Abstract
The block copolypeptide poly(l-homoarginine)60-b-poly(l-leucine)20 (R60L20) was previously found to self-assemble into versatile vesicles with controllable size and encapsulate hydrophilic cargo. These R60L20 vesicles also demonstrated the ability to cross the cell membrane and transport encapsulated cargo into different cell lines. To assess the potential for using the R60L20 vesicles as drug delivery vehicles further, we have investigated their endocytosis and intracellular trafficking behavior. Using drugs that inhibit different endocytosis pathways, we identified macropinocytosis to be a major process by which the R60L20 vesicles enter HeLa cells. Subsequent immunostaining experiments demonstrated that the vesicles entered the early endosomes but not the lysosomes, suggesting that they recycle back to the cell surface. Overall, our studies indicate that the R60L20 vesicles are able to enter cells intact with their cargos, and although some manage to escape from early endosomes, most are trapped within these intracellular compartments.
1. Introduction
Nanoscale drug delivery vehicles are beginning to play important roles in the development of new therapeutics. Encapsulation of drugs into delivery vehicles can improve their pharmacokinetic and pharmacodynamic properties by providing benefits including a longer blood circulation time, targeted delivery, and controlled release. In the area of vesicular nanoencapsulants, many advances have been made with lipid and synthetic polymer-based systems.1–3 These materials strive to include the ability to form structures and sizes that enhance encapsulation as well as delivery, functional surfaces to achieve a variety of purposes (e.g., targeting), and minimal toxicity and immunogenicity. An attribute that is particularly desirable for vesicular nanocarriers is the ability to cross the cell membrane barrier because many therapeutic targets are intracellular. To enhance the cellular uptake of lipid and polymer-based vesicles, they are frequently conjugated to cell-penetrating peptides (CPPs) such as the TAT peptide and oligoarginines.4–6 These functionalized vesicles are readily taken up by cells because of favorable bidentate binding interactions between their multiple guanidinium groups and various anionic species on plasma membranes such as phosphate and sulfate groups (Figure 1A).7–9 However, these conjugations need to be carefully controlled to ensure uniformity for drug delivery applications.
Figure 1.

(A) Proposed bidentate H-bonding interactions between guanidinium and phosphate groups. (B) Schematic of the K60L20 block copolypeptide and vesicle assembly.
Block copolypeptide vesicles have the potential to become effective intracellular drug delivery vehicles.10,11 The amino acid building blocks are naturally occurring, so they may give rise to low toxicity and immunogenicity. The abundance of many natural and synthetic residues also suggests that these materials can be tailored to exhibit a wide variety of chemical properties. We previously investigated the block copolypeptide poly(l-lysine)60-b-poly(l-leucine)20 (K60L20, Figure 1B).12 This polypeptide was synthesized using transition-metal-mediated α-amino acid N-carboxyanhydride polymerization that produces polypeptide segments with narrow molecular weight distributions.13 The K60L20 block copolypeptide demonstrated the ability to self-assemble into vesicles via solvent-based processing using THF and water. These vesicles have high functionality due to the primary amines on the lysine residues and exhibit minimal toxicity when incubated with cells. In addition, the K60L20 vesicles can be extruded through polycarbonate membranes of commensurate pore size to obtain vesicles with uniform diameters ranging from 100 nm to 1 μm, as confirmed with transmission electron microscopy (TEM), and the vesicles can also readily encapsulate hydrophilic cargos.12 Although these properties are desirable for drug carriers, the K60L20 vesicles were not significantly internalized by cells, which is problematic for delivery of drugs with intracellular targets. In contrast with liposome and polymersome systems, we did not conjugate CPPs onto our vesicles to improve their cellular uptake. Instead, we reasoned that replacing lysine residues in K60L20 with arginine residues would preserve the desirable physicochemical properties of the polylysine segments due to the similar charge character between arginine and lysine while imparting to the vesicles the ability to enter cells due to the presence of multiple guanidinium groups in the arginine residues. We synthesized poly(l-homoarginine)60-b-poly(l-leucine)20 (R60L20), which formed vesicles similar to K60L20 and also had the ability to transport cargos across cell membranes and remain inside the cells for up to 48 h (data not shown).13
In this study, we investigated the intracellular trafficking, or transport, properties of 100 nm diameter fluorescein-labeled R60L20 vesicles in HeLa cells. Identification of the intracellular fates has provided insight that allows the design of improved block copolypeptide vesicles for intracellular cargo release.
2. Materials and Methods
Materials
Minimum essential medium (MEM) with Earl's balanced salt solution, penicillin–streptomycin, sodium pyruvate, and phosphate-buffered saline (PBS) were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) and the formalin solution used to fix cells were obtained from Thermo Fisher Scientific (Waltham, MA), and the antibodies for the immunofluorescence experiments were products of Abcam (Cambridge, MA). All other tissue culture reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. HeLa cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). The 48-well tissue culture plates and 8-well chambered coverglass units were purchased from Corning (Lowell, MA) and Lab-Tek (Rochester, NY), respectively. The MTS cell proliferation assay kit was purchased from Promega (Madison, WI).
Synthesis and Processing of Polypeptide Vesicles
As previously described,13 the K60L20 copolypeptide was synthesized by transition-metal-initiated α-amino acid N-carboxyanhydride polymerization using (PMe3)4Co. The polymer was deprotected and then dialyzed exhaustively against DI water to remove any contaminants under pyrogen-free conditions. Fluorescein isothiocyanate (FITC) was conjugated to K60L20 by mixing 1% w/v polypeptide in sodium borate buffer at pH 8.0 with a 6:1 molar ratio of K60L20 to FITC at room temperature for at least 1 h. Following the FITC conjugation, the remaining free primary amines on lysine were converted to guanidinium groups using an excess of the reagent 3,5-dimethyl-1-pyrazolylformaminidium nitrate, as previously described.13 The resulting copolypeptide was purified by dialysis against sterile DI water and then freeze-dried. This poly(l-homoarginine)60-b-poly(l-leucine)20 polypeptide will be referred to as R60L20. Note that both poly(l-homoarginine) and poly(l-arginine) have been used as hydrophilic segments of the block copolypeptide in previous studies and found to yield similar results (data not shown). As previously described,13 polypeptide vesicles were prepared by a two-step THF/water processing procedure under sterile conditions. The resulting vesicles were extruded through a nucleopore polycarbonate membrane with 100 nm diameter pores to obtain a population of vesicles with a low polydispersity index of ~0.13. The size and polydispersity of the vesicles were determined using dynamic light scattering (DLS) measurements.
Cell Culture
The HeLa cell line was maintained in MEM supplemented with 26.2 mM sodium bicarbonate, 10% FBS, 1 mM sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin at a pH of 7.4 in a 37 °C humidified atmosphere with 5% CO2 using standard tissue culture protocols.
Laser Scanning Confocal Microscopy (LSCM)
LSCM images were taken on a Leica Inverted TCS-SP MP Spectral Confocal and Multiphoton Microscope (Heidelberg, Germany) equipped with an argon laser (488 nm blue excitation: JDS Uniphase), a diode laser (DPSS; 561 nm yellow-green excitation: Melles Griot), a helium–neon laser (633 nm red excitation), and a two-photon laser setup consisting of a Spectra-Physics Millenia X 532 nm green diode pump laser and a Tsunami Ti-sapphire picosecond pulsed infrared laser tuned at 768 nm for UV excitation.
Inhibition of Endocytosis Pathways
HeLa cells were seeded at a density of 4 × 104 cells/cm2 onto an eight-well chambered coverglass for at least 12 h before the start of experiment. At the start of the experiment, the cell culture medium was aspirated, and the cells were briefly washed with PBS. In a procedure similar to those reported in the literature,14–16 the cells were then pretreated with various endocytosis inhibitors diluted in cell culture medium at different concentrations for 30 min, except for 5-(N-ethyl-N-isopropyl)amiloride, which was incubated for 10 min. After this incubation period, the medium containing inhibitors was aspirated, and the cells were washed again with PBS to remove any residual amount of inhibitors. The cells were again incubated in cell culture medium containing 100 μM R60L20 polypeptide vesicles for 5 h in a 37 °C humidified atmosphere with 5% CO2. Following this incubation, this medium was aspirated, and the cells were washed with PBS to remove any free-floating polypeptide vesicles that were not internalized before the confocal images were taken.
MTS Cell Proliferation Assay
The MTS cell proliferation assay (CellTiter 96 AQueous non-radioactive cell proliferation assay) was used to quantify any cytotoxic effects that may be associated with using the inhibitor drugs. The procedure described above for inhibiting endocytosis pathways was followed with the exception of seeding HeLa cells on a 48-well plate instead of an 8-well chambered coverglass. At the end of the 5 h incubation period, the medium containing polypeptide vesicles was aspirated. Fresh medium containing 20% MTS was then added to the cells. The cells were placed back in a CO2 incubator for 2 h, and the absorbance at 490 nm was measured with an Infinite F200 plate reader (Tecan Systems, San Jose, CA).
Intracellular Trafficking Experiments
HeLa cells were seeded at a density of 4 × 104 cells/cm2 onto an eight-well chambered coverglass for at least 12 h before the start of the experiment. At the start of the experiment, the cell culture medium was aspirated, and the cells were briefly washed with PBS. The cells were then incubated with the cell culture medium containing 100 μM R60L20 polypeptide vesicles for 5 h in a 37 °C humidified atmosphere with 5% CO2. At the end of this incubation period, the medium containing polypeptide vesicles was aspirated, and the cells were briefly washed with PBS to remove any free floating vesicles. The cells were subsequently fixed with a 10% formalin solution for 1 h at room temperature and washed three times with PBS, with each wash lasting 5 min. After fixation, the cells were permeabilized by a 30 s incubation with PBS containing 0.01% Triton X-100, followed by three more of the 5 min PBS washes. Nonspecific binding sites were then blocked by incubating the fixed and permeabilized cells with a blocking solution containing 5% nonfat milk in PBS for 30 min.
Rabbit polyclonal primary antibodies specific for human early endosome antigen-1 (EEA-1) or lysosomal-associated membrane protein-1 (LAMP-1) were then added at a 1:500 dilution in blocking solution and incubated overnight at 4 °C. Cells were then washed three times in PBS, with each wash lasting 10 min. These washes were followed by incubation with goat polyclonal antibodies against rabbit IgG conjugated with Cy5. These secondary antibodies were added at a 1:500 dilution in blocking solution and incubated for 1 h at room temperature, followed by three more of the 10 min PBS washes before confocal images were taken. For the endosome disruption experiments, the cells were treated with 50 μM chloroquine for 30 min immediately before the fixation step. PBS was then used to wash the cells briefly three times, followed by fixation. Confocal images were taken at the end of each immunofluorescence experiment with each image consisting of two channels: green for vesicles and red for the labeled intracellular compartments (either endosomes or lysosomes).
3. Results and Discussion
To study the mechanism of cellular uptake for the R60L20 vesicles, we first focused on the pathway of internalization, or endocytosis, of the vesicles because the relationships between different mechanisms of endocytosis and the subsequent intra-cellular trafficking properties have been extensively studied. Specifically, three well-understood endocytosis mechanisms were examined: macropinocytosis, caveolae-dependent endocytosis, and clathrin-coated pit (CCP) endocytosis. We suspected that macropinocytosis was the most likely route for endocytosis for two reasons. First, because the surfaces of our vesicles present arginine residues and not biospecific targeting ligands, the vesicles should enter cells through a nonspecific pathway, such as macropinocytosis. Second, macropinosomes are generally >1 μm in diameter, whereas clathrin-coated vesicles are 100–150 nm in diameter and invaginations formed from caveolae-dependent endocytosis are 50–80 nm in diameter. We chose vesicles of 100 nm diameter for our studies because it has been previously shown that liposomes with similar size were more likely to accumulate inside tumors because of the enhanced permeability and retention (EPR) effect.17 Therefore, our 100 nm vesicles should easily fit inside macropinosomes and less likely into clathrin-coated vesicles, whereas they should be unable to enter the caveolae-dependent invaginations.
To determine the cell uptake mechanism, drugs were used to inhibit selectively each of these endocytosis pathways, and their effect on the cellular uptake of R60L20 polypeptide vesicles was observed. Details regarding the inhibitors, their concentrations, their mechanisms of action, and the literature references can be found in the Supporting Information. Note that the least toxic inhibitors were used because cytotoxic effects are known to increase membrane permeability and could confound our endocytosis analysis.18,19 Two inhibitor drugs were chosen for each endocytosis pathway: cytochalasin D and 5-(N-ethyl-N-isopropyl)amiloride for macropinocytosis, nystatin and filipin III for caveolae-dependent endocytosis, and chlorpromazine and dynasore for CCP endocytosis. The results of these inhibition studies demonstrate that inhibition of macropinocytosis (Figures 2B,C) had the most significant effect on the cellular uptake of the R60L20 vesicles, which confirmed our initial hypothesis. Inhibiting either caveolae-mediated endocytosis or CCP endocytosis did not show many inhibitory effects on vesicle uptake (Figures 2D–G). On the basis of these results, it appears that attractive electrostatic interactions initially bind the positively charged R60L20 vesicles to the cell surface. The subsequent favorable bidentate interactions between arginine residues and anions present at the cell surface can then increase the residence time of the vesicles at the cell membranes and potentially modify cell membrane properties. Because they are localized at the cell surface, the R60L20 vesicles are more readily internalized as the cell undergoes macropinocytosis.
Figure 2.
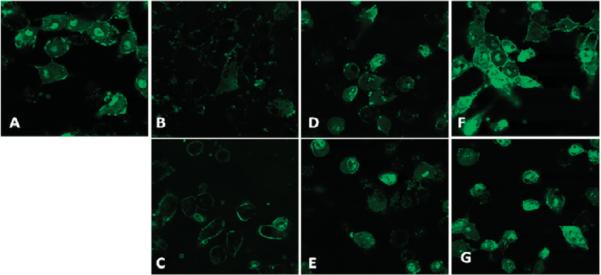
Effects of various endocytosis inhibitors on the uptake of the R60L20 polypeptide vesicles (green) into HeLa cells. (A) Control cells with no drug. Cells treated with (B) cytochalasin D, (C) 5-(N-ethyl-N-isopropyl)amiloride, (D) nystatin, (E) filipin III, (F) chlorpromazine, and (G) dynasore.
The endocytosis inhibition studies described above showed that macropinocytosis was the major pathway for the uptake of R60L20 vesicles into HeLa cells. This result is consistent with other studies performed with polymersomes decorated with TAT peptide on dendritic cells,4 TAT peptide alone, as well as arginine-rich peptides.20,21 Because molecules internalized via macropinocytosis are generally first routed to early endosomes, our vesicles were also expected to be sent to these intracellular compartments, where a sorting decision is typically made to traffic the contents either to lysosomes for degradation or to the cell surface for recycling back into the extracellular media.22 To confirm if the vesicles enter early endosomes and to determine if they are subsequently trafficked to lysosomes, we performed immunofluorescence experiments with antibodies for the early endosome antigen-1 (EEA-1, Figure 3A) and the lysosomal-associated membrane protein-1 (LAMP-1, Figure 3B) markers. Because the fluorescent tag on the polypeptides is fluorescein (green) and the fluorescent label on the secondary antibody is Cy5 (red), colocalization is represented by yellow fluorescent regions.
Figure 3.
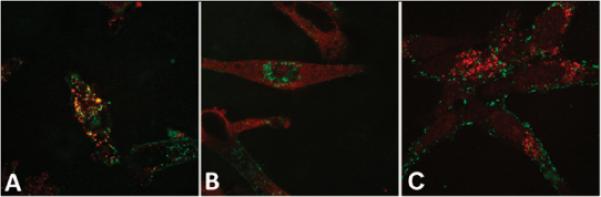
Colocalization studies using R60L20 vesicles (green) with antibodies for (A) EEA-1 (red), (B) LAMP-1 (red), and (C) EEA-1 (red) with chloroquine treatment.
The significant colocalization present in Figure 3A indicates that the vesicles are indeed present in early endosomes, which is consistent with internalization by macropinocytosis. Figure 3B indicates that the vesicles do not traffic to lysosomes, which means that they are possibly recycled back to the cell surface. Such recycling behavior could be advantageous for protein drugs because such drugs could avoid lysosomes, which can inactivate proteins with their harsh and denaturing environments. However, the potential disadvantage is that the vesicles may recycle back to the cell surface without releasing their payload. To address this concern and to confirm the presence of the R60L20 vesicles in endosomes further, the cells were treated with chloroquine, which disrupts endosomes.23 As shown in Figure 3C, the colocalization between the green fluorescently labeled R60L20 vesicles and the red fluorescently labeled early endosomes disappeared upon treatment with chloroquine. Moreover, the green signal remained punctate, that is, not diffuse, even after release of the vesicles into the cytoplasm. This indicated that the vesicles remained intact even after release from the endosomes, which suggests that an encapsulated drug may have difficulty being released from the vesicles. We have previously shown that pH-responsive residues can be incorporated into the hydrophobic domains of polypeptide vesicles.24 Similar modification of the R60L20 vesicles should assist in vesicle disruption and drug release upon endosomal acidification.
Conclusions
In summary, we used inhibitor drugs to identify macropinocytosis as the dominant process by which R60L20 vesicles enter cells. Vesicles internalized via macropinocytosis were first routed to endosomes, and immunostaining experiments were subsequently conducted to confirm these intracellular destinations. Additional immunostaining experiments showed that the vesicles avoid lysosomes, suggesting that vesicles may recycle back to the cell surface. Co-incubation of cells with vesicles and chloroquine enabled the release of vesicles into the cytosol. On the basis of these findings, we can conclude that a mechanism for endosomal disruption should be incorporated into block copolypeptide vesicles to enhance their release into the cytoplasm. Many strategies exist to effect endosomal release, and most rely on a “proton sponge” or buffering capability to cause endosome disruption upon a decrease in endosomal pH.25–27 The development of vesicles with pH switchable endosomal and vesicle disruptive capability is currently underway.
Supplementary Material
Acknowledgment
This work was supported by the UCLA Specialized Program of Research Excellence in Prostate Cancer P50 CA092131-08, the Department of Defense Prostate Cancer Research Program under award number W81XWH-09-1-0584, and the National Science Foundation DMR 0907453. We also acknowledge the use of the SPM facility at the Nano and Pico Characterization Lab at the California NanoSystems Institute.
Footnotes
Supporting Information Available. Selection of drugs for inhibiting endocytosis. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- (1).Budai M, Szogyi M. Acta Pharm. Hung. 2001;71:114–118. [PubMed] [Google Scholar]
- (2).Zhang K, Fang H, Shen G, Taylor JS, Wooley KL. Proc. Am. Thorac. Soc. 2009;6:450–457. doi: 10.1513/pats.200902-010AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. J. Controlled Release. 2006;116:150–158. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- (4).Christian NA, Milone MC, Ranka SS, Li G, Frail PR, Davis KP, Bates FS, Therien MJ, Ghoroghchian PP, June CH, Hammer DA. Bioconjugate Chem. 2007;18:31–40. doi: 10.1021/bc0601267. [DOI] [PubMed] [Google Scholar]
- (5).Tseng YL, Liu JJ, Hong RL. Mol. Pharmacol. 2002;62:864–872. doi: 10.1124/mol.62.4.864. [DOI] [PubMed] [Google Scholar]
- (6).Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D'Souza GG. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sakai N, Matile S. J. Am. Chem. Soc. 2003;125:14348–14356. doi: 10.1021/ja037601l. [DOI] [PubMed] [Google Scholar]
- (8).Frigyes D, Alber F, Pongor S, Carloni P. THEOCHEM. 2001;574:39–45. [Google Scholar]
- (9).Price PA, Williamson MK. J. Biol. Chem. 1985;260:14971–14975. [PubMed] [Google Scholar]
- (10).Schatz C, Louguet S, Le Meins JF, Lecommandoux S. Angew. Chem., Int. Ed. 2009;48:2572–2575. doi: 10.1002/anie.200805895. [DOI] [PubMed] [Google Scholar]
- (11).van Hest JC, Tirrell DA. Chem. Commun. (Cambridge, U. K.) 2001;19:1897–1904. doi: 10.1039/b105185g. [DOI] [PubMed] [Google Scholar]
- (12).Holowka EP, Pochan DJ, Deming TJ. J. Am. Chem. Soc. 2005;127:12423–12428. doi: 10.1021/ja053557t. [DOI] [PubMed] [Google Scholar]
- (13).Holowka EP, Sun VZ, Kamei DT, Deming TJ. Nat. Mater. 2007;6:52–57. doi: 10.1038/nmat1794. [DOI] [PubMed] [Google Scholar]
- (14).Hariton-Gazal E, Rosenbluh J, Graessmann A, Gilon C, Loyter A. J. Cell. Sci. 2003;116:4577–4586. doi: 10.1242/jcs.00757. [DOI] [PubMed] [Google Scholar]
- (15).Shurety W, Bright NA, Luzio JP. J. Cell. Sci. 1996;109:2927–2935. doi: 10.1242/jcs.109.12.2927. [DOI] [PubMed] [Google Scholar]
- (16).Wang L, MacDonald RC. Mol. Ther. 2004;9:729–737. doi: 10.1016/j.ymthe.2004.02.009. [DOI] [PubMed] [Google Scholar]
- (17).Nagayasu A, Uchiyama K, Kiwada H. Adv. Drug Delivery Rev. 1999;40:75–87. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- (18).Ormerod MG, Sun XM, Snowden RT, Davies R, Fearnhead H, Cohen GM. Cytometry. 1993;14:595–602. doi: 10.1002/cyto.990140603. [DOI] [PubMed] [Google Scholar]
- (19).Arends MJ, Wyllie AH. Int. Rev. Exp. Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- (20).Kaplan IM, Wadia JS, Dowdy SF. J. Controlled Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- (21).Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Mol. Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- (22).Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11076–11081. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Sonawane ND, Szoka FC, Jr., Verkman AS. J. Biol. Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- (24).Bellomo EG, Deming TJ. J. Am. Chem. Soc. 2006;128:2276–2279. doi: 10.1021/ja056227h. [DOI] [PubMed] [Google Scholar]
- (25).Akinc A, Thomas M, Klibanov AM, Langer R. J. Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- (26).Shigeta K, Kawakami S, Higuchi Y, Okuda T, Yagi H, Yamashita F, Hashida M. J. Controlled Release. 2007;118:262–270. doi: 10.1016/j.jconrel.2006.12.019. [DOI] [PubMed] [Google Scholar]
- (27).Henry SM, El-Sayed MEH, Pirie CM, Hoffman AS, Stayton PS. Biomacromolecules. 2006;7:2407–2414. doi: 10.1021/bm060143z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


