent (wild-type and DMRΔS) and non-methylated when actively transcribing (DMRΔZ and DMRΔG). It is known that whereas the DMR is methylated in the sperm, a region encompassing the H19 promoter on the paternal chromosome acquires methylation only during early embryogenesis (Brandeis et al. 1993; Tremblay et al. 1995, 1997). Although methylation of the H19 promoter correlates well with its silencing, there might be other epigenetic modifications required (Brenton et al. 1999). The methylated state of the DMR itself does seem to be critical, as demonstrated by biallelic H19 expression in embryos homozygous for the deletion of DNA methyltransferase gene, dnmt (Li et al. 1993). It appears that DMR in its methylated state, as on the paternal chromosome, has the ability to unidirectionally silence the adjacent genes, perhaps by acting as a nucleation center for spread of methylation and hypoacetylation or specifically recruiting enzymes that cause such modifications at the H19 promoter. The nature of such enzymes is presently unknown. Recently identified de novo methylases dnmt3a and dnmt3b, which are required during development (Okano et al. 1999), may have a role to play in this process. Other enzymes that either methylate DNA (dnmt) or recognize methylated DNA (MeCP2 and MBD2) have all been shown to have histone deacetylase activity, demonstrating a strong functional link between methylation, histone deacetylation, and silencing (Nan et al. 1998; Ng et al. 1999; Wade et al. 1999; Fuks et al. 2000). Whether H19 silencing involves any of these processes, remains to be investigated. Interestingly, a 1.1-kb domain (−2.9 to −1.8 kb upstream of H19) within the DMR has been shown to act as a bidirectional silencer in Drosophila (Lyko et al. 1997), an organism that lacks DNA methylation. In mice, transgenes of the H19 region that are devoid of this silencing domain do not exhibit silencing of H19 when paternally inherited (Brenton et al. 1999). The element is an active silencer only in its methylated state (as on the paternal chromosome) and is inactive on the nonmethylated maternal chromosome. The relationship of the 1.1-kb element to act as a bidirectional silencer in Drosophila in an unmethylated state and to act as a unidirectional silencer in mice in a methylated state is presently intriguing.
Thus, whereas the details of the actual molecular events leading to silencing of H19 remain elusive, there are two questions pertaining to H19 silencing. First, how does the DMR direct modifications of the H19 locus during cell differentiation? And second, how does the transcriptional machinery recognize the modified H19 promoter region to be distinct from the unmodified maternal H19 promoter leading to a differential transcriptional response? Our analysis of conditional mutants suggests that these two questions can be temporally divided. Further, our results demonstrate that the original imprint can lead to establishment of new distinctions between the paternal and maternal alleles and that it is these new distinctions that are actually pertinent for allele-specific activation by the transcription machinery of the cells.
Deletion of the DMR on the maternal chromosome led to the activation of maternal Igf2, irrespective of whether the deletion was effected in the germ line during establishment of the imprint, post-fertilization, or in differentiated tissue. Its constant requirement to keep Igf2 silenced is in consonance with the idea that the region harbors an insulating element that prevents the interaction of the upstream Igf2 promoter with downstream enhancers (Schmidt et al. 1999). The observation that deletion of the DMR led to expression of both Igf2 and H19 from the same chromosome (this work; C. Kaffer and M. Srivastava, in prep.) and that insertion of DMR sequences between the H19 promoter and its skeletal muscle enhancers abolished H19 expression in skeletal muscle (C. Kaffer and M. Srivastava, in prep.) strongly supports this idea. Such an insulation prevents the transcription of maternal Igf2. When methylated, as on the paternal chromosome, the insulating function is abrogated, leading to paternal expression of Igf2. The important role of methylation is again evident as homozygous deletion mutants of the DNA methyltransferase gene exhibited complete loss of Igf2 expression (Li et al. 1993).
Presently, however, the role of other epigenetic modifications cannot be ruled out. Also, no trans-acting factors are currently known that work with this insulator to bring about silencing of Igf2. Insulating elements have been reported to regulate gene expression in other systems (Kellum and Schedl 1992; Chung et al. 1993; Geyer 1997). The ability of epigenetic modifications, either directly or in concert with other trans-acting factors, to control the insulating activity in a parent-of-origin-dependent manner appears to be the special feature of the Igf2 locus.
The conclusion that monoallelic expression of the H19 and Igf2 genes occurs via distinct mechanisms is supported by several molecular and biochemical analyses in addition to the genetic studies described here. Early experiments examining nuclease sensitivity at the two genes' promoters suggested that differential regulation might be related to the changes at the H19 promoter (Sasaki et al. 1992; Bartolomei et al. 1993), but that both the maternal and paternal Igf2 promoters were available for active transcription (Sasaki et al. 1992). Further, monoallelic expression of H19 and Igf2 in cell lines is differentially sensitive to drugs that affect histone acetylation and methylation. Maternal Igf2 repression could be alleviated by using inhibitors of histone deacetylase, but alleviation of paternal H19 repression required both an inhibition of histone deacetylation and an inhibition of DNA methylation (Pedone et al. 1999), suggesting that different mechanisms were being used to achieve silencing of these genes.
Our results suggest that imprinted expression of genes may be achieved by taking advantage of mechanisms that are normally used for temporal and spatial regulation of genes as long as the imprint can interfere with this mechanism. Given the variabilty in the mechanisms of gene regulation, the mechanisms that accomplish allele-specific regulation may be very diverse. Association of hypermethylation in some genes with the expressed allele and in others with the nonexpressed allele (Caspary et al. 1998), supports the idea that the distinction of the two alleles is relevant rather than the modification itself. The distinction may be either the original gametic imprint or the changes directed by that imprint. In the case of the DMR, the original state of differential methylation is retained. However, it is possible that for some other locus, the original imprint is lost during development once it has successfully directed certain epigenetic modifications important for allele-specific expression.
It is logical to think that cis elements involved in transcriptional regulation will either be required to establish an open/closed promoter structure at a specific stage and are redundant thereafter, or will be continually required to provide signal to the transcriptional machinery to transcribe/not transcribe a given gene. The DMR between H19 and Igf2 genes happens to contain both kinds of elements. The role in the epigenetic silencing of H19 in a temporal fashion appears to be a unique characteristic of the deleted DMR region. It may represent a new class of mammalian silencers, which are only required to establish a closed promoter structure at a specific stage and are redundant thereafter as has been reported in yeast (Holmes and Broach 1996). Such silencers must have a very different mechanism of action than those that are continually required to keep the promoter shut off. Having dissected the temporal requirements of the DMR in regulating silencing of H19 and Igf2, it is now possible to look for trans-acting factors and details of molecular mechanisms involved in silencing achieved by these two kinds of elements.
To our knowledge, no cis-acting elements involved in transcription, enhancers, insulators, silencers, or LCRs have been dissected in a temporal manner in the mammalian system. We believe that the loxP/cre mediated approach will prove useful for studies investigating the mechanism of such elements. How would enhancer function, for example, be affected in such an experiment? Our studies provide a new perspective on the temporal requirements that may aid the understanding of mechanisms underlying the action of cis elements on genes both within and outside of the realm of the imprinting.
Materials and methods
Generation of conditional mutants of DMR
The targeting vector was constructed by use of DNA derived from 129/SvJ mice. The vector contained sequences extending from −11-kb EcoRI to +6.5-kb XbaI relative to the H19 transcriptional start site. Additionally, it carried a neomycin-resistance gene flanked by loxP sites inserted at the −7.0-kb HindIII site, another loxP site inserted at the −0.8-kb XbaI site, and a diptheria toxin gene as a negative marker. The orientation of all three loxP sites was the same and the insertion of the loxP site at the −0.8-kb XbaI position was engineered to also create a new BglII site at this position. The targeting vector was linearized with NotI and electroporated into mouse R1 embryonic stem cells. Correctly targeted clones were confirmed by Southern hybridization (Fig. 1). Positive clones were electroporated with plasmid pBS185 (GIBCO BRL) directly expressing cre recombinase to excise the Neor gene. These DMRflox alleles were identified by using a PCR-based strategy to detect the presence of loxP sites on either side of the DMR by amplifying the region around the −7.0-kb HindIII site with primers PrA (5′-CAGGCCTGTCCTCACCTGAAC-3′) and PrB (5′-GCCAGCTTGCCTTGGCAACCCCTT-3′), and around the −0.8-kb XbaI site with primers PrC (5′-CCACTGCTGAGTGGTCATG-3′) and PrD (5′-CGTGCGTGCGTATACCATTGCTC-3′). The amplification products were confirmed by sequencing. Clones carrying the DMRflox allele were introduced into C57/BL6-J blastocysts to generate chimeric founder mice. DMRΔG, DMRΔZ, and DMRΔS alleles were obtained by the action of cre recombinase on DMRflox alleles in vivo and screened for the excision by PCR using primers PrA and PrD. The efficiency of the deletion in the muscle and heart was assayed by Southern blotting.
SNuPE assay for allele-specific expression analysis
Liver, heart, and skeletal muscle RNA samples were DNase treated and amplified for H19 and Igf2 by RT–PCR using the Superscript Preamplification System (GIBCO BRL). Absence of any DNA contamination in the RNA samples was evidenced by absence of any amplification from parallel RT minus controls for each RT–PCR reaction. The amplification primers for H19 were 5′-GCACTAAGTCGATTGCACTGG-3′ and 5′-GCCTCAAGCACACGGCCACA-3′. Those for Igf2 were 5′-CCATCAATCTGTGACCTCCTCTTG-3′ and 5′-TGTTGTTCTCAGCCAGCTTTACAC-3′. The PCR products (164 bp for H19 and 574 bp for Igf2) were purified twice using the High Pure PCR Purification Kit (Roche) to eliminate free dNTPs. Approximately 5 ng of the PCR product was used as a template in SNuPE assays for incorporation of dATP or dGTP essentially as described (Kuppuswamy et al. 1991). The primers used for extension were 5′-CGTATGAATGTATACAGCAAGTGTGTAA-3′ for H19 and 5′-ACACCATCGGGCAAGGGGATCTCAGCA-3′ for Igf2. Extended primers were analyzed on an 18% polyacrylamide–6 m urea gel. Incorporation was quantified using the Molecular Dynamics Storm PhosphorImaging System. For each experiment, PCR products amplified from the DNA of castaneus × domesticus F1 mice were used as controls in the SNuPE reaction. Incorporation of dATP/dGTP in F1 DNA was used as a correction factor (F). F × dGTP incorporation was taken as the corrected dGTP incorporation and used to calculate the relative contribution of the paternal H19 allele to total H19 expression [A/(A+G)] and of the maternal Igf2 allele to total Igf2 expression ([G/(A+G)] shown in Table 1.
Bisulphite-based DNA methylation analysis
DNA from wild-type, DMRΔG, and DMRΔZ mutants was digested with BamHI and treated with sodium bisulphite using the CpGenome DNA modification kit (Intergen Company). The region around the H19 promoter (−266 to +338) was amplified by use of a nested PCR strategy. The primer sequences used for this purpose were chosen such that they are completely devoid of CpG residues and were designed such that they would anneal to the modified DNA in which the cytosine residues have been changed to thymidine. The primers for the first PCR were 5′-GTTTTAGATAGGGTTTTAGTAGGTTA-3′ and 5′-CTACTACCAACTATACCTTCACTACC-3′, and those for the nested PCR were 5′-TTAAGGGAGATATTTGGGGATAATGTTA-3′ and 5′-AACTATACCTTCACTACCCAAATCTAAA-3′. DNA from at least four PCR reactions was cloned and sequenced. Paternal clones were identified on the basis of a polymorphism at +167 between castaneus and domesticus DNA. Because DMRΔS DNA has a mix of maternal, undeleted paternal (DMRflox), and deleted paternal DMRΔS alleles, primers were chosen to selectively amplify only the deleted paternal allele. The primers for the first PCR were 5′-TGGAATTGATGGTGGTGTTTGTATTT-3′ and 5′-CTACTACCAACTATACCTTCACTACC-3′, whereas those for the nested PCR were 5′-TTAAGGGAGATATTTGGGGATAATGTTA-3′ and 5′-AACTATACCTTCACTACCCAAATCTAAA-3′. In this case, bisulphite conversion was carried out in agarose beads (Olek et al. 1996) carrying 400 ng of DNA per bead. The PCR products were cloned and sequenced. The sequencing primer for all sequencing reactions was 5′-CTCCCCATTCCTCTCCAACCCTAACTC-3′.
Acknowledgments
We thank Ronald Kahn for providing the mice with MCK-cre transgene and Pierre Vassalli for providing the plasmid pCAGGS-cre. We also thank Paul Love and members of the Pfeifer laboratory for their help with the manuscript.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL kpfeifer@helix.nih.gov; FAX (301) 402-0543.
References
- Araki K, Araki M, Miyazaki J, Vassalli P. Site-specific recombination of a transgene in fertilized-eggs by transient expression of cre recombinase. Proc Natl Acad Sci. 1995;92:160–164. doi: 10.1073/pnas.92.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei MS, Tilghman SM. Genomic imprinting in mammals. Annu Rev Genet. 1997;31:493–525. doi: 10.1146/annurev.genet.31.1.493. [DOI] [PubMed] [Google Scholar]
- Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–155. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- Bartolomei MS, Webber AL, Brunkow ME, Tilghman SM. Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes & Dev. 1993;7:1663–1673. doi: 10.1101/gad.7.9.1663. [DOI] [PubMed] [Google Scholar]
- Brandeis M, Kafri T, Ariel M, Chaillet JR, McCarrey J, Razin A, Cedar H. The ontogeny of allele-specific methylation associated with imprinted genes in the mouse. EMBO J. 1993;12:3669–3677. doi: 10.1002/j.1460-2075.1993.tb06041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan CI, Bartolomei MS. Mechanisms of genomic imprinting. Curr Opin Genet Dev. 1999;9:164–170. doi: 10.1016/S0959-437X(99)80025-2. [DOI] [PubMed] [Google Scholar]
- Brenton JD, Drewell RA, Viville S, Hilton KJ, Barton SC, Ainscough JFX, Surani MA. A silencer element identified in Drosophila is required for imprinting of H19 reporter transgenes in mice. Proc Natl Acad Sci. 1999;96:9242–9247. doi: 10.1073/pnas.96.16.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- Caspary T, Cleary MA, Baker CC, Guan XJ, Tilghman SM. Multiple mechanisms regulate imprinting of the distal chromosome 7 gene cluster. Mol Cell Biol. 1998;18:3466–3478. doi: 10.1128/mcb.18.6.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Whiteley M, Felsenfeld G. A 5′ element of the chicken Beta-globin domain serves as an insulator in human erythroid-cells and protects against position effect in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A. Parental imprinting of autosomal mammalian genes. Curr Opin Genet Dev. 1994;4:265–280. doi: 10.1016/s0959-437x(05)80054-1. [DOI] [PubMed] [Google Scholar]
- Elson DA, Bartolomei MS. A 5′ differentially methylated sequence and the 3′-flanking region are necessary for H19 transgene imprinting. Mol Cell Biol. 1997;17:309–317. doi: 10.1128/mcb.17.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith AC, Sasaki H, Cattanach BM, Surani MA. Parental-origin-specific epigenetic modification of the mouse H19 gene. Nature. 1993;362:751–755. doi: 10.1038/362751a0. [DOI] [PubMed] [Google Scholar]
- Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks F, Burgers WA, Brehm A, Hughes-Davies L, Kouzarides T. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat Genet. 2000;24:88–91. doi: 10.1038/71750. [DOI] [PubMed] [Google Scholar]
- Geyer PK. The role of insulator elements in defining domains of gene expression. Curr Opin Genet Dev. 1997;7:242–248. doi: 10.1016/s0959-437x(97)80134-7. [DOI] [PubMed] [Google Scholar]
- Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA-polymerase-beta gene segment in T-cells using cell-type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Hark AT, Tilghman SM. Chromatin conformation of the H19 epigenetic mark. Hum Mol Genet. 1998;7:1979–1985. doi: 10.1093/hmg/7.12.1979. [DOI] [PubMed] [Google Scholar]
- Holmes SG, Broach JR. Silencers are required for inheritance of the repressed state in yeast. Genes & Dev. 1996;10:1021–1032. doi: 10.1101/gad.10.8.1021. [DOI] [PubMed] [Google Scholar]
- Kellum R, Schedl P. A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol Cell Biol. 1992;12:2424–2431. doi: 10.1128/mcb.12.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Aitchison A, Gregory R, Allen ND, Feil R. Parental allele-specific chromatin configuration in a boundary-imprinting-control element upstream of the mouse H19 gene. Mol Cell Biol. 1999;19:2556–2566. doi: 10.1128/mcb.19.4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppuswamy MN, Hoffmann JW, Kasper CK, Spitzer SG, Groce SL, Bajaj SP. Single nucleotide primer extension to detect genetic diseases: Experimental application to hemophilia B (factor IX) and cystic fibrosis. Proc Natl Acad Sci. 1991;88:1143–1147. doi: 10.1073/pnas.88.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995a;375:34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- Leighton PA, Saam JR, Ingram RS, Stewart CL, Tilghman SM. An enhancer deletion affects both H19 and Igf2 expression. Genes & Dev. 1995b;9:2079–2089. doi: 10.1101/gad.9.17.2079. [DOI] [PubMed] [Google Scholar]
- Li E, Beard C, Jaenisch R. Role For DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Lyko F, Brenton JD, Surani MA, Paro R. An imprinting element from the mouse H19 locus functions as a silencer in Drosophila. Nat Genet. 1997;16:171–173. doi: 10.1038/ng0697-171. [DOI] [PubMed] [Google Scholar]
- Lyons GE, Muhlebach S, Moser A, Masood R, Paterson BM, Buckingham ME, Perriard JC. Developmental regulation of creatine-kinase gene-expression by myogenic factors in embryonic mouse and chick skeletal-muscle. Development. 1991;113:1017–1029. doi: 10.1242/dev.113.3.1017. [DOI] [PubMed] [Google Scholar]
- Nan XS, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Ng HH, Zhang Y, Hendrich B, Johnson CA, Turner BM, Erdjument-Bromage H, Tempst P, Reinberg D, Bird A. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet. 1999;23:58–61. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Olek A, Oswald J, Walter J. A modified and improved method for bisulphite based cytosine methylation analysis. Nucleic Acids Res. 1996;24:5064–5066. doi: 10.1093/nar/24.24.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedone PV, Pikaart MJ, Cerrato F, Vernucci M, Ungaro P, Bruni CB, Riccio A. Role of histone acetylation and DNA methylation in the maintenance of the imprinted expression of the H19 and Igf2 genes. FEBS Lett. 1999;458:45–50. doi: 10.1016/s0014-5793(99)01124-2. [DOI] [PubMed] [Google Scholar]
- Pfeifer K, Leighton PA, Tilghman SM. The structural H19 gene is required for transgene imprinting. Proc Natl Acad Sci. 1996;93:13876–13883. doi: 10.1073/pnas.93.24.13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Jones PA, Chaillet JR, Fergusonsmith AC, Barton SC, Reik W, Surani MA. Parental imprinting—potentially active chromatin of the repressed maternal allele of the mouse insulin-like growth factor-II (Igf2) gene. Genes & Dev. 1992;6:1843–1856. doi: 10.1101/gad.6.10.1843. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Levorse JM, Tilghman SM. Enhancer competition between H19 and Igf2 does not mediate their imprinting. Proc Natl Acad Sci. 1999;96:9733–9738. doi: 10.1073/pnas.96.17.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EA, Spizz G, Perry WM, Vizard D, Weil T, Olson EN. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine-kinase Gene. Mol Cell Biol. 1988;8:2896–2909. doi: 10.1128/mcb.8.7.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsen JL, Duran KL, Bartolomei MS. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes & Dev. 1998;12:3693–3702. doi: 10.1101/gad.12.23.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KD, Saam JR, Ingram RS, Tilghman SM, Bartolomei MS. A paternal-specific methylation imprint marks the alleles of the mouse H19 gene. Nat Genet. 1995;9:407–413. doi: 10.1038/ng0495-407. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Duran KL, Bartolomei MS. A 5′ 2-kilobase-pair region of the imprinted mouse H19 gene exhibits exclusive paternal methylation throughout development. Mol Cell Biol. 1997;17:4322–4329. doi: 10.1128/mcb.17.8.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PA, Gegonne A, Jones PL, Ballestar E, Aubry F, Wolffe AP. Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat Genet. 1999;23:62–66. doi: 10.1038/12664. [DOI] [PubMed] [Google Scholar]