Abstract
Natural killer T (NKT) cells that express the semiinvariant T cell receptor are innate-like lymphocytes whose functions are regulated by self and foreign glycolipid ligands presented by the antigen-presenting, MHC class I-like molecule CD1d. Activation of NKT cells in vivo results in rapid release of copious amounts of effector cytokines and chemokines with which they regulate innate and adaptive immune responses to pathogens, certain types of cancers and self-antigens. The nature of CD1d-restricted ligands, the manner in which they are recognised and the unique effector functions of NKT cells suggest an immunoregulatory role for this T cell subset. Their ability to respond fast and our ability to steer NKT cell cytokine response to altered lipid ligands make them an important target for vaccine design and immunotherapies against autoimmune diseases. This review summarises our current understanding of CD1d-restricted ligand recognition by NKT cells and how these innate-like lymphocytes regulate inflammation.
Introduction
The immune system evolved with the descent of multicellular metazoans as a means to recognise and respond to altered internal milieu (homeostasis). Both internal and external stressors–such as toxic substances or microbial and parasitic infections–are known to incite tissue injury. Containment and removal of the stressor–which are essential for initiating tissue repair–are accomplished initially by the archaic, multi-modular innate immune system. The innate-like lymphocyte module consists of natural killer (NK), B1 B, γδ T, natural killer T (NKT) cells, and others. NKT cells have evolved to jump-start and fine-tune the innate and adaptive immune responses. The adaptive immune system consists of B and T–lymphocytes which are recruited to assist in the healing process should the innate mechanisms fail to contain and clear the inciter. The quick-acting, innate system senses an altered homeostatic state with pattern recognition receptors. In contrast, the slow-responding, adaptive immune system uses antigen-specific receptors that are expressed clonally by B and T lymphocytes–B cell receptors (and Abs), and TCRs, respectively–to sense alterations in the internal milieu. Whilst each module plays a specific role, multiple modules act in concert resulting in an inflammatory response that is essential in maintaining homeostasis (reviewed in ref. 1). In this review, we discuss the current knowledge of a duet between NKT cells and APCs–pivotal to which is the understanding of the TCR-ligand recognition logic–and its impact on inflammation.
NKT cells
NKT cells–which express both NK and T cell phenotypic and functional features–are thymus-derived, innate-like lymphocytes whose functions are regulated by self and non-self lipid ligands presented by CD1d molecules. CD1d molecules are expressed by APCs–such as dendritic cells (DCs), macrophages (Mφ) and B cells–as well as CD4+8+ thymocytes, hepatocytes and intestinal epithelial cells. Hence, under different experimental and pathologic conditions, each of these CD1d+ cell types can present self and microbial lipids and activate NKT cells (2–12).
The majority of NKT cells express an invariant TCR α-chain generated by TRAV11*02 (mouse Vα14i) or TRAV10 (human Vα24i) to TRAJ18 (Jα18) rearrangement. The invariant α-chain pairs predominantly with mouse TRBV13–2*01 (Vβ8.2), TRBV29*02 (Vβ7), TRBV1 (Vβ2) or human TRBV25–1 (Vβ11) β-chain to form a functional semiinvariant TCR. A small subset–referred to as type II NKT cells–expresses a more diverse TCR repertoire but little is known regarding their properties, and hence not discussed here. NKT cells regulate microbial and tumour immunity as well as autoimmune diseases by their ability to rapidly secrete large amounts of immunoregulatory cytokines and to upregulate costimulatory molecules to alert and modulate the effector functions of myeloid and lymphoid cells (13, 14).
CD1d-restricted ligands and distinct modes of NKT cell activation
Exogenous NKT cell agonists
CD1d is a member of the CD1 family of antigen presenting molecules. The original report by Brenner and colleagues demonstrating CD1-restiction of Mycobacterium tuberculosis-reactive T cells and the recognition of Mtb lipids indicated that CD1 molecules present lipid ligands (15–18). Consistent with this is the finding that CD1d assembles with cellular phospholipids and sphingolipids (19–23). CD1d then acquires self and microbial NKT cell agonists in the endo/lysosomes (24). Much of our understanding of NKT cell biology however, has been gleaned from numerous in vitro and in vivo studies that use the marine sponge-derived, synthetic (KRN7000) αGalCer and its analogues as the probe (Fig. 2, 3; (13, 14, 24–29). Sphingomonas spp.–a Gram-negative α-Proteobacteria that lack LPS–synthesise α-glucuronosylceramide and α-galacturonosylceramide (αGalACer) that resemble αGalCer (Fig. 2). αGalACer directly activates NKT cells in a CD1d-restricted manner (30–32). NKT cells activated byαGalACer appear to be important in Sphingomonas-specific immunity because high-dose infection of wild-type mice results in septic shock caused by rapid release of inflammatory cytokines, whilst low-dose infection of NKT cell-deficient mice delays bacterial clearance (30, 31).
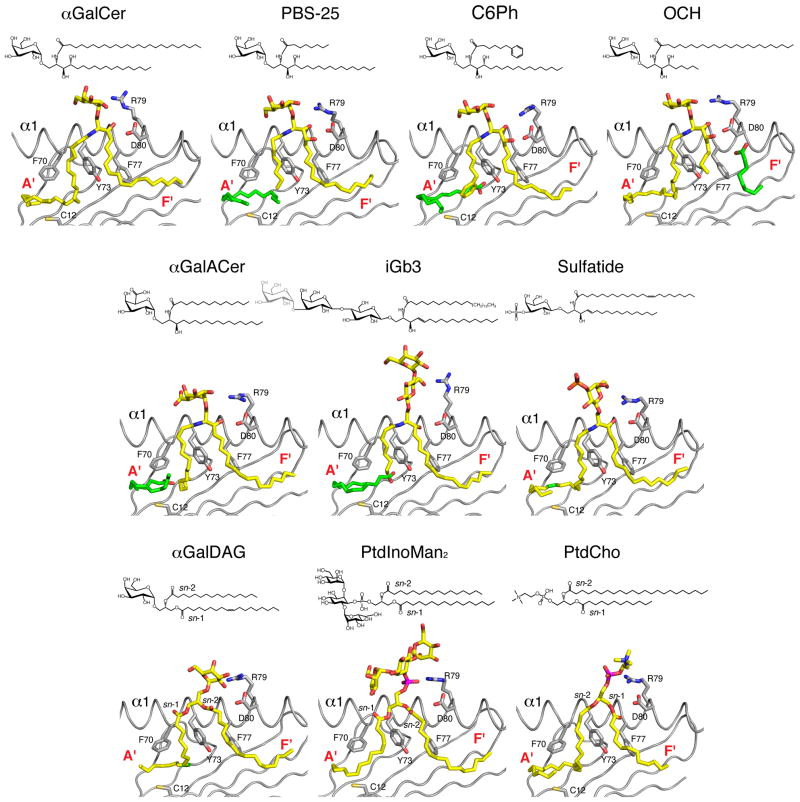
Figure 2. Chemical structure and orientation of ligands in the mCD1d ABG.
Top, chemical structures of the ligands; gray, portions not ordered in the corresponding crystal structures. Bottom, a side view of the ABG with the α2 helix removed for clarity. Ligand, yellow; spacer lipids, green; mouse CD1d heavy chain, gray; unsaturations on the acyl chains of the ligands are also green. Some of the residues involved in defining the ABG and contacting the ligand are highlighted. PDB ID: αGalCer (human), 1ZT4;αGalCer, 1Z5L; C6Ph, 3GML; OCH, 3G08; αGalACer, 2FIK; iGb3, 2Q7Y; sulfatide, 2AKR; αGalDAG, 3ILQ; PtdInoMan2, 2GAZ; PtdCho, 1ZHN.
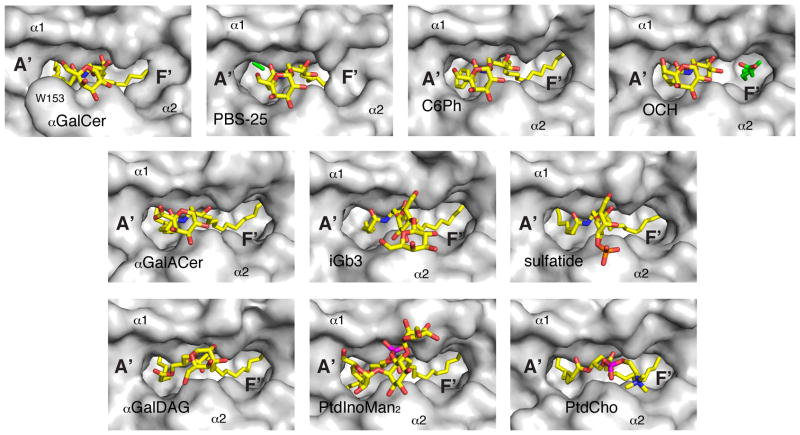
Figure 3. Top view of the CD1d-ligand structures.
A top view of the ABG is shown before NKTCR binding with the protein surface in grey and the ligands in yellow. Note the presence of the pre-formed F′ roof exclusively for αGalCer.
NKT cells also recognise diacylglycerol-based microbial lipids; e.g.,α-galactosyldiacylglycerol (αGalDAG) and phosphatidylinositoltetramannoside (PtdInoMan4) (Fig. 2) (33, 34). These glycolipids are cell-wall components or theirprecursor synthesised by Borrelia burgdorferi (35, 36)–the agent of Lyme disease–and Mtb (34), respectively. Additionally, NKT cells are activated by Helicobacter pylori-derived cholesteryl-6-O-acyl α-glucoside (37). Hence, NKT cells have broad ligand specificity. Whilst how structurally distinct ceramide- and diacylglycerol-based glycolipids are recognised by the semiinvariant NKT cell receptor (NKTCR) is discussed below, how the cholesterylglucoside is recognised remains unknown.
Endogenous NKT cell agonists
NKT cells are also autoreactive–i.e., they react to self lipids presented by the host APCs (38, 39). An initial search for the endogenous NKT cell agonist revealed that neither cells deficient in β-glucosylceramide (βGlcCer) synthase and transiently expressing CD1d nor cell-free CD1d-βGlcCer complexes activate mouse NKT cell-derived hybridomas (40). This finding suggested that one endogenous mouse NKT cell agonist is a cellular, βGlcCer-derived glycosphingolipid (GSL). Notwithstanding, current evidence suggests that both β-linked GSLs–e.g., cellular βGlcCer, isoglobotrihexosylceramide (iGb3; Fig. 2), GD3 and an analogue, β-mannosylceramide (41–44)–as well as glycerophospholipids–e.g., Ptd-inositol, Ptd-ethanolamine and lyso-Ptd-choline (45–47)–are agonists for a subset or all mouse and/or human NKT cells. The identity of other self ligands await identification and characterisation.
The importance of self lipid recognition was realised in studies demonstrating human and mouse NKT cell activation by DCs co-cultivated with either the Gram-positive Staphlococcus aureus or the Gram-negative Salmonella typhimurium. In the case of S. typhimurium, NKT cell activation resulted from bacterial LPS-mediated stimulation of DCs through TLR4 and the secretion of IL-12 (48). This response requires hexosaminidase B (HexB), which converts, amongst other GSLs, the precursor iGb4 to agonistic iGb3 (Fig. 1). These data were interpreted to mean that Salmonella activates NKT cells indirectly through the recognition of self-iGb3 in the presence of IL-12 (31).
Figure 1. Cellular glycolipid gradients.
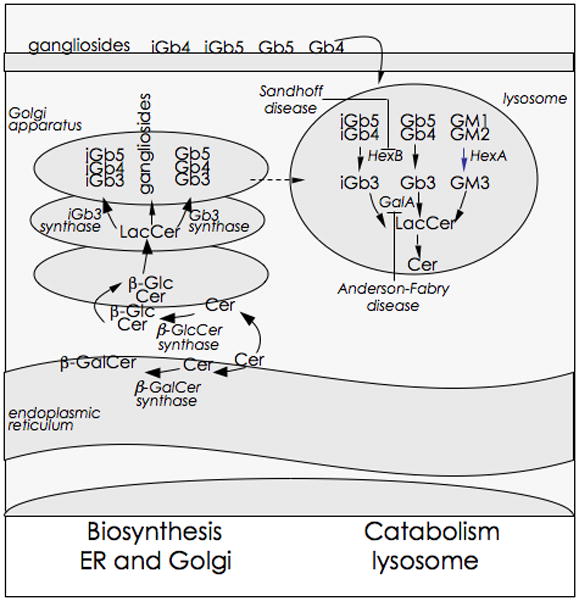
Glycosphingolipid biosynthesis begins with the synthesis of ceramide on the cytosolic leaflet of the ER. The glycosylation of ceramides results in the formation of glycosphingolipids. The major precursors for mammalian glycosphingolipids are β-glucosylceramide (βGlcCer) and β-galactosylceramide (βGalCer). βGluCer is synthesised by βGlcCer synthase whose catalytic site is predisposed to the cytosolic side of the Golgi apparatus. In contrast, βGalCer is synthesised by an ER luminal enzyme βGalCer synthase. As shown, further glycosylation of βGlcCer results in lactosylceramide (LacCer), gangliosides, globosides and isoglobosides as well as lactoneo- and muco-series of glycolipids (not shown). These distribute to various membranous compartments including the lysosomes. The endogenous iNKT cell antigen iGb3 is generated by the enzymatic cleavage of β1–3GalNAc from iGb4 by β-hexosaminidase B (HexB). iGb3 appear to be rapidly catabolised to LacCer by the action of another lysosomal hydrolase, β-galactosidase A (GalA). Microbes, such as Salmonella, and derived products down regulate cellular GalA gene expression, which prevents the catabolism of the NKT cell agonist, iGb3, amongst other glycosphingolipids. Deficiencies in HexB and GalA are know to cause Sandhoff’s and Anderson-Fabry diseases, respectively. These lipid storage diseases impact such fundamental processes as macro-autophagy, mitochondrial function as well as protein and lipid trafficking and thereby alter cellular homeostasis. Put together, cellular lipid homeostasis regulates NKT cell function, which in turn can control inflammation.
Whether iGb3 is the sole endogenous NKT cell agonist has been contentious as NKT cells from iGb3 synthase-deficient mice are fully functional (49). Additionally, only mouse dorsal root ganglion but neither human thymocytes nor DCs appear to synthesise iGb3 (49). Nonetheless, human thymocytes synthesise iGb4 (50) and HexB (44), which can convert iGb4 to iGb3. Moreover, iGb3 is detectable in the absence of the regioisomer Gb3 (51) suggesting that iGb3 is ephemeral and that its levels are regulated by either rapid anabolism to iGb4 or catabolism by the lysosomal α-galactosidase A (GalA; Fig. 1). Indeed, APCs deficient in GalA–which cleaves reducing α-linked Gal residues–cause overt activation of wild type NKT cells, suggesting the accumulation of an agonist (52). GalA also converts iGb3 (and Gb3) to lactosylceramide and therefore, its deficiency increases iGb3 levels in cells up to five-fold (51). A caveat with experiments that use cells or cell lines deficient in lipid metabolic enzymes is that, they are known to cause lipid storage disease, which can in turn alter lipid and protein trafficking within cells (53–57). Nonetheless, these findings have implications for the role of NKT cell responses to microbial infections as Salmonella-infected cells or those stimulated by microbial products that down regulate GalA expression (52) thereby, increasing the levels of iGb3.
NKT cells also respond to a sialylated endogenous lipid when DCs are activated by CpG, a TLR7 ligand, and produce IFN-α (58). They also respond to a combination of inflammatory cytokines such as IL-12 and IL-18 in the absence of a CD1d-restricted agonist (59–61). This latter mechanism is important for immunity to cytomegalovirus (61). Hence, NKT cells have evolved multiple ways to sense microbial stressors including direct recognition of CD1d-restricted exogenous glycolipids. Alternatively, they sense stressors indirectly, either through the recognition of CD1d-self lipid complex or in a CD1d-independent manner, in the presence of inflammatory cytokines.
Structures of CD1d lipid complexes
CD1d is a heterodimer consisting of a heavy chain that is noncovalently associated with the light-chain β2-microglobulin. The heavy chain folds into five domains: the extracellular α1, α2 and α3 domains (Fig. 4A), which are membrane-anchored by the transmembrane region, ending in a short cytoplasmic tail. Solution of the three-dimensional structures of mouse and human CD1d molecules, which differ subtly from each other, in complex with several lipid ligands–αGalCer, αGalACer, OCH, αGalDAG, sulfatide, PtdInoMan2, PtdCho, iGb3 (8, 62–69)–revealed that the α1 and α2 domains of the heavy chain fold into a superdomain to form the Ag-binding groove (ABG; Fig. 2–4). The ABG is laterally confined by two antiparallel α-helices that are supported at the bottom by an 8-stranded antiparallel β-sheet platform. The membrane-proximal immunoglobulin-like α3 domain and the noncovalently associated light chain support the superdomain (Fig. 4A). Therefore, the topology of CD1d resembles peptide-antigen-presenting MHC class I molecules.
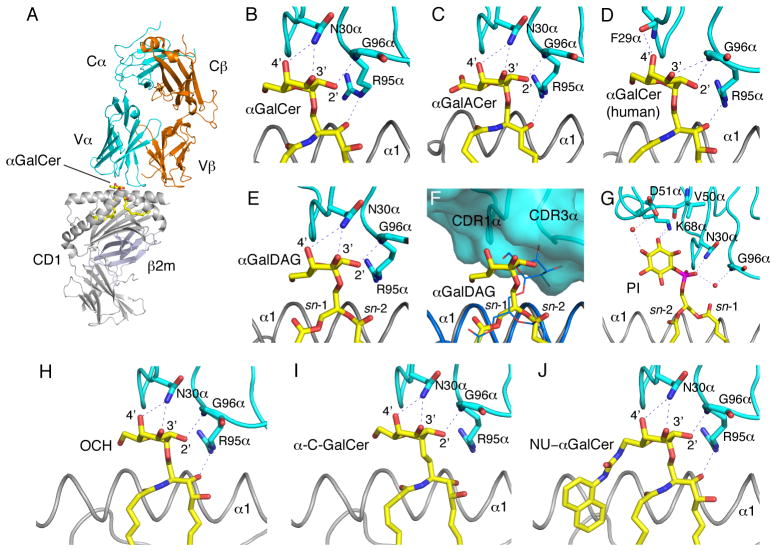
Figure 4. NKTCR/CD1d-lipid interactions.
(A) Structure of the mouse CD1d-αGalCer-NKTCR complex (3HE6). Mouse CD1d, grey; β2m, light blue; TCR α-chain, cyan; TCRβ-chain, orange; αGalCer, yellow. Note how the contacts between the NKTCR and the ligand or CD1d are dominated by the TCR α-chain. (B–J) Conserved interaction of the NKTCR with α-anomeric galactose-containing ligands and with the PtdIno self antigen. The ligands are shown in yellow with CD1d in grey and the TCR α-chain in cyan. Hydrogen bonds between the ligand and conserved residues on the NKTCR are shown as dashed blue lines. (F) Superposition of the mouse CD1d-αGalDAG structure before (blue) and after (ligand in yellow; CD1d, gray; TCR α-chain, cyan) TCR binding. Note how a conformational change of the galactose on the ligand is induced upon binding of the NKTCR α-chain in order to avoid steric clashes and allow for the conserved TCR binding footprint onto CD1d. PDB IDs: (A–B) αGalCer, 3HE6; (C) αGalACer, 3O8X; (D)αGalCer (human), 3HUJ; (E) αGalDAG, 3O9W; (F) αGalDAG, 3O9W and 3ILQ; (G) PtdIno, 3QI9; (H) OCH, 3ARB; (I) α-C-GalCer, 3QUX; (J) NU-αGalCer, 3QUZ.
Display of αGalCer and its analogues
The arrangement of the amino acids that make up the ABG is such that the narrow apical entrance leads into two deep-seated pockets (A′ and F′; Fig. 2, 3). The two pockets are lined predominantly by hydrophobic amino acid residues, and hence permit the binding of hydrocarbon tails of lipid molecules of varying lengths. The N-acyl chain of αGalCer and related compounds–OCH and αGalACer–tucks into the large A′ pocket while the long-chain base of GSLs fits into the F′ pocket (Fig. 2, 3). This binding mode exposes the polar head group out from the ABG (Fig. 3, 4). Moreover, the charged amino acids at the entrance of the ABG form a conserved hydrogen-bond network with polar atoms of the head groups of these α-anomeric GSLs (8, 62, 63, 66–68). The same CD1d residues also form hydrogen-bonds with β-anomeric GSLs, such as sulfatide–a type II NKT cell ligand–and iGb3 (68, 69). This hydrogen-bond network provides stability to the CD1d lipid interaction. Thus, the physicochemical architecture of the ABG dictates how the polar epitope is disposed for recognition by the Vα14i/Vα24i TCR, or the TCR of more diverse type II NKT cells, in the case of sulfatide.
Display of αGalDAG
Microbial αGalDAG antigens are structurally similar to αGalCer in that they also have an α-anomeric galactose attached to a lipid backbone (Fig. 2). However, in contrast to αGalCer, the DAG backbone is characterized by two fatty acids esterified to both the sn-1 and sn-2 position of a glycerol moiety, while the α-anomeric galactose is attached to the sn-3 position. Borrelial αGalDAG lipids bind to CD1d in two different orientations, depending on the nature of the acyl chains linked to sn1- and sn2 positions of glycerol. As a result, the lipid backbone is important in the formation of a TCR epitope as certain αGalDAGs are NKT cell agonists and others not, because they bind in the opposite orientation (65). B. burgdorferi glycolipid 2c (BbGl-2c), which is bound with the sn-1 oleic acid (C18:1) in the A′ pocket and the sn-2 palmitic acid (C16:0) in the F′ pocket is a mouse NKT cell agonist (Fig. 4D), whilst BbGl-2f that binds in a reversed orientation with the sn-2 linked oleic acid (C18:1) in the A′ pocket and the sn-1 linked linoleic acid (C18:2) in the F′ pocket does not activate mouse NKT cells (33). In contrast, BbGl-2f is a human NKT cell agonist (33), but how αGalDAGs are presented by human CD1d is currently unknown. Even though chemical modifications, such as unsaturations do not directly make contact the TCR, but by virtue of affecting the orientation of the hexose sugar contributes to the formation of the NKT cell epitope. Similar changes in the ceramide backbone of αGalCer analogues do not lead to alternative GSL binding orientation, as the ceramide backbone is bound in a conserved orientation through the conserved hydrogen bond network. In sum, αGalDAG presentation reveals for the first time striking differences between mouse and human glycolipid antigen recognition that could not have been appreciated by using strong agonists, such as αGalCer.
Self display: sulfatide and iGb3
Sulfatide binds CD1d in a distinct manner such that the 3′-sulphated galactose is solventexposed and projectsup and away from the ABG as a result of its β linkage (Fig 1, 2; (68). This contrasts the more intimate binding of the galactosyl headgroup of αGalCer to CD1d (8, 63). However, despite differences in the binding mode, sulfatide engages CD1d via a hydrogen-bond network mediated by the same residues involved in stabilizing αGalCer (8, 63, 68).
The first hexose of iGb3–i.e., glucose–akin to sulfatide, is β-linked to ceramide, and hence would be predicted to be solvent exposed in a manner similar to the sulphated galactose. This disposition of the first glucose of iGb3 results in an almost perpendicular exposition of the two terminal galactoses [Glc β(1–4)Gal α(1–3)Gal] of iGb3 out of the ABG as revealed by the structure of the mouse CD1d iGb3 complex (Fig. 2, 3) (69). Nevertheless, the β-linked glucose, which unalike sulfatide lacks a 3′-sulphate and whose 4′-hydroxyl is equatorially disposed, perhaps results in poor binding to CD1d because the 3′-sulfate and the axial 4′-hydroxyl are involved in hydrogen bonding of sulfatide with CD1d.
Taken together, the presentation principles for α- and β-linked glycolipids are distinct. How then the same Vα14i/Vα24i TCRs recognise these structurally distinct agonists remains to be elucidated. Finally, it will be interesting to see why sulfatide and iGb3, which share the same core-structure, are recognised by different NKT cells: sulfatide being a type II NKT cell agonist whilst, the latter a semiinvariant NKT cell agonist.
Display of phosphoglycerolipids
Lyso-Ptd-choline, but not Ptd-choline, is a human NKT cell agonist (45). As it consists of only one acyl-sn1-glycerol, it will be interesting to see into which pocket this single chain lipid binds or, whether two different binding orientation exist of which only one orientation results in an agonist, similar to what has been observed in the case of the borellial DAG ligands (65). As most agonists that are structurally characterized in complex with the NKTCR contain an α-linked galactose that show a conserved TCR binding footprint, it is difficult to predict how the more complex glycolipids, such as PIM4 or iGb3 are recognized and engaged by the same NKTCRs.
NKTCR/CD1d-lipid recognition logic
By contrast to TCR/pMHC complexes–wherein the receptor docks diagonal on the antigen (70)–the NKTCR docks parallel onto the extreme C-terminal end of the CD1d ABG, above the F′ pocket by using three of the six CDRs–CDR1α, CDR3α and CDR2β–while almost excluding CDR2α, CDR1βand CDR3β from the interface (Fig. 4; (71–73). This docking mode enables a lock-and-key interaction with the α-linked galactose epitope that was predicted from biophysical studies of Vα14i TCR ligand binding (71–74). Furthermore, alanine-scanning mutagenesis of the mouse Vα14i TCR as well as the crystal structures of Vα14i-Vβ8.2 and Vα14i-Vβ7 co-complexed with mouse CD1d-αGalCer revealed that the mouse NKTCR interfaces its ligand in a manner similar to the Vα24i TCR ligand interaction (72, 73, 75–77).
The above germline-encoded recognition logic raises the question of how the mouse Vα14i and human Vα24i TCRs recognise structurally distinct ligands such as iGb3, GD3, PtdInoMan4, PtdIno, PtdEtN and lysoPtdCho. Alanine-scan mutants of Vα14i TCR revealed that the NKTCR recognises many α-linked GSLs (αGalCer, OCH, αGalACer, αGalDAG and iGb3, which contains an α-linked terminal galactose) by means of a ‘hot-spot’ of germline-encoded amino acids within CDR1α, CDR3α and CDR2β loops (77). The recent structure of mCD1d-PtdIno bound to an autoreactive Vα14i TCR, in which the β-chain has been mutated to increase affinity toward self-antigens, surprisingly revealed that CDR3α residues do not directly contact the glycolipid, although the conserved TCR footprint on CD1d is maintained, while additional residues in CDR2α contact the phosphoinositol headgroup (Fig. 4; (78). Those interactions are novel and have not been reported for any other Vα14i TCR. Whether recognition of glycolipids by CDR2α residues is unique to self-antigens, however, is currently unknown. Additionally, the recent solution of the Vα14i-Vβ8.2/mouse CD1d-αGalDAG and αGalACer crystal structures revealed that the TCR has the capacity to induce structural changes in both CD1d and the ligand orientation to maintain the conserved TCR footprint (Fig 4B-E; (72). Similar to αGalCer and αGalACer, the TCR contacts αGalDAG exclusively through CDR1α and CDR3α (72). In each of these ternary structures, CDR1α Asn30 hydrogen bonds with the 2′ and 3′ hydroxyls of the galactose or galacturonic acid of the GSL (72). However, for αGalDAG this conserved interaction with the TCR required a re-orientation of the galactose moiety (Fig. 4E; (72). CDR3α residue Gly96 contacts the 2′-OH through a main chain carbonyl, whilst Arg95 contacts the 3″-OH of the ceramide backbone of both αGalCer and αGalACer (72). However, this hydrogen bond interaction is lost in the αGalDAG structure due to the different lipid backbone structure (72). These findings suggest that the interaction of NKTCR with structurally distinct α-linked ligands is accomplished by similar recognition logic, which involves the germline-encoded ‘hot-spot’ composed of amino acids within CDR1α, CDR3α and CDR2β loops.
The recently determined crystal structures of nine ternary complexes with bound αGalCer analogs, such as OCH, C20:2 αGalCer, C20:2 αGluCer, 3′,4″-deoxy αGalCer, 4′,4″-deoxy αGalCer (79), as well as C-glycoside, BnNH-GSL-1′, and naphtylurea (NU)αGalCer (80), provide insights into the mechanisms of TCR binding, as well as illustrates novel and unexpected findings about the flexibility of the antigen presenting molecule CD1d. The successive elimination of individual hydroxyl groups of αGalCer analogues at either the galactose moiety or the phytosphingosine chain disrupts individual H bond interaction between the glycolipid and the TCR (or CD1d, in the case of ceramide modifications) and as such affects their recognition and biological outcome. However, certain 6′-galactose modifications can furthermore induce structural changes in CD1d itself, as demonstrated for the ligand NU-αGalCer (80). The aromatic NU modification is not contacted by the TCR but instead is inserted into the A′ roof, inducing the formation of a small pocket within that roof. It was proposed that the NU group serves as a third anchor in addition to the two alkyl chains that are bound in the A′ and F′ pockets and, as such increases the stability of the CD1d-glycolipid complex, possibly affecting its in vivo activity (80).
NKTCR/ligand binding kinetics: the basis for a synaptic duet and synaptic transmission of effector molecules
The kinetic parameters of NKTCR/ligand interaction have been extensively studied. Surface plasmon resonance and tetramer-binding experiments have revealed high-affinity interaction between Vα14i or Vα24i TCR/CD1d αGalCer (or derived analogues): the relative avidity of this interaction is similar to that of high-affinity interactions between the TCR/pMHC complexes (74, 81–85). Interestingly, the half-life of mouse NKTCR/CD1d αGalCer interaction was unusually long (Table S1; references therein). How these kinetic parameters relate to the rapid and robust NKT cell response remains to be elucidated. In this regard, it is interesting to note that an αGalCer analogue, OCH, which has a shortened long-chain sphingosine base (C9 versus C18) and acyl chain (C24 versus C26; Fig 1) and interacts with the Vα14i and Vα24i TCR with lower relative affinity/avidity compared with αGalCer (Table S1; (64, 82, 83), specifically elicits sustained IL-4 with very little IFN-γ response (86). A similar IL-4-biased response is elicited by a diunsaturated (C20-diene) N-acyl analogue of αGalCer (Fig. 2; (87) whose binding constant is similar to αGalCer but dissociation rate is similar to OCH (Table S1; (64, 83), the structural basis for which is described below. Hence, the relative TCR binding affinities do not seem to be responsible for the observed TH1/TH2 predisposition of structurally related glycolipids. Rather the ability of αGalCer loaded CD1d molecules to accumulate in lipid rafts in vivo, in contrast to CD1d molecules that contain OCH or C20-diene appear to influence the cytokine profile (88, 89).
To or not to roof the F′ pocket
It is surprising that despite conserved NKTCR-ligand binding, the equilibrium binding affinity toward microbial glycolipids can vary up to 600-fold compared to αGalCer (Table S1; (72). Essentially two factors have been identified that affect both the association rate of the TCR as well as the dissociation rate. Firstly, the need to re-orient the galactose of borrelial αGalDAG results in a reduced TCR association (72). Secondly, upon TCR binding onto mouse CD1d-αGalDAG or -αGalACer, the NKTCR induces a structural change in mouse CD1d above the F′ pocket, namely the formation of the F′ roof (72). The F′ roof is already pre-formed upon αGalCer binding to mouse and human CD1d, but not when other known NKT cell agonist bind (Fig 2; (8, 63, 72, 78, 79, 83) and, as such, the TCR does not invest energy into keeping the roof closed upon binding. That results in a more stable complex, indicated by a reduced TCR dissociation rate. The F′ roof is also closed in the previously mentioned ternary complexes of the various αGalCer analogs, as well as in the PtdIno structure (78–80). However, in light of the lack of structures without bound TCR, it is not clear whether the F′ roof is already preformed in those CD1d molecules before TCR engagement. In summary, the agonistic potency of αGalCer and related compounds appears to correlate with the extent to which the F′ roof is pre-formed as well as the ability of the glycolipids to induce further structural changes within CD1d that could enhance CD1d-ligand stability or CD1d-TCR binding stability. Those factors could in turn dictate the biologic outcome upon engaging different ligands, in addition to the pharmacological differences of the glycolipids.
Immune synapse
Conventional T cells and APCs as well as NK cells and target cells form immune synapses in preparation for eliciting an appropriate effector response (90–92). And so do NKT cells and APCs/CD1d-containing planar membrane (93), the specificity of which lies within NKTCR/ligand interactions. Consistent with the kinetic parameters (Table S1), αGalCer and C20-diene efficiently elicit classic immune synapses between NKT cells and the planar membrane by engaging ~10 molecules of CD1d-ligand μm−2. By contrast, OCH forms immune synapses at a tenfold higher concentration (83). Thus, at equal ligand concentration, αGalCer and C20-diene induce very quick and sustained iCa2+ flux (a measure of very early T cell activation) when compared with OCH (83).
Conventional T cells polarise certain cytokine receptors (IFN-γR), cytokines (e.g., IFN-γ and IL-2 but not TNF-α and CCL3; (94–96) and lytic granules to the immune synapse (91). αGalCer-pulsed DCs also form immune synapse with freshly isolated NKT cells within 30 min and polarise IFN-γ to the synapse within 50–60 min (93). Similarly, αGalCer and C20-diene rapidly polarised lytic granules to the immune synapse when compared with OCH (83). In this way they engage in synaptic transmission of effector molecules to modulate inflammatory responses to changes in cellular lipid content.
Conclusions
NKT cells localise to portals on microbial entry around cells that express the lipid presenting molecule–CD1d (7, 97, 98). The stability of CD1d-lipid complexes depends on whether the hydrocarbon chain occupying the F′ pocket permits the formation of a roof. NKTCR interfaces its cognate ligand–CD1d-lipid complexes–in a unique mode, which involves germline-encoded ‘hot spots’ that lie within CDR1α, CDR3α and CDR2β loops. The NKT cell-APC synaptic duet is driven by the binding kinetics of NKTCR/ligand interactions. Synapse formation prepares for effector functions and permits synaptic transmission of certain effector cytokines and lytic granules.
Cellular lipid gradients are tightly regulated. Internal and external stressors are known to alter this gradient (52, 99–101). Because CD1d molecules evolved to present lipids, any alterations in the gradient is displayed at the cell surface for an appraisal by NKT cells. By virtue of sensitive ligand recognition (perhaps based on co-operativity; (82), NKT cells can respond quickly to changes in ligand concentration and/or structure. As such, they are known to regulate autoimmune diseases and microbial immunity, and hence inflammation arising from stressors from within as occurs in autoimmunity or from the outside as in an infection. In this manner, NKT cells can regulate homeostasis.
Supplementary Material
Acknowledgments
We thank Drs. L Van Kaer, Vanderbilt University, and JS Bezbradica, Yale University, for critical reading of the manuscript and helpful suggestions.
Footnotes
Supported by grants from the NIH, AI048224 and AI061721 to SJ and AI074952 to DMZ. DMZ is recipient of a Cancer Research Institute Investigator Award.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 3.Schmieg J, Yang G, Franck RW, Van Rooijen N, Tsuji M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc Natl Acad Sci U S A. 2005;102:1127–1132. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skold M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J Immunol. 2005;175:3584–3593. doi: 10.4049/jimmunol.175.6.3584. [DOI] [PubMed] [Google Scholar]
- 5.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 7.Barral P, Polzella P, Bruckbauer A, van Rooijen N, Besra GS, Cerundolo V, Batista FD. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol. 2010;11:303–312. doi: 10.1038/ni.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, Besra GS, Cerundolo V, Batista FD. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111:2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allan LL, Hoefl K, Zheng DJ, Chung BK, Kozak FK, Tan R, van den Elzen P. Apolipoprotein-mediated lipid antigen presentation in B cells provides a pathway for innate help by NKT cells. Blood. 2009;114:2411–2416. doi: 10.1182/blood-2009-04-211417. [DOI] [PubMed] [Google Scholar]
- 12.Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, Besra GS, Brenner MB. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci U S A. 2008;105:8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 14.Van Kaer L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 15.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 16.Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, Porcelli SA. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 17.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989;341:447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 18.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 19.Cox D, Fox L, Tian R, Bardet W, Skaley M, Mojsilovic D, Gumperz J, Hildebrand W. Determination of cellular lipids bound to human CD1d molecules. PLoS One. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Silva AD, Park JJ, Matsuki N, Stanic AK, Brutkiewicz RR, Medof ME, Joyce S. Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J Immunol. 2002;168:723–733. doi: 10.4049/jimmunol.168.2.723. [DOI] [PubMed] [Google Scholar]
- 21.Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 22.Park JJ, Kang SJ, De Silva AD, Stanic AK, Casorati G, Hachey DL, Cresswell P, Joyce S. Lipid-protein interactions: biosynthetic assembly of CD1 with lipids in the endoplasmic reticulum is evolutionarily conserved. Proc Natl Acad Sci U S A. 2004;101:1022–1026. doi: 10.1073/pnas.0307847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol. 2009;182:4784–4791. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 25.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 26.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 27.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 29.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 31.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 32.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 33.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 34.Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SH, Schaible UE. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroder NW, Schombel U, Heine H, Gobel UB, Zahringer U, Schumann RR. Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi sensu stricto. J Biol Chem. 2003;278:33645–33653. doi: 10.1074/jbc.M305799200. [DOI] [PubMed] [Google Scholar]
- 37.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, Savage PB, Endo S, Yamamura T, Maaskant J, Kitano N, Singh A, Bhatt A, Besra GS, van den Elzen P, Appelmelk B, Franck RW, Chen G, DeKruyff RH, Shimamura M, Illarionov P, Umetsu DT. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 39.Brossay L, Tangri S, Bix M, Cardell S, Locksley R, Kronenberg M. Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J Immunol. 1998;160:3681–3688. [PubMed] [Google Scholar]
- 40.Stanic AK, De Silva AD, Park JJ, Sriram V, Ichikawa S, Hirabyashi Y, Hayakawa K, Van Kaer L, Brutkiewicz RR, Joyce S. Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by β-D-glucosylceramide synthase deficiency. Proc Natl Acad Sci U S A. 2003;100:1849–1854. doi: 10.1073/pnas.0430327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Konek JJ, Illarionov P, Khursigara DS, Ambrosino E, Izhak L, Castillo BF, 2nd, Raju R, Khalili M, Kim HY, Howell AR, Besra GS, Porcelli SA, Berzofsky JA, Terabe M. Mouse and human iNKT cell agonist beta-mannosylceramide reveals a distinct mechanism of tumor immunity. J Clin Invest. 2011 doi: 10.1172/JCI42314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, Ehara H, Sakai T, Serizawa I, Wu L, Wang CR, Joyce S, Van Kaer L. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J Immunol. 2004;173:3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 43.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 45.Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L, Trott DL, Ndonye RM, Veerapen N, Besra GS, Howell AR, Cook ME, Adams EJ, Hildebrand WH, Gumperz JE. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 47.Rauch J, Gumperz J, Robinson C, Skold M, Roy C, Young DC, Lafleur M, Moody DB, Brenner MB, Costello CE, Behar SM. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J Biol Chem. 2003;278:47508–47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 49.Speak AO, Salio M, Neville DC, Fontaine J, Priestman DA, Platt N, Heare T, Butters TD, Dwek RA, Trottein F, Exley MA, Cerundolo V, Platt FM. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci U S A. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Teneberg S, Thapa P, Bendelac A, Levery SB, Zhou D. Sensitive detection of isoglobo and globo series tetraglycosylceramides in human thymus by ion trap mass spectrometry. Glycobiology. 2008;18:158–165. doi: 10.1093/glycob/cwm129. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Thapa P, Hawke D, Kondo Y, Furukawa K, Furukawa K, Hsu FF, Adlercreutz D, Weadge J, Palcic MM, Wang PG, Levery SB, Zhou D. Immunologic glycosphingolipidomics and NKT cell development in mouse thymus. J Proteome Res. 2009;8:2740–2751. doi: 10.1021/pr801040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–228. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gadola SD, Silk JD, Jeans A, Illarionov PA, Salio M, Besra GS, Dwek R, Butters TD, Platt FM, Cerundolo V. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J Exp Med. 2006;203:2293–2303. doi: 10.1084/jem.20060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann B. Fabry disease: recent advances in pathology, diagnosis, treatment and monitoring. Orphanet J Rare Dis. 2009;4:21. doi: 10.1186/1750-1172-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breslow DK, Weissman JS. Membranes in balance: mechanisms of sphingolipid homeostasis. Mol Cell. 2010;40:267–279. doi: 10.1016/j.molcel.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bozza PT, Magalhaes KG, Weller PF. Leukocyte lipid bodies -Biogenesis and functions in inflammation. Biochim Biophys Acta. 2009;1791:540–551. doi: 10.1016/j.bbalip.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolter T, Winau F, Schaible UE, Leippe M, Sandhoff K. Lipid-binding proteins in membrane digestion, antigen presentation, and antimicrobial defense. J Biol Chem. 2005;280:41125–41128. doi: 10.1074/jbc.R500015200. [DOI] [PubMed] [Google Scholar]
- 58.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 60.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. The mechanism of invariant NKT cell responses to viral danger signals. J Immunol (Cutting Edge) 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giabbai B, Sidobre S, Crispin MD, Sanchez-Ruiz Y, Bachi A, Kronenberg M, Wilson IA, Degano M. Crystal structure of mouse CD1d bound to the self ligand phosphatidylcholine: a molecular basis for NKT cell activation. J Immunol. 2005;175:977–984. doi: 10.4049/jimmunol.175.2.977. [DOI] [PubMed] [Google Scholar]
- 63.Koch M, V, Stronge S, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan BA, Nagarajan NA, Wingender G, Wang J, Scott I, Tsuji M, Franck RW, Porcelli SA, Zajonc DM, Kronenberg M. Mechanisms for glycolipid antigen-driven cytokine polarization by Valpha14i NKT cells. J Immunol. 2010;184:141–153. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Li Y, Kinjo Y, Mac TT, Gibson D, Painter GF, Kronenberg M, Zajonc DM. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc Natl Acad Sci U S A. 2010;107:1535–1540. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D, Zajonc DM, Fujio M, Sullivan BA, Kinjo Y, Kronenberg M, Wilson IA, Wong CH. Design of natural killer T cell activators: structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc Natl Acad Sci U S A. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zajonc DM, Ainge GD, Painter GF, Severn WB, Wilson IA. Structural characterization of mycobacterial phosphatidylinositol mannoside binding to mouse CD1d. J Immunol. 2006;177:4577–4583. doi: 10.4049/jimmunol.177.7.4577. [DOI] [PubMed] [Google Scholar]
- 68.Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, Kumar V, Wilson IA. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Vα14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Girardi E, Wang J, Yu ED, Painter GF, Kronenberg M, Zajonc DM. The Valpha14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J Exp Med. 2010;207:2383–2393. doi: 10.1084/jem.20101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cantu C, 3rd, Benlagha K, Savage PB, Bendelac A, Teyton L. The paradox of immune molecular recognition of α-galactosylceramide: low affinity, low specificity for CD1d, high affinity for αβTCRs. J Immunol. 2003;170:4673–4682. doi: 10.4049/jimmunol.170.9.4673. [DOI] [PubMed] [Google Scholar]
- 75.Florence WC, Xia C, Gordy LE, Chen W, Zhang Y, Scott-Browne J, Kinjo Y, Yu KO, Keshipeddy S, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Godfrey DI, Rossjohn J, Richardson SK, Porcelli SA, Howell AR, Hayakawa K, Gapin L, Zajonc DM, Wang PG, Joyce S. Adaptability of the semi-invariant natural killer T-cell receptor towards structurally diverse CD1d-restricted ligands. Embo J. 2009;28:3781. doi: 10.1038/emboj.2009.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mallevaey T, Scott-Browne JP, Matsuda JL, Young MH, Pellicci DG, Patel O, Thakur M, Kjer-Nielsen L, Richardson SK, Cerundolo V, Howell AR, McCluskey J, Godfrey DI, Rossjohn J, Marrack P, Gapin L. T cell receptor CDR2 beta and CDR3 beta loops collaborate functionally to shape the iNKT cell repertoire. Immunity. 2009;31:60–71. doi: 10.1016/j.immuni.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scott-Browne JP, Matsuda JL, Mallevaey T, White J, Borg NA, McCluskey J, Rossjohn J, Kappler J, Marrack P, Gapin L. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 78.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, Patel O, Vivian JP, Matsuda JL, McCluskey J, Godfrey DI, Marrack P, Rossjohn J, Gapin L. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34:315–326. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wun KS, Cameron G, Patel O, Pang SS, Pellicci DG, Sullivan LC, Keshipeddy S, Young MH, Uldrich AP, Thakur MS, Richardson SK, Howell AR, Illarionov PA, Brooks AG, Besra GS, McCluskey J, Gapin L, Porcelli SA, Godfrey DI, Rossjohn J. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity. 2011;34:327–339. doi: 10.1016/j.immuni.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aspeslagh S, Li Y, Yu ED, Pauwels N, Trappeniers M, Girardi E, Decruy T, Van Beneden K, Venken K, Drennan M, Leybaert L, Wang J, Franck RW, Van Calenbergh S, Zajonc DM, Elewaut D. Galactose modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumor metastasis. EMBO J. 2011 doi: 10.1038/emboj.2011.145. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sidobre S, Naidenko OV, Sim BC, Gascoigne NR, Garcia KC, Kronenberg M. The Vα14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J Immunol. 2002;169:1340–1348. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]
- 82.Stanic AK, Shashidharamurthy R, Bezbradica JS, Matsuki N, Yoshimura Y, Miyake S, Choi EY, Schell TD, Van Kaer L, Tevethia SS, Roopenian DC, Yamamura T, Joyce S. Another view of T cell antigen recognition: cooperative engagement of glycolipid antigens by Va14Ja18 natural T (iNKT) cell receptor. J Immunol. 2003;171:4539–4551. doi: 10.4049/jimmunol.171.9.4539. [DOI] [PubMed] [Google Scholar]
- 83.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, Salio M, Denkberg G, Reddington F, Tarlton A, Reddy BG, Schmidt RR, Reiter Y, Griffiths GM, van der Merwe PA, Besra GS, Jones EY, Batista FD, Cerundolo V. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gadola SD, Koch M, Marles-Wright J, Lissin NM, Shepherd D, Matulis G, Harlos K, Villiger PM, Stuart DI, Jakobsen BK, Cerundolo V, Jones EY. Structure and binding kinetics of three different human CD1d-α-galactosylceramide-specific T cell receptors. J Exp Med. 2006;203:699–710. doi: 10.1084/jem.20052369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kjer-Nielsen L, Borg NA, Pellicci DG, Beddoe T, Kostenko L, Clements CS, Williamson NA, Smyth MJ, Besra GS, Reid HH, Bharadwaj M, Godfrey DI, Rossjohn J, McCluskey J. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J Exp Med. 2006;203:661–673. doi: 10.1084/jem.20051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing Th2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 87.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc Natl Acad Sci U S A. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bai L, Sagiv Y, Liu Y, Freigang S, Yu KO, Teyton L, Porcelli SA, Savage PB, Bendelac A. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen alphaGalCer. Proc Natl Acad Sci U S A. 2009;106:10254–10259. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, Jerud ES, Goldberg MF, Baena A, Yu KO, Ndonye RM, Howell AR, Yuan W, Cresswell P, Chang YT, Illarionov PA, Besra GS, Porcelli SA. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roda-Navarro P. Assembly and function of the natural killer cell immune synapse. Front Biosci. 2009;14:621–633. doi: 10.2741/3268. [DOI] [PubMed] [Google Scholar]
- 93.Bezbradica JS, Gordy LE, Stanic AK, Dragovic S, Hill T, Hawiger J, Unutmaz D, Van Kaer L, Joyce S. Granulocyte-macrophage colony-stimulating factor regulates effector differentiation of invariant natural killer T cells during thymic ontogeny. Immunity. 2006;25:487–497. doi: 10.1016/j.immuni.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 94.Clark RH, Stinchcombe JC, Day A, Blott E, Booth S, Bossi G, Hamblin T, Davies EG, Griffiths GM. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat Immunol. 2003;4:1111–1120. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- 95.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 96.Maldonado RA, Irvine DJ, Schreiber R, Glimcher LH. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature. 2004;431:527–532. doi: 10.1038/nature02916. [DOI] [PubMed] [Google Scholar]
- 97.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Libero G, Moran AP, Gober HJ, Rossy E, Shamshiev A, Chelnokova O, Mazorra Z, Vendetti S, Sacchi A, Prendergast MM, Sansano S, Tonevitsky A, Landmann R, Mori L. Bacterial infections promote T cell recognition of self-glycolipids. Immunity. 2005;22:763–772. doi: 10.1016/j.immuni.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Muindi K, Cernadas M, Watts GF, Royle L, Neville DC, Dwek RA, Besra GS, Rudd PM, Butters TD, Brenner MB. Activation state and intracellular trafficking contribute to the repertoire of endogenous glycosphingolipids presented by CD1d [corrected] Proc Natl Acad Sci U S A. 2010;107:3052–3057. doi: 10.1073/pnas.0915056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, Besra GS, Platt FM, Cerundolo V. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.