Abstract
Bioluminescence imaging (BLI) modalities have been developed, refined, and used broadly in the study of small animal models of human biology and disease, including monitoring the fate of transplanted islets in vivo in real time. In order to advance our understanding of the pathophysiology and immunobiology of islet transplantation as they occur in living animals, islet grafts tagged with light-emitting luciferase can be implanted in a mouse islet transplantation model and assessed using in vivo BLI. We have utilized transgenic islets expressing the firefly-luciferase as donor islets in syngeneic and allogeneic islet transplant mouse models for monitoring islets in vivo by BLI after they have been transplanted at different sites of the mice, including the subcapsular renal space and the intrahepatic site via portal vein injection. The sensitive and non-invasive BLI system allows better understanding of the dynamic fate of transplanted islets and the relationships among the islet mass that ultimately engrafts, the quality of graft function, and overall glucose homeostasis. It permits detection of early changes in islet graft function or mass due to rejection to prompt timely therapeutic intervention and change the fate of the graft. This Chapter details some of the procedures for islet isolation, transplantation, and imaging as well as considerations of using the BLI system in the field of islet transplantation research.
Keywords: Islet isolation, islet portal transplantation in mice, bioluminescence imaging
1. Introduction
Islet transplantation can successfully ameliorate long-term glycemic instability and severe hypoglycemic complications in select subjects with type 1 diabetes mellitus (1). The success of islet transplantation depends on engraftment of an adequate mass of viable islets that produce sufficient amounts of insulin. Islets, however, are exquisitely sensitive to mechanical and chemical stresses generated during the isolation, purification and transplantation processes, Islets are also vulnerable to injuries imposed by immediate nonspecific host inflammatory responses, later allo-specific rejection and potential auto-immune reactions post-transplant.
To better understand the dynamic fate of transplanted islets and the relationships among the islet mass that ultimately engrafts, the quality of graft function, and overall glucose homeostasis, it is important to develop sensitive and non-invasive methods of real-time imaging to fate-map the functional mass of islets in vivo. We have applied bioluminescence imaging (BLI) in mouse models of islet transplantation by transplanting transgenic islets expressing the firefly luciferase to recipient mouse liver via portal vein infusion (2, 3). There is a linear relationship between the amount of islets transplanted and the intensity of the luminescent signals detected. BLI is a sensitive method for tracking the fate of islets after transplant and permits detection of early changes in islet graft function or mass due to rejection to prompt timely therapeutic intervention and change the fate of the graft (3).
2. Materials and Methods
2.1. Sources of Luciferase-tagged Islets and Choices of Recipient Mice
Transgenic FVB-Tg(RIP-luc) mice are used as islet donors in each transplant procedure. These mice have the FVB/NJ background (H-2q ) and contain the firefly luciferase gene under the regulation of a rat insulin promoter II (RIP, 760 bp) that specifically and constitutively expresses firefly luciferase in the pancreatic islet beta cells (Xenogen Corp., Alameda, CA).
Wild-type male FVB/NJ (H-2q) and Balb/C (H-2d) mice (Jackson Laboratories, Bar Harbor, ME) are used as islet recipients for isogenic and allogeneic islet transplants.
2.2. Mouse diabetes induction by Streptozotocin treatment
A. Reagents
Streptozotocin (minimum 98% HPLC, Sigma-Aldrich, S0130-IG).
Citric Acid Anhydrous (Sigma Chemial Co., C-4540).
Sodium Citrate Dihydrate (T. Becker, 3646-01)
0.1 M Citric Acid Solution: dissolve 1.9241 g of citric acid in 50 ml distill water. Store at room temperature.
0.1 M Sodium Citrate Solution: Dissolve 1.4705 g of sodium citrate in 50 ml distill water. Store at room temperature.
Citric Acid Buffer: Mix 25.5 ml 0.1 M sodium citrate solution and 22.0 ml 0.1 M citric acid solution and make up vokume to 100 ml with distill water.
B. Procedure
Weight and record body weight of each mouse to be rendered diabetic. Prepare fresh Streptozotocin solution by dissolving 0.065 g of Streptozotocin in 5 ml of Citric Acid Buffer for 20 min at room temperature. Then keep on ice while using.
To render mice diabetic, inject 220 mg/kg of Streptozotocin Solution intraperitoneally.
2.3. Mouse islet isolation
A. Equipment and instruments
50 ml conical tubes
20 ml capacity glass screw top vial (1 per 5 pancreata)
1 glass funnel with 400 μm mesh screen
27 gauge needle
5, 10, 25, 50 ml pipettes
electric pipettor
sterile Pasteur pipette, VWR Cat. 14672-200 size 5 ¾
sterile drawn Pasteur pipette with tubing and 1 ml syringe
60X15 mm Polystyrene (Falcon) Petri dish, 351007 Becton Dickinson
100X15 mm Ten-twenty-nineTM Petri dish, 351029 Becton Dickinson
Cotton tip swab
2 scissors
1 toothed tissue forceps and 1 anatomical forceps
1 mosquito clamp
1 abdominal retractor
B. Reagents
Hank’s Balanced Salt Solution (HBSS 1X with calcium & magnesium without phenol red, Cellgro Cat. # 21-023-CV).
Hank’s Balanced Salt Solution (HBSS 1X with calcium, magnesium & phenol red, Mediatech, Inc., Herndon VA, Cat. # 21-020-CV)
Penicillin-Streptomycin (P/S) 10,000 units/ml and 10,000 μg/ml, Mediatech Cellgro, Cat. No. 30-002-CI, store aliquots of 5 ml in 15 ml conical tubes at −20°C
Fetal Bovine Serum (FBS, HyClone Cat. No. SH 30071.03). If not pretreated, heat at 60°C for 30 min, then store aliquots of 50 ml in 50 ml conical tubes at −20°C until use.
Collagenase, Clostridiopeptidase A from Clostridium histolyticum Type XI (Sigma C7657).
1% Stock Dextran.
HEPES Buffer (Sigma H0887)
Avertin 1.2% solution
C. Working Solutions of Reagents
HBSS+P/S: Add 5 ml of thawed Penicillin/Streptomycin to 1 bottle of 500 ml HBSS 1X with calcium & magnesium without phenolred. Keep on ice. Add 0.5 ml to sterile glass screw top vial (digestion of pancreas) and 40 ml to conical 50 ml tube (rinsing of pancreas) and keep on ice.
Collagenase: Remove collagenase from freezer. Place in desiccator and let it warm up before weighing. Measure 0.0103 g of collagenase and mix with 20 ml of HBSS with Ca, Mg without phenol red +P/S in a 50 ml conical tube and filter it with 10 cc syringe and 0.22 μm filter for injection of 2.5 ml/mouse, sufficient for 6 mice. Keep on ice.
HBSS+P/S+FBS: Add 50 ml thawed FBS and 5 ml of Penicillin/Streptomycin to HBSS 1X with calcium, magnesium & phenol red. Keep on ice.
Dextran Gradient: See separate Dextran Gradient Protocol. Prepare under step 7 in the islet isolation protocol (see below).
Avertin 1.2% Solution: See separate Avertin Protocol.
D. Procedure
-
Pancreas Procurement
Mice, 20–35 g body weight, are anesthetized by an intraperitoneal injection of a weight-adjusted dose of Avertin (see Avertin Protocol). Abdomen and chest are shaved and disinfected with 10% ethanol. The abdominal cavity is entered via a midline incision from the sternum to the symphysis pubis and an abdominal retractor is inserted. After pulling the intestines to the left, the duodenum is folded also to the left. This exposes the bile duct, which is clamped with a mosquito clamp at its junction with the duodenum. The sternum is excised and the mouse exsanguinated by cutting the intrathoracic aorta and vena cava. The liver is retracted to the chest with a wet gauze and the common bile duct is then cannulated at its junction with the cystic duct with a 27 gauge needle, attached to a 3 ml syringe. The tip of the cannula is advanced downward in the bile duct beyond any segmental branches to the liver. By injecting the collagenase over ~ 3 minutes the duodenal and splenic portion of the pancreas should both become distended. After removal of the cannula, the proximal end of the bile duct is cut, and the pancreas excised from the duodenum and other attachments and placed in the pre-cooled screw top vial containing 0.5 ml HBSS+P/S after rinsing in 40 ml HBSS+P/S. Up to five pancreases are collected in one vial, which is kept on ice.
For digestion of the pancreata, the vial is transferred from ice to the 37°C waterbath and shaken once at 5 min. The digestion time depends on the collagenase brand, type and lot and is ca. 11–15 minutes.
To stop the digestion, the digest is transferred to a 50 ml conical tube, containing already 20 ml cold HBSS+P/S+FBS. After shaking the tube fairly hard to break up the pancreas and get a cell suspension, the volume is made up to 50 ml with cold HBSS+P/S+FBS.
Centrifuge at 1500 rpm for 2 min. All samples are centrifuged with a GS-6R Centrifuge (Beckman Coulter) at a temperature of 5°C.
Pour off the supernatant into the waste container. Then add 10 ml HBSS+P/S+FBS and shake the tissue up and down.
Set up the glass funnel with a 400 μm mesh screen and 50 ml conical collection tube. Pour the tissue on the mesh and wash it with another 40 ml of cold HBSS+P/S+FBS through the mesh screen by using a 10 ml syringe with a 19 gauge needle.
Centrifuge at 1500 rpm for 2 min and pour off the supernatant into the waste container.
Now prepare the dextran gradient tube: Add 16 ml gradient (T-3) 1.111 g/ml into the cell pellet, mix well. Gently add 6 ml of gradient (T-2) 1.092 g/ml on top. Add 6 ml of gradient (T-1) 1.083 g/ml on top and then add 6 ml of gradient (T-0) 1.039 on top.
Centrifuge for 20 min. Start with 600 rpm for the first 1 min, then increase rotor speed to 2500 rpm. Set brake off.
The islets should be between the Top (T-0) and (T-1), and (T-1) and (T-2) interfaces. Collect all cells which shine bright under maximum illumination from the interface and transfer into another 50 ml conical tube, containing 10 ml cold HBSS+P/S+FBS and add up to 50 ml.
Wash off the gradient solution by washing the islets 2 times. Centrifuge at 2000 rpm for 1 min with brake high (first wash). Remove 30 ml of the supernatant with the Pasteur pipette. Then mix well and add cold HBSS+P/S+FBS up to 50 ml.
Centrifuge at 1500 rpm for 1 min (second wash). Remove the supernatant completely using the Pasteur pipette.
To do a second gradient, repeat step 7 to 11 with a modification of step 8, centrifuge only 10 min, and a modification of step 10 and 11, wash 3 times, after the second wash remove only 45 ml.
Resuspend the pellet in 10 ml HBSS+P/S+FBS and pour the islet suspension in one 100×15 mm Petri dish, containing already 5 ml of HBSS+P/S+FBS. Wash the conical tube 3 times with 10 ml +P/S+FBS, pouring each wash into the Petri plate. Pick up the islets with a sterile Pasteur pipette and count the islet number. Transfer the islets in 60X15 mm Petri dishes, which contain already 5 ml HBSS+P/S+FBS, and keep the Petri dishes on ice. For culture, use either RPMI 1640 supplemented with 10% FBS+1% P/S, or CMRL 1066 supplemented with 10% FBS, 1% P/S and 1% L-glutamine and transfer to the CO2 incubator (5%CO2, 37°C). Change culture medium every day.
2.4. Intra-portal Islet transplantation
A. Equipment and solution
Dissection Microscope.
25GA butterfly (winged 0.38 × 3 tubing needle).
3ml syringe.
Petri dish filled with sterile saline.
Avitene.
Sterile instrument pack with: small straight edged scissors, small smooth forceps, toothed forceps, gauze pads, and cotton tipped applicators, needle holder, skin stapler.
5-0 Ethicon silk sutures.
Sterile pad.
Shaver.
B. Set Up
Thoroughly wipe down the area and plastic board with 70% ethanol.
Place the syringe and all sterile instruments within reach.
Place sterile pad under microscope optic light.
Roll Avitene into a small ball (about 2cm × 2cm). Place the ball within reach.
C. Procedure
Carefully swirl islets to the center of a Petri dish.
Draw up 0.5 ml sterile saline in 3 ml syringe, attach 25GA butterfly needle to syringe, fill saline in needle tubing. Expel air from syringe and butterfly.
Carefully and slowly aspirate all the islets into butterfly avoiding air bubbles, set aside within easy reach.
Inject 0.3 ml (0.01ml/1gram mouse) Avertin to induce and maintain anesthesia for approximately 20 min.
Shave the abdomen and wipe with a 70% Ethanol soaked gauze pad.
Open skin via long midline incision.
With the toothed forceps lift the muscle layer away from the vital organs and cut the muscle, also along the midline. Be careful not to cut through the rib cage or the diaphragm.
Retract the muscle and skin on each side by suture and tape the end to the pad.
Cover the intestine with wet gauze, carefully move the intestine to the left side to expose the Portal vein.
Hold the tissue adjacent to the portal vein with smooth forceps. This will stabilize the vein during injection. With other hand, insert butterfly needle into portal vein.
While holding butterfly in the portal vein, lay smooth forceps down and use this hand pick up the syringe. Very slowly inject the islets to portal vein.
After all the islets and saline (to make sure all the islets are injected) have been injected into portal vein, put syringe down. Use the same hand to pick up the avitene ball with forceps place the ball on top of needle entrance site. Lay down forceps and pick up dry cotton tipped applicator, push the ball toward needle entrance. At the same time gently withdraw the needle and press the avitene ball to stop bleeding.
Using moist cotton tipped applicator, gently move intestine back to normal position.
Suture the muscle with 5-0 silk. Moisten the muscle layer and approximate the skin. Suture the skin with 5-0 silk.
2.5. Substrate administration and bioluminescence imaging
A. Instruments
In vivo bioluminescence imaging system (IVISR 200, Xenogen Corp., Alemeda, CA).
Gas Anesthesia System (Xenogen).
Computer equipped with the Living Image software (Xenogen).
1 cc syringes.
B. Reagent
Isofluorane
Luciferin (Molecular Therapeutics Inc., Ann Arbor, MI).
C. Procedure
In preparation for BLI, mice are placed in the gas anesthesia chamber that is designed to work with IVISTM and anesthetized with 2.5% isofluorane in air that is delivered by an isoflurane vaporizer. After the mice are anesthetized, they are injected i.p. with substrate luciferin potassium salt (see Note #3) and then placed dorsal side up in the camera chamber where a controlled flow of 2.0% isofluorane in air is administered through a nosecone via the gas anesthesia system. Minutes (see Note #4) after the injection of luciferin, mice are imaged for a one-minute duration on dorsal side at medium-resolution with a field of view (FOV) of 20cm. A gray-scale body image is collected and overlaid by a pseudo-color image representing the spatial distribution of detected photons (Figure 1). Photons per second of light from the liver from dorsal images are quantified using circular regions of interest (ROI), 2 × 2 cm in size. Background images are collected daily and background subtractions are automatically calculated by the Living Image software.
Figure 1.
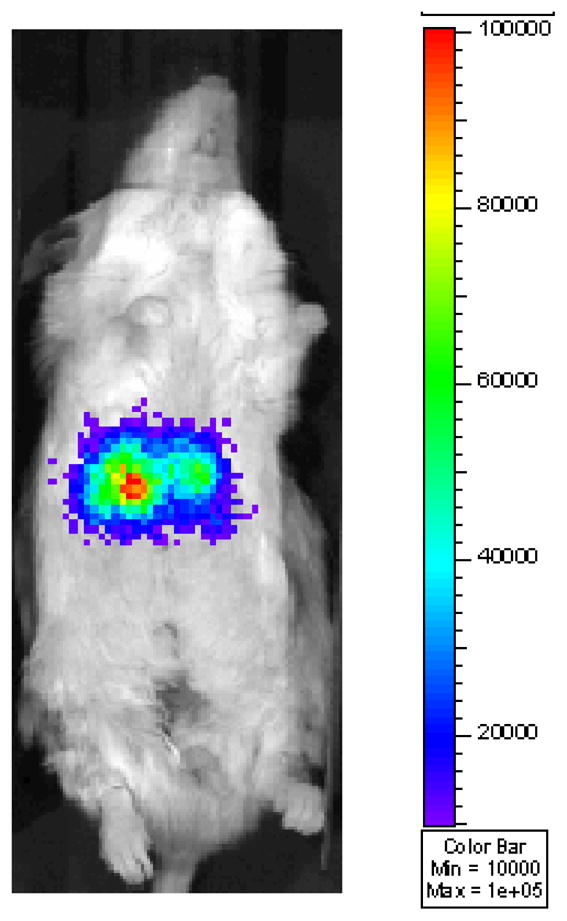
Bioluminescent image of luciferase-positive islets post-intrahepatic transplant. Islets isolated from Tg(RIP-luc) mice were transplanted to a syngeneic mouse via portal vein infusion.
2.6. Data analysis
The BLI signal intensities emitted from the liver region of the recipient mice are analyzed by IGOR IMAGE software (WaveMetrics, CA) and expressed in terms of photons per second. The mean ± standard error of the BLI signal intensity are calculated and the unpaired two-tailed Student’s t-test used for statistical analysis. A probability value of <0.05 is considered to be statistically significant.
Figure 2.
Luciferin administration at 100 ~ 400mg/kg body weight results in a dose dependent increase in luminescence emitted.
Acknowledgments
This work was supported by National Institutes of Health Grant DK063565 (D. B. K.), and the Juvenile Diabetes Research Foundation.
Footnotes
Safety and precautions for handling of streptozotocin: wear a laboratory coat and protective gloves for handing streptozotocin, and a face mask for weighing the powder or weigh inside a fume hood. Waste generated from use of streptozotocin must be stored in suitable containers appropriately labeled as chemical hazard, so they can be properly disposed. The streptozotocin-injected mice must be dept in a study area for at least 6 hours after the injection so that the waste bedding which is contaminated with urinary excretions of streptozotocin can be properly disposed. The animals must then be placed in newly bedded cages before being returned to the animal facility.
Sources of luciferase-tagged islets and choices of recipient mice: transgenic mice with islets expressing luciferase under the β-actin promoter or GAPDH promoter can also be obtained from Xenogen and used as islet donors for transplantation and imaging. Since black animals reduce the sensitivity of BLI significantly as melanin in the skin and fur absorbs light, white or hairless mice are better suited for imaging. Younger mice with lower body weight are preferred as photons can penetrate more efficiently from their internal organs.
The luminescent signal intensities increase with increase amount of luciferin administered. Luciferin at 100 μg/gm body weight generate significant amount of emission of luminescence from the liver region where luciferase-expressing islets are transplanted. Luciferin administration at 100 ~ 400mg/kg body weight results in a dose dependent increase in luminescence emitted (Figure 2)
The magnitude of bioluminescence measured varied with time after the injection of luciferin. Luminescence can be detected in the liver region from the dorsal side of the mouse as early as 2 minutes after the administration of luciferin. The luminescence intensity peaks around 10 minutes, diminishes rapidly within the first hour and completely disappeared by 3~ 4 hours.
BLI measurements are subject to some inherent limitations: Correlation of light emission to islet mass and function must take into consideration the factors that influence light transmission from the bioluminescent source to the CCD camera aperture. Factors influencing BLI measurements include surgical artifacts, motion (mouse positioning at the time of imaging) artifacts, and subject body weight artifacts. It is therefore important to include in the imaging system an internal control such as luminescent beads (4, 5). In vivo BLI measurements may also be influenced by graft site oxygen and ATP levels, re-vascularization and by the amount of luciferin actually reached the graft site (injection artifacts). Careful and consistent luciferin-injection in terms of the amount and the location of injection is required in BLI analysis of islet transplantation.
References
- 1.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation.[see comment] New England Journal of Medicine. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Kaufman DB. Bioluminescence Imaging of Pancreatic Islet Transplants. Curr Med Chem. 2004;4:301–308. [Google Scholar]
- 3.Chen X, Zhang X, Larson CS, Baker MS, Kaufman DB. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation. 2006;81:1421–1427. doi: 10.1097/01.tp.0000206109.71181.bf. [DOI] [PubMed] [Google Scholar]
- 4.Virostko JM. Thesis Submitted to the Faculty of the Graduate School of Vanderbilt University. 2003. Assessment of Pancreatic Islet Transplantation Using In Vivo Bioluminescence Imaging. [Google Scholar]
- 5.Virostko J, Chen Z, Fowler M, Poffenberger G, Powers AC, Jansen ED. Factors influencing quantification of in vivo bioluminescence imaging: application to assessment of pancreatic islet transplants. Molecular Imaging: Official Journal of the Society for Molecular Imaging. 2004;3:333–342. doi: 10.1162/15353500200404133. [DOI] [PubMed] [Google Scholar]



