Abstract
Objective
Among the seven subtypes of juvenile idiopathic arthritis (JIA), oligoarticular JIA (oJIA) and psoriatic JIA (psJIA) display a predilection for onset in early childhood. We examined whether meaningful differences in clinical phenotype justify the distinction between these conditions.
Methods
We performed a chart review to identify children with psoriatic and non-psoriatic oligoarticular-onset JIA. Clinical and demographic features of the two groups of children were compared.
Results
303 met criteria for oJIA and 87 met criteria for oligoarticular-onset psJIA. Both groups had a peak age of onset at 2 – 3 years, though psJIA had appreciable incidence into adolescence. Onset before 5 years of age was observed in 215 (71%) and 38 (44%) children respectively (p < 0.001). Within this age category, children with psJIA demonstrated similar gender ratio and anti-nuclear antibody status to those with oJIA but exhibited a distinctive clinical pattern, with a tendency to involve the wrists and small joints of the hands and feet. Conversely, among all children presenting with oligoarthritis in early childhood, those with wrist or small joint involvement were more likely to have nail pits, psoriasis, or a family history of psoriasis than those without (p < 0.05), supporting the association of this joint pattern with the psoriatic diathesis.
Conclusion
Even taking into account age of onset and number of joints, oJIA and psJIA remain clinically distinct, though important demographic overlap remains. These findings support separate diagnostic categories but justify further investigation into the similarities as well as differences among these children.
Keywords: Juvenile idiopathic arthritis, Psoriatic arthritis, Oligo-articular arthritis
Introduction
Juvenile idiopathic arthritis (JIA) is a heterogeneous set of conditions, currently parsed out by the International League of Associations for Rheumatology (ILAR) group into seven mutually exclusive categories (1). Although the ILAR criteria capture some of the clinical diversity observed in pediatric arthritis, the current system fails to take into account heterogeneity introduced by age of onset (2). Many studies have demonstrated that juvenile-onset arthritis exhibits an age of onset distribution characterized by a peak at 2 – 3 years, in some cases followed by a second peak in mid- to late-adolescence (3-6). Children who develop arthritis within the first 5-6 years of life are distinct from older-onset JIA patients in a number of important respects, including uveitis risk (7), HLA associations (5), and gene expression signatures (8), even within the same JIA category.
Several groups, including ours, have demonstrated that psoriatic JIA (psJIA) displays a similar biphasic age of onset distribution (9-11). Older patients, typically adolescents at disease onset, have an even male-female ratio and exhibit axial joint involvement and enthesitis, suggesting a close relationship with the spondyloarthropathies (9). By contrast, early-onset psJIA peaks at the same time as JIA, and resembles early-onset JIA in its female predominance and tendency to manifest antinuclear antibodies (ANA) (9).
Such similarities have given rise to the suggestion that the psoriatic diathesis is of limited utility in the classification of juvenile arthritis, especially among younger children(2). More broadly, recent work that compared psoriatic- to non-psoriatic arthritis, not stratified by age of onset, found that differences between these populations were modest, again questioning whether psJIA is appropriately included within the JIA classification system (12).
Elimination of psoriatic arthritis from the JIA system would impose a sharp divide between pediatric rheumatology and its adult counterpart, where psoriatic arthritis is well established on the basis of clinical features as well epidemiologic, serologic, histologic and immunopathologic considerations (13-15). Further, earlier work comparing psoriatic and non-psoriatic JIA types as a whole, without consideration of age of onset, found subtle but clear differences in pattern of joint involvement, supporting the retention of psoriatic arthritis as a subcategory within JIA (16). Taking advantage of recent gains in the understanding of psJIA as a heterogeneous disease, we therefore wished to examine the subset of psJIA which is most plausibly controversial, early-onset children with oligoarthritis, to ask whether these patients are better understood as having typical oJIA. We hypothesized that if early-onset oligo-psJIA is identical to oJIA, then clinical, laboratory and demographic features of these children will be similar. If the two conditions represent different entities, then we should most likely be able to identify phenotypic differences between these populations.
Materials and methods
Study design
This was a retrospective study involving children with oligoarticular onset juvenile idiopathic arthritis (JIA). We compared those with oJIA with children having psJIA.
Subjects
The study was conducted at two hospitals: Children’s Hospital Boston (CHB; Boston, MA) and Texas Scottish Rite Hospital for Children (TSRHC; Dallas, TX). Children with psJIA were identified from both locations, while the oJIA cohort was derived exclusively from TSRHC. The study was limited to patients who had oligoarticular onset within the first six months, with or without an extended course; to ensure exclusion of children who might subsequently be classified with polyarticular JIA, we required at least six months of disease.
PsJIA
Children with psJIA in the Boston cohort were identified as described previously (9). Briefly, we reviewed the charts of every patient seen in the rheumatology clinic of Children’s Hospital Boston between January 1997 and February 2005 with a diagnosis code of psoriasis (International Classification of Disease Codes [ICD] – 9 696.1), psoriatic arthritis (ICD-9 696.0), or spondyloarthritis (ICD-9 756.11). Children in the Dallas cohort of patients were identified through a local database of clinical diagnoses of psoriatic arthritis made by attending physicians from January 1985 through December 2008.
Oligoarticular JIA
We identified children through our clinical database as above, limited to patients whose final visit was after the year 2000. Diagnoses searched included pauciarticular and oligoarticular arthritis (17).
In general, we limited the patient population to children who met the ILAR criteria for oJIA or psJIA (1). The only exception to this was children with both dactylitis and nail pits but lacking a personal or family history of psoriasis, who were included as having psJIA, even though technically they would be classified as undifferentiated due to meeting criteria for both psJIA and oJIA. This modification of the ILAR criteria was justified by our previous study of the JIA classification criteria for psoriatic arthritis (18) and affected a total of 10 of 390 (2.6%) patients.
Definitions
The following definitions were used in this chart review. Oligoarticular onset was defined as involvement of less than 5 joints, cumulatively, within the first 6 months of symptoms. A patient was considered to have psoriasis if that diagnosis was made conclusively by a physician, including the attending rheumatologist. First-degree relatives with a history of psoriasis-like rashes were considered to have psoriasis only if there was a history of a definitive diagnosis. The diagnosis of arthritis required either the finding of a swollen joint without any other cause, or the combination of restricted range of motion accompanied by pain or tenderness; these symptoms had to be present for at least six weeks (1, 19). Small peripheral joints included the MCPs, PIPs, and DIPs of the hands, as well as the corresponding joints of the feet; PIP and DIP involvement of the toes was considered together as toe arthritis. Large peripheral joints included the hips, shoulders, elbows, wrists, knees, and ankles. Dactylitis was defined as digital swelling extending beyond the margin of the joints; because dactylitis can reflect tenosynovitis in the absence of synovitis, the latter was not assumed to be present, unless specifically documented (20). Enthesitis was defined as tenderness or swelling at the location of a tendinous insertion into the bone; in practice, entheseal sites were limited to the plantar fascia, Achilles tendon, and tibial tuberosity. ANA values were considered positive if above the upper limits of normal for the laboratory in which the test was performed (generally ≥ 1:40).
Data analysis
We compared categorical data and proportions using the chi-square test or Fisher’s exact test as indicated. Means were compared with the Student t-test and medians with the Mann-Whitney U Test. Odds ratios and 95% confidence intervals were calculated with the Cochran-Mantel-Haenszel test. We set α equal to 0.05. All of the analyses were performed using SPSS Version 16 (Chicago, IL.)
Approval
Institutional Review Board approval for the chart review was obtained at both centers.
Results
Patient population
390 children were included in this study: 303 (78%) had oJIA and 87 (22%) had psJIA. As discussed above, all of the children with oJIA were derived from the Dallas cohort, as were 35/87 (40%) of the children with psJIA. Among the 253 children with early-onset arthritis (age of onset < 5), 215 (85%) had oJIA and 38 (15%) had psJIA. Of those 38, 22 (58%) came from the Dallas cohort. There were no patients present in both the Dallas and the Boston cohorts.
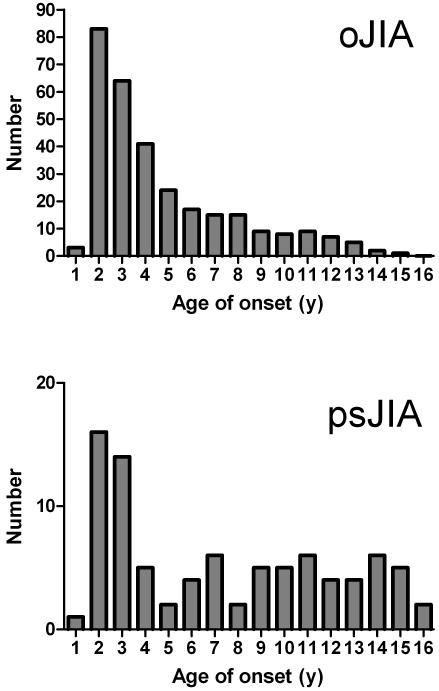
Age of onset distribution of oJIA and psJIA (Figure 1)
Figure 1.
Age of onset of oligoarticular JIA (n=303) and oligo-articular onset psoriatic JIA (n=87). Note similar peak age of onset in early childhood, but higher proportionate frequency of psJIA in later childhood.
The age of onset of children with oligoarticular psoriatic and non-psoriatic JIA is shown in Figure 1. The overall appearances of both curves showed a peak age of onset around 2 – 3 years. The primary difference between the curves is that onset of oligoarticular psJIA continued into adolescence, such that the median age of these patients was substantially higher (6.1 vs. 3.0 years, p < 0.001).
Comparison of children with oJIA vs. psJIA
Demographic and clinical features of children with oligoarticular psoriatic and non-psoriatic JIA are shown in Table 1. Aside from median age of onset, no demographic differences were observed. Children with psJIA were more likely to have small joint and wrist disease, but less likely to have involvement of the knees or of large joints. They were also more likely to extend to a poly-articular course (29.9 vs. 7.6%, p < 0.001), and were more likely to have arthritis or dactylitis at the final visit. Other differences emerged as a direct consequence of the classification criteria, including the absence of psoriasis in the oligoarticular JIA group and a higher prevalence of dactylitis and nail pits in the psJIA group. Measurement of HLA-B27 in our populations was performed with insufficient frequency to analyze.
Table 1.
Comparison of patients with oJIAwith those with oligoarticular psJIA.
| Characteristic | oJIA* | psJIA* | p |
|---|---|---|---|
| N | 303 | 87 | n/a |
| Duration of follow-up (yrs: median, IQR*) |
3.8, 1.7 – 6.9 | 3.4, 1.3 – 5.6 | 0.147 |
| Females (%) | 80.9 | 69.0 | 0.018 |
| Age of onset (yrs: median, IQR) |
3.0, 1.8 – 5.7 | 6.1, 2.2 – 10.8 | < 0.001 |
| Age under five (%) | 71.0 | 43.7 | < 0.001 |
| Extended course (%) | 7.6 | 29.9 | < 0.001 |
| Affected joints (%) | |||
| Any large joint | 97.0 | 81.6 | < 0.001 |
| Shoulder | 0 | 0 | N/A |
| Elbow | 8.9 | 13.8 | 0.181 |
| Wrist | 12.5 | 27.6 | 0.001 |
| Hip | 1.3 | 3.4 | 0.188 |
| Knee | 83.8 | 60.9 | < 0.001 |
| Ankle / subtalar | 38.3 | 49.4 | 0.062 |
| Any small joint | 19.1 | 52.9 | < 0.001 |
| MCP* | 6.6 | 17.2 | 0.002 |
| Hand PIP* | 14.9 | 42.5 | < 0.001 |
| Hand DIP* | 0.7 | 10.3 | < 0.001 |
| MTP* | 2.0 | 14.9 | < 0.001 |
| Foot IP* | 2.3 | 10.3 | 0.003 |
| Extra-articular features (%) |
|||
| Psoriasis | 0 | 60.9 | < 0.001 |
| Nail pits | 3.6 | 48.3 | < 0.001 |
| Dactylitis | 16.8 | 50.6 | < 0.001 |
| Enthesitis | 0 | 27.6 | < 0.001 |
| Uveitis | 29/298, 9.7% | 6/57, 10.5% | 0.854 |
| Laboratory values | |||
| Baseline WBC / μl: mean, SD |
8.9, 2.6 | 8.6, 3.1 | 0.392 |
| Baseline ESR, mm/hr: mean, SD |
26.3, 20.4 | 21.1, 14.6 | 0.013 |
| Baseline platelets x 103 / μl: mean, SD |
375, 110 | 350, 84 | 0.069 |
| ANA | 215/299, 71.9% | 36/73, 49.3% | < 0.001 |
| RF | 8/269, 3.0% | 0/41 | 0.603 |
| HLA-B27 | 17/178, 9.6% | 3/25, 12.0% | 0.719 |
| Treatments (%) | |||
| IAC* | 43.2 | 21.8 | < 0.001 |
| Any traditional | 35.6 | 74.7 | < 0.001 |
| DMARD* | |||
| Any TNF* inhibitor | 7.6 | 18.4 | 0.003 |
Abbreviations: oJIA = oligoarticular juvenile idiopathic arthritis, psJIA = psoriatic juvenile idiopathic arthritis, IQR = inter-quartile range, MCP = metacarptal phalyngeal joint, PIP = proximal intraphalyngeal joint, DIP = distal intraphalyngeal joint, MTP = metatarsal phalyngeal joint, IP = intraphalyngeal joints, IAC = intra-articular corticosteroids, DMARD = disease-modifying anti-rheumatic drug, TNF = tumor necrosis factor.
Comparison of psoriatic and non-psoriatic children with early-onset oligoarticular JIA
We compared the demographic and clinical features of children with early-onset oligoarticular psJIA with those with early-onset oJIA; we used age 5 years as the cutoff based on our previous findings (9). With the exception of laboratory values, most differences between the two groups from the all-ages comparison held when the comparisons were limited to the early-onset children. Specifically, children with early-onset psJIA were less likely to have arthritis affecting the knee (68% vs. 85%, p = 0.012) or any large joint (89.5% vs. 98%, p = 0.02), but were more likely to have involvement of the small joints (58% vs. 22%, p < 0.001) or wrists (34% vs. 13.5%, p = 0.002). Similar differences were observed hen we compared the late-onset oJIA and psJIA patients (data not shown).
Analysis of a composite criterion for psoriatic JIA
Huemer et al. (2002) showed that a composite criterion defined by the presence of one or more of wrist arthritis, small joint involvement, or dactylitis was predictive of psJIA among children with oligoarticular-onset arthritis (16). Reversing our earlier analysis, we pooled our cases to determine whether patients meeting this criterion were more likely to have features of the psoriatic diathesis (Table 3). For the diagnosis of psJIA, the composite criterion yielded a statistically significant OR of 5.6 among the entire group and 9.96 among the early-onset cohort. In part this is definitional, as dactylitis is part of the classification criteria for psJIA. However, as further evidence that the composite criterion meaningfully identifies the psoriatic diathesis, children meeting it were also more likely to have psoriasis (OR for the entire cohort and for the early-onset patients of 3.19 and 3.23 respectively), a first-degree family history of psoriasis (OR for the entire cohort and for the early-onset patients of 5.20 and 23.6 respectively), and nail pits on examination (OR 2.91 and 4.96 for the entire cohort and early-onset group respectively). In contrast, we found no significant differences in the frequencies of features common to early-onset arthritis children (uveitis, ANA positivity, and female gender). Because the composite criterion is not neutral with respect to the case definitions, in that dactylitis is part of the classification criteria for psoriatic JIA, we modified the composite criteria to limit it to small joint or wrist involvement and repeated the above analyses. The results were largely unaffected (data not shown.)
Table 3.
Odds ratios for features of early-onset juvenile arthritis, according to presence vs. absence of the composite criterion.
| Outcome | Entire Group | Age of onset under 5 | ||
|---|---|---|---|---|
| OR, 95% CI | p-value | OR, 95% CI | p-value | |
| psJIA | 5.60, 3.31 – 9.46 | < 0.001 | 9.96, 3.98 – 24.9 | < 0.001 |
| 1° FH psoriasis* | 5.20, 2.27 – 11.9 | < 0.001 | 23.6, 3.07 – 182 | < 0.001 |
| Nail pits | 2.91, 1.60 – 5.29 | < 0.001 | 4.96, 1.76 – 14.0 | 0.001 |
| Psoriasis | 3.19, 1.75 – 5.84 | < 0.001 | 3.23, 1.18 – 8.79 | 0.017 |
| Positive ANA | 0.82, 0.53 – 1.28 | 0.384 | 0.84, 0.46 – 1.53 | 0.572 |
| Uveitis | 0.61, 0.28 – 1.31 | 0.200 | 0.61, 0.27 – 1.40 | 0.243 |
| Female gender | 1.19, 0.73 – 1.96 | 0.486 | 1.72, 0.88 – 3.37 | 0.110 |
FH = family history. 1° FH = parents and siblings.
Discussion
In this study, we compared the clinical features of children with oJIA with the features of children with oligoarticular psJIA. Not unexpectedly, both groups demonstrated a peak age of onset at age 2 – 3 years. We confirmed the findings of Huemer et al. (16), who demonstrated that children with oligoarticular psoriatic arthritis were more likely than their oJIA counterparts to have involvement of the small joints and wrist, as well as dactylitis. Correspondingly, we found that children with oligoarticular psJIA were less likely to have knee and large joint involvement. Additionally, we found that these differences extend even to the most demographically similar clinical subgroup, namely children with early-onset oligoarticular arthritis.
Our data do not demonstrate that psJIA is a pathophysiological entity in its own right, but remain compatible with the hypothesis that the psoriatic diathesis could modulate the phenotype and clinical course of patients who would otherwise have developed another subtype of JIA(2). Indeed, deep similarities between early-onset oligoarticular patients in our psoriatic and non-psoriatic groups, including age of onset, gender ratio, and ANA status, suggest that these conditions are closely related.
However, together with other published data, our findings support the contention that psoriatic JIA is rightly segregated from other types of juvenile-onset arthritis. In adults, the most persuasive evidence that psoriasic arthritis exists as an independent entity is epidemiological: psoriatic patients develop arthritis much more often than the general population and with a gender ratio more balanced than typical for rheumatoid arthritis (13, 14). Further, adult PsA can have distinctive clinical features, including dactylitis, nail pits, and a joint distribution atypical for RA (21). A comparison of psJIA with other types of JIA yields analogous contrasts in many respects, though not all. The frequency of psoriatic arthritis among patients with JIA (approximately 7% in most series, with a range extending to 20%) greatly exceeds that of psoriasis in the general pediatric population (0.5-1%), suggesting more than a chance association (10, 22, 23). The gender ratio of psJIA beginning in late childhood is balanced, though early-onset psJIA exhibits a female predominance similar to early-onset JIA (9). Finally, dactylitis and nail pits are hallmarks of psJIA, even (or especially (9)) among the youngest patients, and we confirm here the findings of Huemer et al. that patients with psJIA exhibit a distinctive pattern of joint involvement even in the subgroup that is demographically most similar to non-psoriatic JIA (16).
The biological basis for clinical differences between psJIA and oJIA remains unknown. However, similarities with adult disease – in particular nail pits and dactylitis – could be informative. Careful imaging studies in adults have implicated enthesitis in the pathogenesis of each of these clinical features, and in adult psoriatic arthritis generally (20, 24). While such studies have yet to be performed in children, it is plausible to suggest that enthesitis may be a hallmark feature of psoriatic arthritis across the age spectrum.
In this context it is remarkable that we observed either dactylitis or nail pits in a number of patients classified as non-psoriatic oligoarthritis (Table 1). This result could represent either the lack of specificity of these findings in children or the difficulty of identifying psoriatic arthritis in this population, where the classic rash may lag for 10 years or more, potentially further obscured by anti-psoriatic DMARDs such as methotrexate or TNF inhibitors (25). In adults, where psoriasis generally precedes psoriatic arthritis, the specificity of dactylitis and nail pits for psoriatic arthritis is in the range of 95-98% (21). If we have indeed misclassified these patients, we expect such misclassification to introduce a conservative bias and therefore not threaten the validity of our findings. By contrast, if these patients are removed from the analysis due to the ambiguity of their classification, then the link between involvement of wrist or small joint and psJIA almost doubles (from OR 4.72 (2.85 – 7.81) to OR 8.26 (4.77 – 14.3), increasing slightly further to 8.30 (5.16 – 13.3) if patients with dactylitis or nail pits are coded as having psJIA.
We were struck by the relatively low percentage of children with oJIA who extended to a persistent course (7.6%). Previous studies have placed the risk of extension at 40 – 50% (26-28). This in unlikely to reflect duration of follow-up, as the median duration of follow-up of the oJIA population was 3.8 years, the period of greatest risk of extension (28). It is possible that the lower risk in our study reflects bias, as children with extended oJIA may have been coded as polyarticular JIA and therefore not captured in this study. It is also possible, however, that more aggressive use of methotrexate and TNF inhibitors prevented extension in children who were at risk. Our overall findings were not altered when we limited the analysis to patients with persistent oligoarticular disease (data not shown).
This study was limited by its retrospective nature. It took place at two hospitals, with possibilities for local differences in case ascertainment, classification and treatment. In addition, differences between the two cities (Boston and Dallas) preclude meaningful demographic comparisons between the respective patient population, oJIA patients were all collected at one center (TSRHC), , while children at psJIA were collected from both centers. At TSRHC, we have, since the year 2000, prospectively collected the core data set on all of our JIA patients, but this was not the case at CHB. Furthermore some of the patients at TSRHC were initially evaluated before the year 2000; thus, much of the information about joint involvement in this study was ascertained directly through a review of the medical record rather than through the flow sheets. However, rheumatologists at both centers documented similarly detailed descriptions of joint involvement and extra-articular features, rendering major systematic bias in patient characterization and classification unlikely. In addition, to ensure consistency in interpretation of findings reported in the medical records, the same investigator (MLS) was responsible for review of the medical records of both the Boston and the Dallas cohorts.
In summary, we compared a cohort of children with oJIA with a cohort of children with oligoarticular psJIA, demonstrating clinical differences that held true even when the comparison were limited to the most demographically homogeneous subset, children with early-onset arthritis. Although the observed similarities between the groups do support the hypothesis that there are important etiologic commonalities among children with early-onset arthritis, we submit that clinical differences justify the preservation of psJIA as a distinct diagnostic entity until the biological subdivisions among patients with juvenile arthritis are better understood.
Table 2.
Comparison of patients with early-onset oJIA with those with early-onset oligoarticular-psJIA.
| Characteristics | oJIA | psJIA | p |
|---|---|---|---|
| N | 215 | 38 | n/a |
| Duration of follow- up (yrs: median, IQR) |
4.0, 1.7 – 7.3 | 5.4, 2.5 – 7.9 | 0.276 |
| Females (%) | 80.9 | 84.2 | 0.632 |
| Age of onset (yrs: median, IQR) |
2.2, 1.7 – 3.2 | 2.1, 1.4 – 2.5 | 0.094 |
| Extended course | 9.8 | 34.2 | < 0.001 |
| Affected joints (%) | |||
| Any large joint | 98.1 | 89.5 | 0.020 |
| Shoulder | 0 | 0 | n/a |
| Elbow | 9.8 | 18.4 | 0.155 |
| Wrist | 13.5 | 34.2 | 0.002 |
| Hip | 0.5 | 0 | 1.000 |
| Knee | 85.1 | 68.4 | 0.012 |
| Ankle/ subtalar | 43.7 | 60.5 | 0.055 |
| Any small joint | 22.3 | 57.9 | < 0.001 |
| MCP | 7.0 | 15.8 | 0.102 |
| Hand PIP | 17.7 | 50 | < 0.001 |
| Hand DIP | 0.5 | 10.5 | 0.002 |
| MTP | 2.3 | 13.2 | 0.008 |
| Foot IP | 3.3 | 10.5 | 0.065 |
| Extra-articular features | |||
| Psoriasis | 0 | 50.0 | < 0.001 |
| Nail pits | 2.8 | 39.5 | < 0.001 |
| Dactylitis | 20.5 | 76.3 | < 0.001 |
| Enthesitis | 0 | 7.9 | 0.003 |
| Uveitis | 23/212, 10.8% | 6/34,17.6% | 0.256 |
| Laboratory values | |||
| Baseline WBC / μl: mean, SD |
9.3, 2.7 | 9.5, 3.1 | 0.748 |
| Baseline ESR, mm/hr: mean, SD |
28.0, 21.0 | 24.2, 15.9 | 0.307 |
| Baseline platelets x 103 / μl: mean, SD |
387, 109 | 396, 83 | 0.669 |
| ANA | 164/212, 77.4% | 23/33, 69.7% | 0.335 |
| RF | 2/188, 1.1% | 0/21 | 1.000 |
| HLA-B27 | 13/113, 11.5% | 1/14, 7.1% | 1.000 |
| Treatment (%) | |||
| IA steroids | 39.5 | 26.3 | 0.121 |
| Any traditional | 40.5 | 73.7 | < 0.001 |
| DMARD | |||
| Any TNF inhibitor | 6.0 | 21.1 | 0.006 |
Acknowledgements
Grant support: Dr. Stoll and Dr. Gotte were supported by Grant Number UL1RR024982, titled, “North and Central Texas Clinical and Translational Science Initiative” (Milton Packer, M.D., PI) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Footnotes
The authors have no conflicts of interest.
References
- 1.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 2.Martini A. Are the number of joints involved or the presence of psoriasis still useful tools to identify homogeneous disease entities in juvenile idiopathic arthritis? J Rheumatol. 2003;30(9):1900–3. [PubMed] [Google Scholar]
- 3.Sullivan DB, Cassidy JT, Petty RE. Pathogenic implications of age of onset in juvenile rheumatoid arthritis. Arthritis Rheum. 1975;18(3):251–5. doi: 10.1002/art.1780180309. [DOI] [PubMed] [Google Scholar]
- 4.Kunnamo I, Kallio P, Pelkonen P. Incidence of arthritis in urban Finnish children. A prospective study. Arthritis Rheum. 1986;29(10):1232–8. doi: 10.1002/art.1780291008. [DOI] [PubMed] [Google Scholar]
- 5.Murray KJ, Moroldo MB, Donnelly P, et al. Age-specific effects of juvenile rheumatoid arthritis-associated HLA alleles. Arthritis Rheum. 1999;42(9):1843–53. doi: 10.1002/1529-0131(199909)42:9<1843::AID-ANR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Berntson L, Andersson Gare B, Fasth A, et al. Incidence of juvenile idiopathic arthritis in the Nordic countries. A population based study with special reference to the validity of the ILAR and EULAR criteria. J Rheumatol. 2003;30(10):2275–82. [PubMed] [Google Scholar]
- 7.Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford) 2007;46(6):1015–9. doi: 10.1093/rheumatology/kem053. [DOI] [PubMed] [Google Scholar]
- 8.Barnes MG, Thompson SD, Griffin TA, Grom AA, Glass DN, Colbert RA. B-cell signature in patients with JIA is associated with age of onset suggesting biologically relevant classification criteria [abstract] Arthritis Rheum. 2009;60(10):S232. [Google Scholar]
- 9.Stoll ML, Zurakowski D, Nigrovic LE, Nichols DP, Sundel RP, Nigrovic PA. Patients with juvenile psoriatic arthritis comprise two distinct populations. Arthritis Rheum. 2006;54(11):3564–3572. doi: 10.1002/art.22173. [DOI] [PubMed] [Google Scholar]
- 10.Southwood TR, Petty RE, Malleson PN, et al. Psoriatic arthritis in children. Arthritis Rheum. 1989;32(8):1007–13. doi: 10.1002/anr.1780320810. [DOI] [PubMed] [Google Scholar]
- 11.Lambert JR, Ansell BM, Stephenson E, Wright V. Psoriatic Arthritis in Childhood. Clin Rheum Dis. 1976;2:339–352. [Google Scholar]
- 12.Butbul YA, Tyrrell PN, Schneider R, et al. Comparison of patients with juvenile psoriatic arthritis and nonpsoriatic juvenile idiopathic arthritis: how different are they? J Rheumatol. 2009;36(9):2033–41. doi: 10.3899/jrheum.080674. [DOI] [PubMed] [Google Scholar]
- 13.Nigrovic PA. Juvenile psoriatic arthritis: bathwater or baby? J Rheumatol. 2009;36(9):1861–3. doi: 10.3899/jrheum.090510. [DOI] [PubMed] [Google Scholar]
- 14.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reece RJ, Canete JD, Parsons WJ, Emery P, Veale DJ. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. 1999;42(7):1481–4. doi: 10.1002/1529-0131(199907)42:7<1481::AID-ANR23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Huemer C, Malleson PN, Cabral DA, et al. Patterns of joint involvement at onset differentiate oligoarticular juvenile psoriatic arthritis from pauciarticular juvenile rheumatoid arthritis. J Rheumatol. 2002;29(7):1531–5. [PubMed] [Google Scholar]
- 17.Gotte AC, Punaro M. Children with persistent oligoarticular juvenile idiopathic arthritis (OJIA) resistant to standard therapy: an under-recognized population [abstract] Arthritis Rheum. 2009;60(10):S749. [Google Scholar]
- 18.Stoll ML, Lio P, Sundel RP, Nigrovic PA. Comparison of Vancouver and International League of Associations for rheumatology classification criteria for juvenile psoriatic arthritis. Arthritis Rheum. 2008;59(1):51–8. doi: 10.1002/art.23240. [DOI] [PubMed] [Google Scholar]
- 19.Brewer EJ, Jr., Bass J, Baum J, et al. Current proposed revision of JRA Criteria. JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Section of The Arthritis Foundation. Arthritis Rheum. 1977;20(2 Suppl):195–9. [PubMed] [Google Scholar]
- 20.Healy PJ, Groves C, Chandramohan M, Helliwell PS. MRI changes in psoriatic dactylitis--extent of pathology, relationship to tenderness and correlation with clinical indices. Rheumatology (Oxford) 2008;47(1):92–5. doi: 10.1093/rheumatology/kem315. [DOI] [PubMed] [Google Scholar]
- 21.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 22.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–41. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 23.Berntson L, Fasth A, Andersson-Gare B, et al. Construct validity of ILAR and EULAR criteria in juvenile idiopathic arthritis: a population based incidence study from the Nordic countries. International League of Associations for Rheumatology. European League Against Rheumatism. J Rheumatol. 2001;28(12):2737–43. [PubMed] [Google Scholar]
- 24.McGonagle D. Imaging the joint and enthesis: insights into pathogenesis of psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii58–60. doi: 10.1136/ard.2004.034264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoll ML, Nigrovic PA. Subgroups within Juvenile Psoriatic Arthritis: a review of the literature. Clinical and Developmental Immunology. 2006 doi: 10.1080/17402520600877802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillaume S, Prieur AM, Coste J, Job-Deslandre C. Long-term outcome and prognosis in oligoarticular-onset juvenile idiopathic arthritis. Arthritis Rheum. 2000;43(8):1858–65. doi: 10.1002/1529-0131(200008)43:8<1858::AID-ANR23>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 27.Al-Matar MJ, Petty RE, Tucker LB, Malleson PN, Schroeder ML, Cabral DA. The early pattern of joint involvement predicts disease progression in children with oligoarticular (pauciarticular) juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46(10):2708–15. doi: 10.1002/art.10544. [DOI] [PubMed] [Google Scholar]
- 28.Felici E, Novarini C, Magni-Manzoni S, et al. Course of joint disease in patients with antinuclear antibody-positive juvenile idiopathic arthritis. J Rheumatol. 2005;32(9):1805–10. [PubMed] [Google Scholar]